Summary
Within the penile microbiome, bacteria associated with seroconversion, immunology, and cells (BASIC species) enhance HIV susceptibility in heterosexual uncircumcised men by inducing foreskin inflammation and HIV target cell recruitment. This phase 1/2 clinical trial randomizes HIV-uninfected Ugandan men (n = 125) to either oral tinidazole, topical metronidazole, topical clindamycin, or topical hydrogen peroxide to define impact on ex vivo foreskin HIV susceptibility, penile immunology, and BASIC species density. Antimicrobials are well tolerated, and 116 (93%) participants complete the protocol. Topical metronidazole and oral tinidazole reduce the inner foreskin tissue density of HIV-susceptible CD4+ T cells (predefined primary endpoint). Antimicrobials also have varying but substantial effects on reducing prepuce inflammation and BASIC species density, reducing density of foreskin T cell subsets, and increasing foreskin epithelial integrity. Immune alterations correlate strongly with changes in the abundance of BASIC species. Clinical interventions targeting the penile microbiota, particularly topical metronidazole, may reduce HIV susceptibility in uncircumcised men.
Keywords: HIV transmission, penile microbiome, clinical trial, antimicrobials, Uganda, genital immunology
Graphical abstract

Highlights
-
•
Penile BASIC bacteria are associated with inflammation and increased HIV risk
-
•
This trial randomizes men to one of four antimicrobials prior to penile circumcision
-
•
Topical metronidazole and oral tinidazole reduce foreskin HIV susceptibility
-
•
Topical clindamycin causes the greatest reduction in BASIC species density
Within the penile microbiome, BASIC bacteria are linked with tissue inflammation and increased HIV risk. In this randomized trial, Galiwango et al. demonstrate that the four antimicrobials tested have variable effects on penile BASIC density, immunology, and HIV susceptibility. The most consistent effects are mediated by topical metronidazole.
Introduction
There were 1.3 million new HIV infections in 2022, more than three times higher than the Joint United Nations Programme on HIV/AIDS (UNAIDS) goal of 375,000 by 2025, despite the availability of several highly effective biomedical tools for HIV prevention.1 Our most effective tools for HIV prevention are antiretroviral drugs, which when used appropriately as treatment can render a person living with HIV non-infectious2 and when used as pre-exposure prevention (PrEP) can reduce the risk of acquisition by >80%.3 However, the global rollout of PrEP faces tremendous challenges in terms of cost, political will, and acceptability, particularly among individuals who may not perceive themselves to be at risk.1 Voluntary medical male circumcision (VMMC; circumcision) is a cheap one-time procedure that reduces HIV risk by over 50% in heterosexual men.4 UNAIDS therefore aimed to perform 25 million circumcision procedures by 2020,5 but this target was missed by 7 million,6 with an important barrier being that many men are hesitant to undergo surgery.7 Exploring alternative, non-surgical, HIV prevention tools for uncircumcised men could enhance HIV prevention efforts in resource-constrained regions.
In uncircumcised men the sub-preputial space is home to a diverse community of bacteria, and the composition of this microbiome changes profoundly after circumcision, with a reduction in overall bacterial load and a shift from anaerobes to common skin-associated aerobes such as corynebacteria and staphylococci.8 HIV risk and foreskin inflammation in uncircumcised men are directly linked to the density of six bacteria associated with seroconversion, immunology, and cells (BASIC) species, namely Prevotella bivia, Prevotella disiens, Peptostreptococcus anaerobius, Dialister micraerophilus, Dialister propionicifaciens, and a genetic near neighbor of Dialister succinatiphilus.9 Based on these observations, BASIC species are hypothesized to increase HIV susceptibility through the induction of penile inflammation, with an elevated tissue density of CD4+ T cells and elevated levels of multiple soluble immune factors, including the chemoattractant cytokine interleukin (IL)-8, proinflammatory cytokines IL-1α/β, and the biomarker of epithelial disruption soluble E-cadherin (sEcad).9,10
There is partial overlap of penile BASIC species with those that cause bacterial vaginosis (BV),11 which is characterized by an increased density and diversity of vaginal anaerobes. Clinical treatment approaches for BV are well defined, and treatment-induced reduction in the density of BV-associated bacteria rapidly reduces vaginal inflammation and epithelial disruption.12,13 However, there are important differences in the species that cause dysbiosis in the vagina and penis,11 and, although antibiotics have been shown to alter the penile microbiome,14 optimal treatment approaches for penile dysbiosis have not been defined and the effect on penile inflammation is unknown. If antimicrobial treatment can reduce BASIC species and inflammation without circumcision, this might provide an alternative for HIV prevention in uncircumcised heterosexual men who are hesitant to undergo a surgical procedure.
We present findings from a phase 1/2 randomized clinical trial that assessed the effects of four widely available antimicrobial products on penile immunology, bacteria, and tissue HIV susceptibility among Ugandan men seeking elective circumcision.15 The antimicrobials tested were tinidazole, an oral imidazole which is effective as BV treatment after a short 2-day course, permitting the first dose to be given as directly observed therapy and reducing potential side effects associated with oral metronidazole such as metallic taste and alcohol intolerance16; topical metronidazole gel and clindamycin cream, both standard treatments for BV17; and topical hydrogen peroxide gel, an antiseptic well tolerated at other mucosal surfaces such as the mouth.18
Results
Demographics and follow-up
A total of 127 men self-identified as interested in participation after reading posted fliers and hearing about the study from research staff in the Entebbe General Hospital Circumcision Clinic. None had been involved in previous research studies or had used antimicrobials within the past month. Two screened men were excluded, one due to positive HIV serology (referred for HIV care) and one due to phimosis (underwent circumcision). A total of 125 HIV-negative uncircumcised males were enrolled, 25 in each of the five trial arms (Table 1). The median age of participants was 24 years (range, 18–49 years), and just over half were married or cohabiting with a female partner. All men reported ever having had insertive vaginal sex, and 47 (38%) reported sexual intercourse within the week preceding enrollment. Antibiotic use within the past 3 months was reported by 32 participants (25.6%), and most men (114; 91%) reported retracting the foreskin to wash the penis at least daily. There were no significant differences between the trial arms in socio-behavioral parameters or prior antibiotic intake. All participants randomized to immediate circumcision (n = 25) underwent the procedure and provided penile swabs and tissues for immune analysis, and of those randomized to one of the 4 intervention arms, 98% (98/100) returned for swabs at the end of the intensive treatment phase and 91% (91/100) returned at 4 weeks for circumcision and to provide samples for immune analysis. Completion rates did not vary by intervention arm (Figure 1).
Table 1.
Demographic and behavioral characteristics by trial arm
| Trial arm |
||||||
|---|---|---|---|---|---|---|
| Control (n = 25)< | Oral tinidazole (n = 25) | Topical clindamycin (n = 25) | Topical H2O2 (n = 25)< | Topical metronidazole (n = 25) | Overall (n = 125)< | |
| Age, years (mean; range)< | 28 (18–49) | 25 (18–39) | 27 (18–40) | 25 (18–40) | 24 (18–36) | 26 (18–49) |
| Secondary or higher education (n, %) | 18 (72%) | 15 (60%) | 16 (64%) | 13 (52%) | 18 (72%) | 80 (64%) |
| Married/cohabiting (n, %)< | 16 (64%) | 13 (52%) | 14 (56%) | 13 (42%) | 11 (44%) | 67 (54%) |
| Recent antibioticsa (n, %)< | 5 (20%) | 6 (24%) | 9 (36%) | 5 (20%) | 7 (28%) | 32 (25.6%) |
| Always use condom with new partner (n, %)< | 11 (44%) | 8 (32%) | 10 (40%) | 6 (24%) | 8 (32%) | 43 (34.4%) |
| >1 sexual partner in past 6 months (n, %) | 11 (44%) | 11 (44%) | 10 (40%) | 14 (56%) | 9 (36%) | 55 (44%) |
| Vaginal sex in past week (n, %) | 11 (44%) | 8 (32%) | 8 (32%) | 10 (40%) | 10 (40%) | 47 (38%) |
| Wash penis at least daily (n, %) | 24 (96%) | 19 (76%) | 25 (100%) | 22 (88%) | 24 (96%) | 114 (91%) |
| Vaginal sex in study period (n, %)b | N/A | 12/22 (55%) | 14/22 (64%)< | 15/24 (63%) | 11/22 (50%)< | 52/90 (58%) |
Defined as any reported antibiotic use within the past 3 months.
Data only available for participants randomized to an intervention arm, and who completed full protocol.
Figure 1.
Antimicrobials and penile immunology recruitment and follow-up
Flow chart illustrates follow-up protocols and retention by intervention groups.
Although no participants with symptoms of a sexually transmitted infection (STI) were recruited, batched first-void urine diagnostics subsequently demonstrated an asymptomatic STI in 6 participants (C. trachomatis in n = 6, with N. gonorrhoeae co-infection in n = 1), 2 from the oral tinidazole arm, 3 from the topical hydrogen peroxide arm, and one from the topical clindamycin arm. One of these participants never returned for surgery and could not be reached for treatment, while the remaining 5 received treatment after circumcision as per Uganda National Guidelines. Participants with an asymptomatic STI were included in the overall trial analysis, with exclusion in a subsequent sensitivity analysis (see in the following). Self-reported product compliance data were collected for all participants presenting for visit 3 (circumcision) in the topical product groups (n = 68): 63 men reported 100% compliance with the study product, and 5 less than complete compliance (clindamycin, n = 1; metronidazole n = 2; hydrogen peroxide n = 2). All participants reported prior vaginal sex at enrollment, but many were not currently in a sexual relationship. Sexual behavior at follow-up was provided by 90/91 participants in the treatment arms. Vaginal sex during the treatment phase was reported by 52/90 (58%) with no difference by intervention groups (Table 1); oral sex during the treatment phase was only reported by 2 participants, and no participants reported anal sex.
Antimicrobial treatments and HIV pseudovirus entry into foreskin-derived CD4+ T cells
The primary trial endpoint was predefined as entry of a clade A CCR5-tropic HIV pseudovirus into inner foreskin-derived CD4+ T cells15 (Figure 2A). Assays were performed for all 116 participants where VMMC was performed, although no data could be analyzed for 2 participants (both controls) due to inadequate cell numbers. Both the proportion and tissue density of foreskin-derived CD4+ T cells susceptible to HIV pseudovirus entry were assessed, with the latter extrapolated from CD3 tissue immunofluorescence (IF) microscopy (Figure 2B). Overall, the median percentage of foreskin-derived CD4+ T cells entered by the pseudovirus was 10.05% (range, 0.82%–33.82%), and the median tissue density was 7.06 cells/mm2 (range, 0.13–83.52 cells/mm2). No trial arm demonstrated a reduced proportion of CD4+ T cells susceptible to viral entry compared to the control group, although this tended to be lower in the oral tinidazole arm (median 9.68% TZ vs. 12.13% control, Mann-Whitney p = 0.053; Figure 2C). However, the tissue density of CD4+ T cells entered by the pseudovirus (calculated by multiplying flow cytometry proportions by IF CD3+ T cell density) was significantly lower in both the oral tinidazole arm (median 4.19 vs. 10.43 cells/mm2 in controls; p = 0.015) and the topical metronidazole arm (4.40 vs. 10.43 cells/mm2; p = 0.0008; Figure 2D), but not the topical clindamycin or hydrogen peroxide arms. These results remained unchanged when participants with an asymptomatic STI were excluded (tinidazole p = 0.025, clindamycin p = 0.281, hydrogen peroxide p = 0.103, metronidazole p < 0.001).
Figure 2.
Impact of antimicrobial interventions on HIV pseudovirus entry into foreskin-derived CD4+ T cells
Clade A HIV entry into foreskin-derived mononuclear CD4+ T cells was assessed by blinded investigators using a direct CCR5-tropic viral entry assay.
(A) Representative flow cytometry plots show entry for (i) mock control (no pseudovirus) and (ii) clade A pseudovirus after gating on inner foreskin-derived lymphocytes, singlets, live cells, and CD4+ CD3+ T cells.
(B) CD3+ T cell density was calculated using immunofluorescence staining of inner foreskin tissues.
(C and D) Co-primary trial endpoints were (C) the relative proportion and (D) the tissue density of inner foreskin CD4+ T cells susceptible to entry by a clade A HIV pseudovirus. TZ, oral tinidazole; CDM, topical clindamycin; MTZ, topical metronidazole; HP, topical hydrogen peroxide. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Impact of antimicrobials on penile soluble immune parameters and tissue E-cadherin
The penile immune parameter most strongly associated with increased HIV acquisition in prospective clinical studies was the chemokine IL-8.9,19 In addition, IL-1β is strongly correlated with vaginal dysbiosis12 and sEcad is a validated marker of penile epithelial disruption.10 Therefore we next focused on the impact of antimicrobial interventions on levels of IL-8, IL-1β, and sEcad in coronal sulcus swabs and explored levels of other immune factors abundant on the coronal sulcus (IL-1α, macrophage inflammatory protein-1β [MIP-1β], resistin, tissue inhibitor of metalloproteinases-1 [TIMP-1], matrix metalloproteinase-9 [MMP-9], and vascular endothelial growth factor [VEGF]). Immune factors were assayed at baseline, after the intensive treatment phase, and at the 4-week circumcision visit.
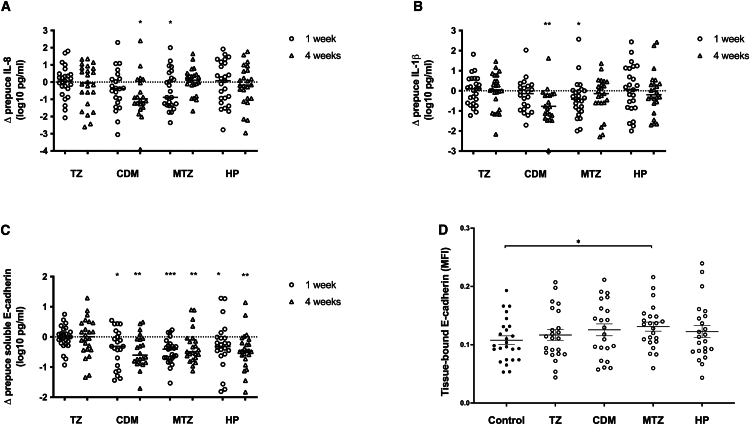
Oral tinidazole had no effects on penile soluble immune factors at either time point, while the three topical antimicrobials had varying impacts (Figures 3A–3C). The most striking change was a decrease in the coronal sulcus levels of sEcad, after both the intensive and maintenance treatment phases. Clindamycin induced reductions in sEcad at both the time points (from median 4.21 at baseline to 3.70 log10 pg/mL at week 1, p = 0.042, and 3.77 log10 pg/mL at week 4, p = 0.001), as did topical metronidazole (4.19–3.83 log10 pg/mL, p = 0.0006; 3.68 log10 pg/mL, p = 0.005) and topical hydrogen peroxide (4.45–3.86 log10 pg/mL, p = 0.011; 4.04 log10 pg/mL, p = 0.004). There were less consistent changes in levels of proinflammatory cytokines: topical clindamycin reduced levels of both IL-1β (0.74 vs. 0.26 log10 pg/mL, Wilcoxon p = 0.007) and IL-8 (1.57 vs. 0.99 log10 pg/mL, p = 0.028) at week 4 only, while topical metronidazole significantly reduced both soluble IL-1β (1.07 vs. 0.35 log10 pg/mL, p = 0.011) and soluble IL-8 (1.44 vs. 0.82 log10 pg/mL, p = 0.042) at week 1 only.
Figure 3.
Impact of antimicrobial interventions on penile soluble immune factors and tissue-bound E-cadherin
(A–C) Change in soluble immune factor levels in the penile prepuce (log10 pg/mL) is shown from baseline to completion of intensive antimicrobial application (either 2 days or 7 days; circles) and to the end of the maintenance phase, prior to circumcision (triangles). Soluble immune factors shown are (A) IL-8, (B) IL-1β, and (C) soluble E-cadherin.
(D) Levels of tissue-bound E-cadherin (mean fluorescence intensity, MFI) in the inner foreskin were quantified at the time of VMMC. TZ, oral tinidazole; CDM, topical clindamycin; MTZ, topical metronidazole; HP, topical hydrogen peroxide. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Other proinflammatory markers assessed included IL-1α, MIP-1β, resistin, TIMP-1, MMP-9, and VEGF; these immune factors were exploratory, and so results are not shown in Figure 3. Clindamycin significantly reduced the soluble levels of resistin at both the time points (2.17 vs. 1.40 log10 pg/mL at week 1, p = 0.007; 1.19 log10 pg/mL at week 4, p = 0.007), while hydrogen peroxide reduced resistin levels at week 4 only (2.49 vs. 1.63 log10 pg/mL, p = 0.021). VEGF levels were reduced in the coronal sulcus of participants receiving metronidazole at week 1 only (2.19 vs. 1.66, log10 pg/mL, p = 0.017). Levels of other immune factors in the coronal sulcus did not vary after any of the antimicrobial interventions.
The reduced levels of sEcad seen in penile secretions from intervention arms would be expected to reflect enhanced inner foreskin epithelial integrity. Therefore, IF microscopy was used to assess the mean fluorescence intensity of tissue-bound E-cadherin in the foreskin tissues that were obtained at the time of circumcision, with an increase in tissue-bound E-cadherin indicating increased epithelial integrity. Tissue-bound E-cadherin was increased in the topical metronidazole arm compared to controls (median 0.135 vs. 0.098, p = 0.035; Figure 3D), with no significant changes in other intervention arms (all p > 0.1).
Foreskin CD4+ T cell subset proportions and density
The inner foreskin is enriched for CD4+ T cell subsets that are highly HIV susceptible, including Th17 cells and CD4+ T cells expressing C-C chemokine receptor 5 [CCR5].20 We next compared both the proportion and tissue density of key T cell subsets between control and antimicrobial groups. Compared to controls, there were no significant differences in any treatment arm in the percentage of inner foreskin-derived CD3+ T cells expressing CD4, CD4/CCR6 (i.e., Th17 cells), or CD4/CCR5 (all p > 0.05). Next, the density of these T cell subsets in the inner foreskin was assessed by applying the T cell subset proportions (%) from flow cytometry to the tissue density of CD3+ T cells that was directly measured by IF microscopy. Compared to controls, the density of CD3+ T cells in the inner foreskin by IF was lower in participants from all intervention arms except topical hydrogen peroxide (control median 203.97 CD3+ T cells/mm2 vs. 135.50/mm2 TZ, p = 0.015; 134.67/mm2 CDM, p = 0.005; 85.58/mm2 MTZ, p < 0.001; 173.50/mm2 HP, p = 0.96; Figure 4A). The calculated inner foreskin tissue density of CD4+ T cells was reduced in the topical clindamycin and metronidazole arms (control median 81.86 CD4+ T cells/mm2 vs. 55.01/mm2 TZ, p = 0.055; 48.34/mm2 CDM, p = 0.017; 39.09/mm2 MTZ, p = 0.003; 72.93/mm2 HP, p = 0.286; Figure 4B), with similar findings for the inner foreskin density of Th17 cells (control median 45.21 Th17 cells/mm2 vs. 17.14/mm2 TZ, p = 0.055; 20.24/mm2 CDM, p = 0.017; 14.89/mm2 MTZ, p = 0.003; 28.95/mm2 HP, p = 0.286; Figure 4C) and CD4/CCR5+ T cells (control median 37.89 CD4/CCR5+ T cells/mm2 vs. 15.04/mm2 TZ, p = 0.033; 19.60/mm2 CDM, p = 0.071; 17.29/mm2 MTZ, p = 0.016; 34.94/mm2 HP, p = 0.602; Figure 4D).
Figure 4.
Impact of antimicrobial interventions on the density of T cell subsets in the inner foreskin
The tissue density of key T cell subsets in the inner foreskin was calculated based on immunofluorescence staining of tissues obtained during VMMC to define CD3+ T cell density, combined with inner foreskin T cell proportions defined by flow cytometry. Subsets illustrated are (A) CD3+ T cells, (B) CD4+ T cells, (C) Th17 cells, and (D) CCR5+ CD4+ T cells. TZ, oral tinidazole; CDM, topical clindamycin; MTZ, topical metronidazole; HP, topical hydrogen peroxide. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Impact of antimicrobial interventions on penile BASIC and control bacterial species
The density of BASIC species in the coronal sulcus was previously linked to HIV acquisition in uncircumcised men,9 while the density of more typical skin bacteria (corynebacteria and staphylococci, here defined as control bacterial genera) was not. We hypothesized that the antimicrobial interventions would decrease coronal sulcus density of BASIC species but not control genera (Figure 5). At baseline the median coronal sulcus density of BASIC species was 5.07 log10 copies/swab, with no difference between study arms (control 4.90, oral tinidazole 4.54, topical clindamycin 5.18, topical metronidazole 5.41, and topical hydrogen peroxide 4.84 log10 copies/swab; all p > 0.1). Oral tinidazole had no impact on the coronal sulcus density of either BASIC or control bacteria, either immediately after treatment or at 4 weeks, while all topical antimicrobials dramatically lowered the absolute density of BASIC bacteria at both week 1 and week 4. The greatest reductions in BASIC density were seen for clindamycin (reductions of 4.99 and 3.61 log10 copies/swab at 1 and 4 weeks, respectively; both p < 0.001), with significant reductions also by metronidazole (reductions of 2.49 and 1.04 log10 copies/swab at 1 and 4 weeks; p < 0.001 and p = 0.008, respectively) and hydrogen peroxide (reductions of 1.40 and 0.61 log10 copies/swab at 1 and 4 weeks, p = 0.001 and p = not significant [NS], respectively; Figure 5A). When pooling the topical antimicrobial groups, greater reductions in the coronal sulcus density of BASIC species were seen after the intensive phase of twice-daily topical application (week 1) than after the maintenance phase of twice-weekly application (week 4; 2.64 vs. 1.63 log10 copies/swab reduction; p = 0.001).
Figure 5.
Impact of antimicrobial interventions on the absolute abundance of BASIC and control species in the coronal sulcus
Change in bacterial density in the penile prepuce (log10 bacterial copies/swab) is shown from baseline to completion of intensive antimicrobial application (either 2 days or 7 days; circles), and to the end of the maintenance phase, prior to circumcision (triangles). Analysis was focused on (A) the combined density of the 6 BASIC species, specifically Peptostreptococcus anaerobius, Prevotella bivia, Prevotella disiens, Dialister propionicifaciens, Dialister micraerophilus, and a genetic near neighbor of Dialister succinatiphilus, and (B) the combined density of two bacterial genera not previously linked to HIV risk, Staphylococcus and Corynebacterium (control species). TZ, oral tinidazole; CDM, topical clindamycin; MTZ, topical metronidazole; HP, topical hydrogen peroxide. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Interestingly, metronidazole application resulted in an increase in the coronal sulcus estimated absolute density of control bacteria at both week 1 (median 4.00 vs. 5.38 log10 copies/swab; p = 0.004; Figure 5B) and week 4 (3.80 vs. 5.23 log10 copies/swab; p = 0.005); likewise, the estimated absolute density of control bacteria tended to increase in the topical clindamycin group at week 1 (3.73 vs. 4.72 log10 copies/swab; p = 0.101; Figure 5B) and increased significantly at week 4 (3.82 vs. 5.10 log10 copies/swab; p = 0.031). No changes in the density of control bacteria were seen in the oral tinidazole or topical hydrogen peroxide groups (both p > 0.1).
Baseline association between penile immunology and the abundance of BASIC species
Overall, antimicrobial interventions reduced HIV entry into target cells, reduced soluble and cellular inflammation, improved epithelial integrity, and reduced BASIC (but not control) species. We hypothesized that effects on penile microbiota were causing these immune changes. To test this hypothesis, we first examined the relationship of soluble immune factors (sEcad, IL-1β, and IL-8) in the coronal sulcus with the abundance of BASIC bacteria at baseline (pre-intervention) and found that each immune factor was correlated with an increased abundance of BASIC species (all Spearman rho ≥0.25, p ≤ 0.005). In contrast, the abundance of control species correlated with reduced sEcad (Spearman’s rho −0.27, p = 0.003), but not with levels of other immune factors (p > 0.05). We then assessed the association between BASIC species and the density of HIV-susceptible T cell subsets in the inner foreskin tissue at the time of circumcision (i.e., at the baseline visit for controls and at the 4-week visit for intervention groups). Absolute abundance of BASIC species correlated positively with the density of CD4+ T cells, Th17 cells, and CD4/CCR5+ T cells (all: Spearman rho >0.25, p < 0.01). No cell associations were observed with control bacteria (all: Spearman rho <0.15, p > 0.1).
Changes in penile bacteria density and correlation with coronal sulcus immune parameters
To further explore the possibility that intervention-associated immune changes were driven by BASIC species, we examined whether changes in the abundance of bacteria between baseline and week 4 correlated with changes in soluble immune factors. The magnitude of decrease in BASIC species correlated significantly with the reduction in each of sEcad, IL-1β, and IL-8 (all: Spearman rho ≥0.35, p < 0.05; Figures 6A–6C). In addition, the magnitude of change in BASIC species correlated with changes in all other soluble immune factors, including resistin (Spearman rho 0.429; p < 0.001), MIP1β, MMP-9, TIMP-1, and VEGF (all: Spearman rho >0.2, p < 0.05). Increases in the absolute abundance of control species were not significantly associated with immune changes (all p > 0.1, data not shown).
Figure 6.
Changes in BASIC species abundance correlate with alterations in penile soluble immune parameters
Changes over the entire study period in the prepuce density of BASIC species were strongly correlated with changes in prepuce levels of the soluble immune factors (A) sEcad, (B) IL-1β, and (C) IL-8. The changes (delta) in immune parameters (y axes) and BASIC species density (x axes) reflect log10-transformed changes in these parameters over the 4-week period from baseline to study completion.
Safety and tolerability
Among 100 participants randomized to an intervention arm, 91 completed the study (Figure 1); no follow-up or tolerability data were available for the 9 participants who did not complete the protocol (n = 2 TZ; n = 3 CDM; n = 2 MTZ; n = 2 HP). Minor adverse effects (nausea and weakness lasting ≤3 days) were reported by 2/23 (8.7%) participants in the tinidazole arm, and no specific side effects were reported in other arms. No participant reported that application of topical product had an adverse effect on sexual relations, and one participant in the clindamycin group reported improved intercourse with product use. Participants with current female sexual partners were directly asked whether their female partner had expressed concerns regarding the study product, and no issues were reported.
Discussion
Inflammatory cytokines and BASIC species in the coronal sulcus are associated with HIV acquisition in uncircumcised men19,21 and also correlate with an increased density of CD4+ T cell target cells in the inner foreskin.9 Circumcision removes the HIV-susceptible tissues of the inner foreskin and reduces both inflammatory cytokine levels and BASIC species in the coronal sulcus,8,19 but some men are reluctant to undergo this surgical procedure.7 This phase 1/2 clinical trial assessed the impact of four antimicrobial treatments on penile immune and bacterial correlates of HIV risk in uncircumcised Ugandan men.15 Both an oral antibiotic (tinidazole) and a topical antibiotic (metronidazole) reduced the density of CD4+ T cells susceptible to HIV pseudovirus entry in the inner foreskin, our predefined primary study endpoint. All three topical antimicrobial interventions dramatically reduced the density of penile BASIC bacteria, with variable reductions in penile inflammatory cytokines and tissue T cell density; in general the most substantial microbial effects were seen with topical clindamycin and the strongest immune effects with topical metronidazole. There was a significant decrease in coronal sulcus concentration of sEcad in all three topical intervention groups, indicating enhanced epithelial integrity10; while increased inner foreskin tissue E-cadherin density was only demonstrated by IF microscopy in the metronidazole group, similar trends were seen for all three topical agents and the lack of significance may relate to statistical power for this secondary endpoint. Overall the strong, prospective association between reduced penile BASIC species, reduced penile inflammation, and reduced biomarkers of epithelial disruption provides further evidence that these key bacterial species are causally related to penile immunology and HIV susceptibility.
This trial demonstrates that clinical interventions targeting the penile microbiome can reduce penile density of BASIC species and have a beneficial effect on penile immune correlates of HIV susceptibility, but the duration of these immune benefits is unknown. Importantly, penile microbiome changes returned to baseline within 12 weeks of antibiotics in a recent pilot clinical trial of concurrent male partner treatment for BV,14 and in keeping with this we observed that BASIC bacterial density began to increase during the “maintenance” phase of antimicrobial treatments. Therefore, it will be important to perform longer-term studies in men to define the optimal approach to induce sustained reductions in BASIC species. Furthermore, it is not known what degree of change in immune or microbial parameters will have an impact on real-life HIV acquisition; the earlier cohort studies linking HIV risk to these factors9,19 collected samples (in retrospect) using suboptimal sampling techniques for immune and microbial analysis, and direct extrapolation of concentrations from these studies would not be valid.
Certain subsets of CD4+ T cells are preferential HIV targets. For instance, not only is there enhanced entry of HIV into Th17 cells22 but also Th17 cells are rapidly depleted from cervicovaginal tissues after HIV/SIV infection.23,24 Therefore the penile microbiome might be expected to increase HIV susceptibility—and interventions targeting the penile microbiome to reduce it—either by altering the proportion of highly susceptible CD4+ T cells in foreskin tissue or by simply reducing the total tissue density of CD4+ T cells. A previous observational study of the penile microbiome demonstrated that BASIC species were associated with increased total density of CD4+ T cells without skewing of subset frequencies.9 In keeping with this, in the current trial we observed that antimicrobial treatments reduced the density of key CD4+ T cell subsets through a reduction in the overall tissue T cell density, rather than through any skewing of T cell subset proportions.
While the most pronounced effect of the antimicrobials tested in this trial was to reduce the density of BASIC species, both topical metronidazole and clindamycin were associated with significant increases in the density of control penile bacteria, including Corynebacterium spp. that have been previously associated with reduced penile inflammation.11 Recent studies of BV demonstrated that the provision of “optimal” vaginal species after antimicrobial treatment, specifically intravaginal treatment with a Lactobacillus crispatus live biotherapeutic, both reduced the frequency of clinical BV recurrence25 and provided a more sustained reduction in vaginal inflammation.26 While it would be interesting to examine the effect of probiotics or live biotherapeutics containing Corynebacterium spp. after therapeutic disruption of the penile microbiome, it is important to remember that the urethra is an alternative site for HIV acquisition in uncircumcised men,27,28 and so potential immune and microbial effects at this tissue site must also be considered.
In conclusion, we demonstrate in this phase 1/2 clinical trial that four different antimicrobial interventions had heterogeneous but substantial effects on penile cellular HIV susceptibility, coronal sulcus cytokine/chemokine levels, and penile epithelial integrity and that these benefits were directly associated with reductions in the density of BASIC species previously linked to HIV acquisition and penile inflammation. The most consistent immune effects were seen with topical metronidazole, but all the antimicrobials tested appeared to be safe and well tolerated. A more granular analysis of microbiome and immune effects may guide the design of future HIV prevention trials in uncircumcised men who are unwilling to undergo circumcision.
Limitations of the study
Despite the clear central findings of this clinical trial, there are weaknesses to our design that merit discussion, and future questions that remain to be addressed. Immune outcomes were focused on immune factors on the coronal sulcus and HIV-susceptible T cells in the inner foreskin, since the former were directly linked to real-world HIV acquisition,9 putatively through their association with the latter cell subsets. We did not assess impact of antimicrobial treatments on the immunology or microbiology of the distal penile urethra, which also contains HIV-susceptible target cells28 and may be colonized by sexually acquired bacteria from female partner(s)29; this would be an interesting focus for future studies. Our primary microbial analysis here was deliberately focused on the overall density of BASIC bacteria and skin-associated control genera. Subsequent analyses will need to define the effects of antimicrobial treatments on individual bacterial species and will provide a more granular understanding of immune-bacterial and tissue-bacterial interactions; for instance, it is plausible that differential antimicrobial effects on individual bacterial species may explain why the most substantial microbial effects were seen with topical clindamycin and yet the strongest immune effects with topical metronidazole. Not only do antimicrobial interventions have dramatic effects on vaginal bacteria and immunology12,13 but also there is sharing of microbiome components between the penis and vagina of heterosexual couples.29 Although stable female partners were invited to participate, since only one female accepted, we were unable to assess the impact of these penile antimicrobial treatments on the vaginal microbiome or immune milieu of participants’ female sexual partners.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| LD-NIR APC-Cy7 | Invitrogen | L34993 |
| CD3-AF700 | Biolegend | 317340 |
| CD4-BV650 | BD Horizon | 563875 |
| Beta 7-PerCP Cy5.5 | Biolegend | 121008 |
| Beta 1-APC | Biolegend | 303008 |
| Alpha 4-PE-Green | Biolegend | 304304 |
| Alpha E-PE-Cy7 | Biolegend | 350212 |
| CD69-BV786 | Biolegend | 310932 |
| CCR5-PE-CF594 | BD Horizon | 562456 |
| CCR6-BV605 | Biolegend | 353420 |
| CD38-PerCP Cy5.5 | Biolegend | 303522 |
| CD25-AF647 | Biolegend | 356128 |
| CD127-BV421 | Biolegend | 351310 |
| CCR4-PE-Cy7 | Biolegend | 359410 |
| CXCR3-BV510 | Biolegend | 353725 |
| CCR6-BV605 | Biolegend | 353420 |
| CCR5-PE-CF594 | BD Horizon | 562456 |
| CCR10-PE-Green | R&D Systems | FAB3478P-100 |
| HLA-DR-BV786 | BD Horizon | 564041 |
| Anti-CD3 | Abcam | ab21703 |
| Anti-E-cadherin | BD | 610182 |
| Fluoromount G mounting media with DAPI | Thermofisher | 00-4959-52 |
| Bacterial and virus strains | ||
| pCMV4-BlaM-Vpr | Cavrois M et al.30 | Addgene, catalog 21950 |
| Q23Δenv gfp nef | Overbaugh lab. | Humes D et al.31 |
| Q259d2.17env | Overbaugh lab. | Long E et al.32 |
| Biological samples | ||
| Blood, penile swabs, penile tissues | Entebbe General Hospital, Uganda | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Collagenase type 1 | Gibco | 17100 |
| DNAse | Invitrogen | 18047–019 |
| pAdVAntage | Promega Corporation | E1711 |
| CO2-independent media | Gibco | 18045088 |
| Lysozyme | Sigma-Alrich | L6876-1G |
| Mutanolysin | Sigma-Alrich | M4782 -5KU |
| Lysostaphin | Sigma-Aldrich | SAE0091-2MG |
| Critical commercial assays | ||
| Multiplex chemiluminescent U-Plex assay (IL-1α, IL-1β, resistin, IL-8, MIP-1β, sEcad, MMP-9, TIMP-1, VEGF) | Meso Scale Discovery (MSD) | N/A (custom) |
| MagMax DNA Multi-Sample Ultra 2.0 Kit | Applied BioSystems | A36570 |
| LiveBLAzer™ FRET-B/G Loading Kit with CCF2-AM | Invitrogen | K1032 |
| Deposited data | ||
| Immune data, BASIC/control taxa data | Mendeley | https://doi.org/10.17632/7bymvktmc3.1 |
| Clinical trial registration | ClinicalTrials.gov | NCT03412071 |
| Software and algorithms | ||
| DADA2 v1.10 modules | https://benjjneb.github.io/dada2/ | Callahan B et al.33 |
| Naive Bayesian Classifier (v.2.12) | https://bioweb.pasteur.fr/packages/pack@rdp_classifier@2.12 | Wang Q et al.34 |
| Inhouse version of the Naive Bayesian Classifier | Liu lab. | Liu CM et al.35 |
| SPSS Statistics | IBM | Ver. 29.0.1.0 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Rupert Kaul, rupert.kaul@utoronto.ca.
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Immune and microbial data have been deposited at Mendeley (https://doi.org/10.17632/7bymvktmc3.1) and are publicly available as of the date of publication. Microscopy data reported in this paper will be shared by the lead contact upon request.
-
•
This study did not generate any code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
Human participants
Biological males over the age of 18 years. The study was approved by the UVRI Research and Ethics Committee (GC/127/17/07/613), Uganda National Council for Science and Technology (HS2299), Uganda National Drug Authority (clinical trial certificate #CTA0041), and University of Toronto Research Ethics Board (RIS #35254). A sample size of 25 participants per study group was selected to provide 80% power to identify interventions associated with ≥33% reduced virus entry.15 Participants meeting selection criteria were randomized to experimental groups.
Method details
Study design
This was a registered, open label, randomized clinical trial (ClinicalTrials.gov #NCT03412071) that enrolled participants from December 2017 to November 2018 through the UVRI-IAVI HIV Vaccine Program and Entebbe General Hospital in Uganda.15 Although recruitment began in December 2017, due to an administrative delay the study was not formally registered at ClinicalTrials.gov until January 2018; no changes were made in the protocol or study endpoints during that period.
Study participants
Participants were 125 HIV-negative uncircumcised Ugandan males seeking VMMC at Entebbe General Hospital for HIV prevention, who were willing to consent to a four-week delay in surgery and random assignment of a treatment intervention. Inclusion criteria were lack of circumcision, age ≥18 years, HIV seronegative, no symptoms or signs of a sexually transmitted infection, and availability for the duration of the study period (4 weeks). Interested men received voluntary HIV counseling and testing as part of circumcision preparation, and those with STI symptoms were referred for Ministry of Health approved syndromic treatment.
Randomization and masking
Information regarding the study, including selection criteria, was posted in the Circumcision Clinic at the Entebbe General Hospital. Interested males met with a research coordinator for further information and HIV serology. Eligible, consenting men were randomized to one of five groups (n = 25/group): (i) control, receiving immediate circumcision, and four intervention groups that deferred surgery for 4 weeks while receiving either (ii) oral tinidazole (TZ) 2g by mouth daily for two days, or topical (iii) 0.75% metronidazole gel (MTZ), (iv) 2% clindamycin cream (CDM) or (v) 1% hydrogen peroxide gel (HP), applied underneath the foreskin twice daily for the first week then twice weekly for three weeks. Participants were instructed to clean and dry their hands, and then to express cream/gel onto their index finger (approximate volume of three peanut seeds) while retracting the foreskin with their other hand; the cream/gel was then generously applied to the head of the penis and under the foreskin prior to protracting the foreskin to cover the glans. The rationale for an intensive and less intense application phase for the topical products was concern regarding the feasibility of long-term intensive product application.
For randomization, 125 preprinted forms with a study ID and study group were computer generated and sealed in opaque envelopes prior to start of enrollment (25/group). Envelopes were shuffled and stored in a folder; each participant removed an envelope and opened it with the study staff. While clinic staff and participants were not masked to product allocation, all laboratory and immune assays were performed by personnel unaware of participant status.
Procedures
At baseline, all participants completed a social-behavioral questionnaire and provided peripheral blood; a self-collected first-catch urine specimen; and clinician-collected swabs from the coronal sulcus (2), inner foreskin (2), and urethral meatus (1). For participants in the intervention groups, follow-up sampling was performed at the time of any intervention dose change (day #3 for tinidazole arm, day #8 for topical treatment arms) and at day #28 (week 4). At week 4 (prior to VMMC), participants completed a questionnaire assessing ease of product use, tolerability, and sexual practices. Foreskin tissues were collected after surgery. Molecular diagnostics for asymptomatic N. gonorrhoeae and C. trachomatis were performed after study completion using first-void urine samples, with directed treatment provided according to Uganda National Guidelines. Participants were deemed lost to follow-up if not traced within 3 days of a scheduled visit.
Outcomes
The primary endpoint was ex vivo HIV entry into inner foreskin-derived CD4+ T cells, using a previously-validated clade A HIV pseudovirus entry assay.20 The sample size of 25 participants per study group provided 80% power to identify reduced virus entry by ≥ 33%.15 Predefined secondary endpoints included treatment acceptability and effects on the penile immune milieu, bacteria, epithelial integrity, and T cell subset proportion and density.
Biological sample collection and processing
Blood (20mL) was collected into acid citrate dextran (ACD) vacutainer tubes, with plasma separated and stored at −80°C. Penile swabs were transported to the lab on ice within 1 h of collection. One swab from each of the inner foreskin and coronal sulcus was frozen “as is” at −80°C, and one from the urethra, inner foreskin and coronal sulcus was re-suspended in PBS (with cOmplete protease inhibitor, Roche) and stored at −80°C. Foreskin tissues were processed as previously described.20 Following circumcision, foreskin tissue was tagged with a suture to distinguish the inner from the outer foreskin and immediately placed into room temperature R10 medium comprising RPMI 1640 media, 10% heat-inactivated fetal bovine serum, 10 U/mL penicillin, 10 μg/mL streptomycin, 250 ng/mL amphotericin B, and 2 mM L-glutamine (Gibco, Invitrogen, Carlsbad, CA). Excess fat, visible blood vessels and clots were excised, and the inner was separated from the outer foreskin. Separately, each portion was sectioned into 0.5cm-wide strips from which 0.25cm2 pieces were then cut. A total of 24 pieces of inner foreskin tissue were each placed in an Eppendorf tube with 0.5mL of 500Uml of collagenase type 1 (GIBCO 17100) and 42.5Uml of DNAse (Invitrogen cat:18047-019). Using scissors, each piece was mechanically cut to a fine paste then enzymatically digested on a 37°C pre-warmed shaker set at 900 r.p.m (Eppendorf Thermomixer; Hamburg, Germany) for 40 min. After 40 min, the cell suspensions were pooled, fetal bovine serum (3mls) added to inhibit ongoing collagenase activity followed by filtration through a 100-μm cell strainer (BD Biosciences, Franklin Lakes, NJ). The filtrate was spun to for 6 min at 1400 rpm to pellet cells which were then washed, resuspended in 20mL of R10 without antibiotics and rested overnight at 37°C, 5% CO2.
BlaM-Vpr pseudovirus entry assay
The HIV-1 pseudovirus fusion assay was adapted from the protocol of Cavrois and colleagues.30 As previously described,20 poly-ethylenimine (Polysciences Inc) was used to transfect plated HEK 293T cells with plasmids containing 20 μg HIV backbone lacking envelope; Q23Δenv gfp,31 10 μg early-transmitted R5-tropic clade A envelope Q 259d2.17 env,32 10 μg pCMV-BlaM-Vpr (#21950, Addgene, Cambridge), and 5 μg of pAdvantage (#E1711, Promega) followed by 48 h of incubation after which the pseudo-virus containing media was harvested and centrifuged to a 100-fold concentration. Viral titration of input virus was set at 50% of maximum infection of reference PBMC stocks. Following an overnight rest, either 200 μL of HIV pseudovirus or 200 μL of RPMI as mock control was added to 10×106 foreskin mononuclear cells in flat-bottom plates, spinoculated (1200 g, 17°C, 2 h) followed by incubation (37°C, 5% CO2, 2 h). Following incubation, cells were washed in CO2 independent media (Invitrogen) to remove extracellular virus that could cleave the dye and loaded with 100ul/well of a 1 μmol/L of CCF2-AM (Invitrogen, #K1032), a cell membrane permeant dye, and incubated at room temperature for 1.5 h to allow cellular diffusion of the dye. Thereafter, the cells were re-washed in CO2-independent media (Gibco, #18045088) was added, and cells were incubated in the dark, at room temperature for 12 h to allow CCF2-AM cleavage by Blam in cell-incorporated replication incompetent pseudovirions. Virus and mock treated cells were stained with a fluorochrome master mix comprising LD-NIR APC-Cy7 (Invitrogen, cat: L34993), CD3-AF700 (Biolegend, cat:317340), CD4-BV650 (BD Horizon, cat:563875), Beta 7-PerCP Cy5.5 (Biolegend, cat:121008), Beta 1-APC (Biolegend, cat:303008), Alpha 4-PE-Green (Biolegend, cat:304304), Alpha E-PE-Cy7 (Biolegend, cat:350212), CD69-BV786 (Biolegend, cat:310932), CCR5-PE-CF594 (BD Horizon, cat:562456), CCR6-BV605 (Biolegend, cat:353420), with uncleaved dye CCF2-AM emitting at 510 and cleaved dye at V450 (Invitrogen, #K1032).
Cytometry-based assessment of foreskin T cell subsets
For assessment of cellular surface HIV susceptibility phenotypic markers, rested foreskin mononuclear cells were washed and stained with a master mix comprising LD-NIR-APC-Cy7 (Invitrogen cat: L3499), CD3-AF700 (Biolegend, cat:317340), CD4-BV650 (BD Horizon, cat:563875), CD38-PerCP Cy5.5 (Biolegend, cat:303522), CD25-AF647 (Biolegend, cat:356128), CD127-BV421 (Biolegend, cat:351310), CCR4-PE-Cy7 (Biolegend, cat:359410), CXCR3-BV510 (Biolegend, cat:353725), CCR6-BV605 (Biolegend, cat:353420), CCR5-PE-CF594 (BD Horizon, cat:562456), CCR10-PE-Green (R&D systems, cat:FAB3478P-100), HLA-DR-BV786 (BD Horizon, cat:564041). CD4+ T cell subsets were defined based on chemokine receptor expression as follows: (a) Th22 subset as CCR4+, CCR6+, CCR10+ and CXCR3-; (b) Th17 subset as CCR4+, CCR6+, CCR10-, CXCR3-; (c) Th1 subset as CCR4-, CXCR3+, CCR6-; (d) Th1.17 subset as CCR4−, CXCR3+, CCR6+. Cells were fixed and immediately acquired on an LSRII multi-color flow cytometer (BD Systems) with data analyzed using FlowJo 10.4.1 (TreeStar, Ashland, OR).
Multiplex ELISA for soluble immune factor quantification
As previously described,27 nine immune factors were quantified in penile swabs using a multiplex chemiluminescent ELISA system (Meso Scale Discovery, MSD; Rockville, MD): IL-1α, IL-1β, resistin, IL-8, MIP-1β (macrophage inflammatory protein beta); soluble E-cadherin (sEcad, a marker of epithelial disruption10), MMP-9 (matrix metalloproteinase-9), TIMP-1 (tissue inhibitor of metalloproteinases-1), and VEGF (vascular endothelial growth factor). Samples from each participant were assayed in duplicate on the same plate, in order to limit plate-plate variation. Standard curves were established from serial dilutions of stock analyte provided by the manufacturer, to enable extrapolation of undiluted analyte concentrations. Any assay with a coefficient of variation >30%, or that exceeded the upper limit of quantification, were re-run. Concentrations below the threshold of detection on the standard curve for any analyte were assigned as the lower limit of detection (LLoD), with the LLoD derived from the mean of all standard curves, irrespective of the coefficient of variation. The established LLoD values were 29.97 pg/mL for E-cadherin, 0.74 pg/mL for IL-1α, 0.07 pg/mL for IL-1β, 0.07 pg/mL for IL-8, 2.09 pg/mL for MIP-1β, 1.37 pg/mL for MMP-9, 0.05 pg/mL for resisitin, 31.67 pg/mL for TIMP-1 and 2.02 pg/mL for VEGF.
Foreskin immunofluorescence microscopy (IF)
IF was performed on frozen foreskin tissue sections as previously described.36 Tissue samples frozen in OCT were cryosectioned at 8μm using a CM1850 cryostat (Leica) and stained for nuclei (DAPI), CD3 (Invitrogen), and tissue E-cadherin (BD). Full tissue section scans were taken with a Leica DM5500B fluorescence microscope using the 20× objective lens (HCX PL FLUOTAR 20×/0.50). T cells were quantified using a deep-learning cell counting algorithm based on the StarDist method.37 Cell density image analysis focused on the tissue area within 300μm of the apical surface of the epithelium, as the total amount of dermis tissue varied greatly between study participants. The threshold of 300μm from the apical surface of the epithelium was selected as this area contained most HIV target cells, enabling comparison of HIV susceptibility between treatment groups. For E-cadherin, the entire epithelium was manually traced from full tissue images, excluding tissue folds and artifacts which were manually excluded. Tissue E-cadherin expression was determined using methods similar to those previously described.38 Briefly, relative fluorescence was used to define the E-cadherin net-like structure, which is influenced by both the brightness and distribution of positive staining. Expression was quantified as the mean fluorescence intensity within this net structure (referred to as MFI). IF was performed by research staff unaware of participant group.
Characterizing coronal sulcus bacteria
Microbiome DNA was extracted from 80uL of eluant from cryopreserved coronal sulcus swabs through a combination of enzymatic and chemical lysis. All samples were treated with 122 μL Tris-EDTA, 45 μL 10mM Tris-HCl, 5 μL 100 mg/mL lysozyme (L6876-1G Sigma-Alrich), 5 μL 25 KUmL mutanolysin (M4782-5KU, Sigma-Alrich), and 3 μL 4U/uL lysostaphin (SAE0091-2MG, Sigma-Aldrich) and incubated at 37°C for 1 h. DNA was then extracted using the MagMax DNA Multi-Sample Ultra 2.0 Kit (A36570, Applied BioSystems) following manufacturer’s instructions, resulting in an 80uL final elution volume. The V3-V4 hypervariable region of the 16S rRNA gene region was amplified using a broad-coverage quantitative PCR and sequenced as described previously.27,39 Briefly, purified amplicons were pooled and sequenced on an Illumina MiSeq platform using a MiSeq v3 600 cycle kit at the George Washington University Genomics Core facility. The paired amplicon sequences were processed with cutadapt to remove primers, Trimmomatic to trim and filter sequences based on low quality regions and length. A negative-extraction control (NEC) was included with each extraction and sequenced in order to assess cross-contamination. No-template controls (NTCs) and positive-template controls (PTCs) were included to assess cross-contamination and verify PCR performance. Samples with under 1000 reads were excluded from analysis.
Finally, we used DADA2 v1.10 modules33 to chimera check and infer Amplicon Sequence Variants (ASV). Genus-level taxonomic classification of the ASVs was performed using a Naive Bayesian Classifier (v.2.12).34 We used an inhouse version of the Naïve Bayesian Classifier35 trained on BASICS 16S rRNA gene sequences to classify ASV at the species level. The resultant sequencing data were used to calculate the prevalence and the proportional abundance of each taxon for each sample (i.e., Number of 16S rRNA gene sequences assigned to a taxon divided by the total number of 16S rRNA sequences). Additional details can be found at: https://github.com/araclab/mb_analysis.
Our initial analysis focused on the combined density in the foreskin prepuce of the six BASIC species, specifically Peptostreptococcus anaerobius, Prevotella bivia, Prevotella disiens, Dialister propionicifaciens, Dialister micraerophilus, and a genetic near neighbor of Dialister succinatiphilus.9 In addition, we analyzed two bacterial genera not previously linked to HIV risk, namely Staphylococcus and Corynebacterium (control taxa). Targeted quantitative polymerase chain reaction was used to estimate total bacterial abundance by targeting the 16S region of the rRNA gene. This total bacterial abundance was multiplied by relative taxon abundance to calculate the estimated absolute abundance of specific taxa, and the combined density of BASIC species and control species were calculated through addition of the relevant taxa.
Statistical analysis
The predefined primary study endpoints (per-cell HIV entry% and the extrapolated tissue density as cells/mm2 of inner foreskin tissue, based on IF) were compared between each intervention group and the untreated control group using the Mann-Whitney U test. The same cross-sectional approach was used to compare secondary endpoints from tissue (available only at the time of surgery) including the tissue cell density of other T cell subsets (CD3+, CD3/CD4 +, CD3/CD4/CCR5+, Th17 cells) and epithelial tissue-bound E-cadherin. The impacts of intensive and maintenance interventions on soluble immune factors and the density of BASIC and control bacterial species were assessed within an individual by Wilcoxon signed-rank test using log10-transformed data. All participants were analyzed according to randomized group (Intention-to-Treat), regardless of reported product compliance. Men with a symptomatic sexually transmitted infection (STI) were not eligible to participate, but STI diagnostics and treatment were performed for all participants after the circumcision visit; participants with an asymptomatic STI were included in the overall analysis, and excluded in a subsequent sensitivity analysis. The sample size was calculated from prior studies examining HIV pseudovirus entry into inner foreskin derived CD4+ T cells20; based on virus entry and variance in those studies, 25 participants per group gave a statistical power of 80% to define antimicrobial approaches reducing virus entry by ≥ 33%.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Additional resources
Deidentified immune and microbial data are available at Mendeley (https://doi.org/10.17632/7bymvktmc3.1). This clinical trial was registered at ClinicalTrials.gov (#NCT03412071).
Acknowledgments
The authors wish to thank all participants, UVRI-IAVI for the infrastructure and support that they provided, and the following UVRI-IAVI staff members: Mathias Wambuzi, Paul Kato Kitandwe, Teddy Nakaweesi, Joel Odenyo, Ruth Amanyi Ababe, Doreen Nassolo, Umar Hassan Nkuutu, Evelyn Nalwanga, Eva Akite, and Edward Kagolo. This work was supported by the Canadian Institutes of Health Research (TMI-138656 and PJT-1806; R.K.), the National Institutes of Health (R01AI123002-01A1; C.M.L.), and the Fogarty HIV Research Training Program of the National Institutes of Health (4D43TW009578-04; R.M.G.), J.L.P. is supported by the Canada Research Chairs Program, and R.K. is supported by the Elisabeth Hofmann Chair in Translational Research at the University of Toronto. The sponsors did not have any role in the design or implementation of the study. This study was registered at ClinicalTrials.gov (NCT03412071).
Author contributions
R.K. and R.M.G. conceived, designed, and initiated the study. R.M.G., R.K., J.L.P., A.A.R.T., and C.M.L. developed the study hypothesis. R.M.G., B.O., and R.K. developed the trial protocol and oversaw clinical trial activities. D.E.P., R.M.G., and R.K. performed statistical analyses. B.B., C.K., and J.M. enrolled participants and collected data at study visits. V.J. and S.Y. prepared pseudovirus stocks. L.B. performed E-cadherin immunofluorescence, and Z.S. performed CD3 immunofluorescence. R.M.G., A.N., V.M.B., T.N., B.K., A.S., and M.M. processed samples and performed flow cytometry. T.P., M.A., and D.E.P. performed microbiota assays. S.H. performed soluble immune factor quantification. R.K. wrote the first draft of the manuscript. All authors contributed to manuscript editing, had full access to all the data in the study, and had final responsibility for the decision to submit for publication. R.M.G. and R.K. directly accessed and verified the data.
Declaration of interests
The authors declare no competing interests.
Published: August 29, 2024
References
- 1.DANGER UNAIDS Global AIDS Update 2022. 2022. https://www.unaids.org/sites/default/files/media_asset/2022-global-aids-update_en.pdf
- 2.Rodger A.J., Cambiano V., Bruun T., Vernazza P., Collins S., van Lunzen J., Corbelli G.M., Estrada V., Geretti A.M., Beloukas A., et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316:171–181. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 3.McCormack S., Dunn D.T., Desai M., Dolling D.I., Gafos M., Gilson R., Sullivan A.K., Clarke A., Reeves I., Schembri G., et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegfried N., Muller M., Deeks J.J., Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst. Rev. 2009;15:CD003362. doi: 10.1002/14651858.CD003362.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS data 2021. 2021. https://www.unaids.org/en/resources/documents/2021/2021_unaids_data
- 6.UNAIDS global AIDS update 2021 Confronting inequalities: lessons for pandemic responses from 40 years of AIDS. 2021. https://www.unaids.org/sites/default/files/media_asset/2021-global-aids-update_en.pdf
- 7.Grabowski M.K., Serwadda D.M., Gray R.H., Nakigozi G., Kigozi G., Kagaayi J., Ssekubugu R., Nalugoda F., Lessler J., Lutalo T., et al. HIV prevention efforts and incidence of HIV in Uganda. N. Engl. J. Med. 2017;377:2154–2166. doi: 10.1056/NEJMoa1702150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C.M., Hungate B.A., Tobian A.A.R., Serwadda D., Ravel J., Lester R., Kigozi G., Aziz M., Galiwango R.M., Nalugoda F., et al. Male circumcision significantly reduces prevalence and load of genital anaerobic bacteria. mBio. 2013;4 doi: 10.1128/mBio.00076-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prodger J.L., Abraham A.G., Tobian A.A., Park D.E., Aziz M., Roach K., Gray R.H., Buchanan L., Kigozi G., Galiwango R.M., et al. Penile bacteria associated with HIV seroconversion, inflammation, and immune cells. JCI Insight. 2021;6 doi: 10.1172/jci.insight.147363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu R., Armstrong E., Constable S., Buchanan L.B., Mohammadi A., Galiwango R.M., Huibner S., Perry M.C., Prodger J.L., Coburn B., Kaul R. Soluble E-cadherin: a marker of genital epithelial disruption. Am. J. Reprod. Immunol. 2023;89 doi: 10.1111/aji.13674. [DOI] [PubMed] [Google Scholar]
- 11.Kaul R., Liu C.M., Park D.E., Galiwango R.M., Tobian A.A.R., Prodger J.L. The penis, the vagina and HIV risk: key differences (aside from the obvious) Viruses. 2022;14:1164. doi: 10.3390/v14061164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong E., Hemmerling A., Miller S., Burke K.E., Newmann S.J., Morris S.R., Reno H., Huibner S., Kulikova M., Liu R., et al. Metronidazole treatment rapidly reduces genital inflammation through effects on bacterial vaginosis-associated bacteria rather than lactobacilli. J. Clin. Invest. 2022;132 doi: 10.1172/JCI152930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joag V., Obila O., Gajer P., Scott M.C., Dizzell S., Humphrys M., Shahabi K., Huibner S., Shannon B., Tharao W., et al. Impact of standard bacterial vaginosis treatment on the genital microbiota, immune milieu, and ex vivo Human Immunodeficiency Virus susceptibility. Clin. Infect. Dis. 2019;68:1675–1683. doi: 10.1093/cid/ciy762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plummer E.L., Vodstrcil L.A., Doyle M., Danielewski J.A., Murray G.L., Fehler G., Fairley C.K., Bulach D.M., Garland S.M., Chow E.P.F., et al. A prospective, open-label pilot study of concurrent male partner treatment for bacterial vaginosis. mBio. 2021;12 doi: 10.1128/mBio.02323-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galiwango R.M., Bagaya B., Mpendo J., Joag V., Okech B., Nanvubya A., Ssetaala A., Muwanga M., Kaul R. Protocol for a randomized clinical trial exploring the effect of antimicrobial agents on the penile microbiota, immunology and HIV susceptibility of Ugandan men. Trials. 2019;20:443. doi: 10.1186/s13063-019-3545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong N.R., Wilson J.D. Tinidazole in the treatment of bacterial vaginosis. Int. J. Womens Health. 2010;1:59–65. doi: 10.2147/ijwh.s4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Workowski K.A., Bachmann L.H., Chan P.A., Johnston C.M., Muzny C.A., Park I., Reno H., Zenilman J.M., Bolan G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 2021;70:1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashed H.T. Evaluation of the effect of hydrogen peroxide as a mouthwash in comparison with chlorhexidine in chronic periodontitis patients: A clinical study. J. Int. Soc. Prev. Community Dent. 2016;6:206–212. doi: 10.4103/2231-0762.183114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prodger J.L., Gray R.H., Shannon B., Shahabi K., Kong X., Grabowski K., Kigozi G., Nalugoda F., Serwadda D., Wawer M.J., et al. Chemokine levels in the penile coronal sulcus correlate with HIV-1 acquisition and are reduced by male circumcision in Rakai, Uganda. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1006025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galiwango R.M., Yegorov S., Joag V., Prodger J., Shahabi K., Huibner S., Muyanja E., Kabuubi B.R., Namuniina A., Nalutaaya A., et al. Characterization of CD4(+) T cell subsets and HIV susceptibility in the inner and outer foreskin of Ugandan men. Am. J. Reprod. Immunol. 2019;82 doi: 10.1111/aji.13143. [DOI] [PubMed] [Google Scholar]
- 21.Liu C.M., Prodger J.L., Tobian A.A.R., Abraham A.G., Kigozi G., Hungate B.A., Aziz M., Nalugoda F., Sariya S., Serwadda D., et al. Penile anaerobic dysbiosis as a risk factor for HIV infection. mBio. 2017;8 doi: 10.1128/mBio.00996-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteiro P., Gosselin A., Wacleche V.S., El-Far M., Said E.A., Kared H., Grandvaux N., Boulassel M.R., Routy J.P., Ancuta P. Memory CCR6+CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin beta-7. J. Immunol. 2011;186:4618–4630. doi: 10.4049/jimmunol.1004151. [DOI] [PubMed] [Google Scholar]
- 23.McKinnon L.R., Nyanga B., Kim C.J., Izulla P., Kwatampora J., Kimani M., Shahabi K., Mugo N., Smith J.S., Anzala A.O., et al. Early HIV-1 infection is associated with reduced frequencies of cervical Th17 cells. J. Acquir. Immune Defic. Syndr. 2015;68:6–12. doi: 10.1097/QAI.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 24.Stieh D.J., Matias E., Xu H., Fought A.J., Blanchard J.L., Marx P.A., Veazey R.S., Hope T.J. Th17 cells are preferentially infected very early after vaginal transmission of SIV in macaques. Cell Host Microbe. 2016;19:529–540. doi: 10.1016/j.chom.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen C.R., Wierzbicki M.R., French A.L., Morris S., Newmann S., Reno H., Green L., Miller S., Powell J., Parks T., Hemmerling A. Randomized trial of Lactin-V to prevent recurrence of bacterial vaginosis. N. Engl. J. Med. 2020;382:1906–1915. doi: 10.1056/NEJMoa1915254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong E., Hemmerling A., Miller S., Burke K.E., Newmann S.J., Morris S.R., Reno H., Huibner S., Kulikova M., Nagelkerke N., et al. Sustained effect of LACTIN-V (Lactobacillus crispatus CTV-05) on genital immunology following standard bacterial vaginosis treatment: results from a randomised, placebo-controlled trial. Lancet. Microbe. 2022;3:e435–e442. doi: 10.1016/S2666-5247(22)00043-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galiwango R.M., Park D.E., Huibner S., Onos A., Aziz M., Roach K., Anok A., Nnamutete J., Isabirye Y., Wasswa J.B., et al. Immune milieu and microbiome of the distal urethra in Ugandan men: impact of penile circumcision and implications for HIV susceptibility. Microbiome. 2022;10:7. doi: 10.1186/s40168-021-01185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganor Y., Zhou Z., Bodo J., Tudor D., Leibowitch J., Mathez D., Schmitt A., Vacher-Lavenu M.C., Revol M., Bomsel M. The adult penile urethra is a novel entry site for HIV-1 that preferentially targets resident urethral macrophages. Mucosal Immunol. 2013;6:776–786. doi: 10.1038/mi.2012.116. [DOI] [PubMed] [Google Scholar]
- 29.Toh E., Xing Y., Gao X., Jordan S.J., Batteiger T.A., Batteiger B.E., Van Der Pol B., Muzny C.A., Gebregziabher N., Williams J.A., et al. Sexual behavior shapes male genitourinary microbiome composition. Cell Rep. Med. 2023;4 doi: 10.1016/j.xcrm.2023.100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavrois M., Neidleman J., Greene W.C. HIV-1 fusion assay. Bio. Protoc. 2014;4 doi: 10.21769/bioprotoc.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long E.M., Rainwater S.M.J., Lavreys L., Mandaliya K., Overbaugh J. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res. Hum. Retroviruses. 2002;18:567–576. doi: 10.1089/088922202753747914. [DOI] [PubMed] [Google Scholar]
- 32.Humes D., Overbaugh J. Adaptation of subtype A human immunodeficiency virus type 1 envelope to pig-tailed macaque cells. J. Virol. 2011;85:4409–4420. doi: 10.1128/JVI.02244-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C.M., Price L.B., Hungate B.A., Abraham A.G., Larsen L.A., Christensen K., Stegger M., Skov R., Andersen P.S. Staphylococcus aureus and the ecology of the nasal microbiome. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1400216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchanan L.B., Shao Z., Jiang Y.C., Lai A., Hope T.J., Carias A.M., Prodger J.L. Quantitative immunofluorescent imaging of immune cells in mucosal tissues. Methods Mol. Biol. 2022;2440:143–164. doi: 10.1007/978-1-0716-2051-9_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt U., Weigert M., Broaddus C., Myers G. Springer International Publishing; 2018. Cell Detection with Star-Convex Polygons; pp. 265–273. [Google Scholar]
- 38.Edfeldt G., Lajoie J., Röhl M., Oyugi J., Åhlberg A., Khalilzadeh-Binicy B., Bradley F., Mack M., Kimani J., Omollo K., et al. Regular use of depot medroxyprogesterone acetate causes thinning of the superficial lining and apical distribution of Human Immunodeficiency Virus target cells in the human ectocervix. J. Infect. Dis. 2022;225:1151–1161. doi: 10.1093/infdis/jiaa514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C.M., Aziz M., Kachur S., Hsueh P.R., Huang Y.T., Keim P., Price L.B. BactQuant: an enhanced broad-coverage bacterial quantitative real-time PCR assay. BMC Microbiol. 2012;12:56. doi: 10.1186/1471-2180-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
Immune and microbial data have been deposited at Mendeley (https://doi.org/10.17632/7bymvktmc3.1) and are publicly available as of the date of publication. Microscopy data reported in this paper will be shared by the lead contact upon request.
-
•
This study did not generate any code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.