Abstract
The management of adenomyosis has undergone significant evolution, moving from traditional surgical interventions like hysterectomy to more conservative methods aimed at preserving fertility. Essential roles have been played by uterine-sparing surgeries and uterine artery embolization. Despite these advancements, there is a growing interest in less invasive alternatives. This review delves into the potential of high-intensity focused ultrasound (HIFU). HIFU employs focused ultrasound waves for precise ablation of adenomyotic lesions. The review conducts a thorough analysis of HIFU principles, safety, efficacy, and its possible synergies with other therapies. HIFU seems to be effective for adenomyosis treatment, demonstrating a favorable adverse effect profile and suitability for fertility preservation. Combining HIFU with hormonal treatment appears to enhance long-term symptom control, presenting a promising and comprehensive approach for managing adenomyosis. The goal of this article is to develop a comprehensive understanding of HIFU’s role in contemporary adenomyosis management and to explore areas requiring further research.
Keywords: adenomyosis, high-intensity focused ultrasound, thermal ablation, non-invasive, fertility preservation, uterus sparing, ultrasonography, magnetic resonance imaging
Introduction
Adenomyosis is a common gynecological disorder characterized by the presence of endometrial glands and stroma within the myometrium, which induces hyperplasia and hypertrophy of the surrounding smooth muscle cells. 1 Histologically, it is defined as the presence of endometrial tissues at least 2.5 mm below the endometrial-myometrial junction. 2 The disease was first described by von Rokitansky in 1860 who found islands of endometrial tissue scattered within the myometrium and was later named “adenomyosis” in 1925. 2 Adenomyosis can be asymptomatic or present as pelvic pain (chronic pelvic pain and/or dysmenorrhea), abnormal uterine bleeding, or subfertility. 3 These symptoms are found in about two-thirds of women with adenomyosis. 4 Over the years, several treatment modalities have been developed to address the disease. 5 For many years, hysterectomy was considered the gold standard treatment for symptomatic adenomyosis. However, it was not a suitable option for women desiring fertility preservation. 4 This led to the introduction of uterus-sparing treatment options such as medical therapy and uterus-sparing surgeries. 4 Although medications, such as oral contraceptive pills, gonadotropin-releasing hormone analogs (GnRH-a), and levonorgestrel intrauterine system (LNG-IUS), are effective in controlling menstrual symptoms of adenomyosis, their therapeutic effect is generally transient and limited to the period of treatment only.4,6 While uterus-sparing surgical procedures, such as complete or partial adenomyomectomy, enable uterus preservation, there are many concerns regarding their impact on pregnancy and fertility outcomes due to uterine scarring and potential compromise of myometrial thickness.2,7 Additionally, it is challenging to remove the adenomyotic lesions completely as the adenomyosis boundaries are often unclear. This translates into high failure and recurrence rates.2,7,8
In view of the limitations of these modalities, there has been a need to develop newer modalities of treatment. Among these methods were uterine artery embolization (UAE) and ablative technologies, which utilize radiofrequency and microwave energies, as well as high-intensity focused ultrasound (HIFU). 8 The effect of HIFU on tissues and living animal organs was first explored in 1942. Its first therapeutic application was in 1960 when Fry brothers used it to induce cortical lesions in the brains of Parkinson’s patients in an attempt to slow down disease progression. 9 Later, most of the interest in HIFU ceased due to the lack of technologies for accurate temperature tracking and organ imaging. 9 In 1980s, this interest was revived when magnetic resonance imaging (MRI) machines were invented. 9 Through the years, HIFU has gained a special interest in the field of oncology for managing malignant neoplasms. 4 The high-intensity ultrasound waves are focused in one point within the cancer mass, generating significant heat enough to induce thermal injury and coagulative necrosis of cancer cells. 4 The ability to concentrate multiple ultrasound waves of high intensity at one focal point inside the lesion allows targeted ablation of the lesion while leaving normal tissues in the beam path unharmed. 10 Recently, HIFU applications expanded further to include some of the gynecological solid tumors, such as uterine fibroids and breast fibroadenomas. 10
Regulatory approvals for HIFU devices used in treating adenomyosis vary by region and device type. MRI-guided HIFU (MRgHIFU) and ultrasound-guided HIFU (USgHIFU) systems are more widely approved and established for the treatment of uterine fibroids compared to their use for adenomyosis. 11 The most frequently used HIFU systems worldwide include the ultrasound-guided JC Focused Ultrasound Tumor Therapeutic System from Chongqing Haifu Technology and the MRI-guided Exablate system by InSightec and Sonalleve system by Profound Medical. 12 The Exablate 2000 and Exablate O.R. by InSightec are approved for treating both uterine fibroids and adenomyosis in Europe, China, Japan, Korea, and Russia. 12 In the United States, the Exablate O.R. received FDA approval for treating uterine fibroids in 2004, and it has CE Marking from the European Economic Area and KFDA approval in Korea. 13 The JC system has broader regulatory approvals for treating adenomyosis, with endorsements in Europe, China, Russia, and South America, as well as additional approvals in Kenya, Peru, Saudi Arabia, Malaysia, and several other countries. 14
This article provides a thorough exploration of HIFU principles and their application in addressing adenomyosis. It aims to present the current evidence regarding the safety and efficacy of HIFU in alleviating adenomyosis symptoms, its potential impact on fertility and pregnancy outcomes, and the synergistic effects of various HIFU combination therapies. Through a comprehensive review, our goal is to illuminate the current understanding of HIFUs application in adenomyosis treatment. This exploration also identifies areas requiring further research, contributing to a more nuanced comprehension of HIFU’s role in adenomyosis management.
Materials and methods
A comprehensive literature search was conducted using the databases PubMed, Scopus, and Cochrane Database. The search utilized keywords such as “focused ultrasound,” “focused ultrasound surgery,” “high-intensity focused ultrasound,” “thermal ablation,” “adenomyosis,” and “adenomyoma,” as well as Medical Subject Headings like “high-intensity focused ultrasound ablation” and “adenomyosis.” The search was filtered to include studies published between January 2014 and January 2024 and written in English to ensure the relevance and accessibility of the evidence.
Inclusion criteria encompassed clinical trials, retrospective or prospective studies, case reports, and reviews that evaluated the use of HIFU in adenomyosis patients. Specifically, studies were included if they assessed outcomes related to lesion volume reduction, symptomatic relief, adverse effects, or pregnancy outcomes. Comparative studies of HIFU against invasive surgical treatments or other thermal ablation methods, as well as those examining adjunctive hormonal therapies, were also considered.
Exclusion criteria involved studies focusing on HIFU for other pelvic pathologies, such as uterine fibroids or endometriosis, studies discussing modalities other than HIFU or treating thermal ablation as a single entity, studies combining HIFU with non-hormonal therapies, and animal studies. Editorials, conference abstracts, and commentaries were also excluded. After an initial retrieval of 347 results, duplicates were removed, and 15 studies were excluded based on the criteria. Ultimately, 72 articles met the inclusion criteria and were incorporated into the review.
The review process involved independent evaluation of all titles by authors SB and LA. The relevance of studies was agreed upon through regular discussions. Titles and abstracts were screened to avoid duplication, and full-text copies of selected papers were reviewed to extract relevant data.
Discussion
HIFU principle
HIFU operates by targeting and destroying specific lesions by generating heat-induced coagulative necrosis. The process starts with the emission of multiple ultrasound beams from a piezoelectric or piezoceramic transducer. The beams then pass through various body tissues to eventually converge at a focal point within the target lesion, which typically measures 5 mm in diameter and 10 mm in length.4,8,10 When several high-intensity focused ultrasound beams converge at one point, the net temperature generated at that point within the lesion increases significantly.4,10 To achieve the desired ablative effect, it is crucial to sustain a temperature above 60°C for 1 s or more. 4 This level of heat triggers protein denaturation and leads to coagulative necrosis.4,8,10,15 The extent of thermal injury is dependent on the achieved temperature and the time of exposure. 10 Additionally, acoustic cavitation plays a vital role in achieving tissue destruction during HIFU procedures. 16 Furthermore, the ultrasound waves can cause blood vessel injury producing a non-perfused volume (NPV) and resulting in persistent tissue necrosis that continues even after the procedure has concluded.4,10 The larger the post-treatment NPV is, the more favorable the long-term outcomes are. 17
HIFU procedure
HIFU offers distinct advantage over alternative ablative techniques, such as radiofrequency waves, microwaves, and cryotherapy, due to its non-invasive nature. Unlike these methods, HIFU eliminates the need to insert an applicator directly into the target tissue to deliver the ablative energy. As a result, there is no risk of injury to other nearby organs or vascular structures related to the applicator insertion procedure. 7
In the practical application of HIFU in clinical settings, the use of an imaging modality is essential for precise lesion targeting and real-time monitoring of the ablative process. It ensures that the ultrasound beams are accurately focused within the lesion (not elsewhere in the surrounding healthy tissue) and that the desired ablative damage is effectively inflicted on the target tissue. 10 Depending on the specific imaging modality employed, HIFU can be categorized into two main approaches: USgHIFU or MRgHIFU. 18
Patient selection and factors affecting outcome
HIFU represents a valuable therapeutic option for women suffering from symptomatic adenomyosis, particularly those desiring fertility preservation or facing contraindications for invasive intervention. 19 The criteria for treatment typically include women aged 18 years or older presenting with significant adenomyosis-related symptoms and confirmed by MRI, with lesion sizes ranging from 3 to 10 cm. 4 Absolute contraindications for HIFU application include suspected malignancy, pregnancy, or acute inflammatory conditions. 19 Pre-treatment evaluation via MRI plays a crucial role in ensuring appropriate patient selection and treatment planning. Key aspects evaluated during this assessment include the sonication path, as well as the lesion’s location, size, type, and vascularity. Additionally, the proximity of the lesion to critical structures, such as bones, is carefully considered. These factors can significantly impact procedural difficulty and outcome.20–22
Evaluation of the sonication path involves assessing the distance from the skin surface to the lesion’s midpoint and identifying any intervening structures that could impede effective beam transmission. Ideally, eligible candidates for HIFU treatment should have an abdominal wall thickness of less than 5 cm. 4 This ensures optimal beam focus within the transducer’s focal length range of 12–15 cm. Additionally, as the fat layer thickens, it significantly increases its acoustic impedance compared to underlying muscles. The greater the difference in acoustic impedance at the fat-muscle interface, the higher the reflection of the ultrasound beams across it. Consequently, less ultrasound energy reaches the target lesion, potentially reducing treatment efficacy. 23 A retrospective analysis of 173 adenomyosis patients by Liu et al. demonstrated that an increase in BMI is associated with a 22% increase in the risk of symptom recurrence after HIFU. 24 Another retrospective analysis conducted by Gong et al. 25 of 245 adenomyosis patients treated with USgHIFU highlighted that a thinner abdominal wall and anteriorly situated adenomyosis correlated with higher rates of non-perfused volume ratio (NPVR) and energy efficiency factor.
Certain structures in the acoustic path, such as air-containing bowel loops, scar tissue, or metallic fragments, 26 pose challenges by reflecting or absorbing ultrasound beams, potentially compromising treatment efficacy.18,26 Techniques to mitigate these challenges include using liquid-filled balloons to displace bowel loops 18 or adjusting the sonication path to avoid scar tissue areas whenever feasible. 21 However, significant scar tissue (>10 mm width) requires careful consideration due to the increased risk of inadequate treatment and inadvertent tissue heating. 8
Lesions located in close proximity to the sacral bone present unique challenges. Such lesions are further away from the transducer which can result in insufficient energy deposition. 21 Moreover, the sacral bone has a higher capacity to absorb ultrasound waves compared to soft tissue, which can lead to significant heating of the bone during treatment. Consequently, nerves in contact with the sacral bone can get irritated due to increased bone temperature, leading to pain or potential damage.20,21 The same is true for other soft tissue structures in contact with the sacrum such as the piriformis muscle and its fascia and the sacral ligaments. 27 Techniques such as angulating the sonication path or treating the lesion in stages might be helpful. 20 However, lesions located too close to the sacrum (<30 mm) may necessitate careful consideration for adjusting the therapeutic dose or, alternatively, switching to a different treatment approach to effectively minimize these risks. 28
Research indicates that certain adenomyosis types exhibit better response rates to HIFU. Table 1 illustrates the adenomyosis classification described by Kishi et al., commonly used in the studies presented in this review. 29 A retrospective cohort study by Zhao et al. of 227 patients showed that adenomyosis type III (intramural) is associated with significantly higher post-treatment NPVR and lesion volume reduction rate compared to other types of adenomyosis (p = 0.018 and 0.0083, respectively). 30 It was also associated with a higher complete remission rate of dysmenorrhea compared to other types (p < 0.05). This observation is attributed to the capacity to achieve complete ablation of type III lesions. Treatment of type I and II lesions requires preserving a safety margin to prevent injury to the adjacent endometrium or serosa. In contrast, treatment of type III lesions, being entirely intramural, does not necessitate such precautions. Consequently, type III lesions are inherently easier to treat and can be fully ablated. 30 A retrospective study compared the HIFU outcomes in patients with internal adenomyosis (n = 238) versus external adenomyosis (n = 167). 31 It was revealed that treating external adenomyosis necessitated notably longer treatment and sonication times, as well as higher energy levels to achieve comparable NPVR levels to those of internal adenomyosis. Furthermore, when using the same treatment settings, internal adenomyosis achieved significantly higher NPVR compared to external adenomyosis. 31 The significant difference in NPVR remained even after accounting for variations in lesion distribution between the two groups. 31 Therefore, it was suggested that distinct pathological characteristics between the two types, rather than the distribution of the lesion, might be the factor that influenced the HIFU ablation efficacy. 31 Notably, besides differences in lesion distribution, external adenomyosis lesions exhibited significantly smaller volumes than internal adenomyosis. The smaller lesion size might have contributed to the increased sonication time and energy required for treating external adenomyosis due to the “damage-damage interference effect,” where the altered acoustic environment due to the necrotic area in the treated lesion enhanced the deposition of ultrasound energy within the adenomyotic lesion.32,33 It was also noted that dysmenorrhea scores were significantly lower in the internal adenomyosis group but this could have been due to the significantly higher incidence of associated endometriosis in the external adenomyosis group.31–33 Gong et al. retrospectively compared HIFU outcomes between symmetric and asymmetric adenomyosis in 321 patients and found that the relief rate of heavy menstrual bleeding was significantly lower in patients with asymmetric external adenomyosis than that of the other groups (p < 0.005). 33 However, this may be attributed to the adenomyotic lesion being more frequently situated in the farther posterior uterine wall in the asymmetric adenomyosis group, as compared to the symmetric adenomyosis group. 33
Table 1.
The “Kishi” classification of adenomyosis. 29
| Type | Description |
|---|---|
| Type I (internal/intrinsic) | Adenomyosis lesion has an intimate relationship with inner structural components of the uterus, such as the endometrium and the junctional zone. |
| Type II (external/extrinsic) | Adenomyosis located in the outer myometrium disrupting the serosa but not affecting the inner components. |
| Type III (intramural) | Subtype III (intramural) adenomyosis resided solitarily in the myometrium. |
| Type IV (indeterminate) | Any lesion that doesn’t meet the previous descriptions, for example, full thickness lesions involving inner components and serosa. |
Additionally, adenomyosis lesions exhibiting high intensity, indicative of increased vascularity, or multiple hyperintense foci on T2-weighted MRI scans are associated with poorer treatment outcomes.25,34,35 Increased blood flow can dissipate the heat generated by ultrasound waves.21,25 Contrast-enhanced MRI can confirm that increased intensity is due to increased lesion vascularity, guiding treatment decisions accordingly. 21 A retrospective study involving 428 adenomyosis patients investigated the impact of hyperintense foci identified via MRI T2WI on treatment outcomes. The results supported that the number of MRI T2WI hyperintense foci is likely to be a predictor of the efficacy of HIFU in adenomyosis treatment. 36 Another study by Du et al. prospectively followed 82 adenomyosis patients after HIFU treatment and found that uterine fibroid symptom (UFS) scores were significantly lower in the group whose pre-treatment MRI hyperintense foci were less than 5 compared to those with more hyperintense foci (p = 0.049). 37 The hyperintense foci within adenomyosis, potentially indicating areas of bleeding or gland secretions, could absorb more ultrasound energy, restricting its penetration into deeper tissues or causing uneven heating. 25 As a result, the temperature needed to effectively ablate the lesion may not be achieved, leading to inadequate treatment and poorer clinical results.21,25
Meticulous evaluation of patient and lesion characteristics is crucial for identifying optimal candidates for HIFU therapy. Factors, such as shorter sonication paths, internal or focal adenomyosis types, low vascularity, fewer hyperintense foci on MRI T2WI, and anteriorly located lesions, are predictive of favorable treatment outcomes (Supplemental Table S1). This approach not only enhances therapeutic efficacy but also ensures appropriate patient referral when HIFU may not be the optimal choice.
Patient preparation
Before the treatment commences, patients undergo pre-treatment imaging, as described earlier. The skin of lower abdominal wall should be shaved prior to procedure, because the air bubbles trapped between hair strands can act as a nidus for heating and result in skin burns. 26 In some medical centers, the patient’s urinary bladder may be cooled with cold normal saline. Additionally, simple analgesics such as acetaminophen or diclofenac may be administered before the procedure for patient comfort.
HIFU treatments are usually completed in one session, often as a day case procedure. During the procedure, the patient lies in a prone position on the treatment table, with continuous intravenous conscious sedation or an epidural analgesia to maintain patient comfort and cooperation. Lee et al. 38 conducted a case-control study involving 68 adenomyosis patients undergoing HIFU, comparing the efficacy of epidural analgesia with ropivacaine against conscious sedation with remifentanil. The findings revealed that the epidural anesthesia group reported a notably lower incidence of severe or very severe intra-operative pain compared to the conscious sedation group (41.9% versus 75.7%, respectively; p = 0.006). Additionally, the epidural anesthesia group exhibited reduced postoperative opioid consumption and higher post-procedure numeric pain rating scale scores compared to the conscious sedation group (0.87 ± 0.18 versus 0.43 ± 0.32, respectively; p < 0.001). 38
USgHIFU versus MRgHIFU
USgHIFU is more cost-effective and faster than MRgHIFU. 8 It provides real-time guidance of the ablation process. 8 The JC Focused Ultrasound Tumor Therapeutic System has been used for adenomyosis treatment since 2012. 18 During the procedure, the patient lies prone on the treatment table with her lower anterior abdominal wall aligned with an aperture in the table. 39 Below this aperture, the HIFU device is positioned. It includes a degassed fluid basin housing a convex-shaped ultrasound probe and a ceramic transducer, which emit diagnostic ultrasound (3.5 MHz) and therapeutic ultrasound (0.8 MHz) waves, respectively.18,39 The transducer measures 12–15 cm in diameter with a focal length of 15 cm, mounted on a six-directional therapeutic planning system allowing a wide range of motion to target the lesion.18,39 A degassed liquid-filled balloon is placed between the lower anterior abdominal wall of the patient and the transducer to displace the bowels away from the sonication path and provide an efficient medium for ultrasound wave transmission.18,39 The device is controlled by a computer control unit, which plans the sonication path and focal point using real-time ultrasound image feeds, ensuring precise lesion targeting and effective ablation.18,39 This treatment system is illustrated in Figure 1. 40 The therapeutic beams can reach an acoustic power of up to 400 W, which are typically concentrated in a focal point 5 mm in diameter and 10 mm in length.4,8,10 Successful ablation is indicated by changes in the echogenicity of the focal point. Following this, the device adjusts to target the next adjacent point in the lesion. This process continues until the entire lesion is ablated.4,8,39 In USgHIFU, contrast-enhanced ultrasound is used for immediate assessment of residual NPV after ablation to determine if further sonication is required. In contrast, MRgHIFU, which uses gadolinium (Gd)-enhanced imaging, does not allow immediate sonication if needed. Immediate thermal ablation carries a risk of Gd conversion into Gd3+, which is toxic to the bone marrow. This difference significantly impacts the overall post-ablation outcome in favor of USgHIFU. 41 However, newer non-Gd imaging modalities in MRI, such as diffusion-weighted imaging, have enabled immediate sonication after NPV assessment in MRgHIFU. 42
Figure 1.
JC focused ultrasound tumor treatment system: (a) computer-operated control system; (b) adjustable HIFU table; (c) integrated therapeutic and diagnostic ultrasound transducer; (d) real-time control monitor displaying treatment progress.
Source: Adapted from: Rodríguez et al. 40 Licensed under CC BY 4.0.
HIFU: high-intensity focused ultrasound.
MRgHIFU offers superior anatomic resolution and tissue localization when compared to USgHIFU. 4 MRgHIFU devices monitor treatment adequacy through real-time temperature mapping changes.4,8,26,43 The temperature data acquired is instrumental in automatically optimizing HIFU delivery parameters, establishing a dynamic feedback loop based on real-time temperature monitoring.4,8,26,43 This critical step is essential for ensuring brief treatment durations while upholding the high-quality ablation of target tissue. 43 In MRgHIFU, the ultrasound transducer device seamlessly integrates into the MRI table, see Figure 2, 44 submersed in a degassed liquid. The sonication phase is followed by a cooling interval in order to allow the temperature of surrounding tissues to drop to safe levels before resuming the procedure. This is essential to reduce harm to the surrounding healthy tissues. The temperature drop is assessed through thermal mapping, and the procedure can be resumed when the thermal map suggests a return of temperature to baseline. 15 After patient preparation and table set-up, the procedure itself might take about 3–5 h for completion depending on the size of the lesion to be ablated. 45
Figure 2.
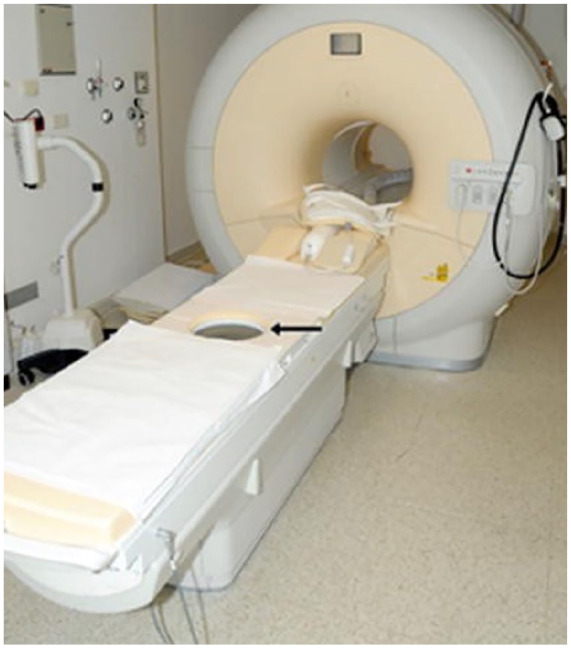
Sonalleve MRgHIFU system: an MRI scanner paired with a focused ultrasound transducer embedded in the surface of the treatment table.
Source: Adapted from: Voogt et al. 44 Licensed under CC BY 4.0.
MRI: magnetic resonance imaging; HIFU: high-intensity focused ultrasound; MRgHIFU: MRI-guided HIFU.
Efficacy and safety
A prospective clinical trial of seven patients with localized adenomyosis performed HIFU through a US transducer during laparoscopy to ablate adenomyosis prior to hysterectomy. Microscopic and macroscopic examination of the uterus in all patients showed HIFU-induced thermal ablation of the targeted adenomyosis in the form of coagulative necrosis and complete loss of cellular viability on electronic microscopy. 46 This trial proved that high-intensity ultrasound is capable of inducing coagulative necrosis of adenomyotic tissues. 46 Further studies followed to assess the efficacy of the technique to produce significant long-term clinical improvement in adenomyosis symptoms.47–51
When reviewing the literature, studies evaluated the efficacy of HIFU by assessing its impact on post-treatment NPV, post-treatment uterine and adenomyosis volumes, menorrhagia and dysmenorrhea, quality of life, and pregnancy outcome.47–51 They also measured the adverse reactions encountered during the treatment.26,27,31–33 Most studies showed a significant reduction in adenomyotic lesions and uterine volumes and a significant reduction in menstrual complaints.47–52
Effect on uterine volume, adenomyosis lesion volume, and menstrual complaints
The collective findings from multiple studies investigating HIFU treatment for adenomyosis reveal its efficacy in reducing both uterine volume and the size of adenomyotic lesions (Supplemental Table S2).47–50,53 These studies reported a collective adenomyosis volume reduction rate that ranged between 45% and 56% within 3–6 months after HIFU treatment.47,48,50 Additionally, these studies proved the efficacy of HIFU in ameliorating associated symptoms and enhancing patients’ quality of life (Supplemental Table S3). The quality of life improvement varied across studies between approximately 50% and 80% within 3–12 months post-HIFU treatment.47–50
Lee et al. 48 conducted a retrospective study examining HIFUs impact on reducing uterine and adenomyosis volumes and alleviating menstrual symptoms and enhancing patients’ quality of life. The study, involving 346 adenomyosis patients treated with USgHIFU at Incheon Christian Hospital, Korea, demonstrated substantial reductions in uterine volume at 3, 6, and 12 months post-treatment—decreasing by 43.99%, 47.01%, and 53.98%, respectively. 48 Additionally, a notable 50% reduction in symptom severity scores (SSS) and an 80% enhancement in quality of life were evident after only 3 months of treatment. 48 Another retrospective study assessed the outcome of USgHIFU for symptomatic uterine fibroids and/or adenomyosis patients (n = 1,807). 47 Eight hundred eighty-nine adenomyosis patients were included and underwent USgHIFU. Uterine volume reduction rates were 44.5%, 50.7%, and 60.1% at 3, 6, and 12 months, respectively. 47 Improvement of SSS (around 50%) during follow-up for patients with adenomyosis were statistically significant (p < 0.001). 47 Similarly, several other retrospective studies showed significant reduction in adenomyosis volume, and significant improvement in adenomyosis symptoms using various scoring systems such as uterine fibroid symptom health-related quality of life questionnaire (UFS-QOL).36,50,54
A meta-analysis by Liu et al. 49 compared the outcome of three methods of thermal ablations—HIFU, percutaneous microwave ablation (PMWA), and radiofrequency ablation (RFA)—in the treatment of adenomyosis . The study reported that the HIFU group showed the lowest lesion volume reduction (45.1%) and NPVR (68.3%) compared to PMWA (74.9%, 82.5%) and RFA (61.3%, 79.2%), respectively. However, the rate of menstrual symptom relief was comparable across all three groups (HIFU/PMWA/RFA: 84.2%/89.7%/89.2%).
Currently, there is lack of comparative studies assessing the outcomes of USgHIFU and MRgHIFU in treating adenomyosis. Nevertheless, Wang et al. 41 compared the outcomes of the two modalities in treating uterine fibroids. They prospectively followed 43 patients who were treated with MRgHIFU and 51 treated with USgHIFU. Their findings indicated significantly higher rates of complete ablation (defined as 100% NPVR at 6 months) in the USgHIFU group compared to the MRgHIFU group (22 cases versus 10 cases, p = 0.031). 41 Moreover, treatment duration was notably longer in the MRgHIFU group than in the USgHIFU group (174.5 ± 42.2 min versus 114.4 ± 39.2 min, p = 0.021). Importantly, neither group experienced major complications necessitating treatment or hospitalization exceeding 48 h. 41 USgHIFU has been studied more extensively in the context of adenomyosis treatment, notably with the JC Focused Ultrasound Tumor Therapeutic System from Chongqing Haifu Technology. Treatment durations in these studies ranged from 1.3 to 3 h.47,48,50,54–57 Reductions in menstrual symptoms varied from around 45% to 60%.47,48,50,54–56 Conversely, studies on MRgHIFU outcomes are limited, mostly utilizing the ExAblate model from Insightec. They are prospective studies of a small number of participants ranging from 10 to 26.58–62 Treatment durations varied from 2 to 4 h.58–60,62 Assessment of menstrual complaints using SSS and UFS-QOL revealed improvements ranging from around 30% to 65% at 12–18 months post-treatment.58,60–63 However, Fan et al. reported lower average symptom improvement rate of around 25% in 10 patients undergoing MRgHIFU with an MRI-compatible HIFU system other than ExAblate. 59
The positive outcomes of HIFU therapy for adenomyosis are tempered by variations in treatment efficacy across different studies, indicating a need for standardized protocols and refined patient selection criteria. However, HIFU consistently reduces both uterine and adenomyotic lesion volumes effectively, with significant improvements in symptoms such as menstrual pain and overall quality of life observed across various follow-up periods. Comparative studies of HIFU against other thermal ablative methods like PMWA and RFA suggest similar improvements in menstrual symptom relief but less effective lesion volume reduction rates. Future research should focus on standardizing treatment protocols and refining patient selection criteria to ensure optimized and consistent efficacy.
Fertility and pregnancy outcome
HIFU could be of great value to women with symptomatic adenomyosis who are seeking fertility. A study that compared HIFU and other uterus-sparing treatments showed higher pregnancy rates after HIFU, with better natural conception rates than laparoscopic excision (LE) surgery, particularly in patients with focal adenomyosis. 50 After the procedure, patients can attempt to conceive much earlier than after surgical treatment, but the exact time of delay in conception is unknown. The overall pregnancy rate after HIFU ranged from 16.7% to 38.8%, varying with adenomyosis types (Supplemental Table S4).49,64,65 External adenomyosis demonstrated lower pregnancy rates than internal adenomyosis.53,55 The association may be attributed to the concurrent presence of endometriosis and pelvic adhesions in the majority of cases of external adenomyosis, thereby increasing the difficulty of conception. 66 Factors like age, adenomyosis type, NPVR, and infertility duration could be correlated with chances of conception. 65
There could be some theoretic concerns regarding the effect of HIFU on pelvic organs, more specifically the ovaries. However, three prospective studies by Otonkoski et al., 67 Lee et al., 68 and Wei et al. 64 compared post-HIFU anti-mullerian hormone levels at 3–6 months to pre-HIFU levels in 74, 79, and 129 women, respectively, and no significant changes were observed. Furthermore, Wei et al. 64 reported that the overall pregnancy rate in the included 129 infertile women with adenomyosis who underwent USgHIFU was 38.8%. Internal adenomyosis had the highest pregnancy rate compared to other types of adenomyosis (59.6% p = 0.001). Moreover, the natural pregnancy rate was significantly higher in the internal adenomyosis group than in external adenomyosis group (74.2% versus 12.5%). 64 It is worth mentioning that no uterine rupture was observed during pregnancy or at delivery. 64
Xiong et al. 65 analyzed retrospectively the data of 27 patients with adenomyosis and primary infertility who underwent USgHIFU. Post HIFU, 10 patients conceived with a total of 11 pregnancies. Several factors were identified to be correlated with reproductive outcomes after HIFU. Younger age, lower BMI, internal adenomyosis, higher NPVR, and shorter duration of infertility were significantly correlated with better reproductive outcomes. This encourages measures such as treatment at an earlier age and reducing body weight to improve the post-HIFU reproductive prospects in adenomyosis patients. It was also suggested that patients with internal adenomyosis can try to conceive naturally after HIFU, while patients with external adenomyosis should be evaluated early for assisted reproductive technology to achieve conception. 65
In the large meta-analysis conducted by Liu et al. 49 comparing the outcomes of different thermal ablation methods in adenomyosis patients, pregnancy rates after HIFU were found to be 16.7% compared to 4.93% after PMWA and 35.8% after RFA. A small prospective study by Huang et al. included 93 adenomyosis patients and compared fertility outcomes of patients who underwent USgHIFU to patients who underwent LE. 51 The study showed that while both treatments achieved significant and comparable relief of dysmenorrhea and menorrhagia, patients treated with HIFU showed significantly higher pregnancy rates and natural conception rates than those who underwent LE surgery (52% versus 30.2%, p = 0.034 and 40% versus 18.6% p = 0.025). 51 Pregnancy complications were not significantly different between the two groups. 51 Notably, in the HIFU treatment group, those with diffuse adenomyotic lesions had significantly lower postoperative pregnancy rates than those with focal adenomyosis. 51
Zhang et al. reported a lower occurrence of uterine ruptures during pregnancy or delivery following HIFU compared to conventional surgical techniques. 8 In a retrospective study by Zhou et al., 69 out of 68 patients treated with HIFU, 54 were conceived and 21 babies were delivered. It was notably associated with a higher rate of miscarriages but there were no severe complications such as uterine rupture. This indicates that HIFU potentially maintains the integrity of the myometrium around the adenomyotic lesion. In a small prospective study involving 23 unintended pregnancies post-HIFU, 12 patients experienced uneventful pregnancies, delivering at full term, while three encountered spontaneous miscarriages. Additionally, one patient had a preterm delivery, and five remained pregnant at the time of study publication. 70
While most literature on pregnancy complications post-HIFU treatment did not report uterine rupture, three case reports highlighted such occurrences. In the first case, a 34-year-old woman underwent HIFU followed by two adenomyomectomies and conceived through IVF 3 months after the last surgery. 71 During the adenomyomectomy, a longitudinal uterine incision was made, removing 220 g of adenomyosis followed by uteroplasty. At 27 weeks of gestation, an emergency cesarean section revealed a 5 × 7 cm dehiscence in the uterine wall. 71 Another case detailed by Liu et al. 72 involved a 34-year-old woman with adenomyosis and two prior cesarean sections. She underwent aggressive HIFU, resulting in a 44.0 × 27.2 × 33.8 mm lesion in the anterior uterine wall. 72 Subsequently, an unintended pregnancy led to an emergency cesarean section at 38 weeks, revealing a 5 × 6 cm full-thickness defect in the same area of HIFU treatment. 72 Indeed, this case carried multiple risk factors for uterine rupture beyond the HIFU treatment alone. Another case report detailed an intrapartum uterine rupture following induction of labor at term in a 40-year-old woman who had conceived 13 months after undergoing USgHIFU for a 6-cm anterior uterine fibroid and diffuse adenomyosis. The patient required an emergency cesarean section due to abnormal fetal heart tracings and was found to have a 3 × 4 cm full-thickness uterine rupture in the anterior wall. 73
HIFU presents a promising option for women with symptomatic adenomyosis seeking fertility, especially those with focal adenomyosis. Factors such as internal adenomyosis, younger age, higher NPVR post-treatment, and shorter infertility duration appear significant in enhancing success rates. Evidence suggests that early intervention for patients with internal adenomyosis may facilitate natural conception attempts, while those with external adenomyosis might benefit from timely evaluation for assisted reproductive technologies. However, women opting for this treatment should be informed of the risk of uterine rupture, especially with extensive procedures, and such pregnancies would require close monitoring. Further research is needed to guide recommendations on optimal selection criteria for HIFU in women desiring pregnancy and to guide their optimal antepartum and intrapartum care.
Safety
The safety profile of HIFU for adenomyosis treatment exhibits variability in reported rates of adverse effects, ranging from 4.5% to 47% (Supplemental Table S5).47,49,54,74,75 Recurrence rates post-treatment spanned approximately 4%–10%, with re-intervention rates ranging from around 2% to 8%.47,49,76 HIFU-related adverse effects are predominantly mild to moderate (grade A or B based on the SIR classification) and mostly short-term in nature.30,48,54 Common adverse effects encompass first- and second-degree skin burns, mild cutaneous edema, sciatic nerve pain, lower abdominal pain, and vaginal discharge. More severe but less frequent adverse effects include hematuria, nerve injury, and bowel dysfunction.47,49,54,74 While no studies directly compared the safety profiles of USgHIFU and MRgHIFU in treating adenomyosis, research examining other pelvic pathologies such as uterine fibroids suggests no significant differences in the safety profiles between the two modalities. 41
Most studies evaluating HIFU safety have explored the USgHIFU, particularly JC System. The reported adverse reactions are predominantly classified as SIR grade A and B. Reports of SIR grade C and D complications are rare, occurring in less than 1% of cases.55,77,78 Lee et al. 47 implemented a retrospective study exploring the safety of USgHIFU in 889 adenomyosis patients. The most frequent adverse reactions encountered where first- and second-degree skin burns, transient hematuria, and sciatic nerve pain. Symptoms recurred in 39 women of the adenomyosis group and 20 women needed hysterectomy eventually. A comprehensive retrospective study, involving 27,053 patients treated between 2011 and 2017, systematically investigated the adverse effects associated with USgHIFU treatment for benign uterine diseases. The majority of adverse events, totaling 12,851 cases, were categorized under SIR grade A and B. 75 Common minor adverse effects included mild lower abdominal pain, sacrococcygeal pain, and abnormal vaginal discharge, attributed to thermally induced inflammation. These symptoms typically resolved within 3 days. 75 Additional minor adverse effects, such as paresthesia, nausea, vomiting, and hematuria, were transient and likely associated with medications administered for conscious sedation and bladder catheterization. 75 Abnormal vaginal discharge and bleeding were associated with treatment of submucosal fibroids and adenomyosis, and found to be related to endometrial thermal injury and necrosis. 75 Grade C and D side effects affected 104 patients. The most frequent adverse effects in these categories were deep skin burns, which were notably associated with individuals having abdominal scars. 79 Bowel perforation was diagnosed in four patients between day 4 and 12 post-treatment, one of whom was treated for adenomyosis, and the remaining cases involved fibroids. In all instances, the bowel was not adequately displaced from the acoustic pathway and the lesions were overtreated. 75 Additionally, four patients experienced acute renal failure, a potential adverse effect that may have been associated with the use of contrast, antibiotics, or non-steroidal anti-inflammatory drugs in the treatment protocol. 75 An interesting case was reported by Ker et al. involving a 38-year-old woman with a uterine fibroid of 13 cm in size who was otherwise healthy and received USgHIFU therapy without any contrast injection. Post-HIFU, the patient developed acute renal insufficiency most likely due to a massive intravascular hemolysis secondary to HIFU-induced red blood cells heat denaturation and mechanical trauma. 80 One notable case reported a patient who developed severe burns affecting the skin, subcutaneous fat, anterior abdominal wall muscles, peritoneum, and uterus 3 days after receiving treatment for adenomyosis with a different US-guided HIFU device (YDME FEP-BY02; Yuande Bio-Medical Engineering, Beijing, China). 81 This patient required a total abdominal hysterectomy and debridement of necrotic abdominal tissues. 81
In a comprehensive meta-analysis of 38 studies, Liu et al. 76 evaluated the incidence of adverse effects and re-intervention associated with different thermal ablative therapies. HIFU group had recurrence and re-intervention rates of 10.2% and 4.0%, respectively. A large meta-analysis by Liu et al. compared the risk of recurrence and re-intervention of adenomyomectomy, UAE, and image-guided thermal ablation, including HIFU, RFA, and microwave ablation. 49 The recurrence rates after adenomyomectomy, UAE, and image-guided thermal ablation were 12.6% (95% CI 8.9%–16.4%), 29.5% (95% CI 17.4%–41.5%), and 10.0% (95% CI 5.6%–14.4%), respectively. The reintervention rates were 2.6% (95% CI 0.9%–4.3%), 12.8% (95% CI 7.2%–18.4%), and 8.2% (95% CI 4.6%–11.9%) after adenomyomectomy, UAE, and image-guided thermal ablation, respectively. 49
Studies exploring the safety profile of MRgHIFU in adenomyosis treatment have generally involved small sample sizes insufficient for meaningful comparisons with USgHIFU. Similar to USgHIFU, reported adverse reactions have predominantly been mild (SIR grade A or B), infrequent, and self-limiting.58,59,60,82 Common adverse effects reported include lower abdominal pain, mild cramps, skin erythema, contact dermatitis from the gel, genital discharge or bleeding, and prolonged first menstrual periods.58,59,61,82 However, there is one documented case of mortality following MRgHIFU. The patient experienced immediate hemodynamic collapse after the procedure and died despite resuscitation efforts. The post-mortem examination revealed 3 L of intraperitoneal hemorrhage and ruptured right uterine and ovarian vessels, likely due to thermal injury to the vessel intima during the procedure. 83
The reported rates of adverse effects following HIFU treatment for adenomyosis vary widely across studies. However, most adverse effects are categorized as SIR grades A and B, with complications of grades C and D occurring in less than 1% of cases. Notably, there is only one documented instance of an SIR grade E complication and one case of mortality (grade F). To enhance patient safety and maintain treatment efficacy, it is crucial to standardize HIFU protocols across different centers, ensuring the adoption of the most effective and safest practices. This approach may help minimize adverse outcomes and optimize the overall benefit of HIFU treatment for adenomyosis.
Combination therapies
Combining HIFU with adjunct hormonal therapies has emerged as a promising approach to managing adenomyosis, aiming to enhance treatment outcomes and reduce recurrence rates (Supplemental Table S6). Studies indicate that when HIFU is combined with hormonal treatments like LNG-IUS or GnRH, there is a significant reduction in uterine volume and improved relief from symptoms like dysmenorrhea and menorrhagia.55,84–90 This combined approach, particularly HIFU with LNG-IUS, demonstrates better long-term efficacy compared to HIFU alone. Yang et al. 91 investigated the effects of HIFU along with GnRH-a and LNG-IUS in 466 adenomyosis patients. Their findings revealed significant reduction in uterine volume post-treatment and notable alleviation in dysmenorrhea and menorrhagia symptoms. Adenomyosis is recognized as an estrogen-dependent condition. 66 GnRH-a has the capacity to suppress endogenous estrogen production, thereby contributing to a reduction in adenomyosis volume. A treatment strategy proposed by Yang et al. involves a three-step approach, wherein patients receive GnRH-a injections following HIFU treatment. Subsequently, when the uterine cavity depth diminishes to less than 9 cm, the insertion of a LNG-IUS is recommended to maintain long-term symptoms control. 91
Zhao et al. 84 conducted a systematic review and meta-analysis incorporating 13 studies involving 1,861 individuals who underwent HIFU with LNG-IUS insertion. Their analysis suggested that while initial uterine volume reduction at 3 months did not significantly differ from HIFU alone, the combined therapy exhibited substantial advantages at 6- and 12-months post-procedure.
Furthermore, Xu et al. 86 reported marked differences in dysmenorrhea scores favoring the HIFU+LNG-IUS group at the 12-month follow-up over HIFU alone and HIFU combined with GnRH-a. Li et al.’s 55 extensive retrospective analysis reinforced these findings, demonstrating improved long-term efficacy in relieving dysmenorrhea and menorrhagia symptoms with combinations involving LNG-IUS at the 3-year mark.
In addition, a study exploring HIFU paired with mifepristone and LNG-IUS revealed significant improvements in uterine volume and menstrual symptoms, surpassing outcomes achieved by HIFU alone or in combination with individual hormonal therapies. 88 Together, these investigations support the potential and advantages of integrating adjunct hormonal therapies with HIFU for comprehensive adenomyosis management.
Challenges and future perspectives
The utilization of HIFU in adenomyosis treatment presents both challenges and promising perspectives. One critical challenge lies in the necessity for studies that compare the outcomes of different treatment protocols, providing insights into optimal HIFU settings. Moreover, larger studies are needed to establish the long-term efficacy of the treatment. Additionally, the perspective of HIFU becoming the recommended method for preserving fertility in symptomatic adenomyosis patients warrants larger studies to evaluate its effect on fertility and pregnancy. Multicentric studies focused on fertility and pregnancy outcomes after HIFU are essential to firmly establish its effectiveness in improving fertility and ensuring safety during pregnancy. Addressing these challenges and advancing research in these directions will contribute significantly to refining HIFUs role in adenomyosis treatment and enhancing its clinical recommendations.
Limitations
While this review offers a detailed overview of the current understanding and application of HIFU for adenomyosis, it is not without limitations inherent to narrative reviews. First, the variability in study designs and methodologies among the included research introduces challenges in directly comparing and generalizing the findings. Many of the studies included had small sample sizes or retrospective designs, which can introduce selection biases and limit the reliability of the reported outcomes. Additionally, the lack of a meta-analysis in this review restricts the ability to quantitatively aggregate data and assess treatment efficacy with greater precision. As a result, while the review provides valuable insights into HIFU treatment for adenomyosis, it does not offer pooled analyses or direct comparisons of study results. Despite these limitations, this review serves as a comprehensive narrative summary that highlights key findings and identifies areas for further investigation to advance the understanding and application of HIFU in the management of adenomyosis.
Conclusion
HIFU is an effective non-invasive treatment modality for symptomatic adenomyosis patients seeking to preserve fertility. The long-term efficacy of the procedure is enhanced with adjunctive therapies such as LNG-IUS, which has been shown to improve symptom relief. The procedure is generally safe, with rare reports of severe complications, making it a promising option for women aiming to conceive after treatment. Compared to other thermal ablation methods, such as PMWA and RFA, HIFU offers an alternative therapy with a favorable safety profile and low re-intervention rate. While PMWA and RFA may achieve a more significant reduction in lesion size, HIFU has demonstrated comparable outcomes in symptom relief. HIFU is particularly effective for patients with thin abdominal walls, anteriorly located adenomyosis, fewer hyperintense foci on MRI T2WI, and focal or type III lesions. Although evidence on pregnancy outcomes following HIFU is limited, its non-invasive nature suggests a potentially lower risk of negatively impacting future pregnancies compared to more invasive treatments. To establish the long-term efficacy of HIFU and evaluate post-treatment pregnancy outcomes comprehensively in adenomyosis, larger multicentric studies involving more extensive patient populations are needed.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057241295593 for High-intensity focused ultrasound in adenomyosis treatment: Insights on safety, efficacy, and reproductive prospects by Shadha Nasser Bahutair and Laila Yahya Alhubaishi in Women’s Health
Acknowledgments
None.
Footnotes
ORCID iD: Shadha Nasser Bahutair  https://orcid.org/0009-0002-6427-1355
https://orcid.org/0009-0002-6427-1355
Supplemental material: Supplemental material for this article is available online.
Declarations
Ethics approval and consent to participate: Not applicable as it is a review based on existing literature.
Consent for publication: Not applicable as it is a review based on existing literature.
Author contribution(s): Shadha Nasser Bahutair: Conceptualization; Investigation; Visualization; Writing – original draft.
Laila Yahya Alhubaishi: Writing – review & editing; Supervision.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Zhai J, Vannuccini S, Petraglia F, et al. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med 2020; 38(2–3): 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horng HC, Chen CH, Chen CY, et al. Uterine-sparing surgery for adenomyosis and/or adenomyoma. Taiwan J Obstet Gynecol 2014; 53(1): 3–7. [DOI] [PubMed] [Google Scholar]
- 3. Gordts S, Grimbizis G, Campo R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis 2018; 109: 380–388. [DOI] [PubMed] [Google Scholar]
- 4. Dong X, Yang Z. High-intensity focused ultrasound therapy. Best Pract Res Clin Obstet Gynaecol 2018; 46: 74–83. [DOI] [PubMed] [Google Scholar]
- 5. Sharara FI, Kheil MH, Feki A, et al. Current and prospective treatment of adenomyosis. J Clin Med 2021; 10: 3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pontis A, D’Alterio MN, Pirarba S, et al. Adenomyosis: a systematic review of medical treatment. Gynecol Endocrinol 2016; 32(9): 696–700. [DOI] [PubMed] [Google Scholar]
- 7. Dong X, Yang Z. High-intensity focused ultrasound ablation of uterine localized adenomyosis. Curr Opin Obstet Gynecol 2010; 22(4): 326–330. [DOI] [PubMed] [Google Scholar]
- 8. Zhang L, Rao F, Setzen R. High intensity focused ultrasound for the treatment of adenomyosis: selection criteria, efficacy, safety and fertility. Acta Obstet Gynecol Scand 2017; 96(6): 707–714. [DOI] [PubMed] [Google Scholar]
- 9. Bachu VS, Kedda J, Suk I, et al. High-intensity focused ultrasound: a review of mechanisms and clinical applications. Ann Biomed Eng 2021; 49(9): 1975–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marinova M, Rauch M, Schild HH, et al. Novel non-invasive treatment with high-intensity focused ultrasound (HIFU). Ultraschall Med 2016; 37(1): 46–55. [DOI] [PubMed] [Google Scholar]
- 11. Diseases and Conditions—UK Focused Ultrasound Foundation, UK Focused Ultrasound Development Status 2023, https://ukfusf.org/diseases-and-conditions/ (accessed 4 August 2024).
- 12. Siedek F, Yeo SY, Heijman E, et al. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU): technical background and overview of current clinical applications (part 1). RöFo 2019; 191(6): 522–530. [DOI] [PubMed] [Google Scholar]
- 13. Aharoni U, Cohn E, Epstein A, et al. InSightec the quest for ROI-return on imagination the operating room of the future: opportunities and challenges. Coller School of Management, Tel Aviv University 2017. [Google Scholar]
- 14. Emily W, Michael B, Sara M, et al. Focused Ultrasound Foundation State of the Field Report 2023, Focused Ultrasound Foundation 2023, (accessed 4 August 2024). [Google Scholar]
- 15. Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. Am J Roentgenol 2004; 183(6): 1713–1719. [DOI] [PubMed] [Google Scholar]
- 16. Bahutair WN, Abuwatfa WH, Husseini GA. Ultrasound triggering of liposomal nanodrugs for cancer therapy: a review. Nanomaterials (Basel) 2022; 12(17): 3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dueholm M. Minimally invasive treatment of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2018; 51: 119–137. [DOI] [PubMed] [Google Scholar]
- 18. Cheung VYT. Current status of high-intensity focused ultrasound for the management of uterine adenomyosis. Ultrasonography 2017; 36(2): 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Recker F, Thudium M, Strunk H, et al. Multidisciplinary management to optimize outcome of ultrasound-guided high-intensity focused ultrasound (HIFU) in patients with uterine fibroids. Sci Rep 2021; 11(1): 22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Napoli A, Alfieri G, Andrani F, et al. Uterine myomas: focused ultrasound surgery. Semin Ultrasound CT MR 2021; 42(1): 25–36. [DOI] [PubMed] [Google Scholar]
- 21. Yoon SW, Lee C, Cha SH, et al. Patient selection guidelines in MR-guided focused ultrasound surgery of uterine fibroids: a pictorial guide to relevant findings in screening pelvic MRI. Eur Radiol 2008; 18(12): 2997–3006. [DOI] [PubMed] [Google Scholar]
- 22. Duc NM, Huy HQ. Influences of screening magnetic resonance imaging parameters on high-intensity focused ultrasound outcome for adenomyosis. Rep Med Imaging 2018; 11: 9–14. [Google Scholar]
- 23. ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 2007; 23(2): 89–104. [DOI] [PubMed] [Google Scholar]
- 24. Liu X, Wang W, Wang Y, et al. Clinical predictors of long-term success in ultrasound-guided high-intensity focused ultrasound ablation treatment for adenomyosis: a retrospective study. Medicine (Baltimore) 2016; 95(3): e2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gong C, Yang B, Shi Y, et al. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperthermia 2016; 32(5): 496–503. [DOI] [PubMed] [Google Scholar]
- 26. Coakley FV, Foster BR, Farsad K, et al. Pelvic applications of MR-guided high intensity focused ultrasound. Abdom Radiol 2013; 38: 1120–1129. [DOI] [PubMed] [Google Scholar]
- 27. Li D, Gong C, Bai J, et al. Analysis of magnetic resonance signal intensity changes in the sacrococcygeal region of patients with uterine fibroids treated with high intensity focused ultrasound ablation. Int J Hyperthermia 2020; 37(1): 404–413. [DOI] [PubMed] [Google Scholar]
- 28. Zheng AQ, Chen JY, Xiao ZB, et al. Sacral injury and influencing factors after ultrasonic ablation of uterine fibroids ⩽30 mm from the sacrum. Diagn Interv Radiol 2023; 29(1): 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kishi Y, Suginami H, Kuramori R, et al. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol 2012; 207(2): 114.e1–114.e7. [DOI] [PubMed] [Google Scholar]
- 30. Zhao Y, Luo S, Liu Y, et al. High intensity focused ultrasound treatment for adenomyosis: comparison of efficacy based on MRI features. Int J Hyperthermia 2023; 40(1): 2197574. [DOI] [PubMed] [Google Scholar]
- 31. Xu F, Lin Z, Wang Y, et al. Comparison of high-intensity focused ultrasound for the treatment of internal and external adenomyosis based on magnetic resonance imaging classification. Int J Hyperthermia 2023; 40(1): 2211268. [DOI] [PubMed] [Google Scholar]
- 32. Chen L, Ter Haar G, Hill CR. Influence of ablated tissue on the formation of high-intensity focused ultrasound lesions. Ultrasound Med Biol 1997; 23(6): 921–931. [DOI] [PubMed] [Google Scholar]
- 33. Gong C, Wang Y, Lv F, et al. Evaluation of high intensity focused ultrasound treatment for different types of adenomyosis based on magnetic resonance imaging classification. Int J Hyperthermia 2022; 39(1): 530–538. [DOI] [PubMed] [Google Scholar]
- 34. Yu J, Jiang L, Su X, et al. Comparison efficacy of ultrasound-guided HIFU for adenomyosis-associated dysmenorrhea with different signal intensity on T2-weighted MR imaging. J Obstet Gynaecol Res 2023; 49(4): 1189–1197. [DOI] [PubMed] [Google Scholar]
- 35. Yu JW, Yang MJ, Jiang L, et al. Factors influencing USgHIFU ablation for adenomyosis with NPVR ⩾ 50%. Int J Hyperthermia 2023; 40(1): 2211753. [DOI] [PubMed] [Google Scholar]
- 36. Gong C, Setzen R, Liu Z, et al. High intensity focused ultrasound treatment of adenomyosis: the relationship between the features of magnetic resonance imaging on T2 weighted images and the therapeutic efficacy. Eur J Radiol 2017; 89: 117–122. [DOI] [PubMed] [Google Scholar]
- 37. Du CC, Wang YQ, Qu DC, et al. Magnetic resonance imaging T2WI hyperintense foci number and the prognosis of adenomyosis after high-intensity focused ultrasound treatment. Int J Gynaecol Obstet 2021; 154(2): 241–247. [DOI] [PubMed] [Google Scholar]
- 38. Lee CS, Lee JY, Ro S, et al. Comparison of effectiveness of epidural analgesia and monitored anesthesia care for high-intensity focused ultrasound treatment of adenomyosis. Int J Hyperthermia 2018; 35(1): 617–625. [DOI] [PubMed] [Google Scholar]
- 39. Tonguc T, Recker F, Ganslmeier J, et al. Improvement of fibroid-associated symptoms and quality of life after US-guided high-intensity focused ultrasound (HIFU) of uterine fibroids. Sci Rep 2022; 12(1): 21155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rodríguez J, Isern J, Pons N, et al. Pregnancy outcomes after ultrasound-guided high-intensity focused ultrasound (USgHIFU) for conservative treatment of uterine fibroids: experience of a single institution. Int J Hyperthermia 2021; 38(2): 9–17. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Wang ZB, Xu YH. Efficacy, efficiency, and safety of magnetic resonance-guided high-intensity focused ultrasound for ablation of uterine fibroids: comparison with ultrasound-guided method. Korean J Radiol 2018; 19(4): 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liao D, Xiao Z, Lv F, et al. Non-contrast enhanced MRI for assessment of uterine fibroids’ early response to ultrasound-guided high-intensity focused ultrasound thermal ablation. Eur J Radiol 2020; 122: 108670. [DOI] [PubMed] [Google Scholar]
- 43. Łoziński T, Filipowska J, Gurynowicz G, et al. Non-invasive therapeutic use of high-intensity focused ultrasound (HIFU) with 3 Tesla magnetic resonance imaging in women with symptomatic uterine fibroids. Ginekol Pol 2017; 88(9): 497–503. [DOI] [PubMed] [Google Scholar]
- 44. Voogt MJ, Trillaud H, Kim YS, et al. Volumetric feedback ablation of uterine fibroids using magnetic resonance-guided high intensity focused ultrasound therapy. Eur Radiol 2012; 22(2): 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pron G. Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) treatment of symptomatic uterine fibroids: an evidence-based analysis. Ont Health Technol Assess Ser 2015; 15(4): 1–86. [PMC free article] [PubMed] [Google Scholar]
- 46. Yang Z, Cao YD, Hu LN, et al. Feasibility of laparoscopic high-intensity focused ultrasound treatment for patients with uterine localized adenomyosis. Fertil Steril 209; 91: 2338–2343. [DOI] [PubMed] [Google Scholar]
- 47. Lee JS, Hong GY, Lee KH, et al. Safety and efficacy of ultrasound-guided high-intensity focused ultrasound treatment for uterine fibroids and adenomyosis. Ultrasound Med Biol 2019; 45(12): 3214–3221. [DOI] [PubMed] [Google Scholar]
- 48. Lee JS, Hong GY, Park BJ, et al. Ultrasound-guided high-intensity focused ultrasound treatment for uterine fibroid & adenomyosis: a single center experience from the Republic of Korea. Ultrason Sonochem 2015; 27: 682–687. [DOI] [PubMed] [Google Scholar]
- 49. Liu L, Wang T, Lei B. Image-guided thermal ablation in the management of symptomatic adenomyosis: a systematic review and meta-analysis. Int J Hyperthermia 2021; 38(1): 948–962. [DOI] [PubMed] [Google Scholar]
- 50. Jeng CJ, Ou KY, Long CY, et al. 500 Cases of high-intensity focused ultrasound (HIFU) ablated uterine fibroids and adenomyosis. Taiwan J Obstet Gynecol 2020; 59(6): 865–871. [DOI] [PubMed] [Google Scholar]
- 51. Huang YF, Deng J, Wei XL, et al. A comparison of reproductive outcomes of patients with adenomyosis and infertility treated with High-Intensity focused ultrasound and laparoscopic excision. Int J Hyperthermia 2020; 37(1): 301–307. [DOI] [PubMed] [Google Scholar]
- 52. Park J, Lee JS, Cho JH, et al. Effects of high-intensity-focused ultrasound treatment on benign uterine tumor. J Korean Med Sci 2016; 31(8): 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li W, Mao J, Liu Y, et al. Clinical effectiveness and potential long-term benefits of high-intensity focused ultrasound therapy for patients with adenomyosis. J Int Med Res 2020; 48(12): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Feng Y, Hu L, Chen W, et al. Safety of ultrasound-guided high-intensity focused ultrasound ablation for diffuse adenomyosis: a retrospective cohort study. Ultrason Sonochem 2017; 36: 139–145. [DOI] [PubMed] [Google Scholar]
- 55. Li X, Zhu X, He S, et al. High-intensity focused ultrasound in the management of adenomyosis: long-term results from a single center. Int J Hyperthermia 2021; 38(1): 241–247. [DOI] [PubMed] [Google Scholar]
- 56. Zhou M, Chen JY, Tang LD, et al. Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril 2011; 95(3): 900–905. [DOI] [PubMed] [Google Scholar]
- 57. Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: two-year follow-up results. Ultrason Sonochem 2015; 27: 677–681. [DOI] [PubMed] [Google Scholar]
- 58. Dev B, Gadddam S, Kumar M, et al. MR-guided focused ultrasound surgery: a novel non-invasive technique in the treatment of adenomyosis-18 month’s follow-up of 12 cases. Indian J Radiol Imaging 2019; 29(3): 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fan TY, Zhang L, Chen W, et al. Feasibility of MRI-guided high intensity focused ultrasound treatment for adenomyosis. Eur J Radiol 2012; 81(11): 3624–3630. [DOI] [PubMed] [Google Scholar]
- 60. Ferrari F, Arrigoni F, Miccoli A, et al. Effectiveness of magnetic resonance-guided focused ultrasound surgery (MRgFUS) in the uterine adenomyosis treatment: technical approach and MRI evaluation. Radiol Med 2016; 121(2): 153–161. [DOI] [PubMed] [Google Scholar]
- 61. Fukunishi H, Funaki K, Sawada K, et al. Early results of magnetic resonance-guided focused ultrasound surgery of adenomyosis: analysis of 20 cases. J Minim Invasive Gynecol 2008; 15(5): 571–579. [DOI] [PubMed] [Google Scholar]
- 62. Jayaram R, Subbarayan K, Mithraprabhu S, et al. Heavy menstrual bleeding and dysmenorrhea are improved by magnetic resonance guided focused ultrasound surgery (MRgFUS) of adenomyosis. Fertil Res Pract 2016; 2(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang Y, Zhou S, Wang J, et al. Efficacy and safety of magnetic resonance-guided focused ultrasound surgery (MRgFUS) ablation in the management of abnormal uterine bleeding due to uterine leiomyoma or adenomyosis. Am J Transl Res 2022; 14(1): 656. [PMC free article] [PubMed] [Google Scholar]
- 64. Wei J, Wang L, Tao H, et al. Comparison of pregnancy outcomes in infertile patients with different types of adenomyosis treated with high-intensity focused ultrasound. Int J Hyperthermia 2023; 40(1): 2238140. [DOI] [PubMed] [Google Scholar]
- 65. Xiong L, Cheng W, Wang Z, et al. Pregnancy outcomes of adenomyotic patients with primary infertility after high-intensity focused ultrasound treatment. Int J Hyperthermia 2023; 40(1): 2264547. [DOI] [PubMed] [Google Scholar]
- 66. Bourdon M, Oliveira J, Marcellin L, et al. Adenomyosis of the inner and outer myometrium are associated with different clinical profiles. Hum Reprod 2021; 36(2): 349–357. [DOI] [PubMed] [Google Scholar]
- 67. Otonkoski S, Sainio T, Mattila S, et al. Magnetic resonance guided high intensity focused ultrasound for uterine fibroids and adenomyosis has no effect on ovarian reserve. Int J Hyperthermia 2023; 40(1): 2154575. [DOI] [PubMed] [Google Scholar]
- 68. Lee JS, Hong GY, Lee KH, et al. Changes in anti-müllerian hormone levels as a biomarker for ovarian reserve after ultrasound-guided high-intensity focused ultrasound treatment of adenomyosis and uterine fibroid. BJOG 2017; 124: 18–22. [DOI] [PubMed] [Google Scholar]
- 69. Zhou CY, Xu XJ, He J. [Pregnancy outcomes and symptom improvement of patients with adenomyosis treated with high intensity focused ultrasound ablation]. Zhonghua Fu Chan Ke Za Zhi 2016; 51(11): 845–849. [DOI] [PubMed] [Google Scholar]
- 70. Lee JS, Kim TE, Kim JH, et al. Unintended pregnancies with term delivery following ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation of uterine fibroid and adenomyosis. Clin Exp Obstet Gynecol 2018; 45(6): 842–844. [Google Scholar]
- 71. Kwack JY, Kwon YS, Im KS, et al. A case report of uterine rupture after repeated conservative treatment for adenomyosis. Clin Exp Obstet Gynecol 2022; 49(8): 178. [Google Scholar]
- 72. Liu Y, Fu N, Lv B, et al. Uterine rupture after high-intensity focused ultrasound ablation of adenomyosis: a case report and literature review. Int J Hyperthermia 2023; 40(1): 2212885. [DOI] [PubMed] [Google Scholar]
- 73. Lai THT, Seto MTY, Cheung VYT. Intrapartum uterine rupture following ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroid and adenomyosis. Ultrasound Obstet Gynecol 2022; 60(6): 816–817. [DOI] [PubMed] [Google Scholar]
- 74. Cai Y, Sun Y, Xu F, et al. Effects of high-intensity focused ultrasound combined with levonorgestrel-releasing intrauterine system on patients with adenomyosis. Sci Rep 2023; 13(1): 9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu Y, Zhang WW, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperthermia 2018; 35(1): 56–61. [DOI] [PubMed] [Google Scholar]
- 76. Liu L, Tian H, Lin D, et al. Risk of recurrence and reintervention after uterine-sparing interventions for symptomatic adenomyosis: a systematic review and meta-analysis. Obstet Gynecol 2023; 141(4): 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem 2015; 27: 671–676. [DOI] [PubMed] [Google Scholar]
- 78. Ko JKY, Seto MTY, Cheung VYT. Thermal bowel injury after ultrasound-guided high-intensity focused ultrasound treatment of uterine adenomyosis. Ultrasound Obstet Gynecol 2018; 52(2): 282–283. [DOI] [PubMed] [Google Scholar]
- 79. Xiong Y, Yue Y, Shui L, et al. Ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation for the treatment of patients with adenomyosis and prior abdominal surgical scars: a retrospective study. Int J Hyperthermia 2015; 31(7): 777–783. [DOI] [PubMed] [Google Scholar]
- 80. Ker CR, Ou KY, Long CY, et al. Acute renal insufficiency and thrombocytopenia after high-intensity focused ultrasound (HIFU) ablation for uterine myomas. Taiwan J Obstet Gynecol 2020; 59(4): 594–597. [DOI] [PubMed] [Google Scholar]
- 81. Hong SH, Hong GS, Lee CW, et al. Complication following ultrasound-guided high-intensity focused ultrasound for the treatment of uterine adenomyosis: case report of CT imaging features. J Korean Soc Radiol 2019; 80(3): 579–584. [Google Scholar]
- 82. Kim KA, Yoon SW, Lee C, et al. Short-term results of magnetic resonance imaging-guided focused ultrasound surgery for patients with adenomyosis: symptomatic relief and pain reduction. Fertil Steril 2011; 95(3): 1152–1155. [DOI] [PubMed] [Google Scholar]
- 83. Bhise SS, Chavan GS, Nanandkar SD, et al. First reported death in india during MRgFUS: a case report. J Indian Acad Forensic Med 2015; 37(2): 204–206. [Google Scholar]
- 84. Zhao TT, Pang LL, Yang LL, et al. Efficacy of high-intensity focused ultrasound combined with LNG-IUS for adenomyosis: a systematic review and meta-analysis. Arch Gynecol Obstet 2023; 308: 351–362. [DOI] [PubMed] [Google Scholar]
- 85. Pang LL, Mei J, Fan LX, et al. Efficacy of high-intensity focused ultrasound combined with GnRH-a for adenomyosis: a systematic review and meta-analysis. Front Public Health 2021; 9: 688264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xu Y, Zhou Z, Wang H, et al. High-intensity focused ultrasound combined with gonadotropin-releasing hormone agonist or levonorgestrel-releasing intrauterine system in treating dysmenorrhea of severe adenomyosis. J Comput Assist Tomogr 2021; 45(2): 224–231. [DOI] [PubMed] [Google Scholar]
- 87. Otgontuya A, Jeng CJ, Wu TN, et al. Comparison of the treatment efficacies of HIFU, HIFU combined with GnRH-a, and HIFU combined with GnRH-a and LNG-IUS for adenomyosis: a systematic review and meta-analysis. Taiwan J Obstet Gynecol 2023; 62(2): 226–238. [DOI] [PubMed] [Google Scholar]
- 88. Zhu H, Ma Q, Dong G, et al. Clinical evaluation of high-intensity focused ultrasound ablation combined with mifepristone and levonorgestrel-releasing intrauterine system to treat symptomatic adenomyosis. Int J Hyperthermia 2023; 40(1): 2161641. [DOI] [PubMed] [Google Scholar]
- 89. Guo Y, Duan H, Cheng J, et al. Gonadotrophin-releasing hormone agonist combined with high-intensity focused ultrasound ablation for adenomyosis: a clinical study. BJOG 2017; 124(Suppl 3): 7–11. [DOI] [PubMed] [Google Scholar]
- 90. Guo Q, Xu F, Ding Z, et al. High intensity focused ultrasound treatment of adenomyosis: a comparative study. Int J Hyperthermia 2018; 35(1): 505–509. [DOI] [PubMed] [Google Scholar]
- 91. Yang X, Zhang X, Lin B, et al. Combined therapeutic effects of HIFU, GnRH-a and LNG-IUS for the treatment of severe adenomyosis. Int J Hyperthermia 2019; 36(1): 486–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057241295593 for High-intensity focused ultrasound in adenomyosis treatment: Insights on safety, efficacy, and reproductive prospects by Shadha Nasser Bahutair and Laila Yahya Alhubaishi in Women’s Health



