Abstract
Background
Extracorporeal membrane oxygenation (ECMO) might be required as a treatment option in patients with critical pulmonary embolism (PE). However, the clinical features and outcomes of the use of ECMO for critical acute PE are still limited. The present study aimed to clarify the clinical characteristics, management strategies and outcomes of patients with acute PE requiring ECMO in the current era using data from a large-scale observational database.
Methods
We analyzed the data of the COMMAND VTE Registry-2: a physician-initiated, multicenter, retrospective cohort study enrolling consecutive patients with acute symptomatic venous thromboembolism (VTE). Among 2035 patients with acute symptomatic PE, there were 76 patients (3.7%) requiring ECMO.
Results
Overall, the mean age was 58.4 years, and 34 patients (44.7%) were men. Cardiac arrest or circulatory collapse at diagnosis was reported in 67 patients (88.2%). The 30-day incidence of all-cause death was 30.3%, which were all PE-related deaths. The 30-day incidence of major bleeding was 54.0%, and the vast majority of bleedings were procedure site-related bleeding events and surgery-related bleeding (22.4%). The 30-day incidence of all-cause death was 6.3% in 16 patients with surgical intervention, 43.8% in 16 patients with catheter intervention, 25.0% in 16 patients with thrombolytic therapy, and 39.3% in 28 patients with anticoagulation only.
Conclusions
The current large real-world VTE registry in Japan revealed clinical features and outcomes of critical acute PE requiring ECMO in the current era, which suggested several unmet needs for future clinical trials.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40560-024-00755-x.
Keywords: ECMO, Acute pulmonary embolism, Surgical pulmonary embolectomy, Prognosis, Mortality, Major bleeding
Background
Pulmonary embolism (PE) is a serious clinical presentation of venous thromboembolism (VTE). Extracorporeal membrane oxygenation (ECMO) could be a treatment option for critical acute PE when cardiac arrest or circulatory collapse is impending or when hemodynamic instability continues despite other treatments [1, 2]. Actually, ECMO has been considered as a bridge therapy to intensive treatment for clot resolution including surgical or catheter embolectomy and systemic thrombolysis [3–5]. A previous study reported that ECMO therapy was associated with a lower mortality risk in critical PE patients and there was a trend toward increasing use of ECMO for patients with massive PE [6], although mortality risk for patients receiving ECMO was still high [6–8]. However, because use of ECMO for critical acute PE has not been so common in daily clinical practice, there is a scarcity of data on patients with PE who were treated with ECMO, which could be important in understanding the unsolved issues and unmet needs in the current treatment of massive PE. Therefore, the present study aimed to clarify the clinical characteristics, management strategies and outcomes in patients with critical acute PE requiring ECMO in a large-scale observational database in Japan.
Methods
Study design
The COMMAND VTE Registry-2 is a physician-initiated, multicenter, retrospective cohort study enrolling consecutive patients with acute symptomatic VTE objectively confirmed by imaging examination or by autopsy among 31 participating centers in Japan between January, 2015 and August, 2020 after the introduction of direct oral anticoagulants (DOACs) for VTE in Japan. The registry design was previously reported in detail [9]. Just briefly, we registered consecutive patients who met the definitions of acute symptomatic VTE diagnosed within 31 days after symptoms onset during the study period [10]. The study was conducted in accordance with the principles of the Declaration of Helsinki. The relevant review boards or ethics committee in all participating centers approved the research protocol (Supplementary Appendix 1, Supplementary Appendix Table). We obtained informed consent in the form of an opt-out on the website of each hospital, because we only used clinical information obtained during routine clinical practice. This method was concordant with the guidelines for epidemiological studies issued by the Ministry of Health, Labor, and Welfare in Japan.
Study population
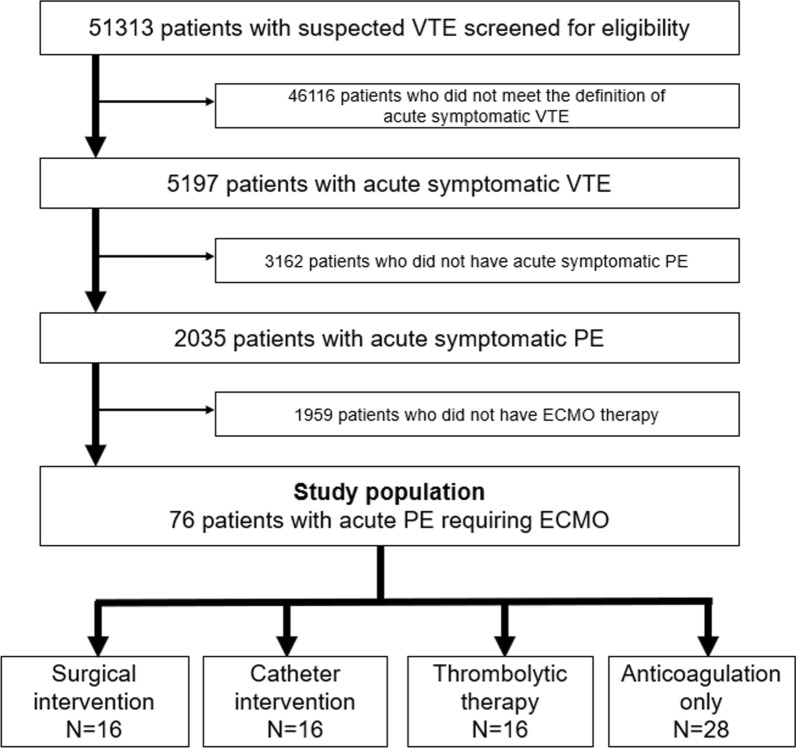
After screening 51,313 patients with suspected VTE for eligibility through chart review by the physicians in each participating center, a total of 5197 patients with acute symptomatic VTE were enrolled in the registry. After excluding 3162 patients without acute symptomatic PE and 1959 PE patients without ECMO use, the current study population consisted of 76 (3.7%) patients with acute PE requiring ECMO (Fig. 1). To investigate the difference depending on different treatment strategies, we divided the current study population into the following 4 subgroups; surgical pulmonary embolectomy (surgical intervention), catheter pulmonary embolectomy (catheter intervention), systemic thrombolysis (thrombolytic therapy) and anticoagulation only. Anticoagulation therapy was defined as oral or parenteral anticoagulation therapy (warfarin, DOAC, or heparin). When multiple treatments were conducted, we exclusively classified them in the following order based on the degree of invasiveness; surgical intervention > catheter intervention > thrombolytic therapy > anticoagulation only. Furthermore, to investigate the difference in baseline characteristics and treatment depending on the vital status at 30 days, we divided the current study population into the survivor and non-survivor groups at 30 days.
Fig. 1.
Patients flowchart in this study. ECMO extracorporeal membrane oxygenation, PE pulmonary embolism, VTE venous thromboembolism
Data collection and definitions of patient characteristics
Data on patient characteristics were collected from the hospital charts or hospital databases according to the prespecified definitions using an electronic case report form in a web-based database system. The physicians in each center were responsible for the entry of data, which were automatically checked for missing or contradictory input and values out of the expected range. The general office of the registry performed additional editing checks.
Patients with active cancer were defined at the time of VTE diagnosis as those receiving treatment for cancer such as chemotherapy or radiotherapy, those scheduled to undergo cancer surgery, those with metastasis to other organs, or those with terminal cancer defined as an expected life expectancy of 6 months or less. History of major bleeding was diagnosed if the patient had a history of International Society of Thrombosis and Hemostasis (ISTH) major bleeding, which consisted of fatal bleeding, symptomatic bleeding in a critical area or organ, and bleeding causing a reduction in the hemoglobin level by at least 2 g/dL or leading to transfusion of at least 2 units of whole blood or red cells. ECMO use at hospital arrival initiated immediately at diagnosis after hospital arrival. In the other group, ECMO was used as the condition worsened after starting initial treatment without ECMO. The detailed definitions of other patient characteristics are provided in Supplementary Appendix 2.
Clinical outcomes
The primary outcome was all-cause death during the 30-day period. The secondly outcome was major bleeding. PE-related death (fatal PE) was adjudicated if it was confirmed by autopsy or if death followed a clinically severe PE, either initially or after recurrent PE events. Major bleeding was defined as ISTH major bleeding [11]. The members of the independent clinical event committee, who were blinded to the patient characteristics, reviewed the detailed clinical courses, and adjudicated the clinical events (Supplementary Appendix 3). If there was inconsistency, final adjudication for clinical events was made based on the full consensus of the committee [9].
Statistical analysis
Considering the small number of patients with ECMO use, we focused on the descriptive statistics in this manuscript. Categorical variables were described as numbers and percentages (%), and continuous variables were described as means and standard deviation or medians and interquartile range (IQR) based on their distributions. The Kaplan–Meier method was used to evaluate the cumulative incidences of clinical outcomes, and we assessed the differences by the log-rank test. Exploratory, we performed univariate logistic regression analysis to evaluate the potential influence of several variables on all-cause death at 30 days, and calculated the odds ratios (OR) with 95% confidence intervals (CI).
All statistical analyses were performed using JMP version 14 (SAS Institute, Cary, NC, USA).
Results
Clinical characteristics
Overall, the mean age was 58.4 years, 34 (44.7%) patients were men, and the mean body mass index was 24.3 kg/m2 (Table 1). Four patients (5.6%) had history of VTE and 5 patients (6.6%) had history of major bleeding. Among 76 patients with ECMO, 16 (21.0%) patients were treated with surgical intervention. Patients who developed PE out of hospital accounted for 60 (79.0%) (Table 2). Patients who presented with cardiac arrest or collapse at diagnosis accounted for 67 (88.2%), and patients who received ECMO at hospital arrival accounted for 61 (80.3%). The median D-dimer level at diagnosis was 28.1 µg/mL and the median BNP level was 225.6 pg/mL.
Table 1.
Clinical characteristics
| Overall | Surgical intervention | Catheter intervention | Thrombolytic therapy | Anticoagulation only | |
|---|---|---|---|---|---|
| Number | 76 | 16 | 16 | 16 | 28 |
| Baseline characteristics | |||||
| Gender, male | 34 (44.7%) | 5 (31.3%) | 7 (43.8%) | 9 (56.3%) | 13 (46.4%) |
| Age (years) | 58.4 ± 14.3 | 61.7 ± 11.8 | 55.5 ± 14.8 | 59.6 ± 17.1 | 57.6 ± 14.0 |
| BMI (kg/m2) | 24.3 ± 4.5 | 24.7 ± 4.5 | 23.8 ± 4.2 | 23.8 ± 4.0 | 24.7 ± 5.0 |
| BMI ≥ 30 kg/m2 | 7 (9.2%) | 2 (12.5%) | 0 (0.0%) | 0 (0.0%) | 5 (17.9%) |
| Comorbidity | |||||
| History of VTE | 4 (5.3%) | 0 (0.0%) | 1 (6.3%) | 2 (12.5%) | 1 (3.6%) |
| History of PE | 2 (2.6%) | 0 (0.0%) | 0 (0.0%) | 1 (6.3%) | 1 (3.6%) |
| History of DVT | 4 (5.3%) | 0 (0.0%) | 1 (6.3%) | 2 (12.5%) | 1 (3.6%) |
| Chronic heart disease | 11 (14.5%) | 2 (12.5%) | 5 (31.3%) | 2 (12.5%) | 2 (7.1%) |
| History of stroke | 2 (2.6%) | 0 (0.0%) | 1 (6.3%) | 1 (6.3%) | 0 (0.0%) |
| COPD/asthma | 4 (5.3%) | 0 (0.0%) | 2 (12.5%) | 0 (0.0%) | 2 (7.1%) |
| History of major bleeding | 5 (6.6%) | 2 (12.5%) | 1 (6.3%) | 1 (6.3%) | 1 (3.6%) |
| Hypertension | 32 (42.1%) | 4 (25.0%) | 7 (43.8%) | 9 (56.3%) | 12 (42.9%) |
| Diabetes | 11 (14.5%) | 0 (0.0%) | 3 (18.8%) | 3 (18.8%) | 5 (17.9%) |
| Dyslipidemia | 13 (17.1%) | 2 (12.5%) | 3 (18.8%) | 3 (18.8%) | 5 (17.9%) |
| Chronic kidney disease | 14 (18.4%) | 2 (12.5%) | 3 (18.8%) | 4 (25.0%) | 5 (17.9%) |
| Dialysis | 1 (1.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (3.6%) |
| Autoimmune disease | 2 (2.6%) | 0 (0.0%) | 1 (6.3%) | 0 (0.0%) | 1 (3.6%) |
| Congenital coagulation defects | 7 (9.2%) | 0 (0.0%) | 1 (6.3%) | 3 (18.8%) | 3 (10.7%) |
| Active cancer | 6 (7.9%) | 2 (12.5%) | 1 (6.3%) | 0 (0.0%) | 3 (10.7%) |
BMI body mass index, VTE venous thromboembolism, PE pulmonary embolism, DVT deep vein thrombosis, COPD chronic obstructive pulmonary disease. The detailed definition of comorbidities is shown in supplementary appendix 2
Table 2.
Initial presentation and laboratory data at diagnosis
| Overall | Surgical intervention | Catheter intervention | Thrombolytic therapy | Anticoagulation only | |
|---|---|---|---|---|---|
| Number | 76 | 16 | 16 | 16 | 28 |
| Initial presentation | |||||
| Out-of-hospital onset | 60 (79.0%) | 11 (68.8%) | 12 (75.0%) | 15 (93.8%) | 22 (78.6%) |
| Arrest/collapse at diagnosis | 67 (88.2%) | 12 (75.0%) | 13 (81.3%) | 15 (93.8%) | 27 (96.4%) |
| Arrest at diagnosis | 52 (68.4%) | 10 (62.5%) | 11 (68.8%) | 7 (43.8%) | 24 (85.7%) |
| Collapse at diagnosis | 15 (19.7%) | 2 (12.5%) | 2 (12.5%) | 8 (50.0%) | 3 (10.7%) |
| Concomitant DVT | 41 (54.0%) | 12 (75.0%) | 10 (62.5%) | 9 (56.3%) | 10 (35.7%) |
| ECMO use at hospital arrival | 61 (80.3%) | 13 (81.3%) | 12 (75.0%) | 12 (75.0%) | 24 (85.7%) |
| Laboratory data at diagnosis | |||||
| WBC (/μL) | 11,250 [9425–15,000] | 10,150 [8937–19,450] | 12,050 [8550–15,750] | 11,350 [9900–14,100] | 11,250 [9425–15,067] |
| Hemoglobin (g/dL) | 12.0 [10.1–14.3] | 11.0 [8.9–13.3] | 13.3 [10.0–14.6] | 14.2 [11.8–16.0] | 11.6 [9.7–13.7] |
| Platelet (109/μL) | 16.7 [11.6–23.7] | 17.0 [10.5–24.3] | 19.2 [14.6–22.6] | 16.1 [12.4–22.3] | 15.8 [10.5–25.6] |
| Total bilirubin (mg/dL) | 0.6 [0.4–0.8] | 0.8 [0.7–1.45] | 0.6 [0.4–0.8] | 0.6 [0.5–0.8] | 0.6 [0.3–0.8] |
| AST (IU/L) | 111 [43–230] | 77 [41–144] | 142 [35–252] | 120 [29–222] | 122 [59–274] |
| ALT (IU/L) | 80 [39–177] | 47 [20–113] | 85 [67–182] | 54 [24–162] | 114 [52–233] |
| Serum creatinine (mg/dL) | 1.03 [0.83–1.33] | 0.94 [0.78–1.10] | 0.96 [0.79–1.16] | 1.13 [0.93–1.33] | 1.09 [0.86–1.45] |
| eGFR (mL/min/1.73m2) | 49.9 [40.1–60.3] | 50.1 [43.6–60.8] | 51.0 [38.6–70.5] | 52.9 [40.7–56.4] | 43.5 [38.7–58.6] |
| D-dimer (μg/mL) | 28.1 [13–49] | 33.2 [12.8–57.3] | 26.3 [14.3–33.5] | 21.5 [10.6–66.0] | 34.5 [9.4–51.9] |
| BNP (pg/mL) | 225.6 [70.1–592.7] | 605.2 [58.6–892.8] | 330.1 [32.5–918.5] | 307.7 [29.5–713.3] | 183.9 [83.0–460.5] |
DVT deep vein thrombosis, ECMO extracorporeal membrane oxygenation, WBC white blood cell, AST aspartate aminotransferase, ALT alanine aminotransferase, eGFR estimated glomerular filtration rate, BNP brain natriuretic peptide. ECMO use at hospital arrival initiated immediately at diagnosis after hospital arrival. In the other group, ECMO was used as the condition worsened after starting initial treatment without ECMO
Clinical outcomes at 30 days
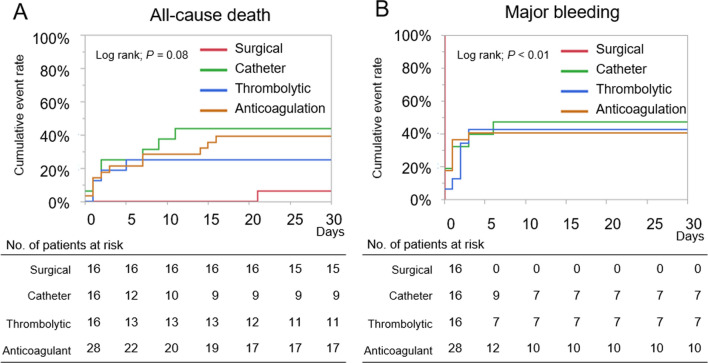
Overall, 17 (30.3%) patients died at 30 days, and all of the deaths were due to PE (PE-related death) (Table 3). Major bleeding occurred in 41 (54.0%) at 30 days, and the most common bleeding site was procedure site bleeding and surgery-related bleeding (N = 17). All patients who received surgical intervention had surgery-related major bleeding events leading to transfusion of 2 or more units of red blood cells during the surgical procedures or within 48 h after surgery, however the surgical intervention group had a numerically lower 30-day incidence of all-cause death compared with other groups (surgical intervention: 6.3%, catheter intervention: 43.8%, thrombolytic therapy: 25.0%, and anticoagulation only: 39.3%). The Kaplan–Meier curves for all-cause death and major bleeding according to the treatment strategies are described in Fig. 2. Most of all-cause death and major bleeding events occurred within 10 and 5 days, respectively.
Table 3.
Clinical outcomes at 30 days
| Overall | Surgical intervention | Catheter intervention | Thrombolytic therapy | Anticoagulation only | |
|---|---|---|---|---|---|
| Number | 76 | 16 | 16 | 16 | 28 |
| All-cause death | 23 (30.3%) | 1 (6.3%) | 7 (43.8%) | 4 (25.0%) | 11 (39.3%) |
| PE-related death | 23 (30.3%) | 1 (6.3%) | 7 (43.8%) | 4 (25.0%) | 11 (39.3%) |
| Major bleeding | 41 (54.0%) | 16 (100%)* | 7 (43.8%) | 6 (37.5%) | 12 (42.9%) |
| Intracranial bleeding | 3 (3.9%) | 0 (0.0%) | 0 (0.0%) | 1 (6.3%) | 2 (7.1%) |
| Respiratory bleeding | 2 (2.6%) | 0 (0.0%) | 1 (6.3%) | 1 (6.3%) | 0 (0.0%) |
| Thoracic cavity/abdominal cavity | 2 (2.6%) | 0 (0.0%) | 0 (0.0%) | 1 (6.3%) | 1 (3.6%) |
| Procedure site bleeding | 17 (22.4%) | 0 (0.0%) | 6 (37.5%) | 3 (18.8%) | 8 (28.6%) |
| Surgery-related bleeding | 17 (22.4%) | 16 (100%) | 0 (0.0%) | 0 (0.0%) | 1 (3.6%) |
PE pulmonary embolism. The detailed definition of clinical outcomes is shown in supplementary appendix 4
*All major bleeding events were bleeding events leading to transfusion of 2 or more units of red blood cells during the surgical procedures or within 48 h after surgery
Fig. 2.
The Kaplan–Meier curves for A all-cause death and B major bleeding according to the treatment strategies
Clinical features comparing the survivors and non-survivors at 30 days
The baseline characteristics, comorbidity, initial presentation, and laboratory data were generally similar between the survivor and non-survivor groups except for brain natriuretic peptide, which was much lower in the survivor group than in the non-survivor group (Table 4). As for treatment, the survivor group included a much higher proportion of surgical intervention than the non-survivor group (28.3 vs. 4.4%, P = 0.009). Univariate regression analysis demonstrated that surgical intervention was significantly associated with a lower risk of 30-day all-cause death (OR, 0.12; 95% CI, 0.01–0.93).
Table 4.
Clinical characteristics comparing the survivor and non-survivor at 30 days
| Survivor | Non-survivor | Univariate odds ratio (95% CI) | |
|---|---|---|---|
| Number | 53 | 23 | |
| Baseline characteristics | |||
| Gender, male | 23 (43.4%) | 11 (47.8%) | 1.20 (0.44–3.19) |
| Age (years) | 59.3 ± 13.6 | 56.4 ± 16.0 | 0.99 (0.95–1.02) |
| BMI (kg/m2) | 24.0 ± 4.0 | 25.1 ± 5.5 | 1.06 (0.94–1.18) |
| BMI ≥ 30 kg/m2 | 3 (5.7%) | 4 (17.4%) | 3.51 (0.72–17.16) |
| Comorbidity | |||
| History of VTE | 3 (5.7%) | 1 (4.4%) | 0.76 (0.07–7.69) |
| History of PE | 1 (1.9%) | 1 (4.4%) | 2.36 (0.14–39.51) |
| History of DVT | 3 (5.7%) | 1 (4.4%) | 0.76 (0.07–7.69) |
| Chronic heart disease | 5 (9.4%) | 6 (26.1%) | 3.39 (0.91–12.55) |
| History of stroke | 2 (3.8%) | 0 (0%) | N/A |
| COPD/asthma | 1 (1.9%) | 3 (13.0%) | 7.8 (0.77–79.56) |
| History of major bleeding | 3 (5.7%) | 2 (8.7%) | 1.59 (0.25–10.20) |
| Hypertension | 23 (43.4%) | 9 (39.1%) | 0.84 (0.31–2.28) |
| Diabetes | 8 (15.1%) | 3 (13.0%) | 0.84 (0.20–3.52) |
| Dyslipidemia | 11 (20.8%) | 2 (8.7%) | 0.36 (0.07–1.79) |
| Chronic kidney disease | 9 (17.0%) | 5 (21.7%) | 1.36 (0.40–4.61) |
| Dialysis | 1 (1.9%) | 0 (0%) | N/A |
| Autoimmune disease | 0 (0%) | 2 (8.7%) | N/A |
| Congenital coagulation defects | 4 (7.6%) | 3 (13.0%) | 1.84 (0.38–8.96) |
| Active cancer | 3 (5.7%) | 3 (13.0%) | 2.50 (0.46–13.44) |
| Initial presentation | |||
| Out-of-hospital onset | 40 (75.5%) | 20 (87.0%) | 2.17 (0.55–8.49) |
| Arrest/collapse at diagnosis | 48 (90.6%) | 19 (82.6%) | 0.49 (0.12–2.04) |
| Arrest at diagnosis | 35 (66.0%) | 17 (73.9%) | 1.46 (0.49–4.34) |
| Collapse at diagnosis | 13 (24.5%) | 2 (8.7%) | 0.29 (0.06–1.42) |
| ECMO use at hospital arrival | 44 (83.0%) | 17 (73.9%) | 0.57 (0.18–1.88) |
| Laboratory data | |||
| WBC (/μL) | 13,200 [1000–15190] | 10,800 [9125–14750] | 1.00 (0.99–1.00) |
| Hemoglobin (g/dL) | 12 [9.7–14.3] | 12.1 [10.9–14.6] | 1.04 (0.88–1.23) |
| Platelet (109/μL) | 16.2 [12.6–24.4] | 17.3 [9.3–20.4] | 0.98 (0.93–1.04) |
| Serum creatinine (mg/dL) | 1.01 [0.81–1.19] | 1.09 [0.92–1.38] | 1.18 (0.70–1.98) |
| eGFR (mL/min/1.73m2) | 50.5 [40.8–60.7] | 48.1 [36.3–56.6] | 0.98 (0.95–1.01) |
| Total bilirubin (mg/dL) | 0.6 [0.4–0.9] | 0.65 [0.4–0.8] | 1.03 (0.69–1.54) |
| D-dimer (μg/mL) | 28.8 [12.5–53.9] | 23.4 [12.8–36.8] | 0.99 (0.98–1.01) |
| BNP (pg/mL) | 143.8 [36.5–4442.6] | 800.1 [230.9–1365.1] | 1.00 (1.00–1.01) |
| Treatment | |||
| Surgical intervention | 15 (28.3%) | 1 (4.4%) | 0.12 (0.01–0.93) |
| Catheter intervention | 10 (18.9%) | 7 (30.4%) | 1.88 (0.61–5.79) |
| Thrombolytic therapy | 13 (24.5%) | 8 (34.8%) | 1.64 (0.57–4.75) |
| Anticoagulation only | 17 (32.1%) | 11 (47.8%) | 1.94 (0.71–5.28) |
BMI body mass index, VTE venous thromboembolism, PE pulmonary embolism, DVT deep vein thrombosis, COPD chronic obstructive pulmonary disease, ECMO extracorporeal membrane oxygenation, WBC white blood cell, eGFR estimated glomerular filtration rate, BNP brain natriuretic peptide, N/A not applicable. The detailed definition of comorbidities is shown in supplementary appendix 2. The odds ratio indicates the risk of death based on the number of survivors
Discussion
The present study demonstrated the detailed epidemiological snapshot of patients with critical acute PE requiring ECMO in Japan. The present study from Japan showed a relatively higher prevalence of ECMO for critical PE (3.7%) compared with reports from the Western countries; previous studies reported that prevalence of ECMO was 0.28% among high-risk PE in the United States, and 0.2% among acute PE in Germany [6, 7]. The present study also showed that patients with ECMO were older (mean age: 58.4 years) and less often men (44.7%) than those in the Western countries; previous studies reported that the mean age was 42 years and the proportion of men was 67.6% in the United States, and the median age was 55 years and the proportion of men was 61.8% in Germany [6, 7]. Other recent reports between 2015 and 2022 in the United States showed that about 2.2% of PE patients with high risk received ECMO therapy [12, 13]. Another study showed that patients with ECMO for acute PE had better outcomes compared with those supported for other indications [14]. There was no significant differences of mortality and bleeding events rate between patients treated with ECMO after systemic thrombosis and those who were not [15]. The use of ECMO on high-risk patients with pulmonary embolism may have become more common in recent years.
The proportion of surgical intervention among patients with ECMO in the present study (21.1%) was comparable to that in United States (17.1%) and in Germany (20.4%) [6, 7]. Notably, the present study also showed a lower incidence rate of mortality (30.3%) in patients with critical acute PE requiring ECOM compared with that in United States (61.6%) and in Germany (61.8%) [6, 7]. In our study, 85.7% of patients had arrest or collapse when ECMO was started. In German cohort, 55.5% of patients had shock and 45.2% of patients needed cardiopulmonary resuscitation [7]. A recent study in France showed 79% of high-risk PE patients with ECMO had arrest at ECMO use and 90-day mortality rate was 59% [15]. Another study showed hospital mortality rate of 47.2% in PE patients with ECMO between 2010 and 2019 [14]. Mortality rate of patients requiring ECMO for acute PE remains still high. These differences could be partly due to the difference in demographics, practice pattern, emergency medical system, and access to medical care.
The current Japanese Circulation Society (JCS) guidelines have recommended to consider ECMO use for patients with cardiac arrest or collapse due to critical PE, and combination with anticoagulation and thrombolysis therapy, or surgical embolectomy, or catheter-directed treatment depending on the available treatment strategies in each institution [16]. A previous study reported that additional ECMO use for patients with failed fibrinolysis resulting in no reperfusion was associated with unfavorable outcomes compared with ECMO use and surgical intervention [8]. Another study also reported that ECMO was a valuable supportive treatment in conjunction with reperfusion treatment, but not as a stand-alone treatment especially for patients with arrest [13]. Present study showed patients with catheter intervention, thrombolytic therapy and anticoagulation only were at a numerically higher risk of mortality compared with surgical intervention, which seemed to be consistent with the previous studies [7, 17]. These findings might suggest that these treatment options other than surgical intervention seemed not to be enough for cases of huge clots resulting in cardiac arrest or collapse. When the amount of thrombus is extremely large, there is concern that sufficient effects cannot be expected from thrombolytic therapy or anticoagulation only and that there is an increased risk of bleeding complications. Surgical intervention under ECMO might be the most effective treatment option in terms of removal of clots. Actually, a previous study reported the utility of a protocolized strategy for acute PE including appropriate surgical intervention for massive PE [18]. On the other hand, availability of surgical intervention could vary widely depending on each institution. Additional treatments for patients with ECMO depended on the patients’ background, medical institution system, and predicted neurological prognosis. In our study, patients with anticoagulation only had the highest rate of arrest at diagnosis (85.7%) in each treatment. In Japan, there are no approved specific devices for use in pulmonary artery thrombus aspiration or thrombus disruption. Thus, alternative treatment options with more easy availability could be unmet needs for critical PE requiring ECMO, which might include upcoming new catheter treatment [19].
The present study had several limitations. First, the study was based on an observational study and treatment strategies, such as implementation of ECMO and several invasive treatments, were determined at the discretion of the attending physicians. Indications for ECMO have been strongly influenced by economics and other non-medical factors. Thus, the results were hypothesis generating and should be interpreted with caution. Second, the absolute number of patients with ECMO was small (N = 76), although it was derived from a large observational database of patients with VTE. Due to lack of adequate statistical power, we could not conduct detailed analyses including multivariable analysis. Third, there is no detailed data of the amount of thrombus in pulmonary artery, its distribution or neurological outcomes. Most of the patients with ECMO had arrest or collapse in this study. Our study, like others, included a large number of patients with severe acute pulmonary embolism. Therefore, the results should be regarded as exploratory.
Conclusions
The current large real-world VTE registry in Japan revealed clinical features and outcomes of critical acute PE requiring ECMO in the current era, which suggested several unmet needs for future clinical trials.
Supplementary Information
Acknowledgements
The authors appreciate the support and collaboration of the co-investigators participating in the COMMAND VTE Registry-2.
Abbreviations
- PE
Pulmonary embolism
- VTE
Venous thrombo-embolism
- ECMO
Extracorporeal membrane oxygenation
- DOACs
Direct oral anticoagulants
- ISTH
International Society of Thrombosis and Hemostasis
- IQR
Interquartile range
- OR
Odds ratios
- CI
Confidence intervals
Author contributions
Kensuke Takabayashi: conceptualization, methodology, software, formal analysis, investigation, writing—original draft; Yugo Yamashita: conceptualization, methodology, software, formal analysis, investigation, writing—review and editing; Takeshi Morimoto: conceptualization, methodology, writing—review and editing; Takeshi Kimura: conceptualization, methodology, writing—review and editing. All other authors contributed to the investigation and supervision. All authors read and approved the final manuscript.
Funding
The COMMAND VTE Registry-2 is partially supported by JSPS KAKENHI (grant number: JP21K16022). The research funding body had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Availability of data and materials
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. However, if the relevant review board or ethics committee approve data sharing and all investigators of the COMMAND VTE Registry-2 provide consent, the deidentified participant data will be shared on a request basis through the principal investigator. Study protocol will also be available. The data will be shared as Excel files via e-mail during the proposed investigation period.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the amended Declaration of Helsinki, and the relevant board or ethics committee in all 31 participating centers.
Consent for publication
The review board waived the requirement for written informed consent because of using clinical information obtained in routine clinical practice.
Competing interests
Dr. Yamashita received lecture fees from Bayer Healthcare, Bristol-Myers Squibb, Pfizer, and Daiichi-Sankyo, and grant support from Bayer Healthcare and Daiichi-Sankyo. Dr. Morimoto reports lecturer's fees from Bristol-Myers Squibb, Daiichi-Sankyo, Japan Lifeline, Kowa, Kyocera, Novartis, and Toray; manuscript fees from Bristol-Myers Squibb and Kowa; advisory board for Sanofi. Dr. Kaneda received lecture fees from Bristol-Myers Squibb, Pfizer, and Daiichi-Sankyo. Dr. Nishimoto received lecture fees from Bayer Healthcare, Bristol-Myers Squibb, Pfizer, and Daiichi-Sankyo. Dr. Ikeda N. received lecture fees from Bayer Healthcare, Bristol-Myers Squibb, and Daiichi-Sankyo. Dr. Ikeda S. received lecture fees from Bayer Healthcare, Bristol-Myers Squibb and Daiichi-Sankyo. All other authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rivers J, Pilcher D, Kim J, Bartos JA, Burrell A. Extracorporeal membrane oxygenation for the treatment of massive pulmonary embolism. An analysis of the ELSO database. Resuscitation 2023; 191:109940. [DOI] [PubMed]
- 2.Belohlavek J. Pulmonary embolism, part II: management. Exp Clin Cardiol. 2013;18:139–47. [PMC free article] [PubMed] [Google Scholar]
- 3.Corsi F, Lebreton G, Brechot N, Hekimian G, Nieszkowska A, Trouillet JL, et al. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care. 2017;21:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh YN, Oh DK, Koh Y, Lim CM, Huh JW, Lee JS, et al. Use of extracorporeal membrane oxygenation in patients with acute high-risk pulmonary embolism: a case series with literature review. Acute Crit Care. 2019;34:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kmiec L, Philipp A, Floerchinger B, Lubnow M, Unterbuchner C, Creutzenberg M, et al. Extracorporeal membrane oxygenation for massive pulmonary embolism as bridge to therapy. ASAIO J. 2020;66:146–52. [DOI] [PubMed] [Google Scholar]
- 6.Elbadawi A, Mentias A, Elgendy IY, Mohamed AH, Syed MH, Ogunbayo GO, et al. National trends and outcomes for extra-corporeal membrane oxygenation use in high-risk pulmonary embolism. Vasc Med. 2019;24:230–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobohm L, Sagoschen I, Habertheuer A, Barco S, Valerio L, Wild J, et al. Clinical use and outcome of extracorporeal membrane oxygenation in patients with pulmonary embolism. Resuscitation. 2022;170:285–92. [DOI] [PubMed] [Google Scholar]
- 8.Meneveau N, Guillon B, Planquette B, Piton G, Kimmoun A, Gaide-Chevronnay L, et al. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: a multicentre series of 52 cases. Eur Heart J. 2018;39:4196–204. [DOI] [PubMed] [Google Scholar]
- 9.Kaneda K, Yamashita Y, Morimoto T, Chatani R, Nishimoto Y, Ikeda N, et al. Anticoagulation strategies and long-term recurrence in patients with venous thromboembolism in the era of direct oral anticoagulants. Eur J Intern Med. 2023;118:59–72. [DOI] [PubMed] [Google Scholar]
- 10.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386–9. [DOI] [PubMed] [Google Scholar]
- 11.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T, Pugliese S, Sethi SS, Parikh SA, Goldberg J, Alkhafan F, et al. Contemporary management and outcomes of patients with high-risk pulmonary embolism. J Am Coll Cardiol. 2024;83:35–43. [DOI] [PubMed] [Google Scholar]
- 13.Farmakis IT, Sagoschen I, Barco S, Keller K, Valerio L, Wild J, et al. Extracorporeal Membrane Oxygenation and Reperfusion Strategies in High-Risk Pulmonary Embolism Hospitalizations. Crit Care Med 2024;52(10):e512–21. [DOI] [PubMed]
- 14.Scott EJ, Young S, Ratcliffe SJ, Wang XQ, Mehaffey JH, Sharma A, et al. Venoarterial extracorporeal life support use in acute pulmonary embolism shows favorable outcomes. Ann Thorac Surg. 2024;118:253–60. [DOI] [PubMed] [Google Scholar]
- 15.Levy D, Saura O, Passarelli MT, Lucenteforte M, Lebreton G, Bougle A, et al. Thrombolysis before venoarterial ECMO for high-risk pulmonary embolism: a retrospective cohort study. Intensive Care Med. 2024;50:1287–97. [DOI] [PubMed] [Google Scholar]
- 16.Group JJW. Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). Circ J. 2011;75:1258–81. [DOI] [PubMed] [Google Scholar]
- 17.Karami M, Mandigers L, Miranda DDR, Rietdijk WJR, Binnekade JM, Knijn DCM, et al. Survival of patients with acute pulmonary embolism treated with venoarterial extracorporeal membrane oxygenation: a systematic review and meta-analysis. J Crit Care. 2021;64:245–54. [DOI] [PubMed] [Google Scholar]
- 18.Pasrija C, Shah A, George P, Kronfli A, Raithel M, Boulos F, et al. Triage and optimization: a new paradigm in the treatment of massive pulmonary embolism. J Thorac Cardiovasc Surg. 2018;156:672–81. [DOI] [PubMed] [Google Scholar]
- 19.Sista AK, Horowitz JM, Tapson VF, Rosenberg M, Elder MD, Schiro BJ, et al. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. JACC Cardiovasc Interv. 2021;14:319–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. However, if the relevant review board or ethics committee approve data sharing and all investigators of the COMMAND VTE Registry-2 provide consent, the deidentified participant data will be shared on a request basis through the principal investigator. Study protocol will also be available. The data will be shared as Excel files via e-mail during the proposed investigation period.