Abstract
Background:
Ascites is common in advanced gastrointestinal cancers with peritoneal metastases (PM) and negatively impacts patient survival. No study to date has specifically evaluated the relationship between ascites, PM and survival outcomes in metastatic colorectal cancer (mCRC) and metastatic gastric cancer (mGC).
Objectives:
This study aims to investigate and elucidate the relationship between malignant ascites, PM and survival outcomes in both mCRC and mGC patients.
Design:
This is a retrospective analysis of prospectively collected clinical trial data of mCRC and mGC patients with PM.
Methods:
We performed two pooled analyses, firstly of two Italian randomized trials enrolling patients with mCRC eligible for systemic therapy (TRIBE2; VALENTINO), and secondly of gastric cancer and peritoneal metastasis (GCPM) patients who underwent bi-directional therapeutic treatment comprising systemic and peritoneal-directed therapies.
Results:
Of 900 mCRC patients, 39 (4.3%) had PM with malignant ascites. Compared to the group without PM, median progression-free and overall survival were significantly inferior in the ascites group (hazard ratio (HR) for progression-free survival (PFS) 1.68, 95% confidence interval (CI): 1.21–2.35, p = 0.007; HR for overall survival (OS) 2.14, 95% CI: 1.57–3.01, p < 0.001), but not in the group of PM without ascites (HR for PFS 1.10, 95% CI: 0.91 – 1.34; HR for OS 1.04, 95% CI: 0.84 – 1.30). Of 170 patients with GCPM, those with ascites had higher median Peritoneal Cancer Index scores (23 vs 9, p < 0.001). Median OS was significantly inferior among those with ascites compared to those without (13.0 vs 21.0 months, HR 1.71, 95% CI: 1.16–2.52, p = 0.007).
Conclusion:
Ascites identifies a subgroup of patients with PM and poor outcomes, for whom tailored research are needed.
Keywords: chemotherapy, gastric cancer, intraperitoneal chemotherapy, medical oncology, precision oncology, prognostic biomarker, systemic treatment, targeted therapy
Introduction
The peritoneal cavity is a common site for metastasis among intraperitoneal tumours, including gynaecological and gastrointestinal malignancies. In metastatic gastric cancer (mGC), the incidence of synchronous and metachronous peritoneal metastases (PM) ranges between 12.9% and 26.5% and between 7% and 32%, respectively. 1 In metastatic colorectal cancer (mCRC), the prevalence of synchronous PM ranges from 4%–5% to 20% based on autopsy findings.2,3
PM may be diagnosed via imaging as part of routine staging or as incidental intra-operative findings in asymptomatic patients with mCRC and mGC. Among symptomatic patients, common symptoms include abdominal pain and distension, due to the presence of malignant ascites. 4 Malignant ascites is a significantly challenging clinical problem, often leading to hospitalization and clinical deterioration. 5 In addition, patients with malignant ascites are usually excluded from locoregional treatment strategies such as cytoreductive surgery (CRS), with or without hyperthermic intraperitoneal chemotherapy (HIPEC).6,7
Malignant ascites is recognized as an adverse prognostic factor across various cancer types. In mCRC patients, peritoneal involvement is acknowledged as a negative prognostic factor and is incorporated into staging criteria (IVC AJCC VIII ed.). 8 Emerging data indicate that the presence of malignant ascites in mCRC and mGC confers resistance to systemic therapy and poorer prognosis.9,10 Our previous research have also demonstrated its negative prognostic impact in patients with microsatellite instability high mCRC treated with immune-checkpoint blockade. 11
Conversely, studies evaluating the role of HIPEC in both mGC and mCRC suggest that the peritoneal carcinomatosis burden, commonly quantified by the peritoneal cancer index (PCI) score, rather than the presence or absence of malignant ascites per se, has significant prognostic implications on survival outcomes.12,13
Regardless of the conflicting data within existing literature, to date, no large-scale study has specifically studied and reported the relationship between malignant ascites and peritoneal carcinomatosis burden, along with its clinical implications. This study aims to investigate and elucidate the relationship between malignant ascites, PM and survival outcomes in both mCRC and mGC patients.
Patients and methods
Study design and patients’ population
We reviewed prospective cohorts of mCRC and gastric cancer patients. First, we performed an individual data pooled analysis of two Italian randomized controlled trials which enrolled patients with mCRC eligible for initial therapy.14,15 The phase III TRIBE-2 trial randomized 679 treatment-naïve mCRC patients to receive the triplet FOLFOXIRI/bevacizumab or sequential-doublet regimens plus bevacizumab; the phase II VALENTINO trial evaluated the effect of maintenance treatment with panitumumab with or without 5-fluorouracil/leucovorin after FOLFOX/panitumumab induction in 229 patients with RAS wild-type mCRC. The inclusion and exclusion criteria of these trials, as well as study protocols, have previously been published.14,15 Clinical and biological characteristics were extracted from trial Case Report Forms. The presence of PM and ascites at baseline was evaluated by means of conventional imaging techniques (i.e. abdominal computed tomography (CT) or magnetic resonance imaging scan) by expert radiologists at each participating centre. The presence of PM was coded as a polytomous categorical variable encompassing three values: no PM, PM without ascites or PM with ascites. The malignant nature of the ascites was assumed, as patients with decompensated liver cirrhosis, renal impairment and active nephrotic syndrome were excluded from these trials.
Next, to cross-validate the prognostic relevance of malignant ascites in advanced gastrointestinal cancers, we conducted a pooled analysis of patients with gastric cancer and peritoneal metastasis (GCPM) who underwent bi-directional therapeutic treatment comprising systemic and peritoneal-directed therapies (catheter-based intraperitoneal chemotherapy (IPC) and/or pressurized intraperitoneal aerosol chemotherapy respectively) in two centres, including a tertiary oncology centre in Singapore, as well as a tertiary surgical oncology centre in Verona. Clinico-pathological data, including PCI and the presence or absence of ascites based on diagnostic laparoscopy, were collected.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the ‘Fondazione IRCCS Istituto Nazionale dei Tumori’ of Milan as well as the Domain Specific Review Board (DSRB) of National Healthcare Group of Singapore (DSRB approval number 2012/00429).
The reporting of this study conforms to the STROBE statement (Supplemental File). 16
Statistical analysis
To evaluate interactions between groups according to the presence of ascites and PM coded as a polytomous categorical variable, Chi-Square or Fisher’s exact test was used, as appropriate. In order to test the presence of outcome differences between groups, survival analysis was performed using the Kaplan–Meier method and Cox’s proportional hazards regression. In the colorectal cohort, progression-free survival (PFS) was defined as the time from the study enrolment to progression of disease or death from any cause, whichever occurred first. Overall survival (OS) was defined as the time from study enrolment, or date of diagnosis of PM, to death from any cause for the colorectal and gastric cohorts, respectively. Univariable Cox regression analysis was used to assess the effect of different baseline factors on PFS and OS; variables significantly associated with survival outcomes at the univariable analysis were then fitted in a multivariable Cox’s proportional hazards regression model. Hazard ratios (HRs) with the corresponding 95% confidence intervals (CIs) were provided for Cox’s proportional hazard regression models. The interaction test was performed to check the presence of a subgroup effect of ascites in different treatment arms. All statistical tests were two-tailed, and a p < 0.05 was considered statistically significant. Statistical analyses were performed using R software (version 4.1.2) and R Studio (version 1.4.1717).
Results
Study population
Colorectal cohort
The colorectal cancer cohort consisted of 900 patients. Among these, 725 patients (80.6%) presented without PM, 136 (15.1%) patients with PM but without ascites, whereas 39 (4.3%) patients had PM with ascites at diagnosis.
Patients’ and disease characteristics, in addition to treatments received, in the entire dataset and according to the presence or absence of PM and ascites, are reported in Table 1. Three groups were considered: (1) patients without PM, (2) patients with PM but without ascites and (3) patients with PM and ascites. The presence of malignant ascites and PM was significantly associated with worse Eastern Cooperative Oncology Group Performance Status (ECOG PS; 1 or more vs 0), higher prevalence of right-sided tumours and BRAF mutations, and lower frequency of liver metastases. Patients with PM and no ascites were more likely to be female, with RAS wild-type/BRAF mutated tumours and without lung metastases at presentation. Treatment type, as expected, was equally distributed among the three subgroups.
Table 1.
Colorectal cancer cohort.
| Characteristics, no. (%) | Total (N = 900) | (A) No PM (N = 725) | (B) PM w/o ascites (N = 136) | (C) PM with ascites (N = 39) | (A) vs (B) | (B) vs (C) | (A) vs (C) |
|---|---|---|---|---|---|---|---|
| Sex | 0.022 | 0.968 | 0.148 | ||||
| Male | 535 (59.4) | 447 (61.7) | 69 (50.7) | 19 (48.7) | |||
| Female | 365 (40.5) | 278 (38.3) | 67 (49.3) | 20 (51.3) | |||
| Age | 0.385 | 1.000 | 0.757 | ||||
| <70 | 714 (79.3) | 570 (78.6) | 112 (82.4) | 32 (82.1) | |||
| ⩾70 | 186 (20.7) | 155 (21.4) | 24 (17.6) | 7 (17.9) | |||
| ECOG PS | 0.162 | 0.049 | <0.001 | ||||
| 0 | 740 (82.2) | 609 (84.0) | 107 (78.7) | 24 (61.5) | |||
| ⩾1 | 160 (17.8) | 116 (16.0) | 29 (21.3) | 15 (38.5) | |||
| Primary tumour resection | 0.019 | 0.017 | 0.209 | ||||
| Yes | 485 (53.9) | 382 (52.7) | 87 (64.0) | 16 (41.0) | |||
| No | 415 (46.1) | 343 (47.3) | 49 (36.0) | 23 (59.0) | |||
| Site of origin | 0.001 | 0.744 | 0.019 | ||||
| Right | 294 (32.7) | 215 (29.7) | 60 (44.1) | 19 (48.7) | |||
| Left | 606 (67.3) | 510 (70.3) | 76 (55.9) | 20 (51.3) | |||
| Synchronous metastasis | 1.000 | 0.188 | 0.166 | ||||
| Yes | 774 (86.0) | 621 (85.7) | 116 (85.3) | 37 (94.9) | |||
| No | 126 (14.0) | 104 (14.3) | 20 (14.7) | 2 (5.1) | |||
| Liver mets | <0.001 | 0.964 | <0.001 | ||||
| Yes | 703 (78.1) | 597 (82.3) | 83 (61.0) | 23 (59.0) | |||
| No | 197 (21.9) | 128 (17.7) | 53 (39.0) | 16 (41.0) | |||
| Nodal mets | 0.544 | 0.352 | 0.132 | ||||
| Yes | 286 (31.8) | 223 (30.8) | 46 (33.8) | 17 (43.6) | |||
| No | 614 (68.2) | 502 (69.2) | 90 (66.2) | 22 (56.4) | |||
| Lung mets | 0.002 | 0.800 | 0.259 | ||||
| Yes | 300 (33.3) | 260 (35.9) | 30 (22.1) | 10 (25.6) | |||
| No | 600 (66.7) | 465 (64.1) | 106 (77.9) | 29 (74.4) | |||
| RAS status | 0.005 | 0.598 | 0.057 | ||||
| Wild-type | 324 (36.0) | 246 (33.9) | 58 (42.6) | 20 (51.3) | |||
| Mutated | 439 (48.8) | 376 (51.9) | 50 (36.8) | 13 (33.3) | |||
| Unknown | 137 (15.2) | 103 (14.2) | 28 (20.6) | 6 (15.4) | |||
| BRAF status | <0.001 | 0.030 | <0.001 | ||||
| Wild-type | 736 (81.8) | 606 (83.6) | 107 (78.7) | 23 (59.0) | |||
| Mutated | 77 (8.5) | 43 (5.9) | 23 (16.9) | 11 (28.2) | |||
| Unknown | 87 (9.7) | 76 (10.5) | 6 (4.4) | 5 (12.8) | |||
| MSS status | 0.830 | 0.409 | 0.498 | ||||
| MSS | 688 (76.4) | 553 (76.3) | 104 (76.5) | 31 (79.5) | |||
| MSI | 31 (3.4) | 25 (3.4) | 6 (4.4) | 0 (0.0) | |||
| Unknown | 181 (20.2) | 147 (20.3) | 26 (19.1) | 8 (20.5) | |||
| Chemotherapy | 0.122 | 0.107 | 0.399 | ||||
| Doublet | 560 (62.2) | 446 (61.5) | 93 (68.4) | 21 (53.8) | |||
| Triplet | 333 (37.0) | 274 (37.8) | 41 (30.1) | 18 (46.2) | |||
| Never treated | 7 (0.8) | 5 (0.7) | 2 (1.5) | 0 (0.0) | |||
| Biologic agent | 0.218 | 0.509 | 0.971 | ||||
| Bevacizumab | 671 (74.6) | 546 (75.3) | 95 (69.9) | 30 (77.0) | |||
| Panitumumab | 229 (25.4) | 179 (24.7) | 41 (30.1) | 9 (23.0) |
Patient and disease characteristics overall and according to the three subgroup of patients: (A) no PM; (B) PM without ascites; (C) PM with ascites.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; MSI, microsatellite instability; MSS, microsatellite stability; PM, peritoneal metastases.
Gastric cohort
The gastric cancer cohort consisted of 170 patients − 74 (43.5%) were treated with bidirectional therapy using catheter-based IPC in a tertiary institution in Singapore, while 96 (56.5%) were treated with pressurized intraperitoneal aerosolized chemotherapy (PIPAC) in a tertiary surgical oncology centre in Verona, Italy. Patients’ and disease characteristics are reported in Table 2. As all these patients were required to have confirmed PM in order to receive bidirectional therapy, only two groups were considered: (1) patients with GCPM without ascites and (2) patients with GCPM with ascites.
Table 2.
Gastric cancer cohort.
| Characteristics | Total (N = 170) | GCPM without ascites (N = 65) | GCPM with ascites (N = 105) | p |
|---|---|---|---|---|
| Sex, no. (%) | 0.525 | |||
| Male | 71 (41.8) | 17 (26.2) | 46 (43.8) | |
| Female | 99 (58.2) | 48 (73.8) | 59 (56.2) | |
| Median age (range), years | 60 (22–82) | 59 (27–81) | 60 (22–82) | 0.510 |
| Ethnicity, no. (%) | 0.019 | |||
| Caucasian | 95 (55.9) | 45 (69.2) | 50 (47.6) | |
| Asian | 74 (43.5) | 20 (30.8) | 54 (51.4) | |
| Others | 1 (0.6) | 0 (0) | 1 (1.0) | |
| BMI, median (range) | 22 (15–29) | 22 (16–29) | 22 (15–28) | 0.450 |
| Synchronous metastases, no. (%) | 0.019 | |||
| Yes | 142 (83.5) | 60 (92.3) | 82 (78.1) | |
| No | 28 (16.5) | 5 (7.3) | 23 (21.9) | |
| Diagnosis of GCPM, no. (%) | 0.031 | |||
| Positive peritoneal washing cytology | 6 (3.5) | 5 (7.7) | 1 (0.1) | |
| Macroscopic PM | 164 (96.5) | 60 (92.3) | 104 (99.9) | |
| Type of IP-directed chemo, no. (%) | 0.011 | |||
| PIPAC | 96 (56.5) | 45 (69.2) | 51 (48.6) | |
| Catheter-based IPC | 74 (43.5) | 20 (30.8) | 54 (51.4) | |
| Median cycles of IP-directed treatment (range) | 3 (0–36) | 2 (1–19) | 3 (0–36) | 0.053 |
| Median PCI (interquartile range) | 18 (9–28) | 9 (4–14) | 23 (16–33) | <0.001 |
Patient and disease characteristics overall and according to two subgroups of patients: GCPM without ascites and GCPM with ascites.
BMI, body mass index; GCPM, gastric cancer and peritoneal metastasis; IP, intra-peritoneal; IPC, intraperitoneal chemotherapy; PCI, peritoneal cancer index; PIPAC, pressurized intraperitoneal aerosolized chemotherapy; PM, peritoneal metastasis.
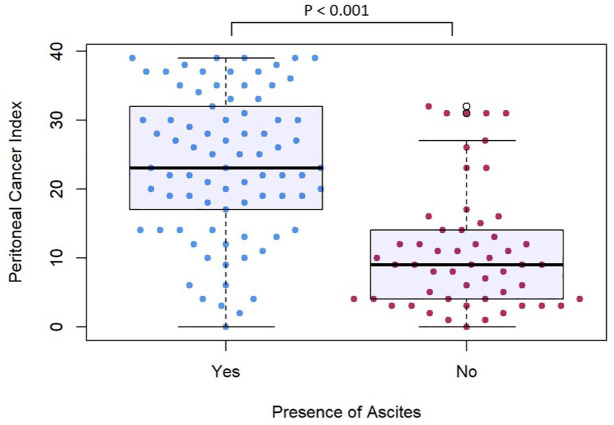
Majority (96.5%) of patients were diagnosed with GCPM with macroscopic disease seen on diagnostic laparoscopy. Among the 135 patients with available information on PCI at diagnosis of GCPM, those with ascites had higher median PCI scores (p < 0.001, Figure 1).
Figure 1.
Peritoneal cancer index in patients with gastric cancer peritoneal metastases with and without ascites.
Survival outcomes according to the presence of PM and ascites
Colorectal cohort
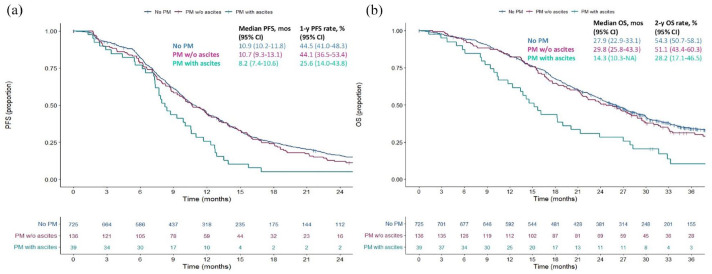
The median follow-up time was 39.87 months (interquartile range: 32.6–47.9). Patients with PM but no ascites had similar PFS and OS to those without PM (mPFS 10.7 vs 10.9 months, HR 1.10, 95% CI: 0.91–1.34; mOS 29.8 vs 27.9 months, HR 1.04, 95% CI: 0.84–1.30), whereas significantly inferior PFS and OS were observed only in patients with PM and ascites (mPFS 8.2, HR 1.68, 95% CI: 1.21–2.35, p = 0.007; mOS 14.3 months; HR 2.14, 95% CI: 1.57–3.01, p < 0.001; Figure 2). Table 3 shows HRs as analysed by means of Cox Regression Model. Multivariate analysis confirmed an independent effect of ascites on both survival outcomes (adjusted HR for PFS 1.60; 95% CI: 1.14–2.24, p < 0.001; for OS 1.78, 95% CI: 1.26–2.51, p = 0.006).
Figure 2.
Kaplan–Meier curves showing cumulative progression-free survival (a) and overall survival (b) according to the three different cohorts of colorectal cancer patients: no PM, PM without ascites, PM with ascites.
OS, overall survival; PFS, progression-free survival; PM, peritoneal metastases.
Table 3.
Colorectal cancer cohort.
| Characteristics | Progression-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable models | Multivariable model | Univariable models | Multivariable model | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Sex | 0.242 | 0.228 | ||||||
| Male | Ref. | Ref. | ||||||
| Female | 0.92 (0.79–1.06) | 0.90 (1.11–0.77) | ||||||
| Age, years | 0.446 | 0.601 | ||||||
| <70 | Ref. | Ref. | ||||||
| ⩾70 | 1.07 (0.90–1.27) | 1.17 (0.97–1.41) | ||||||
| ECOG PS | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| 0 | Ref. | Ref. | Ref. | Ref. | ||||
| ⩾1 | 1.65 (1.38–1.97) | 1.57 (1.30–1.88) | 1.84 (1.52–2.23) | 1.88 (1.54–2.29) | ||||
| Primary tumour resection | 0.003 | 0.006 | <0.001 | 0.014 | ||||
| No | Ref. | Ref. | Ref. | Ref. | ||||
| Yes | 0.81 (0.71–0.93) | 0.82 (0.71–0.95) | 0.76 (0.65–0.89) | 0.80 (0.68–0.96) | ||||
| Site of origin | 0.049 | 0.002 | <0.001 | <0.001 | ||||
| Right | Ref. | Ref. | Ref. | Ref. | ||||
| Left | 0.86 (0.74–0.99) | 0.79 (0.68–0.92) | 0.71 (0.60–0.84) | 0.68 (0.57–0.81) | ||||
| Synchronous mets | 0.107 | 0.01 | 0.459 | |||||
| No | Ref. | Ref. | Ref. | |||||
| Yes | 1.18 (0.96–1.45) | 1.37 (1.08–1.74) | 1.11 (0.85–1.44) | |||||
| Liver mets | 0.008 | 0.001 | 0.003 | <0.001 | ||||
| No | Ref. | Ref. | Ref. | Ref. | ||||
| Yes | 1.26 (1.06–1.49) | 1.34 (1.13–1.60) | 1.36 (1.11–1.66) | 1.50 (1.21–1.87) | ||||
| Nodal mets | 0.537 | 0.034 | 0.041 | |||||
| No | Ref. | Ref. | Ref. | |||||
| Yes | 1.05 (0.90–1.22) | 1.20 (1.01–1.42) | 1.20 (1.01–1.43) | |||||
| Lung mets | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| No | Ref. | Ref. | Ref. | Ref. | ||||
| Yes | 1.28 (1.11–1.48) | 1.35 (1.17–1.57) | 1.33 (1.13–1.57) | 1.46 (1.23–1.72) | ||||
| RAS status | 0.234 | 0.058 | ||||||
| Wild-type | Ref. | Ref. | ||||||
| Mutated | 1.06 (0.96–1.17) | 1.11 (0.99–1.24) | ||||||
| BRAF status | 0.094 | <0.001 | 0.016 | |||||
| Wild-type | Ref. | Ref. | Ref. | |||||
| Mutated | 1.10 (0.98–1.22) | 1.24 (1.10–1.40) | 1.16 (1.03–1.31) | |||||
| MSS status | 0.247 | 0.401 | ||||||
| MSS | Ref. | Ref. | ||||||
| MSI | 1.05 (0.97–1.15) | 1.04 (0.94–1.15) | ||||||
| Chemotherapy | 0.002 | 0.001 | 0.282 | |||||
| Doublet | Ref. | Ref. | Ref. | |||||
| Triplet | 0.79 (0.69–0.92) | 0.78 (0.68–0.91) | 0.91 (0.77–1.08) | |||||
| Biologic agent | 0.724 | 0.022 | 0.726 | |||||
| Bevacizumab | Ref. | Ref. | Ref. | |||||
| Panitumumab | 0.97 (0.83–1.14) | 0.81 (0.67–0.97) | 0.96 (0.79–1.18) | |||||
| PM | 0.007 | <0.001 | <0.001 | 0.006 | ||||
| No PM | Ref. | Ref. | Ref. | Ref. | ||||
| PM without ascites | 1.10 (0.91–1.34) | 1.14 (0.93–1.39) | 1.04 (0.84–1.30) | 1.10 (0.88–1.38) | ||||
| PM with ascites | 1.68 (1.21–2.35) | 1.60 (1.14–2.24) | 2.14 (1.57–3.01) | 1.78 (1.26–2.51) | ||||
Cox proportional hazards regression models for both progression-free survival and overall survival. Both univariable and multivariable model outcomes are shown.
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; MSI, microsatellite instability; MSS, microsatellite stability; PM, peritoneal metastases.
We also investigated the effects of specific treatment types. In the subgroup of patients with ascites, the HR for PFS and OS according to triplet FOLFOXIRI versus doublet FOLFOX regimen were 0.89 (95% CI: 0.47–1.72) and 1.31 (95% CI: 0.66–2.58). The p for interaction between ascites and chemotherapy regimen was not significant (respectively 0.600 for PFS, 0.408 for OS). Analogously, in the subgroup of patients with RAS wild-type status, the biological agent used (bevacizumab or panitumumab) did not significantly affect the survival outcomes in patients with ascites (PFS: HR for panitumumab vs bevacizumab 0.88, 95% CI: 0.33–2.35; OS: HR 0.70, 95% CI: 0.25–2.01). The interaction test between ascites and biological agent was coherently not significant (p = 0.868). The Kaplan–Meier curves displaying the impact of chemotherapy backbone and biological agents used in the groups of patients with and without ascites are shown in Supplemental Figure 1. The HRs for survival outcomes in the three different subgroups (no PM, PM without ascites versus PM with ascites) and according to the treatment type are shown in Supplemental Table 1.
Supplemental Figure 2 shows RECIST v1.1 tumour response according to the presence or absence of ascites and PM. Overall response rate resulted numerically, but not statistically, inferior in the ascites group, whereas no differences were observed between the PM without ascites and the no PM group (OR 0.59, 95% CI: 0.3–1.15 for ascites vs no PM; p = 0.122. OR 0.95, 95% CI: 0.64–1.41 for PM without ascites vs no PM, p = 0.806). No differences were observed in disease control rate between the three groups.
Gastric cohort
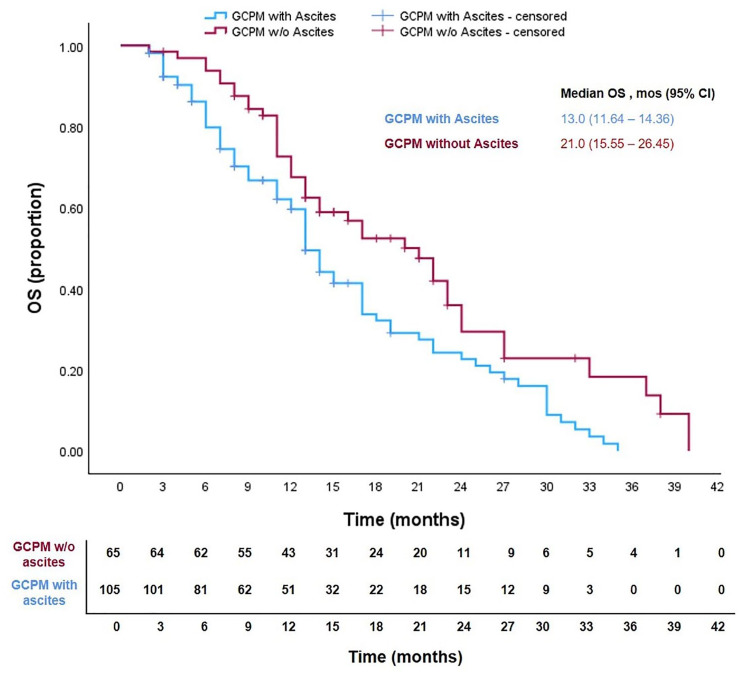
Among GCPM patients, median OS was significantly inferior among those with ascites compared to those without ascites (Figure 3, 13.0 vs 21.0 months, HR 1.71, 95% CI: 1.16–2.52, p = 0.007). The results of the univariate and multivariate analyses for factors associated with OS as analysed by means of Cox Regression Model are presented in Supplemental Table 2. Univariate analysis revealed that presence of ascites and PCI score were significantly associated with OS. A multivariate model using these two factors demonstrated that only PCI score was significantly associated with OS (HR 2.58, 95% CI 1.58–4.24, p < 0.001).
Figure 3.
Kaplan–Meier curves showing overall survival (OS) of patients with gastric cancer peritoneal metastases (GCPM) with and without ascites.
Discussion
In our study, we aimed to elucidate the relationship between PM and malignant ascites, and its implications on survival outcomes. In our cohort of CRC patients undergoing systemic therapy, those with concurrent PM and ascites at diagnosis had inferior survival outcomes compared to those without ascites. This subgroup of patients was more likely to have features associated with poorer prognosis, such as BRAF mutations and right-sided tumour location. 17 The underlying reasons for the pronounced tropism for serous cavities remain to be fully understood; whether it signifies the cancer’s intrinsic biological aggressiveness or merely indicates more advanced disease and, therefore, increased metastatic burden. Notably, patients with PM and ascites were less likely to have liver and lung involvement, potentially suggesting distinct patterns of disease dissemination influenced by tumour biology. This hypothesis is supported by several preclinical works that revealed a specific biology of peritoneal clones from colorectal cancer (mucinous and signet-ring cell histology, higher TIMP-2, IGF-1 and HIF-1A protein expression).3,18
A recent report of CRC patients who underwent CRS/HIPEC demonstrated that patients with ascites had significantly higher PCI scores, although higher PCI score, but not presence of ascites per se, was associated with poorer outcomes. 12 Similarly, in our GCPM cohort of patients who underwent bidirectional therapy, PCI score emerged as the sole prognostic factor associated with OS. While not prognostic for survival when adjusted for PCI, patients with ascites were shown to have significantly higher median PCI scores compared to those without ascites, implying that the presence of ascites reflects an increased extent of peritoneal carcinomatosis.
Findings from our study suggest that the presence of malignant ascites identifies a subgroup of mCRC and mGC patients with increased PM burden with a particularly dismal prognosis. Several potential biological mechanisms may contribute to the poorer survival outcomes observed in this subgroup of patients. The occurrence of malignant ascites in advanced gastrointestinal cancers is driven by a complex interplay of factors such as increased vascular permeability of the peritoneal microenvironment promoted by the presence of vascular endothelial growth factor (VEGF) and lymphatic obstruction by peritoneal carcinomatosis. Inflammatory cytokines (such as IL-6 and IL-8) present within ascitic fluid promote angiogenesis and chemoresistance, thereby providing a pro-survival environment for tumour cells. 19 Other paracrine factors present within malignant ascites, including a mixture of inflammatory cytokines (such as tumour necrosis factor-α, interferon-γ and IL-1β), chemokines and growth factors, also contribute to a tumourigenic environment for PM.20,21 Notably, some of these paracrine factors are not exclusive to gastrointestinal cancers – for example, bevacizumab and tocilizumab, targeting VEGF and IL-6, respectively, have been studied in the setting of ovarian cancer treatment.22,23 Non-cancer cells within malignant ascites, such as macrophages and cancer-associated fibroblasts, also contribute to the progression of PM by inducing an immune-suppressed microenvironment through impairing CD8+ T cells proliferation.24 –26 Lastly, ascites can also result in anorexia and malnutrition, which will also impair patients’ fitness to tolerate prolonged systemic anti-cancer therapy.
Improved treatment options are urgently needed to improve outcomes for patients with PM and concomitant ascites. Intensification of systemic treatment or employing targeted biological agents is a potential strategy. However, the efficacy of FOLFOXIRI with anti-VEGF bevacizumab on survival in our CRC cohort appeared unaffected by the presence or absence of ascites, as indicated by negative interaction tests. Hence, a judicious balance between potential adverse events and treatment efficacy is paramount in such cases. From a biologic standpoint, PM are constituted by a hypoxic environment that hampers chemotherapy penetration and immune cell infiltration. This immunosuppressive niche is associated with the presence of cavity-resident M2-polarized macrophages potentially contributing to decreased response to Programmed-Death-1 inhibition in the presence of ascites.11,27 Combining anti-angiogenic drugs with cytotoxic chemotherapy could theoretically restore the physiological oxygenation in the peritoneal niche, thereby increasing drug and immune cells penetration. 4 However, despite this biological rationale, the use of the antiangiogenic drug bevacizumab did not improve the survival outcomes over the anti-EGFR directed therapies in RAS wild-type subgroup with ascites, albeit this subgroup of patients was relatively small in our cohort. Beyond systemic therapy alone, multimodal approaches integrating systemic chemotherapy, intra-peritoneal directed chemotherapy and/or CRS have been explored in both colorectal and gastric cancers in attempts to improve poor outcomes among patients with PM. Despite initial optimism derived from limited experience in selected patients, HIPEC did not improve prognosis in colorectal cancer patients with macroscopic PM, in the face of higher rate of perioperative complications. 28 Moreover, complete CRS remains challenging especially in cases with substantial ascites, limiting the achievement of curative intent.6,29 In the context of GCPM, various peritoneal-directed approaches including HIPEC, PIPAC and catheter-based IPC have been evaluated as potential treatment options, with heterogeneous outcomes in published studies.30 –35 Emerging approaches, which include newer surgical and intraperitoneal approaches, nanoparticles technologies and Chimeric Antigen Receptor-T Cell, are undergoing early-phase investigation.4,36
Our study has some obvious limitations. Firstly, the inclusion of patients from clinical trials in the CRC cohort may introduce selection bias, as individuals with poor Performance Status (with potentially higher disease burden and increased risk for malignant ascites) were excluded from the TRIBE-2 and VALENTINO trials. In our CRC cohort, a greater proportion of patients with PM and ascites had poorer performance status (ECOG PS ⩾ 1 vs 0) compared to those without PM and PM without ascites (38.5% vs 21.3% vs 16%, Table 1). Yet, despite the potential selection bias, our results demonstrate that CRC patients with PM and ascites have poorer outcomes compared to those without ascites. Secondly, the lack of centralised reporting of imaging scans is a limitation of our study, as the presence of mild ascites may not be systematically reported and could be overlooked by some radiologists. However, the presence of PM and ascites was assessed at each centre by expert radiologists, ensuring a high quality of the imaging reports. The sensitivity of CT in detecting and measuring PM is lower compared to surgical laparoscopy, which is often used to determine PCI, a commonly used metric for quantifying PM. 37 PCI was not available in our CRC cohort of patients receiving systemic therapy, as surgical laparoscopy was not part of standard disease evaluation or treatment protocol; peritoneal-directed therapies in CRC such as HIPEC are not routine practice in our Italian centres. To address this limitation, we therefore sought to corroborate our findings by independently validating the prognostic significance of malignant ascites in our cohort of GCPM patients undergoing bidirectional treatment in our centres, for whom PCI scores were available. We recognise that the absence of PCI scores in our CRC cohort precludes direct comparison with the GCPM cohort. However, independent and consistent findings from both cohorts of patients suggest that ascites is a terminal and biologically different event to the occurrence of PM itself, which occurs across both colorectal and gastric cancer patients as a pan-gastrointestinal cancer phenomenon.
Conclusion
In conclusion, our study of patients with colorectal or gastric cancer and PM suggests that the presence of malignant ascites could represent a critical milestone in the progression of PM, indicative and reflective of increased peritoneal carcinomatosis burden, with correspondingly poorer survival outcomes.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241289517 for Unveiling the prognostic significance of malignant ascites in advanced gastrointestinal cancers: a marker of peritoneal carcinomatosis burden by Leonardo Provenzano, Yong Xiang Gwee, Veronica Conca, Sara Lonardi, Silvia Bozzarelli, Emiliano Tamburini, Alessandro Passardi, Alberto Zaniboni, Federica Tosi, Giuseppe Aprile, Vincenzo Nasca, Alessandra Boccaccino, Margherita Ambrosini, Guglielmo Vetere, Martina Carullo, Marcello Guaglio, Luigi Battaglia, Joseph Jonathan Zhao, Daryl Kai Ann Chia, Wei Peng Yong, Patrick Tan, Jimmy So, Guowei Kim, Asim Shabbir, Chin-Ann Johnny Ong, Francesco Casella, Chiara Cremolini, Maria Bencivenga, Raghav Sundar and Filippo Pietrantonio in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241289517 for Unveiling the prognostic significance of malignant ascites in advanced gastrointestinal cancers: a marker of peritoneal carcinomatosis burden by Leonardo Provenzano, Yong Xiang Gwee, Veronica Conca, Sara Lonardi, Silvia Bozzarelli, Emiliano Tamburini, Alessandro Passardi, Alberto Zaniboni, Federica Tosi, Giuseppe Aprile, Vincenzo Nasca, Alessandra Boccaccino, Margherita Ambrosini, Guglielmo Vetere, Martina Carullo, Marcello Guaglio, Luigi Battaglia, Joseph Jonathan Zhao, Daryl Kai Ann Chia, Wei Peng Yong, Patrick Tan, Jimmy So, Guowei Kim, Asim Shabbir, Chin-Ann Johnny Ong, Francesco Casella, Chiara Cremolini, Maria Bencivenga, Raghav Sundar and Filippo Pietrantonio in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359241289517 for Unveiling the prognostic significance of malignant ascites in advanced gastrointestinal cancers: a marker of peritoneal carcinomatosis burden by Leonardo Provenzano, Yong Xiang Gwee, Veronica Conca, Sara Lonardi, Silvia Bozzarelli, Emiliano Tamburini, Alessandro Passardi, Alberto Zaniboni, Federica Tosi, Giuseppe Aprile, Vincenzo Nasca, Alessandra Boccaccino, Margherita Ambrosini, Guglielmo Vetere, Martina Carullo, Marcello Guaglio, Luigi Battaglia, Joseph Jonathan Zhao, Daryl Kai Ann Chia, Wei Peng Yong, Patrick Tan, Jimmy So, Guowei Kim, Asim Shabbir, Chin-Ann Johnny Ong, Francesco Casella, Chiara Cremolini, Maria Bencivenga, Raghav Sundar and Filippo Pietrantonio in Therapeutic Advances in Medical Oncology
Supplemental material, sj-jpeg-4-tam-10.1177_17588359241289517 for Unveiling the prognostic significance of malignant ascites in advanced gastrointestinal cancers: a marker of peritoneal carcinomatosis burden by Leonardo Provenzano, Yong Xiang Gwee, Veronica Conca, Sara Lonardi, Silvia Bozzarelli, Emiliano Tamburini, Alessandro Passardi, Alberto Zaniboni, Federica Tosi, Giuseppe Aprile, Vincenzo Nasca, Alessandra Boccaccino, Margherita Ambrosini, Guglielmo Vetere, Martina Carullo, Marcello Guaglio, Luigi Battaglia, Joseph Jonathan Zhao, Daryl Kai Ann Chia, Wei Peng Yong, Patrick Tan, Jimmy So, Guowei Kim, Asim Shabbir, Chin-Ann Johnny Ong, Francesco Casella, Chiara Cremolini, Maria Bencivenga, Raghav Sundar and Filippo Pietrantonio in Therapeutic Advances in Medical Oncology
Supplemental material, sj-jpeg-5-tam-10.1177_17588359241289517 for Unveiling the prognostic significance of malignant ascites in advanced gastrointestinal cancers: a marker of peritoneal carcinomatosis burden by Leonardo Provenzano, Yong Xiang Gwee, Veronica Conca, Sara Lonardi, Silvia Bozzarelli, Emiliano Tamburini, Alessandro Passardi, Alberto Zaniboni, Federica Tosi, Giuseppe Aprile, Vincenzo Nasca, Alessandra Boccaccino, Margherita Ambrosini, Guglielmo Vetere, Martina Carullo, Marcello Guaglio, Luigi Battaglia, Joseph Jonathan Zhao, Daryl Kai Ann Chia, Wei Peng Yong, Patrick Tan, Jimmy So, Guowei Kim, Asim Shabbir, Chin-Ann Johnny Ong, Francesco Casella, Chiara Cremolini, Maria Bencivenga, Raghav Sundar and Filippo Pietrantonio in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
ORCID iDs: Alessandro Passardi  https://orcid.org/0000-0002-7099-240X
https://orcid.org/0000-0002-7099-240X
Guglielmo Vetere  https://orcid.org/0000-0001-6943-2897
https://orcid.org/0000-0001-6943-2897
Chiara Cremolini  https://orcid.org/0000-0002-0520-4841
https://orcid.org/0000-0002-0520-4841
Raghav Sundar  https://orcid.org/0000-0001-9423-1368
https://orcid.org/0000-0001-9423-1368
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Leonardo Provenzano, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Yong Xiang Gwee, Department of Haematology–Oncology, National University Cancer Institute, Singapore, Singapore.
Veronica Conca, Department of Translational Research and New Technologies in Medicine, University of Pisa, Pisa, Italy.
Sara Lonardi, Medical Oncology 3 Unit, Veneto Institute of Oncology IOV-IRCCS, Padua, Italy.
Silvia Bozzarelli, Medical Oncology and Hematology Unit, Humanitas Cancer Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy.
Emiliano Tamburini, Medical Oncology Unit, Cardinale G. Panico Hospital, Tricase, Italy.
Alessandro Passardi, Department of Medical Oncology, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) ‘Dino Amadori’, Meldola, Italy.
Alberto Zaniboni, Unity of Oncology, Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy.
Federica Tosi, Department of Hematology, Oncology and Molecular Oncology, Niguarda Cancer Center, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy.
Giuseppe Aprile, Department of Oncology, San Bortolo General Hospital, Azienda ULSS8, Vicenza, Italy.
Vincenzo Nasca, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Alessandra Boccaccino, Department of Translational Research and New Technologies in Medicine, University of Pisa, Pisa, Italy.
Margherita Ambrosini, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Guglielmo Vetere, Unity of Oncology, University Hospital of Pisa, Pisa, Italy.
Martina Carullo, Unity of Oncology, University Hospital of Pisa, Pisa, Italy.
Marcello Guaglio, Colorectal Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Luigi Battaglia, Colorectal Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Joseph Jonathan Zhao, Department of Haematology–Oncology, National University Cancer Institute, Singapore, Singapore.
Daryl Kai Ann Chia, Department of Surgery, University Surgical Cluster, National University Hospital, Singapore, Singapore.
Wei Peng Yong, Department of Haematology–Oncology, National University Cancer Institute, Singapore, Singapore; Singapore Gastric Cancer Consortium, Singapore, Singapore; Cancer Science Institute of Singapore, National University of Singapore, Singapore, Singapore.
Patrick Tan, Singapore Gastric Cancer Consortium, Singapore, Singapore; Cancer Science Institute of Singapore, National University of Singapore, Singapore, Singapore; Cancer and Stem Cell Biology Program, Duke-NUS Medical School, Singapore, Singapore; Genome Institute of Singapore, Agency for Science, Technology and Research, Singapore, Singapore; SingHealth/Duke-NUS Institute of Precision Medicine, National Heart Centre Singapore, Singapore, Singapore; Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
Jimmy So, Department of Surgery, University Surgical Cluster, National University Hospital, Singapore, Singapore; Singapore Gastric Cancer Consortium, Singapore, Singapore; Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore; Division of Surgical Oncology, National University Cancer Institute, Singapore, National University Health System, Singapore, Singapore.
Guowei Kim, Department of Surgery, University Surgical Cluster, National University Hospital, Singapore, Singapore; Division of Surgical Oncology, National University Cancer Institute, Singapore, National University Health System, Singapore, Singapore.
Asim Shabbir, Department of Surgery, University Surgical Cluster, National University Hospital, Singapore, Singapore; Division of Surgical Oncology, National University Cancer Institute, Singapore, National University Health System, Singapore, Singapore.
Chin-Ann Johnny Ong, Department of Sarcoma, Peritoneal and Rare Tumors (SPRinT), Division of Surgery and Surgical Oncology, National Cancer Centre Singapore, Singapore, Singapore.
Francesco Casella, Upper GI Surgery Unit, Verona University Hospital, Verona, Italy.
Chiara Cremolini, Department of Translational Research and New Technologies in Medicine, University of Pisa, Pisa, Italy; Unity of Oncology, University Hospital of Pisa, Pisa, Italy.
Maria Bencivenga, The N.1 Institute for Health, National University of Singapore, Singapore, Singapore; Department of Surgery, Dentistry, Pediatrics and Gynaecology, Upper GI Surgery Unit, University of Verona, Verona, Italy.
Raghav Sundar, Department of Haematology–Oncology, National University Cancer Institute, 1E Kent Ridge Road, Singapore 119228, Singapore; Singapore Gastric Cancer Consortium, Singapore, Singapore; Cancer and Stem Cell Biology Program, Duke-NUS Medical School, Singapore, Singapore; Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore; The N.1 Institute for Health, National University of Singapore, Singapore, Singapore.
Filippo Pietrantonio, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Via G.Venezian, 1, Milan 20133, Italy.
Declarations
Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the ‘Fondazione IRCCS Istituto Nazionale dei Tumori’ of Milan as well as the Domain Specific Review Board (DSRB) of National Healthcare Group of Singapore (DSRB approval number 2012/00429). All the patients provided written informed consent before any study-related procedures.
Consent for publication: Not applicable.
Author contributions: Leonardo Provenzano: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing – original draft.
Yong Xiang Gwee: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing – original draft; Writing – review & editing.
Veronica Conca: Conceptualization; Data curation; Investigation; Methodology; Project administration; Writing – original draft.
Sara Lonardi: Data curation.
Silvia Bozzarelli: Data curation.
Emiliano Tamburini: Data curation.
Alessandro Passardi: Data curation.
Alberto Zaniboni: Data curation.
Federica Tosi: Data curation.
Giuseppe Aprile: Data curation.
Vincenzo Nasca: Data curation.
Alessandra Boccaccino: Data curation.
Margherita Ambrosini: Data curation.
Guglielmo Vetere: Data curation.
Martina Carullo: Data curation.
Marcello Guaglio: Data curation.
Luigi Battaglia: Data curation.
Joseph Jonathan Zhao: Data curation.
Daryl Kai Ann Chia: Data curation.
Wei Peng Yong: Data curation.
Patrick Tan: Data curation.
Jimmy So: Data curation.
Guowei Kim: Data curation.
Asim Shabbir: Data curation.
Chin-Ann Johnny Ong: Data curation.
Francesco Casella: Data curation.
Chiara Cremolini: Data curation.
Maria Bencivenga: Data curation; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Raghav Sundar: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Visualization; Writing – original draft; Writing – review & editing.
Filippo Pietrantonio: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
F.P. reported receiving institutional research grants from BMS, Incyte, Agenus, Amgen, Lilly and AstraZeneca, and personal fees from BMS, MSD, Amgen, Merck-Serono, Pierre-Fabre, Servier, Bayer, Takeda, Astellas, Johnson&Johnson, Rottapharm, Ipsen, AstraZeneca, GSK, Daiichi-Sankyo, Seagen outside the submitted work. C.C. received honoraria from Amgen, Bayer, Merck, Roche and Servier; has consulting or advisory role at Amgen, Bayer, MSD and Roche; was a speakers bureau member at Servier; received research funding from Bayer, Merck and Servier; and received travel and accommodation expenses from Roche and Servier outside the submitted work. S.L. reports research funding from Amgen,Merck Serono, Bayer, Roche, Eli Lilly, AstraZeneca, Bristol Myers Squibb; honoraria from Roche, Eli Lilly, Bristol Myers Squibb, Servier, Merck Serono, Pierre Fabre, GlaxoSmithKline, Amgen; participation in advisory board for Amgen, Merck Serono, Eli Lilly, AstraZeneca, Incyte, Daiichi-Sankyo, Bristol Myers Squibb, Servier, Merck Sharp & Dohme. M.G. reports honoraria from Roche, Amgen. W.P.Y. has consulting or advisory role at AbbVie/Genentech, Amgen, Bristol Myers Squibb, Ipsen, Novartis, AstraZeneca; is a speakers’ bureau member at Lilly, Sanofi/Aventis, Taiho Pharmaceutical, Eisai, Bayer, MSD Oncology; received travel and accommodations expenses from Pfizer. P.T. has stocks in Tempus Healthcare, Auristone Pte Ltd. R.S. reports grants from National Medical Research Council (NMRC/CIRG23Jul-0035 and NMRC/MOH-000627) during the conduct of the study, as well as other support from Bristol Myers Squibb, Merck, Eisai, Bayer, Taiho, Novartis, Eli Lilly, Roche, AstraZeneca, DKSH, MSD, Paxman Coolers, Natera, Astellas, GSK, Ipsen, Pierre-Fabre, Tavotek, Sanofi, Daichii Sankyo, Beigene, CytoMed and Auristone outside the submitted work.
Availability of data and materials: None.
References
- 1. Gwee YX, Chia DKA, So J, et al. Integration of genomic biology into therapeutic strategies of gastric cancer peritoneal metastasis. J Clin Oncol 2022; 40(24): 2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Segelman J, Granath F, Holm T, et al. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 2012; 99(5): 699–705. [DOI] [PubMed] [Google Scholar]
- 3. Hugen N, van de Velde CJH, de Wilt JHW, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 2014; 25(3): 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cortes-Guiral D, Hubner M, Alyami M, et al. Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers 2021; 7(1): 91. [DOI] [PubMed] [Google Scholar]
- 5. Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol 2007; 18(5): 945–949. [DOI] [PubMed] [Google Scholar]
- 6. Randle RW, Swett KR, Swords DS, et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol 2014; 21(5): 1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiao J, Li C, Yu G, et al. Efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) in the management of malignant ascites. World J Surg Oncol 2020; 18(1): 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stillwell AP, Ho YH, Veitch C. Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases. World J Surg 2011; 35(3): 684–692. [DOI] [PubMed] [Google Scholar]
- 9. Zheng LN, Wen F, Xu P, et al. Prognostic significance of malignant ascites in gastric cancer patients with peritoneal metastasis: a systemic review and meta-analysis. World J Clin Cases 2019; 7(20): 3247–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hallam S, Tyler R, Price M, et al. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open 2019; 3(5): 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuca G, Cohen R, Lonardi S, et al. Ascites and resistance to immune checkpoint inhibition in dMMR/MSI-H metastatic colorectal and gastric cancers. J Immunother Cancer 2022; 10(2): e004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Said I, Ubink I, Ewalds RSG, et al. In patients undergoing CRS/HIPEC for colorectal adenocarcinoma with peritoneal metastases, presence of ascites on computed tomography imaging is not a prognostic marker for survival. Ann Surg Oncol 2022; 29(8): 5256–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White MG, Kothari A, Ikoma N, et al. Factors associated with resection and survival after laparoscopic HIPEC for peritoneal gastric cancer metastasis. Ann Surg Oncol 2020; 27(13): 4963–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cremolini C, Antoniotti C, Rossini D, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2020; 21(4): 497–507. [DOI] [PubMed] [Google Scholar]
- 15. Pietrantonio F, Morano F, Corallo S, et al. Maintenance therapy with panitumumab alone vs panitumumab plus fluorouracil-leucovorin in patients with RAS wild-type metastatic colorectal cancer: a phase 2 randomized clinical trial. JAMA Oncol 2019; 5(9): 1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370(9596): 1453–1457. [DOI] [PubMed] [Google Scholar]
- 17. Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol 2016; 17(12): 1709–1719. [DOI] [PubMed] [Google Scholar]
- 18. Varghese S, Burness M, Xu H, et al. Site-specific gene expression profiles and novel molecular prognostic factors in patients with lower gastrointestinal adenocarcinoma diffusely metastatic to liver or peritoneum. Ann Surg Oncol 2007; 14(12): 3460–3471. [DOI] [PubMed] [Google Scholar]
- 19. Kersy O, Loewenstein S, Lubezky N, et al. Omental tissue-mediated tumorigenesis of gastric cancer peritoneal metastases. Front Oncol 2019; 9: 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Grevenstein WM, Hofland LJ, Jeekel J, et al. The expression of adhesion molecules and the influence of inflammatory cytokines on the adhesion of human pancreatic carcinoma cells to mesothelial monolayers. Pancreas 2006; 32(4): 396–402. [DOI] [PubMed] [Google Scholar]
- 21. Cao L, Hu X, Zhang J, et al. The role of the CCL22-CCR4 axis in the metastasis of gastric cancer cells into omental milky spots. J Transl Med 2014; 12: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sjoquist KM, Espinoza D, Mileshkin L, et al. REZOLVE (ANZGOG-1101): a phase 2 trial of intraperitoneal bevacizumab to treat symptomatic ascites in patients with chemotherapy-resistant, epithelial ovarian cancer. Gynecol Oncol 2021; 161(2): 374–381. [DOI] [PubMed] [Google Scholar]
- 23. Pasquier J, Gosset M, Geyl C, et al. CCL2/CCL5 secreted by the stroma induce IL-6/PYK2 dependent chemoresistance in ovarian cancer. Mol Cancer 2018; 17(1): 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito M, Nakano M, Ariyama H, et al. Macrophages are primed to transdifferentiate into fibroblasts in malignant ascites and pleural effusions. Cancer Lett 2022; 532: 215597. [DOI] [PubMed] [Google Scholar]
- 25. Vokurka M, Lacina L, Brabek J, et al. Cancer-associated fibroblasts influence the biological properties of malignant tumours via paracrine secretion and exosome production. Int J Mol Sci 2022; 23(2): 964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chow A, Schad S, Green MD, et al. Tim-4(+) cavity-resident macrophages impair anti-tumor CD8(+) T cell immunity. Cancer Cell 2021; 39(7): 973.e9–988.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruiz Hispan E, Pedregal M, Cristobal I, et al. Immunotherapy for peritoneal metastases from gastric cancer: rationale, current practice and ongoing trials. J Clin Med 2021; 10(20): 4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quenet F, Elias D, Roca L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021; 22(2): 256–266. [DOI] [PubMed] [Google Scholar]
- 29. Ba M, Chen C, Long H, et al. Cytoreductive surgery and HIPEC for malignant ascites from colorectal cancer — a randomized study. Medicine (Baltimore) 2020; 99(33): e21546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chia DKA, So JBY. Recent advances in intra-peritoneal chemotherapy for gastric cancer. J Gastric Cancer 2020; 20(2): 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chia DKA, Sundar R, Kim G, et al. Outcomes of a phase II study of intraperitoneal paclitaxel plus systemic capecitabine and oxaliplatin (XELOX) for gastric cancer with peritoneal metastases. Ann Surg Oncol 2022; 29(13): 8597–8605. [DOI] [PubMed] [Google Scholar]
- 32. Bonnot PE, Piessen G, Kepenekian V, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J Clin Oncol 2019; 37(23): 2028–2040. [DOI] [PubMed] [Google Scholar]
- 33. Kitayama J, Ishigami H, Yamaguchi H, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol 2014; 21(2): 539–546. [DOI] [PubMed] [Google Scholar]
- 34. Ishigami H, Yamaguchi H, Yamashita H, et al. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer 2017; 20(Suppl 1): 128–134. [DOI] [PubMed] [Google Scholar]
- 35. Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol 2018; 36(19): 1922–1929. [DOI] [PubMed] [Google Scholar]
- 36. Lu CS, Lin JK, Chen WS, et al. Intraperitoneal ziv-aflibercept effectively manages refractory ascites in colorectal cancer patients. Oncotarget 2017; 8(22): 36707–36715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koh JL, Yan TD, Glenn D, et al. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 2009; 16(2): 327–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241289517 for Unveiling the prognostic significance of malignant ascites in advanced gastrointestinal cancers: a marker of peritoneal carcinomatosis burden by Leonardo Provenzano, Yong Xiang Gwee, Veronica Conca, Sara Lonardi, Silvia Bozzarelli, Emiliano Tamburini, Alessandro Passardi, Alberto Zaniboni, Federica Tosi, Giuseppe Aprile, Vincenzo Nasca, Alessandra Boccaccino, Margherita Ambrosini, Guglielmo Vetere, Martina Carullo, Marcello Guaglio, Luigi Battaglia, Joseph Jonathan Zhao, Daryl Kai Ann Chia, Wei Peng Yong, Patrick Tan, Jimmy So, Guowei Kim, Asim Shabbir, Chin-Ann Johnny Ong, Francesco Casella, Chiara Cremolini, Maria Bencivenga, Raghav Sundar and Filippo Pietrantonio in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241289517 for Unveiling the prognostic significance of malignant ascites in advanced gastrointestinal cancers: a marker of peritoneal carcinomatosis burden by Leonardo Provenzano, Yong Xiang Gwee, Veronica Conca, Sara Lonardi, Silvia Bozzarelli, Emiliano Tamburini, Alessandro Passardi, Alberto Zaniboni, Federica Tosi, Giuseppe Aprile, Vincenzo Nasca, Alessandra Boccaccino, Margherita Ambrosini, Guglielmo Vetere, Martina Carullo, Marcello Guaglio, Luigi Battaglia, Joseph Jonathan Zhao, Daryl Kai Ann Chia, Wei Peng Yong, Patrick Tan, Jimmy So, Guowei Kim, Asim Shabbir, Chin-Ann Johnny Ong, Francesco Casella, Chiara Cremolini, Maria Bencivenga, Raghav Sundar and Filippo Pietrantonio in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359241289517 for Unveiling the prognostic significance of malignant ascites in advanced gastrointestinal cancers: a marker of peritoneal carcinomatosis burden by Leonardo Provenzano, Yong Xiang Gwee, Veronica Conca, Sara Lonardi, Silvia Bozzarelli, Emiliano Tamburini, Alessandro Passardi, Alberto Zaniboni, Federica Tosi, Giuseppe Aprile, Vincenzo Nasca, Alessandra Boccaccino, Margherita Ambrosini, Guglielmo Vetere, Martina Carullo, Marcello Guaglio, Luigi Battaglia, Joseph Jonathan Zhao, Daryl Kai Ann Chia, Wei Peng Yong, Patrick Tan, Jimmy So, Guowei Kim, Asim Shabbir, Chin-Ann Johnny Ong, Francesco Casella, Chiara Cremolini, Maria Bencivenga, Raghav Sundar and Filippo Pietrantonio in Therapeutic Advances in Medical Oncology
Supplemental material, sj-jpeg-4-tam-10.1177_17588359241289517 for Unveiling the prognostic significance of malignant ascites in advanced gastrointestinal cancers: a marker of peritoneal carcinomatosis burden by Leonardo Provenzano, Yong Xiang Gwee, Veronica Conca, Sara Lonardi, Silvia Bozzarelli, Emiliano Tamburini, Alessandro Passardi, Alberto Zaniboni, Federica Tosi, Giuseppe Aprile, Vincenzo Nasca, Alessandra Boccaccino, Margherita Ambrosini, Guglielmo Vetere, Martina Carullo, Marcello Guaglio, Luigi Battaglia, Joseph Jonathan Zhao, Daryl Kai Ann Chia, Wei Peng Yong, Patrick Tan, Jimmy So, Guowei Kim, Asim Shabbir, Chin-Ann Johnny Ong, Francesco Casella, Chiara Cremolini, Maria Bencivenga, Raghav Sundar and Filippo Pietrantonio in Therapeutic Advances in Medical Oncology
Supplemental material, sj-jpeg-5-tam-10.1177_17588359241289517 for Unveiling the prognostic significance of malignant ascites in advanced gastrointestinal cancers: a marker of peritoneal carcinomatosis burden by Leonardo Provenzano, Yong Xiang Gwee, Veronica Conca, Sara Lonardi, Silvia Bozzarelli, Emiliano Tamburini, Alessandro Passardi, Alberto Zaniboni, Federica Tosi, Giuseppe Aprile, Vincenzo Nasca, Alessandra Boccaccino, Margherita Ambrosini, Guglielmo Vetere, Martina Carullo, Marcello Guaglio, Luigi Battaglia, Joseph Jonathan Zhao, Daryl Kai Ann Chia, Wei Peng Yong, Patrick Tan, Jimmy So, Guowei Kim, Asim Shabbir, Chin-Ann Johnny Ong, Francesco Casella, Chiara Cremolini, Maria Bencivenga, Raghav Sundar and Filippo Pietrantonio in Therapeutic Advances in Medical Oncology