Abstract
Purpose
Not all patients benefit from transarterial chemoembolization (TACE) due to the heterogeneity of the tumour burden in intermediate-stage hepatocellular carcinoma (HCC). To compare the outcomes of transarterial chemoembolization (TACE) combined with molecular-targeted agents plus immune checkpoint inhibitors (TACE-MTAs-ICIs) with those of TACE for patients with unresectable hepatocellular carcinoma (uHCC) that were beyond the up-to-seven criteria.
Patients and methods
Between January 2019 and July 2022, 130 patients diagnosed with uHCC beyond the up-to-seven criteria were retrospectively identified, including 47 patients who received TACE-MTAs-ICIs and 83 patients who received TACE alone. The primary endpoints were overall survival (OS) and progression-free survival (PFS); the secondary endpoints included tumour response and adverse events (AEs).
Results
There were 43 matched patients. The median OS and PFS times in the TACE-MTAs-ICIs group were significantly longer than those in the TACE group (OS: 27.2 vs. 15.9 months, p = 0.007; PFS: 15.4 months vs. 4.8 months, p < 0.001). The objective response rate (ORR) in the TACE-MTAs-ICIs group was higher than that in the TACE group (65.1% vs. 37.2%, p = 0.010). Reversible AEs (grade 3 or 4) occurred differently in TACE-MTAs-ICIs and TACE groups (83.7% vs. 51.2%, p = 0.001). Univariate and multivariate analyses revealed that TACE-MTAs-ICIs treatment was an independent favourable prognostic factor for both PFS and OS (p < 0.001).
Conclusion
For uHCC patients beyond the up-to-seven criteria, TACE-MTAs-ICIs provided superior ORR and OS. Early combined TACE and systemic treatment should shift for patients who are beyond these criteria.
Keywords: Carcinoma, hepatocellular, chemoembolization, therapeutic, molecular-targeted therapy, immune checkpoint inhibitors
KEY MESSAGES
The ORR and median OS reached 65.1% and 27.2 months, respectively, in the treatment model of TACE-MTAs-ICIs for patients with unresectable HCC that were beyond the up-to-seven criteria.
TACE-TKI-ICI yielded a synergistic effect on these patients and was an independent favourable prognostic factor for PFS and OS.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and represents a major global healthcare challenge [1]. Most HCC patients are initially diagnosed at an intermediate or advanced stage, which means that they miss the opportunity for curative treatment [2]. Transarterial chemoembolization (TACE) has been recommended as the primary treatment for patients with intermediate-stage HCC [3, 4]. Due to the heterogeneity of tumour burden and liver function in intermediate-stage HCC, not all patients experience the same benefits from TACE [5].
The up-to-seven criteria (the sum of the size (cm) of the largest tumour and the number of tumours) are commonly used to define Barcelona Clinic Liver Cancer (BCLC) B-stage HCC [6–8], and the efficacy of TACE alone is unsatisfactory for HCC patients meeting the beyond the up-to-seven criteria [9, 10]. The objective response rate (ORR) was 37.8% in patients treated with TACE alone [10]. TACE refractoriness also occurred earlier in patients beyond the up-to-seven criteria than in patients within the criteria (the median time to untreatable progression was 25.7 vs. 14.6 months, p = 0.005) [11]. A proof-of-concept study reported that switching patients who were beyond the up-to-seven criteria to lenvatinib contributed to improved prognosis [median overall survival (OS): 37.9 vs. 21.3 months, p < 0.01] [9]. In addition, the TACE procedure could exacerbate microenvironmental hypoxia and stimulate vascular endothelial growth factor (VEGF) and fibroblast growth factor [12–14], which compromised the clinical benefit. Therefore, a previous study explored whether TACE combined with molecular-targeted agents (MTAs) could improve the clinical outcomes of intermediate-stage HCC patients. In the specific subgroup of patients who were beyond the up-to-seven criteria, no significant improvement was found. As for systemic therapies, one prior study showed that the ORR at 6 weeks of atezolizumab plus bevacizumab in patients with HCC beyond the up-to-seven criteria was 42.5% according to the modified response evaluation criteria in solid tumours (mRECIST) [15]. Therefore, for intermediate-stage patients beyond the up-to-seven criteria, treatment remains challenging. Notably, immune checkpoint inhibitors (ICIs) have exhibited promising clinical benefits and play a vital role in the long-term remission of tumours [16, 17]. Considering the secondary enhanced PD-1/PD-L1 expression after TACE [18], in theory, combining ICIs with TACE and MTAs can reduce neovascularization and prolong immune activation in patients with HCC, which results in a synergistic effect on the antitumor response [16, 17]. Based on these findings, we hypothesized that combined TACE with MTAs and ICIs would provide a superior clinical benefit for patients with HCC beyond the up-to-seven criteria.
Therefore, we conducted this retrospective study to evaluate the efficacy and safety of combining TACE with MTAs plus ICIs for HCC patients beyond the up-to-seven criteria.
Patients and methods
This retrospective study was approved by the local institutional ethics review board. Written informed consent was not required for this retrospective study. The study was conducted by the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of our institution. The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Patients
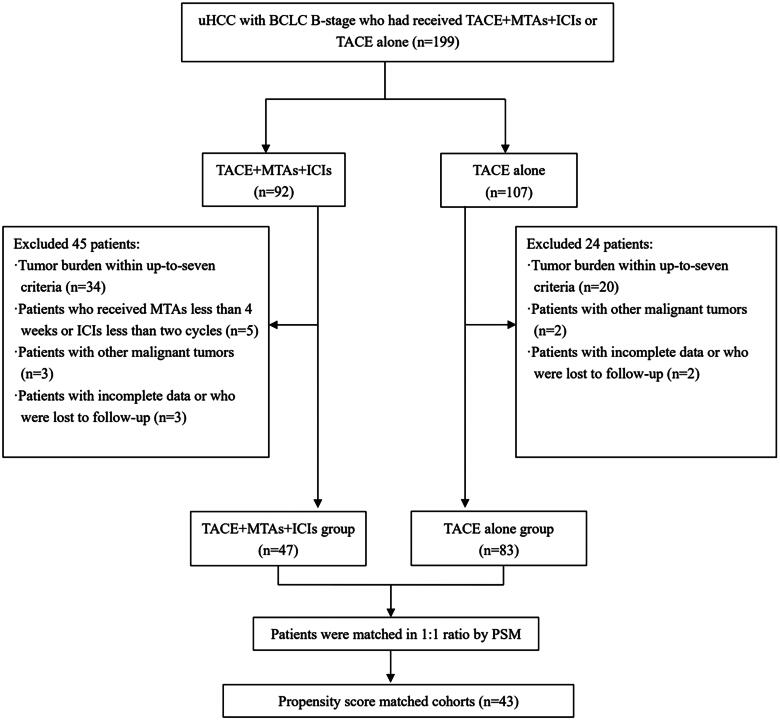
Between January 2019 and June 2022, the medical records of 199 patients were reviewed. HCC was confirmed pathologically or clinically based on the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China [3]. The inclusion criteria were as follows: (1) BCLC B-stage patients; (2) Eastern Cooperative Oncology Group (ECOG) performance status score of 0; and (3) Child-Pugh class A or B. The exclusion criteria were as follows: (1) tumour burden within the up-to-seven criteria; (2) patients who received MTAs less than 4 weeks or ICIs less than two cycles; (3) patients with other malignant tumours; and (4) patients with incomplete data or who were lost to follow-up. In this study, there were overlapping population with our previous research (21 patients who treated with TACE alone) [14]. A total of 130 patients were ultimately included in this study (Figure 1).
Figure 1.
Patient enrolment flowchart. Abbreviations: uHCC, unresectable hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization; MTAs, molecular targeted agents; ICIs, immune check-point inhibitors; PSM, propensity score matching.
Base class, albumin-bilirubin (ALBI) grade, α-fetoprotein (AFP) level, ECOG performance status, number of tumours, maximum tumour diameter, and subclassification of BCLC B-stage [7].
TACE procedure
TACE was performed before systemic therapy. The TACE procedure was performed in a superselective fashion. A 5-F catheter was introduced under local anaesthesia, and then, angiography was used to identify the tumour number, size, and location. Then, a chemotherapeutic agent (lobaplatin, 30 to 50 mg) was infused through the hepatic artery. Subsequently, a super-selective microcatheter was inserted into the feeding artery of the tumours. The embolization procedure using a microcatheter was conducted employing a standard technique involving lipiodol (5 to 20 mL) combined with epirubicin (10 to 20 mg). The feeding arteries were blocked by gelatin sponges or particles. The TACE procedure was performed by two doctors with over 10 years of experience.
Systematic therapy
MTAs or ICIs were suspended during the TACE procedure, and patients recovered after TACE. Atezolizumab (1200 mg), sintilimab (200 mg), or camrelizumab (200 mg) were injected intravenously approximately every 3 weeks. For MTAs, bevacizumab was injected intravenously at 7.5 mg/kg, and lenvatinib was administered orally at a dose of 8 mg for patients < 60 kg or 12 mg for patients ≥ 60 kg daily. Termination or changes to the therapeutic regimen were considered based on disease progression, unacceptable adverse events (AEs), patient refusal, or clinician decision.
Follow-up and assessments
Overall survival (OS) was defined from the date of the initiation of therapy to the date of death for any reason; progression-free survival (PFS) was defined from the date of the initiation of therapy to the date of progression. Enhanced CT or MRI was implemented at 2–3-month intervals. Iodixanol is used for dynamic three-phase scans in enhanced CT, with scans conducted at 25 ∼ 30 s, 60 ∼ 75 s, and 120 ∼ 180 s for the arterial, portal venous, and delayed phases, respectively. Gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid is used for dynamic four-phase enhanced MRI scans, and post-contrast images are obtained at 15 s, 45 s, 3 min, and 15 min after contrast injection for arterial, portal venous, delayed, and hepatobiliary phases. All image evaluations were performed by a radiologist with over 8 years of experience. Tumour responses were assessed based on the mRECIST [19]. The objective response rate (ORR) was defined as the proportion of patients who achieved a complete response (CR) or partial response (PR). The disease control rate (DCR) was defined as the rate of objective response plus stable disease (SD).
Treatment-related toxicity was observed and recorded according to the National Cancer Institute Common Toxicity Criteria Adverse Events (CTCAE) version 5.0. Liver function, blood coagulation profile, and serum alpha-fetoprotein (AFP) levels were examined approximately 1 month later. The end date of the follow-up was 31 October 2022.
Statistical analysis
A propensity score matching (PSM) analysis was conducted between the TACE-MTAs-ICIs group and the TACE group to minimize study bias, and the caliper value was set at 0.02. The variables were as follows: age, sex, Child-Pugh class, AFP, BCLC B substage, and aetiology. OS and PFS were estimated using the Kaplan–Meier method, and the differences were assessed for significance using the log-rank test. The Cox proportional hazards regression model was used to determine the factors associated with survival. Factors with p < 0.1 in the univariate analysis were input into the multivariate analysis. Categorical data are expressed as percentages and frequencies and were compared between the two groups using the χ2 test or Fisher’s exact test as appropriate. A two-tailed p value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS (Version 25.0, Chicago, IL) and GraphPad Software (Prism 8.0.1, San Diego, California).
Results
Patient characteristics
A total of 47 patients and 83 patients treated with TACE-MTAs-ICIs and TACE alone, respectively, were enrolled in this study. The clinical characteristics of all the patients (n = 130) are given in Table 1. After the PSM analysis, 43 pairs of patients were matched (Figure 1). The two groups were similar in each clinical variable after PSM. The average number of TACE procedures was 3.2 (range, 1–11) and 3.6 (range, 1–11) in the TACE-MTAs-ICIs and TACE groups, respectively.
Table 1.
Patient characteristics before and after propensity score matching.
| Variables | Before PSM |
After PSM |
||||
|---|---|---|---|---|---|---|
| TACE-MTAs-ICIs | TACE | p value | TACE-MTAs-ICIs | TACE | p value | |
| (n = 47) | (n = 83) | (n = 43) | (n = 43) | |||
| Age (years) | 0.222 | 0.664 | ||||
| ≥ 60 | 18 (38.3%) | 41 (49.4%) | 18 (41.9%) | 20 (46.5%) | ||
| < 60 | 29 (61.7%) | 42 (50.6%) | 25 (58.1%) | 23 (53.5%) | ||
| Sex | 0.102 | 1.000 | ||||
| Male | 43 (91.5%) | 67 (80.7%) | 40 (93.0%) | 39 (90.7%) | ||
| Female | 4 (8.5%) | 16 (19.3%) | 3 (7.0%) | 4 (9.3%) | ||
| Aetiology | 0.041 | 0.178 | ||||
| HBV | 39 (83.0%) | 55 (66.3%) | 36 (83.7%) | 40 (93.0%) | ||
| Others | 8 (17.0%) | 28 (33.7%) | 7 (16.3%) | 3 (7.0%) | ||
| Child-Pugh Class | 0.743 | 0.763 | ||||
| A | 39 (83.0%) | 69 (83.1%) | 36 (83.7%) | 37 (86.0%) | ||
| B | 8 (17.0%) | 14 (16.9%) | 7 (16.3%) | 6 (14.0%) | ||
| BCLC B substage | 0.703 | 1.000 | ||||
| B2 | 44 (93.6%) | 79 (95.2%) | 42 (97.7%) | 42 (97.7%) | ||
| B3 | 3 (6.4%) | 4 (4.8%) | 1 (2.3%) | 1 (2.3%) | ||
| Tumour diameter (cm) | 0.042 | 0.074 | ||||
| ≥ 7 | 34 (72.9%) | 45 (54.2%) | 31 (72.1%) | 23 (53.5%) | ||
| < 7 | 13 (17.7%) | 38 (45.8%) | 12 (27.9%) | 20 (46.5%) | ||
| Tumours number | 0.646 | 0.510 | ||||
| < 3 | 19 (40.4%) | 37 (44.6%) | 16 (37.2%) | 19 (44.2%) | ||
| ≥ 3 | 28 (59.6%) | 46 (55.4%) | 27 (62.8%) | 24 (55.8%) | ||
| ALBI | 0.330 | 0.357 | ||||
| 1 | 12 (24.5%) | 28 (33.7%) | 12 (27.9%) | 16 (37.2%) | ||
| 2 | 35 (74.5%) | 55 (66.3%) | 31 (72.1%) | 27 (62.8%) | ||
| AFP (ng/mL) | 0.226 | 0.666 | ||||
| < 400 | 22 (46.8%) | 48 (57.8%) | 20 (46.5%) | 22 (51.2%) | ||
| ≥ 400 | 25 (53.2%) | 35(42.2%) | 23 (53.5%) | 21 (48.8%) | ||
Abbreviations: PSM, propensity score matching; HBV, hepatitis B virus; ALBI, albumin-bilirubin; AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer.
Survival analysis
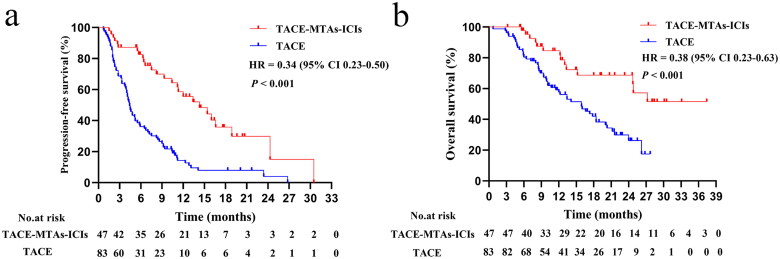
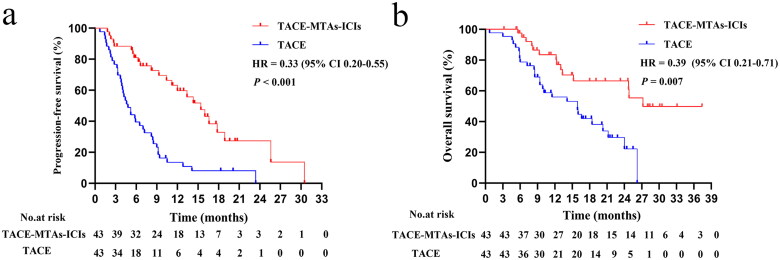
The median follow-up was 21.6 months. Before PSM, 14 patients (29.8%) in the TACE-MTAs-ICIs group and 51 patients (61.4%) in the TACE group died. The median PFS was 14.3 months in the TACE-MTAs-ICIs group and 4.4 months in the TACE group (p < 0.001) (Figure 2(a)), and the corresponding median OS was not reached and 15.9 months, respectively (p < 0.001) (Figure 2(b)). After PSM, the median PFS was 15.4 months in the TACE-MTAs-ICIs group and 4.8 months in the TACE group (p < 0.001) (Figure 3(a)), and the corresponding median OS was 27.2 and 15.9 months, respectively (p = 0.007) (Figure 3(b)).
Figure 2.
Kaplan–Meier survival for progression-free survival before PSM (a). Kaplan–Meier survival for survival before PSM (b).
Figure 3.
Kaplan–Meier survival for progression-free survival before PSM (a), Kaplan–Meier survival for overall survival after PSM (b).
After PSM, multivariate analysis indicated that the AFP level [hazard ratio (HR) = 2.96; 95% CI, 1.55–5.71; p = 0.001] and treatment method (HR = 0.31; 95% CI, 0.15–0.61; p = 0.001) were independent predictive factors for OS (Table 2). Multivariate analysis showed that the treatment method (HR = 0.23; 95% CI, 0.13–0.41; p < 0.001) was an independent predictive factor of PFS (Table 3).
Table 2.
Predictive factor analysis for overall survival (after matching).
| Variables | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (≥ 60 years) | 0.77 (0.42–1.43) | 0.411 | ||
| Sex (male) | 1.06 (0.38–2.99) | 0.907 | ||
| BCLC (B3) | 4.81 (0.60–38.48) | 0.139 | ||
| Child-Pugh class (B) | 1.08 (0.48–2.43) | 0.862 | ||
| ALBI (2) | 1.16 (0.61–2.20) | 0.657 | ||
| AFP (≥ 400 ng/mL) | 2.58 (1.35–4.93) | 0.004 | 2.96 (1.55–5.71) | 0.001 |
| Tumours number (≥ 3) | 1.59 (0.84–3.03) | 0.156 | ||
| Tumour diameter (≥ 7 cm) | 0.93 (0.50–1.71) | 0.809 | ||
| TACE-MTAs-ICIs | 0.35 (0.18–0.70) | 0.003 | 0.31 (0.15–0.61) | 0.001 |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; ALBI, albumin-bilirubin; AFP, α‐fetoprotein.
Table 3.
Predictive factor analysis for progression-free survival (after matching).
| Variables | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (≥ 60 years) | 0.86 (0.52–1.43) | 0.579 | ||
| Sex (male) | 0.82 (0.35–1.92) | 0.652 | ||
| BCLC (B3) | 1.23 (0.17–9.03) | 0.836 | ||
| Child-Pugh class (B) | 1.16 (0.58–2.28) | 0.092 | 1.23 (0.60–2.54) | 0.118 |
| ALBI (2) | 1.02 (0.60–1.74) | 0.945 | ||
| AFP (≥ 400 ng/mL) | 1.00 (0.61–1.65) | 0.998 | ||
| Tumours number (≥ 3) | 1.36 (0.82–2.27) | 0.234 | ||
| Tumour diameter (≥ 7 cm) | 0.78 (0.47–1.29) | 0.780 | ||
| TACE-MTAs-ICIs | 0.30 (0.18–0.51) | < 0.001 | 0.23 (0.13-0.41) | < 0.001 |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; ALBI, albumin-bilirubin; AFP, α‐fetoprotein.
Tumour response
The tumour responses in the two groups after PSM are shown in Table 4. The ORR was significantly higher in the TACE-MTAs-ICIs group than in the TACE group (65.1% vs. 37.2%, p = 0.010). In addition, DCR was similar between the TACE-MTAs-ICI group and the TACE group (76.7% vs. 69.8%, p = 0.465).
Table 4.
Tumour response based on the modified response evaluation criteria in solid tumours.
| Unmatched group |
Matched group |
|||||
|---|---|---|---|---|---|---|
| TACE-MTAs-ICIs (n = 47) | TACE (n = 83) | p value | TACE-MTAs-ICIs (n = 43) | TACE (n = 43) | p value | |
| CR | 2 (4.3%) | 1 (1.2%) | 0.296 | 2 (4.7%) | 1 (2.3%) | 1.000 |
| PR | 28 (59.6%) | 34 (41.0%) | 0.041 | 26 (60.5%) | 15 (34.9%) | 0.018 |
| SD | 5 (10.6%) | 21 (25.3%) | 0.045 | 5 (11.6%) | 14 (32.6%) | 0.019 |
| PD | 12 (25.5%) | 27 (32.5%) | 0.403 | 10 (23.3%) | 13 (30.2%) | 0.465 |
| ORR | 30 (63.8%) | 35 (42.2%) | 0.018 | 28 (65.1%) | 16 (37.2%) | 0.010 |
| DCR | 35 (74.5%) | 56 (67.5%) | 0.403 | 33 (76.7%) | 30 (69.8%) | 0.465 |
Abbreviations: CR, complete response; PR, partial response; SD, stable response; ORR, objective response rate; PD, progressive disease; DCR, disease control rate; CI, confidence interval.
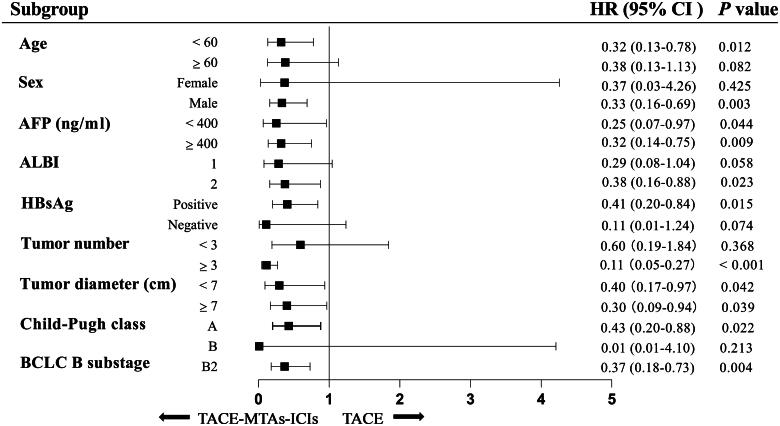
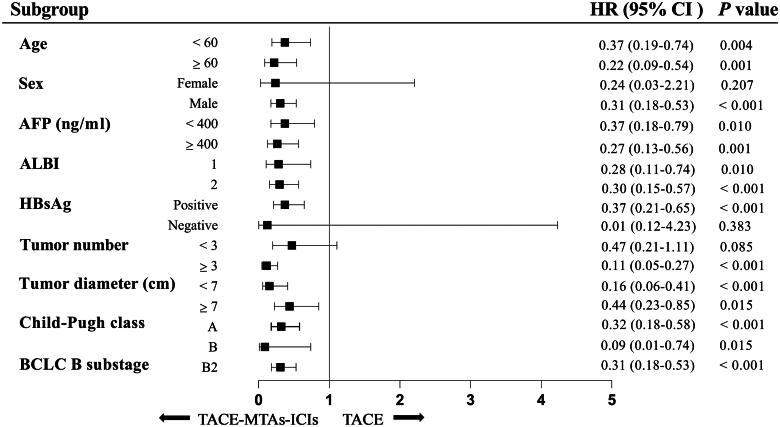
Subgroup analysis
After PSM, the subgroup analyses of the factors associated with OS and PFS are shown in Figure 4 and Figure 5 as forest plot. The results indicated that TACE-MTAs-ICIs treatment could confer superior survival and PFS benefits for most subgroups.
Figure 4.
Forest plot for overall survival of the two groups of patients. Abbreviations: AFP, α-fetoprotein; ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; HR, hazard ratio.
Figure 5.
Forest plot for progression-free survival of the two groups of patients. Abbreviations: AFP, α-fetoprotein; ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; HR, hazard ratio.
AEs (grades 3 and 4)
Treatment‐related AEs are shown in Table 5. The incidence rates of grade 3 and 4 AEs were higher in the TACE-MTAs-ICIs group than in the TACE group (83.7% vs. 51.2%, p = 0.001). These AEs resolved or were eliminated after conservative treatment. No treatment‐related deaths occurred. In addition, dose reduction and treatment interruption of MTAs were observed in 27.9% (12 of 43) of patients in the TACE-MTAs-ICIs group. Treatment interruption was performed in one patient in the TACE-MTA-ICI group because of hypothyroidism. After receiving symptomatic treatment, the patient resumed the previous treatment.
Table 5.
Treatment-related adverse events (grade 3 or 4) (after matching).
| Adverse events | TACE-MTAs-ICIs (n = 43) | TACE (n = 43) | p value |
|---|---|---|---|
| All | 36 (83.7%) | 22 (51.2%) | 0.001 |
| Hypertension | 6 (13.2%) | 0 | 0.026 |
| Elevated AST | 12 (27.9%) | 9 (20.9%) | 0.451 |
| Elevated ALT | 13 (30.2%) | 9 (20.9%) | 0.323 |
| Hand-foot-skin reactions | 1 (2.3%) | 0 | >0.999 |
| Abdominal pain | 2 (4.7%) | 2 (4.7%) | >0.999 |
| Fever | 1 (2.3%) | 2 (4.7%) | >0.999 |
| Hypothyroidism | 1 (2.3%) | 0 | >0.999 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase;
Discussion
TACE is recommended as the best treatment for intermediate HCC in many countries [4]. However, HCC with BCLC B-stage is a highly heterogeneous, posing unique challenges for therapeutic modalities, and TACE might not be the most effective treatment for patients who are beyond the up-to-seven criteria [7, 9]. HCC patients who exceed the up-to-seven criteria are less likely to respond to TACE alone due to its high tumour burden [20]. In this study, compared to TACE alone, the triple treatment modality improved the tumour response rate (ORR: 65.1%) and clinical benefit (median OS: 27.2 months; median PFS: 15.4 months) in HCC patients who were beyond the up-to-seven criteria. Survival increased by a median of 11.3 months with the triple treatment modality.
The initial and optimal responses after TACE predict survival well. Tumours larger than 5 cm and ≥ 4 tumours are less likely to achieve a complete response to TACE [21]. Attaining an effective response at the initial TACE remains the strongest indicator of a favourable outcome in HCC, even after repeat sessions. Notably, repeated TACE procedures could impact liver function. Therefore, it is important to consider early initiation of systemic therapy to prevent any compromise in liver function, especially for HCC patients beyond the up-to-seven criteria, which could enhance overall treatment outcomes [5]. The TACTICS trial showed the benefit of sorafenib followed by TACE as a treatment option to improve the clinical outcome of patients with HCC compared with TACE alone (median PFS: 25.2 vs. 13.5 months, p = 0.006; OS at 1 year: 96.2% vs. 77.2%) [22]. Similarly, compared to monotherapy with lenvatinib, the combination of TACE and lenvatinib improved the median OS (17.8 vs. 11.5 months, p < 0.001) and median PFS (10.6 vs. 6.4 months, p < 0.001), as observed in the LAUNCH study [23]. Although phase III trials of immunotherapy with nivolumab or pembrolizumab did not meet the primary endpoint of OS, a clinical benefit was still observed [24, 25]. Combination therapies based on immunotherapy have been shown to improve survival rates and provide durable responses in HCC patients [26]. In particular, what we should learn from these studies is that the idea of treatment stage migration may be worth pursuing [4], and the early combination of TACE with MTAs and ICIs could result in extraordinary clinical benefits for intermediate-stage patients with a high tumour burden in the dawn of a promising new era for HCC treatment. Notably, TACE can also exacerbate microenvironmental hypoxia in tumour cells and trigger the upregulation of hypoxia-related factors, including VEGF and fibroblast growth factor [12, 13]. In addition, TACE can lead to locoregional tumour necrosis and release tumour antigens, which stimulates tumour-specific immune responses and neutralizes immune evasion [27]. MTAs may counteract hypoxia-induced angiogenesis after TACE and adjust the tumour immune microenvironment, boosting the immune response to PD-1 inhibitors in HCC [28].
Based on the potential mechanisms above, as in the protocol in our study, following the TACE procedure, combined MTAs and ICIs may cause a maximal surge of synergistic antitumor activity. Compared with TACE alone, triple therapy led to a higher tumour response rate (ORR: 65.1% vs. 37.2%, p = 0.010), a longer median PFS (15.4 vs. 4.8 months, p < 0.001), and a better median OS (27.2 vs. 15.9 months, p = 0.007) in HCC patients who were beyond the up-to-seven criteria. Not all intermediate-stage HCC patients benefit from TACE or combined therapy because it is a heterogeneous disease not only in terms of tumour burden but also in terms of liver function. In addition, it is important to preserve liver function and achieve a high objective response to prolong overall survival [5]. One study found that being beyond the up-to-seven criteria was associated with a decrease in the Child-Pugh grade after TACE [29]. In this study, the majority of the patients’ liver function was Child-Pugh A, which may be the maximum adaptation of the competitive risk of liver function damage caused by combined therapy or TACE. The combination of TACE with MTAs plus ICIs seems to be clinically feasible and safe [30–32]. Achieving a high ORR and preserving liver function are equally critical to prolong overall survival with HCC, especially on the background of liver cirrhosis [5]. In our study, the triple therapy group had a higher incidence of all treatment‐related AEs (grade ≥ 3 or 4). Overall, they were controllable and manageable. All approved molecularly targeted agents and immune checkpoint inhibitors are indicated only for patients with Child-Pugh A liver function [33]. Thus, a higher proportion of Child-Pugh A patients is associated with greater safety benefits from triple therapy.
This study had several natural limitations. First, the MTAs or ICIs used in the combined treatment group were not uniform. However, in general, the targets of ICIs or MTAs partially overlap. Therefore, it did not stifle the superiority of the triple modality. Second, nearly 90% of aetiologies were hepatitis B. The copies of the virus were monitored, and no reversal was found. Comprehensive monitoring in subsequent studies is required to further confirm the safety of the triple treatment protocol. Although a PSM analysis was used in this study, the potential selection bias could not be avoided entirely. Survival may be competitive, but the balance between liver function damage and patient tumour response benefit is not homogeneous in different cultural settings.
In conclusion, for uHCC patients beyond the up-to-seven criteria, TACE-MTAs-ICIs provided superior ORR and OS. Early combined TACE and systemic treatment should shift for patients who are beyond these criteria.
Supplementary Material
Glossary
Abbreviations
- Aes
adverse events
- AFP
α-fetoprotein
- ALBI
albumin-bilirubin
- BCLC
Barcelona Clinic Liver Cancer
- CI
confidence interval
- CR
complete response
- DCR
disease control rate
- ECOG
Eastern Cooperative Oncology Group
- HCC
hepatocellular carcinoma
- HRs
hazard ratios
- ICIs
immune checkpoint inhibitors
- mRECIST
modified response evaluation criteria in solid tumours
- MTAs
molecular-targeted agents
- ORR
objective response rate
- OS
overall survival
- PFS
progression-free survival
- PR
partial response
- PSM
propensity score matching
- SD
stable disease
- TACE
transarterial chemoembolization
- VEGF
vascular endothelial growth factor.
Funding Statement
This study was funded by the Interventional Medicine Research Fund of Jiangsu Medical Association (SYH-3201140-0049 to Jin-Xing Zhang) and the Jiangsu Province Capability Improvement Project through Science, Technology and Education (JSDW202243 to Hai-Bin Shi).
Author contributions
Wen Chen, Hai-Tao Yan, Jin-Xing Zhang, and Hai-Bin Shi were involved in the study conception and design; Wen Chen, Hai-Tao Yan, Chun-Gao Zhou, Jin Liu, and Sheng Liu were involved in the analysis and interpretation of the data; Wen Chen, Hai-Tao Yan, and Yuan Cheng were involved in the drafting of the paper; Qing-Quan Zu was involved in revising the manuscript for intellectual content; all authors were involved in the final approval of the version to be published; and all authors agreed to be accountable for all aspects of the work.
Compliance with ethical standards
The study protocol followed the World Medical Association Declaration of Helsinki guidelines and was approved by the Ethics Committee of the First Affiliated Hospital with Nanjing Medical University (Ethical review no. 2021-SR-332). For this retrospective study, informed consent was waived by Ethics Committee of the First Affiliated Hospital with Nanjing Medical University.
Disclosure statement
The authors have no conflicts of interest or financial ties to disclose. The authors alone are responsible for the content and writing of the article.
Data availability statement
The data that support the findings of this study are available from the corresponding author [Q.Q. Z.], upon reasonable request.
References
- 1.Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi: 10.1016/S0140-6736(22)01200-4. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 3.Xie DY, Ren ZG, Zhou J, et al. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudo M, Han KH, Ye SL, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer. 2020;9(3):245–260. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hucke F, Pinter M, Graziadei I, et al. How to STATE suitability and START transarterial chemoembolization in patients with intermediate-stage hepatocellular carcinoma. J Hepatol. 2014;61(6):1287–1296. doi: 10.1016/j.jhep.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Arizumi T, Ueshima K, et al. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: proposal of Modified Bolondi’s Subclassification (Kinki Criteria). Dig Dis. 2015;33(6):751–758. doi: 10.1159/000439290. [DOI] [PubMed] [Google Scholar]
- 8.Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32(4):348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M, Ueshima K, Chan S, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child-Pugh A liver function: a proof-of-concept study. Cancers (Basel). 2019;11(8):1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silk T, Silk M, Wu J.. Up to seven criteria in selection of systemic therapy for hepatocellular carcinoma. World J Gastroenterol. 2022;28(23):2561–2568. doi: 10.3748/wjg.v28.i23.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arizumi T, Minami T, Chishina H, et al. Time to transcatheter arterial chemoembolization refractoriness in patients with hepatocellular carcinoma in Kinki criteria stages B1 and B2. Dig Dis. 2017;35(6):589–597. doi: 10.1159/000480208. [DOI] [PubMed] [Google Scholar]
- 12.Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99(10):2037–2044. doi: 10.1111/j.1349-7006.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han G, Berhane S, Toyoda H, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response-based approach. Hepatology. 2020;72(1):198–212. doi: 10.1002/hep.31022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YX, Zhang JX, Zhou CG, et al. Comparison of the efficacy and safety of transarterial chemoembolization with or without lenvatinib for unresectable hepatocellular carcinoma: a retrospective propensity score-matched analysis. J Hepatocell Carcinoma. 2022;9:685–694. doi: 10.2147/JHC.S373250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraoka A, Kumada T, Tada T, et al. Early experience of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma BCLC-B stage patients classified as beyond up to seven criteria - Multicenter analysis. Hepatol Res. 2022;52(3):308–316. doi: 10.1111/hepr.13734. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 17.Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montasser A, Beaufrère A, Cauchy F, et al. Transarterial chemoembolisation enhances programmed death-1 and programmed death-ligand 1 expression in hepatocellular carcinoma. Histopathology. 2021;79(1):36–46. doi: 10.1111/his.14317. [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Lencioni R.. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. doi: 10.1016/j.jhep.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol. 2012;56(4):886–892. doi: 10.1016/j.jhep.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Kim BK, Kim SU, Kim KA, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62(6):1304–1310. doi: 10.1016/j.jhep.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase iii, randomized clinical trial (LAUNCH). J Clin Oncol. 2023;41(1):117–127. doi: 10.1200/JCO.22.00392. [DOI] [PubMed] [Google Scholar]
- 24.Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomized, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 25.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, Phase III Trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 26.Rimassa L, Finn RS, Sangro B.. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79(2):506–515. doi: 10.1016/j.jhep.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi C, Chen L, Lin Z, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021;74(5):2544–2560. doi: 10.1002/hep.31921. [DOI] [PubMed] [Google Scholar]
- 29.Eso Y, Takai A, Takahashi K, et al. Combination of Mac-2 Binding protein glycosylation isomer and up-to-seven criteria as a useful predictor for Child-Pugh grade deterioration after transarterial chemoembolization for hepatocellular carcinoma. Cancers (Basel). 2019;11(3):405. doi: 10.3390/cancers11030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib Plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi: 10.3389/fimmu.2022.848387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia WL, Zhao XH, Guo Y, et al. Transarterial chemoembolization combined with apatinib with or without PD-1 inhibitors in BCLC stage C hepatocellular carcinoma: a multicenter retrospective study. Front Oncol. 2022;12:961394. doi: 10.3389/fonc.2022.961394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu JY, Wu JY, Li YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for neoadjuvant treatment of resectable hepatocellular carcinoma with high risk of recurrence: a multicenter retrospective study. Front Oncol. 2022;12:985380. doi: 10.3389/fonc.2022.985380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [Q.Q. Z.], upon reasonable request.