ABSTRACT
Background
This study aimed to investigate the impact of Urolithin A (UA) on muscle endurance, muscle strength, inflammatory levels, oxidative stress, and protein metabolism status in resistance-trained male athletes.
Method
An 8-week randomized, double-blind, placebo-controlled study was conducted with twenty resistance-trained male athletes. Participants were supplemented with 1 g of UA daily. Muscle strength and muscle endurance measures were assessed, and fasting venous blood samples and morning urine samples were collected to evaluate their oxidative stress levels, inflammatory markers, and protein metabolism status.
Results
There were no significant differences observed in terms of dietary energy intake and composition between the two assessments conducted within a 24-hour period. After 8 weeks of UA supplementation, compared to baseline measurements, the UA group exhibited increases in 1RM bench press and squat, although these changes were not statistically significant (Δ = 3.00 ± 0.17 kg, p = 0.051, Δ = 1.35 ± 2.73 kg, p = 0.499). However, significant improvements were noted in Maximum Voluntary Isometric Contraction (MVIC) and repetitions to failure (RTF) performance (Δ = 36.10 ± 0.62 NM, p = 0.000; Δ = 2.00 ± 0.56, p = 0.001). When compared to the placebo group, the UA supplementation for 8 weeks led to an increase in 1RM bench press and squat, although statistical significance was not reached (Δ = 3.50 ± 0.79 kg, p = 0.462; Δ = 2.55 ± 1.36 kg, p = 0.710). Furthermore, the group receiving UA supplementation, compared to the placebo group, showed significant improvements in MVIC and RTF (Δ = 43.50 ± 0.77 NM, p = 0.048; Δ = 2.00 ± 1.22, p = 0.011), indicating that the UA group exhibited superior performance enhancements in these metrics compared to the placebo group. After 8 weeks of UA supplementation, the UA group showed a significant decrease in 3-methylhistidine (3-MH) compared to baseline measurement (Δ=-2.38 ± 1.96 μmol/L, p = 0.049). Additionally, the UA group exhibited a significant increase in C-reactive protein (CRP) compared to baseline (Δ = 0.71 ± 0.21 mg/L, p = 0.001). However, there was no significant changes observed in Interleukin-6 (IL-6) (Δ=-1.00 ± 1.01 pg/mL, p = 0.076), or superoxide dismutase (SOD) (Δ=-0.004 ± 0.72 U/mL, p = 0.996) compared to baseline in the UA group. When compared to the placebo group, there was no significant difference observed in 3-MH in the UA group (Δ=-3.20 ± 0.31 μmol/L, p = 0.36). In terms of inflammation markers, the UA group exhibited a significant decrease in CRP (Δ=-0.79 ± 0.38 mg/L, p = 0.032) compared to the placebo group, whereas there was a decrease in IL-6 without statistical significance (Δ=-1.75 ± 0.45 pg/mL, p = 0.215). Furthermore, the UA group showed a significant decrease in SOD compared to the placebo group (Δ=-4.32 ± 0.90 U/mL, p = 0.041).
Conclusions
After 8 weeks of UA supplementation at 1 g/day, resistance-trained male athletes showed improvements in muscle strength and endurance. Additionally, UA supplementation was also associated with reduced oxidative stress levels and a decrease in inflammation response levels.
KEYWORDS: Urolithin A, ergogenic aid, exercise performance, oxidative stress
1. Introduction
Muscle endurance and muscle strength are critical determinants of athletic performance [1]. However, prolonged high-intensity exercise can lead to muscle fatigue and damage [2,3], limiting an athletes’ performance and recovery capacity [4]. To enhance training efficacy and recovery, sports nutritionists have been exploring new nutritional supplements and natural agents [5–9]. Urolithin A (UA), a metabolite derived from plant polyphenols, has gained considerable attention in recent years [10]. UA, a natural compound, interacts with intestinal microbiota through tannins found in foods such as pomegranates, berries, and nuts [11,12]. Studies have showed that UA can stimulate mitochondrial autophagy and improve muscle function in animals [13,14]. Additionally, it enhances mitochondrial gene expression in skeletal muscles, demonstrating significant anti-fatigue effects across various age groups [13,15–18]
UA has been established as safe and bioavailable in humans, as evidenced by improved cellular health after oral administration for four weeks in the elderly (aged 61–85) [17]. Furthermore, research has indicated that long-term UA consumption can enhance muscle endurance in older individuals (aged 65–90) [19]. A recent study even suggests that UA can improve muscle strength, exercise performance, and mitochondrial health in middle-aged individuals (aged 51.03 ± 7.16 and 52.07 ± 5.56) [20]. Recent research emphasizes UA’s antioxidant, anti-inflammatory, improved mitochondrial function, and cell apoptosis-inducing properties [15].
However, current UA research primarily focuses on middle-aged and elderly individuals [13–19], leaving a gap in empirical studies regarding UA’s impact on muscle function in athletes. Hence, we have designed a randomized, double-blind, placebo-controlled trial to investigate the effects of UA on muscle endurance, muscle strength, oxidative stress, and protein breakdown metabolism in male athletes undergoing resistance training. By examining UA’s potential role in athletic performance and muscle recovery, we aim to provide athletes with more effective training aids and promote improved competitive performance. This study anticipates revealing UA’s potential to enhance muscle endurance, boost muscle strength, reduce oxidative stress, and inhibit markers of protein breakdown in athletes. These findings will provide scientific evidence for athletes to optimize their training and competition performance, as well as inspire innovative concepts for the development of new sports nutrition supplements and rehabilitation products.
2. Materials and methods
2.1. Participants
We recruited twenty male individuals for this study (average age: 24.1 ± 1.59 years, average height: 177.4 ± 5.92 cm, average weight: 84.55 ± 2.72 kg, average training experience: 4.40 ± 1. 07 years), all of whom had engaged in long-term resistance training. Eligibility criteria required participants to demonstrate the ability to lift 1.25 times their body weight in the bench press and 1.75 times their body weight in the squat exercises. Additionally, participants needed to have a consistent history of training with loads exceeding 80% of their one-repetition maximum (1RM) for both the bench press and squat exercises and to have been actively following a structured resistance exercise program for a minimum of three years.
Participants were instructed to consume two capsules of UA(Mitopure, USA) daily(1 g/day), each containing 250 mg of UA, after both breakfast and dinner on a daily basis. Exclusion criteria included any current or recent skeletal muscle injuries, current or past use of anabolic steroids, and diagnosed untreated metabolic disorders. Participants were also advised to refrain from consuming alcohol, caffeine, and tobacco throughout the entire study duration, which spanned eight weeks, inclusive of two testing weeks. Furthermore, participants were instructed not to engage in strenuous exercise within 48 hours before both the initial and final assessment tests.
Throughout the study, participants were required to abstain from or discontinue the use of all dietary supplements, such as creatine and pre-workout products. To ensure no interference from previous supplement use, a four-week washout period, comprising a four-week supplementation washout followed by a one-week exercise washout, was implemented prior to the formal commencement of the study. Ethical guidelines were followed in accordance with the Declaration of Helsinki, and the study received approval from the Institutional Review Board of Beijing Sport University (2021038 h).
2.2. Procedures
This study employed a double-blind, randomized, placebo-controlled trial design. Following the introduction to the study protocol, participants were randomly assigned to one of two groups: the UA group or the placebo group. The UA powder contained within the capsules was sourced from the Timeline Mito Pure brand in the United States.
The study spanned a total duration of 8 weeks, with the initial week designated as the assessment week. During this first week, the study’s content was thoroughly explained to both the participants and their coaches, and baseline measurements were taken. These measurements included Bench Press/Squat 1RM, Repetitions to Failure (RTF) performance, Rating of Perceived Exertion (RPE), Maximum Voluntary Isometric Contraction (MVIC) Test for the Quadriceps, Cognitive ability, C-reactive protein (CRP), Interleukin-6 (IL-6), Reactive Oxygen Species (SOD), and 3-Methylhistidine (3-MH).
Following the completion of these assessments, the capsules were distributed to the participants. To guide the participants through the intervention, serval trained coaches was engaged by the research team. Daily questionnaires were sent via social media platforms to ensure that participants adhered to the prescribed capsule ingestion regimen Fasting venous blood samples were collected again from the participants during the 4th week and on the final testing day. Additionally, urine samples were collected on the final testing day (see Figure 1 for a visual representation).
Figure 1.
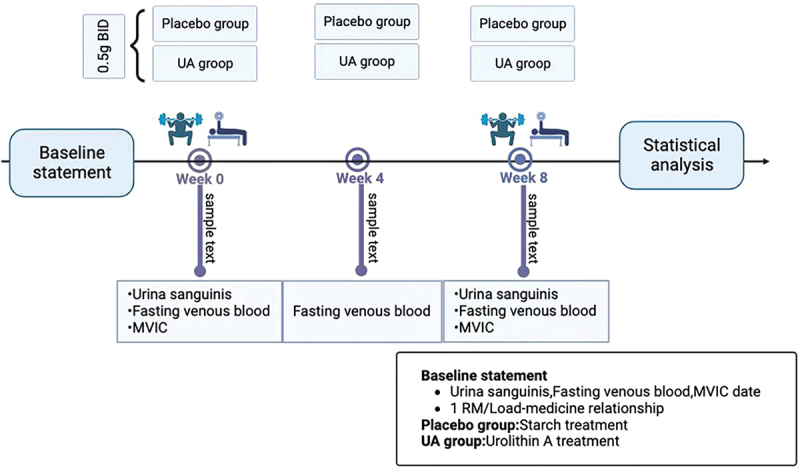
Overview of testing sessions.
BID: Bis in die
All assessments were conducted concurrently between 2:00 PM and 5:00 PM to minimize the potential influence of diurnal variations on participant performance. To maintain blinding, an independent pharmacist prepared and managed all capsule supplements, ensuring that neither the researchers nor the participants were aware of the capsule contents. UA powder was carefully removed from the capsules and replaced with an equivalent weight of cornstarch as a placebo, a widely recognized inert substance that does not affect human physiological processes and is relevant to this study. Participants were not informed of the contents of the capsules they consumed, nor were they aware that they were taking a placebo. The capsules were made to appear identical to those containing UA, ensuring the maintenance of a double-blind procedure throughout the study. In order to minimize the potential placebo effect, the placebo group consumed an equivalent volume of water when taking the capsules.
Professional coaches oversaw the participants’ training program during the study period to ensure consistent training intensity across all participants. Failure to meet the requisite number of training sessions, set at four per week, resulted in exclusion from the study. The blinding of the study was meticulously managed and implemented by independent statisticians uninvolved in the actual experiment. To maintain the integrity of blinding, the coaches, assessors, and statistical analysts operated independently. The coaches provided interventions to the participants based on a predetermined numerical intervention scheme, conducted consistently by the same skilled coach. Interventions were assigned to the participants using sealed envelopes, with the coaches unaware of each participant’s group assignment. Assessors were solely responsible for evaluating outcome measures and had no involvement in participant recruitment, grouping, or management. Statisticians were only aware that there were two groups but were uninformed regarding the specific group allocation for each participant.
In the present study, during the subject selection process, we confirmed the lifestyle habits of the potential participants and excluded those with a history of chronic consumption of alcohol, caffeine, tobacco, and other dietary supplements. Additionally, at the baseline phase of the study, participants were provided detailed instructions and requested to refrain from using specific substances that could potentially influence the study outcomes. To ensure compliance with these stipulations throughout the experimental period, researchers also conducted regular interviews with each participant to discuss their personal habits and lifestyle, including their adherence to the study’s directives. This approach ensured that all participants complied with the requirements of the research protocol throughout the duration of the study.
2.3. Training protocol
The training program lasted for 8 weeks, encompassing four weekly training sessions, each targeting specific muscle groups – chest, back, legs, and shoulder-arm muscles. Each session comprised 4–5 exercises. This intervention was closely supervised by a team of experienced coaches, each with expertise in these specific exercises. To ensure adequate supervision, each coach was responsible for overseeing three participants. All training exercises took place at the same fitness facility, utilizing equipment from a consistent brand throughout the entire study.
During the initial 4 weeks of the training program, participants completed 4 sets of 10–12 repetitions, exercising at an intensity level of 60–70% of their 1RM. In the subsequent 4 weeks, the participants’ training regimen consisted of 4 sets of 6–8 repetitions, with an increased intensity of 70–80% of their 1RM. This progression aimed to gradually increase the training load and stimulate muscle adaptation.
2.4. Daily recordings of dietary intake
Throughout the study’s duration, all participants were instructed to maintain their habitual dietary intake. Additionally, participants received both written and verbal instructions to ensure the consistency of their usual dietary patterns. To accurately assess daily total energy intake, data was sourced from the National Nutrient Database for Standard Reference, provided by the United States Department of Agriculture. Dietary composition and intake were recorded 24 hours prior to two specific testing sessions [21]. The dietary intake was measured in terms of energy, expressed as calories per day, and macronutrients, including carbohydrates, proteins, and fats, expressed as grams per day.
2.5. Baseline statement
2.5.1. Bench press/deep squat 1RM
The one-repetition maximum (1RM) test, which measures the maximum weight that can be lifted in a single bench press or deep squat, stands as a classic gauge of absolute strength [22,23]. The 1RM assessments for both the bench press and squat primarily consist of three essential steps, pivotal in establishing baseline strength levels among participants and informing resistance loads in muscle training regimens.
(1) Initial Warm-up: Participants, under the guidance of a certified coach, begin with a light warm-up. For the bench press, this involves lifting approximately 25 ± 2.5 kg, while for the squat, it is 35 ± 2.5 kg. They perform 10–15 repetitions, a manageable task. The professional coach adjusts the load as per individual requirements. (2) Progressive Warm-up Sets: Following the initial warm-up, the load is increased by 10–15%. Participants then complete approximately 6–8 repetitions, followed by a rest period lasting 2–5 minutes. Subsequently, there is another increment in the load by 5–10%, enabling participants to complete 3–4 repetitions. These are considered conservative attempts, not pushing for maximal effort. (3) 1RM Assessment Attempts: Participants receive a 2–5 minutes’ rest period. Afterward, the load is increased by 5–10%, following the same procedure as in step 2. This allows participants to complete 1 or 2 repetitions. If they successfully complete these attempts, they rest for another 2–5 minutes and then proceed to increase the load by 5–10% for subsequent bench press or deep squat 1RM attempts. In cases of failure, participants rest for 2–5 minutes and slightly reduce the load before making a final attempt. The overall determination of the bench press or deep squat 1RM should be completed within a maximum of five attempts.
2.5.2. Repetitions to failure (RTF) performance
The measurement of maximal repetitions during a 60% 1RM bench press test is a classic method for evaluating isotonic muscular endurance in the upper limbs. This test assesses the number of movements muscles can perform under isotonic contraction at a specified load within a given timeframe [24]. Muscular endurance during this test is characterized by three main indicators: weight, time and repetitions. For the bench press muscular endurance test, it is recommended to utilize a weight set at 60–70% of an individual’s 1RM as the optimal load. Participants are instructed to execute the test continuously without pauses, with verbal cues provided by a professional coach to maintain the movement rhythm. To determine the level of fatigue for each participant, two criteria have been established: (1) if the participant pauses for more than 10 seconds during the lifting or lowering phases or if there is significant distortion in the movement. (2) if the participant voluntarily decides to discontinue further repetitions. The final number of repetitions achieved by each participant is recorded.
2.5.3. MVIC test for the quadriceps
In this study, the American Isometric Force Testing System (Biodex Medical Systems Inc., Shirley, NY, USA) was employed to measure the knee extension moment generated by the knee extension muscles of the participant’s legs. This measurement was taken with a sampling frequency of 2000 hz and a moment accuracy of ± 1%. The knee joint was set at a 75° angle for the test, with corresponding settings for test time, test frequency, and interval time. Each maximum knee extension torque test took five seconds and was repeated three times [25].
2.5.4. Blood index
On the initial baseline assessment day, a 5 ml fasting venous blood sample was collected from each participant. Additional fasting venous blood samples of 5 ml were collected from each participant on the Monday morning of the fourth week and on the final day of testing. These samples were then centrifuged at 2000 revolutions per minute (rpm) for 15 minutes using a high-speed centrifuge and stored in a −80 °C freezer. Subsequently, serum samples were analyzed using an automated biochemical analyzer and a spectrophotometer. The levels of CRP, IL-6, and SOD were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) method with double antibodies, and the respective values were recorded.
2.5.5. Urine index 3-methylhistamine (3-MH)
Urine samples were collected in the morning on two occasions: the initial baseline assessment day and the final testing day. Participants fasted for at least 12 hours and cleanse the external genitalia with water and soap before urine specimen collection. Approximately 30 ml of midstream urine was collected using a disposable plastic urine cup and transferred into a VACUETTE collection tube for coagulation induction. This tube was left undisturbed for 1 hour and then centrifuged at 3000 rpm for 5 minutes to separate the urine. The separated urine was transferred into a 1 ml tube and stored at −20°C for further analysis. The levels of 3-MH were measured using an ELISA method.
2.6. Statistical analysis
All results are presented as mean ± standard deviation (S.D.). Statistical significance was set at an alpha level of p ≤ 0.05 for all tests. Data were initially assessed for normality using the Shapiro – Wilk test. A 2 condition × 5 times (sets of repetitions to exhaustion) ANOVA was used to analyze bench press and deep squat results. The sphericity assumption (Mauchly’s test) was met, and the Bonferroni post hoc test was used to determine specific pairwise differences when appropriate. However, due to non-normal distribution, muscle endurance data were analyzed using the Friedman test. Subsequently, Dunn’s pairwise post hoc tests were conducted with a Bonferroni correction applied to identify specific pairwise differences. All statistical analyses were performed with Prism 7.0 (GraphPad) and SPSS 22.0 software.
3. Result
3.1. 24-hour dietary intake assessment
There were no significant differences observed in the dietary energy intake and composition during the 24-hour period preceding each of the two assessment sessions among the participants (Figure 2).
Figure 2.
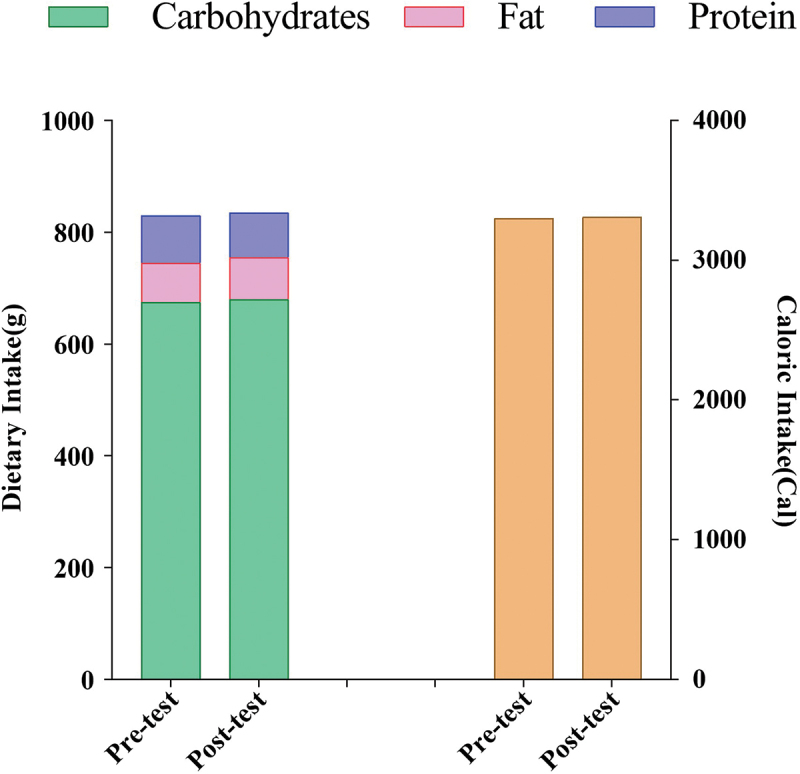
Mean of dietary intake 24 h before either testing session.
3.2. Muscle endurance and muscle strength
After 8 weeks of UA supplementation, compared to the baseline measurements, the UA group exhibited increased 1RM bench press and squat values, although these increases were not statistically significant (Δ = 3.00 ± 0.17, p = 0.051 for bench press; Δ = 1.35 ± 2.73, p = 0.499 for squat) (Figure 3a,b). Significant improvements were observed in MVIC and RTF, with notable increases (Δ = 36.10 ± 0.62, p = 0.000 for MVIC, Δ = 2.00 ± 0.56, p = 0.001 for RTF) (Figure 3c,d).
Figure 3.
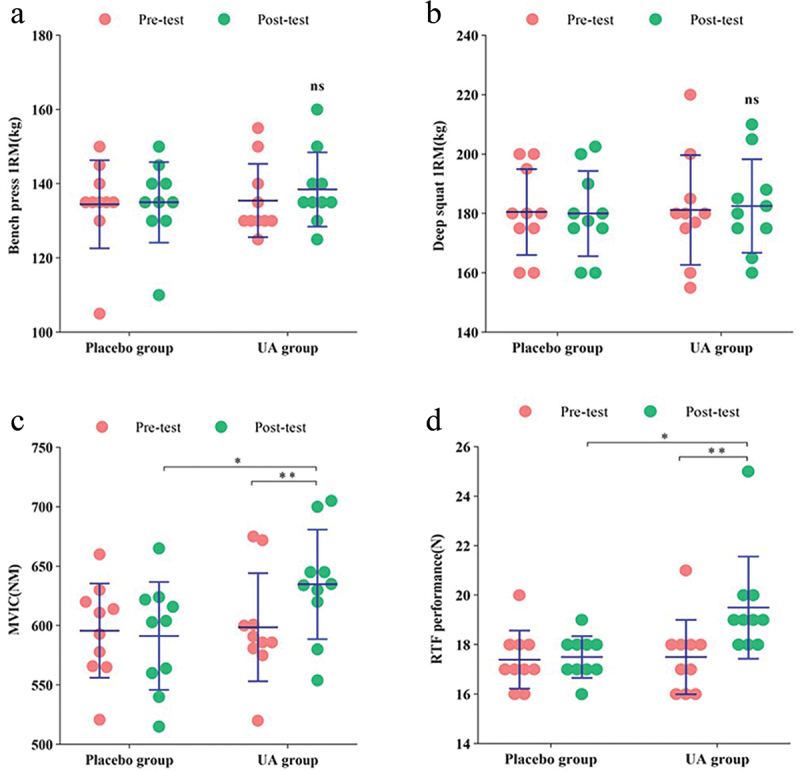
Changes in muscle strength and endurance indices after 6 weeks of supplementation in the placebo group and UA group. (a) Bench press 1RM. (b) Deep squat 1RM. (c) MVIC. (d) RTF performance. */** Significant difference (p<0.05).
Compared to the placebo group, after 8 weeks of UA supplementation, the UA group showed increased 1RM bench press and squat values, although these increases were not statistically significant (Δ = 3.50 ± 0.79, p = 0.462 for bench press; Δ = 2.55 ± 1.36, p = 0.710 for squat) (Figure 3a,b). However, significant improvements were observed in MVIC and RTF, with substantial increases (Δ = 43.50 ± 0.77, p = 0.048 for MVIC; Δ = 2.00 ± 1.22, p = 0.011 for RTF) (Figure 3c,d).
3.3. Blood and urine index
After 8 weeks of UA supplementation, compared to the baseline measurements, the UA group showed a significant decrease in 3-MH levels (Δ=-2.38 ± 1.96, p = 0.049) (Figure 4a). The UA group exhibited a significant increase in CRP levels compared to the baseline (Δ = 0.71 ± 0.21, p = 0.001) (Figure 4b). There were no significant differences observed in IL-6 levels compared to the baseline in the UA group (Δ=-1.00 ± 1.01, p = 0.076) (Figure 4c). Similarly, there were no significant differences observed in SOD levels compared to the baseline in the UA group (Δ=-0.004 ± 0.72, p = 0.996) (Figure 4d).
Figure 4.
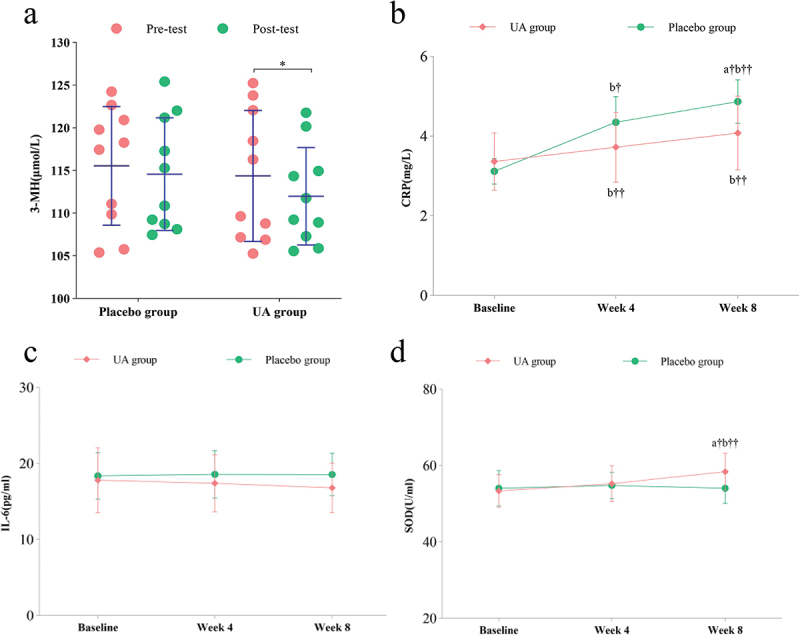
6 weeks of supplementation, the changes in urinary and blood indicators were assessed in both the placebo and UA groups (a)3-mh. (b)CRP (c)IL-6. (d)SOD. *Significant difference (p<0.05). †significant difference (p<0.05). (a) comparison between the two groups; (b) compared to the baseline.
Compared to the placebo group, there were no significant differences observed in 3-MH levels in the UA group (Δ=-3.20 ± 0.31, p = 0.363) (Figure 4a). However, the UA group showed a significant decrease in CRP levels compared to the placebo group (Δ=-0.79 ± 0.38, p = 0.032) (Figure 4b). Compared to the placebo group, the UA group exhibited a decrease in IL-6 levels, although not statistically significant (Δ=-1.75 ± 0.45, p = 0.215) (Figure 4c). Lastly, the UA group exhibited a significant decrease in SOD levels compared to the placebo group (Δ=-4.35 ± 0.90, p = 0.041) (Figure 4d).
4. Discussion
In our study, we were the first to discover that after 8 weeks of UA supplementation, there was a significant improvement in Maximum Voluntary Isometric Contraction and repetitions to failure performance in the UA group compared to baseline measurements and the placebo group. These results effectively demonstrate the beneficial impact of UA supplementation on enhancing exercise performance.
Furthermore, after 8 weeks of UA supplementation, the UA group showed a significant decrease in 3-methylhistidine and a significant increase in C-reactive protein compared to baseline measurements. Additionally, the UA group exhibited a significant decrease in CRP and SOD levels compared to the placebo group. Overall, after 8 weeks of daily 1 g UA supplementation, certain indicators of muscle strength and endurance in resistance-trained male athletes significantly improved. This suggests that UA, as a sports supplement, positively impacts exercise performance, likely due to reduced oxidative stress levels and a decrease in inflammation response levels.
A favorable anabolic metabolic state is recognized as one of the prerequisites for their continuous progress. We chose a duration of 8 weeks for the study, taking into consideration the fact that the included participants have been consistently engaged in regular physical activity. Their long-term muscle performance has exhibited stability, and we deemed 8 weeks as a safe and effective duration that minimizes potential disruptions from exercise interventions.
Similar to our findings, recent research recruited 66 participants who were randomly assigned to either the UA intervention group (n = 33) or the placebo group (n = 33). The participants had an average age of 71.7 ± 4.94 years. Compared to the placebo, UA significantly improved muscle endurance of the tibialis anterior in the leg and the first dorsal interosseus in the hand skeletal muscles at 2 months, as evidenced by an increase in the number of muscle contractions from baseline until fatigue. Additionally, at 4 months, UA supplementation led to a reduction in the plasma levels of several acylcarnitines, ceramides, and CRP compared to the placebo.
Overall, this randomized clinical trial found that UA supplementation is safe and well-tolerated in the assessed population. Although the improvements in the 6-minute walk distance and maximal ATP production in hand muscles were not significant compared to the placebo group, long-term supplementation with UA benefited muscle endurance and plasma biomarkers. These findings suggest that UA may mitigate age-related muscle decline [16].
All participants exhibited good tolerability throughout the intervention period and one month following the completion of the testing, with no adverse events reported. This study did not show improved muscle strength. However, the UA group demonstrated significantly better performance in terms of knee joint MVIC and RTF performance compared to the placebo group. Current research suggests that UA acts as a mitochondrial activator [17]. UA can activate the Nrf2-ARE signaling pathway, upregulate the expression of GSTs, enhance cellular autophagy, and regulate mitochondrial quality control [13,26,27]. Additionally, studies have shown that UA can activate the PINK1/Parkin signaling pathway, promoting the aggregation and degradation of damaged mitochondria [28] thereby improving muscle strength. Furthermore, UA can activate AMPK to promote fatty acid oxidation [25], increase the expression of PPARγ, and transcriptionally regulate its downstream target genes [29], thereby promoting fatty acid oxidation through both pathways, improving insulin sensitivity, and enhancing muscle endurance.
Intense exercise often induces elevated levels of oxidative stress and inflammatory responses in athletes [30], the ability to swiftly restore themselves from this state to a resting level is a primary manifestation of athletes’ recovery status, as well as a prerequisite for their subsequent training sessions. [31]. In this study, we observed that compared to the placebo, UA was found to improve exercise-induced oxidative stress and lower systemic inflammation levels, although the differences were not statistically significant. The study indicates that UA is capable of scavenging and neutralizing reactive oxygen species such as Peroxyl Radical and superoxide radicals, thereby reducing cellular oxidative stress levels [32]. Furthermore, UA can activate the Nrf2 antioxidant pathway, upregulating the expression of a series of antioxidant enzymes to enhance cellular antioxidant defenses [33]. Additionally, UA has been reported to inhibit ROS-generating enzyme activities, reducing the occurrence of oxidative stress and protecting cells from oxidative damage [32,34]. Based on the aforementioned studies, it can be concluded that UA exhibits comprehensive and robust antioxidant properties, which our research also confirms.
We further investigated the impact of UA on the metabolic state of protein breakdown and synthesis in the body. The results revealed that UA significantly reduces the concentration of 3-MH in athletes. The urinary concentration of 3-MH serves as a reliable indicator of skeletal muscle protein breakdown in human subjects [35]. Previous studies have reported UA’s ability to regulate cell cycle and apoptosis [36], which our research has confirmed. The analysis suggests that UA may affect cell cycle-related protein kinase complexes, such as CDKs (cyclin-dependent kinases) and cyclins. UA inhibits the activity of CDKs and reduces the expression of cell cycle proteins like cyclin D1, thereby causing cell cycle arrest in the G1 phase [36]. Furthermore, studies have found that UA can regulate the cell cycle by increasing the levels of cell cycle inhibitory proteins p21 and p27 [34,37]. Additionally, UA increases the Bax/Bcl-2 ratio [38], leading to loss of mitochondrial membrane potential, release of cytochrome c, and activation of caspase cascade reactions, ultimately resulting in cell apoptosis. Moreover, UA activates the JNK (c-Jun N-terminal kinase) [35] and p38 MAPK (mitogen-activated protein kinase) signaling pathways [36], further promoting cell apoptosis. By accelerating cell apoptosis and enhancing overall cellular activity, UA improves the body’s protein synthesis status.
5. Conclusion
In conclusion, our study demonstrates that an 8-week supplementation of UA (1 g/day) can enhance muscle strength and endurance in male athletes undergoing resistance training. Additionally, it improves oxidative stress levels and lowers inflammation response in athletes.
6. Limitations and future perspectives
This study has certain limitations. The inclusion of participants from a single gender and the limited range of exercise types represent the primary limitations of this research, providing avenues for future investigations. Furthermore, all participants were instructed to maintain their usual dietary habits, which introduces diet as a potential confounding factor. Currently, UA has been widely utilized in the middle-aged and elderly population, and this study provides empirical evidence for its application in the field of sports. It also offers a novel choice for sports nutrition experts in terms of supplement selection.
Acknowledgments
We gratefully acknowledge the participants involved in this study.
Funding Statement
This work was supported by the 14th five-year Education Plan of Jiangsu Province [C/2022/01/78].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contributions
The study and methodology were conceived using HTZ, HKZ, HZY, and JQL. GS, HTZ, GS, JQL, HZY and JT were involved in data collection and data treatment, writing was conducted by HTZ and HKZ, all authors reviewed critically the manuscript under DZ, NL and CL supervision.
Data availability statement
Datasets used in this study are available from the corresponding author under reasonable request.
Publication statement
This manuscript has not been published elsewhere.
References
- 1.Mujika I, Rønnestad BR, Martin DT.. Effects of increased muscle strength and muscle mass on endurance-cycling performance. Int J Sport Physiol. 2016;11(3):283–15. doi: 10.1123/ijspp.2015-0405 [DOI] [PubMed] [Google Scholar]
- 2.Appell HJ, Soares JM, Duarte JA. Exercise, muscle damage and fatigue. Sports Med. 1992;13(2):108–115. doi: 10.2165/00007256-199213020-00006 [DOI] [PubMed] [Google Scholar]
- 3.Balnave CD, Thompson MW. Effect of training on eccentric exercise-induced muscle damage. J Appl Physiol. 1985/1993;75(4):1545–1551. doi: 10.1152/jappl.1993.75.4.1545 [DOI] [PubMed] [Google Scholar]
- 4.Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24(5):512–520. doi: 10.1249/00005768-199205000-00004 [DOI] [PubMed] [Google Scholar]
- 5.Thomas DT, Erdman KA, Burke LM. American college of sports medicine joint position statement. Nutrition and athletic performance. Med Sci Sports Exerc. 2016;48(3):543–568. doi: 10.1249/MSS.0000000000000852 [DOI] [PubMed] [Google Scholar]
- 6.Malsagova KA, Kopylov AT, Sinitsyna AA, et al. Sports nutrition: diets, selection factors, recommendations. Nutrients. 2021;13(11):3771. doi: 10.3390/nu13113771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothschild JA, Kilding AE, Plews DJ. What should I eat before exercise? Pre-exercise nutrition and the response to endurance exercise: current prospective and future directions. Nutrients. 2020;12(11):3473. doi: 10.3390/nu12113473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin DS. Sports dietetics: impact beyond playing fields. J Acad Nutr Diet. 2017;117(9):1337. doi: 10.1016/j.jand.2017.06.368 [DOI] [PubMed] [Google Scholar]
- 9.Arazi H, Aboutalebi S, Taati B, et al. Effects of short-term betaine supplementation on muscle endurance and indices of endocrine function following acute high-intensity resistance exercise in young athletes. J Int Soc Sports Nutr. 2022;19(1):1–16. doi: 10.1080/15502783.2022.2041988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiNicolantonio JJ, McCarty MF, O’Keefe JH. Nutraceutical activation of Sirt1: a review. Open Heart. 2022;9(2):e002171. doi: 10.1136/openhrt-2022-002171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toney AM, Fox D, Chaidez V, et al. Immunomodulatory role of urolithin a on metabolic diseases. Biomedicines. 2021;9(2):192. doi: 10.3390/biomedicines9020192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu ZH, Cao M, Wang YX, et al. Urolithin a attenuates Helicobacter pylori-induced damage in vivo. J Agric Food Chem. 2022;70(38):11981–11993. doi: 10.1021/acs.jafc.2c03711 PMID 36106620. [DOI] [PubMed] [Google Scholar]
- 13.Ryu D, Mouchiroud L, Andreux PA, et al. Urolithin a induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22(8):879–888. doi: 10.1038/nm.4132 PMID 27400265. [DOI] [PubMed] [Google Scholar]
- 14.Huang JR, Zhang MH, Chen YJ, et al. Urolithin a ameliorates obesity-induced metabolic cardiomyopathy in mice via mitophagy activation. Acta Pharmacol Sin. 2023;44(2):321–331. doi: 10.1038/s41401-022-00919-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Amico D, Andreux PA, Valdés P. Impact of the natural compound urolithin a on health, disease, and aging. Trends Mol Med. 2021;27(7):687–699. doi: 10.1016/j.molmed.2021.04.009 [DOI] [PubMed] [Google Scholar]
- 16.Liu S, D’Amico D, Shankland E, et al. Effect of urolithin a supplementation on muscle endurance and mitochondrial health in older adults: a randomized clinical trial. JAMA Network Open. 2022. Jan 4;5(1):e2144279. doi: 10.1001/jamanetworkopen.2021.44279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreux PA, Blanco-Bose W, Ryu D, et al. The mitophagy activator urolithin a is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019. Jun;1(6):595–603. doi: 10.1038/s42255-019-0073-4 [DOI] [PubMed] [Google Scholar]
- 18.Singh A, D’Amico D, Andreux PA, et al. Urolithin a improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep Med. 2022. May 17;3(5):100633. doi: 10.1016/j.xcrm.2022.100633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, D’Amico D, Shankland E, et al. Effect of urolithin a supplementation on muscle endurance and mitochondrial health in older adults: a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2144279. doi: 10.1001/jamanetworkopen.2021.44279 PMID 35050355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A, D’Amico D, Andreux PA, et al. Urolithin a improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Reprod Med. 2022;3(5):100633. doi: 10.1016/j.xcrm.2022.100633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knüppel S, Norman K, Boeing H. Is a single 24-hour dietary recall per person sufficient to estimate the population distribution of usual dietary intake? J Nutr. 2019;149(9):1491–1492. doi: 10.1093/jn/nxz118 [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Medina L, Pallarés JG, Pérez CE, et al. Estimation of relative load from bar velocity in the full back squat exercise. Sports Med Int Open. 2017;1(2):E80–E88. doi: 10.1055/s-0043-102933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo DI, Kim E, Fahs CA, et al. Reliability of the one-repetition maximum test based on muscle group and gender. J Sports Sci Med. 2012;11(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan MJ, Oxford SW. The effect of caffeine ingestion on mood state and bench press performance to failure. J Strength Cond Res. 2011;25(1):178–185. doi: 10.1519/JSC.0b013e318201bddb [DOI] [PubMed] [Google Scholar]
- 25.Fuzari HK, Dornelas de Andrade A, Rodrigues MA, et al. Whole body vibration improves maximum voluntary isometric contraction of knee extensors in patients with chronic kidney disease: a randomized controlled trial. Physiother Theory Pract. 2019. May;35(5):409–418. doi: 10.1080/09593985.2018.1443537 [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Yan F, Xu Z, et al. Urolithin a protects human dermal fibroblasts from uva-induced photoaging through NRF2 activation and mitophagy. J Photochem Photobiol B. 2022;232:112462. doi: 10.1016/j.jphotobiol.2022.112462 [DOI] [PubMed] [Google Scholar]
- 27.Hassanein EHM, Sayed AM, Hussein OE, et al. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. Oxid Med Cell Longev. 2020;2020:1675957. doi: 10.1155/2020/1675957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang EF, Hou Y, Palikaras K, et al. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of alzheimer’s disease. Nat Neurosci. 2019;22(3):401–412. doi: 10.1038/s41593-018-0332-9 PMID 30742114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han QA, Yan C, Wang L, et al. Urolithin a attenuates ox-ldl-induced endothelial dysfunction partly by modulating microRNA-27 and erk/ppar-gamma pathway. Mol Nutr Food Res. 2016;60(9):1933–1943. doi: 10.1002/mnfr.201500827 [DOI] [PubMed] [Google Scholar]
- 30.Pingitore A, Lima GP, Mastorci F, et al. Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Nutrition. 2015;31(7–8):916–922. doi: 10.1016/j.nut.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 31.Powers SK, Radak Z, Ji LL. Exercise-induced oxidative stress: past, present and future. J Physiol. 2016;594(18):5081–5092. doi: 10.1113/JP270646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boakye YD, Groyer L, Heiss EH. An increased autophagic flux contributes to the anti-inflammatory potential of urolithin a in macrophages. Biochim Biophys Acta Gen Subj. 2018;1862(1):61–70. doi: 10.1016/j.bbagen.2017.10.006 PMID 29031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin C, Wang Y, Peng Y, et al. Urolithin a inhibits inflammation and oxidative stress induced by high lipid in hepatocytes via activating Nrf2 pathway and autophagy. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2021;37(11):973–980 PMID 34809736. [PubMed] [Google Scholar]
- 34.Esselun C, Theyssen E, Eckert GP. Effects of urolithin a on mitochondrial parameters in a cellular model of early alzheimer disease. Int J Mol Sci. 2021;22(15). doi: 10.3390/ijms22158333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cegielski J, Brook MS, Phillips BE, et al. The combined oral stable isotope assessment of muscle (COSIAM) reveals D-3 creatine derived muscle mass as a standout cross-sectional biomarker of muscle physiology vitality in older age. Geroscience. 2022;44(4):2129–2138. doi: 10.1007/s11357-022-00541-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vicinanza R, Zhang Y, Henning SM, et al. Pomegranate juice metabolites, ellagic acid and urolithin A, synergistically inhibit androgen-independent prostate cancer cell growth via distinct effects on cell cycle control and apoptosis. Evid-Based Compl Alt. 2013;2013:1–12. doi: 10.1155/2013/247504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-González C, Ciudad CJ, Izquierdo-Pulido M, et al. Urolithin a causes p21 up-regulation in prostate cancer cells. Eur J Nutr. 2016;55(3):1099–1112. doi: 10.1007/s00394-015-0924-z PMID 25962506. [DOI] [PubMed] [Google Scholar]
- 38.El-Wetidy MS, Ahmad R, Rady I, et al. Urolithin a induces cell cycle arrest and apoptosis by inhibiting Bcl-2, increasing p53-p21 proteins and reactive oxygen species production in colorectal cancer cells. Cell Stress Chaperones. 2021;26(3):473–493. doi: 10.1007/s12192-020-01189-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets used in this study are available from the corresponding author under reasonable request.


