Abstract
The porcine enteric coronaviruses (PECs) currently reported include porcine epidemic diarrhea virus (PEDV), porcine deltacoronavirus (PDCoV), transmissible gastroenteritis virus (TGEV), and swine acute diarrhea syndrome coronavirus (SADS-CoV). In the absence of effective treatment, they can cause similar clinical characteristics including weight loss, sleepiness, vomiting, anorexia and fatal diarrhea in neonatal piglets, resulting in significant economic losses to the global pig industry. Although many studies on drugs for treating and combating PECs have been issued. There are still no specific drug targeting PECs and used in clinical production. Therefore, it is necessary to sort out and summarize the research on the treatment and anti PECs drugs, and further development of low toxicity and high efficiency drugs is needed. Here, we review the latest progress of anti PECs drugs, focus on the mechanism of anti PECs reaction of drug components, and try to clarify new strategies for effective control and elimination of PECs. These comprehensive and profound insights will help to further investigate, prevent and control the transmission of PECs infection.
Keywords: PEDV, TGEV, SADS-CoV, PDCoV, therapy, antiviral drugs
1. Introduction
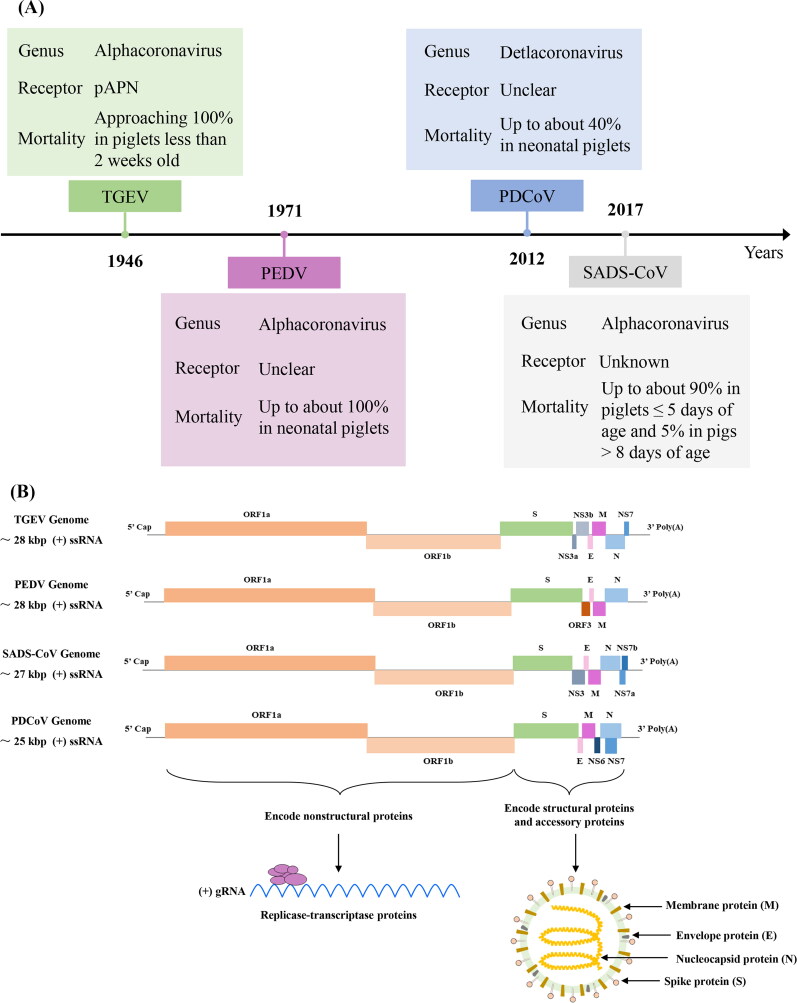
The 2019 coronavirus (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) has given rise to extensive research explorations in human society regarding the preventive control and cross-species transmission mechanisms of coronaviruses in animals (Zhai et al. 2021; Suo et al. 2023). As the largest single stranded positive strand RNA virus widely existing in nature, which belongs to order Nidovirales, family Coronaviridae, genus Coronavirus in virus taxonomy (Gao et al. 2022). According to the genome standard, four coronavirus genera can be distinguished, α coronavirus, β coronavirus, γ coronavirus and δ coronavirus (Gao et al. 2022). So far, four different porcine enteric coronaviruses (PECs) have been identified, including transmissible gastroenteritis virus (TGEV), porcine epidemic diarrhea virus (PEDV), porcine deltacoronavirus (PDCoV) and swine acute diarrhea syndrome coronavirus (SADS-CoV) (Duan et al. 2022). Among the four known PECs, PDCoV belongs to δ coronavirus, PEDV, TGEV and SADS-CoV belong to α coronavirus (Figure 1 A) (Walker et al. 2020; Chen and Burrough 2022). It can infect pigs of different ages, and the incidence rate and mortality of piglets are high, which is one of the most difficult problems in the world pig industry (Liu and Wang 2021). PECs mainly affect the digestive tract of piglets, and clinical symptoms include weight loss, sleepiness, vomiting, anorexia, watery diarrhea, and even death (Duan et al. 2022). The pathological features were necrosis and exfoliation of intestinal cells and injury of intestinal villi (Chen and Burrough 2022). Notably, a new zoonotic coronavirus, SARS-CoV-2, emerged in humans (Arun Krishnan et al. 2020). Many scientists are interested in the possibility of its occurrence and the pathogenicity of domestic animals, including pigs as hosts of different coronaviruses and one of the most important food producing animals that may have a significant impact on public health (Turlewicz-Podbielska and Pomorska-Mól 2021). Pigs are not susceptible to SARS-CoV-2 infection and do not play any role in the epidemiology of SARS-CoV-2 disease (Turlewicz-Podbielska and Pomorska-Mól 2021). Fortunately, the infectivity of PECs into humans has not yet been effectively confirmed.
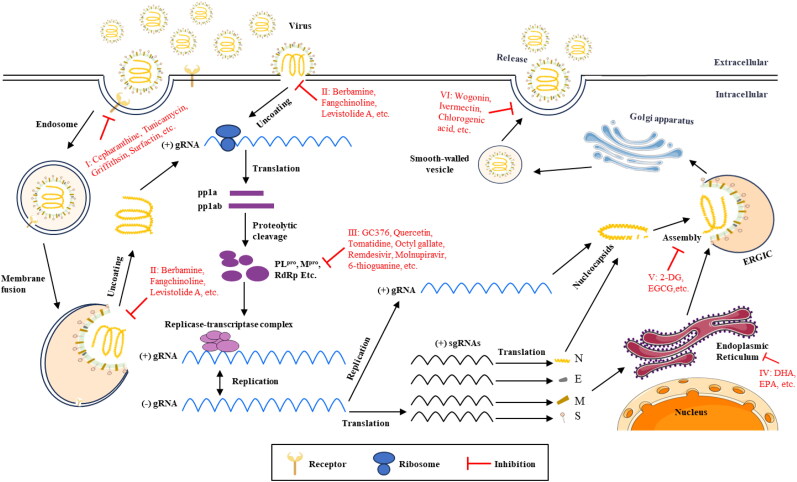
Figure 1.
The time flow chart (A) illustrates the initial report of porcine enteric coronavirus and the schematic diagram (B) depicts the genome composition and viral particle structure.
In recent decades, due to advancements in farming technology and the expansion of farming operations, various pathogenic microorganisms, such as PECs, have become widespread worldwide. This poses a significant challenge to the health and safety of the breeding industry (Zhang et al. 2022c). Previous studies found that there was no virus neutralization cross reaction among PEDV, TGEV and PDCoV (Ma et al. 2016; Luo et al. 2017). Therefore, the prevention of these very similar PECs requires the development of separate virus specific vaccine products (Ma et al. 2016; Wang et al. 2019b). TGEV vaccine has existed for a long time (Gerdts and Zakhartchouk 2017). Effective vaccines for PDCoV and PEDV are still under development, while SADS-CoV has not been reported (Li et al. 2019a). In particular, the high genetic diversity of PEDV hinders the development of effective PEDV vaccines (Gerdts and Zakhartchouk 2017; Wang et al. 2019b). Thus, screening for safe, inexpensive, and broad-spectrum coronavirus antiviral drugs remains important. As we all know, virus infection is a multi-step process, including adsorption, cell entry, peeling, biosynthesis, assembly and release (Zeng et al. 2022a). In addition to the viral proteins of PECs, the role of many host factors (including receptors) in these processes has also been confirmed. An in-depth understanding of the interaction between these virus particles (or host factors) and drugs will help to clarify the pathogenesis of PECs and accelerate the development of commercial anti PECs drugs.
More coronaviruses will be discovered as research technology and global commercial trade evolve. An appropriate summary will benefit us in more calmly responding to the harm that the newly emerging coronavirus brings to humans or the livestock industry. In this review, we focus on describing the mechanism of action of drugs against PECs virus particle infection documented in the literature. Finally, we try to propose new strategies for effective control and elimination of PECs.
2. Porcine epidemic diarrhea virus
The genome size of PEDV is approximately 28 kb, encoding four structural proteins: spike protein (S), membrane protein (M), envelope protein (E), and nucleocapsid protein (N) (Figure 1 B). PEDV was first reported in the UK in the 1970s and subsequently emerged in East Asia around 2010, with reports of its presence now widespread globally (Figure 1 A). This virus is highly virulent and contagious to piglets, exhibiting a mortality rate of up to 100% in piglets aged 1 to 7 days (Figure 1 A). Research indicates that PEDV can replicate in the primary target cells of its natural host and can efficiently infect human, monkey, and bat cells. There is speculation that PEDV may be transmitted through bat coronaviruses, initially causing diarrhea in fattening and adult pigs before subsequently infecting piglets. To date, only pigs have been documented as infected with PEDV, with no evidence of impact on public health safety. Although numerous studies have investigated drugs targeting PEDV, there are currently no accurate and highly effective commercial treatments available.
2.1. Bacteria- and bacterial metabolites-based PEDV inhibitors
2.1.1. Streptomyces inhibitors
Kim and his colleagues isolated three new metabolites, xiamycin C, D and E (Table 1), from Streptomyces sp (#HK18). which have inhibitory activity against PEDV replication. Among them, xiamycin D showed the strongest inhibitory effect on PEDV replication (EC50 = 0.93 μmol/L in Ref. Kim et al. 2016a) with low cytotoxicity (CC50 = 56.03 μmol/L, Table 1). Quantitative real-time PCR data revealed the inhibitory effect of xiamycin D on genes encoding essential structural proteins (GP6 nucleocapsid, GP2 spike, and GP5 membrane) for PEDV replication in a dose-dependent manner. Western blotting data also showed a dose-dependent inhibition of GP2 spike and GP6 nucleocapsid protein synthesis of PEDV by xiamycin D (Kim et al. 2016a).
Table 1.
Summary of natural product extract cocktail- and natural metabolites-based PECs inhibitors.
| Species (or others) | Inhibitor | Testing model | Main phases of action | Toxicity CC50 value of drugs in vitro assay (μg/mL or others) | Viruses | Ref. |
|---|---|---|---|---|---|---|
| Streptomyces | Xiamycin C | In vitro | Replication | 76.90 ± 3.29 μmol/L | PEDV | (Kim et al. 2016a) |
| Xiamycin D | In vitro | Replication | 56.03 ± 3.45 μmol/L | PEDV | (Kim et al. 2016a) | |
| Xiamycin E | In vitro | Replication | 98.74 ± 1.52 μmol/L | PEDV | (Kim et al. 2016a) | |
| Bacillus subtilis | Surfactin | In vitro and In vivo | Attachment, entry | > 50 | PEDV | (Yuan et al. 2018) |
| Surfactin | In vitro and In vivo | Attachment, entry | > 50 | TGEV | (Yuan et al. 2018) | |
| Surfactin | In vitro | Attachment, entry | > 20 | TGEV | (Wang et al. 2017b) | |
| Compound 1 (surfactin analogues) | In vitro | Attachment, entry | 847.2 ± 124.9 | PEDV | (Yuan et al. 2019) | |
| B. subtilis OKB105 | In vitro | Attachment, entry | – | TGEV | (Wang et al. 2017b) | |
| Bacillus licheniformis | B. licheniformis fermented products | In vitro and In vivo | Replication | > 150 | PEDV | (Peng et al. 2019) |
| Lactic acid bacteria | Lact. plantarum 22 F and 25 F | In vitro | Replication | – | PEDV | (Sirichokchatchawan et al. 2018) |
| Pediococcus strains 72 N and 77 F | In vitro | Replication | – | PEDV | (Sirichokchatchawan et al. 2018) | |
| Lactobacillus agilis (L3) | In vitro | Attachment | 1 × 108 CFU/mL | PEDV | (Chen et al. 2022b) | |
| Lactobacillus salivarius (L4) | In vitro | Attachment | 1 × 108 CFU/mL | PEDV | (Chen et al. 2022b) | |
| Lactobacillus salivarius (L4) | In vitro | Replication | – | PEDV | (Dong et al. 2021) | |
| Ln esenteroides (L5) | In vitro | Replication | – | PEDV | (Chang-Liao et al. 2020) | |
| L. acidophilus S-layer protein | In vitro | Attachment, replication | > 512 | PEDV | (Zhang et al. 2019) | |
| Lactobacillus plantarum metabolites | In vitro and In vivo | Attachment | > 1/8 times of the stock solution | PEDV | (Huang et al. 2021) | |
| L. plantarum exopolysaccharides | In vitro and In vivo | Attachment | > 1.35 × 103 | PEDV | (Huang et al. 2021) | |
| Lp-1 strain | In vitro | Replication | < 1/4-fold by Lp-1 | TGEV | (Wang et al. 2019a) | |
| LP-1S | In vitro | Replication | < 1/4-fold by LP-1S | PEDV | (Kan et al. 2023) | |
| Extracellular polysaccharide | In vitro | Replication | > 400 | PEDV | (Chen et al. 2023a) | |
| Limosilactobacillus reuteri (L6) | In vitro and In vivo | Replication | – | PEDV | (Huang et al. 2023) | |
| Lactobacillus rhamnosus | Lactobacillus rhamnosus GG | In vivo | Replication | – | PEDV | (Xu et al. 2024b) |
| Australian green treefrog | Caerin1.1 | In vitro | Attachment | > 200 | PEDV | (Guo et al. 2018) |
| Epimedium koreanum Nakai | Extract E7 | In vitro and In vivo | Replication | > 1.5 × 103 | PEDV | (Cho et al. 2012) |
| Extract E7 | In vitro | Replication | > 1.5 × 103 | TGEV | (Cho et al. 2012) | |
| Lonicera japonica Thunberg | Extract L8 | In vitro | Replication | > 20 fold-diluted | PEDV | (Cho et al. 2012) |
| Polysaccharide from Ginkgo biloba exocarp | P9 | In vitro | Attachment, entry | > 100 | PEDV | (Lee et al. 2015) |
| Griffithsia spp. | Griffithsin | In vitro | Attachment | > 10 | PEDV | (Li et al. 2019b) |
| In vitro | Attachment | 167.034 | PDCoV | (Tang et al. 2022) | ||
| Pogostemon cablin polysaccharides | PCP10 | In vitro | Replication | > 1250 | PEDV | (Chen et al. 2020b) |
| PCP11 | In vitro | Replication | > 625 | PEDV | (Chen et al. 2020b) | |
| PCP12 | In vitro | Attachment, entry, replication | > 5000 | PEDV | (Chen et al. 2020b) | |
| PCP13 | In vitro | Attachment, entry, replication | > 2500 | PEDV | (Chen et al. 2020b) | |
| Aloe | Aloe extract | In vitro and In vivo | Replication | > 1.6 × 104 | PEDV | (Xu et al. 2020) |
| Stixis scandens | S20 | In vitro | Replication | > 40 | PEDV | (Trinh et al. 2021) |
| Lactuca indica L. | L14 | In vitro | Replication | > 630 | PEDV | (Trinh et al. 2021) |
| Glochidion eriocarpum Champ. | G15 | In vitro | Replication | > 40 | PEDV | (Trinh et al. 2021) |
| Anisomeles indica (L.) Kuntze | A26 | In vitro | Replication | > 40 | PEDV | (Trinh et al. 2021) |
| Pericampylus glaucus (Lam.) Merr. | P27 | In vitro | Replication | > 160 | PEDV | (Trinh et al. 2021) |
| Mahonia bealei (Fortune) Carrière | M28 | In vitro | Replication | > 160 | PEDV | (Trinh et al. 2021) |
| Gnetum montanum Markgr | G29 | In vitro | Replication | > 160 | PEDV | (Trinh et al. 2021) |
| Tacca chantrieri André | T30 | In vitro | Replication | > 310 | PEDV | (Trinh et al. 2021) |
| Crinum asiaticum L. | C31 | In vitro | Replication | > 80 | PEDV | (Trinh et al. 2021) |
| Mallotus barbatus Müll.Arg | M32 | In vitro | Replication | > 40 | PEDV | (Trinh et al. 2021) |
| Croton kongensis Gagnep. | C33 | In vitro | Replication | > 40 | PEDV | (Trinh et al. 2021) |
| Tinospora sinensis (Lour.) Merr. | T34 | In vitro | Replication | > 80 | PEDV | (Trinh et al. 2021) |
| Aristolochia xuanlienensis | A35 | In vitro | Replication | > 1250 | PEDV | (Trinh et al. 2021) |
| Aristolochia acuminata Lam. | A36 | In vitro | Replication | > 80 | PEDV | (Trinh et al. 2021) |
| Alpinia genus | Alpinia zerumbet extract | In vitro | Attachment, replication | > 500 | PEDV | (Narusaka et al. 2021) |
| Portulaca oleracea | Portulaca oleracea extract | In vitro | Attachment, replication | > 2.5 × 104 | PEDV | (Liu et al. 2021) |
| Portulaca oleracea extract | In vitro | Replication | > 2.5 × 104 | PEDV | (Zhang et al. 2023d) | |
| Alpiniae oxyphyllae fructus | Alpiniae oxyphyllae fructus polysaccharide 3 | In vitro | Attachment, entry | > 5000 | PEDV | (Chen et al. 2021b) |
| Alpiniae oxyphyllae fructus polysaccharide 3 | In vitro | Attachment, entry | > 5000 | PEDV | (Luo et al. 2022) | |
| Alpiniae oxyphyllae fructus polysaccharide 3 | In vitro | Replication | > 400 | PEDV | (Wu et al. 2023) | |
| M. oleifera | M.oleifera extract | In vitro | Replication | > 2000 | PEDV | (Cao et al. 2022) |
| Hypericum japonicum | Hypericum japonicum extract | In vitro and In vivo | Later of replication stage | > 500 | PEDV | (Rao et al. 2023) |
| Licorice | Licorice extract | In vitro and In vivo | Attachment, internalization, replication | 473.3 | PEDV | (Bai et al. 2024) |
| Traditional Chinese medicine compound | Lizhong decoction | In vitro and In vivo | Replication | > 4 × 104 | PEDV | (Chen et al. 2024b) |
| Insects product | Black soldier fly extract | In vivo | Replication | > 50 mg/kg | PEDV | (Yu et al. 2024) |
| Fungal product | Yeast polysaccharides | In vivo | Replication | > 20 mg/kg | PEDV | (Li et al. 2024a) |
| – | Attapulgite | In vitro | Attachment | – | PEDV | (Wang et al. 2024c) |
| Enterococcus faecium | NCIMB 10415 | In vitro | Attachment, replication | – | TGEV | (Chai et al. 2013) |
| Antimicrobial peptide | Bovine antimicrobial peptide-13 | In vitro and In vivo | Replication | > 62.5 | TGEV | (Liang et al. 2020) |
| mReg3a | In vitro | Replication | > 62.5 | PEDV | (Bai et al. 2021) | |
| mReg3a | In vitro | Replication | > 62.5 | TGEV | (Bai et al. 2021) | |
| Cimicifuga | Cimicifuga rhizoma polysaccharide | In vitro | Replication | > 250 | TGEV | (Tan et al. 2024) |
Note: “–”, no data provided.
2.1.2. Bacillus subtilis inhibitors
Surfactin is a cyclic lipopeptide naturally produced by various strains of Bacillus subtilis, the structure consists of a seven amino acid peptide loop and a hydrophobic fatty acid chain. Surfactin acts as an antiviral agent by inhibiting viral membrane fusion and has antiviral activity against a variety of enveloped viruses, including herpes simplex virus (HSV-1, HSV-2), vesicular stomatitis virus (VSV), monkey immunodeficiency virus (SIV) (Huang et al. 2006). Yuan et al. found that surfactin from Bacillus subtilis can suppress the proliferation of PEDV and TGEV in epithelial cells at a relatively low concentration range (15 to 50 μg/mL), without cytotoxicity (Table 1) or viral membrane disruption. Experiments demonstrate that surfactin treatment significantly reduces the rate at which the virus fuses to the cell membrane, and the incorporation of small amounts of surfactin hinders the formation of negative curvature by lamellar-phase lipids (Yuan et al. 2018). Whereafter, Yuan et al. found that daily oral surfactin doses in excess of 20 mg/kg body weight (bw) will significantly increase the anti-PEDV property of jejunal contents in BALB/c mouse assays. Therefore, oral dose was set to 20 mg/kg bw to explore whether surfactin (Table 1) can protect piglets from PEDV challenge. In the surfactin treatment group, the piglets were orally administered surfactin every 6 h starting at the age of 1 day, and the daily dose was 20 mg/kg bw. After PEDV challenge, no death was observed within 48 h. All piglets were sacrificed at 48 h post-infection (hpi). Jejunal hyperemia and colonic tympanites could be seen in the PEDV group piglets. In contrast, in the surfactin treatment group, there were no obvious pathological changes in the intestines. The histopathological results for the jejuna further confirmed that jejunal hyperemia was observed only in the piglets in the PEDV group. Finally, the level of the PEDV genome in jejunal tissue was quantified by qRT-PCR. PEDV was undetectable in the surfactin treatment group, as well as the blank group (Yuan et al. 2018). Next, Yuan’s group obtained 10 surface protein analogues through chemical synthesis and evaluated them to determine their anti-PEDV activity, hemolysis activity and critical micelle concentration. Among them, Compound 1 [SLP5 in Ref. Yuan et al. 2019] has lower hemolysis activity than surfactin and has the same antiviral activity. In addition, Compound 1 has a higher safe and effective concentration range than surfactin. Similar to surfactin, Compound 1 has direct antiviral effects on PEDV (Yuan et al. 2019).
Huang et al. found that oral immunization of piglets with Bacillus subtilis spores (B.s) plus whole inactivated PEDV WIV enhanced anti-PEDV capacity on mucosal surfaces and reduced plaque neutralization tests in serum and intestinal fluids. Antigen-specific IgG titers were increased in serum and IgA titers were increased in saliva, feces and nasal rinse fluid. Increased area of ileal Peyer’s patches and number of intraepithelial lymphocytes in piglets. The percentage of CD4+CD8+ memory T cells in intestinal mucosa-associated lymphocytes was upregulated, and the proliferation of antigen-specific memory T cells was enhanced. It is suggested that B.s can enhance potential immunity through oral immune upregulation of memory CD4+CD8+ T cells (Huang et al. 2019). Another study found that the number of T lymphocytes and monocytes in the blood and colostrum increased significantly after sows were fed 4,4′-diaponeurosporene-producing Bacillus subtilis (BS-Dia) on day 80 of gestation. The proliferative activity of T lymphocytes in colostrum also increased significantly. In addition, the levels of transforming growth factor β (TGF-β), interleukin 6 (IL-6), lysozyme and lactoferrin were significantly increased. It is worth noting that piglets from sows fed BS-Dia during pregnancy did not show diarrhea symptoms or intestinal pathological changes 48 h after being infected with PEDV, and the PEDV loads in the jejunum and ileum were significantly reduced. The piglets of sows that did not take BS-Dia orally during pregnancy showed obvious diarrhea symptoms, and there was extensive PEDV colonization in the jejunum and ileum. These results indicate that oral administration of BS-Dia to pregnant sows can significantly improve innate lactogenic immunity and thereby prevent neonatal piglets from being infected with PEDV (Liu et al. 2023).
Luo and his colleagues used crossbred (Duroc × Landrace × Yorkshire) weanling piglets with an average weight of 6.62 ± 0.36 kg to explore the effects of adding synbiotics (SYB, consist of S. cerevisiae, Lactobacilli, B. subtilis, and their fermentation extract, such as β-glucan, mannan oligosaccharide, and various metabolites in Ref. Luo et al. 2024) to the diet on the growth performance, immune function and intestinal barrier function of PEDV-challenged piglets. The rats were fed with diets containing 0.1% SYB and 0.2% SYB for 21 days respectively. On the 22nd day of feeding, 40 mL of 5.6 × 103 TCID50/mL PEDV virus liquid was administered to each head. On the 26th day of feeding, adding SYB to the diet could inhibit the decrease in average daily feed intake and average daily weight gain caused by PEDV challenge, among which 0.1% SYB had the best alleviation effect. In addition, the levels of serum interleukin (IL)-10, immunoglobulin M, complement component 4 and jejunal mucosa IL-4 in the diet supplemented with 0.1% SYB were significantly increased, and the serum diamine oxidase activity was significantly reduced. In addition, 0.1% SYB increased the mRNA expression of cludin-1, occludens protein-1, mucin 2, interferon-γ, interferon regulatory factor-3, signal transduction factors and transcription activators, and occludin protein expression in the jejunal mucosa. Downregulating toll-like receptor 3 (TLR3) and tumor necrosis factor (TNF)-α mRNA expression. Adding 0.2% SYB also had a positive effect on piglets, but the effect was not as good as 0.1% SYB. These results show that adding 0.1% SYB to the diet can improve the growth performance of pigs, reduce inflammation and intestinal barrier damage by improving innate immune function and reducing PEDV genome copy number, and has a good protective effect against PEDV infection (Luo et al. 2024).
2.1.3. Bacillus licheniformis inhibitors
Bacillus licheniformis (B. licheniformis, Table 1) is commonly used as probiotic and its secondary metabolites are attractive anti‑microbial candidate. Peng et al. showed by in vitro experiments revealed that while B. licheniformis crude extracts exhibited no toxicity in Vero cells (CC50 > 150 μg/mL, Table 1), co‑cultivation of B. licheniformis crude extracts with PEDV significantly reduced viral infection and replication (Peng et al. 2019). Next, Peng et al. showed by in vivo, PEDV‑infected piglets supplemented with air‑dried solid state fermentative cultivate containing BLFP (5 kg/ton feed) showed milder clinical symptoms and decreased viral shedding. Importantly, no significant systemic pathological lesions and no reduction in average daily gain were noted in pigs supplemented with the BLFP, which suggests that it is safe for use in pigs (Peng et al. 2019).
2.1.4. Lactic acid bacteria inhibitors
Sirichokchatchawan et al. isolated from pig feces seven Lactic acid bacteria (LAB) [Ent. faecium 79 N and 40 N, Lact. plantarum 22, 25 and 31 F, Ped.acidilactici 72 N and Ped. pentosaceus 77 F in Ref. Sirichokchatchawan et al. 2018]. Among them, Lact. plantarum (22 F and 25 F) and Pediococcus strains 72 N and 77 F could reduce infectivity of the PEDV in the Vero cells (Sirichokchatchawan et al. 2018). Similarly, Chen and his colleagues isolated some LAB strains from the feces of nursing piglets that showed the protective effects of against PEDV infection. Among L3 and L4 strains [YM22 and YM33 in Ref. Chen et al. 2022b, Table 1 (L3) and (L4)] the intracellular extracts or cell wall fractions are more effective in preventing PEDV than other strains. L3 and L4 strains did not interact directly with virions, but had high adhesion capacity to Vero cells. Thereby, L3 and L4 could compete with PEDV viral particles to attach to cell receptors, preventing the virus from invading cells. Meanwhile, L3 and L4 could inhibited the increased TNF-α and IL-8 mRNA expression by PEDV-infected cells (Chen et al. 2022b). Another study found that when porcine jejunum intestinal epithelial (IPEC-J2) cells were treated with L4 before infection with PEDV, the expression levels of ITGA1, ITGA5, ITGB5, FAK, PIK3R1, PIK3CA and AKT1 mRNA were significantly increased at different times after infection. L4 may upregulate the FAK/PI3K/Akt signaling pathway in IPEC-J2 cells to resist PEDV infection (Dong et al. 2021).
Table 3.
Summary of inorganic substance-based PECs inhibitors.
| Inhibitor | Testing model | CC50 (μmol/L or others) | Main phases of action | Viruses | Ref. |
|---|---|---|---|---|---|
| Lithium chloride (LiCl) | In vitro | > 5.0 × 104 | Entry, replication | TGEV | (Ren et al. 2011) |
| In vitro | > 1.5 × 104 | Entry, replication | PEDV | (Li et al. 2018) | |
| In vitro | > 6 × 104 | Entry, replication | PDCoV | (Zhai et al. 2019) | |
| Graphene oxide | In vitro | > 50 μg/mL | Entry, replication | PEDV | (Ye et al. 2015) |
| Te/BSA NPs | In vitro | > 30 μg/mL | Entry | PEDV | (Zhou et al. 2020a) |
| Ag2S nanoclusters | In vitro | > 184 μg/mL | Replication | PEDV | (Du et al. 2018a) |
| Silver nanoparticle-modified graphene oxide nanocomposites | In vitro | > 8.0 μg/mL | Entry, replication | PEDV | (Du et al. 2018b) |
| Au@Ag nanorods | In vitro | > 0.16 | Entry, replication | PEDV | (Du et al. 2020) |
| Zinc sulfide nanoparticles | In vitro | > 1.20 mg/mL | Entry, replication | PEDV | (Zhou et al. 2020b) |
| Gly-CDs | In vitro | > 0.90 mg/mL | Entry, replication | PEDV | (Tong et al. 2020) |
| Selenium Nano-Particles | In vitro | > 10 μg/mL | Replication | PDCoV | (Ren et al. 2022d) |
| Zinc chloride | In vitro | 321 | Replication, release | TGEV | (Wei et al. 2012) |
| Zinc sulfate | In vitro | 343 | Replication, release | TGEV | (Wei et al. 2012) |
| Ag nanoparticles (Ag NPs) | In vitro | > 12.5 μg/mL | Entry, replication | TGEV | (Lv et al. 2014) |
| NM-300 | In vitro | > 12.5 μg/mL | Entry, replication | TGEV | (Lv et al. 2014) |
| Silver nanowires (XFJ011) | In vitro | > 12.5 μg/mL | Entry, replication | TGEV | (Lv et al. 2014) |
| Silver colloids (XFJ04) | In vitro | > 12.5 μg/mL | Entry, replication | TGEV | (Lv et al. 2014) |
Kefir is an acidic and mildly alcoholic fermented milk product that is believed to have many beneficial activities, such as hypocholesterolemic activity, antibacterial and antifungal activities, antitumor activity, immunomodulatory activity, and quickening of wound healing (Bourrie et al. 2016). Numerous bacterial strains with specific properties, such as hypocholesterolemic effect, antiallergenic effect, immunoregulatory effects, and antipathogenic activities, have been isolated from kefir grains (Prado et al. 2015). Moreover, Chang-Liao et al. screened twenty-nine LAB strains with anti-PEDV potential from kefir grains, which were isolated and identified as Enterococcus durans, Lactobacillus kefiri, Lactococcus lactis, and Leuconostoc mesenteroides. Among them, the intracellular extracts of Ln. mesenteroides showed higher anti-PEDV activities than that of the other species. Next, the antiviral activity of an Ln. mesenteroide strain named L5 (YPK30 in Ref. Chang-Liao et al. 2020, Table 1), which showed a higher growth rate than that of the other strains, and was further evaluated. The results showed that the intracellular extract of L5 up-regulatory effect on the expression of myxovirus resistance 1 (MX1) and interferon-stimulated gene 15 (ISG15) genes in Vero cells, thus possessed prophylactic, therapeutic, and direct-inhibitory effects against PEDV in vitro (Chang-Liao et al. 2020).
Surface layer (S-layer) proteins are crystalline arrays of proteinaceous subunits forming the outermost component of the cell wall in the Lactobacillus acidophilus (L. acidophilus). Zhang et al. found that L. acidophilus S-layer proteins (Table 1) in Vero cells could reduce PEDV infection, mainly via inhibiting the attachment of viral particles and by reducing apoptosis in Vero cells at the later stages of PEDV infection. Mechanistically L. acidophilus S-layer proteins protect against PEDV-induced apoptosis by reducing caspase-8 and caspase-3 activation during the late stages of infection (Zhang et al. 2019).
Both Lactobacillus plantarum metabolite (LPM) and L. plantarum exopolysaccharide (LPE) have strong inhibitory effect on PEDV. Extraction of LPM with trichloroacetic acid resulted in LPE at a concentration of 2.71 mg/mL. LPE could adhere to PEDV to prevent its adsorption to Vero cells. The best inhibitory effect was obtained when LPE at a concentration of 1.35 mg/mL (Table 1) was incubated with PEDV. But the inhibition of PEDV will completely lost at LPE concentrations below 0.3375 mg/mL. LPE inhibits PEDV replication by attenuating the inflammatory response and inducing early apoptosis of damaged cells, but cannot regulate the immune function of cells (Huang et al. 2021). Next, Huang’s team pre-fed 3-day-old piglets with LPM for 2 d and then conducted PEDV infection experiment. There was no sign of severe gastroenteritis or intestinal wall thinning in the LPM pretreated group, and the viral load was significantly lower than that in the PEDV-infected group. Immunohistochemistry (IHC) was used to detect PEDV positive antigen in jejunum. The colonization level of PEDV positive antigen on villi in LPM pretreatment group was significantly lower than that in positive control group. These results suggest that LPM has a good inhibitory effect on PEDV in vivo (Huang et al. 2021). Using IPEC-J2 cells as a model, Kan and his research team found that Lactiplantibacillus plantarum supernatant (LP-1S, Table 1) can reduce PEDV-induced loss of calcium channel proteins (TRPV6 and PMCA1b), alleviate intracellular Ca2+ accumulation caused by PEDV infection, and promote intracellular and extracellular Ca2+ concentration balance, thereby inhibiting the proliferation of PEDV (Kan et al. 2023).
Limosilactobacillus reuter (L. reuteri) is one of the dominant LAB species in the intestine of piglets. Researchers have isolated and identified 9 strains of L. reuteri from pig feces, among which L6 (LRC8 in Ref. Huang et al. 2023, Table 1) has a higher inhibitory rate against PEDV than other strains. Subsequently, the research team found that L6 and its metabolites could significantly down-regulate the mRNA expression levels of inflammatory cytokines in Vero cells, and could see preventive, therapeutic, competitive and direct inhibitory effects on PEDV. In addition, the L6 strain effectively alleviated clinical symptoms and intestinal damage in PEDV-infected piglets (Huang et al. 2023).
Chen and his colleagues utilized CRISPR-Cas9 technology to uncover the biological function of extracellular polysaccharide (EPS) derived from Lacticaseibacillus casei (L.casei), which possesses antioxidant and anti-inflammatory properties. Furthermore, EPS was found to suppress the generation of reactive oxygen species (ROS) in IPEC-J2 cells. Moreover, EPS demonstrated an immunomodulatory impact on PEDV infection by promoting the activation of IL-10, a receptor involved in type III interferon (IFN) signaling, leading to the inhibition of PEDV replication (Chen et al. 2023a).
Yang et al. used RT-qPCR to examine the relative mRNA expression of inflammation-related factors in PEDV-infected and uninfected pigs at different ages and found that IL-6 and TNF-α mRNA expression was significantly higher in infected piglets than in infected older pigs (Yang et al. 2023a). Coincidentally, a previous study by Yang and his colleagues found that PEDV infection altered the distribution of intestinal microbes, with Lactobacillus and Shigella having the greatest impact. The absolute and relative abundance of Lactobacillus increased and the absolute and relative abundance of Shigella decreased in the gut of 1-week-old PEDV-infected piglets compared to non-PEDV-infected piglets. In contrast, the trend of Lactobacillus and Shigella in the intestine of 2/4-week-old piglets was opposite, with the absolute and relative abundance of Lactobacillus decreasing and the absolute and relative abundance of Shigella increasing. Combined with the differences in mortality of PEDV infection in piglets at different ages, it was hypothesized that LAB had an inhibitory effect on PEDV infection in newborn piglets (Yang et al. 2020b). In addition, it has been shown that LAB have strong anti-inflammatory effects (Saez-Lara et al. 2015; Chen et al. 2022b). Therefore, Yang and his colleagues first arranged for newborn piglets to be infected with PEDV orally for 7 d after oral administration of LAB (Table 4), and continued to observe and record all piglets and collect samples after 14 d of euthanasia. Analysis of clinical data from the piglet experiment showed that the lactobacillus preparation reduced mortality and diarrhea caused by PEDV. Notably, piglets in the treated group had lower intestinal viral loads, almost no positive staining for PEDV-N protein on intestinal immunohistochemical analysis, and intestinal villi as healthy as those in the uninfected group. In addition, piglets in the treated group showed suppressed inflammatory response, enhanced intestinal barrier and significantly upregulated type III interferon levels (Yang et al. 2023a). Similarly, a recent study found that Lactobacillus delbrueckii, Lactobacillus johnsonii, and Lactococcus lactis can protect piglets infected by PEDV from less intestinal damage, less epithelial cell necrosis, and less severe damage. Lactobacilli can inhibit the replication of PEDV in the intestinal tract of piglets. Short-chain fatty acid content was assessed through targeted metabolomics and it was found that acetic acid exhibits resistance to PEDV. In addition, sodium acetate modulated increased NK cell and macrophage counts in mesenteric lymph nodes, increased NK cells in the spleen and macrophages in the blood, enhancing innate immune defense against PEDV. In addition, acetic acid can increase the number of CD8+ IFN-γ T cells in the blood, spleen and mesenteric lymph, CD4+ IFN-γ T cells in the mesenteric lymph node and spleen, and the number of CD4+ IL-4+ T cells in the blood. Further studies found that acetic acid increased the expression of ZO-1 through the P13K/AKT signaling pathway. These results indicate that acetic acid produced by lactic acid bacteria can regulate the intestinal barrier and immune function and alleviate PEDV infection (Sun et al. 2024b). Combined with the previous literature studies on LAB inhibition of PEDV in vitro (Sirichokchatchawan et al. 2018; Chen et al. 2022b), this suggests that LAB has great potential value in the prevention of PEDV infection in pigs.
Table 4.
Drug treatment of PECs infection in piglets.
| Drugs | Virus strains | Days of age | Delivery | Infective dose | Drug dose | Therapeutic effect | Ref. |
|---|---|---|---|---|---|---|---|
| Epimedium koreanum Nakai extract | KPEDV-9 and PEDV sm98 | > 4 d | Oral challenge | –, 10 LD50 | Basal diet supplemented with 0.6% | E7-treated piglets exhibited no symptoms of disease, including diarrhea, and biopsy results indicated clean intestines. | (Cho et al. 2012) |
| MYCI | PEDV | 3 d | Oral challenge | 3 mL, 1 × 103.5 TCID50/ mL | 60 mg | MYCI treatment significantly enhanced the average daily weight gain of virus-challenged piglets and mitigated the severe intestinal villous atrophy and crypt hyperplasia induced by viral invasion. | (Kim et al. 2015) |
| Homoharringtonine | PEDV CV777 | 3 ∼ 5 d | Injected intramuscularly | –, 2 × 103 PFU | 0.2 mg/kg | HHT treatment can significantly reduce viral load and alleviate clinical symptoms. | (Dong et al. 2018) |
| Surfactin | PEDV CV777 | 1 d | Oral administration | – | 20 mg/kg | The survival rate of piglets was 100% (3/3), no obvious pathological damage was observed in the intestines, and no viral genome was detected in the jejunal tissue. | (Yuan et al. 2018) |
| B. licheniformis‑fermented products | PEDV PT-P6 | 28 d | Orally challenged | –, 5 × 105 TCID50/ mL | 5 kg/ton feed | The clinical symptoms of piglets treated were mild and virus shedding was reduced. | (Peng et al. 2019) |
| Aloe extract | PEDV GDS01 | 4 d | Orally challenged | 2 mL, 1 × 107 PFU | 100 mg/kg | It can reduce the viral load and pathological changes in pig intestines and protect piglets from the fatal challenge of infection by the highly pathogenic PEDV variant GDS01. | (Xu et al. 2020) |
| Puerarin | PEDV YN15 | 7 d | Oral administration | 3.3 mL, 1 × 104.5 TCID50/ mL | 0.5 mg/kg | PR can alleviate the decline in cell proliferation and growth performance of PEDV-infected piglets and has antiviral and anti-inflammatory effects. | (Wu et al. 2020) |
| 7 d | Oral administration | 3.3 mL, 1 × 104.5 TCID50/ mL | 0.5 mg/ kg | PR can reduce the incidence of PEDV-infected piglets, enhance anti-inflammatory function, improve antioxidant capacity, enhance the intestinal mucosal barrier, and increase the abundance of beneficial intestinal bacteria. | (Wu et al. 2021) | ||
| Phenanthridine Derivatives | PEDV NK-2 | 1 d | Orally challenged | 5 mL,103 MID/mL | 50 mg/kg | Treated piglets had reduced intestinal damage, reduced viral load and reduced mortality. | (Chen et al. 2021a) |
| L. plantarum CQ2017RC | PEDV CV777 | 3 d | Oral administration | 10 mL, 107 PFU/mL | – | It can improve the intestinal morphology of infected piglets and inhibit virus replication in the gastrointestinal tract. | (Huang et al. 2021) |
| Limosilactobacillus reuteri | PEDV strain JS-2013 | 21 d | Oral administration | 4 mL, 1 × 106 TCID50/ mL | 5 mL, 3 × 108 CFU/mL | Effectively alleviated the clinical symptoms and intestinal damage of infected piglets. | (Huang et al. 2023) |
| Monolaurin (ML) | PEDV Yunnan strain, KT021228 | 7 d | Orally administrated | –, 1 × 104.5 TCID50/ mL | 100 mg/kg | The recovery of intestinal villi in infected piglets following treatment alleviates diarrhea, enhances intestinal function, diminishes viral replication, and exerts anti-inflammatory effects. | (Zhang et al. 2021a) |
| 7 d | Orally administrated | –, 1 × 104.5 TCID50/ mL | 100 mg/kg | ML can facilitate the recovery of piglets following infection by restoring the integrity of the intestinal barrier, enhancing protein utilization efficiency, and boosting the body’s antioxidant capacity and immune defense functions. | (Wang et al. 2023a) | ||
| Bacillus subtilis spores (B.s) | PEDV WIV | 5 d | Orally administrated | –, 100 μg/dose | 109 CFU/test | The treated piglets exhibited improved antiviral immunity. | (Huang et al. 2019) |
| Buddlejasaponin IVb | PEDV AH-2018-HF1 | 3 d | Orally administrated | 1 mL, 1 × 106 TCID50/ mL | 1 mg/kg | The clinical symptoms of piglets were mild, the lung lesions and viral load were basically the same as those in the non-challenged control group, and the levels of intestinal inflammatory factors were also lower. | (Sun et al. 2022) |
| Cepharanthine | PEDV ZJXS11 | 3 d | Orally challenged | 1 mL, 1 × 102.2 LD50 | 11.1 mg/kg | Treatment resulted in a reduction of viral load in the intestinal tract of piglets, as well as alleviation of pathological damage. | (Dong et al. 2022) |
| Hypericum japonicum extract | PEDV G2 | 8 d | Oral administration | 5 mL, l × 105 PFU | 1.28 g/kg | HJ can reduce viral titers in the intestines of infected piglets, improve intestinal pathology, and inhibit piglet diarrhea by regulating intestinal flora. | (Rao et al. 2023) |
| Lactic acid bacteria (LAB) | PEDV GS | 7 d | Orally infected | –, 109 copies/pig | 109 CFU | After treatment, the inflammatory response of piglets was suppressed, the intestinal barrier and anti-viral immunity were enhanced, and the diarrhea, virus copy number and mortality of infected piglets were reduced. | (Yang et al. 2023a) |
| Acetic acid produced by lactic acid bacteria. | PEDV | 7 d | Feeding | 2 mL, 1.0 × 105.25 TCID50/mL | 2 g/kg | Piglets in the treatment group can mitigate PEDV infection by enhancing both intestinal barrier function and immune response. | (Sun et al. 2024b) |
| Octyl gallate (OG) | PEDV strain HM2017 | 5 d | Orally challenged | 3 mL, 1.33 × 106 TCID50/mL | 250 mg/kg | None of the piglets died following treatment, and 75% of the infected piglets exhibited significant relief in clinical symptoms, pathological lesions, and viral loads in both the jejunum and ileum. | (Su et al. 2023) |
| Pemetrexed acts | PEDV-LJX | 7 d | Feeding | 15 mL, 1.35 × 106 TCID50/mL | 0.5 mg/kg | No significant pathological changes were observed in the gastrointestinal tract of piglets, and the intestinal viral load was reduced, which can effectively alleviate diarrhea caused by PEDV. | (Zhang et al. 2024b) |
| Ellagic acid | PEDV | 7 d | Orally administered | 1 mL, 1.0 × 106 TCID50/mL | 20 mg/kg | EA has been shown to reduce oxidative stress and intestinal inflammation in piglets. It enhances antiviral function by modulating the interferon pathway and concurrently activating JAK2/STAT3 signaling. | (Song et al. 2024) |
| Lithocholic acid | PEDV CV777 | 11 d | Orally gavaging | 1 mL, 103.5 PFU ml−1 | Basal diet supplemented with 0.02% | LCA enhances the expression of SLA-I in porcine intestinal epithelial cells via FXR receptors, subsequently attracting a greater number of CD8+ CTLs to combat PEDV infection. | (Xing et al. 2024) |
| Licorice extract | PEDV HM2017 | 4 d | Orally administered | 3 mL, 1 × 104.8 TCID50 /mL | 250 mg/kg | The survival rate of the treatment group was 80% (4/5), and there was a significant alleviation of clinical symptoms, pathological lesions, and viral loads in the jejunum and ileum. | (Bai et al. 2024) |
| Lizhong decoction | PEDV- LN-P15 | 3 d | orally administered | 5 mL, 1 × 105.48 TCID50 /mL | 1 g/kg | LZD was able to decrease the viral titers in the infected piglets’ intestinal and visceral tissues, ameliorate their intestinal pathology, cause a significant increase in body weight growth and increase the survival rate of piglets by 40% (2/5). | (Chen et al. 2024b) |
| Hyperoside | PEDV HM2017 | 3 d | Orally given | 2 mL, 1 × 106.125 TCID50/mL | 500 mg/kg | The survival rate of PEDV-infected piglets following Hyperoside treatment was 75% (3/4), and there was a significant reduction in viral load. | (Wang et al. 2024a) |
| Synbiotics | PEDV | 26 ± 1 d | Orally administrated | 40 mL, 5.6 × 103 TCID50/mL | Basal diet supplemented with 0.1% | Improve pig growth performance, improve innate immune function and reduce viral genome copy number, reduce inflammatory response and intestinal barrier damage. | (Luo et al. 2024) |
| Lactobacillus rhamnosus GG | PEDV | 7 d | Orally administrated | 3 mL, 1 × 106 TCID50/mL | 50 mg/kg | LGG has the potential to improve the intestinal morphology of piglets infected with PEDV, enhance their intestinal antioxidant capacity, and mitigate jejunal mucosal inflammation as well as lipid metabolism disorders. | (Xu et al. 2024b) |
| Black soldier fly extract | PEDV | 7 d | Orally administrated | –, 1 × 106 TCID50/mL | 500 mg/kg | BFE can enhance the morphological indicators of intestinal tissue in piglets, mitigate the oxidative stress induced by PEDV infection, and promote the mRNA expression levels of antiviral-related genes in the ileum. | (Yu et al. 2024) |
| Yeast polysaccharides | PEDV | 7 d | Orally administrated | –, 1 × 106 TCID50/mL | 20 mg/kg | YP has been shown to inhibit viral replication, improve intestinal morphology, enhance antioxidant capacity, reduce inflammation, and regulate intestinal metabolism in piglets infected with PEDV. | (Li et al. 2024a) |
| PA-824 | PEDV AH-2018-HF1 | 2 d | Orally administrated | 1 mL, 4 × 105 TCID50/mL | 50 mg/kg | It can effectively alleviate clinical symptoms, intestinal pathological changes, and inflammatory reactions in piglets, while significantly reducing the viral load in both pig feces and intestinal tissues. | (Li et al. 2024b) |
| Berbamine | PEDV AH-2018-HF1 | 3 d | orally administered | 10 mL, 1 × 106 TCID50/mL | 100 mg/kg | BBM can effectively mitigate intestinal damage caused by PEDV infection in piglets, leading to a reduction in both viral load and cytokine levels, including IL-6, IL-8, IL-1β, and TNF-α. | (Xiang et al. 2024) |
| Benzoic acid | PEDV ZJ08 | 1 d | orally administered | 1 mL, 104 PFU/mL | 0.15g | BA promotes the differentiation of intestinal goblet cells by mediating the Wnt/Notch/MAPK pathway, which subsequently enhances the mucus barrier and protects piglets from PEDV. | (Liu et al. 2024b) |
| Andrographolide | PEDV FS202201 | 3 d | orally administered | –, 1 × 105 TCID50/mL | 10 mg/kg | AND treatment alleviated clinical symptoms, enhanced intestinal integrity, and increased the survival rate of infected piglets by 16.7%. | (He et al. 2024) |
| Ergosterol peroxide | PDCoV CHN-HN-1601 | 7 d | Oral administration | 5 mL, 1 × 106 TCID50/mL | 2.5 mg/kg | EP treatment can reduce the incidence of diarrhea, alleviate intestinal lesions, and reduce viral loads in feces and tissues. | (Duan et al. 2021c) |
| Niacin | PDCoV CHN-HN-17 | 28 d | Oral administration | 10 mL, 1 × 107 TCID50/mL | 40 mg | Niacin could partly alleviate diarrhea, intestinal barrier damages, intestinal immune response and colonic microflora disruption in PDCoV-infected weaned piglets. | (Chen et al. 2022a) |
| APB-13 | TGEV HN-2012 | 4 d | Oral administration | 10 mL, 1 × 108 TCID50/mL | 10 g/kg | APB-13 can enhance digestive enzyme activity, improve digestibility, and promote piglet growth performance and survival rates by correcting intestinal microbial disorders. | (Liang et al. 2020) |
| Eugenol | TGEV TS strain | 21 d | Oral administration | –, 2.8 × 109 PFU | 400 mg/kg | Reduce pyroptosis of intestinal epithelial cells and reduce intestinal damage in infected piglets. | (Wang et al. 2023d) |
| Polygonum Cillinerve polysaccharide | TGEV | 28 d | Orally administered | 15 mL, 1 × 106 TCID50/mL | – | PCP has a cure rate of 50% (4/8). | (Duan et al. 2024a) |
| Quercetin | SADS-CoV | 2 d | Orally administered | 10 mL, 5 × 106 TCID50/mL | 10 mg/kg | Quercetin has been shown to alleviate clinical symptoms and intestinal pathological damage while also reducing the expression levels of inflammatory factors. | (Feng et al. 2024) |
Note: “–”, no data provided.
2.1.5. Lactobacillus rhamnosus inhibitors
Lactobacillus rhamnosus GG (LGG) is a probiotic strain known for its safety. Xu et al. investigated the impact of LGG on intestinal health in nursing piglets exposed to PEDV. The group receiving LGG treatment was administered continuously for 8 days before and after infection. Samples were collected for analysis on the 3rd day post-inoculation. The findings indicated that LGG supplementation could enhance intestinal morphology, boost antioxidant capacity, reduce jejunal mucosal inflammation, and mitigate lipid metabolism disorders in PEDV-infected piglets. These effects may be attributed to alterations in the expressions of the TNF signaling pathway, PPAR signaling pathway, and fat digestion and absorption pathway (Xu et al. 2024b).
2.1.6. Antimicrobial peptides inhibitors
Antimicrobial peptides (AMPs) are important components of the animal nonspecific immune system and have a wide range of antimicrobial activities against microorganisms such as bacteria, viruses, fungi and parasites. Also, AMPs can modulate host immune responses such as chemokines, cytokine production and pro-inflammatory responses (Hölzl et al. 2008). Caerin 1.1 (Table 1) is a peptide with 25 residues (GLLSV LGSVA KHVLP HVVPV IAEHLNH2) from the granular glands in the skin of the Australian green treefrog (Haney et al. 2009). Caerin 1.1 has been shown to have a complete inhibitory effect on HIV by preventing viral fusion to target cells and disrupting the HIV envelope. Moreover, Caerin 1.1 is also very effective in inhibiting the transfer of HIV from dendritic cells (DCs) to T cells (Vancompernolle et al. 2015). Guo et al. found that at a very low concentration, Caerin1.1 also had the ability to disrupt the integrity of PEDV virus particles and prevent the release of the virus, leading to a significant reduction in PEDV infection without interfering with the binding process between PEDV and cellular receptors (Guo et al. 2018).
2.2. Plants- and plant extract cocktails-based PEDV inhibitors
Over the past years, there have been many reports about the anti-PEDV activity of plants and plant extract cocktails. These plants and plant extract cocktails are of particular interest to researchers due to their easy availability and low toxicity. Although plant extract cocktails have considerable potential as virus control agents, further extensive, specific screening and development are required. Cho et al. found two herbal extracts, Epimedium koreanum Nakai and Lonicera japonica Thunberg [KIOM 198 and KIOM 124 in Ref. Cho et al. 2012, Table 1 (E7) and (L8)], from 333 natural oriental herbal medicines for that significant anti-PEDV effects. The plaque and cytopathic effects (CPE) inhibition assay in vitro showed that E7 had more potent antiviral activity than L8. Additionally, E7 also exhibited a similar extent of antiviral effect against TGEV (Cho et al. 2012). Cho et al. used germ-free piglets to dieted with E7 (Table 1) for 4 d, and then were orally infected with PED viruses (Table 4). At 24 hpi, the piglets fed with E7 had normal feces, whereas the challenge group piglets that were not fed E7 had diarrhea. Furthermore, in the presence of E7, the intestine of piglet was free of disease symptom, and no virus was detected. However, from 48 h of post-infection, viral number in the E7-dieted piglets was increased, but it was still 10-fold lower than the challenge group piglets that were not fed E7. Despite the fact that the underlying mechanism of E7 action has not been detailed, Cho and his colleagues assume E7 exerts strong antiviral effect through modulating immune response such as macrophage and lymphocyte stimulation (Cho et al. 2012). Moreover, Kim et al. treated PEDV-infected 3-day-old piglets with a daily oral milk substitute containing 60 mg which use of extracts of medicinal herbs Taraxaumi mongolicum, Viola yedoensis Makino, Rhizoma coptidis, and Radix isatidis (MYCI) for 7 days. Furthermore, MYCI mixture alleviated severe intestinal villus atrophy and crypt hyperplasia caused by PEDV attack in piglets. Herb extract improved growth performance impairment and intestinal damage in newborn piglets attacked by toxic PEDV. MYCI mixtures can be used as prophylactic or therapeutic agents for PED (Kim et al. 2015).
Lee and his colleagues investigated in vitro anti-PEDV effect of polysaccharide from Ginkgo biloba exocarp, and found that the polysaccharide (P9, Table 1) exhibited potent antiviral activity against PEDV reducing the formation of a visible CPE (IC50 = 1.7 ± 1.3 μg/mL), compared to ribavirin and it did not show cytotoxicity at 100 μg/mL (CC50 > 100 μg/mL, Table 1). Polysaccharide also showed effective inhibitory effects when added at the viral attachment and entry steps. Moreover, polysaccharide effectively inactivated PEDV infection in time-, dose- and temperature-dependent manners (Lee et al. 2015). Griffithsin [Table 1 (G6)], a high-mannose-specific lectin from Griffithsia spp. marine red algae, showed exerts antiviral activity against multiple enveloped viruses. Li et al. found that Griffithsin had potent anti-PEDV activity via prevention of viral attachment and cell-to-cell spread, and it exhibited a stronger effect on PEDV infection when it was added during early stages of infection (Li et al. 2019b). Pogostemon cablin (Blanco) Benth is widely used in China as a traditional Chinese medicine for the treatment of diarrhea, vomiting, nausea and fever. Polysaccharide is an important component of pogostemon cablin (Blanco) Benth. Chen et al. obtained four pogostemon cablin polysaccharides (PCP1.1, PCP1.2, PCP2.1 and PCP2.2 in Ref. Chen et al. 2020b, named as PCP10, PCP11, PCP12 and PCP13, Table 1) with the anti-PEDV activities from the dry overground parts of pogostemon cablin (Blanco) benth by water extraction and alcohol precipitation method. PCP10 and PCP11 inhibited PEDV replication, while PCP12 and PCP13 inhibited PEDV penetration and replication. Furthermore, those PCP10-13 showed anti-oxidative effects, which were important to the anti-PEDV activities (Chen et al. 2020b).
Previous studies reported that not only the antiviral effects of the whole extracts of aloe, but also for their isolated compounds have significant antiviral activity, such as catechin hydrate, kaempferol, aloin and emodin. Xu et al. found that aloe extract (Ae) can hamper completely the proliferation of PEDV at a non-cytotoxic concentration of 16 mg/mL (Table 1) in Vero and IPEC-J2 cells in vitro. Furthermore, time course analysis indicated the extract exerted its inhibition at the late stage of the viral life cycle. Moreover, the extract can inactivate PEDV directly but did not act on the viral genome and S1 protein. Because, it was reported that emodin from Ae are directly virucidal to enveloped viruses and are related to the partially disruption of viral envelopes, indicating that the direct inactivation PEDV by Ae might relate to emodin’s destruction of the virus envelope (Xu et al. 2020). Next, Xu et al. confirmed that the relative safe concentration of Ae at 100 mg/kg in BALB/c mouse assays. Therefore, oral dose was set to 100 mg/kg bw to explore whether Ae can protect piglets from PEDV challenge. The results show that no piglets died in the Ae treatment-PEDV challenge groups (4 piglets/group) in two days. In contrast, PEDV challenge without Ae treatment group (4 piglets/group), two piglets died in two days. This indicates that Ae could protect newborn piglets from lethal challenge with highly pathogenic PEDV variant GDS01 infection (Table 4). To determine the gross pathological and histological changes in piglets after PEDV infection, all piglets from each group were necropsied at 2 dpi. In the PEDV challenge without Ae treatment group, the small intestinal tract, where yellow watery contents accumulated, were transparent, thin-walled, and gas-distended. No lesions or slight lesions were observed in the mock and Ae treatment-PEDV challenge groups. As shown that abruption of jejunum villus was observed in the PEDV challenge without Ae treatment group, whereas the jejunum was normal in the mock and Ae treatment-PEDV challenge groups. Consistent with the histopathological results, more PEDV antigen was detected in the cytoplasm of the villous enterocytes of the PEDV challenge without Ae treatment piglets by immunohistochemical analysis. Collectively, Ae could reduce virus load and pathological change in intestinal tract of piglets, indicating that Ae efficiently inhibited PEDV infection in vivo. Although Xu et al. confirmed that Ae could inhibit PEDV in vivo, slight diarrhea was found in Ae-treatmented piglets, which Xu et al. speculates might be related with Aloe vera ingredient emodin, which used as a laxative (Xu et al. 2020).
Trinh and his colleagues evaluated the antiviral activity of ethanolic and aqueous extracts of 17 traditional Vietnamese medicinal plants against PEDV based on a cytopathic effect-based assay and found that 14 of them inhibited the cytopathic effect of PEDV (Table 1). Among them, the ethanolic extract of Stixis scandens (Table 1) was identified as the most effective extract with minimum inhibitory concentration (MIC) of 0.15 μg/mL (Trinh et al. 2021). Narusaka et al. Proanthocyanidins (PACs, Table 1) extracted from Alpinia zerumbet effectively inactivated the viral particle activity of PEDV in a dose-dependent manner. The results of the cytopathic effect assay showed that 0.1 mg/ml of PACs significantly reduced the titer of PEDV (Narusaka et al. 2021). Liu’s group found that the water extract of Portulaca oleracea (WEPO, Table 1) could significantly inhibit PEDV replication by 92.73% on human lung mucosal epithelial (VH) cells and 63.07% on Vero cells. Furthermore, WEPO inhibited PEDV infection mainly during the adsorption phase and down-regulated the expression of S protein in a dose-dependent manner. In addition, the WEPO to inhibit PEDV replication in VH cells by down-regulating the cytokine levels (TNF-α, IL-22 and IFN-α) and inhibiting the NF-κB signaling pathway activated by PEDV (Liu et al. 2021). Next, the research team further studied the anti-PEDV action mechanism of WEPO in Vero cells. WEPO can inhibit Vero cell pyroptosis caused by PEDV and reduce the increase in inflammatory factors caused by infection. It mainly acts through the Caspase-1/GSDMD pathway (Zhang et al. 2023d). Alpiniae oxyphyllae frucus (Table 1), derived from the dry ripe fruit of Alpinia oxyphyla Miq., is a fructus polysaccharide with a wide range of activities including immunomodulatory, antineoplastic, antiviral and antioxidant activity. Chen et al. found that Alpiniae oxyphyllae fructus polysaccharide 3 (AOFP3) could significantly reduce the PEDV titer in IPEC-J2 cells and prevent the damage of IPEC-J2 cells caused by PEDV infection. Furthermore, AOFP3 showed antioxidant activity in inhibiting PEDV propagation (Chen et al. 2021b). Next, the anti-PEDV mechanism of AOFP3 was further explored and found that AOFP3 competitively inhibited the adsorption of PEDV on IPEC-J2 cells by blocking the binding of PEDV S protein to porcine aminopeptidase in IPEC-J2 cells. Moreover, AOFP3 reduced the penetration of PEDV into host cells by decreasing cholesterol levels in IPEC-J2 cells (Luo et al. 2022). In addition, a recent study found that AOFP3 significantly reduced PEDV’s replication by down-regulating the activity of PEDV RNA-Dependent RNA polymerase (RdRp) and reducing the expression of heterogeneous nuclear ribonucleoprotein A1 (Wu et al. 2023). Cao et al. found that an aqueous leaf extract of M.oleifera (MOE, Table 1) exhibited antiviral activity in response to PEDV infection at the stage of PEDV replication instead of attachment or internalization. Mechanistically, MOE suppressed the oxidative stress and the expression of inflammatory cytokines induced by PEDV infection and upregulated the expression of anti-apoptotic proteins, which further led to less cell apoptosis (Cao et al. 2022).
Rao’s team found that Hypericum japonicum extract (HJ, Table 1) had antiviral effects against PEDV in vitro and in vivo. In in vitro assays, HJ could directly inactivate different PEDV strains. Notably, at non-cytotoxic concentrations HJ could inhibit the proliferation of PEDV strains in Vero or IPI-FX cells at various stages of the viral life cycle, especially in the late stage (Rao et al. 2023). Next, Rao and his colleagues also investigated the antiviral effect of Hypericum japonicum extract (HJ) in newborn piglets infected with PEDV. First, at 3 days of age, piglets in the treatment group (n = 7) were given HJ orally at a dose of 1.28 g/kg body weight twice daily, while piglets in the model group (n = 7) were fed with the same amount of water. Next, after 6 d of administration (Table 4), all piglets were orally dosed with 5 mL of DMEM containing PEDV-G2 solution. Finally, all piglets were euthanized 48 h after infection, and follow-up studies with collected samples revealed that HJ reduced virus titers in the intestines of infected piglets and improved histopathological damage in their intestines compared to the model group. At the same time, HJ increased the number of intestinal probiotic flora in the treated piglets. Therefore, HJ may play a protective role in PEDV-induced injury by directly inhibiting viral proliferation and modulating the intestinal microbiota (Rao et al. 2023).
One study found that licorice extract (Le, Table 1) inhibited PEDV replication in a dose-dependent manner in vitro. It mainly inhibits the attachment, internalization and replication stages of PEDV-infected Vero cells (Bai et al. 2024). Subsequently, the in vivo treatment experiment of Le (Table 4) in piglets found that the survival rate of the Le treatment group was 80%, and the clinical symptoms, pathological lesions, and viral loads in the jejunum and ileum were significantly alleviated (Bai et al. 2024). Similarly, another study showcased the anti-PEDV effects of Lizhong decoction (LZD, Table 1) both in vitro and in vivo. The study identified a total of 648 compounds in LZD, including 144 alkaloids and 128 terpenes. The inhibitory impact of LZD on PEDV primarily targets the replication stage of the virus life cycle. Furthermore, LZD notably reduces the apoptosis rate of IPEC-J2 cells and Vero cells during PEDV infection. Noteworthy, LZD can decrease the virus titer in the intestinal and visceral tissues of PEDV-infected piglets (Table 4), enhance the intestinal pathology of piglets, and elevate the survival rate of piglets (Chen et al. 2024b).
2.3. Small molecule inhibitors affect PEDV adsorption
Viral entry is the initial step in the viral life cycle and involves multiple steps that primarily rely on the interaction between viral proteins and the membrane of the host cell. Generally, coronaviruses attach to specific receptors on the cell surface and then enter the host cell through two pathways: either by directly fusing with the plasma membrane or by using the endosomal pathway, which involves releasing the viral genome into the cytoplasm. Targeting viral entry through endosomal acidification, particularly for small molecules, is a promising strategy to combat porcine enterocoronavirus infection.
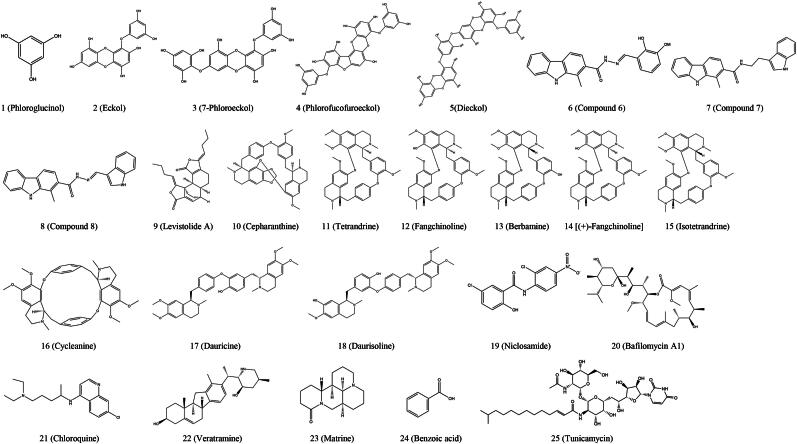
Kwon et al. conducted a study to evaluate the antiviral activity of five phlorotannins [compounds 1 to 5, Figure 2 (1 to 5)] isolated from Ecklonia cava against PEDV. In the medications-treatment assay, compounds 1to 5 (excluding compound 2) showed antiviral activities with a 50% inhibitory concentration (IC50, Table 2) ranging from 10.8 ± 1.4 to 22.5 ± 2.2 μmol/L against PEDV. Hemagglutination inhibition revealed that compounds 1 to 5 completely blocked the binding of viral spike protein to sialic acids at concentrations below 36.6 μmol/L. Furthermore, compounds 4 and 5 among the five phlorotannins inhibited viral replication with IC50 values (in Ref. Kwon et al. 2013) of 12.2 ± 2.8 and 14.6 ± 1.3 μmol/L, respectively, in the post-treatment assay. Notably, compounds 4 and 5 inhibited viral entry through hemagglutination inhibition and viral replication by inhibiting viral RNA and viral protein synthesis, but not viral protease (Kwon et al. 2013).
Figure 2.
Chemical structures of small molecule inhibitors that mainly inhibit porcine enteric coronavirus adsorption.
Table 2.
Small molecule inhibitors against PECs.
| Number of chemical structures in Fig. | Inhibitor | Testing model | Activity IC50/EC50 (μmol/L or others) | Toxicity CC50 value of drugs in vitro assay (μmol/L or others) | Main phases of action | Viruses | Ref. |
|---|---|---|---|---|---|---|---|
| Small molecule inhibitors that mainly inhibit PECs adsorption. | |||||||
| Figure 2 (1) | Phloroglucinol | In vitro | – | 374.4 ± 4.0 | Entry | PEDV | (Kwon et al. 2013) |
| Figure 2 (2) | Eckol | In vitro | 22.5 ± 2.2 | 388.3 ± 2.6 | Entry | PEDV | (Kwon et al. 2013) |
| Figure 2 (3) | 7-Phloroeckol | In vitro | 18.6 ± 2.3 | 446.2 ± 3.8 | Entry | PEDV | (Kwon et al. 2013) |
| Figure 2 (4) | Phlorofucofuroeckol | In vitro | 10.8 ± 1.4 | 579.0 ± 4.3 | Entry, replication | PEDV | (Kwon et al. 2013) |
| Figure 2 (5) | Dieckol | In vitro | 16.6 ± 3.0 | 490.6 ± 1.6 | Entry, replication | PEDV | (Kwon et al. 2013) |
| Figure 2 (6) | Compound 6 | In vitro | – | < 10 | Attachment | PEDV | (Chen et al. 2021c) |
| Figure 2 (7) | Compound 7 | In vitro | – | > 40 | Attachment | PEDV | (Chen et al. 2021c) |
| Figure 2 (8) | Compound 8 | In vitro | – | > 60 | Attachment | PEDV | (Chen et al. 2021c) |
| Figure 2 (9) | Levisrolide A | In vitro | – | > 100 | Attachment, entry, replication | PEDV | (Zeng et al. 2022b) |
| Figure 2 (10) | Cepharanthine | In vitro and In vivo | 2.53 | 29.92 | Attachment, entry | PEDV | (Dong et al. 2022) |
| In vitro | 0.2 | 10.98 | Attachment, replication | PEDV | (Leng et al. 2024) | ||
| In vitro | – | 17 | Attachment, replication | PDCoV | (Sun et al. 2024c) | ||
| In vitro | – | 16.18 | Entry | SADS-CoV | (Chen et al. 2022c) | ||
| In vitro | 1.03 | > 12.5 | Attachment, replication | SADS-CoV | (Leng et al. 2024) | ||
| Figure 2 (11) | Tetrandrine | In vitro | 3.50 | 24.78 | Attachment, entry | PEDV | (Dong et al. 2022) |
| In vitro | – | 30.03 | Attachment, replication | PEDV | (Qian et al. 2024) | ||
| In vitro | 0.44 | 11.71 | Attachment, replication | PEDV | (Leng et al. 2024) | ||
| In vitro | 2.19 | > 12.5 | Attachment, replication | SADS-CoV | (Leng et al. 2024) | ||
| Figure 2 (12) | Fangchinoline | In vitro | 6.69 | 30.19 | Attachment, entry | PEDV | (Dong et al. 2022) |
| In vitro | 3.54 | 17 | Attachment, entry | PEDV | (Zhang et al. 2023a) | ||
| In vitro | 0.67 | 37.49 | Replication | PEDV | (Zhang et al. 2023c) | ||
| In vitro | 0.2 | 12.35 | Attachment, replication | PEDV | (Leng et al. 2024) | ||
| In vitro | 2.23 | > 12.5 | Attachment, replication | SADS-CoV | (Leng et al. 2024) | ||
| Figure 2 (13) | Berbamine | In vitro | 9.00 | 20 | Attachment, entry | PEDV | (Zhang et al. 2023a) |
| In vitro | 0.5 | > 25 | Attachment, replication | PEDV | (Leng et al. 2024) | ||
| In vitro | 5.84 | > 25 | Attachment, replication | PEDV | (Leng et al. 2024) | ||
| Figure 2 (14) | (+)-Fangchinoline | In vitro | 4.68 | 16 | Attachment, entry | PEDV | (Zhang et al. 2023a) |
| Figure 2 (15) | Isotetrandrine | In vitro | 1.67 | > 25 | Attachment, replication | PEDV | (Leng et al. 2024) |
| In vitro | 3.67 | > 50 | Attachment, replication | SADS-CoV | (Leng et al. 2024) | ||
| Figure 2 (16) | Cycleanine | In vitro | 0.34 | > 12.5 | Attachment, replication | PEDV | (Leng et al. 2024) |
| In vitro | 2.77 | > 12.5 | Attachment, replication | SADS-CoV | (Leng et al. 2024) | ||
| Figure 2 (17) | Dauricine | In vitro | 1.7 | > 25 | Attachment, replication | PEDV | (Leng et al. 2024) |
| In vitro | 0.84 | > 12.5 | Attachment, replication | SADS-CoV | (Leng et al. 2024) | ||
| Figure 2 (18) | Daurisoline | In vitro | 0.82 | > 12.5 | Attachment, replication | PEDV | (Leng et al. 2024) |
| In vitro | 2.8 | > 12.5 | Attachment, replication | SADS-CoV | (Leng et al. 2024) | ||
| Figure 2 (19) | Niclosamide | In vitro | 0.246 | 25.29 | Entry | PEDV | (Wang et al. 2023g) |
| Figure 2 (20) | Bafilomycin A1 | In vitro | – | > 1.0 | Entry | PEDV | (Wang et al. 2023g) |
| Figure 2 (21) | Chloroquine | In vitro | – | > 25 | Entry | PEDV | (Wang et al. 2023g) |
| Figure 2 (22) | Veratramine | In vitro | ≤ 5 | >60 | Entry, replication | PEDV | (Chen et al. 2024a) |
| Figure 2 (23) | Matrine | In vitro | – | > 1.0 mg/mL | Entry, attachment, replication | PEDV | (Qiao et al. 2024) |
| Figure 2 (24) | Benzoic acid | In vitro and In vivo | – | > 4 × 103 | Entry | PEDV | (Liu et al. 2024b) |
| Figure 2 (25) | Tunicamycin | In vitro | 25.3 ng/mL | > 2 μg/mL | Attachment, entry | SADS-CoV | (Chen et al. 2023b) |
| Small molecule inhibitors that regulate viral proteins or proteases to inhibit PECs replication. | |||||||
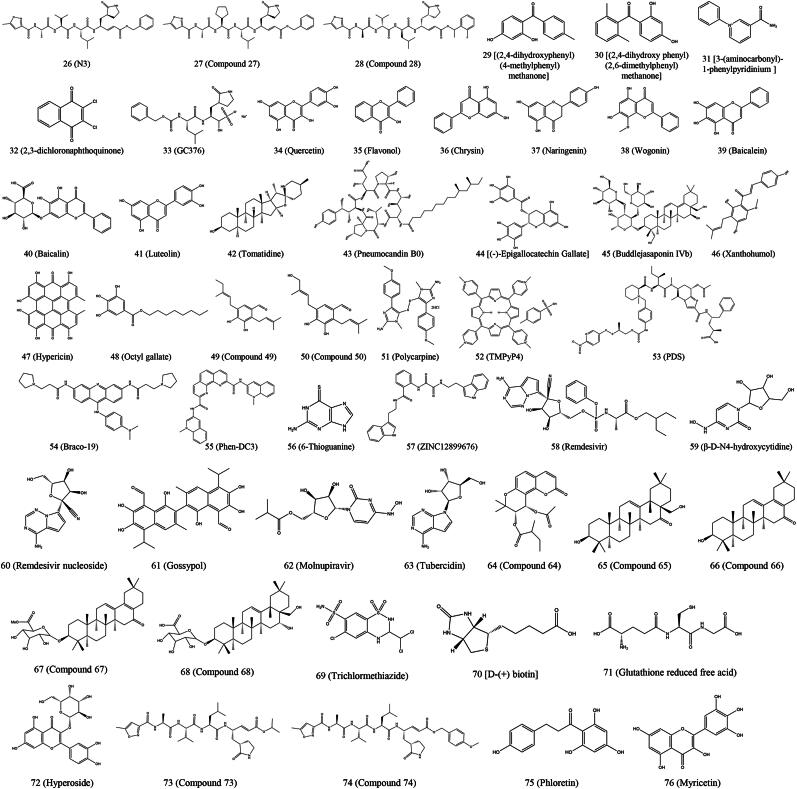
| Figure 3 (26) | N3 | In vitro | – | – | Replication | PEDV | (Wang et al. 2017a) |
| Figure 3 (27) | Compound 27 | In vitro | – | – | Replication | PEDV | (Wang et al. 2017a) |
| Figure 3 (28) | Compound 28 | In vitro | – | – | Replication | PEDV | (Wang et al. 2017a) |
| Figure 3 (29) | Compound 29 | In vitro | 37.8 | 533.8 | Replication | PEDV | (Shi et al. 2018) |
| Figure 3 (30) | Compound 30 | In vitro | 23.4 | 522.3 | Replication | PEDV | (Shi et al. 2018) |
| Figure 3 (31) | 3-(aminocarbonyl)-1-phenylpyridinium | In vitro | 0.1877 | 73.8 | Replication | PEDV | (Zhou et al. 2021) |
| Figure 3 (32) | 2,3-dichloronaphthoquinone | In vitro | 6.765 | 21.79 | Replication | PEDV | (Zhou et al. 2021) |
| Figure 3 (33) | GC376 | In vitro | 1.11 ± 1.13 | > 200 | Replication | PEDV | (Ye et al. 2020) |
| Figure 3 (34) | Quercetin | In vitro | ≤ 3 | > 400 | Replication | PEDV | (Li et al. 2020d) |
| In vitro | – | > 400 μg/mL | Replication | SADS-CoV | (Zheng et al. 2022) | ||
| In vitro | – | > 600 | Attachment, replication | SADS-CoV | (Feng et al. 2024) | ||
| Figure 3 (35) | Flavonol | In vitro | 20.37 | 463.8 | Replication | PEDV | (Liang et al. 2024) |
| Figure 3 (36) | Chrysin | In vitro | 2.484 ± 0.59 μg/mL | 83.56 ± 2.12 μg/mL | Replication | PEDV | (Gong et al. 2023) |
| Figure 3 (37) | Naringenin | In vitro | 4.505 ± 2.25 μg/mL | 61.86 ± 0.97 μg/mL | Replication | PEDV | (Gong et al. 2023) |
| Figure 3 (38) | Wogonin | In vitro | – | > 475 | Replication | PEDV | (Wang et al. 2023d) |
| Figure 3 (39) | Baicalein | In vitro | ≤ 11.2 | > 400 | Replication | PEDV | (Li et al. 2024c) |
| In vitro | – | 758.7 μg/mL | Replication | PDCoV | (Liu et al. 2024a) | ||
| Figure 3 (40) | Baicalin | In vitro | ≤ 13 | > 400 | Replication | PEDV | (Li et al. 2024c) |
| Figure 3 (41) | Luteolin | In vitro | ≤ 68.5 | > 238 | Replication | PEDV | (Wang et al. 2024b) |
| Figure 3 (42) | Tomatidine | In vitro | 3.447 | 45.68 | Replication | PEDV | (Wang et al. 2020) |
| Figure 3 (43) | Pneumocandin B0, | In vitro | 3.476 | 43.00 | Replication | PEDV | (Wang et al. 2020) |
| Figure 3 (44) | (-)-Epigallocatechin gallate | In vitro | 8.764 | 99.04 | Replication | PEDV | (Wang et al. 2020) |
| Figure 3 (45) | Buddlejasaponin IVb | In vitro | 8.136 | 89.77 | Replication | PEDV | (Wang et al. 2020) |
| In vitro and In vivo | 6.943 | 84.56 | Replication, release | PEDV | (Sun et al. 2022) | ||
| Figure 3 (46) | Xanthohumol | In vitro | 7.51 | 57.04 ± 2.11 | Replication | PEDV | (Lin et al. 2021) |
| Figure 3 (47) | Hypericin | In vitro | 5.90 ± 0.26 | 56.73 ± 9.4 | Replication | PEDV | (Zhang et al. 2021b) |
| In vitro | – | 97.06 ± 9.4 | Replication | TGEV | (Zhang et al. 2021b) | ||
| Figure 3 (48) | Octyl gallate (OG) | In vitro and In vivo | 242.15 | > 40 | Replication | PEDV | (Su et al. 2023) |
| Figure 3 (49) | Compound 49 | In vitro | 7.5 ± 0.7 | > 20 | Replication | PEDV | (Cho et al. 2019) |
| Figure 3 (50) | Compound 50 | In vivo | 8.0 ± 2.5 | > 20 | Replication | PEDV | (Cho et al. 2019) |
| Figure 3 (51) | Polycarpine | In vivo | 5.68 ± 0.80 | 50.20 ± 1.19 | Replication | PEDV | (Zhang et al. 2024a) |
| Figure 3 (52) | TMPyP4 | In vitro | – | > 60 | Replication | PEDV | (Li et al. 2023c) |
| Figure 3 (53) | PDS | In vitro | – | > 60 | Replication | PEDV | (Li et al. 2023c) |
| Figure 3 (54) | Braco-19 | In vitro | – | > 10 | Replication | PEDV | (Li et al. 2023c) |
| Figure 3 (55) | Phen-DC3 | In vitro | – | > 60 | Replication | PEDV | (Li et al. 2023c) |
| Figure 3 (56) | 6-thioguanine | In vivo | 13.7 ± 1.7 | – | Replication | PEDV | (Chu et al. 2018) |
| Figure 3 (57) | ZINC12899676 | In vitro | – | > 10 | Replication | PEDV | (Wang et al. 2022c) |
| Figure 3 (58) | Remdesivir | In vivo | – | > 10 | Replication | PDCoV | (Brown et al. 2019) |
| In vitro | – | > 250 | Replication | PEDV | (Xie et al. 2021) | ||
| In vitro | – | > 40 | Replication | SADS-CoV | (Zhou et al. 2023) | ||
| Figure 3 (59) | β-D-N4-hydroxycytidine | In vitro | – | > 125 | Replication | PEDV | (Xie et al. 2021) |
| Figure 3 (60) | Remdesivir nucleoside | In vitro | – | > 250 | Replication | PEDV | (Xie et al. 2021) |
| Figure 3 (61) | Gossypol | In vitro | 0.99 | > 10 | Replication | PEDV | (Wang et al. 2022d) |
| In vitro | 2.55 | > 10 | Replication | SADS-CoV | (Wang et al. 2022d) | ||
| In vitro | 1.06 | > 5 | Replication | PDCoV | (Wang et al. 2022d) | ||
| Figure 3 (62) | Molnupiravir | In vivo | 12.30 | > 96 | Replication | PEDV | (Huang et al. 2013) |
| Figure 3 (63) | Tubercidin | In vitro | 0.2487 | 14.23 | Replication | PEDV | (Wang et al. 2024d) |
| In vivo | – | 14.32 | Replication | SADS-CoV | (Wang et al. 2024d) | ||
| Figure 3 (64) | Compound 64 | In vitro | – | > 20 | Replication | PEDV | (Yang et al. 2015a) |
| Figure 3 (65) | Compound 65 | In vitro | – | 12.47 ± 0.97 | Replication | PEDV | (Yang et al. 2015b) |
| Figure 3 (66) | Compound 66 | In vitro | – | 9.32 ± 1.19 | Replication | PEDV | (Yang et al. 2015b) |
| Figure 3 (67) | Compound 67 | In vitro | – | 13.72 ± 1.35 | Replication | PEDV | (Yang et al. 2015b) |
| Figure 3 (68) | Compound 68 | In vitro | – | 2.25 ± 0.11 | Replication | PEDV | (Yang et al. 2015b) |
| Figure 3 (69) | Trichlormethiazide | In vitro | 8.754 mg/mL | > 0.094 mg/mL | Replication | PEDV | (Deejai et al. 2017) |
| Figure 3 (70) | D- (+) biotin | In vitro | 0.925 mg/mL | > 0.094 mg/mL | Replication | PEDV | (Deejai et al. 2017) |
| Figure 3 (71) | Glutathione reduced free acid | In vitro | 2.722 mg/mL | > 1.5 mg/mL | Replication | PEDV | (Deejai et al. 2017) |
| Figure 3 (72) | Hyperoside | In vitro | – | > 20 | Replication | PEDV | (Su et al. 2021) |
| In vitro | 2.588 μg/ml | 25.15 μg/ml | Replication | PEDV | (Wang et al. 2024a) | ||
| Figure 3 (73) | Compound 73 | In vitro | – | > 100 | Replication | PDCoV | (Wang et al. 2022a) |
| Figure 3 (74) | Compound 74 | In vitro | – | > 100 | Replication | PDCoV | (Wang et al. 2022a) |
| Figure 3 (75) | Phloretin | In vivo | 65.4 ± 4.26 | 440.6 ± 4.2 | Replication | TGEV | (Duan et al. 2024b) |
| Figure 3 (76) | Myricetin | In vivo | 31.19 | > 1000 | Replication | TGEV | (Fan et al. 2024) |
| Small molecule inhibitors that regulate host factors to inhibit PECs replication. | |||||||
| Figure 4 (77) | Glycyrrhizin | In vitro | – | >800 | Entry, replication | PEDV | (Huan et al. 2017) |
| In vitro | – | >5 | Replication | PEDV | (Gao et al. 2020) | ||
| Figure 4 (78) | Chem-80,048,685 | In vitro | 39.03 | 116.7 | Attachment, replication | PEDV | (Wang et al. 2023b) |
| Figure 4 (79) | Ouabain | In vitro | – | > 10 nmol/L | Attachment | PEDV | (Xiong et al. 2023) |
| In vitro | 0.147 ± 0.028 | > 10 | Replication | TGEV | (Yang et al. 2017) | ||
| In vitro | – | > 0.2 | Replication | TGEV | (Yang et al. 2022a) | ||
| Figure 4 (80) | PST2238 | In vitro | – | > 10 | Attachment | PEDV | (Xiong et al. 2023) |
| Figure 4 (81) | Pemetrexed acts | In vitro and In vivo | – | > 256 | Replication | PEDV | (Zhang et al. 2024b) |
| Figure 4 (82) | SP2509 | In vitro | 0.919 | 4.763 | Replication | PEDV | (Zhao et al. 2024) |
| Figure 4 (83) | Triacetyl resveratrol | In vitro | 42.5 | > 200 | Replication | PEDV | (Wang et al. 2022e) |
| Figure 4 (84) | Andrographolide | In vitro and In vivo | – | > 50 | Replication | PEDV | (He et al. 2024) |
| Figure 4 (85) | PA-824 | In vitro and In vivo | 18.4 | 233.2 | Replication | PEDV | (Li et al. 2024b) |
| Figure 4 (86) | NAD+ | In vitro | 17.63 | 184.3 | Replication | PEDV | (Li et al. 2024b) |
| Figure 4 (87) | Lovastatin | In vitro | 19.67 | 115.2 | Replication | PEDV | (Li et al. 2024b) |
| Figure 4 (88) | Hyodeoxycholic acid | In vitro | 18.22 | 174.45 | Replication | PEDV | (Li et al. 2024b) |
| Figure 4 (89) | Rapamycin | In vitro | – | > 0.1 | Replication | PEDV | (Ko et al. 2017) |
| Figure 4 (90) | Homoharringtonine | In vivo | 0.112 | 5.582 | Replication | PEDV | (Dong et al. 2018) |
| In vitro and In vivo | – | > 1.0 | Replication | PEDV | (Li and Wang 2020) | ||
| Figure 4 (91) | Hydroxychloroquine | In vitro and In vivo | – | > 100 | Replication | PEDV | (Li and Wang 2020) |
| Figure 4 (92) | Cinchonine | In vitro | – | > 200 | Replication | PEDV | (Ren et al. 2022a) |
| Figure 4 (93) | Erastin | In vitro | – | > 8 | Replication | PEDV | (Zhang et al. 2023b) |
| Figure 4 (94) | (1S,3R)-RSL3 | In vitro | – | > 6 | Replication | PEDV | (Li et al. 2023b) |
| Figure 4 (95) | Quercetin7-rhamnoside | In vitro | 0.014 μg/mL | > 100 μg/mL | Replication | PEDV | (Song et al. 2011) |
| Figure 4 (96) | Emodin | In vitro | 2.1 | > 100 | Replication | PEDV | (Li et al. 2021) |
| In vitro | – | > 12.5 μg/mL | Attachment, replication | SADS-CoV | (Zheng et al. 2022) | ||
| Figure 4 (97) | Gossypol-Acetic acid | In vitro | 2.9 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (98) | Gynostemma Extract | In vitro | 2.7 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (99) | Oridonin | In vitro | 3.0 | 35 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (100) | Licochalcone A | In vitro | 4.0 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (101) | Amphotericin B | In vitro | 2.91 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (102) | Demethylzeylasteral | In vitro | 2.37 | 38.6 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (103) | Tubeimoside I | In vitro | 4.21 | 74.8 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (104) | Harmine hydrochloride | In vitro | 1.33 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (105) | Betulonic acid | In vitro | < 1.25 | 61.9 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (106) | Ursonic acid | In vitro | 2.13 | 41.0 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (107) | 3′-Hydroxypterostilbene | In vitro | 4.29 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (108) | Tannic acid | In vitro | 4.37 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (109) | (E)-Cardamonin | In vitro | 2.15 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (110) | Harmine | In vitro | 1.96 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (111) | Esculetin | In vitro | 5.97 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (112) | Lithocholic acid | In vitro | 2.37 | > 100 | Replication | PEDV | (Li et al. 2021) |
| In vitro | – | > 25 | Replication | PDCoV | (Kong et al. 2021) | ||
| Figure 4 (113) | Nordihydroguaiaretic acid | In vitro | 5.00 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (114) | Efonidipine | In vitro | 5.58 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (115) | Tabersonine hydrochloride | In vitro | 4.30 | 82.6 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (116) | Protoporphyrin IX | In vitro | < 1.25 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (117) | Proanthocyanidins | In vitro | 2.19 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (118) | Caffeic Acid Phenethyl Ester | In vitro | 1.74 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (119) | Grape seed Extract | In vitro | 2.42 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (120) | 7-Ethylcamptothecin | In vitro | < 1.25 | > 100 | Replication | PEDV | (Li et al. 2021) |
| Figure 4 (121) | Puerarin | In vivo | – | – | Replication | PEDV | (Wu et al. 2020) |
| Figure 4 (122) | Magnolol | In vitro | 28.21 | 57.28 | Replication | PEDV | (Wang et al. 2023e) |
| Figure 4 (123) | Ergosterol peroxide | In vitro | – | 327.6 | Entry, replication, release | PEDV | (Liu et al. 2022) |
| In vitro and In vivo | – | > 248 | Attachment, entry, replication | PDCoV | (Duan et al. 2021a) | ||
| In vitro and In vivo | – | > 248 | Attachment, entry, replication | PDCoV | (Duan et al. 2021b) | ||
| In vivo | – | – | Replication | PDCoV | (Duan et al. 2021c) | ||
| Figure 4 (124) | Monolaurin | In vivo | – | – | Replication | PEDV | (Zhang et al. 2021a) |
| Figure 4 (125) | Ellagic acid | In vitro | – | > 80 | Replication | PEDV | (Song et al. 2024) |
| Figure 4 (126) | Compound 126 | In vitro and In vivo | 1.89 ± 0.25 | > 100 | Replication | PEDV | (Chen et al. 2021a) |
| Figure 4 (127) | Compound 127 | In vitro | 3.26 ± 0.23 | > 100 | Replication | PEDV | (Chen et al. 2021a) |
| Figure 4 (128) | Compound 128 | In vitro and In vivo | 0.72 ± 0.11 | > 100 | Replication | PEDV | (Chen et al. 2021a) |
| Figure 4 (129) | Palmitic acid | In vitro | – | > 50 μmol/L | Replication | PEDV | (Mao et al. 2022) |
| In vitro | – | > 200 | Replication | PEDV | (Suo et al. 2023) | ||
| Figure 4 (130) | Lauric acid | In vitro | – | > 200 | Replication | PEDV | (Suo et al. 2023) |
| In vitro | – | > 200 | Replication | TGEV | (Suo et al. 2023) | ||
| Figure 4 (131) | Sodium butyrate | In vitro | – | > 200 | Replication | PDCoV | (Suo et al. 2023) |
| In vitro | – | > 1000 | Replication | PEDV | (He et al. 2023) | ||
| Figure 4 (132) | Docosahexaenoic acid | In vitro | – | > 200 | Replication | PEDV | (Suo et al. 2023) |
| In vitro | – | > 200 | Replication | TGEV | (Suo et al. 2023) | ||
| In vitro | – | > 200 | Replication | PDCoV | (Suo et al. 2023) | ||
| Figure 4 (133) | Eicosapentaenoic acid | In vitro | – | > 200 | Replication | PEDV | (Suo et al. 2023) |
| In vitro | – | > 200 | Replication | TGEV | (Suo et al. 2023) | ||
| In vitro | – | > 200 | Replication | PDCoV | (Suo et al. 2023) | ||
| Figure 4 (134) | Linoleic acid | In vitro | – | > 50 μg/mL | Replication | PEDV | (Yang et al. 2023b) |
| Figure 4 (135) | Ribavirin | In vitro | – | > 200 | Replication | PEDV | (Kim and Lee 2013) |
| Figure 4 (136) | 2-Deoxy-D-glucose | In vivo | – | > 1.0 × 104 | Replication | PEDV | (Wang et al. 2014) |
| Figure 4 (137) | A77 1726 | In vitro | – | > 200 | Replication | PEDV | (Li et al. 2020b) |
| Figure 4 (138) | Formic acid | In vitro | – | 1.5869 mg/mL | Replication | PEDV | (Gómez-García et al. 2021) |
| Figure 4 (139) | Ursonic acid | In vitro | – | > 32 | Replication | PEDV | (Yang et al. 2024) |
| Figure 4 (140) | Panax notoginseng saponins | In vitro | – | > 512 μg/mL | Replication | PEDV | (Hu et al. 2024) |
| Figure 4 (141) | Salinomycin | In vitro | – | < 20 | Entry, replication | PEDV | (Yuan et al. 2021) |
| Figure 4 (142) | Nicotinamide | In vitro | – | > 2.5 × 103 | Replication | PEDV | (Li et al. 2023a) |
| In vitro | – | > 500 | Replication | PDCoV | (Li et al. 2023a) | ||
| Figure 4 (143) | Epigallocatechin-3-gallate | In vitro | 14.95 | > 100 | Attachment, entry, replication, assembly | PEDV | (Huan et al. 2021) |
| Figure 4 (144) | Ivermectin | In vitro | 7.05 | 25.34 | Replication, release | PEDV | (Wang et al. 2023h) |
| In vitro | 4.63 | > 10 | Replication | PEDV | (Xu et al. 2024a) | ||
| Figure 2 (13) | Berbamine | In vitro and In vivo | 177.1 | 1.789 | Replication | PEDV | (Xiang et al. 2024) |
| Figure 4 (145) | Avermectin B1 | In vitro | 8.98 | 219.3 | Replication, release | PEDV | (Wang et al. 2023h) |
| Figure 4 (146) | Doramectin | In vitro | 6.26 | 130.4 | Replication, release | PEDV | (Wang et al. 2023h) |
| Figure 4 (147) | Chenodeoxycholic acid | In vitro | – | > 200 | Replication | PDCoV | (Kong et al. 2021) |
| Figure 4 (148) | Methyl-β-cyclodextrin | In vitro | – | > 3000 | Attachment, entry | PDCoV | (Jeon and Lee 2018) |
| Figure 4 (149) | 25-hydroxycholesterol | In vitro | – | > 25 | Entry | PDCoV | (Ke et al. 2021) |
| In vitro | – | > 100 | Attachment, entry | PDCoV | (Zhang et al. 2022a) | ||
| Figure 4 (150) | 17-AAG | In vitro | 0.1426 | 36.34 | Replication | PDCoV | (Zhao et al. 2022) |
| Figure 4 (151) | VER-82576 | In vitro | 1.106 | 41.58 | Replication | PDCoV | (Zhao et al. 2022) |
| Figure 4 (152) | Diammonium glycyrrhizinate | In vitro | – | > 1250 μg/mL | Entry, replication | PDCoV | (Zhai et al. 2019) |
| Figure 4 (153) | Chlorogenic acid | In vitro | 69 ± 11 | 5598 ± 229 | Replication, release | PDCoV | (Shi et al. 2024) |
| Figure 4 (154) | Compound 154 | In vitro | 17.34 ± 7.20 | > 800 | Replication | PDCoV | (Sun et al. 2024a) |
| Figure 4 (155) | Selenomethionine | In vitro | – | > 16 | Replication | PDCoV | (Ren et al. 2022c) |
| Figure 4 (156) | Curcumin | In vitro | 5.979 | 408 | Replication | PDCoV | (Wang et al. 2023f) |
| In vitro | – | 77.96 ± 1.005 | Attachment, entry | TGEV | (Li et al. 2020d) | ||
| Figure 4 (157) | Niacin | In vitro | – | > 1 mg/mL | Replication | PDCoV | (Chen et al. 2022a) |
| Figure 4 (158) | Lycorine | In vitro | – | > 10 | Replication | PDCoV | (Fang et al. 2021) |
| Figure 4 (159) | Resveratrol | In vitro | – | > 10 | Replication | PDCoV | (Fang et al. 2021) |
| Figure 4 (160) | Digitoxin | In vitro | 0.373 ± 0.032 | > 10 | Replication | TGEV | (Yang et al. 2017) |
| Figure 4 (161) | Oleandrin | In vitro | 0.166 ± 0.008 | > 10 | Replication | TGEV | (Yang et al. 2017) |
| Figure 4 (162) | AG1024 | In vitro | – | > 50 | Replication | TGEV | (Yang et al. 2022a). |
| Figure 4 (163) | All-trans retinoic acid | In vivo | – | > 100 | Replication | TGEV | (Pu et al. 2022a) |
| In vivo | – | > 100 | Replication | TGEV | (Pu et al. 2022a) | ||
| Figure 4 (164) | Polygonum Cillinerve polysaccharide | In vitro | – | > 500 | Replication | TGEV | (Pan et al. 2021) |
| In vivo | – | > 62.5 μg/mL | Replication | TGEV | (Duan et al. 2024a) | ||
| Figure 4 (165) | (+)-Catechin | In vitro | – | > 640 | Replication | TGEV | (Liang et al. 2015) |
| Figure 4 (166) | Eugenol | In vitro | – | > 800 | Replication | TGEV | (Wang et al. 2022b) |
| Figure 4 (167) | L-leucine | In vitro | – | > 1.0 × 104 | Replication | TGEV | (Du et al. 2021) |
| Figure 4 (168) | Tyrphostin A9 | In vitro | – | > 6.0 | Replication | TGEV | (Dong et al. 2020). |
| Figure 4 (169) | Phlorizin | In vitro | – | > 200 | Replication | TGEV | (Yang et al. 2020c) |
| Figure 4 (170) | 2-bromopalmitate | In vitro | – | > 10 | Replication | SADS-CoV | (Luo et al. 2021) |
| Figure 4 (171) | CP-724714 | In vitro | 0.91 ± 0.18 | > 20 | Replication | SADS-CoV | (Zhou et al. 2023) |
| In vitro | 2.13 ± 0.6 | > 20 | Replication | PEDV | (Zhou et al. 2023) | ||
| In vitro | 0.84 ± 0.22 | > 80 | Replication | PDCoV | (Zhou et al. 2023) | ||
| In vitro | 2.53 ± 0.24 | > 80 | Replication | TGEV | (Zhou et al. 2023) | ||
| Figure 4 (172) | Lonafarnib | In vitro | 2.378 | > 64 | Replication | SADS-CoV | (Zhou et al. 2023) |
| Figure 4 (173) | Sorafenib | In vitro | 0.4628 | > 64 | Replication | SADS-CoV | (Zhou et al. 2023) |
| Figure 4 (174) | Gemcitabine | In vitro | – | 88.48 | Replication | SADS-CoV | (Chen et al. 2022c) |
| Figure 4 (175) | Mycophenolate mofetil | In vitro | – | > 100 | Replication | SADS-CoV | (Chen et al. 2022c) |
| Figure 4 (176) | Mycophenolic acid | In vitro | – | > 100 | Replication | SADS-CoV | (Chen et al. 2022c) |
| Figure 4 (177) | Methylene blue | In vitro | – | > 25 | Entry, replication | SADS-CoV | (Chen et al. 2022c) |
| Figure 4 (178) | LJ001 | In vitro | – | 146.4 | Replication | PDCoV | (Zhang et al. 2020) |
| In vitro | – | 146.4 | Replication | TGEV | (Zhang et al. 2020) | ||
| Figure 4 (179) | Melatonin | In vitro | – | > 3 × 103 | Replication | PEDV, PDCoV, TGEV | (Zhai et al. 2021) |
| Figure 4 (180) | Indole | In vitro | – | > 1 × 103 | Replication | (Zhai et al. 2021) | |
| Figure 4 (181) | Tryptamine | In vitro | – | > 5 × 102 | Replication | (Zhai et al. 2021) | |
| Figure 4 (182) | L- tryptophan | In vitro | – | > 3 × 103 | Replication | (Zhai et al. 2021) | |
| Figure 4 (183) | Rifampicin | In vitro | – | > 200 | Replication | PEDV | (Wei et al. 2024) |
| In vitro | – | > 200 | Replication | SADS-CoV | (Wei et al. 2024) | ||
Note: “–”, no data provided.
Graphene oxide (GO, Table 3) and its derivatives have been widely explored for their antimicrobial properties due to their high surface-to-volume ratios and unique chemical and physical properties (Mao et al. 2021). Ye et al. found that GO significantly suppressed the infection of PEDV for a 2log reduction in virus titers at noncytotoxic concentrations (CC50 > 50 μg/mL). Mechanically, the potent antiviral activity of both GO and reduced GO (rGO) can be attributed to the unique single-layer structure and negative charge. First, GO exhibited potent antiviral activity when conjugated with PVP, a nonionic polymer, but not when conjugated with PDDA, a cationic polymer. Additionally, the precursors graphite and graphite oxide showed much weaker antiviral activity than monolayer GO and rGO, suggesting that the nanosheet structure is important for antiviral properties. Furthermore, GO inactivated virus by structural destruction prior to viral entry (Ye et al. 2015).
Some carbazole derivatives synthesized by Chen et al. show activity against PEDV in cell assays. Among compounds 6 to 8 [No.5, No.7 and No.18 in Ref. Chen et al. 2021c, Figure 2 (6 to 8)], compound 6 is highly cytotoxic, no follow-up tests were performed. Compound 7 (CC50 > 40 μmol/L, Table 2) and compound 8 (CC50 > 60 μmol/L, Table 2) were less cytotoxic and both dose-dependently resisted PEDV attachment, but did not affect the entry phase (Chen et al. 2021c). Levistolide A [LA, Figure 2 (9)], a natural product polymerized by two molecular (Z)-ligustilide. Zeng et al. found that LA (CC50 > 100 μmol/L, Table 2) could inhibit PEDV from attaching to the cellular membrane or penetrating the Vero cells, and can suppress PEDV replication via inducing ROS generation (Zeng et al. 2022b).
Bis-benzylisoquinoline alkaloids belong to the large family of isoquinoline alkaloids and have antiviral, antibacterial, antifungal, antiparasitic, anti-inflammatory and anti-allergic effects (Costa et al. 2008; Sakurai et al. 2015; Paudel et al. 2016). Dong’s group found that cepharanthine (CEP, Figure 2 (10)), tetrandrine (TET, Figure 2 (11)) and fangchinoline (FAN, Figure 2 (12)) had anti-PEDV activities with IC50 values of 2.53, 3.50 and 6.69 µmol/L, respectively (Table 2). These three compounds block all processes of the viral cycles, but early application of the compounds before or during virus infection was advantageous over application at a late stage of virus replication. FAN performs its inhibitory function more efficiently by interfering with the process of virus entry and attachment or by directly attenuating the virus. CEP had a more notable effect on virus entry, and its SI index was the highest among the three compounds (Dong et al. 2022). Thus, Dong et al. first evaluated the toxicity and dose of CEP in BALB/c mice and found that 100 mg/kg CEP orally was safe for mice. The equivalent dose conversion table was used to convert the safe dose for mice to data for piglets, yielding a safe dose of 11.1 mg/kg for newborn piglets. Subsequently, after oral PEDV inoculation, piglets were given a safe dose of 11.1 mg/kg CEP twice a day, 2 mL each time, at the onset of clinical symptoms. After 3 consecutive days of oral administration, piglets were euthanized. Viral load in the treated group was significantly lower than in the untreated group. No intestinal damage was observed on autopsy in the piglets of each group. However, microscopic pathological sections showed mild to moderate shortening, blunting and premature stripping of the jejunum and ileum tissues in the piglets challenged but receiving no CEP treatment, while the jejunum remained normal in the CEP-treated group. These results confirmed that CEP could protect piglets against PEDV infection in vivo (Dong et al. 2022). Similarly, another study reported the inhibitory effect of berbamine [BBM, Figure 2 (13)], FAN, and (+)-fangchinoline [+FAN, Figure 2 (14)] on PEDV activity with 50% inhibitory concentration of 9.00 µmol/L, 3.54 µmol/L, and 4.68 µmol/L (Table 2), respectively, and these compounds also mainly inhibited the invasive phase of PEDV. Mechanistically, BBM, FAN and + FAN inhibited PEDV entry by increasing lysosomal pH to inhibit endonuclease body flux as well as cathepsin L (CTSL) and CTSB activities. In addition, the low activity of CTSL and CTSB fails to cleave the PEDV-S protein exposing the fusion structural domain, thereby inhibiting membrane fusion. Ultimately, the viral genome cannot be transferred into the cytoplasm (Zhang et al. 2023a). Notably, a study also found that FAN plays a role in the attachment and internalization phases of the virus life cycle, but FAN mainly affects the replication phase of PEDV. Further experimental results showed that FAN blocks PEDV infection by blocking the expression of autophagy associated proteins in cells (Zhang et al. 2023c). Recently, a study to develop anti-SARS-CoV-2 drugs also found that CEP, TET, BBM, FAN, isotetrandrine (Figure 2 (15)), cycleanine (Figure 2 (16)), dauricine (Figure 2 (17)) and daurisoline (Figure 2 (18)) have anti-PEDV and anti-SADS-CoV effects in Vero cells (Table 2) (Leng et al. 2024). Additionally, another study showed that TET can effectively inhibit the proliferation of PEDV in both Vero cells and IPEC-J2 cells (Qian et al. 2024).
Niclosamide (NIC, Figure 2 (19)) is a deworming drug widely used in animals and humans. Wang and his colleagues used PEDV-expressing renilla luciferase (PEDV-Rluc) to screen NIC from an FDA-approved drug library in Vero cells, which significantly reduced the luciferase activity of PEDV-Rluc. It can effectively inhibit viral RNA synthesis, protein expression and viral progeny production of classical and mutated PEDV strains in a dose-dependent manner. In addition, NIC significantly inhibited the entry stage of PEDV infection by influencing virus internalization rather than virus attachment to cells (Table 2). The combination of NIC with other endosomal acidification inhibitors, such as V-ATPase inhibitor bafilomycin A1 (Figure 2 (20)) and lysosomotropic compound chloroquine (Figure 2 (21)), would lead to enhanced inhibition of PEDV entry (Wang et al. 2023g).
Veratramine [VAM, Figure 2 (22)] is a drug with multiple biological activities such as antihypertensive, antitumor, and antiarrhythmic properties. One study found that VAM significantly inhibited PEDV infection in a dose-dependent manner. It mainly affects the entry stage of PEDV virus, and the IC50 for the replication of PEDV HLJBY strain and PEDV CV777 strain is both less than or equal to 5 µmol/L (Table 2). Further research showed that VAM inhibits the macrophage proliferation pathway by inhibiting the PI3K/Akt pathway, thus having anti-PEDV properties against the entry stage of PEDV (Chen et al. 2024a).
A recent study discovered that matrine [MT, Figure 2 (23)], a naturally occurring alkaloid, can effectively target the S protein of PEDV to hinder viral attachment and entry. Interestingly, when MT was administered to cells before, during, and after infection, it exhibited anti-PEDV activity and a virucidal effect. Moreover, MT notably enhanced the apoptosis of PEDV-infected cells and suppressed PEDV infection by activating the MAPK signaling pathway (Qiao et al. 2024). Similarly, another study employed in vivo piglet and in vitro intestinal organoid models to investigate the effects of benzoic acid [BA, Figure 2 (24)] on the intestinal barrier and the differentiation of intestinal stem cells. The in vivo results demonstrated that oral administration of BA significantly increased the number of goblet cells (GC) in the small intestine and enhanced the mucus barrier, thereby providing partial protection against PEDV infection in piglets. Furthermore, in vitro experiments demonstrated that BA facilitates GC differentiation by modulating the Wnt/Notch/MAPK pathway, leading to a significant increase in the number of MUC2+ GCs (Liu et al. 2024b).
2.4. Small molecule inhibitors that regulate viral proteins or proteases to inhibit PEDV replication
2.4.1. Main protease inhibitors
N3 (Figure 3 (26)) is used as a potent inhibitor for coronavirus researches, especially its α, β-unsaturated ester group is commonly used to irreversibly inactivate cysteine proteases. Wang et al. constructed the eutectic structure of PEDV main protease (Mpro) in combination with N3 revealed in detail the irreversible inhibition mechanism of the active site of this enzyme. Based on lead N3 permitted further rational optimization of a Michael acceptorbased peptidomimetic inhibitor to target the substrate-binding pocket of PEDV Mpro. Wang and his colleagues designed a series of 17 new compounds (M1 to M17 in Ref. Wang et al. 2017a) derived from the backbone structure of compound 26 with altered side chains. Among compounds M1 to M17, compound 27 and compound 28 (M2 and M17 in Ref. Wang et al. 2017a) [Figure 3 (27 and 28)] showed the most potent antiviral activity against PEDV Mpro in enzymatic activity and inhibition assays (Wang et al. 2017a).
Figure 3.
Chemical structures of small molecule inhibitors that regulate viral proteins or proteases to inhibit PECs replication.
Shi et al. found that both (2,4-dihydroxyphenyl) (4-methylphenyl) methanone (Figure 3 (29)) and (2,4-dihydroxy phenyl) (2,6-dimethylphenyl) methanone (Figure 3 (30)) exhibited in a dose-dependent manner inhibition of PEDV. Next, they tested compounds 29 and 30 for inhibition of PEDV viral replication. They found that compounds 29 and 30 could target the PEDV Mpro and block PEDV replication with IC50 and CC50 values of 37.8 μmol/L (CC50 = 533.8 μmol/L, Table 2) and 23.4 μmol/L (CC50 = 522.3 μmol/L, Table 2), respectively. Virtual analysis demonstrates the ability of compounds 29 and 30 to block PEDV replication by binding to the conserved His41 in PEDV Mpro via hydrogen bonding and hydrophobic forces. Importantly, they also found that compounds 29 and 30 also targeted the porcine reproductive and respiratory syndrome virus (PRRSV) 3 C-like serine protease (3CLpro) and inhibited PRRSV replication in MARC-145 cells, showing IC50 and CC50 values of 144.3 μmol/L (CC50 = 479.9 μmol/L) and 115.9 μmol/L (CC50 = 482.8 μmol/L), respectively (Shi et al. 2018). Similarly, Zhou and his colleagues established a high-throughput antiviral inhibitor screening platform based on fluorescence resonance energy transfer (FRET) to identify drugs targeting the PEDV Mpro from compound libraries, a low-molecular-weight (low-MW) fragmentbased library containing 1,590 compounds (Specs, Delf, Netherlands), to be used to treat piglets with porcine epidemic diarrhoea. Their findings indicate that 3-(aminocarbonyl)-1-phenylpyridinium (Figure 3 (31)) and 2,3-dichloronaphthoquinone (Figure 3 (32)) are of low molecular weight and greatly inhibited the activity of PEDV Mpro enzyme (Table 2). The results of putative molecular docking models indicate that 31 and 32, contact the conserved active sites (Cys144, Glu165, Gln191) of Mpro via hydrogen bonds. Moreover, Zhou et al. found that 31 (CC50 = 60.48 μg/mL in Ref. Zhou et al. 2021) and 32 (CC50 = 36.7 μg/mL in Ref. Zhou et al. 2021) also exhibited antiviral capacity against feline infectious peritonitis virus (FIPV) (Zhou et al. 2021).
GC376 (Figure 3 (33)) is a dipeptidyl bisulfite adduct salt, which can treat highly pathogenic feline infectious peritonitis recovery caused by FIPV (Kim et al. 2016b). Further research by Ye et al. found that GC376 (IC50 = 1.11 ± 1.13 μmol/L, Table 2) could target FIPV Mpro to inhibit viral infection. Delightfully, PEDV Mpro shares 62% sequence homology with FIPV Mpro, and GC376 can also target the Mpro of PEDV to significantly inhibit PEDV infection. Furthermore, the structure of the PEDV Mpro in complex with GC376 was determined at 1.65 Å. Four residues of PEDV Mpro, Cys 144, His 162, Gln 163 and Glu 165, can interact with the hydrogen bonds of GC376 (Ye et al. 2020).
Quercetin (Figure 3 (34)), a flavonoid molecule, is commonly found in vegetables, fruits, and Chinese herbs. Which has been widely used to antioxidative, anticancer, antibacterial, and antiviral effects (Li et al. 2020d). Li et al. found that quercetin inhibited PEDV infection of Vero cells in a dose-dependent manner; which did not inhibit PEDV entry into cells but could interfere with the early stages of viral replication. A molecular docking study indicated that quercetin might bind the active site (Cys144, Asn141 and His162) and binding pocket of PEDV Mpro. Surface plasmon resonance (SPR) analysis revealed that quercetin exhibited a binding affinity to PEDV Mpro. Based on the results of the FRET assay, quercetin was proven to exert an inhibitory effect on PEDV Mpro. Thus, quercetin might block the recognition and binding of PEDV Mpro to its substrates, thereby inhibiting PEDV replication (Li et al. 2020d). A recent study also found that flavonol (Figure 3 (35)) can target Mpro to inhibit the proliferation of PEDV in IPEC-J2 cells (Liang et al. 2024). Furthermore, chrysin (Figure 3 (36)) and naringenin (Figure 3 (37)) are two natural flavonoid compounds. Chrysin is the main active ingredient extracted from wisteria and Chinese red pine, and naringenin is a secondary metabolite of citrus plants of Rutaceae. A study found that both chrysin and naringenin showed strong anti-PEDV activity and could inhibit viral titers, mRNA and protein levels in the prevention of PEDV infection and the post-virus entry stage. Molecular docking analysis showed that chrysin and naringenin mainly interact with viral replicase proteins Mpro and papain-like protease 2 (PLP-2) through hydrogen bonds and hydrophobic forces. In addition, molecular dynamics analysis showed that the complexes formed by chrysin or naringenin with Mpro and PLP-2 have high stability. These results suggest that chrysin and naringenin may exert antiviral effects by interacting with the viral Mpro protein or PLP-2 protein, thereby affecting their role in the formation of PEDV non-structural proteins or interfering with viral replication (Gong et al. 2023). Moreover, Wang et al. found that wogonin (Figure 3 (38)) showed antiviral activity against PEDV, interacting with PEDV particles and inhibiting the internalization, replication and release of PEDV (Table 2). Molecular docking model shows that wogonin can be combined with Mpro. Furthermore, the interaction between wogonin and Mpro was validated in silico via microscale thermophoresis and SPR analyses. In addition, the results of a FRET assay indicated that wogonin exerted an inhibitory effect on Mpro (Wang et al. 2023d). In addition, a recent study used molecular docking analysis to find that baicalein (Figure 3 (39)) and baicalin (Figure 3 (40)) have good binding activity to PEDV Mpro. Moreover, baicalein and baicalin have good in vitro inhibitory effects on PEDV Mpro, with IC50 values of 9.50 ± 1.02 μmol/L and 65.80 ± 6.57 μmol/L respectively. The research team demonstrated that baicalein and baicalin mainly inhibit the early replication stage after PEDV enters Vero cells or LLC-PK1 cells (Li et al. 2024c). Similarly, Wang and colleagues reported the antiviral effects of luteolin (Figure 3 (41)) in PEDV-infected Vero and IPEC-J2 cells, identifying IC50 values of 23.87 μmol/L and 68.5 μmol/L, respectively. Furthermore, the three-dimensional docking model indicated that luteolin was effectively positioned within the groove of the Mpro active pocket, and FRET assays corroborated that luteolin inhibits PEDV Mpro activity (Wang et al. 2024b).
Wang and his colleagues screened an FDA-approved library of 911 natural products and found that four of the compounds [tomatidine (Figure 3 (42)), pneumocandin B0 (Figure 3 (43)), (-)-epigallocatechin gallate (Figure 3 (44)), buddlejasaponin IVb (Figure 3 (45))] produced negligible cytotoxicity (Table 2) and inhibited PEDV in a dose-dependent manner. Next, the authors chose tomatidine for further study as it had the highest selectivity index (SI = 13.25, in Ref. Wang et al. 2020) and the lowest price. Molecular docking and molecular dynamics analysis predicted interactions between tomatidine and the active pocket of PEDV Mpro, which were confirmed by fluorescence spectroscopy and isothermal titration calorimetry (ITC). The inhibiting effect of tomatidine on Mpro was determined using cleavage visualization and FRET assay. It indicates that tomatidine inhibits PEDV replication mainly by targeting Mpro. Meanwhile, the results show that tomatidine does not obstruct the attachment, invasion and release phases of PEDV in Vero cells, and also does not directly virucidal and kills PEDV virions. In addition, tomatidine also has antiviral activity against TGEV, PRRSV, encephalo myocarditis virus (EMCV) and seneca virus A (SVA) in vitro (Wang et al. 2020).
Xanthohumol (Figure 3 (46)) is an amylated chalcone from the hops plant (Humulus lupulus) that inhibits cervical cancer, colorectal cancer, and prostate cancer. Lin et al. found that xanthohumol was a potent pan-inhibitor for various coronaviruses by targeting Mpro, for example, betacoronavirus SARS-CoV-2 (IC50 value of 1.53 µmol/L, in Ref. Lin et al. 2021), and alphacoronavirus PEDV (IC50 value of 7.51 µmol/L, Table 2). In addition, xanthohumol pretreatment can limit the replication of PEDV in Vero-E6 cells (Lin et al. 2021). Similarly, Zhang et al. demonstrated that hypericin (Figure 3 (47)) was for the first time shown to have an inhibitory effect on PEDV or TGEV by targeting Mpros, and found that the viral replication and egression were significantly reduced with hypericin post-treatment in Vero cells or ST cells (Zhang et al. 2021b).
Based on molecular docking, Su et al. found that octyl gallate [OG, Figure 3 (48)] had a strong binding affinity with the Mpro active site of PEDV. Next, biolayer interference and FRET experiments further demonstrated that OG could directly interact with PEDV Mpro (KD = 549 nM) and inhibit the activity of Mpro (IC50 = 22.15 µmol/L). In the experiment of PEDV-infected Vero E6 cells, the addition of 40 µmol/L OG (Table 2) could significantly reduce PEDV-N protein expression and virus titer. Further studies found that OG had no effect on PEDV binding, internalization or budding in Vero E6 cells. Therefore, OG inhibits PEDV replication by interfering with the activity of Mpro, thereby inhibiting PEDV replication. Notably, OG also has strong resistance to TGEV, PDCoV and SADS-CoV in in vitro experiments. Due to the high conserved nature of Mpro, OG, as a potential broad-spectrum anti-porcine enteric coronavirus drug, is highly likely to act on Mpro. At the same time, these results suggest that OG has a potential ability to resist other coronaviruses, such as SARS-CoV-2 (Su et al. 2023). In addition, Su and his colleagues determined through the toxicity of OG in mouse feeding that piglets in the treatment group were given 250 mg/kg of OG orally every 6 h before feeding. One day after the treatment group took oral OG, all infected piglets were orally infected with PEDV. Then, all piglets were observed and recorded at 12 h intervals until euthanized 48 h after infection. Animal experiments showed that the severity of clinical symptoms and diarrhea of piglets in the treatment group was less. Virus shedding and tissue distribution of all experimental piglets were evaluated. Virus excretion in feces, and virus distribution in jejunum and ileum of OG-treated piglets were lower than those of the PEDV challenge group without OG treatment piglets. These results suggest that OG has a protective effect against PEDV infection in piglets (Su et al. 2023).
Cho’s group isolated 16 compounds from leaves of Sabia limoniacea. Among them, compounds 49 and 50 [compound 15 and compound 16 in Ref. Cho et al. 2019, Figure 3 (49 and 50)] showed antiviral activity, and their IC50 values for PEDV replication were 7.5 ± 0.7 μmol/L and 8.0 ± 2.5 μmol/L (Table 2), respectively. Molecular docking results indicate that compounds 47 and 48 may exert anti-PEDV activity by targeting Mpro (Cho et al. 2019). In addition, a study found that the IC50 value of polycarpine (Figure 3 (51)) derived from marine natural substances against PEDV Mpro was 0.12 ± 0.05 μmol/L. Further studies found that polycarpine pretreatment could inhibit the proliferation of PEDV in Vero cells (Zhang et al. 2024a).
G-quadruplexes are non-canonical nucleic acid structures formed in G-rich DNA and RNA (Varshney et al. 2020). Currently, extensive research has shown that G-quadruplexes can regulate many biological processes of the virus and have been identified as promising therapeutic approaches (Zhao et al. 2021; Qin et al. 2022). Li et ai. used multiple biophysical techniques and molecular biology analyses to show that S-PQS and NSP5-PQS in the S and Nsp5 genes of the PEDV genome are capable of folding into G4 structures. Importantly, four G4 stabilizers [TMPyP4, PDS, Braco-19 and Phen-DC3, Figure 3 (52 to 55)] were found to exert antiviral activity during PEDV infection (Table 2). Among them, TMPyP4 (Figure 3 (52)) and PDS (Figure 3 (53)), significantly inhibited the transcription, translation and proliferation of PEDV in vitro. In particular, TMPyP4 was shown to inhibit gene expression by targeting the G4 structure of the Nsp5 gene (Li et al. 2023c).
2.4.2. Papain-like protease inhibitors
Alpha coronaviruses have both papain like protease 1 (PL1Pro) and papain like protease 2 (PL2Pro). Like other coronaviral PLpros, PEDV PL2Pro is not only a deubiquitination (DUB) protein, but also a multifunctional protein that plays a role in regulating the host’s antiviral immune response. The DUB activity of PL2Pro is required to suppress host immunity by blocking type 1 interferon signaling. One study found that the chemotherapeutic agent 6-thioguanine [6TG, Figure 3 (56)] had competitive inhibition of papayin-like enzymes in SARS and MERS coronaviruses, but had non-competitive inhibition with PEDV PL2Pro. Therefore, the results suggest that 6TG can exert broad-spectrum inhibition on coronavirus by acting on PL2Pro through different mechanisms (Chu et al. 2018).
2.4.3. NTPase inhibits
Wang and colleagues identified potential PEDV replicase enzymes (PLP2、Mpro、RdRp、NTPase and NendoU) inhibitors by virtually screening of 187,119 compounds contained in the ZINC natural products database in order to discover antiviral compounds against PEDV, and seven compounds (M2 to M8 in Ref. Wang et al. 2022c) had high binding potential to NTPase and showed drug-like property. Among them, ZINC12899676 [M4 in Ref. Wang et al. 2022c, Figure 3 (57)] was inhibited the NTPase activity of PEDV by targeting its active pocket in a dose-dependent manner and reduced PEDV replication in Vero cells. The IUPAC for ZINC12899676 is N-[2-(1H-indol-3-yl) ethyl]-N’-[2-[2-(1H-indol-3-yl) ethylcarb amoyl] phenyl] oxamide. Furthermore, ZINC12899676 also significantly inhibited PEDV replication in IPEC-J2 cells (Wang et al. 2022c).
2.4.4. RdRp inhibitors
More and more studies have shown that RNA-dependent RNA polymerase (RdRp) plays a crucial role in viral RNA synthesis and is an ideal therapeutic target for pan-coronavirus. Remdesivir [RDV, Figure 3 (58)] is a 1′-cyano-substituted adenosine nucleotide analogue prodrug which acts as a non-obligated chain terminator by targeting the highly conserved active site of viral polymerase and shows broad-spectrum antiviral activity against an array of RNA viruses, such as HCV, MERS virus, SARS-CoV virus, Ebola virus and SARS-CoV-2 virus. β-D-N4-Hydroxycytidine [NHC, Figure 3 (59)] is also an orally available ribonucleoside analogue with broad-spectrum antiviral activity against RNA viruses, including influenza, Ebola, SARS-CoV-2 and Venezuelan equine encephalitis (VEE) virus. Xie et al. reported that RDV, NHC and RDV nucleoside [RDV-N, Figure 3 (60)] showed antiviral activity against PEDV in Vero cells. Among them, RDV-N was the most active agent with EC50 of 0.31 μmol/L (in Ref. Xie et al. 2021), and more potent than RDV (EC50 = 0.74 μmol/L, in Ref. Xie et al. 2021) and NHC (EC50 = 1.17 μmol/L, in Ref. Xie et al. 2021). Moreover, RDV-N exhibited a good safety profile in cells and in mice (Xie et al. 2021). Notably, a study demonstrated that gossypol (Figure 3 (61)) directly blocks SARS-CoV-2 RdRp, thereby inhibiting the replication of SARS-CoV-2 in both cellular and mouse models of infection. Moreover, that the RdRp inhibitor gossypol has broad-spectrum anti-coronavirus activity against alphacoronaviruses (PEDV and SADS-CoV, Table 2), betacoronaviruses (SARS-CoV-2), gammacoronaviruses (avian infectious bronchitis virus), and deltacoronaviruses (PDCoV, Table 2) is showed (Wang et al. 2022d). In addition, another study found that a nucleotide analogue drug, molnupiravir (Figure 3 (62)), inhibited PEDV replication in a dose-dependent way in Vero cells (Table 2). Further studies showed that molnupiravir inhibits PEDV RdRp activity and can reverse changes in the intracellular transcriptional environment caused by PEDV infection (Huang et al. 2013). A recent study discovered that the nucleoside analog tubercidin (Figure 3 (63)) exhibited effective antiviral properties against PEDV infection. Molecular docking analysis indicated that tubercidin efficiently binds to the RdRp of different viruses including PEDV and SADS-CoV. Time of addition assay revealed that tubercidin notably inhibited viral post-entry events without affecting other stages (Wang et al. 2024d).
2.4.5. Nucleocapsid protein inhibitors
Yang et al. extracted thirteen coumarin compounds (compound 1–13 in Ref. Yang et al. 2015a) from parsnip root with methanol. Among them, the compound 64 [compound 5 in Ref. Yang et al. 2015a, Figure 3 (64)] with the carranopyran coumarin skeleton showed the strongest inhibitory effect on PEDV replication (Yang et al. 2015a). Next, Yang et al. evaluated the antiviral activity of fifteen oleanane triterpenes against PEDV replication. Among them, compounds 65 to 68 (6, 9, 11, and 13 in Ref. Yang et al. 2015b) [Figure 3] showed most potent inhibitory effects on PEDV replication. A CPE assay, RT-PCR analysis, Sabia limoniacea and their antiviral activities western blot analysis, and immunofluorescence assay indicated that four representative triterpenes [Table 2 (65 to 68)] showed potent and promising inhibitory effects on PEDV replication by targeting key structural protein (GP6 nucleocapsid and GP2 spike protein) synthesis and relevant gene (GP6 nucleocapsid, GP2 spike, and GP5 membrane protein) expression (Yang et al. 2015b).
Deejai et al. identified 1286 compounds of FDA-approved drug database that could virtually bind to the RNA-binding region of the PEDV-N protein. Among them, three compounds, trichlormethiazide (Figure 3 (69)), D-(+) biotin (Figure 3 (70)), and glutathione reduced free acid (Figure 3 (71)) successfully bound to the N protein, in vitro, with the IC50 at 8.754 mg/mL, 0.925 mg/mL, and 2.722 mg/mL. Antiviral activity in PEDV-infected Vero cells demonstrated that the effective concentration of trichlormethiazide, D-(+) biotin, and glutathione in inhibiting PEDV replication were 0.094, 0.094 and 1.5 mg/mL (Deejai et al. 2017).
Research has found that the binding of PEDV N protein and p53 in the cell nucleus maintains a sustained high levels expression of p53, thereby activating the p53 DREAM pathway. This leads to S-phase arrest in the cell cycle of Vero E6 cells and promotes viral replication. The key structural domains NS171-N194 and G183RG185 of the N protein that interact with p53 are critical for inducing S-phase arrest. Su and his colleagues screened small molecule drugs targeting the PEDV N protein NS171-N194 domain using molecular docking. They found that hyperoside (Figure 3 (72)) can antagonize N protein-induced S-phase arrest and inhibit viral replication by interfering with the interaction between N protein and p53. Consistent with the results in Vero E6 cells, Su and his colleagues reached the same conclusion in pig intestinal cells. Therefore, inhibiting the interaction between PEDV N protein and p53 can effectively inhibit PEDV replication in cells (Su et al. 2021). Next, the natural hyperoside extracted from hawthorn by other members of the laboratory team also had a strong inhibitory effect on the replication of PEDV in cells (Wang et al. 2024a). Subsequently, the research team used 3-day-old piglets as an experimental model and fed hyperoside at a dose of 500 mg/kg bw every 6 h one day before inoculation with the PEDV strain. The results showed that hyperoside significantly reduced the viral load in the intestines of piglets and protected 3 out of 4 piglets from death caused by PEDV infection. Mechanistically, hyperoside can interfere with the interaction between PEDV N protein and p53 (Wang et al. 2024a).
2.5. Small molecule inhibitors that regulate host factors to inhibit PEDV replication
Coronaviruses require the hijacking of host factors to complete their life cycle within the host cell. However, studies on the host factors involved in porcine enteric coronavirus replication remain unclear. In general, viral entry into cells requires binding to host receptors that activate signaling pathways to regulate an intracellular environment conducive to viral proliferation. Internal and external ligands or receptors for viral entry into the cell will be responsible for activating intracellular kinases in downstream signaling pathways. Therefore, antiviral drugs often target host signaling pathways that act to hijack or inactivate/activate host factors.
2.5.1. Drugs competitively affect the binding of PEDV to host factors
Glycyrrhizin [GLY, Figure 4 (77)] is the major component of licorice root extracts, which is a glycosylated saponin, containing one molecule of glycyrretinic acid and two molecules of glucuronic acid. GLY is a competitive inhibitor of high mobility group box-1 (HMGB1) and inhibits the cytokine activity of HMGB1, which functions as a damage associated molecular pattern (DAMP) molecule and activates pro-inflammatory signalling pathways by activating pattern recognition receptors. GLY inhibits the entry and replication of PEDV in Vero cells. GLY attenuates the pro-inflammatory response by inhibiting HMGB1, confirmed to be associated with TLR4 and RAGE (Huan et al. 2017). Another study found that GLY inhibited PEDV infection and reduced the secretion of pro-inflammatory cytokines via the HMGB1/TLR4 mitogen-activated protein kinase (MAPK) p38 pathway.PEDV infection of Vero cells had no effect on TLR4 transcription and translation levels, but TLR4 could regulate the expression of pro-inflammatory cytokines to regulate PEDV infection.HMGB1 could regulate p38 phosphorylation via GLY competitively inhibits HMGB1, which in turn reduces p38 phosphorylation (Gao et al. 2020).
Figure 4.
Chemical structures of small molecule inhibitors that regulate host factors to inhibit PECs replication.
Over the past years, researchers engineered an african green monkey genome-scale CRISPR/Cas9 knockout (VeroCKO) library containing 75,608 single guide RNAs targeting 18,993 protein-coding genes. Subsequently, the VeroCKO library was used to identify the key host factors that promote PEDV infection in Vero cells. Results revealed that knockdown of the trimeric motif 2 (TRIM2) and the solute carrier family 35 member A1 (SLC35A1) inhibited PEDV replication. Through virtual screening and molecular docking methods, the researchers found that chem-80,048,685 (Figure 4 (78)), which could target SLC35A1 significantly inhibits PEDV attachment and late replication (Table 2) (Wang et al. 2023b).
Na+/K+-ATPase (NKA) is a channel protein embedded in the phospholipid bilayer of the cell membrane. It has physiological functions such as ATPase activity and maintaining osmotic pressure inside and outside the cell. NKA consists of 4 α subunits, 3 β subunits and γ subtype. The α1 isoform (ATP1A1) is widely expressed in eukaryotic cells. One study found that the host protein ATP1A1 is involved in PEDV attachment and co-localizes with the PEDV S1 protein early in infection. Inhibiting the expression of host ATP1A1 protein using siRNA notably decreases cell susceptibility to PEDV, whereas overexpression of ATP1A1 significantly boosts PEDV infection. Subsequent experiments revealed that ATP1A1 inhibitors like ouabain (Figure 4 (79)) and PST2238 (Figure 4 (80)) bind specifically to ATP1A1, impeding the internalization and degradation of the ATP1A1 protein, consequently leading to a considerable reduction in PEDV infection rates in host cells (Xiong et al. 2023). In another recent study, researchers discovered that PEDV N protein participates in the regulation of Na+/H+ exchanger 3 (NHE3) activity by interacting with Ezrin, and then the expression of NHE3 on the cell membrane is significantly reduced during viral infection. The research team used molecular docking technology to find that pemetrexed acts (Figure 4 (81)) can target the PEDV N-Ezrin interaction region, thereby interfering with the inhibitory effect of PEDV infection on NHE3 expression. Subsequently, the research team showed that pemetrexed acts could restore Na+ concentration imbalance in PEDV-infected IPEC-J2 cells and that pemetrexed acts could alleviate diarrhea symptoms in PEDV-infected piglets (Zhang et al. 2024b).
A study found that SP2509 (Figure 4 (82)), a specific antagonist of lysine-specific demethylase 1 (LSD1), effectively inhibits PEDV infection at a concentration of 1 μmol/L (Table 2). The inhibitory action of SP2509 is focused on hindering PEDV internalization and replication, rather than attachment and release. The authors also observed that knockdown of LSD1 can impede PEDV replication in Huh-7 cells, suggesting that the inhibitory mechanism of SP2509 on PEDV is closely linked to the activity of LSD1 (Zhao et al. 2024).
2.5.2. Medicines inhibit PEDV by inducing cell apoptosis
Triacetyl resveratrol [TCRV, Figure 3 (83)] is a novel natural derivative of resveratrol. It has been found that TCRV has a strong inhibitory effect on PEDV in Vero cells and IPEC-J2 cells. Mechanistically, TCRV could promote the expression and activation of apoptosis-related proteins and release mitochondrial cytochrome C into cytoplasm. This is in contrast to PEDV-induced apoptosis, where the TCRV-activated cystatin protease pathway triggers early apoptosis in PEDV-infected cells, thereby inhibiting PEDV infection (Wang et al. 2022e). A recent study investigated the activity and mechanism of action of andrographolide [AND, Figure 4 (84)], an extract from the herb Andrographis paniculata, against PEDV both in vivo and in vitro. The in vitro experiments demonstrated that AND treatment significantly inhibited the activation of the JAK2-STAT3 pathway induced by PEDV, which in turn promoted cell apoptosis and suppressed viral proliferation. Additionally, three-day-old piglets infected with PEDV were treated with AND, and the clinical symptoms, intestinal morphology, and viral load were assessed. The in vivo experiments indicated that AND treatment alleviated clinical symptoms, improved intestinal damage, and increased the survival rate of infected piglets by 16.7% (He et al. 2024).
Lithium chloride (LiCl, Table 3) has been used as a drug for treatment of bipolar disorder, depression, Alzheimer’s disease and diabetes, is reported to have potent antiviral activity against some DNA and RNA viruses. Li et al. found that LiCl effectively inhibited the entry and replication of PEDV in Vero cells, and the expression of viral RNA and protein of PEDV was suppressed in a dose-dependent manner. Moreover, LiCl at a concentration of 15 mM significantly inhibited early and late apoptosis of cells induced by PEDV (Li et al. 2018).
A recent study discovered that four drugs, namely PA-824 (Figure 4 (85)), NAD+ (Figure 4 (86)), lovastatin (Figure 4 (87)), and hyodeoxycholic acid (Figure 4 (88)), exhibit significant inhibitory effects on PEDV in Vero cells (Table 2). Among these, PA-824, identified with the highest SI index, was chosen for in vivo experiments. The findings indicated that PA-824 effectively mitigates clinical symptoms, intestinal pathological damage, and inflammatory responses in suckling piglets post PEDV infection (Table 4). In vitro antiviral assessments demonstrated that PA-824 can directly target virus particles and impede the adsorption, internalization, and replication phases of the virus. Mechanistic analysis revealed that PA-824 suppresses the apoptosis signaling pathway by inhibiting PEDV-induced p53 activation (Li et al. 2024b).
2.5.3. Medicines inhibit PEDV by inducing cell autophagy
Ko et al. found that rapamycin (Figure 4 (89)) activated autophagy at dose that does not affect IPEC-J2 cells viability and tight junction permeability. Pretreatment with rapamycin significantly restricted infection with PEDV, while this effect disappeared when autophagy was blocked. Furthermore, co-localization of PEDV and autophagosomes suggests that PEDV could be a target of autophagy. Therefore, preventing PEDV infection in IPEC-J2 cells at the early stage of autophagy, which might be an effective strategy for the restriction of PEDV (Ko et al. 2017).
High trichothecene [HHT, Figure 4 (90)] inhibited PEDV replication in Vero cell culture at an IC50 values of 0.112 µmol/L (Table 2). The addition of 400 nmol/L HHT within 6 h of PEDV infection of Vero cells resulted in less than 30% infection of virus-infected cells and a significant reduction in PEDV-N protein. 500 nmol/L concentration of HHT completely inhibited PEDV infection (Dong et al. 2018). Next, Dong et al. treated PEDV-infected piglets at a safe dose of 0.05 mg/kg of HHT at 3–5 days of age and significantly reduced the viral load in the intestine and blood of the piglets. Moreover, HHT-treated piglets showed no histopathological changes or diarrheal symptoms (Dong et al. 2018). Another study found that hydroxychloroquine [HCQ, Figure 4 (91)] and HHT could synergistically exert antiviral effects. HCQ is an autophagy inhibitor chloroquine hydroxy derivative that acts as an anti-inflammatory agent by downregulating inflammatory activation to reduce tissue damage. HCQ reduced PEDV-induced autophagy and inflammatory responses in a dose-dependent manner. Viral titers in Vero cells treated with 50 μmol/L HCQ and 150 nmol/L HHT were reduced 30-fold and 3.5-fold, respectively, and the bound viral titers of both were reduced by 200-fold. Therefore, the combination of HHT and HCQ had an additive effect in inhibiting PEDV infection in vitro and was significantly more effective than the two treatments alone (Li and Wang 2020).
Ren et al. found that cinchonine (Figure 4 (92)) inhibited PEDV replication in Vero CCL81 cells in a dose-dependent manner. Mechanically, cinchonine can significantly induce the transformation of LC3-I [Microtubule-associated protein 1 light chain 3 (LC3) is a marker of autophagosome] to LC3-II in Vero cells to activate autophagy, thereby inhibiting PEDV replication, which disappeared when autophagy inhibitor 3MA pretreatment attenuated autophagy (Ren et al. 2022a).
2.5.4. Medicines inhibits PEDV by regulating cellular reactive oxygen species
Ferroptosis is a non-apoptotic form of cell death that is dependent on intracellular iron accumulation and leads to elevated toxic lipid peroxide reactive oxygen species (ROS). Erastin (Figure 4 (93)) is a classical activator of ferroptosis, and treatment of Vero cells with erastin by zhang et al. had no significant effect on PEDV adhesion, invasion and release had no significant effect, but it mainly inhibited the replication phase of PEDV in Vero cells. Mechanistically, in Vero cells, the regulation of NRF2, ACSL4 and Gpx4 genes by erastin was shown to stimulate the onset of ferroptosis, while in the PEDV-infected group the regulation was opposite to the onset of ferroptosis. erastin treatment reversed the regulation of PEDV-infected gene expression, and in turn erastin then inhibited the replication of PEDV in Vero cells (Zhang et al. 2023b). Another study found that the compound (1S,3R)-RSL3 (Figure 4 (94)) induced the activation of ferroptosis by regulating glutathione peroxidase 4, and upregulated ROS levels, inhibiting PEDV replication in Vero cells, but had no significant effect on adhesion, invasion, and release stages (Li et al. 2023b).
Quercetin7-rhamnoside [Q7R, Figure 4 (95)] is a flavonoid that inhibits PEDV replication at a 50% inhibitory concentration of 0.014 μg/mL (Table 2). Q7R does not directly interact with or inactivate PEDV particles, altering the infectivity of the virus. Pre-addition of the drug Q7R to Vero cells also did not reduce PEDV infection. Q7R addition within 1-4 h after PEDV virus infection directly reduced the formation of visible CPE. In contrast, Q7R did not inhibit PEDV infection after 6 h of virus addition. This suggests that Q7R exerts its inhibitory effect in the early stages of viral replication after infection (Choi et al. 2009). Infection of Vero cells with PEDV resulted in increased ROS production, starting at 2 hpi on and reaching a peak at 6 h. Q7R did not reduce CPE formation. Q7R did not reduce the increased ROS and its antiviral activity was not related to its antioxidant capacity. Song et al. suggested that the inhibition of PEDV production by Q7R was not only due to its general role as an antioxidant but was also highly specific (Song et al. 2011). Li and his colleagues, using reverse genetics, constructed a full-length cDNA clone of the cell-adapted PEDV strain YN150, replacing the open reading frame 3 (ORF3) in the viral genome with the nanoluciferase (NLuc) gene to construct a recombinant PEDV expressing NLuc (rPEDV NLuc). Compared with wild-type PEDV, rPEDV-NLuc produced similar plaque morphology in vitro and showed similar growth kinetics. Levels of luciferase activity were stably detected in rPEDV-NLuc-infected cells and showed a strong positive correlation with viral titer. Based on NLuc expression representing a direct readout of PEDV replication, anti-PEDV compounds were screened from a library of 803 natural products identified by quantifying NLuc activity, and twenty-five compounds [Figure 4 (96 to 120)] were identified that significantly inhibited PEDV replication (Table 2). Meanwhile, Li et al. found that seven of the 25 identified compounds were natural antioxidants, including betulonic acid, ursonic acid, esculetin, lithocholic acid [LCA, Figure 4 (103)], nordihydroguaiaretic acid, caffeic acid phenethyl ester, and grape seed extract. All of the antioxidants could potently reduce PEDV-induced oxygen species production, which, in turn, inhibit PEDV replication in a dose-dependent manner (Li et al. 2021). Furthermore, Xing et al. found that LCA has a protective effect on PEDV-infected piglets (Table 4). Mechanistically, the addition of LCA improved the distribution of intestinal T cell populations. LCA increases the expression of SLA-I in porcine intestinal epithelial cells through the farnesol X receptor, thereby recruiting CD8+ CTLs to exert antiviral effects (Xing et al. 2024).
Puerarin [PR, Figure 4 (121)], an isoflavone extracted from Pueraria lobata, is an excellent antioxidant, anti-inflammatory and antibacterial agent. Researchers inoculated 7-day-old piglets with PEDV virus via oral inoculation after 4 d of prior oral administration of PR at a dose of 0.5 mg/kg. PR attenuated the reduction in growth performance of PEDV-infected piglets and inhibited PEDV replication and the expression of several cytokines. PR restored the abundance of 29 proteins in the ileum that were altered by PEDV infection. Moreover, PR restored the expression of several interferon-stimulated genes and selectively upregulated the expression of guanylate binding protein. In addition, PR inhibited PEDV-induced NF-κB activation (Wu et al. 2020). Wu’s group next found that puerarin decreased aspartate aminotransferase and alkaline phosphatase activities, the ratio of serum aspartate aminotransferase to serum alanine aminotransferase, the number of leukocytes and neutrophils, and the plasma concentrations of IL-6, IL-8, and TNF-α, as well as the protein abundance of heat shock protein-70 in PEDV-infected piglets. Puerarin increased plasma D-xylose concentrations but decreased intestinal fatty acid binding protein concentrations and diamine oxidase activity in PEDV-infected piglets. Puerarin increased the activities of total superoxide dismutase, glutathione peroxidase and catalase in the intestine and plasma of PEDV-infected piglets, while decreasing myeloperoxidase activity and hydrogen peroxide concentration. Puerarin decreased the mRNA level of glutathione S-transferase ω2 but increased the level of nuclear factor erythrogenic lineage 2-related factor 2. Puerarin increased the abundance of total fungal bacteria (16S rRNA), Enterococcus spp., Lactobacillus spp. and Enterobacteriaceae in the intestine, but decreased the abundance of Clostridium perfringens-like bacteria in the cecum. These data suggest that puerarin improved intestinal function and reduced the incidence of PEDV infection in PEDV-infected piglets (Wu et al. 2021).
Magnolol [MAG, Figure 4 (122)] is a hydroxylated biphenyl neolignan, which is the main component of magnolia officinalis bark in traditional Chinese medicine. The compound has been endowed with antioxidant, anti-inflammatory, anti-tumor, antibiotic, and antiviral properties. Wang and his colleagues tested MAG for PEDV inhibition in Vero cells. The results showed that MAG had anti-PEDV activity in vitro, with IC50 and CC50 values of 28.21 and 57.28 µmol/L, respectively (Table 2). In particular, PEDV proliferation was significantly inhibited during the replication phase of the virus life cycle (Wang et al. 2023e).
Ergosterol peroxide [EP, Figure 4 (123)], a sterol naturally present in medicinal mushrooms, lichens, sponges, etc., has been reported to exhibit bioactivities against neoplasms, inflammation, oxidation and antiviral activity. A study reported that EP (CC50 = 327.6 μmol/L, Table 2) could significantly inhibit multiple stages of the PEDV life cycle in Vero cells, including internalization, replication and release, possesses the ability to directly inactivate PEDV. However, it did not affect PEDV attachment. Furthermore, EP mitigated the decrease in mitochondrial membrane potential caused by PEDV infection, and can inhibit PEDV-induced apoptosis by interfering with PEDV-induced ROS production and p53 activation (Liu et al. 2022).
Nanoparticles show great antiviral potential due to their structural properties that make them well adapted to bind to host recognition sites and thus inhibit viral entry into cells (Du et al. 2018a). Ag2S nanocrystals (Ag2S NCs, Table 3), also called quantum dots, are an ideal class of narrowband-gap near-infrared fluorescent materials, which have been used in optical and electronic devices, biomarkers and biological imaging due to their low or no toxicity to biological tissues, good chemical stability and excellent optical amplitude limiting properties (Gui et al. 2014). Dun’s group has shown that Ag2S NCs significantly inhibit PEDV virion replication and viral protein expression at non-cytotoxic concentrations (Table 3). Mechanistically, Ag2S NCs treatment inhibited viral negative-strand RNA synthesis and viral budding. Ag2S NCs treatment also positively regulates IFN-stimulated gene (ISG) production and proinflammatory cytokine expression (Du et al. 2018a). Next, Du and his colleagues self-assembled silver nanoparticle-modified graphene oxide (GO-AgNPs) nanocomposites via interfacial electrostatic forces. It was found that GO-AgNPs nanocomposites exhibited better inhibitory effects than AgNPs and GO. Mechanistic studies showed that GO-AgNPs nanocomposites could prevent the virus from entering the host cells. Meanwhile, GO-AgNPs nanocomposite treatment enhanced the production of IFN-α and ISGs to directly inhibit the proliferation of viruses (Du et al. 2018b). Du’s group also has successfully synthesized Au@Ag nanorods (Au@AgNRs) via coating AuNRs with silver and demonstrated their anti-PEDV replication activity. Mechanistically, Au@AgNRs inhibit PEDV entry and reduce mitochondrial membrane potential and caspase-3 activity. In addition, massive viral proliferation can lead to the production of ROS in cells, and released Ag+ and exposed AuNR have excellent antiviral activity upon stimulation of ROS via Au@AgNRs to ensure long-term inhibition of PEDV replication cycle (Du et al. 2020). In order to mimic cell surface receptors, Zhou and his colleagues synthesized bovine serum albumin (BSA)- coated tellurium nanoparticles (Te BSA NPs, Table 3) with a unique triangular star shape (Te) using a heparan sulfate (HS) analogue, mercaptoethane sulfonate (MES), as a surface modifier, and demonstrated the antiviral effect of Te BSA nanostars on PEDV (Zhou et al. 2020a). Next, Zhou’s group has also successfully synthesized GSH-modified zinc sulfide nanoparticles (GSH-ZnS NPs, Table 3) and mainly explored their antiviral activity against porcine reproductive and respiratory syndrome virus (PRRSV). Mechanistically, GSH-ZnS NPs could reduce PRRSV-induced ROS production to prevent PRRSV proliferation. Furthermore, GSH-ZnS NPs was found to have similar antiviral effects against PEDV (Zhou et al. 2020b). Similar to the antiviral assay with GSH-ZnS NPs, Tong and his colleagues synthesized a kind of highly biocompatible carbon dots using glycyrrhetinic acid (Gly-CDs) as raw material by hydrothermal method. Using PRRSV as a model, Gly-CDs were found to significantly inhibit PRRSV proliferation. Gly-CDs inhibited PRRSV invasion and replication, stimulated antiviral natural immune responses, and suppressed the accumulation of intracellular ROS caused by PRRSV infection. Proteomic analysis showed that Gly-CDs can stimulate cellular regulation of expression of host-restricted factors such as DDX53 and NOS3, which are directly associated with PRRSV proliferation. Moreover, Gly-CDs was also significantly inhibited the proliferation of PEDV in vitro (Tong et al. 2020).
2.5.5. Medicines inhibit PEDV by enhance innate immunity
Buddlejasaponin IVb, a natural triterpene saponin isolated from the Pleurotus ostreatus, has attracted much attention due to its anti-inflammatory, analgesic, lipid-lowering and antitumor activities (Li et al. 2016). Sun et al. found that buddlejasaponin IVb could inhibit the NF-κB signaling pathway activated by PEDV by downregulating the levels of cytokines (IL-6, IL-8, IL-1β, TNF-α) induced by PEDV or LPS. Moreover, buddlejasaponin IVb mainly inhibits the replication and release stages of PEDV in a dose-dependent manner (Table 2) (Sun et al. 2022). Next, Sun et al. used oral challenge to infect 3-day-old piglets with PEDV. Among the piglets in the pharmacological intervention group, buddlejasaponin IVb was administered intramuscularly every 6 h after 12 h of PEDV infection at concentrations of 0.5 mg/kg and 1 mg/kg, respectively. At 48 -72 h after PEDV inoculation, piglets in the PEDV alone infected group showed obvious clinical signs and died successively. In contrast, piglets in the 0.5 mg/kg treatment group showed mild clinical signs, and piglets treated with 1 mg/kg showed no obvious clinical signs. Detection of intestinal virus content in piglets by RT-qPCR revealed that the levels of PEDV virus in the feces and intestine of piglets in the 0.5 mg/kg and 1 mg/kg treatment groups were significantly lower than those in the PEDV alone infected group, and decreased in a dose-dependent manner. This demonstrate that buddlejasaponin IVb can significantly reduce the replication of PEDV in piglets. In addition, in a dose-dependent manner, intestinal damage was significantly less in the buddlejasaponin IVb-treated group of piglets than in the PEDV alone infected group. The mRNA levels of inflammatory factors IL-1β, IL-6, IL-8 and TNF-α in intestinal tissues were measured by RT-qPCR; inflammatory factors in intestinal tissues of piglets treated with buddlejasaponin IVb were reduced in a dose-dependent manner, and piglets treated with 0.5 mg/kg and 1 mg/kg buddlejasaponin IVb the mRNA levels of inflammatory factors in intestinal tissues were significantly lower than in the PEDV alone infected group, which is consistent with the results found in the in vitro experiments above (Sun et al. 2022).
Monolaurin [ML, Figure 4 (124)], also known as glycerol monolaurate or lauroyl, is a natural compound mainly found in coconut oil and human breast milk (Barker et al. 2019). Zhang and colleagues orally administered ML at a dose of 100 mg/kg bw for 7 days before piglets were infected with PEDV (Table 4). The results showed that ML did not significantly affect the growth performance of piglets, but ML administration alleviated diarrhea caused by PEDV infection. ML administration promotes the recovery of intestinal villi, thereby improving intestinal function. Meanwhile, PEDV replication was significantly inhibited with ML administration, and PEDV-induced IL-6 and IL-8 expression was decreased. Proteomic analysis showed that 38 proteins were differentially expressed between PEDV and ML+PEDV groups, and were significantly enriched in interferon-related pathways. This suggests that ML could promote the restoration of homeostasis by regulating the interferon pathway (Zhang et al. 2021a). Next, the team further investigated the effects of ML on blood biochemistry, intestinal barrier function, antioxidant function and antiviral gene expression in PEDV-infected piglets. The results showed that ML was enough to significantly reduce plasma urea nitrogen and total protein content in PEDV-infected piglets, and these results suggested that ML might improve protein utilization efficiency. In addition, ML significantly reduced the number of uninfected macrophages and Hb in the blood of PEDV-infected piglets, and increased the number of leukocytes and eosinophils in the blood, suggesting that ML could improve the immune defense function of the organism. In addition, ML was able to regulate the elevated D-xylose content and reduced the content of intestinal fatty acid binding protein, which in turn repaired the intestinal barrier and absorption function damaged by PEDV infection. In the presence of PEDV infection, ML significantly increased the activities of superoxide dismutase and catalase in the blood and colon, suggesting that ML has the ability to enhance antioxidant capacity. In addition, ML reversed the expression levels of ISG15, Mx1, IFIT3, and IL-29 in the intestine that were upregulated by PEDV infection. These results suggest that ML administration may promote the restoration of homeostasis in vivo by modulating the interferon pathway (Wang et al. 2023a).
Ellagic acid [EA, Figure 4 (125)] is a polyphenol dilactone that is widely present in various fruits and nuts and has various biological functions such as antioxidant, anti-inflammatory, antiviral and antibacterial. Song and his research team administered 20 mg/kg·bw EA orally to 7-day-old piglets for 4 d in advance. Subsequently, PEDV strains were administered orally at a dose of 1 mL of 1 × 106 TCID50 per pig. The results showed that EA treatment significantly increased the proportion of leukocytes in the blood and the concentrations of IL-6, IL-1b and IL-10 in the serum, but decreased the contents of IL-6, IL-1b, TNF-a and CXCL2 in the jejunum and gene expression. In addition, EA intervention significantly increased the total superoxide dismutase (T-SOD) activity in the ileum of piglets. Importantly, EA can promote the restoration of intestinal homeostasis by regulating the interferon pathway associated with JAK2/STAT3 signaling activation. In addition, in vitro experiments show that EA can increase the vitality of PEDV-infected pig intestinal epithelial cells (IPEC-1) and reduce intestinal edema in PEDV-infected piglets (Song et al. 2024).
2.5.6. Mortalin inhibitors
Mortalin, also known as mtHsp70/PBP74/Grp75/HSPA9, is a member of the heat-shock protein (Hsp) 70 family. As a molecular chaperone, mortalin interacts with other proteins and functions in regulating the cellular stress response, cell proliferation, and apoptosis. Furthermore, mortalin also to play multiple roles in membrane-mediated macromolecular transport, endocytosis and exocytosis, and played a role in the viral infection. Fan et al. found that mortalin significantly inhibited the replication of PEDV through restricting virus entry. Mechanistically, a biochemical interaction between the carboxyl terminus of mortalin and clathrin heavy chain (CLTC) was been found, and mortalin could induce CLTC degradation via the proteasome pathway, thereby inhibiting clathrin-mediated endocytosis of PEDV into host cells. In addition, artificial alteration of mortalin expression affected the cell entry of transferrin. Notably, this similar antiviral mechanism of mortalin is widely applicable to other viruses, such as VSV, rotavirus (RV) and TGEV (Fan et al. 2019). Chen and his colleagues designed and synthesized 12 phenanthridine derivatives and evaluated the inhibitory effect of these compounds on PEDV in African green monkey embryonic kidney cell line (Marc145 cells). Among them, three compounds (Compound 1、Compound 2 and Compound 4 in Ref. Chen et al. 2021a) [Figure 4 (Compound 122、Compound 123 and Compound 124)] reduced the production of PEDV mRNA by up to 70%. Compound 126 and compound 128 exhibited good anti-PEDV activity with EC50 values of 1.89 and 0.72 μmol/L, respectively, and no cytotoxicity at high concentrations of 100 μmol/L (Table 2). Notably, these phencyclidine derivatives may down-regulate the expression of host cell heat shock cognate 70 (Hsc70) in PEDV-infected host cells, which in turn suppress the replication of PEDV mainly by destabilizing the mRNA of Hsc70 (Chen et al. 2021a). Therefore, they predicted that these compounds might also be effective in vivo. The dose of administration was converted from the 25% LD50 value of compounds 126 and 128 determined in mice. After 15 h of oral administration of the PEDV strain to all infected groups of piglets (n = 7), three piglets were treated with compound 126 at a dose of 50 mg/kg every 24 h for 72 h, and three additional piglets were treated with the same dose of compound 127, with two infected pigs serving as positive controls that were not treated. Ninety-six hours after inoculation with the PEDV strain, the negative control developed severe watery stools, lethargy and anorexia, and died within the next 12 h. However, the clinical signs were much milder in the two phenanthridine derivatives treatment groups, and none of the piglets died during the entire experimental period. Histopathological observations of small intestine samples from the positive control group showed significantly less intestinal damage in piglet samples treated with compounds 126 and 127 compared to those from the positive control group. Immunofluorescence analysis showed that PEDV replication was significantly inhibited in the intestine of the two phenanthridine derivatives treatment groups compared to the positive control group. These results tentatively suggest that compounds 126 and 127 can inhibit PEDV replication in vivo and reduce PEDV-related mortality (Chen et al. 2021a).
2.5.7. Fatty acids
Fatty acids (FAs) are widely involved in the viral life cycle. Palmitoylation is a common protein post-translational modification, in which fatty acids [primarily palmitic acid, Figure 4 (129)] form a thioester bond with cysteine residues. The palmitoylation of proteins greatly affects their transport, stability, and interactions with other proteins (Mcbride and Machamer 2010). Mao and his colleagues analyzed the differential gene expression profile of PEDV strains infecting porcine intestinal 2-D cells at 12, 24, and 36 h through RNA-Seq. They discovered that highly-passaged PEDV strains significantly suppressed the expression of genes related to lipid metabolism, such as fatty acid binding protein 1 (FABP1) and FABP2, which play important roles in fatty acid transport and metabolism in cells. Treatment with palmitic acid [PA, Figure 4 (129)] led to a significant decrease in PEDV replication in 2-D intestinal cells or IPEC-J2 cells. These results suggest that PEDV may manipulate the host’s lipid metabolism to benefit its replication (Mao et al. 2022). Moreover, another study compared five FAs [lauric acid (LA, Figure 4 (130)), sodium butyrate [NaB, Figure 4 (131)], PA, docosahexaenoic acid [DHA, Figure 4 (132)], and eicosapentaenoic acid [EPA, Figure 4 (133)] against PEDV, TGEV and PDCoV. The results showed that LA, PA, DHA and EPA could significantly inhibit the proliferation of PEDV in Vero cells (Table 2). LA, DHA and EPA can significantly inhibit the proliferation of TGEV in PK-15 cells (Table 2). NaB, DHA and EPA can significantly inhibit the proliferation of PDCoV in LLC-PK1 cells (Table 2). Further in-depth exploration of the in vitro antiviral experiments of DHA and EPA found that DHA and EPA reduced endoplasmic reticulum stress and inhibited PEDV, TGEV and PDCoV infection in vero cells, PK-15 cells and LLC-PK1 cells respectively. In addition, DHA and EPA increase host antioxidant levels and reduce inflammatory responses. These results indicate that DHA and EPA enhance host immunity and have the potential to serve as broad-spectrum anti-coronavirus drugs (Suo et al. 2023). Furthermore, He et al. in vitro experiments showed that when IPEC-J2 cells were pretreated with different concentrations of butyrate, PEDV replication was inhibited and PEDV N levels were reduced in a butyrate dose-dependent manner. Butyrate treatment activated the IFN response and interferon-stimulated gene (ISG) expression. Further experiments showed that butyrate exerts its antiviral effects by inducing GPR43-mediated IFN production in intestinal epithelial cells (He et al. 2023).
Linoleic acid (Figure 4 (134)) is an essential fatty acid. To improve pork quality and reduce lipid synthesis in growing pigs, farm workers often add linoleic acid or conjugated linoleic acid (CLA) to feed additives (Go et al. 2012). Moreover, PEDV infection causes a severe inflammatory response in host cells, and linoleic acid has the ability to inhibit inflammation (Chen et al. 2020a). A recent study showed that linoleic acid stimulates increased expression of transforming growth factor β1 (TGF-β1) in Peripheral blood mononuclear cells. In addition, linoleic acid increased the expression level of TGF-β1 in dendritic cells (DCs) of PEDV-infected pigs. Further studies showed that TGF-β1 overexpression in Vero-E6 cells or IPEC-J2 cells could inhibit PEDV infection. When TGF-β1 was knocked down in IPEC-J2 cells, it resulted in a significant increase in the viral copy number of PEDV infection. Thus, linoleic acid stimulation leads to TGF-β1 production in DCs, which inhibits PEDV infection (Yang et al. 2023b).
2.5.8. Others
Ribavirin (Figure 4 (135)), 1-β-d-ribofuranosyl-1, 2, 4-triazole-3-carboxamide, also known as Virazole, is a synthetic guanosine analog that exhibits antiviral drug against a broad range of both DNA and RNA viruses in vitro. Kim and his colleagues found that the antiviral activity of ribavirin on PEDV replication was primarily exerted at early times post-infection. Treatment with ribavirin resulted in marked reduction of viral genomic and subgenomic RNA synthesis, viral protein expression, and progeny virus production in a dose-dependent manner. Mechanically, ribavirin against PEDV revealed that the addition of guanosine to the ribavirin treatment significantly reversed the antiviral effects, suggesting that depletion of the intracellular GTP pool by inhibiting IMP dehydrogenase may be essential for ribavirin activity (Kim and Lee 2013).
The unfolded protein response (UPR) is cyto-protective machinery elicited towards an influx of large amount of protein synthesis in the endoplasmic reticulum (ER). Wang’s group found that PEDV infection induced UPR in Vero cells. 2-Deoxy-D-glucose [2-DG, Figure 4 (136)], an ER stress inducer, might inhibit viral infection by affecting viral protein translation in the early stages of PEDV infection. Moreover, 2-DG treatment may affect viral assembly. Thus, induction of UPR using 2-DG or glucose/mannose analogs has therapeutic potential to block viral replication (Wang et al. 2014).
Leflunomide is an anti-inflammatory drug primarily used for treating rheumatoid arthritis. After ingestion, leflunomide is quickly converted in the liver and gastrointestinal tract into its active metabolite, A77 1726 (Figure 4 (137)). It was found that A77 1726 effectively restricted PEDV replication by inhibiting Janus kinases (JAKs) and Src kinase activities but not by inhibiting dihydroorotate dehydrogenase (DHO-DHase) and p70 S6 kinase activities. Overexpression of Src, JAK2 or its substrate STAT3 enhanced PEDV replication and attenuated antiviral activity of A77 1726 (Li et al. 2020b).
Organic acids are one of the most effective substitutes for antibiotics and provide a solution to the problem of drug resistance. Using Vero cells, Gómez-García and colleagues found that 1.2 mg/mL (Table 2) formic acid (Figure 4 (138)) effectively reduced PEDV replication in Vero cells. However, the exact mechanism of action was not reported (Gómez-García et al. 2021). Similarly, another study found that ursonic acid (Figure 4 (139)) has antiviral activity against PEDV replication in Vero cells (Yang et al. 2024).
Panax notoginseng saponins [PNS, (Figure 4 (140))] are bioactive extracts from Panax notoginseng plant. A recent study found that PNS can inhibit PEDV replication in a dose-dependent manner in Vero cells. Further mRNA-seq analysis found that PNS has anti-PEDV activity, especially during the genome replication stage. PNS can upregulate the expression of genes such as IFIT1, IFIT3, CFH, IGSF10, SPP1, PLCB4 and FABP4. At the same time, it resulted in decreased expression of IL-1α, TNFRSF19, CDH8, DDIT3, GADD45A, PTPRG, PCK2, and ADGRA2 (Hu et al. 2024).
Salinomycin (Figure 4 (141)), a monocarboxylic ionophore isolated from Streptomyces albus, has been widely used as an anticoccidiosis agent in chickens (Antoszczak et al. 2014). Salinomycin was first suggested by Yuan et al. as a coronavirus therapeutic for treatment of piglets infected by PEDV. It did not directly interact with or inactivate PEDV virions, nor did it affect the attachment of PEDV virions in Vero cells, but it inhibited PEDV entry and replication in a dose-dependent manner. Mechanistically, salinomycin significantly ameliorated the activation of Erk1/2, JNK and p38MAPK signaling pathways that are associated with PEDV infection. This implied that salinomycin inhibits PEDV replication by altering MAPK pathway activation (Yuan et al. 2021).
A study found the effect of nicotinamide [NAM, Figure 4 (142)] on the propagation of PEDV and PDCoV in vitro. The results showed that NAM could significantly inhibit PEDV and PDCoV proliferation in a dose-dependent manner (Table 2). Its main role is in the replication phase of the virus life cycle (Table 2). In addition, NAM treatment was found to inhibit PEDV and PDCoV replication by down-regulating transcription factor expression through activation of ERK1/2/MAPK pathway (Li et al. 2023a).
Huan et al. found that epigallocatechin-3-gallate [EGCG, Figure 4 (143)] can inhibit PEDV with IC50 of 14.95 μmol/L and CC50 > 100 μmol/L in Vero cells (Table 2). EGCG, a polyphenol in green tea, has been demonstrated to inhibit PEDV attachment, entry, replication and assembly in a dose-dependent manner to against PEDV infection. But it had no effect on PEDV release (Huan et al. 2021).
Ivermectin [IVM, Figure 4 (144)] is an FDA-approved anthelmintic drug for the treatment of helminth infections. One study found that IVM can inhibit PEDV infection of different genotypes in Vero cells. It shows strong anti-PEDV activity when added at the same time as PEDV infection or after virus infection. Further RNA sequencing results showed that IVM induced cell cycle arrest, which is consistent with IVM significantly inhibiting the late stage of viral infection by affecting virus release. In addition, ivermectin derivatives, avermectin B1 (Figure 4 (145)) and doramectin (Figure 4 (146)), can also effectively inhibit PEDV infection. Interestingly, IVM showed enhanced anti-PEDV effects when used in combination with niclosamide (Wang et al. 2023h). Another study utilized nanostructured lipid carriers (NLCs) to create IVM-NLCs particles with increased solubility and enhanced pharmacological efficacy. These particles notably suppressed PEDV proliferation, reduced reactive oxygen species accumulation, and mitigated mitochondrial dysfunction caused by PEDV infection. Additionally, IVM-NLCs led to a significant decrease in the apoptosis rate of Vero cells induced by PEDV (Xu et al. 2024a).
BBM has been reported to primarily inhibit the invasion phase of PEDV (Zhang et al. 2023a). However, a recent study demonstrated that BBM significantly inhibits PEDV proliferation in Vero and IPEC-J2 cells in a dose-dependent manner, primarily targeting the replication phase of the PEDV life cycle. Furthermore, in vivo experiments indicate that BBM effectively reduces intestinal damage caused by PEDV infection in piglets, thereby decreasing viral load and cytokine levels, including IL-6, IL-8, IL-1β, and TNF-α (Xiang et al. 2024).
2.6. Others
Black soldier fly is a high-quality protein raw material that can serve as a functional feed additive to enhance the intestinal health of piglets. Yu and colleagues conducted a study using Black soldier fly extract (BFE) to assess its impact on intestinal function in PEDV-infected piglets. Piglets in the treatment group received 500 mg/kg bw of BFE orally for 8 days before and after infection. Samples were collected on day 3 after virus vaccination for analysis. The findings revealed that BFE was able to reverse the decrease in plasma D-xylose concentration and intestinal villus height caused by PEDV infection. Furthermore, BFE was found to elevate plasma and intestinal antioxidant enzyme activity, such as total superoxide dismutase and catalase. In summary, dietary supplementation of BFE can ameliorate intestinal tissue damage and decrease oxidative stress triggered by PEDV infection in piglets. BFE was also observed to significantly boost the mRNA expression of virus-related genes in the ileum (Yu et al. 2024). Additionally, the researchers assessed the protective effects of yeast polysaccharides (YP) on intestinal damage in PEDV-infected piglets. The treatment group received 20 mg/kg bw YP orally 8 days before and after infection, and analysis of samples collected on the third day post-infection revealed that YP could mitigate the damage caused by PEDV on intestinal villus morphology in piglets. YP also demonstrated the ability to enhance antioxidant capacity and the activities of glutathione peroxidase, superoxide dismutase, and catalase in the serum and small intestine of PEDV-infected piglets. Furthermore, YP inhibited PEDV replication in the jejunum, ileum, and colon, while also alleviating intestinal inflammation and regulating intestinal metabolism (Li et al. 2024a).
Attapulgite is recognized for its effectiveness as an antidiarrheal and gastrointestinal mucosal protectant. Wang et al. discovered that attapulgite effectively captured and adsorbed the virus in vitro, inhibiting PEDV and rendering it non-infectious. Comprised mainly of MgO, Al2O3, SiO2, CaO and Fe2O3, attapulgite’s elements, combined with its porous structure, exhibit a strong adsorption capacity and affinity for PEDV. The virus titer was reduced from 10−5.613 TCID50/mL to 10−2.90 TCID50/mL, representing a 102.6-fold decrease. Powdery attapulgite displays typical rod-like crystals and powdery rod-like crystals. The powdery rod crystals demonstrated higher adsorption of proteins, impurities, and viruses, indicating superior adsorption capacity compared to the rod crystals. The authors noted that acidified modified materials exhibited weaker antiviral efficacy in comparison to powdered samples subjected to ultrasonic disintegration, which displayed the most potent antiviral effects (Wang et al. 2024c).
3. Porcine deltacoronavirus
The genome size of PDCoV is approximately 25–26 kb (Figure 1 B). PDCoV has been proven to be highly pathogenic, with mortality rates of up to 40% in suckling pigs (Figure 1 A). First reported in Hong Kong, China, in 2012, PDCoV initially did not attract much attention due to its clinical symptoms being similar to those of PEDV and TGEV. However, in 2014, the detection rate of PDCoV in clinical samples from breeding farms in Ohio, USA, was as high as 92.9%. Subsequently, PDCoV strains have been identified, isolated, and reported in various countries worldwide. Notably, a study detected PDCoV strains in plasma samples from three Haitian children with acute undifferentiated febrile illness, indicating a potential threat to human public health security. This article reviews the current status of drug research against PDCoV, offering valuable insights for the screening of PDCoV drugs and the development of new prevention strategies.
3.1. Small molecule inhibitors that regulate viral proteases to inhibit PDCoV replication
3.1.1. Mpro inhibitors
In one study, 25 derivatives were designed based on the inhibitor N3, which targets SARS Mpro [M1 to M25 in Ref. Wang et al. 2022a. M1 and M25, Table 2 (compound 73) and (compound 74)]. Among them, N3, compound M1 (Figure 3 (73)) and compound M25 (Figure 3 (74)) could significantly reduce the activity of PDCoV Mpro in vitro experiments (Wang et al. 2022a).
3.1.2. RdRp inhibitors
Remdesivir is an adenosine analogue monophosphate amide prodrug with potent activity against a range of RNA virus families, including the filoviridae, paramyxoviridae, pulmonaviridae and orthocoronaviridae, by targeting viral RdRp. Brown’s group found that RDV could target RdRp to significantly inhibit PDCoV, and that RDV could alsoagainst endemic human CoVs OC43 (HCoV-OC43) and 229E (HCoV-229E). This suggests that RDV had a wide range of anti-coronavirus activities (Brown et al. 2019).
3.2. Small molecule inhibitors that regulate host factors to inhibit PDCoV replication
3.2.1. Target-G protein-coupled receptor
In the small intestine bile acids emulsify fats to form micelles to aid in their absorption. Bile acids are produced in the liver from cholesterol and are further modified by the microbiota in the intestine. Bile acids also act as endocrine molecules that regulate a variety of metabolic processes, including glucose, lipid and energy homeostasis, through interactions with the gut microbiota. Kong’s group found that physiological concentrations of bile acids chenodeoxycholic acid [CDCA, Figure 4 (147)] and LCA had antiviral activity against PDCoV in LLC-PK1 and IPEC-J2. In IPEC-J2 cells, CDCA and LCA induced the production of IFN-λ3 and ISG15 via G protein-coupled receptor (GPCR), thereby inhibiting PDCoV replication at the post-entry stage (Kong et al. 2021).
3.2.2. Cholesterol inhibitors
According to one study, the attachment and internalization phase, during which PDCoV enters, involves both cellular and viral cholesterol as important participants. Methyl-β-cyclodextrin [MβCD, Figure 4 (148)] could operate as an antiviral in a dose-dependent way prior to or in the early stages of viral infection because it depletes cholesterol (Jeon and Lee 2018). In addition, a study found that 25-Hydroxycholesterol [25HC, Figure 4 (149)], a key enzyme in the regulation of cholesterol metabolism, was able to inhibit PDCoV infection by inhibiting viral entry in IPI-FX cells, a porcine ileal epithelial cell line (Ke et al. 2021). Another study found that 25-Hydroxycholesterol, a derivative of cholesterol, also inhibits PDCoV infection by regulating cholesterol metabolism. Further investigation revealed that 25HC might regulate the cholesterol metabolism disorder caused by PDCoV infection by transforming growth factor β1 expression down, which mainly restricted PDCoV from entering the early and middle stages of the later stage to inhibit infection (Zhang et al. 2022a).
3.2.3. Heat shock proteins inhibitors
Heat shock proteins (HSPs) are a class of proteins that are produced by cells in response to stress. Many also act as molecular chaperones and play important roles in numerous cellular processes, and they are also key host cell factors for viral replication (Shan et al. 2020). HSP90 is a highly conserved ATP-dependent homodimeric protein consisting of an N-terminal ATPase structural domain, an intermediate client protein recognition domain, and a C-terminal dimeric structural domain (Hoter et al. 2018). HSP90 inhibitors are effective inhibitors of replication of a variety of viruses. For example, the HSP90 inhibitors 17-AAG (Figure 4 (150)) and AT-533 regulated the promoter activity of the HSV-1 alpha gene (Wang et al. 2018). And 17-AAG significantly inhibited the transmission of middle east respiratory syndrome coronavirus (MERS-CoV), SARS-CoV-2 and SARS-CoV (Li et al. 2020a). Zhao et al. found that 17-AAG and VER-82576 (Figure 4 (151)) inhibited PDCoV in the early stages of replication. 17-AAG and PDCoV inhibition by VER-82576 may be achieved by targeting the host cytokine HSP90AB1 but not HSP90AA1. Because, there was no significant difference in HSP90AB1 mRNA and protein levels in 17-AAG and VER-82577 treated cells compared to control cells. However, both 17-AAG and VER-82576 dose-dependently inhibited the expression of TNF-α, IL-6 and IL-12 to varying degrees (Zhao et al. 2022).
3.2.4. Porcine aminopeptidase N protein inhibitors
Previous studies have shown that CEP has good antiviral activity against PEDV and SADS-CoV. A recent study found that CEP has moderate affinity for the PDCoV receptor porcine aminopeptidase N (pAPN) protein. And molecular docking analysis predicts that CEP can form hydrogen bonds with the amino acid residues (R740, N783 and R790) in the PDCoV and pAPN binding regions. This indicates that the anti-PDCoV activity of CEP is based on acting directly on cells and preventing the virus from attaching to host cells. In addition, CEP can also affect the proliferation of the virus in vitro by downregulating the excessive immune response caused by PDCoV and inducing autophagy (Sun et al. 2024c).
3.2.5. Medicines inhibit PDCoV by inducing cell apoptosis
Diammonium glycyrrhizinate [DG, Figure 4 (152)] is an antiviral agent that induces host action. zhai et al. found that DG inhibited PDCoV replication in LLC-PK1 cells in a dose-dependent manner and its antiviral effect occurred early in PDCoV replication. DG also inhibited viral attachment to cells. In addition, DG inhibited PDCoV-induced apoptosis in LLC-PK1 cells (Zhai et al. 2019).
Lithium chloride (LiCl, Table 3) has been shown to have an inhibitory effect on PEDV, which has been described above. Similar to PEDV, zhai et al. found that LiCl inhibited PDCoV replication in LLC-PK1 cells in a dose-dependent manner. The antiviral effect of LiCl occurred at an early stage of PDCoV replication. In addition, LiCl inhibited PDCoV-induced apoptosis in LLC-PK1 cells (Zhai et al. 2019).
Selenium nanoparticles (SeNPs, Table 3) are highly active, low toxic and readily absorbed by humans and showed good antiviral activity against dengue virus (DENV) in Hela and HepG2 cells. SeNPs can reduce mitochondrial dysfunction in disease states and thus reduce apoptosis (Ramya et al. 2015). Ren et al. found that selenomethionine inhibited PDCoV replication in vitro and selenium could reduce oxidative stress induced by viral infection and increase the levels of various cytokines in host cells and improve cellular immunity levels to inhibit viral replication (Ren et al. 2022c). Next, Ren et al. found that 4 μg/mL of SeNPs significantly reduced PDCoV replication on ST cells. Mechanistically, SeNPs attenuated PDCoV-induced mitochondrial division and antagonized PDCoV-induced apoptosis by reducing Cyt C release and activation of Caspase 9 and Caspase 3 (Ren et al. 2022d).
Ergosterol peroxide (EP) is abundant in Cryptoporus volvatus, and previous studies have shown that EP has a variety of biological properties, including antibacterial, antitumorigenic and immunomodulatory activities (He et al. 2017). Moreover, it also has an inhibitory effect on PEDV (Liu et al. 2022). Duan et al. found that EP inhibited PDCoV infection in LLC-PK1 cells in a dose-dependent manner (Table 2). EP blocked viral attachment and entry and exerted its inhibitory effect mainly in the early and middle stages of the PDCoV replication cycle. In addition, EP directly inactivated PDCoV infectivity and inhibited PDCoV-induced apoptosis. Mechanistically, EP treatment reduced PDCoV infection-induced phosphorylation of IκBα and p38 MAPK and mRNA levels of cytokines (IL-1β, IL-6, IL-12, TNF-α, IFN-α, IFN-β, Mx1 and PKR). EP can inhibit in vitro the activation of NF-κB and p38/MAPK signaling pathways by downregulating PDCoV infection and modulate the host immune response (Duan et al. 2021a). Next, Duan’s group found that the autophagy inducing rapamycin drug increased PDCoV N expression in LLC-PK1 cells, while the autophagy inhibiting wortmannin drug decreased PDCoV N expression. Moreover, PDCoV infection activates the p38 signaling pathway, which triggers autophagy to promote viral replication. Interestingly, EP can exert its antiviral effects in vitro and in vivo by attenuating PDCoV-induced p38 activation to inhibit the autophagic response (Duan et al. 2021b). Next, Duan et al. used 7-day-old piglets infected with PDCoV by oral administration in the presence or absence of EP. administration of EP reduced the incidence of diarrhea, attenuated intestinal damage, and decreased viral load in feces and tissues. Mechanistically, EP reduces PDCoV-induced apoptosis in small intestinal cells, enhances tight junction protein expression, and maintains intestinal barrier integrity. EP shows immunomodulatory effects by inhibiting PDCoV-induced activation of pro-inflammatory cytokines and IκBα and NF-κB p65, and by upregulating IFN-I expression. In addition, Duan et al. speculated that EP inhibited PDCoV replication and attenuated PDCoV-induced apoptosis through the p38/MAPK signaling pathway (Duan et al. 2021c). In addition, a study found that chlorogenic acid [CGA, Figure 4 (153)], the main active component of honeysuckle, can significantly inhibit the replication of PDCoV in both LLC-PK1 cells and ST cells. CGA mainly affects the early stages of viral replication and viral release. Mechanistically, CGA can inhibit virus release by directly targeting cell apoptosis caused by PDCoV infection (Shi et al. 2024).
Researchers such as Sun designed and synthesized more than two dozen dihydropterinone derivatives. Among them, compound 154 [compound W8 in Ref. Sun et al. 2024a, Figure 4 (154)] has strong anti-PDCoV activity and low cytotoxicity (Table 2). Mechanistically, compound 154 exerts antiviral effects by inhibiting apoptosis and inflammatory factors caused by viral infection in vitro (Sun et al. 2024a).
3.2.6. Medicines inhibit PDCoV by enhance cell immunity/inflammatory responses
Selenium is an essential trace element for living organisms and has been shown to have antiviral effects. ren’s scientific team used LLC-PK cells to study the antiviral activity of selenomethionine [SeMet, Figure 4 (155)] against PDCoV. It was found that 16 µmol/L Se-Met (Table 2) significantly inhibited PDCoV replication in LLC-PK cells, enhanced MAVS protein expression and IRF-3 phosphorylation, and increased intracellular IFN-α and IFN-β production and antioxidant capacity. The specific mechanism was attributed to the increased antioxidant capacity of SeMet and the activation of the MAVS pathway to enhance immunity (Ren et al. 2022c). Next, Ren’s team systematically investigated the endogenous immune mechanism of SeMet and found that STAT3/miR-125b-5p1/HK2 signalling is essential for the exertion of SeMet anti-PDCoV replication function. Meanwhile, HK2, a key rate-limiting enzyme of the glycolytic pathway, was able to control PDCoV replication in LLC-PK1 cells, suggesting a strategy for viruses to evade innate immunity using glucose metabolism pathways (Ren et al. 2022b). Another study found that IL-6, NR3C2, BCHE and PTGS2 were identified as the most likely targets of curcumin (Figure 4 (156)) by network pharmacological analysis. Furthermore, curcumin inhibited PDCoV replication in LLC-PK1 cells in a dose-dependent manner upon infection. Mechanistically, curcumin inhibited PDCoV-induced IFN-β secretion by inhibiting the RIG-I pathway and reduced inflammation by inhibiting IRF3 or NF-κB protein expression (Wang et al. 2023f).
Niacin acid [niacin, NA, Figure 4 (157)], also known as vitamin B3, usually includes both niacin and nicotinamide forms and is a component of the coenzyme nicotinamide adenine dinucleotide. One study found that niacin alleviated clinical symptoms of diarrhea in weaned piglets infected with PDCoV and alleviated intestinal villi shedding and atrophy (Table 4). In addition, niacin decreased the content of serum IL-1β and IL-6, and decreased the mRNA expression of TNF-α, IL-10 and IL-12 in ileum of piglets infected with PDCoV. Niacin decreased the mRNA expression of NOD1, NOD2, STAT2, IFN-γ and PKR in weaned piglets infected with PDCoV. In addition, the expression of IL-6 mRNA decreased in PDCoV-infected IPEC-J2 cells, and the expression of 2 ‘-5′ -oligoadenylate synthetase, IFN-α and PKR increased after niacin treatment (Table 2). In addition, niacin decreased the expression of ile-induced NOS, nuclear factor-κB, inhibitor kappa B, histone deacetylase Sirtuin 1, histone deacetylase 7 and histone H3 serine s10 phosphorylated protein, and increased the expression of G-protein-coupled receptors in PDCoV-infected piglets. In addition, niacin can improve the intestinal flora of PDCoV-infected piglets and increase the relative abundance of lactobacillus. Niacin partially alleviates diarrhea, intestinal barrier damage, intestinal immune response, and colon microflora disruption in weaned piglets infected with PDCoV. Niacin may reduce inflammation by inhibiting the activation of TLR2/TLR4-NF-κB signaling pathway in the intestinal tract of weaned piglets infected with PDCoV and regulating histone acetylation via GPR109A (Chen et al. 2022a). A recent study observed that treatment with baicalin led to reduced phosphorylation of PI3K, AKT, and p65 proteins, along with decreased mRNA levels of pro-inflammatory cytokines like IL-1β, IL-6, IL-8, and TNF-α in swine testicle (ST) cells. These findings indicate that baicalin may hinder PDCoV replication by blocking the PI3K-Akt-NF-κB protein signaling pathway, consequently suppressing the inflammatory response (Liu et al. 2024a).
3.3. Others
Since Griffithsin can specifically bind to N-linked high mannose oligosaccharides, its potent antiviral activity against enveloped viruses has been outlined above. Studies have shown that Griffithsin exhibited a stronger effect on anti-PEDV infection when it was added during early stages of infection (Li et al. 2019b). Similarly, a study showed that Griffithsin (Table 1) inhibits PDCoV infection at the adsorption and permeation step. Mechanistically, Griffithsin binds to the spike protein on the surface of PDCoV virions, wrapping the virus and blocks its entry (Tang et al. 2022). This may also be the main mechanism of action of Griffithsin against PEDV infection in Vero cells.
Fang et al. successfully constructed an infectious clone of PDCoV strain CHN-HN-2014 based on a bacterial artificial chromosome (BAC) reverse genetics system combined with one-step homologous recombination. Based on the established infectious clone and CRISPR/Cas9 technology, a PDCoV virus expressing nanoluciferase (Nluc) was constructed by replacing the NS6 gene. Lycorine (Figure 4 (158)) and resveratrol (Figure 4 (159)) were found to be highly sensitive and easily quantifiable for rapid antiviral screening of Nluc-containing viruses. These results indicated that lycorine and resveratrol could better inhibit the proliferation of PDCoV (Fang et al. 2021).
4. Transmissible gastroenteritis virus
The genome sequence length of TGEV is approximately 28–30 kb (Figure 1 B). TGEV was first reported in the United States in 1946, and was eventually studied and reported around the world. The mortality rate of piglets infected with TGEV between 1 and 14 days of age is as high as 100% (Figure 1 A). In recent years, epidemiology has found that the detection rate of TGEV in clinical breeding diarrhea samples is low. This may be closely related to the identified key receptors for TGEV infection of host cells, as well as long-term prevention, treatment and control.
4.1. Bacteria- and bacterial metabolites-based TGEV inhibitors
4.1.1. Enterococcus faecium inhibitors
Probiotics are defined as live microbial food supplements that have health-promoting properties. Potential mechanisms for beneficial effects include production of antimicrobial agents, modulation of the immune response and promotion of innate host defense mechanisms. Enterococcus faecium NCIMB 10415 (E. faecium, Table 1) is authorized in the EU as a probiotic feed additive for sows, piglets and several other farm animals. chai et al. found that E. faeciumz was protective against TGEV infection in swine testicle (ST) cells. The viral yield of cultures treated with E. faeciumz was reduced by three log10 units and the expression of viral structural proteins was decreased. Using transmission electron microscopy, TGEV particles were observed to adhere to the surface of E. faeciumz, which may be a means of preventing infection by its direct interference with viral attachment, capture by adsorption of surface components of probiotic bacteria or inactivation of viral particles. Increased NO production and increased expression of IL-6 and IL-8 in E. faeciumz-treated cells suggest that stimulation of cellular defense is the mechanism against TGEV infection (Chai et al. 2013).
4.1.2. Bacillus subtilis inhibitors
Bacillus subtilis is a probiotic with good anti-microbial properties, and one of its secretions, surfactin, is considered a versatile weapon against most plant pathogens, especially against enveloped viruses. Wang et al. found that B. subtilis OKB105 (Table 1) and its surfactant effectively inhibited TGEV entry into IPEC-J2. Empty plaque experiments showed that the surface the active agent could reduce TGEV plaque production in a dose-dependent manner (Table 1). B. subtilis could attach TGEV particles to its surface, thus decreasing the amount of virus bound to host cells. Moreover, the inhibitory effect of B. subtilis is closely related to the competition of TGEV for viral entry receptors, including the epidermal growth factor receptor (EGFR) and aminopeptidase N (APN) proteins. In addition, B. subtilis can enhance the resistance of IPEC-J2 cells by upregating the expression of TLR6 and reducing the proportion of apoptotic cells (Wang et al. 2017b).
4.1.3. Lactobacillus plantarum inhibitors
Wang’s group used the supernatant of an isolated Lactobacillus plantarum Lp-1 strain (Lp-1s, Table 1) to explore its anti-TGEV effect in IPEC-J2 cells. They found that Lp-1s induced large amounts of interferon-β in IPEC-J2 cells during the early phase of TGEV infection and increased the levels of phosphorylated signal transducers and transcriptional activators and their nuclear translocations during the late phase of infection. This leads to upregulation of ISGs expression and increases transcription and protein expression of antiviral proteins, resulting in anti-TGEV effects (Wang et al. 2019a).
4.1.4. Lactobacillus casei inhibitors
Lactobacillus casei (L. casei) is one of the probiotic bacteria in mammals. In one study, it was found that administration of L. casei to mice resulted in sustained upregulation of the antimicrobial peptide mReg3a gene. Subsequently, they used Escherichia coli to induce the expression of mReg3a, and found that tight junction proteins ZO-1 and E-cadherin the expression of cadherin was significantly increased. This suggests that mReg3a promotes cell proliferation and wound healing. Most importantly, mReg3a can also inhibit TGEV and PEDV infection (Bai et al. 2021).
4.1.5. Antimicrobial peptide inhibitors
Bovine antimicrobial peptide-13 (APB-13, Table 1) has antibacterial, antiviral and immunological functions. liang et al. found that APB-13 showed the highest viral inhibition of TGEV at 74.1%. The log10TCID50 of 62.5 µg/mL APB-13 was 3.63 lower than that of the viral control, and significantly reduced the expression of viral gene transcripts and protein content. Then, Liang et al. selected 4-day-old piglets fed APB-13 (10 g/kg, Table 4) continuously for 6 d and then orally inoculated with TGEV. piglets were infected and in vivo TGEV shedding was detected on the first, second, third and fourth days after infection, and the rate of virus shedding was significantly lower in the APB-13 group than in the virus control group. Morphological histological sections of the jejunum showed long, thin and intact jejunal villi in the TGEV+APB-13 group compared to the TGEV group. These results indicated that APB-13 reduced the production of TGEV particles, APB-13 had an antiviral effect on TGEV in vivo (Liang et al. 2020).
4.2. Plants- and plant extract cocktails-based TGEV inhibitors
A recent study discovered that cimicifuga rhizoma polysaccharide (CRP), primarily consisting of glucose and galactose, exhibits a notable inhibitory impact on TGEV. In comparison to the TGVE group, CRP considerably reduces cell apoptosis and prevents the decline in mitochondrial membrane potential. Moreover, network pharmacology analysis predicts that CRP targets AKT1, MMP9, HSP90AA1, CASP3, MMP9, and EGFR to enhance immunity and combat TGEV. Molecular docking simulations demonstrate CRP’s binding potential to these targets, indicating its promising immune-enhancing and anti-TGEV properties (Tan et al. 2024).
4.3. Small molecule inhibitors affect TGEV adsorption
Curcumin is the major polyphenolic compound of the edible spice turmeric due to its multiple biological effects such as antitumor, anti-inflammatory, immunomodulatory, antioxidant, antibacterial, antifungal, antiparasitic, and antiviral activities (Moghadamtousi et al. 2014). Notably, Li et al. found that curcumin strongly inhibited TGEV proliferation and viral protein expression in a dose-dependent manner (Table 2). Moreover, curcumin has a temperature- and time-dependent direct TGEV virucidal ability, which acts mainly in the early stages of TGEV replication. Curcumin showed excellent inhibition of TGEV adsorption, with 40 µmol/L of curcumin resulting in a 3.55 logTCID50 mL−1 reduction in viral titer (Li et al. 2020c).
4.4. Small molecule inhibitors that regulate viral proteases to inhibit TGEV replication
Phloretin (Figure 3 (75)), a naturally occurring dihydrochalcon glycoside, can inhibit the proliferation of TGEV in PK-15 cells in a dose-dependent manner, with its anti-TGEV activity primarily occurring during the internalization and replication stages. Additionally, phloretin reduces the expression levels of pro-inflammatory cytokines induced by TGEV infection. Through network pharmacology and molecular docking techniques, the authors identified potential key targets of phloretin’s anti-TGEV effects, including AR, CDK2, INS, ESR1, ESR2, EGFR, PGR, PPARG, PRKACA, and MAPK14. Furthermore, phloretin can target the Mpro of TGEV. However, when the Mpro residue (S242) is mutated, the binding between phloretin and Mpro is disrupted, leading to the emergence of drug-resistant TGEV strains (Duan et al. 2024b).
Myricetin (Figure 3 (76)) is a natural flavonoid that is widely found in fruits, vegetables, and tea. A recent study demonstrated that myricetin effectively inhibits TGEV-induced CPE in a dose-dependent manner. Moreover, at a concentration of 100 µmol/L, myricetin directly inactivates TGEV and inhibits its intracellular replication phase. Molecular docking results indicate that myricetin binds to the Cys102 residue of PLpro through conventional hydrogen bonds (Fan et al. 2024).
4.5. Small molecule inhibitors that regulate host factors to inhibit TGEV replication
4.5.1. Medicines inhibits TGEV by inducing cell apoptosis
In the biomedical field, the antibacterial, antifungal and antiviral properties of silver ions and silver compounds have been extensively studied and used, but silver nanoparticles have shown superior efficacy due to their promising antibacterial potential and have been used for wound healing against bacteria (Sondi and Salopek-Sondi 2004). Lv et al. found four representative silver nanomaterials, including spherical silver nanoparticles (Ag NPs and NM-300, Table 3), two silver nanowires (XFJ011, Table 3), and silver colloids (XFJ04, Table 3), Ag NPs and silver nanowires significantly reduced TGEV infection in ST cells at safe concentrations. Mechanistically, Ag NPs and silver nanowires reduced the number of TGEV-induced apoptotic cells through regulating the p38/mitochondria-caspase-3 signaling pathway (Lv et al. 2014).
Lithium chloride (LiCl, Table 3) has been found to be effective against several DNA viruses, such as HSV and poxvirus. Also, inhibition of RNA virus infections has been reported, such as PEDV and PDCoV as described above. Ren et al. found that LiCl could inhibit TGEV infection in a dose-dependent manner. In addition, RT-qPCR assays targeting the S and Mpro genes of TGEV viruses confirmed the inhibitory effect of LiCl on TGEV infection and transcription. Similar to the anti-PEDV effect (Li et al. 2018), both early and late apoptosis induction by TGEV infection were effectively inhibited by LiCl (Ren et al. 2011). Combined with the above-mentioned studies of LiCl against PEDV (Li et al. 2018) and PDCoV (Zhai et al. 2019), it was shown that it could inhibit PECs infection by exerting anti-apoptotic effects.
Cardenolides, also known as cardiac glycosides, are C (23) -steroids that binds to and acts as an allosteric inhibitor of Na+/K+-ATpase, a membrane-binding protein that establishes and maintains high internal K+ and low internal Na+ cellular concentrations in most animal cells (Yang et al. 2017). These Na+/K+-ATpase enzymes consist of two subunits (α and β) and a helper subunit γ. α-subunits bind ATP and Na+ and K+ ions, as well as allosteric binding of cardenolides (Habeck et al. 2016). Stimulant steroids such as ouabain, digitoxin (Figure 4 (160)), or oleandrin (Figure 4 (161)) have been reported to alleviate cancer disease in humans, or to reduce replication of murine leukemia virus, hemagglutinating virus of Japan, and HSV-1 in vitro experiments. Yang’s group found that some cardenolides exhibited dose-dependent anti-TGEV activity in ST cells. These cardenolides reduced the expression of TGEV nucleocapsid and spike proteins, blocked TGEV infection-induced apoptosis and CPE, and inhibited Na+/K+-ATpase activity. Notably, the expression of cardenolides’ cell receptor Na+/K+-ATpase in knockdown ST cells significantly reduced the susceptibility of ST cells to TGEV infection (Yang et al. 2017). Next, Yang et al. further showed that cardenolides delivered anti-TGEV activity by targeting the Na+/K+-ATpase and its associated PI3K-PDK1-RSK2 signaling. Upon Na+/K+-ATpase α1 knockdown, ST cells were less sensitive to TGEV infection and PI3K-PDK1-RSK2 signaling was attenuated. cardenolides this anti-TGEV activity was induced by JAK1 protein hydrolysis and mediated through upstream activation of Ndfip1/2 and its effector NEDD4 (Yang et al. 2020a). Furthermore, Yang’s group found that AG1024 (Figure 4 (162)), an insulin receptor (IR) and insulin-like growth factor 1 receptor (IGF-1R) inhibitor, diminishes JAK1 protein levels and exerts anti-coronaviral activities with EC50 values of 5.2 ± 0.3 µmol/L (in Ref. Yang et al. 2022a) against TGEV. However, TGEV infection of ST cells did not trigger the IR and IGF-1R signaling pathways, and AG1024 inhibited TGEV replication and downregulated JAK1 protein levels independently of IR and IGF-1R. Indeed, AG1024-induced JAK1 protein hydrolysis is mediated by the activation of upstream Ndfip1/2 and its effector NEDD4-like E3 ligase Itch. In addition, ouabain, which was reported to mediate JAK1 proteolysis causing anti-coronaviral activity by activation of Ndfip1/2 and NEDD4 E3 ligase, Yang et al. found that additively inhibited anti-coronaviral activity (such as TGEV and human flu coronavirus OC43) and JAK1 diminishment in combination with AG1024 (Yang et al. 2020a).
All-trans retinoic acid [ATRA, Figure 4 (163)] is the active metabolite of vitamin A. Pu and colleagues investigated the protective effect of ATRA on TGEV-infected IPEC-J2 cells. They found that ATRA alleviates TGEV-induced damage in IPEC-J2 cells by upregulating the mRNA expression of ZO-1, Occludin, and Mucin-1. Moreover, ATRA reduced TGEV-induced apoptosis in IPEC-J2 cells by downregulating Caspase-3 expression. Additionally, ATRA treatment inhibited TGEV-induced ROS and MDA production, as well as the upregulation of P38MAPK phosphorylation levels. Overall, ATRA alleviates TGEV-induced apoptosis in IPEC-J2 cells by enhancing antioxidant capacity and inhibiting cell damage (Pu et al. 2022a). The study also found that ATRA was able to decrease the inflammatory response caused by TGEV by suppressing the release of pro-inflammatory cytokines such as IL-1b, IL-6, IL-8, and TNF-a. Furthermore, ATRA treatment effectively prevented the TGEV-induced increase in IкBa and NF-κB p65 phosphorylation levels, as well as the translocation of NF-κB p65 into the nucleus. Moreover, ATRA treatment led to a significant decrease in the expression of TLR3, TLR7, RIG-I, and MDA5 in TGEV-infected IPEC-J2 cells, indicating its potential to mitigate TGEV-induced damage by dampening inflammatory reactions (Pu et al. 2022b).
Polygonum Cillinerve polysaccharide [PCP, Figure 4 (164)] can significantly reduce the apoptosis rate induced by TGEV and reduce the replication of TGEV. Mechanistically, it effectively inhibits cell apoptosis by regulating and increasing the expression levels of Bcl-2 and Bax genes, increasing the expression of Bcl-2 protein, reducing the expression of Cyto-c protein, and reducing the amount of caspase 3 cleavage (Pan et al. 2021). Next, the research team also found that half of the piglets survived TGEV infection when treated with PCP. High-throughput sequencing technology was used to identify PCP interference in TGEV-infected PK15 cells, and the results showed that miR-181 is related to the target genes of key proteins in the apoptosis pathway (Duan et al. 2024a).
4.5.2. Medicines inhibits TGEV by regulating cellular reactive oxygen species
About 70% of green tea extracts are catechins, which are monomeric flavonoids. Catechins have a variety of pharmacological activities and can inhibit different types of viruses in different ways. (+)-catechin (Figure 4 (165)) is one of the catechins in green tea, and Liang’s group found that (+)-catechin treatment exerted a dose-dependent rescue effect in TGEV-infected ST cells. (+)-catechin treatment restricted TGEV RNA replication and attenuated intracellular TGEV infection-induced reactive oxygen species (ROS) activation (Liang et al. 2015).
Eugenol (Figure 4 (166)) is a natural plant essential oil with good antioxidant properties. Wang and his research team found that adding eugenol could alleviate TGEV-induced intestinal and IPEC-J2 cell damage. Mechanistically, eugenol reduces TGEV-induced oxidative stress in intestinal epithelial cells by reducing ROS levels. In addition, eugenol can also inhibit TGEV-induced intestinal cell apoptosis (Wang et al. 2022b). Subsequently, the research team found that eugenol scavenged the TGEV-induced increase in ROS, thereby preventing TGEV-induced NLRP3 inflammatory vesicle activation and pyroptosis, thereby inhibiting TGEV-induced intestinal epithelial cell pyroptosis. By adding eugenol to the diet of weaned piglets, TGEV-induced intestinal damage in piglets can be significantly reduced (Wang et al. 2023d).
4.5.3. Medicines inhibit TGEV by enhance cell immunity
One study found that TGEV infection of IPEC-J2 cells induced downregulation of mTOR and its downstream p70 S6K and 4E-BP1, STAT1 and ISGs, which was blocked by the mTOR activator MHY1485 but not by the mTOR inhibitor RAPA. At the same time, MHY1485 activates mTOR to reduce TGEV levels, and vice versa. L-leucine [Leu, Figure 4 (167)] can reverse the inhibition of STAT1 and ISGs by activating mTOR and its downstream p70 S6K and 4E-BP1 in TGEV-infected IPEC-J2 cells. These results indicate that Leu alleviates TGEV infection by activating the mTOR signaling pathway in cells and promoting the expression of STAT1 and ISGs (Du et al. 2021).
4.5.4. JNK1 and p38 inhibitors
Tyrphostin A9 (Figure 4 (168)) is a tyrosin-like receptor tyrosine kinase inhibitor (RTKI), which was found by Dong et al. to potently inhibit TGEV infection. Tyrphostin A9 was also have potent antiviral activity against the replication of mouse hepatitis virus (MHV), PEDV and FIPV. Further studies identified p38 and JNK1, the downstream molecules of receptor tyrosine kinase required for TGEV replication, as targets of tyrphostin A9, whose inhibitory activity against TGEV infection was mainly mediated through the p38 mitogen-activated protein kinase signaling pathway (Dong et al. 2020).
4.5.5. Na+-glucose co-transporter1 (SGLT1) inhibitors
Na+-glucose co-transporter1 (SGLT1) is predominantly expressed in the mucosa of the small intestine and plays a crucial role in the absorption of Na+ and glucose. The surface Na+/H+ exchanger 3 (NHE3) serves as a pivotal regulatory site for controlling electroneutral Na+ absorption. Yang and colleagues discovered that TGEV infection can impede NHE3 translocation and diminish sodium and hydrogen exchange activity via the SGLT1-mediated p38MAPK/AKt2 signaling pathway, impacting cellular electrolyte absorption and resulting in diarrhea. Nevertheless, phlorizin (Figure 4 (169)) can notably enhance the phosphorylation levels of MAPKAPK-2 and EZRIN, the key downstream proteins of the p38MAPK/AKt2 signaling pathway, by suppressing the expression of SGLT1. This leads to a significant increase in the expression and activity of NHE3 on the cell surface, ultimately alleviating diarrhea induced by TGEV infection (Yang et al. 2020c).
4.6. Others
Zinc (Zn) is an important trace element and the use of high levels of dietary Zn as zinc oxide in swine nutrition reduces the incidence and severity of non-specific diarrhea after weaning (Zhang and Guo 2009). Wei et al. explored the effects of two zinc salts, zinc chloride (ZnCl2, Table 3) and zinc sulfate (ZnSO4, Table 3), on swine testicle (ST) cells infected with TGEV. It was found that ZnCl2 and ZnSO4 did not exhibit direct virucidal effects and did not affect the adsorption of TGEV to ST cells. However, application of ZnCl2 and ZnSO4 at different time points during virus infiltration and after virus cell entry significantly reduced virus titers and the synthesis of TGEV RNA and viral proteins. This suggests that ZnCl2 and ZnSO4 can mediate antiviral effects by inhibiting virus penetration or efflux or intracellular phases of the virus life cycle (Wei et al. 2012).
5. Swine acute diarrhea syndrome coronavirus
SADS-CoV was initially identified as an important pathogen causing infection and death of newborn piglets in Guangdong, China, in 2017 (Figure 1 A). As of now, there have been no reports of the virus in other countries. The genome size of SADS-CoV is approximately 27 kb (Figure 1 B). The virus can lead to mortality rates of up to 90% in newborn piglets between 1 to 5 d old, with reduced mortality rates in piglets over 8 d old (Figure 1 A). SADS-CoV has the ability to infect various cell lines from different species, such as humans, bats, pigs, monkeys, chickens, mice, and non-human primates. Furthermore, researchers compared the identified SADS-CoV genome sequence with the bat coronavirus HKU2-CoV (GenBank: NC/U 009988.1), revealing a similarity of over 90%, indicating a possible bat origin for SADS-CoV and subsequent transmission to piglets. Considering the potential for cross-species transmission and the threat to human health, it is crucial to screen for drugs that can prevent SADS-CoV outbreaks.
5.1. Small molecule inhibitors affect SADS-CoV adsorption
Tunicamycin (Figure 2 (25)), an N-linked glycoprotein inhibitor, was found in one study to inhibit SADS-CoV attachment to host cells. Subsequent research suggested that the SADS-CoV receptor may be an N-linked glycoprotein rather than Neu5Gc or Neu5Ac. Furthermore, the authors discovered that exogenous trypsin, endogenous serine proteases, CTSL, CTSB, and lysosomal acidification can induce SADS-CoV entry into cells (Chen et al. 2023b).
5.2. Small molecule inhibitors that regulate host factors to inhibit SADS-CoV replication
5.2.1. ZDHHC17 inhibitor
CRISPR-Cas9-based screening systems have recently been used to identify major host factors for coronavirus infection (Wei et al. 2021). Luo’s group performed a genome-wide CRISPR knockdown library screen for SADS-CoV in Hela cells. Knockdown of zinc finger DHHC-type palmitoyltransferase 17 (ZDHHC17 or ZD17) in Hela cells was found to significantly reduce SADS-CoV replication, indicating that ZD17 is an important host factor for SADS-CoV infection. Further studies demonstrated that the DHHC structural domain of ZD17, which is involved in palmitoylation, is important for SADS-CoV genomic RNA replication. Notably, Luo et al. found that treatment of infected cells with the palmitoylation inhibitor 2-bromopalmitate [2-BP, Figure 4 (170)] significantly inhibited SADS-CoV infection (Luo et al. 2021). These results suggest that screening for drugs targeting ZD17 may well inhibit SADS-CoV infection.
5.2.2. Receptor tyrosine kinase inhibitor
HER2, a member of the EGFR family which is involved in proliferation and differentiation signaling. Studies have shown that effective SADS-CoV infection requires activation of HER2 and its cascade Ras-Raf-Mek-Erk signaling pathway (Zhang et al. 2022b). CP-724714 (Figure 4 (171)), an inhibitor of the receptor tyrosine kinase, is also a potent HER2 inhibitor (Nami et al. 2019). CP-724714 was shown to inhibit SADS-CoV infection in vitro in a dose-dependent manner to inhibit SADS-CoV infection (Table 2). Further validation showed that CP-724714 mainly acts in the post-entry phase of the SADS-CoV infection cycle. Moreover, the Ras-Raf-Mek-Erk axis is an important intracellular cascade for HER2 phosphorylation activation. Further validation showed that inhibitors targeting Ras [Lonafarnib, Figure 4 (172)] and Raf [Sorafenib, Figure 4 (173)] were effective in limiting SADS-CoV infection. Notably, researchers found that CP-724714 possessed broad-spectrum anti-pig diarrhea coronavirus activity against PEDV, PDCoV, and TGEV infections in vitro in a dose-dependent manner (Table 2). These results highlight the potential utility of CP-724714 or antivirals targeting HER2 and its cascade Ras-Raf-Mek-Erk signaling pathway as host-targeted SADS-CoV and other related coronavirus therapies (Zhou et al. 2023).
5.3. Others
Earliest, the broad-spectrum antiviral drug remdesivir was found to effectively block SADS-CoV replication in an in vitro assay by Edwards et al. (Edwards et al. 2020). Chen’s group, in an effort to quickly find specific drug treatments for SADS-CoV infection, used Huh7 cells and an approved drug library to screen for antiviral drugs by measuring virus-induced CPE and the level of viral RNA expression in the cells. Of a total of 3523 compounds tested, five of them, gemcitabine (Figure 4 (174)), mycophenolate mofetil (Figure 4 (175)), mycophenolic acid (Figure 4 (176)), methylene blue (Figure 4 (177)), and cepharanthine, were shown to inhibit SADS-CoV in a dose-dependent manner (Table 2). Further cellular experiments confirmed that cepharanthine and methylene blue blocked viral entry, and gemcitabine, mycophenolate mofetil, mycophenolic acid and methylene blue could inhibit viral replication after SADS-CoV entry (Chen et al. 2022c).
Zheng’s group found that aloe extract (Ae, Table 1) strongly inhibited SADS-CoV in Vero and IPI-FX cells in vitro. Furthermore, the analysis focused on the anti-SADS-CoV activity of emodin (Figure 4 (96)) from Ae in cells, which did not directly impair SADS-CoV infectivity but inhibited SADS-CoV during the entire phase of the viral replication cycle. Emodin could significantly reduce the attachment of viral particles to the cell surface and induced activation of TLR3, IFN-λ3 and ISG15 expression in IPI-FX cells to inhibit viral replication. Interestingly, Zheng et al. found that quercetin treated Vero cells significantly reduced the expression of SADS-CoV N protein, but showed no anti-SADS-CoV activity in IPI-FX cells (Zheng et al. 2022). Recently, a study found through in vitro experiments that quercetin has a concentration-dependent effect on the proliferation of SADS-CoV and acts on the adsorption and replication stages of the virus life cycle (Table 2). Mechanistically, quercetin disrupts the viral regulation of the P53 gene and inhibits host cell cycle progression induced by SADS-CoV infection. Subsequently, in vivo experiments showed that quercetin can effectively alleviate the clinical symptoms and intestinal pathological damage of SADS-CoV-infected piglets, leading to a reduction in the expression levels of inflammatory factors such as TLR3, IL-6, IL-8, and TNF-α (Feng et al. 2024).
6. Conclusions and perspectives
Thus far, hundreds of drugs that inhibit PECs infection have been screened. Most of the screening of anti-PECs drugs is based on a single effect on host cells or interfering with the virus itself, but its specific mechanism of action and whether it has clinical application value are still unclear. Although a small number of studies have preliminary analyzed the mechanism of drugs interfering with the proliferation of PECs in vivo and in vitro, no clinical trial has produced completely satisfactory results. Like other coronaviruses, PECs genomes encode structural and nonstructural proteins, among which Mpro has been identified as attractive targets for anti-PECs drug development. In addition, a large number of studies have shown that the active site of viral proteins may be conserved in PEDV, TGEV, PDCoV and SADS-CoV. Even the active sites in PECs and human coronavirus proteins are very similar. This provides the possibility to develop and design broad-spectrum PECs inhibitors. Therefore, among the currently reported compounds with antiviral activity against PECs, some compounds have inhibitory effects on two or three of PEDV, TGEV, PDCoV and SADS-CoV, such as rhodanine derivative LJ001 (Figure 4 (178)) significantly inhibits TGEV and PDCoV replication in ST cells. When the concentration of LJ001 was 3.125 μmol/L and 12.5 μmol/L (Table 2), the virus titers of TGEV and PDCoV were significantly decreased, and LJ001 had obvious inhibition on the infection of TGEV and PDCoV during the replication phase of the virus life cycle (Zhang et al. 2020). Another study found strong activity of indoles and their derivatives against porcine coronaviruses, including melatonin (Figure 4 (179)), indole (Figure 4 (180)), tryptamine (Figure 4 (181)) and L-tryptophan (Figure 4 (182)). Melatonin, a ubiquitous and multifunctional molecule, was found to inhibit TGEV, PEDV, and PDCoV infections in PK-15, Vero, and LLC-PK1 cells, respectively, by reducing viral entry and replication (Zhai et al. 2021). Additionally, a study showed that rifampicin (Figure 4 (183)) effectively inhibits PEDV and SADS-CoV infection in Vero cells (Wei et al. 2024). Going forward, the potential of developing a broad-spectrum drug targeting PECs using a single small molecule, which could be beneficial in managing mixed infections in clinical settings.
It is worth noting that many researchers choose to re-screen drugs for treating PECs from FDA-approved and clinical trial drugs, which can not only reduce development costs and shorten development time. We also think this might be a quicker and more practical approach. Additionally, targeting host factors with antiviral drugs is another strategy to pharmacologically limit viral proliferation within host cells. While the virus invades and proliferates in the host cell, the host cell is also trying its best to establish a complex signaling network to identify, control and eliminate the invading virus. Interestingly, bile acids (BAs) were found to increase the infectivity of PEDV in Vero cells and IPEC-J2 cells in one study (Su et al. 2019). Another study reported that BAs had antiviral activity against PDCoV and reduced its replication in LLC-PK1 and IPEC-J2 cells (Kong et al. 2021). Furthermore, researchers found that SADS-CoV infection of piglets resulted in a significant increase in small intestinal bile acids. In turn, BAs are dependent on lipid raft-mediated enhancement of SADS-CoV replication through foveal membrane-mediated endocytosis to enhance SADS-CoV entry and through in vivo acidification to enhance SADS-CoV replication. In particular, cholic acid (CA) enhances SADS-CoV replication by acting on stem cell-derived porcine intestinal analogs early in the infection (Yang et al. 2022b). These seemingly contradictory results suggest that 1. bile acids may regulate the replication of different PECs in very different ways. 2. different PECs invade host cells in different infectious ways. 3. Perhaps the difference in the virus infected cells is also an important reason for the difference in the interaction of the same drug with different PECs. Therefore, more in-depth exploration of the interaction between host and viral proteins, and a summary of the similarities or differences in the immune evasion mechanism of PECs in infected host cells, will be more conducive to the precise development of targeted drugs and broad-spectrum anti-PECs drugs.
Of course, the cost of developing antiviral drugs also needs to be considered, which is an unavoidable problem in clinical application. We find that the antiviral effects of natural products are increasingly favored by scientific researchers. Because natural products come from abundant sources, they have fewer side effects, a lower risk of developing drug resistance, and they are less expensive. Therefore, scientifically and systematically screen antiviral natural products, analyze their structure-activity relationships, identify the antiviral targets of natural products, reveal the binding mode of small molecules and targets, and transform and optimize active antiviral natural products based on the binding mode, which has great significance.
Most of the existing research uses in vitro cell experiments that have the advantages of relatively simple operation, controllable growth environment, and short test cycle. However, confoundingly, PECs often replicate at very low levels in porcine intestinal cell lines in vitro, leading to a large number of studies conducted in non-porcine intestinal cell lines. This increases the uncertainty of whether the selected antiviral drugs can effectively alleviate the harm of PECs in clinical breeding. Therefore, there is a need to further develop technologies such as 3D and organoids to better restore the intestinal ecological environment of piglets in vitro, which is more conducive to screening anti-PECs drugs. In addition, PECs mainly harm newborn piglets, and establishing a good piglet experimental model is one of the important conditions for screening anti-PECs drugs. We found that in clinical breeding, a single drug, especially a small molecule compound, is difficult to effectively protect newborn piglets. We believe that compound prescriptions similar to cocktail therapy can not only target viral proteins to directly affect their proliferation in host cells, but also stimulate the interactive protein network system formed by multiple host factors in newborn piglets to work together to reshape the effects of viral infection. The resulting disorder of cellular homeostasis is more conducive to alleviating the harm of PECs to newborn piglets. In addition, effective anti-PECs drugs can disinfect and treat PECs in adult pigs in daily breeding, especially the preventive effect of drugs in sows.
Although researchers have reported many anti-PECs drugs (Figure 5), there are currently no licensed drugs for commercial production. PECs are prone to mutagenesis and rapid spread, which increases the difficulty of vaccine development. Therefore, the research and development of low-toxic, high-efficiency, broad-spectrum anti-PECs drugs still has considerable development prospects. From a long-term perspective, developing effective anti-PECs drugs requires a lot of basic work. For example, there are more and more analyzes on the genome function of PECs, high-throughput screening of antiviral drug databases and computer-based drug design, etc. These will help us more accurately discover anti-PECs drug candidates or produce effective anti-PECs lead compounds. Researchers need to fully evaluate drug candidates. In particular, further increasing the therapeutic efficacy of drug candidates in in vivo clinical trials. To provide sufficient evidence to ensure the safety and effectiveness of these drugs on PECs, thereby promoting the healthy development of the pig industry.
Figure 5.
The primary action pathways of representative anti-PECs drugs and the infection process of PECs. I: Cepharanthine binds to PDCoV and pAPN, thereby preventing the virus from attaching to host cells. Tunicamycin serves as an inhibitor of N-bioglycoprotein, an N-linked glycoprotein that functions as a receptor for SADS-CoV on infected cells. Griffithsin has been shown to inhibit the adhesion of PEDV and TGEV, while surfactin also inhibits the adhesion of both PEDV and PDCoV. II: Berbamine and Fangchinoline inhibited membrane fusion by disrupting endonucosomal flux and reducing the activity of CTSL and CTSB, thereby preventing membrane fusion and the subsequent transfer of the viral genome into the cytoplasm. Levistolide a inhibits PEDV from attaching to cell membranes or penetrating cells. III: GC376, Quercetin, Tomatidine and Octyl gallate have inhibitory effects on Mpro. Remdesivir and Molnupiravir have inhibitory effects on RdRp. 6-thioguanine has an inhibitory effect on Plpro. IV: DHA and EPA can reduce endoplasmic reticulum stress and inhibit PEDV, TGEV and PDCoV infection of cells. V: 2-DG and EGCG can affect virus assembly. VI: Wogonin can combine with PEDV Mpro to affect virus replication and can also interfere with the virus release stage. Chlorogenic acid inhibits viral release by directly targeting apoptosis caused by PDCoV infection. Ivermectin can affect virus release and significantly inhibit the late stage of PEDV infection.
Funding Statement
This work was supported by the National Natural Science Foundation of China (32373067), and the Key R&D Program of Hubei Jiangxia Laboratory (JXBS021), and the Fundamental Research Funds for the Central Universities (2662022DKPY007)
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Antoszczak M, Popiel K, Stefańska J, Wietrzyk J, Maj E, Janczak J, Michalska G, Brzezinski B, Huczyński A., 2014. Synthesis, cytotoxicity and antibacterial activity of new esters of polyether antibiotic - salinomycin. Eur J Med Chem. 76:435–444. doi: 10.1016/j.ejmech.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Arun Krishnan R, Elizabeth Thomas R, Sukumaran A, Paul JK, Vasudevan DM.. 2020. COVID-19: current trends in invitro diagnostics. Indian J Clin Biochem. 35(3):285–289. doi: 10.1007/s12291-020-00906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai W, Zhu Q, Wang J, Jiang L, Guo D, Li C, Xing X, Sun D.. 2024. Licorice extract inhibits porcine epidemic diarrhea virus in vitro and in vivo. J Gen Virol. 105(3):001964. doi: 10.1099/jgv.0.001964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Huang Y, Li Y, Zhang B, Xiao C, Hou X, Yu L.. 2021. The murine Reg3a stimulated by Lactobacillus casei promotes intestinal cell proliferation and inhibits the multiplication of porcine diarrhea causative agent in vitro. Front Microbiol. 12:675263. doi: 10.3389/fmicb.2021.675263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker LA, Bakkum BW, Chapman C.. 2019. The clinical use of monolaurin as a dietary supplement: a review of the literature. J Chiropr Med. 18(4):305–310. doi: 10.1016/j.jcm.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourrie BC, Willing BP, Cotter PD.. 2016. The microbiota and health promoting characteristics of the fermented beverage kefir. Front Microbiol. 7:647. doi: 10.3389/fmicb.2016.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Won JJ, Graham RL, Dinnon KH, 3rd, Sims AC, Feng JY, Cihlar T, Denison MR, Baric RS, Sheahan TP.. 2019. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Zhang S, Huang Y, Zhang S, Wang H, Bao W.. 2022. The aqueous leaf extract of M. oleifera inhibits PEDV replication through suppressing oxidative stress-mediated apoptosis. Animals (Basel). 12(4):458. doi: 10.3390/ani12040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Burwinkel M, Wang Z, Palissa C, Esch B, Twardziok S, Rieger J, Wrede P, Schmidt MF.. 2013. Antiviral effects of a probiotic Enterococcus faecium strain against transmissible gastroenteritis coronavirus. Arch Virol. 158(4):799–807. doi: 10.1007/s00705-012-1543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Liao WP, Lee A, Chiu YH, Chang HW, Liu JR.. 2020. Isolation of a Leuconostoc mesenteroides strain with anti-porcine epidemic diarrhea virus activities from kefir grains. Front Microbiol. 11:1578. doi: 10.3389/fmicb.2020.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DZ, Fan SR, Yang BJ, Yao HC, Wang YT, Cai JY, Jing CX, Pan ZH, Luo M, Yuze YQ, et al. 2021a. Phenanthridine derivative host heat shock cognate 70 down-regulators as porcine epidemic diarrhea virus inhibitors. J Nat Prod. 84(4):1175–1184. doi: 10.1021/acs.jnatprod.0c01252. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhao P, Zhang C, Ming X, Zhang C, Jung YS, Qian Y.. 2024a. Veratramine inhibits porcine epidemic diarrhea virus entry through macropinocytosis by suppressing PI3K/Akt pathway. Virus Res. 339:199260. doi: 10.1016/j.virusres.2023.199260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui Y, Wang Z, Liu G.. 2020a. Identification and characterization of PEDV infection in rat crypt epithelial cells. Vet Microbiol. 249:108848. doi: 10.1016/j.vetmic.2020.108848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Nai Z, Qin Z, Li G, He X, Wang W, Tian Y, Liu D, Jiang X.. 2023a. The extracellular polysaccharide inhibit porcine epidemic diarrhea virus with extract and gene editing Lacticaseibacillus. Microb Cell Fact. 22(1):225. doi: 10.1186/s12934-023-02226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen X, Qu Q, Lin Y, Chen R, Zhu Y, Lv W, Guo S.. 2024b. Lizhong decoction inhibits porcine epidemic diarrhea virus in vitro and in vivo. J Ethnopharmacol. 333:118428. doi: 10.1016/j.jep.2024.118428. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li P, Zhen R, Wang L, Feng J, Xie Y, Yang B, Xiong Y, Niu J, Wu Q, et al. 2022a. Effects of niacin on intestinal epithelial Barrier, intestinal Immunity, and microbial community in weaned piglets challenged by PDCoV. Int Immunopharmacol. 111:109054. doi: 10.1016/j.intimp.2022.109054. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu X, Zheng JN, Yang LJ, Luo Y, Yao YL, Liu MQ, Xie TT, Lin HF, He YT, et al. 2023b. N-linked glycoproteins and host proteases are involved in swine acute diarrhea syndrome coronavirus entry. J Virol. 97(10):e0091623. doi: 10.1128/jvi.00916-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Luo Q, Li S, Li C, Liao S, Yang X, Zhou R, Zhu Y, Teng L, Chen H, et al. 2020b. Antiviral activity against porcine epidemic diarrhea virus of Pogostemon cablin polysaccharide. J Ethnopharmacol. 259:113009. doi: 10.1016/j.jep.2020.113009. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Luo Q, Zhu Y, Du H, Liao S, Yang Y, Chen H.. 2021b. Inhibition of porcine epidemic diarrhea virus by Alpiniae oxyphyllae fructus polysaccharide 3. Res Vet Sci. 141:146–155. doi: 10.1016/j.rvsc.2021.10.026. [DOI] [PubMed] [Google Scholar]
- Chen YM, Burrough E.. 2022. The effects of swine coronaviruses on ER stress, autophagy, apoptosis, and alterations in cell morphology. Pathogens. 11(8):940. doi: 10.3390/pathogens11080940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YM, Limaye A, Chang HW, Liu JR.. 2022b. Screening of lactic acid bacterial strains with antiviral activity against porcine epidemic diarrhea. Probiotics Antimicrob Proteins. 14(3):546–559. doi: 10.1007/s12602-021-09829-w. [DOI] [PubMed] [Google Scholar]
- Chen YZ, You YC, Wang SQ, Jiang L, Tian LL, Zhu SZ, An XP, Song LH, Tong YG, Fan HH.. 2022c. Antiviral drugs screening for swine acute diarrhea syndrome coronavirus. Int J Mol Sci. 23(19):11250. doi: 10.3390/ijms231911250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Chen J, Wei X, Hua H, Hu R, Ding N, Zhang J, Song D, Ye Y, Tang Y, et al. 2021c. Antiviral activities of carbazole derivatives against porcine epidemic diarrhea virus in vitro. Viruses. 13(12):2527. doi: 10.3390/v13122527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HM, Ha TK, Dang LH, Pham HT, Tran VO, Huh J, An JP, Oh WK.. 2019. Prenylated phenolic compounds from the leaves of Sabia limoniacea and their antiviral activities against porcine epidemic diarrhea virus. J Nat Prod. 82(4):702–713. doi: 10.1021/acs.jnatprod.8b00435. [DOI] [PubMed] [Google Scholar]
- Cho WK, Kim H, Choi YJ, Yim NH, Yang HJ, Ma JY.. 2012. Epimedium koreanum Nakai water extract exhibits antiviral activity against porcine epidermic diarrhea virus in vitro and in vivo. Evid Based Complement Alternat Med. 2012:985151–985110. doi: 10.1155/2012/985151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Kim JH, Lee CH, Ahn YJ, Song JH, Baek SH, Kwon DH.. 2009. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 81(1):77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HF, Chen CC, Moses DC, Chen YH, Lin CH, Tsai YC, Chou CY.. 2018. Porcine epidemic diarrhea virus papain-like protease 2 can be noncompetitively inhibited by 6-thioguanine. Antiviral Res. 158:199–205. doi: 10.1016/j.antiviral.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa HF, Bezerra-Santos CR, Barbosa Filho JM, Martins MA, Piuvezam MR.. 2008. Warifteine, a bisbenzylisoquinoline alkaloid, decreases immediate allergic and thermal hyperalgesic reactions in sensitized animals. Int Immunopharmacol. 8(4):519–525. doi: 10.1016/j.intimp.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Deejai N, Roshorm YM, Kubera A.. 2017. Antiviral compounds against nucleocapsid protein of porcine epidemic diarrhea virus. Anim Biotechnol. 28(2):120–130. doi: 10.1080/10495398.2016.1232268. [DOI] [PubMed] [Google Scholar]
- Dong HJ, Wang ZH, Meng W, Li CC, Hu YX, Zhou L, Wang XJ.. 2018. The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Viruses. 10(11):601. doi: 10.3390/v10110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Yu R, Wang X, Chen B, Si F, Zhou J, Xie C, Li Z, Zhang D.. 2022. Bis-benzylisoquinoline alkaloids inhibit porcine epidemic diarrhea virus in vitro and in vivo. Viruses. 14(6):1231. doi: 10.3390/v14061231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Ding N, Zhang Y, Tan Z, Ding X, Zhang Q, Jiang L.. 2021. Alterations of suckling piglet jejunal microbiota due to infection with porcine epidemic diarrhea virus and protection against infection by Lactobacillus salivarius. Front Vet Sci. 8:771411. doi: 10.3389/fvets.2021.771411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Xie W, Liu Y, Sui B, Zhang H, Liu L, Tan Y, Tong X, Fu ZF, Yin P, et al. 2020. Receptor tyrosine kinase inhibitors block proliferation of TGEV mainly through p38 mitogen-activated protein kinase pathways. Antiviral Res. 173:104651. doi: 10.1016/j.antiviral.2019.104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Chen D, Yu B, He J, Yu J, Mao X, Luo Y, Zheng P, Luo J.. 2021. L-leucine promotes STAT1 and ISGs expression in TGEV-infected IPEC-J2 cells via mTOR activation. Front Immunol. 12:656573. doi: 10.3389/fimmu.2021.656573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Liang J, Dong N, Lu J, Fu Y, Fang L, Xiao S, Han H.. 2018a. Glutathione-capped Ag2S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl Mater Interfaces. 10(5):4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]
- Du T, Lu J, Liu L, Dong N, Fang L, Xiao S, Han H.. 2018b. Antiviral activity of graphene oxide-silver nanocomposites by preventing viral entry and activation of the antiviral innate immune response. ACS Appl Bio Mater. 1(5):1286–1293. doi: 10.1021/acsabm.8b00154. [DOI] [PubMed] [Google Scholar]
- Du T, Zhang J, Li C, Song T, Li P, Liu J, Du X, Wang S.. 2020. Gold/silver hybrid nanoparticles with enduring inhibition of coronavirus multiplication through multisite mechanisms. Bioconjug Chem. 31(11):2553–2563. doi: 10.1021/acs.bioconjchem.0c00506. [DOI] [PubMed] [Google Scholar]
- Duan C, Ge X, Wang J, Wei Z, Feng WH, Wang J.. 2021a. Ergosterol peroxide exhibits antiviral and immunomodulatory abilities against porcine deltacoronavirus (PDCoV) via suppression of NF-kappaB and p38/MAPK signaling pathways in vitro. Int Immunopharmacol. 93:107317. doi: 10.1016/j.intimp.2020.107317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C, Liu Y, Hao Z, Wang J.. 2021b. Ergosterol peroxide suppresses porcine deltacoronavirus (PDCoV)-induced autophagy to inhibit virus replication via p38 signaling pathway. Vet Microbiol. 257:109068. doi: 10.1016/j.vetmic.2021.109068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C, Luo Y, Liang X, Wang X.. 2022. A review of bioactive compounds against porcine enteric coronaviruses. Viruses. 14(10):2217. doi: 10.3390/v14102217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C, Wang J, Liu Y, Zhang J, Si J, Hao Z, Wang J.. 2021c. Antiviral effects of ergosterol peroxide in a pig model of porcine deltacoronavirus (PDCoV) infection involves modulation of apoptosis and tight junction in the small intestine. Vet Res. 52(1):86. doi: 10.1186/s13567-021-00955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Li H, Tan X, Liu N, Wang X, Zhang W, Liu Y, Ma W, Wu Y, Ma L, et al. 2024a. Polygonum cillinerve polysaccharide inhibits transmissible gastroenteritis virus by regulating microRNA-181. Vet J. 304:106083. doi: 10.1016/j.tvjl.2024.106083. [DOI] [PubMed] [Google Scholar]
- Duan Y, Li H, Huang S, Li Y, Chen S, Xie L.. 2024b. Phloretin inhibits transmissible gastroenteritis virus proliferation via multiple mechanisms. J Gen Virol. 105(5). doi: 10.1099/jgv.0.001996. [DOI] [PubMed] [Google Scholar]
- Edwards CE, Yount BL, Graham RL, Leist SR, Hou YXJ, Dinnon KH, Sims AC, Swanstrom J, Gully K, Scobey TD, et al. 2020. Swine acute diarrhea syndrome coronavirus replication in primary human cells reveals potential susceptibility to infection. Proc Natl Acad Sci USA. 117(43):26915–26925. doi: 10.1073/pnas.2001046117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B, Zhu L, Chang X, Zhou J, Guo R, Zhao Y, Shi D, Niu B, Gu J, Yu Z, et al. 2019. Mortalin restricts porcine epidemic diarrhea virus entry by downregulating clathrin-mediated endocytosis. Vet Microbiol. 239:108455. doi: 10.1016/j.vetmic.2019.108455. [DOI] [PubMed] [Google Scholar]
- Fan J, Xi P, Liu H, Song X, Zhao X, Zhou X, Zou Y, Fu Y, Li L, Jia R, et al. 2024. Myricetin inhibits transmissible gastroenteritis virus replication by targeting papain-like protease deubiquitinating enzyme activity. Front Microbiol. 15:1433664. doi: 10.3389/fmicb.2024.1433664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Zhang H, Sun H, Wang G, Xia S, Ren J, Zhang J, Tian L, Fang L, Xiao S.. 2021. Construction, characterization and application of recombinant porcine deltacoronavirus expressing nanoluciferase. Viruses. 13(10):1991. doi: 10.3390/v13101991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Yi H, Zheng X, Liu X, Gong T, Wu D, Song Z, Zheng Z, Peng Q.. 2024. Quercetin inhibition of porcine intestinal alpha coronavirus in vitro and in vivo. BMC Vet Res. 20(1):134. doi: 10.1186/s12917-024-03984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Zhang Y, Kang Y, Xu W, Jiang L, Guo T, Huan C.. 2020. Glycyrrhizin inhibits PEDV infection and proinflammatory cytokine secretion via the HMGB1/TLR4-MAPK p38 pathway. Int J Mol Sci. 21(8):2961. doi: 10.3390/ijms21082961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Fang D, Liang Y, Deng X, Chen N, Zeng M, Luo M.. 2022. Circular RNAs as emerging regulators in COVID-19 pathogenesis and progression. Front Immunol. 13:980231. doi: 10.3389/fimmu.2022.980231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts V, Zakhartchouk A.. 2017. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet Microbiol. 206:45–51. doi: 10.1016/j.vetmic.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go G, Wu G, Silvey DT, Choi S, Li X, Smith SB.. 2012. Lipid metabolism in pigs fed supplemental conjugated linoleic acid and/or dietary arginine. Amino Acids. 43(4):1713–1726. doi: 10.1007/s00726-012-1255-5. [DOI] [PubMed] [Google Scholar]
- Gómez-García M, Puente H, Argüello H, Mencía-Ares Ó, Rubio P, Carvajal A., 2021. In vitro assessment of antiviral effect of natural compounds on porcine epidemic diarrhea coronavirus. Front Vet Sci. 8:652000. doi: 10.3389/fvets.2021.652000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M, Xia X, Chen D, Ren Y, Liu Y, Xiang H, Li X, Zhi Y, Mo Y.. 2023. Antiviral activity of chrysin and naringenin against porcine epidemic diarrhea virus infection. Front Vet Sci. 10:1278997. doi: 10.3389/fvets.2023.1278997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui R, Wan A, Liu X, Yuan W, Jin H.. 2014. Water-soluble multidentate polymers compactly coating Ag2S quantum dots with minimized hydrodynamic size and bright emission tunable from red to second near-infrared region. Nanoscale. 6(10):5467–5473. doi: 10.1039/c4nr00282b. [DOI] [PubMed] [Google Scholar]
- Guo N, Zhang B, Hu H, Ye S, Chen F, Li Z, Chen P, Wang C, He Q.. 2018. Caerin1.1 Suppresses the growth of porcine epidemic diarrhea virus in vitro via direct binding to the virus. Viruses. 10(9):507. doi: 10.3390/v10090507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck M, Tokhtaeva E, Nadav Y, Ben Zeev E, Ferris SP, Kaufman RJ, Bab-Dinitz E, Kaplan JH, Dada LA, Farfel Z, et al. 2016. Selective assembly of Na,K-ATPase α2β2 heterodimers in the heart. J Biol Chem. 291(44):23159–23174. doi: 10.1074/jbc.M116.751735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney EF, Hunter HN, Matsuzaki K, Vogel HJ.. 2009. Solution NMR studies of amphibian antimicrobial peptides: linking structure to function? Biochim Biophys Acta. 1788(8):1639–1655. doi: 10.1016/j.bbamem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- He H, Fan X, Shen H, Gou H, Zhang C, Liu Z, Zhang B, Wuri N, Zhang J, Liao M, et al. 2023. Butyrate limits the replication of porcine epidemic diarrhea virus in intestine epithelial cells by enhancing GPR43-mediated IFN-III production. Front Microbiol. 14:1091807. doi: 10.3389/fmicb.2023.1091807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Su D, Liu Q, Gao W, Kang Y.. 2017. Mushroom lectin overcomes hepatitis B virus tolerance via TLR6 signaling. Sci Rep. 7(1):5814. doi: 10.1038/s41598-017-06261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C,, Zhang R,, Yang L,, Xiang Bin. 2024. Andrographolide inhibits porcine epidemic diarrhea virus by inhibiting the JAK2-STAT3 pathway and promoting apoptosis. Veterinary Microbiology. 298:110235 10.1016/j.vetmic.2024.110235. [DOI] [PubMed] [Google Scholar]
- Hölzl MA, Hofer J, Steinberger P, Pfistershammer K, Zlabinger GJ.. 2008. Host antimicrobial proteins as endogenous immunomodulators. Immunol Lett. 119(1-2):4–11. doi: 10.1016/j.imlet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Hoter A, El-Sabban ME, Naim HY.. 2018. The HSP90 family: structure, regulation, function, and implications in health and disease. Int J Mol Sci. 19(9):2560. doi: 10.3390/ijms19092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li Y, Zhu H, Wang D, Zhou J, Ni Y, Guo R, Fan B, Li B.. 2024. In vitro suppression of porcine epidemic diarrhea virus by Panax notoginseng saponins: assessing antiviral potential. Arch Virol. 169(5):89. doi: 10.1007/s00705-024-06020-8. [DOI] [PubMed] [Google Scholar]
- Huan C, Xu W, Ni B, Guo T, Pan H, Jiang L, Li L, Yao J, Gao S.. 2021. Epigallocatechin-3-gallate, the main polyphenol in green tea, inhibits porcine epidemic diarrhea virus in vitro. Front Pharmacol. 12:628526. doi: 10.3389/fphar.2021.628526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan CC, Wang HX, Sheng XX, Wang R, Wang X, Mao X.. 2017. Glycyrrhizin inhibits porcine epidemic diarrhea virus infection and attenuates the proinflammatory responses by inhibition of high mobility group box-1 protein. Arch Virol. 162(6):1467–1476. doi: 10.1007/s00705-017-3259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Wang J, Wang Y, Zhang E, Li Y, Yu Q, Yang Q.. 2019. Upregulation of CD4(+)CD8(+) memory cells in the piglet intestine following oral administration of Bacillus subtilis spores combined with PEDV whole inactivated virus. Vet Microbiol. 235:1–9. doi: 10.1016/j.vetmic.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Huang S, Yu Q, Xie L, Ran L, Wang K, Yang Y, Gan L, Song Z.. 2021. Inhibitory effects of Lactobacillus plantarum metabolites on porcine epidemic diarrhea virus replication. Res Vet Sci. 139:32–42. doi: 10.1016/j.rvsc.2021.07.002. [DOI] [PubMed] [Google Scholar]
- Huang XQ, Lu ZX, Zhao HZ, Bie XM, Lu FX, Yang SJ.. 2006. Antiviral activity of antimicrobial lipopeptide from Bacillus subtilis fmbj against Pseudorabies Virus, Porcine Parvovirus, Newcastle Disease Virus and Infectious Bursal Disease Virus in vitro. Int J Pept Res Ther. 12(4):373–377. doi: 10.1007/s10989-006-9041-4. [DOI] [Google Scholar]
- Huang Z, Zhang W, Su L, Ma G, Guo J, Zhao Y, Huang W, Zhang W, El-Ashram S, Li Z.. 2023. Isolation of Limosilactobacillus reuteri strain with anti-porcine epidemic diarrhea virus from swine feces. Probiotics Antimicrob Proteins. 17. doi: 10.1007/s12602-023-10138-7. [DOI] [PubMed] [Google Scholar]
- Huang ZX, Zhou ST, Yang ZB, Wang Z.. 2013. Molnupiravir inhibits porcine epidemic diarrhea virus infection in vitro. Viruses. 15(6):1317. doi: 10.3390/v15061317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JH, Lee C.. 2018. Cholesterol is important for the entry process of porcine deltacoronavirus. Arch Virol. 163(11):3119–3124. doi: 10.1007/s00705-018-3967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Z, Zhang S, Liao G, Niu Z, Liu X, Sun Z, Hu X, Zhang Y, Xu S, Zhang J, et al. 2023. Mechanism of Lactiplantibacillus plantarum regulating Ca(2+) affecting the replication of PEDV in small intestinal epithelial cells. Front Microbiol. 14:1251275. doi: 10.3389/fmicb.2023.1251275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke W, Wu X, Fang P, Zhou Y, Fang L, Xiao S.. 2021. Cholesterol 25-hydroxylase suppresses porcine deltacoronavirus infection by inhibiting viral entry. Virus Res. 295:198306. doi: 10.1016/j.virusres.2021.198306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HB, Lee CY, Kim SJ, Han JH, Choi KH.. 2015. Medicinal herb extracts ameliorate impaired growth performance and intestinal lesion of newborn piglets challenged with the virulent porcine epidemic diarrhea virus. J Anim Sci Technol. 57(1):33. doi: 10.1186/s40781-015-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ha TK, Oh WK, Shin J, Oh DC.. 2016a. Antiviral indolosesquiterpenoid Xiamycins C-E from a halophilic Actinomycete. J Nat Prod. 79(1):51–58. doi: 10.1021/acs.jnatprod.5b00634. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lee C.. 2013. Ribavirin efficiently suppresses porcine nidovirus replication. Virus Res. 171(1):44–53. doi: 10.1016/j.virusres.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Liu H, Galasiti Kankanamalage AC, Weerasekara S, Hua DH, Groutas WC, Chang KO, Pedersen NC.. 2016b. Reversal of the progression of fatal coronavirus infection in cats by a broad-spectrum coronavirus protease inhibitor. PLoS Pathog. 12(3):e1005531. doi: 10.1371/journal.ppat.1005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S, Gu MJ, Kim CG, Kye YC, Lim Y, Lee JE, Park BC, Chu H, Han SH, Yun CH.. 2017. Rapamycin-induced autophagy restricts porcine epidemic diarrhea virus infectivity in porcine intestinal epithelial cells. Antiviral Res. 146:86–95. doi: 10.1016/j.antiviral.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Niu X, Liu M, Wang Q.. 2021. Bile acids LCA and CDCA inhibited porcine deltacoronavirus replication in vitro. Vet Microbiol. 257:109097. doi: 10.1016/j.vetmic.2021.109097. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Ryu YB, Kim YM, Song N, Kim CY, Rho MC, Jeong JH, Cho KO, Lee WS, Park SJ.. 2013. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorg Med Chem. 21(15):4706–4713. doi: 10.1016/j.bmc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Park JS, Lee SW, Hwang SY, Young BE, Choi HJ.. 2015. Porcine epidemic diarrhea virus infection: inhibition by polysaccharide from Ginkgo biloba exocarp and mode of its action. Virus Res. 195:148–152. doi: 10.1016/j.virusres.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Leng L, Xu Z, Hong B, Zhao B, Tian Y, Wang C, Yang L, Zou Z, Li L, Liu K, et al. 2024. Cepharanthine analogs mining and genomes of Stephania accelerate anti-coronavirus drug discovery. Nat Commun. 15(1):1537. doi: 10.1038/s41467-024-45690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chu H, Liu X, Chiu MC, Zhao X, Wang D, Wei Y, Hou Y, Shuai H, Cai J, et al. 2020a. Human coronavirus dependency on host heat shock protein 90 reveals an antiviral target. Emerg Microbes Infect. 9(1):2663–2672. doi: 10.1080/22221751.2020.1850183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CC, Wang XJ.. 2020. Three kinds of treatment with Homoharringtonine, Hydroxychloroquine or shRNA and their combination against coronavirus PEDV in vitro. Virol J. 17(1):71. doi: 10.1186/s12985-020-01342-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wu M, Li Z, Zhang Q, Zhang X, Zhang Y, Zhao D, Wang L, Hou Y, Wu T.. 2024a. Effect of supplementation with yeast polysaccharides on intestinal function in piglets infected with porcine epidemic diarrhea virus. Front Microbiol. 15:1378070. doi: 10.3389/fmicb.2024.1378070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Gao DS, Li YT, Wang YS, Liu HY, Zhao J.. 2018. Antiviral effect of lithium chloride on porcine epidemic diarrhea virus in vitro. Res Vet Sci. 118:288–294. doi: 10.1016/j.rvsc.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Li H, Bi Z, Song D, Zhang F, Lei D, Luo S, Li Z, Gong W, Huang D, et al. 2019a. Significant inhibition of re-emerged and emerging swine enteric coronavirus in vitro using the multiple shRNA expression vector. Antiviral Res. 166:11–18. doi: 10.1016/j.antiviral.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li H, Qiu Y, Li J, Zhou Y, Lv M, Xiang H, Bo Z, Shen H, Sun P.. 2024b. PA-824 inhibits porcine epidemic diarrhea virus infection in vivo and in vitro by inhibiting p53 activation. J Virol. 98(7):e0041323. doi: 10.1128/jvi.00413-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yu X, Zhang H, Cheng H, Hou L, Zheng Q, Hou J.. 2019b. In vitro antiviral activity of Griffithsin against porcine epidemic diarrhea virus. Virus Genes. 55(2):174–181. doi: 10.1007/s11262-019-01633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang L, Pan L, Zhou P, Yu R, Zhang Z, Lv J, Guo H, Wang Y, Xiao S, et al. 2023a. Nicotinamide efficiently suppresses porcine epidemic diarrhea virus and porcine deltacoronavirus replication. Viruses. 15(7):1591. doi: 10.3390/v15071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang M, Zheng H, Zhou P, Liu Z, Jongkaewwattana A, Luo R, He Q.. 2021. Construction of a recombinant porcine epidemic diarrhea virus encoding nanoluciferase for high-throughput screening of natural antiviral products. Viruses. 13(9):1866. doi: 10.3390/v13091866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sun J, Prinz RA, Liu X, Xu X.. 2020b. Inhibition of porcine epidemic diarrhea virus (PEDV) replication by A77 1726 through targeting JAK and Src tyrosine kinases. Virology. 551:75–83. doi: 10.1016/j.virol.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bao Y, Li Y, Duan X, Dong S, Lin J, Chang X, Tan Y, Zhang H, Shan H.. 2023b. RSL3 inhibits porcine epidemic diarrhea virus replication by activating ferroptosis. Viruses. 15(10):2080. doi: 10.3390/v15102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang J, Liu Y, Luo X, Lei W, Xie L.. 2020c. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J Gen Virol. 101(10):1079–1084. doi: 10.1099/jgv.0.001466. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu H, Chen L, Tan L.. 2016. A simple and sensitive UHPLC-MS/MS method for quantification of buddlejasaponin IV in rat plasma and its application to a pharmacokinetic study. J Pharm Biomed Anal. 120:374–382. doi: 10.1016/j.jpba.2015.12.044. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhu Y, Wang Y, Feng Y, Li D, Li S, Qin P, Yang X, Chen L, Zhao J, et al. 2023c. Characterization of RNA G-quadruplexes in porcine epidemic diarrhea virus genome and the antiviral activity of G-quadruplex ligands. Int J Biol Macromol. 231:123282. doi: 10.1016/j.ijbiomac.2023.123282. [DOI] [PubMed] [Google Scholar]
- Li Z, Cao H, Cheng Y, Zhang X, Zeng W, Sun Y, Chen S, He Q, Han H.. 2020d. Inhibition of porcine epidemic diarrhea virus replication and viral 3C-like protease by quercetin. Int J Mol Sci. 21(21):8095. doi: 10.3390/ijms21218095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhu L, Wang L, Huang Y, Zhang Y, Zhao D, Wang L, Yi D, Hou Y, Wu T.. 2024c. Identification of two flavonoids antiviral inhibitors targeting 3C-like protease of porcine epidemic diarrhea virus. Front Microbiol. 15:1357470. doi: 10.3389/fmicb.2024.1357470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Xu W, Gou F, Qin L, Yang H, Xiao J, Li L, Zhang W, Peng D.. 2024. Antiviral activity of flavonol against porcine epidemic diarrhea virus. Virology. 597:110128. doi: 10.1016/j.virol.2024.110128. [DOI] [PubMed] [Google Scholar]
- Liang W, He L, Ning P, Lin J, Li H, Lin Z, Kang K, Zhang Y.. 2015. (+)-Catechin inhibition of transmissible gastroenteritis coronavirus in swine testicular cells is involved its antioxidation. Res Vet Sci. 103:28–33. doi: 10.1016/j.rvsc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zhang X, Lian K, Tian X, Zhang M, Wang S, Chen C, Nie C, Pan Y, Han F, et al. 2020. Antiviral effects of Bovine antimicrobial peptide against TGEV in vivo and in vitro. J Vet Sci. 21(5):e80. doi: 10.4142/jvs.2020.21.e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zang R, Ma Y, Wang Z, Li L, Ding S, Zhang R, Wei Z, Yang J, Wang X.. 2021. Xanthohumol is a potent pan-inhibitor of coronaviruses targeting main protease. Int J Mol Sci. 22(22):12134. doi: 10.3390/ijms222212134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wei Z, Shi C, Xia L, Zhang Y, Wang J, Wang M, Yin S, Ren X.. 2024a. Antiviral effect of baicalein on Porcine Deltacoronavirus infection by regulating the inflammatory responses through PI3K-Akt-NF-kappaB signaling pathway in cultured cells. Biosci Rep. 7:BSR20231930. doi: 10.1042/BSR20231930. [DOI] [PubMed] [Google Scholar]
- Liu P, Zhang Q, Yang C, Wang X, Li Y, Li J, Yang Q.. 2023. Feeding with 4,4’-diaponeurosporene-producing Bacillus subtilis enhances the lactogenic immunity of sow. BMC Vet Res. 19(1):280. doi: 10.1186/s12917-023-03846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wang HY.. 2021. Porcine enteric coronaviruses: an updated overview of the pathogenesis, prevalence, and diagnosis. Vet Res Commun. 45(2-3):75–86. doi: 10.1007/s11259-021-09808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Tang R, Li Y, Zhong Q, Cao Y, Yang Q.. 2024b. A novel function of benzoic acid to enhance intestinal barrier defense against PEDV infection in Piglets. Vet Microbiol. 295:110152. doi: 10.1016/j.vetmic.2024.110152. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang X, Wang J, Zhang J, Duan C, Wang J.. 2022. Ergosterol peroxide inhibits porcine epidemic diarrhea virus infection in vero cells by suppressing ROS generation and p53 activation. Viruses. 14(2):402. doi: 10.3390/v14020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhao L, Xie Y, Chen Z, Yang S, Yin B, Li G, Guo H, Lin S, Wu J.. 2021. Antiviral activity of portulaca oleracea L. extracts against porcine epidemic diarrhea virus by partial suppression on myd88/NF-kappab activation in vitro. Microb Pathog. 154:104832. doi: 10.1016/j.micpath.2021.104832. [DOI] [PubMed] [Google Scholar]
- Luo L, Gu Z, Pu J, Chen D, Tian G, He J, Zheng P, Mao X, Yu B.. 2024. Synbiotics improve growth performance and nutrient digestibility, inhibit PEDV infection, and prevent intestinal barrier dysfunction by mediating innate antivirus immune response in weaned piglets. J Anim Sci. 102:skae023. doi: 10.1093/jas/skae023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Zhang C, Chen Y, Chen H, Yang Y.. 2022. Alpiniae oxyphyllae fructus polysaccharide 3 inhibits porcine epidemic diarrhea virus entry into IPEC-J2 cells. Res Vet Sci. 152:434–441. doi: 10.1016/j.rvsc.2022.09.011. [DOI] [PubMed] [Google Scholar]
- Luo SX, Fan JH, Opriessnig T, Di JM, Liu BJ, Zuo YZ.. 2017. Development and application of a recombinant M protein-based indirect ELISA for the detection of porcine deltacoronavirus IgG antibodies. J Virol Methods. 249:76–78. doi: 10.1016/j.jviromet.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Tan CW, Xie SZ, Chen Y, Yao YL, Zhao K, Zhu Y, Wang Q, Liu MQ, Yang XL, et al. 2021. Identification of ZDHHC17 as a potential drug target for swine acute diarrhea syndrome coronavirus infection. Mbio. 12(5):e0234221. doi: 10.1128/mBio.02342-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X, Wang P, Bai R, Cong Y, Suo S, Ren X, Chen C.. 2014. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials. 35(13):4195–4203. doi: 10.1016/j.biomaterials.2014.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhang Y, Liang X, Oglesbee M, Krakowka S, Niehaus A, Wang G, Jia A, Song H, Li J.. 2016. Two-way antigenic cross-reactivity between porcine epidemic diarrhea virus and porcine deltacoronavirus. Vet Microbiol. 186:90–96. doi: 10.1016/j.vetmic.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Huang X, Shan Y, Xu J, Gao Q, Xu X, Zhang C, Shi F, Yue M, He F, et al. 2022. Transcriptome analysis revealed inhibition of lipid metabolism in 2-D porcine enteroids by infection with porcine epidemic diarrhea virus. Vet Microbiol. 273:109525. doi: 10.1016/j.vetmic.2022.109525. [DOI] [PubMed] [Google Scholar]
- Mao M, Zhang W, Huang Z, Huang J, Wang J, Li W, Gu S.. 2021. Graphene oxide-copper nanocomposites suppress cariogenic streptococcus mutans biofilm formation. int J Nanomedicine. 16:7727–7739. doi: 10.2147/IJN.S303521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcbride CE, Machamer CE.. 2010. Palmitoylation of SARS-CoV S protein is necessary for partitioning into detergent-resistant membranes and cell-cell fusion but not interaction with M protein. Virology. 405(1):139–148. doi: 10.1016/j.virol.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K.. 2014. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nami B, Maadi H, Wang Z.. 2019. The effects of pertuzumab and its combination with trastuzumab on HER2 homodimerization and phosphorylation. Cancers (Basel). 11(3):375. doi: 10.3390/cancers.11030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narusaka M, Hatanaka T, Narusaka Y.. 2021. Inactivation of plant and animal viruses by proanthocyanidins from Alpinia zerumbet extract. Plant Biotechnol (Tokyo). 38(4):453–455. doi: 10.5511/plantbiotechnology.21.0925a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Zhou Y, Duan X, Cui J, Liu J, Song X, Ma W, Zhang W, Liu Y, Fan Y.. 2021. The inhibitory effect Polygonum Cillinerve polysaccharide on transmissible gastroenteritis virus of swine. Res Vet Sci. 140:47–55. doi: 10.1016/j.rvsc.2021.08.005. [DOI] [PubMed] [Google Scholar]
- Paudel KR, Karki R, Kim DW.. 2016. Cepharanthine inhibits in vitro VSMC proliferation and migration and vascular inflammatory responses mediated by RAW264.7. Toxicol In Vitro. 34:16–25. doi: 10.1016/j.tiv.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Peng JY, Horng YB, Wu CH, Chang CY, Chang YC, Tsai PS, Jeng CR, Cheng YH, Chang HW.. 2019. Evaluation of antiviral activity of Bacillus licheniformis-fermented products against porcine epidemic diarrhea virus. AMB Express. 9(1):191. doi: 10.1186/s13568-019-0916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado MR, Blandón LM, Vandenberghe LPS, Rodrigues C, Castro GR, Thomaz-Soccol V, Soccol CR., 2015. Milk kefir: composition, microbial cultures, biological activities, and related products. Front Microbiol. 6:1177. doi: 10.3389/fmicb.2015.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu J, Chen D, Tian G, He J, Huang Z, Zheng P, Mao X, Yu J, Luo J, Luo Y, et al. 2022a. All-trans retinoic acid attenuates transmissible gastroenteritis virus-induced apoptosis in IPEC-J2 cells via inhibiting ROS-mediated P(38)MAPK signaling pathway. Antioxidants (Basel). 11(2):345. doi: 10.3390/antiox11020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu J, Chen D, Tian G, He J, Huang Z, Zheng P, Mao X, Yu J, Luo J, Luo Y, et al. 2022b. All-trans retinoic acid attenuates transmissible gastroenteritis virus-induced inflammation in IPEC-J2 cells via suppressing the RLRs/NF-kappaB signaling pathway. Front Immunol. 13:734171. doi: 10.3389/fimmu.2022.734171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian B, Hu Y, Liu C, Zheng D, Han X, Gong M, Zou Y, Zeng D, Liao K, Miao Y, et al. 2024. Tetrandrine (TET) inhibits African swine fever virus entry into cells by blocking the PI3K/Akt pathway. Virus Res. 339:199258. doi: 10.1016/j.virusres.2023.199258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao WT, Yao X, Lu WH, Zhang YQ, Malhi KK, Li HX, Li JL.. 2024. Matrine exhibits antiviral activities against PEDV by directly targeting Spike protein of the virus and inducing apoptosis via the MAPK signaling pathway. Int J Biol Macromol. 270(Pt 2):132408. doi: 10.1016/j.ijbiomac.2024.132408. [DOI] [PubMed] [Google Scholar]
- Qin G, Zhao C, Liu Y, Zhang C, Yang G, Yang J, Wang Z, Wang C, Tu C, Guo Z, et al. 2022. RNA G-quadruplex formed in SARS-CoV-2 used for COVID-19 treatment in animal models. Cell Discov. 8(1):86. doi: 10.1038/s41421-022-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya S, Shanmugasundaram T, Balagurunathan R.. 2015. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J Trace Elem Med Biol. 32:30–39. doi: 10.1016/j.jtemb.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Rao H, Su W, Zhang X, Wang Y, Li T, Li J, Zeng X, Li P.. 2023. Hypericum japonicum extract inhibited porcine epidemic diarrhea virus in vitro and in vivo. Front Pharmacol. 14:1112610. doi: 10.3389/fphar.2023.1112610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Zeng W, Jiang C, Li C, Zhang C, Cao H, Li W, He Q.. 2022a. Inhibition of porcine epidemic diarrhea virus by cinchonine via inducing cellular autophagy. Front Cell Infect Microbiol. 12:856711. doi: 10.3389/fcimb.2022.856711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Meng F, Yin J, Li G, Li X, Wang C, Herrler G.. 2011. Action mechanisms of lithium chloride on cell infection by transmissible gastroenteritis coronavirus. PLoS One. 6(5):e18669. doi: 10.1371/journal.pone.0018669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Ding T, He H, Wei Z, Shi R, Deng J.. 2022b. Mechanism of selenomethionine inhibiting of PDCoV replication in LLC-PK1 cells based on STAT3/miR-125b-5p-1/HK2 signaling. Front Immunol. 13:952852. doi: 10.3389/fimmu.2022.952852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Jia G, He H, Ding T, Yu Y, Zuo Z, Hu Y, Zhong Z, Yu S, Deng H, et al. 2022c. Antiviral effect of selenomethionine on porcine deltacoronavirus in pig kidney epithelial cells. Front Microbiol. 13:846747. doi: 10.3389/fmicb.2022.846747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Yu Y, Zhang X, Wang Q, Deng J, Chen C, Shi R, Wei Z, Hu H.. 2022d. Exploration of PDCoV-induced apoptosis through mitochondrial dynamics imbalance and the antagonistic effect of SeNPs. Front Immunol. 13:972499. doi: 10.3389/fimmu.2022.972499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Lara MJ, Gomez-Llorente C, Plaza-Diaz J, Gil A.. 2015. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. Biomed Res Int. 2015:505878–505815. doi: 10.1155/2015/505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Kolokoltsov AA, Chen CC, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, Davey RA.. 2015. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 347(6225):995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q, Ma F, Wei J, Li H, Ma H, Sun P.. 2020. Physiological functions of heat shock proteins. Curr Protein Pept Sci. 21(8):751–760. doi: 10.2174/1389203720666191111113726. [DOI] [PubMed] [Google Scholar]
- Shi C, Liang W, Guo M, Yuan J, Zu S, Hu H.. 2024. Chlorogenic acid inhibits porcine deltacoronavirus release by targeting apoptosis. Int Immunopharmacol. 127:111359. doi: 10.1016/j.intim. p.2023.111359. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lei Y, Ye G, Sun L, Fang L, Xiao S, Fu ZF, Yin P, Song Y, Peng G.. 2018. Identification of two antiviral inhibitors targeting 3C-like serine/3C-like protease of porcine reproductive and respiratory syndrome virus and porcine epidemic diarrhea virus. Vet Microbiol. 213:114–122. doi: 10.1016/j.vetmic.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichokchatchawan W, Temeeyasen G, Nilubol D, Prapasarakul N.. 2018. Protective effects of cell-free supernatant and live lactic acid bacteria isolated from thai pigs against a pandemic strain of porcine epidemic diarrhea virus. Probiotics Antimicrob Proteins. 10(2):383–390. doi: 10.1007/s12602-017-9281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondi I, Salopek-Sondi B.. 2004. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Song JH, Shim JK, Choi HJ.. 2011. Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virol J. 8(1):460. doi: 10.1186/1743-422X-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Deng C, Chen Q, Zhao S, Li P, Wu T, Hou Y, Yi D.. 2024. Protective effects and mechanisms of ellagic acid on intestinal injury in piglets infected with porcine epidemic diarrhea virus. Front Immunol. 15:1323866. doi: 10.3389/fimmu.2024.1323866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Shi D, Xing X, Qi S, Yang D, Zhang J, Han Y, Zhu Q, Sun H, Wang X, et al. 2021. Coronavirus porcine epidemic diarrhea virus nucleocapsid protein interacts with p53 to induce cell cycle arrest in S-phase and promotes viral replication. J Virol. 95(16):e0018721. doi: 10.1128/JVI.00187-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Yin B, Xing X, Li Z, Zhang J, Feng S, Li L, Zhao F, Yang X, Yu S, et al. 2023. Octyl gallate targeting the 3C-like protease exhibits highly efficient antiviral activity against swine enteric coronavirus PEDV. Vet Microbiol. 281:109743. doi: 10.1016/j.vetmic.2023.109743. [DOI] [PubMed] [Google Scholar]
- Su YF, Hou YX, Wang QH.. 2019. The enhanced replication of an S-intact PEDV during coinfection with an S1 NTD-del PEDV in piglets. Vet Microbiol. 228:202–212. doi: 10.1016/j.vetmic.2018.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Zhang Z, Xing J, Ma S, Ge Y, Xia L, Diao X, Li Y, Wei Z, Wang Z.. 2024a. Synthesis and pharmacodynamic evaluation of Dihydropteridone derivatives against PDCoV in vivo and in vitro. Bioorg Chem. 146:107322. doi: 10.1016/j.bioorg.2024.107322. [DOI] [PubMed] [Google Scholar]
- Sun MJ, Xing JH, Yan QS, Zou BS, Wang YJ, Niu TM, Yu T, Huang HB, Zhang D, Zhang SM, et al. 2024b. The acetic acid produced by Lactobacillus species regulates immune function to alleviate PEDV infection in piglets. Probiotics Antimicrob Proteins. doi: 10.1007/s12602-024-10243-1. [DOI] [PubMed] [Google Scholar]
- Sun P, Wang M, Li J, Qiu Y, Li H, Lv M, Bo Z, Shen H, Li L.. 2022. Inhibitory effect of Buddlejasaponin IVb on porcine epidemic diarrhea virus in vivo and in vitro. Vet Microbiol. 272:109516. doi: 10.1016/j.vetmic.2022.109516. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu Z, Shen S, Zhang M, Liu L, Ghonaim AH, Li Y, Zhang S, Li W.. 2024c. Inhibition of porcine deltacoronavirus entry and replication by Cepharanthine. Virus Res. 340:199303. doi: 10.1016/j.virusres.2023.199303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo X, Wang J, Wang D, Fan G, Zhu M, Fan B, Yang X, Li B.. 2023. DHA and EPA inhibit porcine coronavirus replication by alleviating ER stress. J Virol. 97(11):e0120923. doi: 10.1128/jvi.01209-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Cui J, Liu N, Wang X, Li H, Liu Y, Zhang W, Ma W, Lu D, Fan Y.. 2024. Study on the immune-enhancing and inhabiting transmissible gastroenteritis virus effects of polysaccharides from Cimicifuga rhizoma. Microb Pathog. 192:106719. doi: 10.1016/j.micpath.2024.106719. [DOI] [PubMed] [Google Scholar]
- Tang R, Guo L, Fan Q, Zhang L, Wang Y, Zhang X, Shi D, Wu Y, Shi H, Liu J, et al. 2022. Porcine deltacoronavirus infection is inhibited by Griffithsin in cell culture. Vet Microbiol. 264:109299. doi: 10.1016/j.vetmic.2021.109299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong T, Hu H, Zhou J, Deng S, Zhang X, Tang W, Fang L, Xiao S, Liang J.. 2020. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small. 16(13):e1906206. doi: 10.1002/smll.201906206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh TBN, Le DH, Nguyen TTK, Nguyen VT, Nguyen MH, Muller M, Pham HT, Le VP, Nguyen TKN.. 2021. In vitro antiviral activities of ethanol and aqueous extracts of Vietnamese traditional medicinal plants against Porcine Epidemic Diarrhea virus: a coronavirus family member. Virusdisease. 32(4):797–803. doi: 10.1007/s13337-021-00709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlewicz-Podbielska H, Pomorska-Mól M.. 2021. Porcine coronaviruses: overview of the state of the art. Virol Sin. 36(5):833–851., doi: 10.1007/s12250-021-00364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancompernolle S, Smith PB, Bowie JH, Tyler MJ, Unutmaz D, Rollins-Smith LA.. 2015. Inhibition of HIV infection by caerin 1 antimicrobial peptides. Peptides. 71:296–303. doi: 10.1016/j.peptides.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Varshney D, Spiegel J, Zyner K, Tannahill D, Balasubramanian S.. 2020. The regulation and functions of DNA and RNA G-quadruplexes. Nat Rev Mol Cell Biol. 21(8):459–474. doi: 10.1038/s41580-020-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PJ, Siddell SG, Lefkowitz EJ, Mushegian AR, Adriaenssens EM, Dempsey DM, Dutilh BE, Harrach B, Harrison RL, Hendrickson RC, et al. 2020. Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses. Arch Virol. 165(11):2737–2748. doi: 10.1007/s00705-020-04752-x. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang Q, Ji C, Hu Y, Yi D, Wu T, Wang L, Zhao D, Hou Y.. 2023a. Effects of monolaurin on intestinal barrier, blood biochemical profile, immunity and antioxidant function in porcine epidemic diarrhoea virus-infected piglets. Br J Nutr. 131(2):185–192. doi: 10.1017/S0007114523001721. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen C, Wang Z, Han X, Shi P, Zhou K, Liu X, Xiao Y, Cai Y, Huang J, et al. 2022a. The structure of the porcine deltacoronavirus main protease reveals a conserved target for the design of antivirals. Viruses. 14(3):486. doi: 10.3390/v14030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen C, Yang K, Xu Y, Liu X, Gao F, Liu H, Chen X, Zhao Q, Liu X, et al. 2017a. Michael acceptor-based peptidomimetic inhibitor of main protease from porcine epidemic diarrhea virus. J Med Chem. 60(7):3212–3216. doi: 10.1021/acs.jmedchem.7b00103. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu H, Yang Y, Tan Y, Sun L, Guo Z, Zeng X, Wang Z, Li S, Yin L, et al. 2023b. Genome-scale CRISPR screen identifies TRIM2 and SLC35A1 associated with porcine epidemic diarrhoea virus infection. Int J Biol Macromol. 250:125962. doi: 10.1016/j.ijbiomac.2023.125962. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun H, Su M, Li Z, Li L, Zhao F, Zhang Y, Bai W, Yu S, Yang X, et al. 2024a. Natural hyperoside extracted from hawthorn exhibits antiviral activity against porcine epidemic diarrhea virus in vitro and in vivo. Virology. 594:110037. doi: 10.1016/j.virol.2024.110037. [DOI] [PubMed] [Google Scholar]
- Wang J, Zeng X, Gou J, Zhu X, Yin D, Yin L, Shen X, Dai Y, Pan X.. 2024b. Antiviral activity of luteolin against porcine epidemic diarrhea virus in silico and in vitro. BMC Vet Res. 20(1):288. doi: 10.1186/s12917-024-04053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zeng X, Yin D, Yin L, Shen X, Xu F, Dai Y, Pan X.. 2023d. In silico and in vitro evaluation of antiviral activity of wogonin against main protease of porcine epidemic diarrhea virus. Front Cell Infect Microbiol. 13:1123650. doi: 10.3389/fcimb.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Chen D, Yu B, He J, Mao X, Huang Z, Yan H, Wu A, Luo Y, Zheng P, et al. 2023d. Eugenol alleviates TGEV-induced intestinal injury via suppressing ROS/NLRP3/GSDMD-dependent pyroptosis. J Agric Food Chem. 71(3):1477–1487. doi: 10.1021/acs.jafc.2c05833. [DOI] [PubMed] [Google Scholar]
- Wang K, Ran L, Yan T, Niu Z, Kan Z, Zhang Y, Yang Y, Xie L, Huang S, Yu Q, et al. 2019a. Anti-TGEV miller strain infection effect of Lactobacillus plantarum supernatant based on the JAK-STAT1 signaling pathway. Front Microbiol. 10:2540. doi: 10.3389/fmicb.2019.02540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Tang Y, Wu X, Liang H, Chen D, Yu B, He J, Mao X, Huang Z, Yan H, et al. 2022b. Eugenol attenuates transmissible gastroenteritis virus-induced oxidative stress and apoptosis via ROS-NRF2-ARE signaling. Antioxidants (Basel). 11(9):1838. doi: 10.3390/antiox11091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Bai J, Liu X, Wang M, Wang X, Jiang P.. 2020. Tomatidine inhibits porcine epidemic diarrhea virus replication by targeting 3CL protease. Vet Res. 51(1):136. doi: 10.1186/s13567-020-00865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wang X, Liu X, Sun M, Liang X, Bai J, Jiang P.. 2022c. Natural compound ZINC12899676 reduces porcine epidemic diarrhea virus replication by inhibiting the viral NTPase activity. Front Pharmacol. 13:879733. doi: 10.3389/fphar.2022.879733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Vlasova AN, Kenney SP, Saif LJ.. 2019b. Emerging and re-emerging coronaviruses in pigs. Curr Opin Virol. 34:39–49. doi: 10.1016/j.coviro.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wen Y, Qian B, Tang F, Zhang X, Xu X, Zhou Y, Dai J, Wang A, Xue F.. 2024c. Virological evaluation of natural and modified attapulgite against porcine epidemic diarrhoea virus. Virol J. 21(1):120. doi: 10.1186/s12985-024-02396-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zheng G, Chen Z, Wang Y, Zhao C, Li Y, Yuan Y, Duan H, Zhu H, Yang X, et al. 2024d. Drug repurposing screens identify Tubercidin as a potent antiviral agent against porcine nidovirus infections. Virus Res. 339:199275. doi: 10.1016/j.virusres.2023.199275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li W, Wen Z, Wang C, Liu W, Zhang Y, Liu J, Ding T, Shuai L, Zhong G, et al. 2022d. Gossypol broadly inhibits coronaviruses by targeting RNA-dependent RNA polymerases. Adv Sci (Weinh). 9(35):e2203499. doi: 10.1002/advs.202203499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen B, Yu R, Si F, Xie C, Li Z, Dong S, Zhang D.. 2023e. Magnolol, a Neolignan-like drug, inhibits porcine epidemic diarrhea virus replication in cultured cells. Pathogens. 12(2):263. doi: 10.3390/pathogens12020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu W, Zhu L, Yang Q.. 2017b. Bacillus subtilis and surfactin inhibit the transmissible gastroenteritis virus from entering the intestinal epithelial cells. Biosci Rep. 37(2):BSR20170082. doi: 10.1042/BSR20170082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu Y, Li K, Yang M, Wang Q, Hao Z.. 2022e. Triacetyl resveratrol inhibits PEDV by inducing the early apoptosis in vitro. Int J Mol Sci. 23(23):14499. doi: 10.3390/ijms232314499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang X, Zhang J, Shan Q, Zhu Y, Xu C, Wang J.. 2023f. Prediction and verification of curcumin as a potential drug for inhibition of PDCoV replication in LLC-PK1 cells. Int J Mol Sci. 24(6):5870. doi: 10.3390/ijms24065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang H, Li D, Zhao C, Li S, Qin P, Li Y, Yang X, Du W, Li W, et al. 2023g. Identification of niclosamide as a novel antiviral agent against porcine epidemic diarrhea virus infection by targeting viral internalization. Virol Sin. 38(2):296–308. doi: 10.1016/j.virs.2023.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li JR, Sun MX, Ni B, Huan C, Huang L, Li C, Fan HJ, Ren XF, Mao X.. 2014. Triggering unfolded protein response by 2-Deoxy-D-glucose inhibits porcine epidemic diarrhea virus propagation. Antiviral Res. 106:33–41. doi: 10.1016/j.antiviral.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Qin P, Zhao C, Li Y, Li S, Fan F, Li D, Huang H, Duan H, Yang X, et al. 2023h. Evaluating anti-viral effect of Ivermectin on porcine epidemic diarrhea virus and analyzing the related genes and signaling pathway by RNA-seq in vitro. Virology. 587:109877. doi: 10.1016/j.virol.2023.109877. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang R, Li F, Wang Y, Zhang Z, Wang Q, Ren Z, Jin F, Kitazato K, Wang Y.. 2018. Heat-shock protein 90alpha is involved in maintaining the stability of VP16 and VP16-mediated transactivation of alpha genes from herpes simplex virus-1. Mol Med. 24(1):65. doi: 10.1186/s10020-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Alfajaro MM, Deweirdt PC, Hanna RE, Lu-Culligan WJ, Cai WL, Strine MS, Zhang SM, Graziano VR, Schmitz CO, et al. 2021. Genome-wide CRISPR screens reveal host factors critical for SARS-CoV-2 infection. Cell. 184(1):76–91 e13. doi: 10.1016/j.cell.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R, Li L, Chen H, Wang X, Chen Y, Liu X.. 2024. Inhibition of porcine reproductive and respiratory syndrome virus replication by rifampicin in vitro. Front Vet Sci. 11:1439015. doi: 10.3389/fvets.2024.1439015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Burwinkel M, Palissa C, Ephraim E, Schmidt MF.. 2012. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Vet Microbiol. 160(3-4):468–472. doi: 10.1016/j.vetmic.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Luo Q, Chen Y, Liao S, Chen H, Chen Y, Qin Y.. 2023. Inhibiting mechanism of Alpiniae oxyphyllae fructus polysaccharide 3 against the replication of porcine epidemic diarrhea virus. Virology. 587:109848. doi: 10.1016/j.virol.2023.109848. [DOI] [PubMed] [Google Scholar]
- Wu M, Yi D, Zhang Q, Wu T, Yu K, Peng M, Wang L, Zhao D, Hou Y, Wu G.. 2021. Puerarin enhances intestinal function in piglets infected with porcine epidemic diarrhea virus. Sci Rep. 11(1):6552. doi: 10.1038/s41598-021-85880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Zhang Q, Yi D, Wu T, Chen H, Guo S, Li S, Ji C, Wang L, Zhao D, et al. 2020. Quantitative proteomic analysis reveals antiviral and anti-inflammatory effects of puerarin in piglets infected with porcine epidemic diarrhea virus. Front Immunol. 11:169. doi: 10.3389/fimmu.2020.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H, Qiao J, Lin H, Li J, Li Y, Sun H, Wang X, Bi R, Zhang Z, Bo Z, et al. 2024. Berbamine inhibits porcine epidemic diarrhea virus in vitro and in vivo. Vet Microbiol. 298:110244. doi: 10.1016/j.vetmic.2024.110244. [DOI] [PubMed] [Google Scholar]
- Xie Y, Guo X, Hu T, Wei D, Ma X, Wu J, Huang B, Shen J.. 2021. Significant inhibition of porcine epidemic diarrhea virus in vitro by remdesivir, its parent nucleoside and beta-D-N(4)-hydroxycytidine. Virol Sin. 36(5):997–1005. doi: 10.1007/s12250-021-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing JH, Niu TM, Zou BS, Yang GL, Shi CW, Yan QS, Sun MJ, Yu T, Zhang SM, Feng XZ, et al. 2024. Gut microbiota-derived LCA mediates the protective effect of PEDV infection in piglets. Microbiome. 12(1):20. doi: 10.1186/s40168-023-01734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, Liu X, Liang T, Ban Y, Liu Y, Zhang L, Xu Z, Song C.. 2023. The alpha-1 subunit of the Na(+)/K(+)-ATPase (ATP1A1) is a host factor involved in the attachment of porcine epidemic diarrhea virus. Int J Mol Sci. 24(4):4000. doi: 10.3390/ijms24044000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Gao S, Zuo Q, Gong J, Song X, Liu Y, Xiao J, Zhai X, Sun H, Zhang M, et al. 2024a. Enhanced in vitro antiviral activity of ivermectin-loaded nanostructured lipid carriers against porcine epidemic diarrhea virus via improved intracellular delivery. Pharmaceutics. 16(5):601. doi: 10.3390/pharmaceutics16050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Liu Y, Peng P, Liu Y, Huang M, Ma Y, Xue C, Cao Y.. 2020. Aloe extract inhibits porcine epidemic diarrhea virus in vitro and in vivo. Vet Microbiol. 249:108849. doi: 10.1016/j.vetmic.2020.108849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Zhang Q, Wu M, Zhang Y, Li Z, Li H, Yu C, Zhang X, Zhao D, Wang L, et al. 2024b. Lactobacillus rhamnosus GG powder supplementation alleviates intestinal injury in piglets challenged by porcine epidemic diarrhea virus. Front Cell Infect Microbiol. 14:1371916. doi: 10.3389/fcimb.2024.1371916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, Chang HY, Hsu HY, Lee YZ, Chang HS, Chen IS, Lee SJ.. 2017. Identification of anti-viral activity of the cardenolides, Na(+)/K(+)-ATPase inhibitors, against porcine transmissible gastroenteritis virus. Toxicol Appl Pharmacol. 332:129–137. doi: 10.1016/j.taap.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, Hsu HY, Chang HY, Lee YZ, Lee SJ.. 2020a. Natural cardenolides suppress coronaviral replication by downregulating JAK1 via a Na(+)/K(+)-ATPase independent proteolysis. Biochem Pharmacol. 180:114122. doi: 10.1016/j.bcp.2020.114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, Lee YZ, Hsu HY, Zhao GH, Lee SJ.. 2022a. Tyrphostin AG1024 suppresses coronaviral replication by downregulating JAK1 via an IR/IGF-1R independent proteolysis mediated by Ndfip1/2_NEDD4-like E3 ligase itch. Pharmaceuticals (Basel). 15(2):241. doi: 10.3390/ph15020241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Dhodary B, Quy Ha TK, Kim J, Kim E, Oh WK.. 2015a. Three new coumarins from Saposhnikovia divaricata and their porcine epidemic diarrhea virus (PEDV) inhibitory activity. Tetrahedron. 71(28):4651–4658. doi: 10.1016/j.tet.2015.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Ha TK, Dhodary B, Pyo E, Nguyen NH, Cho H, Kim E, Oh WK.. 2015b. Oleanane triterpenes from the flowers of Camellia japonica inhibit porcine epidemic diarrhea virus (PEDV) replication. J Med Chem. 58(3):1268–1280. doi: 10.1021/jm501567f. [DOI] [PubMed] [Google Scholar]
- Yang QY, Yang YL, Tang YX, Qin P, Wang G, Xie JY, Chen SX, Ding C, Huang YW, Zhu SJ.. 2022b. Bile acids promote the caveolae-associated entry of swine acute diarrhea syndrome coronavirus in porcine intestinal enteroids. PLoS Pathog. 18(6):e1010620. doi: 10.1371/journal.ppat.1010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Li S, Lu Y, Jansen CA, Savelkoul HFJ, Liu G.. 2023a. Oral administration of Lactic acid bacteria inhibits PEDV infection in young piglets. Virology. 579:1–8. doi: 10.1016/j.virol.2022.12.005. [DOI] [PubMed] [Google Scholar]
- Yang S, Li Y, Wang B, Yang N, Huang X, Chen Q, Geng S, Zhou Y, Shi H, Wang L, et al. 2020b. Acute porcine epidemic diarrhea virus infection reshapes the intestinal microbiota. Virology. 548:200–212. doi: 10.1016/j.virol.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang C, Huang X, Jansen CA, Savelkoul HFJ, Liu G.. 2023b. Linoleic acid stimulation results in TGF-beta1 production and inhibition of PEDV infection in vitro. Virology. 581:89–96. doi: 10.1016/j.virol.2023.03.004. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gao Y, Sun H, Bai J, Zhang J, Zhang L, Liu X, Sun Y, Jiang P.. 2024. Ursonic acid from medicinal herbs inhibits PRRSV replication through activation of the innate immune response by targeting the phosphatase PTPN1. Vet Res. 55(1):67. doi: 10.1186/s13567-024-01316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yu Q, Song H, Ran L, Wang K, Xie L, Huang S, Niu Z, Zhang Y, Kan Z, et al. 2020c. Decreased NHE3 activity and trafficking in TGEV-infected IPEC-J2 cells via the SGLT1-mediated P38 MAPK/AKt2 pathway. Virus Res. 280:197901. doi: 10.1016/j.virusres.2020.197901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G, Wang X, Tong X, Shi Y, Fu ZF, Peng G.. 2020. Structural basis for inhibiting porcine epidemic diarrhea virus replication with the 3C-like protease inhibitor GC376. Viruses. 12(2):240. doi: 10.3390/v12020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Shao K, Li Z, Guo N, Zuo Y, Li Q, Lu Z, Chen L, He Q, Han H.. 2015. Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl Mater Interfaces. 7(38):21571–21579. doi: 10.1021/acsami.5b06876. [DOI] [PubMed] [Google Scholar]
- Yu C, Wu M, Sun L, Li H, Xu Z, Zhang Q, Yi D, Wang L, Zhao D, Hou Y, et al. 2024. Effect of supplementation with black soldier fly extract on intestinal function in piglets infected with porcine epidemic diarrhea virus. Animals (Basel). 14(10):1512. doi: 10.3390/ani14101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Huang X, Zhai R, Ma Y, Xu A, Zhang P, Yang Q.. 2021. In vitro antiviral activities of salinomycin on porcine epidemic diarrhea virus. Viruses. 13(4):580. doi: 10.3390/v13040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Zhang S, Peng J, Li Y, Yang Q.. 2019. Synthetic surfactin analogues have improved anti-PEDV properties. PLoS One. 14(4):e0215227. doi: 10.1371/journal.pone.0215227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Zhang S, Wang Y, Li Y, Wang X, Yang Q.. 2018. Surfactin inhibits membrane fusion during invasion of epithelial cells by enveloped viruses. J Virol. 92(21):e00809-18. doi: 10.1128/JVI.00809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S, Li Y, Zhu W, Luo Z, Wu K, Li X, Fang Y, Qin Y, Chen W, Li Z, et al. 2022a. The advances of broad-spectrum and hot anti-coronavirus drugs. Microorganisms. 10(7):1294. doi: 10.3390/microorganisms10071294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Ren J, Li Z, Jiang C, Sun Q, Li C, Li W, Li W, He Q.. 2022b. Levistolide A inhibits PEDV replication via inducing ROS generation. Viruses. 14(2):258. doi: 10.3390/v14020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X, Wang N, Jiao H, Zhang J, Li C, Ren W, Reiter RJ, Su S.. 2021. Melatonin and other indoles show antiviral activities against swine coronaviruses in vitro at pharmacological concentrations. J Pineal Res. 71(2):e12754. doi: 10.1111/jpi.12754. [DOI] [PubMed] [Google Scholar]
- Zhai X, Wang S, Zhu M, He W, Pan Z, Su S.. 2019. Antiviral effect of lithium chloride and diammonium glycyrrhizinate on porcine deltacoronavirus in vitro. Pathogens. 8(3):144. doi: 10.3390/pathogens8030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BK, Guo YM.. 2009. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr. 102(5):687–693. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]
- Zhang C, Chen H, Sun L, Zhao P, Qi C, Yang Y, Si A, Qian Y, Jung YS.. 2023a. Bis-benzylisoquinoline alkaloids inhibit porcine epidemic diarrhea virus by disrupting virus entry. Pathogens. 12(6):845. doi: 10.3390/pathogens12060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li Y, Yang R, Xiao L, Dong S, Lin J, Liu G, Shan H.. 2023b. Erastin inhibits porcine epidemic diarrhea virus replication in Vero cells. Front Cell Infect Microbiol. 13:1142173. doi: 10.3389/fcimb.2023.1142173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang G, Wang X, Zhu Y, Wang J.. 2022a. 25-Hydroxycholesterol mediates cholesterol metabolism to restrict porcine deltacoronavirus infection via suppression of transforming growth factor beta1. Microbiol Spectr. 10(6):e0219822. doi: 10.1128/spectrum.02198-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Shi H, Feng S, Feng T, Chen J, Zhang X, Han Y, Liu J, Wang Y, et al. 2022b. Swine acute diarrhea syndrome coronavirus replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. Virology. 565:96–105. doi: 10.1016/j.virol.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhao L, Bai Y, Li S, Zhang M, Wei B, Wang X, Xue Y, Li L, Ma G, et al. 2024a. An ascidian Polycarpa aurata-derived pan-inhibitor against coronaviruses targeting Mpro. Bioorg Med Chem Lett. 103:129706. doi: 10.1016/j.bmcl.2024.129706. [DOI] [PubMed] [Google Scholar]
- Zhang K, Lin S, Li J, Deng S, Zhang J, Wang S.. 2022c. Modulation of innate antiviral immune response by porcine enteric coronavirus. Front Microbiol. 13:845137. doi: 10.3389/fmicb.2022.845137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Yi D, Ji C, Wu T, Wang M, Guo S, Wang L, Zhao D, Hou Y.. 2021a. Monolaurin confers a protective effect against porcine epidemic diarrhea virus infection in piglets by regulating the interferon pathway. Front Immunol. 12:797476. doi: 10.3389/fimmu.2021.797476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang J, Liu X, Kan Z, Zhang Y, Niu Z, Hu X, Zhang L, Zhang X, Song Z.. 2024b. Pemetrexed alleviates piglet diarrhea by blocking the interaction between porcine epidemic diarrhea virus nucleocapsid protein and Ezrin. J Virol. 98(1):e0162523. doi: 10.1128/jvi.01625-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Shen H, Wang M, Fan X, Wang S, Wuri N, Zhang B, He H, Zhang C, Liu Z, et al. 2023c. Fangchinoline inhibits the PEDV replication in intestinal epithelial cells via autophagic flux suppression. Front Microbiol. 14:1164851. doi: 10.3389/fmicb.2023.1164851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li P, Zheng Q, Hou J.. 2019. Lactobacillus acidophilus S-layer protein-mediated inhibition of PEDV-induced apoptosis of Vero cells. Vet Microbiol. 229:159–167. doi: 10.1016/j.vetmic.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen H, Zou M, Oerlemans R, Shao C, Ren Y, Zhang R, Huang X, Li G, Cong Y.. 2021b. Hypericin inhibit alpha-coronavirus replication by targeting 3CL protease. Viruses. 13(9):1825. doi: 10.3390/v13091825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Yang S, Yin B, Zhao Z, Huang Z, Wu J, Lin S, Wang X.. 2023d. Water extract of portulaca oleracea inhibits PEDV infection-induced pyrolysis by caspase-1/GSDMD. Curr Issues Mol Biol. 45(12):10211–10224. doi: 10.3390/cimb45120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xia L, Yuan Y, Li Q, Han L, Yang G, Hu H.. 2020. Rhodanine derivative LJ001 inhibits TGEV and PDCoV replication in vitro. Virus Res. 289:198167. doi: 10.1016/j.virusres.2020.198167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Qin G, Niu J, Wang Z, Wang C, Ren J, Qu X.. 2021. Targeting RNA G-quadruplex in SARS-CoV-2: a promising therapeutic target for COVID-19? Angew Chem Int Ed Engl. 60(1):432–438. doi: 10.1002/anie.202011419. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Qu S, Tang W, He T, Li P, Zheng X.. 2024. SP2509, a specific antagonist of LSD1, exhibits antiviral properties against porcine epidemic diarrhea virus. BMC Vet Res. 20(1):187. doi: 10.1186/s12917-024-04052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xiao D, Zhang L, Song D, Chen R, Li S, Liao Y, Wen Y, Liu W, Yu E, et al. 2022. HSP90 inhibitors 17-AAG and VER-82576 inhibit porcine deltacoronavirus replication in vitro. Vet Microbiol. 265:109316. doi: 10.1016/j.vetmic.2021.109316. [DOI] [PubMed] [Google Scholar]
- Zheng SM, Wang XW, Hu HQ, Xia YB, Diao XY, Qiu WJ, Xue CY, Cao YC, Xu ZC.. 2022. Emodin from aloe inhibits swine acute diarrhea syndrome coronavirus in cell culture. Front Vet Sci. 9:978453. doi: 10.3389/fvets.2022.978453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li C, Zhang R, Li Q, Sun Y, Feng Y, Lan T, Ma J.. 2023. Identification of a receptor tyrosine kinase inhibitor CP-724714 inhibits SADS-CoV related swine diarrhea coronaviruses infection in vitro. Virol Sin. 38(5):778–786. doi: 10.1016/j.virs.2023.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Han Y, Li M, Ye G, Peng G.. 2021. Two inhibitors against the 3C-like proteases of swine coronavirus and feline coronavirus. Virol Sin. 36(6):1421–1430. doi: 10.1007/s12250-021-00415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Jiang X, Tong T, Fang L, Wu Y, Liang J, Xiao S.. 2020a. High antiviral activity of mercaptoethane sulfonate functionalized Te/BSA nanostars against arterivirus and coronavirus. RSC Adv. 10(24):14161–14169. doi: 10.1039/d0ra01387k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Tong T, Jiang X, Fang L, Wu Y, Liang J, Xiao S.. 2020b. GSH-ZnS nanoparticles exhibit high-efficiency and broad-spectrum antiviral activities via multistep inhibition mechanisms. ACS Appl Bio Mater. 3(8):4809–4819. doi: 10.1021/acsabm.0c00332. [DOI] [PubMed] [Google Scholar]