ABSTRACT
Objective
This study investigates how astaxanthin (AST) counters tert-butyl hydroperoxide (tBHP)-induced cellular damage in C28/I2 chondrocytes, focusing on the circ-HP1BP3/miR-139-5p/SOD1 signaling pathway and its use in sustained-release microspheres for osteoarthritis treatment.
Methods
We employed a variety of techniques including real-time quantitative PCR, Western blot, ELISA, and dual-luciferase reporter gene assays to explore AST's molecular effects. Additionally, the efficacy of AST-loaded sustained-release microspheres was evaluated in vitro and in a mouse model of osteoarthritis.
Results
AST significantly enhanced SOD1 expression, reducing apoptosis and inflammation in damaged cells. The AST-loaded microspheres showed promising in vitro drug release, improved cell viability, and reduced oxidative stress. In the osteoarthritis mouse model, they effectively decreased joint inflammation and increased the expression of chondrocyte markers.
Conclusion
Astaxanthin effectively mitigates oxidative stress and inflammation in chondrocytes via the circ-HP1BP3/miR-139-5p/SOD1 pathway. The development of AST-loaded microspheres offers a novel and promising approach for osteoarthritis therapy, potentially extending to osteoarthritis treatment.
KEYWORDS: Astaxanthin, C28/I2 cells, tBHP, Circ-HP1BP3, Mir-139-5p, SOD1, sustained-release microspheres, osteoarthritis
Introduction
Osteoarthritis (OA) is a chronic degenerative joint disease that extensively affects the elderly population worldwide, being one of the leading causes of disability in adults [1–3]. According to a report by the World Health Organization, OA affects approximately 10% of males and 18% of females globally [4–6]. With the aging population, these numbers are expected to continue rising [7, 8]. The primary characteristic of OA is the gradual degeneration of cartilage, leading to symptoms such as pain, swelling, and limited range of motion, significantly impacting the quality of life for patients [9]. Despite the availability of various treatment strategies such as nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, and moderate exercise therapy, these treatments often offer only temporary pain relief and come with significant side effects [10]. Therefore, the current focus of research is on developing new treatment methods without side effects, particularly those capable of delaying or reversing cartilage degeneration [11].
Oxidative stress plays a critical role in the pathophysiology of OA, especially in the damage and degenerative changes of chondrocytes [12]. The overproduction of free radicals and reactive oxygen species (ROS) directly damages cell lipids, proteins, and nucleic acids, leading to cellular dysfunction and death [13–15]. tBHP, as an organic peroxide, is used experimentally to simulate this oxidative damage in order to study the specific effects of oxidative stress on chondrocytes [16, 17]. Under the influence of oxidative stress, cells activate a series of signaling pathways, including but not limited to NF-κB and MAPK pathways, which further regulate the expression of inflammation-related genes [18, 19] Additionally, oxidative stress can activate stress response proteins within cells such as heat shock proteins and antioxidant enzyme systems, which can alleviate damage to some extent. However, under chronic and excessive oxidative stress conditions, this protective effect is often insufficient to counteract the damage [20–22]. Therefore, understanding and intervening in these processes are crucial for developing new strategies to treat OA [23, 24]. By establishing a tBHP-induced C28/I2 cell damage model, researchers can closely observe and analyze how oxidative stress triggers cell death and inflammation, providing a basis for the development of treatment methods targeting these mechanisms [25].
Astaxanthin (AST), a potent natural carotenoid derived from marine organisms such as shrimp and algae, is renowned for its exceptional antioxidant capabilities [26–28]. This compound can penetrate cell membranes, effectively eliminate free radicals inside and outside the cells, thereby protecting cells from oxidative stress damage. AST significantly reduces oxidative injury by decreasing the generation of ROS and increasing the activity of antioxidant enzymes like superoxide dismutase (SOD) and glutathione peroxidase (GPX) [29–31]. In numerous animal studies, the application of AST not only demonstrated the ability to alleviate arthritic inflammation but also improve the structure and function of joint cartilage, indicating its potential effects in combating OA [32, 33]. Moreover, AST possesses the functionality of regulating cell signaling pathways, being able to suppress the production of inflammatory factors by influencing key inflammatory regulatory factors such as NF-κB, thereby alleviating cellular and tissue inflammation [34–36]. This multifaceted protective mechanism renders AST a promising natural compound for treating various diseases caused by oxidative stress and inflammation, including but not limited to OA [35, 37, 38]. Further research will help clarify the potential of AST in clinical applications, particularly how it exerts therapeutic effects by modulating specific molecular targets such as miR-139-5p and SOD1.
This study delves into investigating the protective effects mediated by AST through a specific molecular mechanism – the circ-HP1BP3/miR-139-5p/SOD1 signaling pathway – aiming to elucidate its specific mechanisms in alleviating tBHP-induced damage in C28/I2 cells. Real-time quantitative PCR (qPCR), Western blot, ELISA, and other technical approaches were employed to meticulously examine the expression levels of SOD1, circHP1BP3, miR-139-5p, along with changes in inflammatory factors and oxidative stress markers within the cells. Additionally, by establishing a mouse model of OA, this study evaluated the therapeutic efficacy of AST-loaded sustained-release microspheres, a novel sustained-release microsphere system designed to enhance the bioavailability and sustained release capability of AST for improved therapeutic outcomes. These findings not only contribute to understanding the mechanisms of action of AST but also potentially offer experimental and theoretical support for developing new strategies to treat OA, laying a foundation for future clinical applications.
Materials and methods
Experimental materials
All chemicals and reagents used in this study, including the human normal chondrocyte cell line C28/I2 (Shanghai Cell Bank, Chinese Academy of Sciences), DMEM culture medium and fetal bovine serum (Gibco, USA), trypsin (Abcam, USA), penicillin–streptomycin mixture (Abcam, USA), DMSO (Abcam, USA), AST (Sigma, USA), cell apoptosis detection kit (Cell Signaling Technology, Beijing), TRIZOL and Lipofectamine 3000 transfection reagent (Invitrogen, USA), reverse transcription kit (Biorad Biotechnology, China), and SYBR Green fluorescent dye PCR kit (Applied Biosystems, USA) were employed. All antibodies used, including type II collagen, proteoglycan, matrix metalloproteinase 13, SOD1, and β-actin antibodies, as well as the HRP-conjugated anti-rabbit IgG (Abcam, USA), were obtained from the same source. Additional materials included GPX4 antibody, OPN antibody, COL2A1 antibody, Bcl-2, and Bax antibody IgG (Abcam, USA).
Cell culture and grouping
In this study, C28/I2 human chondrocytes were utilized as the experimental model, sourced from the Shanghai Cell Bank of the Chinese Academy of Sciences. The cell culture process involved the use of DMEM medium (Gibco, USA) containing 10% fetal bovine serum and 1% penicillin–streptomycin (Sigma, USA), maintained at 37°C with 5% CO2 in a constant temperature incubator (Thermo Fisher Scientific, USA). The cells were seeded into 6-well plates at a density of 5 × 104 cells per well and grown until reaching 90% confluence, at which point they were divided into three groups for treatment: the control group continued to be cultured under standard conditions without any additional treatment; the tBHP group was exposed to 100μM tBHP (Sigma, USA) for 24 h to induce oxidative stress damage; and the tBHP + AST group pre-treated with 20μM AST (Sigma, USA) for 2 h before being co-treated with 100μM tBHP for 24 h. Each experimental group was replicated three times to ensure the reliability of the results. During the experiments, the growth status and morphology of the cells were observed and documented using an inverted microscope.
Real-time quantitative PCR (qPCR)
qPCR analysis utilized TRIZOL reagent (Invitrogen, USA) for total RNA extraction from C28/I2 cells, followed by RNA quantification using a NanoDrop instrument (Thermo Fisher Scientific, USA) to ensure a constant RNA amount of 2 µg for the reverse transcription reaction. Reverse transcription was carried out using a kit from Bao Ri Medical Biotechnology Company (China), with a reaction volume of 20 µL and specific program settings including an initial 2-minute denaturation at 94°C, followed by 40 cycles (94°C for 30 s, 60°C for 30 s). SYBR Green PCR reagent kit (ABI, USA) was employed for qPCR analysis, targeting genes such as circHP1BP3, SOD1, and miR-139-5p, with GPADH as the reference gene. Primers were provided by Shanghai Sangon Biotech Limited. The qPCR was performed on an ABI Prism 7500 sequence detection system (Applied Biosystems, USA) with a protocol involving 2 min of initial denaturation at 94°C, followed by 40 cycles (30 s denaturation at 94°C, annealing and extension at 60°C for 30 s). The experimental results were determined using the ΔΔCT method normalized to GPADH for target gene expression. The primer sequences involved in this study are listed in Table 1.
Table 1.
Primer sequences used for PCR.
| Genes | Forward primer sequence (5’–3’) | Reverse primer sequence (5’–3’) |
|---|---|---|
| circHP1BP3 (hsa_circ_0002437) | AACTTGATGAAGCAGAAGATGATT | AAAAGACAGTAGCCCTCCGATA |
| SOD1 | GGTGGGCCAAAGGATGAAGAG | CCACAAGCCAAACGACTTCC |
| miR-139-5p | TCTACAGTGCACGTGTCTCCAGT | CCAGTGCAGGGTCCGAGGTA |
| GAPDH | CAAATTCCATGGCACCGTCA | GACTCCACGACGTACTCAGC |
Cell transfection
In this study, we employed Lipofectamine 3000 transfection reagent (Invitrogen, USA) following the manufacturer's guidelines to transfect C28/I2 cells. The transfection materials used included the circ-HP1BP3 overexpression plasmid (PLCDH-ciR-circ-HP1BP3, circ-HP1BP3), siRNA targeting circ-HP1BP3 (si-Circ-HP1BP3), miR-139-5p mimic, miR-139-5p inhibitor (anti-miR-139-5p), and shRNA targeting circ-HP1BP3 (pLKO.1-circ-HP1BP3) along with their respective negative controls, all provided by China Ribo Biological Technology Company. Transfection was performed when the cell confluency reached 70%–80%, and 48 h later, the transfection efficiency was assessed using the SYBR Green PCR kit (ABI, USA) on the ABI Prism 7500 Sequence Detection System through qRT-PCR. The 2-ΔΔCT method was utilized, with GAPDH as the housekeeping gene for normalization of gene expression levels.
Western blot
In this study, Western blot analysis was utilized to quantitatively assess the expression levels of the target proteins. Initially, C28/I2 cells were subjected to specific treatments, followed by centrifugation at 1000 g for 5 min to collect the cell pellet, which was then washed three times with phosphate-buffered saline (PBS) to remove any residual culture medium. Subsequently, lysis buffer (containing protease inhibitors from Sigma-Aldrich, USA) was added to the cells on ice for 15 min to extract total cellular proteins. The protein samples were then boiled for 5 min to undergo denaturation. Subsequently, 50 µg of protein from each sample was separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (PVDF, Millipore, USA). After blocking the membrane with 5% non-fat milk for 1 h, it was then incubated overnight at 4°C with the respective diluted primary antibodies (specific for the target protein and β-actin, purchased from Abcam, USA). Following this, the membrane was washed three times with PBS-T (0.1% Tween-20) and then incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG secondary antibody (from Sigma-Aldrich, USA) at room temperature for 2 h. After washing the membrane, signal visualization was conducted using the enhanced chemiluminescence (ECL) detection system (from GE Healthcare, USA) and exposure and imaging were performed under a microscope. The grayscale intensity of the protein bands was analyzed using Image J software (from the National Institutes of Health, USA) and normalized to β-actin as an internal control for assessing protein expression levels.
Detection of inflammatory factors IL-6, IL-1β, and TNF-α
In the inflammation factor detection section of this study, after cell treatment, we carefully collected the culture supernatant of C28/I2 cells to measure the levels of key inflammatory mediators IL-6, IL-1β, and TNF-α. This step was carried out using ELISA assay kits specifically designed for these inflammatory factors (R&D Systems, USA), following the manufacturer's instructions strictly. At least three independent experimental repeats were set for each group to ensure data reliability and reproducibility. The culture supernatant collection occurred 48 h after specific cell treatments (e.g. control, mimic treatment, and inhibitor treatment) to ensure that the production and secretion of inflammatory factors reached detectable levels. During analysis, each sample was handled under sterile conditions to avoid any possible cross-contamination, ensuring the accuracy of the experimental results.
Detection of oxidative stress-related markers
In this study, to evaluate the impact of cell treatment on oxidative stress status, particular attention was paid to changes in levels of ROS and malondialdehyde (MDA). We utilized specialized assay kits to measure these indicators. Following cell treatment, C28/I2 cell pellets were collected by centrifugation at 1000 g for 5 min, and the levels of ROS and MDA were determined using ROS and MDA assay kits (Abcam, Cambridge, UK) following the manufacturer's detailed instructions. To ensure the reliability and reproducibility of the experimental results, each treatment group was subjected to at least three independent experimental replicates. Specifically, immediately after cell collection, cells were lysed with the buffer provided in the assay kit, and the quantitative analysis of ROS and MDA content in the lysates was performed using the appropriate biochemical methods as directed in the instructions. All measurements were conducted at room temperature, and proper quantitative calculations were carried out based on standard curves.
H2DCFDA staining for ROS level detection
Following the kit's instructions, H2DCFDA (TargetMol, Shanghai, China, T15458) was diluted in a serum-free medium at a ratio of 1:1000 to a final concentration of 10 μM. After specific treatments, C28/I2 cells were collected in flow cytometry tubes and incubated with the diluted H2DCFDA (1 × 106 cells/mL) at 37 °C in the dark for 30 min. The analysis was then performed using a BD Aria flowIII cytometer, and the results were analyzed with FlowJo v10 software [39].
Detection of intracellular superoxide anion and catalase levels
According to the product instructions, dihydroethidium (DHE, Cat. No. S0063, Beyotime) and the Catalase Assay Kit (Cat. No. S0051, Beyotime) were used to detect superoxide anion and catalase activity, respectively. Superoxide Anion Detection: 10 μl of DHE working solution (5 μM, diluted in PBS; stock solution 10 mM, dissolved in DMSO, Beyotime, Cat. No. S0063) was added to 100 μl of washed cell samples and incubated at 37°C for 30 min. After incubation, red fluorescence signals were detected using a microplate reader with an excitation wavelength of 300–310 nm and an emission wavelength of 610 nm. Catalase Activity Detection: The Catalase Assay Kit (Beyotime, Cat. No. S0051) was used for catalase activity detection. According to the product instructions, an appropriate amount of the sample was added to the reaction solution in the kit, where hydrogen peroxide was catalyzed by peroxidase to oxidize the substrate, generating a red product. After the reaction, the absorbance (A520) of the samples was measured at 520 nm using a microplate reader.
Dual-luciferase reporter gene assay
To investigate the regulatory role of miR-139-5p on SOD1 expression, this study constructed wild-type (WT) and mutant-type (MUT) SOD1 3'UTR sequences containing the miR-139-5p binding sites in the circ-HP1BP3 dual-luciferase reporter gene vectors (pmirGLO, Promega, Madison, WI, USA). These constructs were utilized to validate the direct interaction between miR-139-5p and its target gene SOD1. C28/I2 cells were cultured to 70%–80% confluency before transfection and then transfected using Lipofectamine 3000 transfection reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Specifically, cells were co-transfected with miR-139-5p mimic or control miRNA mimic, along with the corresponding WT and MUT reporter gene vectors. After 48 h of transfection, the fluorescence and Renilla luciferase activities were measured using the dual-luciferase reporter assay system (Promega, Madison, WI, USA) following the manufacturer's guidelines to evaluate the regulatory effect of miR-139-5p on the target gene's 3'UTR region. Each transfection group was performed with three technical replicates, and at least three independent biological replicates were conducted to ensure result reliability. The measurement of luciferase activity was standardized against the internal control of Renilla luciferase activity to minimize the impact of transfection efficiency and changes in cellular conditions.
Ago2-RIP experiment
In this study, we conducted an Ago2 RNA immunoprecipitation (RIP) experiment to validate the interaction between miR-139-5p and its target genes, circ-HP1BP3, and SOD1 mRNA. Initially, C28/I2 cells were subjected to specific treatments, followed by collection and lysis using RIP lysis buffer (Millipore, Billerica, MA, USA). The lysate was then incubated on ice for 5 min to ensure complete cell lysis. Subsequently, the cell lysate was mixed with magnetic beads coated with Ago2 antibody (Abcam, Cambridge, UK) or control IgG antibody (Santa Cruz Biotechnology, Dallas, TX, USA), and incubated at 4°C with gentle rotation for 3 h to enrich RNA associated with the Ago2 complex. After the incubation, the magnetic beads were separated using a magnet, and the precipitate was collected for further RNA extraction for subsequent qRT-PCR analysis.
The extracted RNA was purified using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions and reverse transcribed using a reverse transcription kit (Takara Bio, Shiga, Japan). Subsequently, qRT-PCR was performed on a LightCycler® 480 real-time PCR system (Roche, Basel, Switzerland) using a SYBR Green PCR kit (Qiagen, Hilden, Germany) to quantify the expression levels of circ-HP1BP3, miR-139-5p, and SOD1. Each sample was subjected to at least three technical replicates, and at least three biological replicates were set up to ensure the accuracy and reproducibility of the data.
RNA pull-down experiment
In this study, to thoroughly investigate the direct interaction of miR-139-5p with circ-HP1BP3 and SOD1 mRNA, we employed RNA pull-down technology. The specific experimental procedure was as follows: initially, C28/I2 cells were collected after receiving specific treatments, then treated with cell lysis buffer containing protease inhibitors, RNase inhibitors, and phospholipase inhibitors (all inhibitors provided by Sigma-Aldrich, St. Louis, MO, USA) to ensure complete cell lysis. Post-lysis, the supernatant was collected after centrifugation at 12000g for 15 min and kept for later use. Subsequently, streptavidin-coated magnetic beads (Invitrogen, Carlsbad, CA, USA) were pre-washed and then mixed with 2× binding and wash buffer, biotinylated miR-139-5p probe (Integrated DNA Technologies, Coralville, IA, USA), and sufficient DEPC water to reach a total volume of 800 µL. The mixture was incubated overnight at 4°C to allow the beads to thoroughly bind with the target RNA. After the incubation, 500 µL of cell lysis buffer was added to the bead-RNA mixture, followed by a further 1-hour incubation at room temperature to promote extensive interaction. Post-incubation, the supernatant was removed by low-speed centrifugation and washed with PBS, ultimately extracting the bead-bound RNA using TRIZOL (Invitrogen, Carlsbad, CA, USA).
For quantitative analysis of circ-HP1BP3 and SOD1 mRNA expression levels, qRT-PCR technology was utilized, employing SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) following the manufacturer's instructions. Each sample was assayed in at least three technical replicates, with appropriate controls set to ensure experiment accuracy.
Preparation of sustained-release microspheres loaded with AST
In this study, we employed an innovative approach to prepare sustained-release microspheres loaded with AST, aiming to achieve effective drug release through a core–shell structure. Initially, the shell solution was prepared, composed of chitosan and nano-hydroxyapatite, both supplied by Sigma-Aldrich (St. Louis, MO, USA). The core solution was prepared by mixing AST (also sourced from Sigma-Aldrich) with corn protein, with AST serving as the active ingredient and corn protein providing a suitable carrier matrix.
Subsequently, utilizing coaxial electrostatic spraying technology with the device (Fluidnatek LE-10, Bioinicia, Valencia, Spain), the above two solutions were sprayed into particles to form sustained-release microspheres with a core–shell structure. The application of this technique ensured the uniformity and stability of the microspheres, while allowing precise control over their size and drug-loading capacity.
The surface morphology of the microspheres was assessed by scanning electron microscopy (SEM, FEI Quanta 200, Hillsboro, OR, USA) to evaluate their shape, size, and surface texture. At least three batches of microspheres were prepared under each set of conditions to ensure the repeatability and representativeness of the experiment. ImageJ software (National Institutes of Health, USA) was used to process and analyze SEM images to quantify the average diameter and surface roughness of the microspheres.
Animal and the OA model
In order to investigate the therapeutic effects of AST on a mouse model of OA, eighteen healthy male C57BL/6 mice, aged 8 weeks and weighing between 18 and 22 grams, were obtained from Beijing VitalHua Experimental Animal Technology Co., Ltd., with the product code 213. The mice were housed under laboratory conditions with a controlled temperature of 22 ± 2°C, relative humidity between 50% and 60%, and a 12-h light cycle. They were provided with ample food and water supply, as well as sufficient space to reduce stress and ensure their welfare.
The experimental groups were as follows: Control group, OA model group, and OA + AST treatment group, each consisting of 6 mice. In the OA group and OA + AST group mice, after anesthesia, a medial joint incision was made to expose the left joint cavity, followed by a transection of the tibial collateral ligament, and finally, the joint incision was closed. In the Control group, only the joint cavity was exposed without further intervention [40].
One week after surgery, the OA + AST group mice will receive treatment with astaxanthin-loaded sustained-release microspheres administered via intra-articular injection. These microspheres were prepared using coaxial electrohydrodynamic atomization technique with the Fluidnatek LE-10 equipment (Bioinicia, Valencia, Spain), ensuring a good core–shell structure of the microspheres for controlled drug release. The treatment dosage was set at 5 mg/kg body weight every other day for a continuous 4-week period. Mice in the control group and OA group received microspheres containing saline as a control treatment (Figure S1). Throughout the entire experiment, daily observations were made on the general health, activity levels, food and water intake of the mice, while recording changes in body weight. The typical symptoms of OA model development, such as joint swelling, erythema, and mobility impairment, were monitored. At the end of the experiment, joint tissues were collected for histopathological examination.
Immunohistochemical staining
In this study, to investigate the impact of AST on the expression of SOD1 protein in a mouse model of OA, we employed immunohistochemical staining technique. Initially, joint tissues were sampled from the control group, OA model group, and OA group treated with AST. The samples were fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Microtome was used to obtain 4–5 µm thick sections, followed by dewaxing and hydration steps. Subsequently, antigen retrieval was performed in citrate buffer solution to enhance the binding efficiency of the subsequent antibodies. Then, endogenous peroxidase was blocked with 3% H2O2, non-specific binding sites were blocked in PBS containing 5% goat serum, and the sections were incubated overnight with rabbit anti-SOD1 primary antibody (Sigma-Aldrich, catalog number SAB2108604) to label the target protein. SOD1 expression was visualized in brown deposition form using an HRP-conjugated secondary antibody and DAB substrate. Finally, the sections were counterstained with hematoxylin–eosin, nuclear blueing was performed with lithium carbonate, and observation and analysis were carried out using an optical microscope. Based on the intensity and extent of staining, differences in SOD1 protein expression among different experimental groups were evaluated. Through these meticulous experimental steps, we aim to gain a deeper understanding of how AST alleviates the inflammatory response and oxidative stress in rheumatoid arthritis by regulating SOD1 expression, providing a scientific basis for the application of AST in disease treatment.
Hematoxylin and eosin (HE) staining
Initially, the collected joint tissue samples were fixed in 4% formaldehyde solution (catalog number: HT501128, Sigma-Aldrich) at room temperature for 24 h. The fixed samples were then dehydrated through a series of alcohol gradients (catalog number: HX6831762, Thermo Fisher Scientific), followed by clearing with xylene (catalog number: T5118, Sigma-Aldrich). Subsequently, the processed samples were embedded in paraffin wax (catalog number: P6148, Sigma-Aldrich) and sliced into 4–5 μm-thick consecutive sections using a rotary microtome (Leica Microsystems).
Next, the sections were stained in hematoxylin solution (catalog number: GHS316, Merck) for a few minutes, rinsed, and counterstained with eosin solution (catalog number: 230251, Sigma-Aldrich). After staining, the sections underwent additional dehydration and clearing in alcohol gradients and xylene, followed by sealing with a mounting medium (catalog number: F6182, Sigma-Aldrich).
Finally, the stained tissue sections were observed and analyzed under a microscope (model: BX53, Olympus). In this process, cell nuclei stained with hematoxylin appeared blue-purple, while cytoplasm and collagen fibers stained with eosin appeared red. This method effectively demonstrates morphological changes in tissues, providing reliable morphological evidence for further pathological analysis and evaluation of therapeutic effects.
Statistical analysis
In this study, the experimental data of all cells were subjected to at least three independent experiments to ensure the repeatability and reliability of the results. The data were initially preprocessed using Microsoft Excel software, followed by detailed statistical analysis and graphical representation using SPSS 26.0 software (IBM, Armonk, New York, USA) and GraphPad Prism 8.0 software (GraphPad Inc., San Diego, California, USA). All experimental data were presented as mean ± standard deviation (SD). Depending on the data distribution and the needs of comparison, independent samples t-test and one-way analysis of variance (ANOVA) were employed to statistically analyze the differences between two or more groups of data. To further investigate specific differences between groups, Tukey's post-hoc analysis was conducted for results showing significant differences in the analysis of variance. A P-value less than 0.05 was considered statistically significant in all statistical tests.
Results
AST alleviates tBHP-induced cell damage
Recent studies have demonstrated that AST significantly ameliorates tBHP-induced C28/I2 cell damage by inhibiting cell apoptosis, reducing cell inflammatory response, increasing the expression of type II collagen and proteoglycan, as well as inhibiting matrix metalloproteinase 13 [41]. This study clearly establishes the protective effect of AST against tBHP-induced cell damage in C28/I2 cells. Experimental data reveals that cells treated with AST exhibit significantly enhanced survival capabilities compared to those solely treated with tBHP (Figure 1A). Further flow cytometry analysis indicates that AST significantly reduces the rate of tBHP-induced cell apoptosis (Figure 1B). Regarding the inflammatory response, AST markedly decreases the levels of inflammatory factors such as IL-6, IL-1β, and TNF-α (Figure 1C), suggesting its protective role through anti-inflammatory mechanisms. Moreover, astaxanthin significantly reduces the generation of reactive oxygen species (ROS) and malondialdehyde (MDA), while decreasing the activity of oxidized glutathione (GSSG) and enhancing the activities of superoxide dismutase (SOD), glutathione peroxidase (GPX), and reduced glutathione (GSH) (Figure 1D). Meanwhile, further flow cytometry and immunofluorescence analyses indicated that astaxanthin significantly reduced the total ROS levels, as well as the levels of superoxide anion and hydrogen peroxide, in tBHP-induced cells (Figure 1E–F). This mechanism alleviates oxidative stress. In terms of extracellular matrix protein expression, AST elevates the levels of type II collagen and proteoglycan, concurrently suppressing matrix metalloproteinase 13 (MMP13) expression (Figure 1G), supporting its potential role in cartilage cell injury repair. Further experimental analysis reveals the complex role of AST in modulating the apoptosis process of C28/I2 cells. Western blot data demonstrates a significant increase in the expression level of Bcl-2 (an anti-apoptotic protein) in the AST-treated group within the tBHP-induced cell model (Figure 1H), while the expression of Bax (a pro-apoptotic protein) is notably reduced, indicating AST's ability to balance pro-apoptotic and anti-apoptotic signals, thereby enhancing cell survival. Investigation into the impact of AST on cartilage-specific proteins shows an increase in COL2A1 (type II collagen alpha 1 chain, a major component of cartilage) expression in the AST-treated group (Figure 1I), promoting maintenance of cartilage cell structural integrity. Concurrently, AST decreases the expression levels of OPN (osteopontin, associated with inflammation and tissue remodeling processes) in tBHP-induced C28/I2 cells, further indicating its alleviative effect on inflammation induced by cartilage cell damage. Oxidative stress is a crucial factor affecting cartilage cell survival, and the protein expression level of the antioxidant enzyme GPX4 reflects the cartilage cell's antioxidant stress resistance. Data reveals that AST significantly rescues the inhibited expression of GPX4 by tBHP, restoring the cartilage cell's antioxidant stress resistance (Figure 1I). The experimental data presented confirms that AST promotes cell antioxidant stress resistance, regulates cell apoptosis pathways and inflammatory factors, modulates protein levels, enhances cell antioxidant stress resistance, and promotes structural protein expression, effectively protecting cartilage cells.
Figure 1.
Protective effect of AST against tBHP-induced C28 / I2 cell damage. (A) Effect of AST treatment on the viability of tBHP-induced C28 / I2 cells. (B) Effect of AST treatment on tBHP-induced apoptosis in C28 / I2 cells. (C) Effect of AST treatment on tBHP-induced inflammatory factors IL-6, IL-1β, and TNF-α in C28 / I2 cells. (D) The effect of astaxanthin treatment on the levels of ROS, MDA, SOD, GPX, GSH, and GSSG in tbhp-induced C28/I2 cells. (E) Effect of astaxanthin treatment on tBHP-induced ROS levels in C28/I2 cells. (F) The effect of astaxanthin treatment on superoxide anion and catalase levels in tBHP-induced C28/I2 cells. (G) The effect of astaxanthin treatment on the expression levels of type II collagen, proteoglycans, and matrix metalloproteinase 13 in tbhp-induced C28/I2 cells. (H) The effect of astaxanthin treatment on the expression levels of Bcl-2 and Bax in tbhp-induced C28/I2 cells. (I) The effect of astaxanthin treatment on the expression levels of COL2A1, OPN, and GPX4 in tbhp-induced C28/I2 cells. *p < 0.05; **p < 0.01; ***p < 0.001.
circHP1BP3: A key mediator of AST protection mechanism
The results from treatment with Actinomycin D and RNase R further emphasize the higher stability of circHP1BP3 compared to its linear counterpart, HP1BP3 mRNA (Figure 2B and C). This implies that circHP1BP3 can exert its function more durably within the cell. Additionally, nuclear-cytoplasmic fractionation experiments demonstrated that circHP1BP3 is predominantly located in the cytoplasm (Figure 2D), aligning with its potential role as a miRNA sponge.
Figure 2.
AST regulates tBHP-induced damage in C28 / I2 cells via circHP1BP3. (A) Effect of AST treatment on tBHP-induced circHP1BP3 expression in C28 / I2 cells. (B) Effect of actinomycin D on the expression of linear HP1BP3 and circular circHP1BP3 in C28 / I2 cells. (C) Effect of exonuclease RNase R on the expression of linear HP1BP3 and circular circHP1BP3 in C28 / I2 cells. (D) circHP1BP3 expression in the nucleus and in the cytoplasm. (E) Effect of circHP1BP3 silencing on cell viability. (F) Effects of circHP1BP3 silencing on cell apoptosis. (G) Effect of circHP1BP3 silencing on the inflammatory cytokines IL-6, IL-1β, and TNF-α. (H) Effect of circHP1BP3 silencing on ROS, MDA, and SOD1 levels. (I) Effect of circHP1BP3 silencing on ROS levels. (J) The effect of circHP1BP3 silencing on superoxide anion and catalase levels in C28/I2 cells. (K) Effect of circHP1BP3 silencing on the expression of type collagen, proteoglycan, and matrix metalloproteinase 13. (L) Effect of circHP1BP3 silencing on Bcl-2 and Bax expression. (M) Effect of circHP1BP3 silencing on COL2A1 and OPN expression. (N) Effects of circHP1BP3 silencing on GPX4 expression. *p < 0.05; **p < 0.01; ***p < 0.001.
circHP1BP3, a circular RNA, has been shown to be involved in tert-butyl hydroperoxide-induced C28/I2 cell damage and AST-mediated cell damage protection [42]. In order to investigate the pivotal role of circHP1BP3 as a stable circular RNA in the protective effect provided by AST to C28/I2 cells, we measured the changes in circHP1BP3 levels. Real-time qPCR results showed a significant decrease in circHP1BP3 expression in the tBHP-treated group compared to the control group, indicating the downregulation of circular RNA expression in oxidant stress-induced cell injury inhibition. AST was able to markedly reverse this decreased expression (Figure 2A), not only demonstrating the critical role of AST in enhancing cellular antioxidant capacity but also suggesting that circHP1BP3 may be a key molecular target of its action.
To investigate the role of circHP1BP3 in chondrocytes, we utilized RNA interference to specifically silence circHP1BP3, resulting in a significant decrease in cell viability (Figure 2E), an increase in apoptosis rate (Figure 2F), and a significant elevation in the levels of inflammatory factors IL-6, IL-1β, and TNF-α (Figure 2G). Meanwhile, the silencing of circHP1BP3 resulted in increased levels of ROS and MDA, along with decreased SOD1 activity (Figure 2H), as well as elevated levels of total ROS, superoxide anion, and catalase (Figure 2I–J). Additionally, silencing circHP1BP3 led to decreased levels of type II collagen and proteoglycan, and increased levels of matrix metalloproteinase 13 (MMP13) (Figure 2K), further confirming the important role of circHP1BP3 in regulating the cellular response to oxidative stress. These findings not only validate the central role of circHP1BP3 in AST-mediated protective mechanisms but also provide new therapeutic strategies for targeting circular RNAs, potentially significant for treating oxidative stress-related diseases such as OA. Further protein expression analysis revealed that AST regulates Bcl-2 and Bax expression by modulating circHP1BP3, thereby inhibiting apoptosis, as evidenced by a significant increase in Bcl-2 expression and a decrease in Bax expression in the AST-treated group (Figure 2L), suggesting the involvement of AST in apoptosis-related pathways via circHP1BP3 regulation. Moreover, alterations in COL2A1 and OPN expression (Figure 2M) indicated the potential role of AST through circHP1BP3 modulation in maintaining chondrocyte function and cartilage repair, holding promise for degenerative joint diseases like rheumatoid arthritis. The increased expression of GPX4 protein (Figure 2N) demonstrated that AST enhances cellular antioxidant defense capability by regulating circHP1BP3, crucial for mitigating cell damage caused by ROS. In summary, these data collectively reveal AST's ability to regulate various aspects of cellular physiology to combat damage.
Bioinformatics prediction of the interaction between circHP1BP3 and miR-139-5p
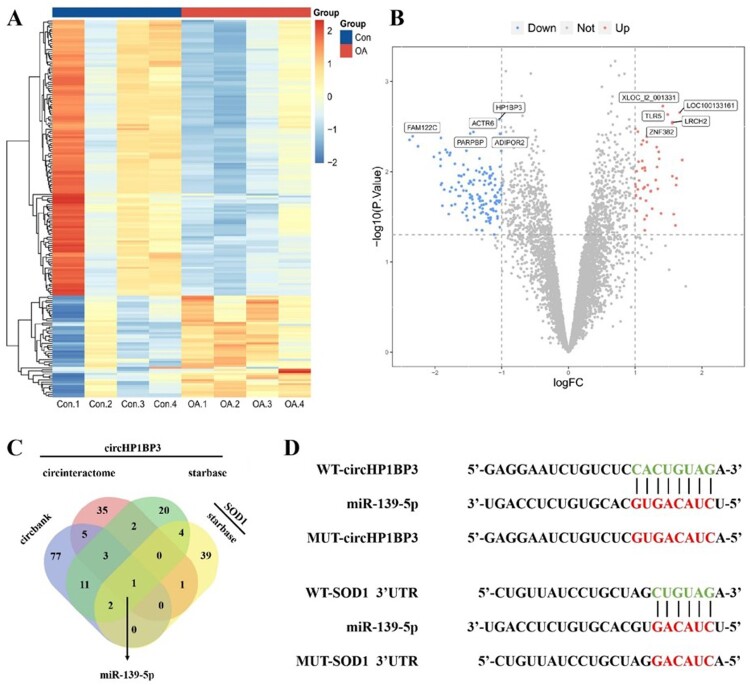
Through in-depth analysis, we observed that the expression of circHP1BP3 in C28/I2 cells was influenced under oxidative stress conditions. This influence was also reflected in the comparison of samples from OA patients, indicating a potential significant role of circHP1BP3 in pathological conditions (Figure 3A–B). To further investigate the function of circHP1BP3, we used bioinformatics tools to predict its potential interactions with miRNAs. Our analysis revealed that both circHP1BP3 and SOD1's 3'UTR contain binding sites for miR-139-5p, suggesting that miR-139-5p may play a critical role in regulating the interaction between these two molecules (Figure 3C–D). These findings lay a solid theoretical foundation for subsequent experimental validation.
Figure 3.
Potential binding miRNAs of circ-HP1BP3 and SOD1. (A) Heatmap showing the difference in gene expression between the control (Con) and OA groups. (B) Volcano plot highlights the significant changes in gene expression between the control and OA groups. Genes upregulated in the OA group are shown in red, downregulated in blue and those with no significant changes in gray. (C) Wayn diagram showing the predicted overlap of circHP1BP3 with the 3 ′ UTR region of SOD1 at the miR-139-5p binding site, suggesting that these molecules are regulated by a targeting interaction of miR-139-5p. (D) Schematic diagram showing the WT and MUT sequences of circHP1BP3 and SOD1 3'UTR, indicating that miR-139-5p has complementary sequence interactions with the WT form of both, while the binding site of the MUT form is disrupted by mutation.
Experimental validation of the direct interaction of miR-139-5p with circHP1BP3 and SOD1
Following the successful establishment of overexpression and knockdown models of miR-139-5p (Figure 4A), the dual-luciferase reporter gene assay clearly demonstrated the impact of miR-139-5p on the activity of the target genes, providing us with direct molecular evidence (Figure 4B) of its regulatory role. Combining the results of AGO2-RIP and RNA pull-down experiments, we have confirmed the direct interaction between circHP1BP3 and miR-139-5p, highlighting the crucial role of this interaction in the cell protective effect provided by AST (Figure 4C–D). Western blot and qRT-PCR analyses (Figure 4E and F) revealed that miR-139-5p downregulates the expression of SOD1, consistent with the findings of the reporter gene assay in Figure 4B. Figure 4G shows the effect of miR-139-5p on cytokine expression. Overexpression of miR-139-5p significantly increased the expression of various inflammatory cytokines (IL-6, IL-1β, TNF-α, IL-8, IL-2), indicating that miR-139-5p has a pro-inflammatory role. Figures 4H and I–K show oxidative stress-related indicators, including MDA, total ROS levels, superoxide anion, and catalase levels, demonstrating that overexpression of miR-139-5p can elevate these oxidative stress factors, suggesting that miR-139-5p may have a role in counteracting oxidative stress.
Figure 4.
circ-HP1BP3 regulates the expression of SOD1 by targeting the binding to miR-139-5p. (A) Changes in miR-139-5p mRNA levels in C28 / I2 cells transfected with miR-139-5p mimics (miR-139-5p), miR-139-5p inhibitor (anti-miR-139-5 p) and their negative controls. (B) Fluorescein detection after cotransfection of C28 / I2 cells containing WT-or MUT-circ-HP1BP3, SOD1 3- ′ -UTR and miR-139-5P mimic, miR-139-5P inhibitor or miR-NC enzyme activity. (C) The Ago 2-RIP experiment. (D) Rna pull-down experiments. (E) Changes in SOD1 mRNA and protein levels after transfection of miR-139-5p mimics, miR-139-5p inhibitor (anti-miR-139-5p) in C28 / I2 cells and their negative control. (F) The mRNA and protein levels of SOD1 were measured in the circ-HP1BP3 and miR-139-5p over and circ-5 expression groups using empty transfected vector and miR-NC as control group. (G-I) The levels of IL-6, IL-1β, TNF-α, IL-8, IL-2, MDA and ROS of circ-HP1BP3 and miR-139-5p were measured using empty transfection vector and miR-NC as control group. (J) ROS levels were measured in the overexpression circ-HP1BP3 and miR-139-5p groups, using empty transfection vector and miR-NC as control groups. (K) Superoxide anion and catalase levels were measured in the circ-HP1BP3 overexpression and miR-139-5p groups, with empty vector and miR-NC as control groups. *p < 0.05; **p < 0.01; ***p < 0.001.
Chitosan-loaded sustained-release microspheres for effective repair of chondrocyte damage
This study investigates the potential application of chitosan-loaded sustained-release microspheres in repairing cartilage cell damage. The appearance of the chitosan-loaded microspheres was observed using transmission electron microscopy, revealing smooth surfaces, high sphericity, good dispersion, relatively uniform particle size, minimal aggregation, as shown by the red triangle (Figure 5A). Particularly, it was found through the measurement of inflammatory factors that the chitosan sustained-release microspheres significantly reduced the expression of inflammatory factors such as IL-6, IL-1β, and TNF-α compared to cells treated only with tBHP, directly reflecting their effectiveness in reducing the inflammatory response (Figure 5B). Moreover, there was no significant difference between the group treated solely with microspheres and the control group, ruling out any direct effect of the microspheres themselves (Figure S2). Figure 5C demonstrates the impact of chitosan sustained-release microspheres on oxidative stress-related indicators, showing a significant reduction in the generation of ROS and MDA, and GSSG activity, while an increase in SOD, GSH, and GPX activity was observed after the addition of chitosan microspheres, highlighting the critical role of chitosan sustained-release microspheres in enhancing cellular antioxidant defense mechanisms. Meanwhile, after the addition of astaxanthin microspheres, the total ROS levels, superoxide anion, and catalase levels were significantly reduced (Figure 5D–E). These findings collectively demonstrate that chitosan sustained-release microspheres can effectively alleviate cartilage cell damage induced by tBHP and reveal their potential protective mechanism through regulating the expression of inflammatory factors and oxidative stress responses, especially by improving cellular antioxidant defense capabilities.
Figure 5.
Repair effect of AST-loaded sustained-release microspheres on chondrocyte damage. (A) Transmission electron microscopy was used to assess the biological functionality of the microspheres, with red triangles marking chondrocyte microspheres. (B) Effects of AST-loaded sustained-release microspheres on the level of inflammation in chondrocytes. (C) Effects of AST-loaded sustained-release microspheres on the level of oxidative stress in chondrocytes. (D) Effect of astaxanthin-loaded sustained-release microspheres on ROS levels in chondrocytes. (E) The effect of astaxanthin-loaded sustained-release microspheres on superoxide anion and catalase levels in chondrocytes. *p < 0.05; **p < 0.01; ***p < 0.001.
AST improves oxidative stress and chondrocyte marker expression in an OA model
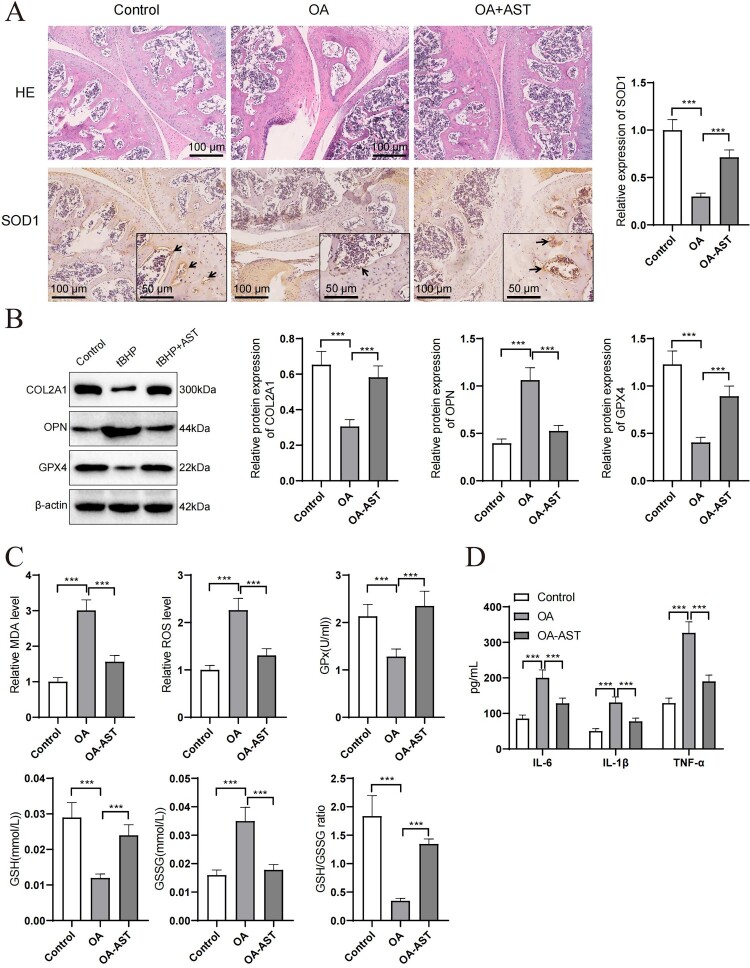
When assessing the therapeutic effects of AST on an OA model, experimental results indicate a significant impact of AST on the expression of key proteins. As shown in Figure 6, the expression of SOD1 decreased in OA model mice, but showed significant recovery following AST treatment, suggesting a potential antioxidant protective effect of AST (Figure 6A). Similarly, the expression of COL2A1 significantly decreased in the OA model, while the AST treatment group exhibited increased expression levels, indicating a positive role of AST in maintaining chondrocyte integrity. The expression of GPX4 protein, as a critical antioxidant enzyme, was significantly higher in the AST treatment group compared to the OA model group, further reinforcing the role of AST in reducing oxidative stress damage. Additionally, osteopontin (OPN), as a marker of bone metabolism, exhibited reduced expression after AST treatment following an increase in the OA model, suggesting that AST may operate through regulating bone metabolism participants (Figure 6B). Moreover, AST treatment reduced the levels of MDA and ROS in mouse joint tissues (Figure 6C), and also decreased the levels of inflammatory factors in mouse tissues (Figure 6D). In summary, the application of AST in the OA model not only enhances the expression of key antioxidant enzymes and reduces oxidative stress, but also improves the molecular marker expression in cartilage tissue. These findings provide scientific grounds for the potential of AST as a therapeutic strategy in rheumatoid arthritis treatment.
Figure 6.
Effects of OA on expression of SOD1, COL2A1, GPX4, and OPN in joint tissues in a mouse model of OA. (A) HE staining and immunohistochemical staining of joint tissues in the control group (Control), OA model group and OA group receiving AST treatment (OA + AST) were shown. SOD1 expression was shown by staining intensity, and the measurement results showed that AST treatment significantly improved the expression of SOD1. Black arrows represent inflammatory cell infiltration, and black triangles indicate SOD1-positive cells. (B) Western blot analysis demonstrated the protein expression levels of COL2A1, OPN and GPX4 in the joint tissues of mice in different experimental groups. Compared with the OA model group, the expression of COL2A1 decreased and GPX4 and OPN increased in the OA + AST group, indicating that AST has a significant regulatory effect on the expression of these markers. (C) Evaluation of MDA, ROS, GPX, GSH, and GSSG levels in joint tissues of mice from different groups indicated a significant reduction in oxidative stress in the AST treatment group. (D) Analysis of inflammatory factor expression levels in joint tissues of mice revealed a marked decrease in inflammatory factors in the AST treatment group, demonstrating its anti-inflammatory effect. Beta-actin was used as a reference protein to ensure consistency of protein loading. Each group of experimental animals contained six individuals. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Articular cartilage injuries have emerged as a threat to human health and quality of life due to the aging population [43]. Therefore, exploring the mechanisms of joint injury repair can offer new perspectives for clinical treatment. The occurrence and progression of OA are linked to various factors such as oxidative stress, synovial inflammation, chondrocyte apoptosis, and degradation of chondrocyte extracellular matrix, with oxidative stress playing a crucial role [44, 45].
ROS-induced oxidative stress is a key factor in the induction of OA [46]. Henrotin et al. suggested that during OA progression, oxidative phosphorylation is the main source of ROS, and chondrocytes express NOX, NOS, as well as NO and O2, which are major producers of ROS. These reactive oxygen species generate various derivatives, including H2O2, ONOO−, and OH˙ [47, 48]. ROS can directly cause cartilage degradation by cleaving collagen, aggregating proteoglycans, and activating MMPs [49, 50].
Recently, AST has been widely recognized for its potent antioxidant and anti-inflammatory capabilities in preventing and treating OA [51, 52]. This study investigated the effect of AST on the repair mechanisms of chondrocyte damage. We induced C28/I2 cell injury by using tBHP to establish a chondrocyte damage model and confirmed that tBHP induced C28/I2 cell damage by downregulating circHP1BP3. AST was found to ameliorate tBHP-induced cell damage and protect chondrocytes [53]. AST, a non-vitamin A carotenoid isolated from salmon and crustacean products, is believed to possess strong antioxidant and anti-inflammatory effects [54]. Due to its high free radical scavenging activity, AST's antioxidant activity exceeds that of other carotenoids such as zeaxanthin, lutein, lycopene, and β-carotene by 10 times; it surpasses α-tocopherol by 100 times [55]. SOD1, as an antioxidant enzyme, significantly decreases during joint injuries and plays a crucial role in OA [56]. The research findings demonstrate that silencing CircHP1BP3 can inhibit the protective effect of astaxanthin against tBHP-induced C28/I2 cell damage. This was confirmed by measuring apoptosis, IL-6, IL-1β, TNF-α, MDA, ROS, matrix metalloproteinase 13 expression levels, superoxide dismutase, GSH, GPX, GSSG activities, as well as the levels of type II collagen and proteoglycans. Astaxanthin restored the downregulation of expression induced by tBHP by modulating circHP1BP3 levels, indicating that astaxanthin improves tBHP-induced cell damage by regulating circHP1BP3 levels, enhancing SOD1 activity, and reducing inflammatory response.
MicroRNAs regulate mRNA and protein levels through complementary base pairing and binding to mRNA sites [57, 58]. circRNAs, containing abundant microRNA response elements, act as competitive endogenous RNA molecules, serving as sponges for microRNAs [59, 60]. In this study, circ-HP1BP3 circRNA also functions as a sponge for microRNAs. Using Venn diagram analysis, miRNAs predicted to have target binding sites within circHP1BP3 and SOD1 genes were identified. The results revealed that both circHP1BP3 and SOD1's 3'UTR regions contain binding sites for miR-139-5p. Dual-luciferase reporter assays, AGO2 RNA immunoprecipitation, and pull-down experiments further confirmed the targeting interaction between miR-139-5p and circHP1BP3 as well as SOD1. The binding of miR-139-5p to circHP1BP3 can modulate SOD1 expression by sequestering miR-139-5p. These experimental findings demonstrate that circHP1BP3 influences SOD1 expression levels by targeting miR-139-5p, ultimately regulating chondrocyte function. The study reveals that miR-139-5p negatively regulates SOD1 mRNA and protein expression in C28/I2 cells. Overexpression of circHP1BP3 or treatment with AST significantly increased SOD1 mRNA and protein levels while markedly reducing levels of IL-6, IL-1β, TNF-α, IL-8, IL-2, MDA, and ROS, indicating that high levels of circHP1BP3 can effectively repair chondrocyte damage. AST alleviates chondrocyte injury induced by tBHP, promoting cell proliferation and suppressing extracellular matrix (ECM) degradation [61, 62]. However, AST exhibits poor solubility and stability when exposed to oxygen, light, and high temperatures simultaneously [63, 64]. Effective drug delivery systems can enhance the bioavailability of AST, achieve localized sustained release effects, and aid in chondrocyte repair [64, 65]. Therefore, in this study, we prepared sustained-release microspheres loaded with AST and co-cultured them with chondrocytes to improve its bioavailability. Analysis of IL-6, IL-1β, TNF-α, IL-8, IL-2, MDA, and ROS levels demonstrated that sustained-release AST microspheres significantly ameliorated tBHP-induced chondrocyte injury. Silencing circ-HP1BP3 inhibited the beneficial effects of sustained-release AST microspheres on chondrocyte improvement, suggesting that the microspheres regulate tBHP-induced chondrocyte injury by modulating circHP1BP3.
Previous studies have demonstrated that combining an efficient drug delivery system with a bioengineering scaffold can further accelerate the repair of damaged tissue and the growth of new tissue [66]. Gelatin, a hydrolyzed product of collagen, is known for its low immunogenicity, excellent biocompatibility, and complete absorption in the body [67, 68]. Research indicates that it promotes the adhesion, proliferation, and differentiation of chondrocytes on the scaffold and is widely used as a scaffold material in cartilage repair [69]. In future studies, sustained-release microspheres loaded with AST will be mixed with gelatin in a certain proportion to prepare AST-loaded sustained-release microsphere-gelatin composite scaffolds, exploring their application in tissue-engineered cartilage construction.
While the current results confirm the protective characteristics of AST and the circHP1BP3/miR-139-5p/SOD1 axis on chondrocytes, there are key limitations to consider. One limitation is the lack of inclusion of healthy patients and comparison of expression between healthy control groups and patients with OA. Additionally, various cell types are involved in OA, including synovial macrophages [70, 71] and anterior cruciate ligament cells [72], all of which influence the progression of OA. Therefore, another restriction of our experiment is the validation using only a single cell type. Although our use of tBHP-treated C28/I2 cells demonstrated the role of AST in vitro, the complex inflammatory microenvironment of OA should be further considered. These limitations will be addressed and explored in future in-depth research. In conclusion, this study establishes a theoretical basis for the application of AST in the treatment of OA.
Conclusion
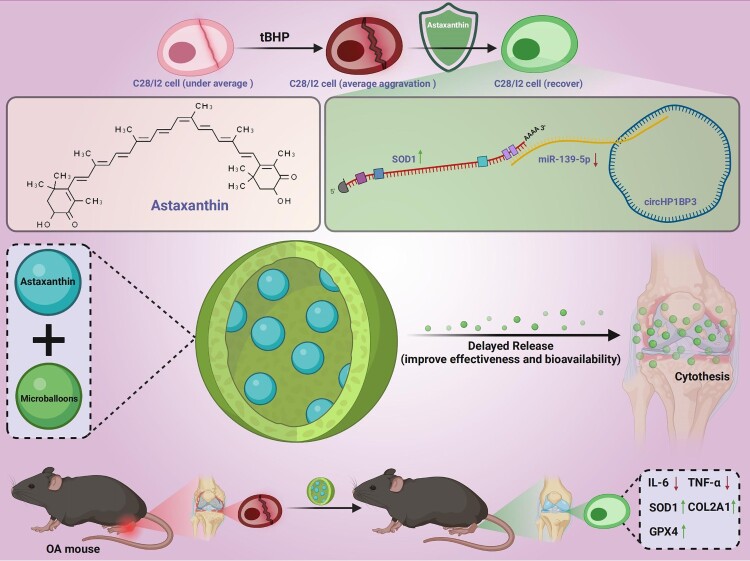
This study confirms that AST significantly improves the survival rate of chondrocytes, reduces the expression of inflammatory factors, and enhances cellular antioxidant capacity by regulating the interaction between circHP1BP3 and miR-139-5p, as illustrated in Figure 7. Furthermore, the use of AST sustained-release microspheres further demonstrates its potential value in repairing damaged chondrocytes. Additionally, AST alleviates joint damage in OA mice, restoring the expression of SOD1 in joint tissues of OA mice. These findings suggest that AST may serve as a potential therapeutic agent for treating oxidative stress-related diseases such as OA.
Figure 7.
Therapeutic effects of AST on OA via regulation of the circ-HP1BP3/miR-139-5p/SOD1 pathway.
Supplementary Material
Funding Statement
This study was supported by Young Talent Development Plan of Changzhou Health Commission (CZQM2022009); Changzhou Health Talents Overseas Training Grant Project (GW2023021).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data can be provided as needed.
Ethical statement
All experimental procedures were conducted following animal ethics and welfare guidelines, with prior approval from the Institutional Animal Care and Use Committee (IACUC).
Author contributions
Wenwei Liang: Conceptualization, Investigation, Data curation, Writing – original draft. Gang Liu: Methodology, Investigation, Writing – review & editing. Weibo Zhou: Formal analysis, Visualization, Validation. Wei Chen: Investigation, Resources, Software. Yaojun Lu: Data curation, Project administration. Hao Wu: Supervision, Writing – review & editing. Yao Qin: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. Chunhui Zhu: Conceptualization, Project administration, Supervision, Writing – review & editing. All authors read and approved the final manuscript.
References
- 1.Yao Q, Wu X, Tao C, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):56. doi: 10.1038/s41392-023-01330-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin Z, Chang B, Wei Y, et al. Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy. Biomed Pharmacother. 2022;151:113092. doi: 10.1016/j.biopha.2022.113092 [DOI] [PubMed] [Google Scholar]
- 3.Mintarjo JA, Poerwanto E, Tedyanto EH.. Current Non-surgical management of knee osteoarthritis. Cureus. 2023;15(6):e40966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shevroja E, Reginster JY, Lamy O, et al. Update on the clinical use of Trabecular Bone Score (TBS) in the management of osteoporosis: results of an expert group meeting organized by the European society for clinical and economic aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), and the International Osteoporosis Foundation (IOF) under the auspices of WHO Collaborating Center for Epidemiology of musculoskeletal health and aging. Osteoporos Int. 2023;34(9):1501–1529. doi: 10.1007/s00198-023-06817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin S, Tyrrell J, Thomas EL, et al. Disease consequences of higher adiposity uncoupled from its adverse metabolic effects using Mendelian randomisation. Elife. 2022:11:e72452. doi: 10.7554/eLife.72452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Encho H, Uchida K, Nakamura J, et al. Association between locomotive syndrome and anemia among community-dwelling older adults. Geriatr Gerontol Int. 2023;23(6):426–429. doi: 10.1111/ggi.14593 [DOI] [PubMed] [Google Scholar]
- 7.Park J, Lee M, Lee H, et al. National trends in rheumatoid arthritis and osteoarthritis prevalence in South Korea, 1998-2021. Sci Rep. 2023;13(1):19528. doi: 10.1038/s41598-023-46279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diseases GBD, Injuries C . Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403(10440):2133–2161. doi: 10.1016/S0140-6736(24)00757-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu P, Feng J, Hu Y, et al. Botanical drug extracts combined With biomaterial carriers for osteoarthritis cartilage degeneration treatment: A review of 10 years of research. Front Pharmacol. 2022;12:789311. doi: 10.3389/fphar.2021.789311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlesworth J, Fitzpatrick J, Perera NKP, et al. Osteoarthritis- a systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet Disord. 2019;20(1):151. doi: 10.1186/s12891-019-2525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinohara M, Akagi R, Watanabe A, et al. Time-Dependent change in cartilage repair tissue evaluated by magnetic resonance imaging up to 2 years after atelocollagen-assisted autologous cartilage transplantation: data from the CaTCh study. Cartilage. 2022;13(3):19476035221109227. doi: 10.1177/19476035221109227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Contreras PC, Kluz PN, Hines MR, et al. Intersections between mitochondrial metabolism and redox biology mediate posttraumatic osteoarthritis. Curr Rheumatol Rep. 2021;23(5):32. doi: 10.1007/s11926-021-00994-z [DOI] [PubMed] [Google Scholar]
- 13.Hajam YA, Rani R, Ganie SY, et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. 2022;11(3):552. doi: 10.3390/cells11030552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahola S, Langer T.. Ferroptosis in mitochondrial cardiomyopathy. Trends Cell Biol. 2023;34(2):150–160. doi: 10.1016/j.tcb.2023.06.002 [DOI] [PubMed] [Google Scholar]
- 15.Shaito A, Aramouni K, Assaf R, et al. Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front Biosci (Landmark Ed). 2022;27(3):105. doi: 10.31083/j.fbl2703105 [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Guo X, Zeng Y, et al. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci Rep. 2023;13(1):15515. doi: 10.1038/s41598-023-42760-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Huan Y, Liu S, et al. Diphenyl diselenide alleviates tert-butyl hydrogen peroxide-induced oxidative stress and lipopolysaccharide-induced inflammation in Rat glomerular mesangial cells. Int J Mol Sci. 2022;23(19):11215. doi: 10.3390/ijms231911215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ardizzone A, Repici A, Capra AP, et al. Efficacy of the radical scavenger, tempol, to reduce inflammation and oxidative stress in a murine model of atopic dermatitis. Antioxidants (Basel). 2023;12(6):1278. doi: 10.3390/antiox12061278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen QTN, Fang M, Zhang M, et al. Crataegus laevigata suppresses LPS-induced oxidative stress during inflammatory response in human keratinocytes by regulating the MAPKs/AP-1, NFkappaB, and NFAT signaling pathways. Molecules. 2021;26(4):869. doi: 10.3390/molecules26040869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitada M, Koya D.. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17(11):647–661. doi: 10.1038/s41574-021-00551-9 [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Xu C, Xiao W, et al. Unravelling the role of NFE2L1 in stress responses and related diseases. Redox Biol. 2023;65:102819. doi: 10.1016/j.redox.2023.102819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu C, Fan X, Yu W.. Functional diversity of mammalian small heat shock proteins: A review. Cells. 2023;12(15):1947. doi: 10.3390/cells12151947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan R, Peng X, Xie L, et al. Importance of Bmal1 in Alzheimer's disease and associated aging-related diseases: mechanisms and interventions. Aging Cell. 2022;21(10):e13704. doi: 10.1111/acel.13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Yang J, Xiang R, et al. Research and publication trends on knee osteoarthritis and cellular senescence: a bibliometric analysis. Front Physiol. 2023;14:1269338. doi: 10.3389/fphys.2023.1269338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo X, Yu B, Yan M, et al. Endogenous development models and paths selection of rural revitalization from the perspective of ecological environment advantages: A case study of Nanshi village, China. Int J Environ Res Public Health. 2022;19(19):11979. doi: 10.3390/ijerph191911979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Kumar R, Diksha, et al. Astaxanthin: A super antioxidant from microalgae and its therapeutic potential. J Basic Microbiol. 2022;62(9):1064–1082. doi: 10.1002/jobm.202100391 [DOI] [PubMed] [Google Scholar]
- 27.Nishida Y, Nawaz A, Hecht K, et al. Astaxanthin as a novel mitochondrial regulator: A New aspect of carotenoids, beyond antioxidants. Nutrients. 2022;14(1):107. doi: 10.3390/nu14010107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara KY, Yagi S, Hirono-Hara Y, et al. A method of solubilizing and concentrating astaxanthin and other carotenoids. Mar Drugs. 2021;19(8):462. doi: 10.3390/md19080462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y, Osewe M, Anastacia C, et al. Agricultural supply-side structural reform and path optimization: evidence from China. Int J Environ Res Public Health. 2023;20(1):113. doi: 10.3390/ijerph20010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Chen X, Baker JS, et al. Astaxanthin promotes mitochondrial biogenesis and antioxidant capacity in chronic high-intensity interval training. Eur J Nutr. 2023;62(3):1453–1466. doi: 10.1007/s00394-023-03083-2 [DOI] [PubMed] [Google Scholar]
- 31.Kang Y, Xu L, Dong J, et al. Programmed microalgae-gel promotes chronic wound healing in diabetes. Nat Commun. 2024;15(1):1042. doi: 10.1038/s41467-024-45101-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill WS, Dohnalek MH, Ha Y, et al. A multicenter, randomized, double-blinded, placebo-controlled clinical trial to evaluate the efficacy and safety of a krill Oil, astaxanthin, and oral hyaluronic acid complex on joint health in people with mild osteoarthritis. Nutrients. 2023;15(17):3769. doi: 10.3390/nu15173769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadanangode Narayanaswam N, Caston E, Satish Kumar RC, Vijayakumar TM, Vanangamudi VS, Pankaj N, et al. A randomized interventional clinical trial assessing the safety and effectiveness of PeaNoc XL tablets in managing joint pain and inflammation in arthritis patients. F1000Res. 2023;12:895. doi: 10.12688/f1000research.138477.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oydanich M, Khouri AS.. Risk of aerosolization and the importance of corneal hysteresis measurements in glaucoma patients during the COVID-19 Era. Int Ophthalmol. 2023;43(2):359–361. doi: 10.1007/s10792-022-02455-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohandel Z, Farkhondeh T, Aschner M, et al. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed Pharmacother. 2022;145:112179. doi: 10.1016/j.biopha.2021.112179 [DOI] [PubMed] [Google Scholar]
- 36.Medoro A, Davinelli S, Milella L, et al. Dietary astaxanthin: A promising antioxidant and anti-inflammatory agent for brain aging and adult neurogenesis. Mar Drugs. 2023;21(12):643. doi: 10.3390/md21120643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao V, Bhushan R, Kumari P, et al. Chemobrain: a review on mechanistic insight, targets and treatments. Adv Cancer Res. 2022;155:29–76. doi: 10.1016/bs.acr.2022.04.001 [DOI] [PubMed] [Google Scholar]
- 38.Rostami S, Alyasin A, Saedi M, et al. Astaxanthin ameliorates inflammation, oxidative stress, and reproductive outcomes in endometriosis patients undergoing assisted reproduction: A randomized, triple-blind placebo-controlled clinical trial. Front Endocrinol (Lausanne). 2023;14:1144323. doi: 10.3389/fendo.2023.1144323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Y, Yan W, Sun L, et al. PiRNA hsa_piR_019914 promoted chondrocyte anabolic metabolism By inhibiting LDHA-dependent ROS production. Cartilage. 2024;15(3):303–314. doi: 10.1177/19476035231181094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan Y, Shen K, Yu H, et al. Baicalein limits osteoarthritis development by inhibiting chondrocyte ferroptosis. Free Radical Biol Med. 2023;196:108–120. doi: 10.1016/j.freeradbiomed.2023.01.006 [DOI] [PubMed] [Google Scholar]
- 41.Chen WP, Xiong Y, Shi YX, et al. Astaxanthin reduces matrix metalloproteinase expression in human chondrocytes. Int Immunopharmacol. 2014;19(1):174–177. doi: 10.1016/j.intimp.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 42.Zhu CH, Zhang Y, Song HH, et al. Protective effect of astaxanthin on tert-butyl hydrogen peroxide-induced chondrocyte damage. Zhongguo Zuzhi gongcheng Yanjiu. 2022;26(11):1648–1655. doi: 10.12307/2022.346 [DOI] [Google Scholar]
- 43.Corradetti B, Taraballi F, Minardi S, et al. Chondroitin sulfate immobilized on a biomimetic scaffold modulates inflammation while driving chondrogenesis. Stem Cells Transl Med. 2016;5(5):670–682. doi: 10.5966/sctm.2015-0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ansari MY, Ahmad N, Haqqi TM.. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. 2020;129:110452. doi: 10.1016/j.biopha.2020.110452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeo C, Ahn CR, Kim JE, et al. Chaenomeles Fructus (CF), the fruit of chaenomeles sinensis alleviates IL-1beta induced cartilage degradation in Rat articular chondrocytes. Int J Mol Sci. 2022;23(8):4360. doi: 10.3390/ijms23084360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Zhang W, Liu T, et al. The physiological metabolite α-ketoglutarate ameliorates osteoarthritis by regulating mitophagy and oxidative stress. Redox Biol. 2023;62:102663. doi: 10.1016/j.redox.2023.102663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henrotin Y, Kurz B, Aigner T.. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13(8):643–654. doi: 10.1016/j.joca.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 48.Scott JL, Gabrielides C, Davidson RK, et al. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 2010;69(8):1502–1510. doi: 10.1136/ard.2009.119966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajagopalan S, Meng XP, Ramasamy S, et al. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98(11):2572–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen SV, Oury TD, Ostergaard L, et al. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279(14):13705–13710. doi: 10.1074/jbc.M310217200 [DOI] [PubMed] [Google Scholar]
- 51.Chang MX, Xiong F.. Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases: recent advances and future directions. Molecules. 2020;25(22):5342. doi: 10.3390/molecules25225342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farruggia C, Kim MB, Bae M, et al. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J Nutr Biochem. 2018;62:202–209. doi: 10.1016/j.jnutbio.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 53.Liu Q, Zhang X, Hu X, et al. Circular RNA related to the chondrocyte ECM regulates MMP13 expression by functioning as a MiR-136 ‘sponge’ in human cartilage degradation. Sci Rep. 2016;6:22572. doi: 10.1038/srep22572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu K, Yan W, Dai Z, et al. Astaxanthin extract from shrimp (Trachypenaeus curvirostris) by-products improves quality of ready-to-cook shrimp surimi products during frozen storage at -18 degrees C. Foods. 2022;11(14):2122. doi: 10.3390/foods11142122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM.. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46(2):185–196. doi: 10.1080/10408690590957188 [DOI] [PubMed] [Google Scholar]
- 56.Koike M, Nojiri H, Kanazawa H, et al. Superoxide dismutase activity is significantly lower in end-stage osteoarthritic cartilage than non-osteoarthritic cartilage. PLoS One. 2018;13(9):e0203944. doi: 10.1371/journal.pone.0203944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loeser RF, Collins JA, Diekman BO.. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–420. doi: 10.1038/nrrheum.2016.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z, Huang S.. Inhibition of miR-191 contributes to radiation-resistance of two lung cancer cell lines by altering autophagy activity. Cancer Cell Int. 2015;15(1):16. doi: 10.1186/s12935-015-0165-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulcheski FR, Christoff AP, Margis R.. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J Biotechnol. 2016;238:42–51. doi: 10.1016/j.jbiotec.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 60.Zhou M, Xiao MS, Li Z, et al. New progresses of circular RNA biology: from nuclear export to degradation. RNA Biol. 2021;18(10):1365–1373. doi: 10.1080/15476286.2020.1853977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sztretye M, Dienes B, Gonczi M, et al. Astaxanthin: A potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid Med Cell Longevity. 2019;2019:3849692. doi: 10.1155/2019/3849692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fakhri S, Abbaszadeh F, Dargahi L, et al. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol Res. 2018;136:1–20. doi: 10.1016/j.phrs.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 63.Zhang C, Tan X, Lv C, et al. Shrimp ferritin greatly improves the physical and chemical stability of astaxanthin. J Food Sci. 2021;86(12):5295–5306. doi: 10.1111/1750-3841.15945 [DOI] [PubMed] [Google Scholar]
- 64.Sun J, Wei Z, Xue C.. Recent research advances in astaxanthin delivery systems: fabrication technologies, comparisons and applications. Crit Rev Food Sci Nutr. 2023;63(19):3497–3518. doi: 10.1080/10408398.2021.1989661 [DOI] [PubMed] [Google Scholar]
- 65.Zhao X, Wang K, Zhao J, et al. Physical and oxidative stability of astaxanthin microcapsules prepared with liposomes. J Sci Food Agric. 2022;102(11):4909–4917. doi: 10.1002/jsfa.11854 [DOI] [PubMed] [Google Scholar]
- 66.Bharathi R, Ganesh SS, Harini G, et al. Chitosan-based scaffolds as drug delivery systems in bone tissue engineering. Int J Biol Macromol. 2022;222(Pt A):132–153. doi: 10.1016/j.ijbiomac.2022.09.058 [DOI] [PubMed] [Google Scholar]
- 67.Shi M, Gao Y, Lee L, et al. Adaptive gelatin microspheres enhanced stem cell delivery and integration With diabetic wounds to activate skin tissue regeneration. Front Bioeng Biotechnol. 2022;10:813805. doi: 10.3389/fbioe.2022.813805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan J, Lee CS, Kim S, et al. Trb3 controls mesenchymal stem cell lineage fate and enhances bone regeneration by scaffold-mediated local gene delivery. Biomaterials. 2021;264:120445. doi: 10.1016/j.biomaterials.2020.120445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao S, Meng H, Zhao J, et al. Injectable adipose-derived stem cells-embedded alginate-gelatin microspheres prepared by electrospray for cartilage tissue regeneration. J Orthop Translat. 2022;33:174–185. doi: 10.1016/j.jot.2022.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donahue RP, Link JM, Meli VS, et al. Stiffness- and bioactive factor-mediated protection of self-assembled cartilage against macrophage challenge in a novel Co-culture system. Cartilage. 2022;13(1):19476035221081466. doi: 10.1177/19476035221081466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samavedi S, Diaz-Rodriguez P, Erndt-Marino JD, et al. A three-dimensional chondrocyte-macrophage coculture system to probe inflammation in experimental osteoarthritis. Tissue Eng, Part A. 2017;23(3-4):101–114. doi: 10.1089/ten.tea.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Long D, Xu Y, Mao G, et al. tRNA-derived fragment TRF365 regulates the metabolism of anterior cruciate ligament cells by targeting IKBKB. Cell Death Discov. 2022;8(1):19. doi: 10.1038/s41420-021-00806-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data can be provided as needed.