Abstract
Background
Malignant mesothelioma is an aggressive cancer with poor prognosis. Programmed cell death protein-1 (PD-1) and its ligand 1 (PD-L1) immune checkpoint inhibitors (ICIs) have recently presented as a viable option in some first line but primarily as a second-line treatment of advanced-stage malignant mesothelioma (asMM). Therefore, this systematic review and meta-analysis aims to assess the safety and efficacy of PD-1/L-1 ICIs in advanced-stage malignant mesothelioma.
Methods
PubMed, Scopus, and Cochrane databases were searched for all studies assessing the safety and efficacy of anti PD-1/PD-L1 agents. Primary outcomes were objective response rate (ORR) and disease control rate (DCR). Secondary outcomes were median progression free (mPFS) and overall survival (mOS). Safety outcomes were treatment- (TRAEs) and immune-related adverse events (IRAEs). A random-effects meta-analysis was performed to pool medians and to derive event rates.
Results
A total of 15 studies were included with total of 1064 asMM patients. ORR and DCR were 16% and 57%, respectively. A pooled mPFS was 4.53 (CI: 3.40–5.65) and mOS was 10.51 (CI: 9.03-12.00). Overall TRAEs had an event rate of 0.69 (0.50–0.83) whereas IRAEs had an event rate of 0.28 (0.15–0.46). There were no significant differences between pembrolizumab, nivolumab primarily, and avelumab subgroups for all the outcomes. Additionally, meta-regression found no covariate to be a significant factor in ORR and DCR.
Conclusion
In this meta-analysis we found that anti-PD1/PD-L1 treatment could be useful in pretreated asMM as they had at least comparable or greater mPFS, mOS, ORR, and DCR than other second-line agents currently being used.
Registration number
This systematic review was registered at PROSPERO prior to the literature search, CRD42023442350.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-13127-3.
Keywords: PD-1, PD-L1, Immune checkpoint inhibitors, Malignant mesothelioma
Background
Malignant mesothelioma (MM) is an aggressive cancer with a poor prognosis that typically results from being exposed to asbestos fibres [1]. It commonly develops in the outer lining of the chest cavity or, less often, in the lining of the abdominal cavity, the sac around the heart, or the testes. There are three main histological subtypes of mesothelioma, each with decreasing levels of survival rates: epithelioid, biphasic, and sarcomatoid [2, 3]. Palliative chemotherapy is often recommended for many individuals diagnosed with MM who are not eligible for surgery due to factors such as advanced stage, advanced age, underlying health conditions, or limited physical well-being. The combination of cisplatin and pemetrexed is a standard first-line treatment for unresectable malignant pleural mesothelioma which was approved in 2004 [4]. From 1990 to 2017, global mesothelioma deaths varied between 18,200 and 32,400 [5]. However, one study by Driscoll et al. [6] showed an estimate of 43,000 deaths. Currently, the burden of global mesothelioma deaths is shouldered by high-income countries [7]. However, a group of studies led by Takahashi found that a rise in global mesothelioma deaths is inevitable, especially in the developing countries [8–10]. To date, there have been no randomized phase 3 clinical trials that have demonstrated any improvement in the overall survival of patients with malignant mesothelioma after the progression of the disease, regardless of the use of new drugs or drug combinations [11, 12].
Recently, modest improvements in progression-free and overall survival were observed with bevacizumab as compared to chemotherapy alone [13]. Over the past few years, the treatment landscape of numerous solid tumors has been significantly transformed by the introduction of immune checkpoint inhibitors (ICIs). Although cytotoxic T lymphocyte antigen-4 (CTLA-4) directed agents did not show any benefit in advanced-stage malignant mesothelioma (asMM) [12], programmed cell death protein-1 (PD-1) and programmed death ligand 1 (PD-L1) antagonists have shown benefit in increasing survival [14]. Studies such as those by Sahin et al. [15]and Guven et al. [16] demonstrate that novel combinatorial strategies have led to incremental improvements in patient survival in other solid tumors, and these findings are shaping the framework for treatment in malignant mesothelioma. Studies have emphasized the role of molecular biomarkers in guiding immune-oncology (IO) therapy for advanced cancers, including MM [17]. However, the heterogeneity and low mutation burden in MM present challenges in pinpointing reliable predictors of response. Additionally, Rizzo et al. [18] identified potential markers beyond PD-L1 that may influence the efficacy of IO therapies, suggesting an expanding research focus for this cancer.
The findings from various clinical phase I or III trials have shown varying effectiveness of different second line and onward ICI monotherapies in treating MM. Nivolumab demonstrated median progression-free survival (mPFS) and median overall survival (mOS) durations ranging from 2.6 to 5.9 months and 9.2 to 17.3 months, respectively [19–22]. Similarly, Pembrolizumab achieved comparable outcomes with mPFS and mOS durations of 2.1 to 5.4 months and 10 to 11.5 months, respectively [23–25]. Avelumab, on the other hand, resulted in mPFS and mOS durations of 4.1 and 10.7 months, respectively [26]. However, it is essential to note that these outcomes were observed in carefully selected patients, and their reproducibility in a broader population of non-selected patients in routine therapeutic settings remains uncertain.
Due to the inconsistent efficacy outcomes of PD-1/PD-L1 ICIs across various trials, this systematic review and meta-analysis aims to synthesize existing evidence on the safety and efficacy of second-line PD-1 and PD-L1 ICI monotherapies in patients with advanced-stage malignant mesothelioma (asMM) who have already undergone treatment. By compiling data from multiple studies, we aim to provide a more comprehensive understanding of these therapies’ potential benefits and risks, ultimately offering valuable insights to guide clinical decisions and support the development of more effective treatment strategies for asMM.
Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systemic Review and Meta-analysis (PRISMA) [27] and Cochrane Collaboration guidelines [28]. A protocol was registered at PROSPERO prior to the literature search, CRD42023442350.
Data sources and search strategy
Two independent (AZ and AM) investigators conducted a systematic literature search using electronic databases, including PubMed, Scopus, and Cochrane Central, from inception to July 2023. Online databases such as www.clinicaltrials.gov, medRxiv.org, and conference proceedings and presentations were also searched to identify grey literature. The following keywords were used: pleural neoplasms, mesothelioma, avelumab, nivolumab, durvalumab, and pembrolizumab. A detailed search strategy used for each database is shown in the supplementary material (Table S1).
Screening of studies
All articles initially retrieved from the systematic search of the electronic databases were transferred to Endnote Reference Library (Version X7.5; Clarivate Analytics, Philadelphia, Pennsylvania) software, where duplicates were identified and removed. Two researchers (AM and AAR) independently shortlisted the remaining articles based on the titles and abstracts and subsequently screened the full texts of the articles to assess relevance. Any discrepancy was settled by consulting a third reviewer (AZ) until a consensus was reached. The reference list of included articles was also sifted manually to identify relevant articles. All discrepancies between the reviewers were resolved by discussion.
Inclusion and exclusion criteria
Articles were shortlisted based on the following eligibility criteria (a) PD-1 or PD-L1 immune checkpoint inhibitors (ICIs) monotherapy in patients with advanced-stage malignant mesothelioma (asMM), (b) studies with at least one outcome of interest, (c) retrospective and prospective cohorts, and randomized controlled trials (RCTs). The primary outcomes included efficacy outcomes which were objective response rate (ORR), disease control rate (DCR), median progression free survival (mPFS), PFS at 6- and 12-month interval, median overall survival (mOS), and OS at 6- and 12-month interval The safety outcomes secondary outcomes which were treatment- and immune-related adverse events divided in two cohorts: (a) adverse event grade 1–5 and (b) adverse event grade 3–5. Articles in languages other than English were excluded. Review articles, editorials, and commentaries were also excluded.
Data extraction and quality assessment
Two independent reviewers (AZ and AM) conducted data extraction of the relevant articles shortlisted. In each study following data was extracted: (a) study name and year, (b) study design, (c) the number of patients in each group, (d) general patient characteristics (age and gender), (e) tumor histology, (f) all the outcomes of interest. Two independent reviewers performed a quality assessment to gauge the validity and reliability of the included studies. The risk of bias in the included non-randomized studies was evaluated independently by two investigators (AA and AB) using the Risk Of Bias In Non-randomised Studies - of Interventions (ROBINS-I) tool [29]. Further, the score assigned to each study was categorized into ratings. The risk of bias-2 tool (RoB-2) of the Cochrane collaboration [30] was used to evaluate quality of the included randomized controlled trials. Any disagreement was resolved by consensus.
Statistical analysis
This meta-analysis was conducted using Review Manager (RevMan) [Computer program] Version 5.4 Cochrane Collaboration, Comprehensive Meta Analysis Version 3.3.070, and OpenMetaAnalyst. A random-effects model was used to calculate event rates for dichotomous variables, whereas the Mantel-Haenszel (MH) method was used to pool medians and 95% CI for median PFS and median OS. A p-value less than 0.05 was considered significant in all cases. The Higgins I2 index was utilized to examine heterogeneity among the included studies. The I2 values of 0–25% were labeled as low, 25–50% as mild, 50–75% as moderate, and 75% above as critical. For each clinical outcome, forest plots were generated to show the relative effect sizes of the comparison groups. Publication bias assessment was carried out by performing Egger’s regression test. A subgroup analysis was performed for all outcomes based on the type of immune checkpoint inhibitor to evaluate their individual impact on the overall effect size. Additionally, meta-regression was performed for primary outcomes and various covariates.
Results
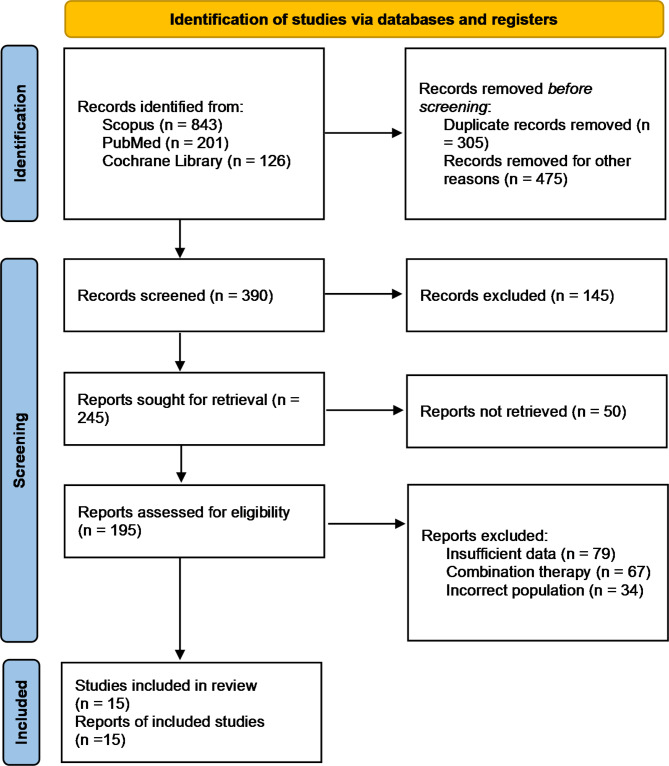
A total of 1170 potentially relevant citations were identified and screened from the initial search. After the removal of duplicated studies, we retrieved 195 full-text articles for evaluation of which 15 studies fulfilled the set selection criteria. The PRISMA flow chart of the study selection is shown in Fig. 1. From the 15 studies, two were RCTs [20, 23], four were phase 2 trials [21, 24, 31, 32], two were phase 1b trials [26, 33], and seven were retrospective cohorts [34–40]. Of the 14, six assessed efficacies of pembrolizumab, seven evaluated nivolumab, and one tested avelumab. A total of 1133 patients with advanced-stage malignant mesothelioma were identified. The mean age of the patients was 66.8 (10.2) years and 74.5% were males. Study characteristics are shown in Table 1 and detailed patient characteristics are shown in Table 2. All the included studies had a low risk of bias. Detailed quality assessment is shown in Figures S1 and S2.
Fig. 1.
PRISMA flowchart
Table 1.
General characteristics of the included studies
| Study Name | Type | Sample size | Patient Population | Mean Age | Male % |
|---|---|---|---|---|---|
| Pembrolizumab | |||||
| Alley 2017 (34) | Phase 1b trial | 25 | PD-L1b positive MPM | 65 (12.6) | 68 |
| Metaxas 2018 (37) | Retrospective | 93 | MPM | 63.8 (14) | 91 |
| Ahmadzada 2020 (35) | Retrospective | 98 | MPM | - | 92 |
| Popat 2020 (23) | RCTc | 73 | MPM | 68.3 (6.5) | 79.4 |
| Yap 2021 (24) | Phase 2 trial | 118 | MPMa | 64.3 (2.3) | 72 |
| Marmarelis 2023 (36) | Retrospective | 24 | Diffuse MPM | 61.7 (14.4) | 41.7 |
| Nivolumab | |||||
| Okada 2019 (31) | Phase 2 trial | 34 | MPM | 64.3 (8.4) | 85 |
| Scherpereel 2019 (21) | Phase 2 trial | 63 | MPM | 71.2 (9.5) | 75 |
| Cantini 2020 (39) | Retrospective | 107 | Pretreated MPM | 64 (9.9) | 87 |
| Nakamura 2020 (40) | Retrospective | 35 | Postoperative recurrence MPM | 68 (5.2) | 88.6 |
| Fennell 2021 (20) | RCT | 221 | Mesothelioma | 69.7 (6.7) | 76 |
| Yoneda 2021 (41) | Retrospective | 11 | MPM | 71 (8.8) | 72.7 |
| Assie 2022 (38) | Retrospective | 109 | MPM | 69 (7.5) | 67.9 |
| Avelumab | |||||
| Hassan 2019 (26) | Phase 1b trial | 53 | Mesothelioma | 62.5 (11.3) | 60 |
| Durvalumab | |||||
| Canvo 2022 | Phase 2 trial | 69 | Mesothelioma | 69.9 (8.9) | 63.8 |
Table 2.
Baseline patient and tumor characteristics
| Study Name | ECOGa | PD-L1b expression | Histology | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | ≥ 2 | Pos | Neg | Epithelioid | Biphasic | Sarcomatoid | Not specified | |
| Pembrolizumab | |||||||||
| Alley 2017 (34) | 9 | 16 | 25 | 0 | 18 | 2 | 2 | 3 | |
| Metaxas 2018 (37) | 11 | 55 | 27 | 67 | 10 | 15 | 1 | ||
| Ahmadzada 2020 (35) | 21 | 55 | 18 | 31 | 45 | 74 | 8 | 8 | 8 |
| Popat 2020 (23) | 21 | 51 | 1 | 33 | 36 | 66 | 7 | ||
| Yap 2021 (24) | 44 | 74 | 77 | 31 | 82 | 9 | 10 | 17 | |
| Marmarelis 2023 (36) | 5 | 15 | 1 | 6 | 11 | 18 | 4 | 1 | 1 |
| Nivolumab | |||||||||
| Okada 2019 (31) | 13 | 21 | 20 | 12 | 27 | 4 | 3 | ||
| Scherpereel 2019 (21) | 19 | 42 | 0 | 21 | 31 | 52 | 11 | ||
| Cantini 2020 (39) | 20 | 68 | 6 | 11 | 22 | 78 | 22 | 7 | |
| Nakamura 2020 (40) | 11 | 21 | 3 | 32 | 2 | 1 | |||
| Fennell 2021 (20) | 44 | 177 | 60 | 101 | 195 | ||||
| Yoneda 2021 (41) | 4 | 6 | 1 | 11 | |||||
| Assie 2022 (38) | 91 | 14 | 90 | 11 | 8 | ||||
| Avelumab | |||||||||
| Hassan 2019 (26) | 14 | 39 | 22 | 21 | 43 | 2 | 2 | ||
| Durvalumab | |||||||||
| Canvo 2022 (33) | 43 | 26 | 22 | 21 | 62 | 4 | 3 | ||
aEastern Cooperative Oncology Group
bProgrammed death-ligand 1
Primary outcomes
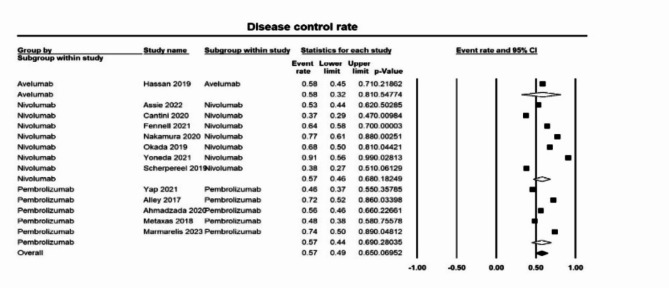
A total of 13 studies were included in the outcome objective response rate (ORR). A pooled overall event rate of 0.16 ([0.13–0.20]; p < 0.05, I2 = 48%) (Fig. 2). For nivolumab, the ORR was 0.15 [0.11–0.20] and 0.17 [0.13–0.23] for pembrolizumab. Thirteen studies evaluating disease control rate (DCR) found an overall event rate of 0.57 ([0.49–0.65]; p = 0.07 I2 = 46%) (Fig. 3). No difference was seen in DCRs of nivolumab (0.57 [0.46–0.68]), pembrolizumab (0.57 [0.44–0.69]), and avelumab (0.58 [0.32–0.81]).
Fig. 2.
Forest plot of objective response rate
Fig. 3.
Forest plot of disease control rate
Secondary outcomes
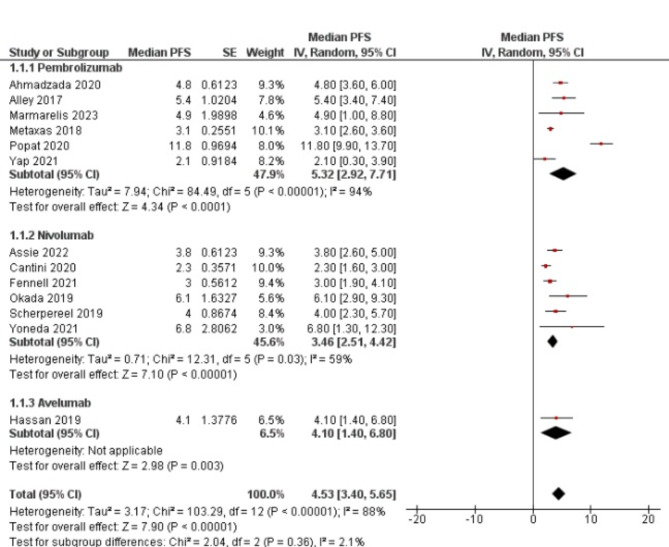
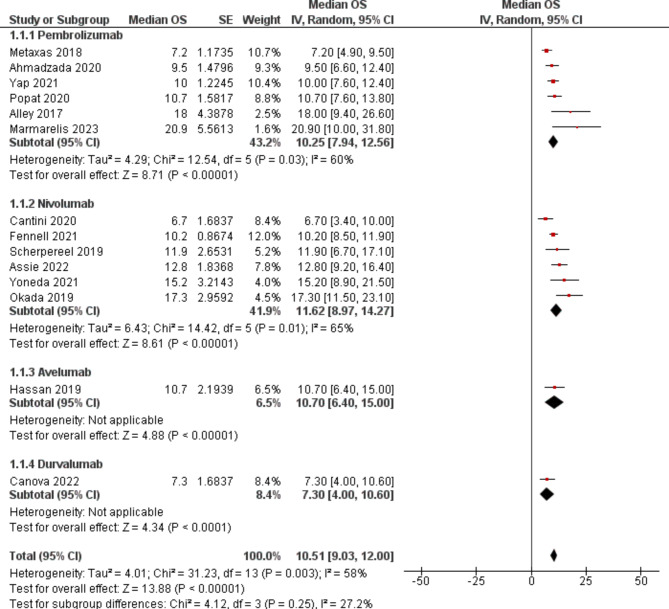
Median progression free survival (mPFS) was assessed in 13 studies. An aggregate mPFS was found to be 4.53 ([3.40–5.65]; p < 0.00001, I2 = 88%) (Fig. 4). No difference was found between the pembrolizumab (5.32 [2.92–7.71]), nivolumab (3.46 [2.51–4.42]), and avelumab (4.10 [1.40–6.80]) subgroups. 6- and 12-month progression free survival was found to be (event rate 0.41 95% CI [0.25–0.59]) (Figure S3) and (event rate 0.18 95% CI [0.11–0.28]) (Figure S4). Similarly, median overall survival (mOS) was found in 14 studies. Pooled mOS was 10.51 ([9.03-12.00]; p < 0.00001, I2 = 58%) across the studies (Fig. 5). No significant difference was observed between pembrolizumab (10.25 [7.94–12.56]), nivolumab (11.62 [8.97–14.27]), avelumab (10.70 [6.40–15.00]), and durvalumab (7.3 [4.00-10.60) subgroups. 6- and 12-month overall survival was found to be (event rate 0.70 95% CI [0.60–0.79]) (Figure S5) and (event rate 0.44 95% CI [0.37–0.51]) (Figure S6).
Fig. 4.
Forest plot of median progression free survival
Fig. 5.
Forest plot of median overall survival
Safety outcomes
Treatment-related adverse events grade 1–5 were found to be nine studies (Table 3). An overall event rate of 0.69 (0.50–0.83) was found (Figure S7). In grades 3–5, the event rate was 0.15 (0.10–0.23) (Figure S8). An overall event rate of immune-related adverse events grade 1–5 was found to be 0.28 (0.15–0.46) (Figure S9) and 0.05 (0.03–0.11) in grades 3–5 (Figure S10).
Table 3.
Treatment- and immune-related adverse events
| Subgroup | Treatment-related adverse events grade 1–5 (event rate and 95% confidence interval) | Treatment-related adverse events grade 3–5 (event rate and 95% confidence interval) |
|---|---|---|
| Avelumab (26) | 0.75 (0.39–0.94) | 0.09 (0.02–0.32) |
| Nivolumab (20,31,32,38) | 0.77 (0.60–0.88) | 0.17 (0.09–0.30) |
| Pembrolizumab (23,24,34,37) | 0.56 (0.37–0.74) | 0.15 (0.10–0.23) |
| Immune-related adverse events grade 1–5 (event rate and 95% confidence interval) | Immune-related adverse events grade 3–5 (event rate and 95% confidence interval) | |
| Avelumab (26) | 0.21 (0.04–0.65) | 0.05 (0.02–0.15) |
| Nivolumab (20,32,41) | 0.39 (0.16–0.67) | 0.02 (0.00-0.1) |
| Pembrolizumab (24,34,35) | 0.22 (0.08–0.46) | 0.07 (0.04–0.11) |
Meta-regression and publication bias
Meta-regression was performed for the primary outcomes against the following covariates: mean age, mean male sex percentage, percentage of PD-1 positive patients, and percentage of ECOG status 0 patients. For ORR and DCR, none of the covariates were found to be a significant predictor. Bubble plots are added to the supplementary material Figures S11-12. There was no significant publication bias detected in all the outcomes. Results of Egger’s regression test is shown in supplementary Table S2.
Discussion
This meta-analysis was performed by pooling all the data of relevant studies todate evaluating safety and efficacy of anti PD-1/PD-L1 immune checkpoint inhibitors as a monotherapy in patients with pre-treated advanced-stage malignant mesothelioma (asMM). In this meta-analysis, we found an objective response rate of 0.16 (0.13–0.20) and a disease control rate of 0.57 (0.49–0.65). Additionally, we observed a median progression free survival of 4.53 (3.40–5.65) and a median overall survival of 10.80 (9.26–12.35). For the safety outcomes, treatment- and immune-related adverse events grade 1–5 had an event rate of 0.69 (0.50–0.83) and 0.28 (0.15–0.46), respectively.
In this meta-analysis, the majority of studies lacked in reporting asbestos exposure in patients with asMM as greater efficacy of anti PD-1/PD-L1 is noted in tumors with an etiology related to carcinogens exposure [41, 42]. Additionally, MM is inherently resistant to cytotoxic chemotherapy [43] and second line treatments have not exclusively shown an increase in overall or progression free survival. The choice of second line agents is based on various factors: choice of patient, response with first-line therapy, and performance status.
The Third Italian Consensus Conference on MM highlighted the need for new treatment options in the second-line setting, as there is currently a lack of approved agents. This situation presents an excellent opportunity to evaluate and test novel drugs. In cases where patients are unable to participate in a clinical trial, single-agent chemotherapy can be considered as a treatment option for those who are medically suitable, although best supportive care remains a valid choice [44]. Vinorelbine and gemcitabine monotherapy are most commonly used in practice for second-line treatment of MM as stated by international guidelines reporting an ORR of 7–16% [45–47]. Moreover, the ESMO clinical practice guidelines suggested the role of platinum-premetrexed or premetrexed alone as a second-line agent as a meta-analysis reported a median overall survival of 7.93 and 7.78 months, respectively [48, 49].
As a second-line agent, our study found anti-PD1/PD-L1 agents to have an ORR of 16% and a DCR of 57% which is consistent with previous meta-analysis [50]. These findings are also found in the PROMISE-meso phase III trial which found pembrolizumab to have a greater ORR than chemotherapy (22% vs. 6%). Our findings were found to be superior to that of gemcitabine and vinorelbine monotherapy in MM patients [46, 47].
Additionally, we found a median PFS of 4.53 months and a median OS of 10.80 months with no significant difference between pembrolizumab, nivolumab, and avelumab. Previous meta-analysis did not pool these parameters however, they found a similar range of mPFS (2.1 to 5.9 months) and mOS (6.7 to 20.9 months) [50]. These findings are consistent with a recent study by Marmarelis et al. reporting an mPFS and mOS of 4.9 and 20.9 months, respectively. Median PFS was comparable to studies evaluating vinorelbine monotherapy for MM (4.2 months) [47] however, mOS was significantly better in our study as compared to gemcitabine (13.8 months) and vinorelbine monotherapy (9.3 months) [47, 51].
We also measured progression free and overall survival at 6- and 12-month intervals. The PFS decreased from an event rate of 0.41 to 0.18 and OS decreased from 0.70 to 0.44. This decline in survival rate could be attributed to various factors. With increasing age, there is decreasing patient survival rate with approximately 6% survival in patients between ages 65 to 74 years. Since pleural mesothelioma is more difficult to remove during surgery as they attack chest linings, they have shorter survival rates as compared to peritoneal mesothelioma. This could explain the decrease in survival rates since the majority of our studies had patients with pleural mesothelioma [52, 53].
In our study, we found a 69% occurrence of grade 1–5 treatment-related adverse events grade, however, only 15% were of a grade 3 or above. Similarly, serious immune-related adverse events were found in only 5% of the patients with asMM. These findings are consistent with RCTs with pembrolizumab and nivolumab monotherapies as interventions [20, 23]. Previous meta-analysis on anti PD-1/PD-L1 agents did not take adverse effects of the drugs into account [50]. Through meta-regression, our study did not find PD-L1 expression to be a significant predictor of increased ORR or DCR. These findings differ from those found by Cantini et al., as they demonstrated PD-L1 positive tumors to be linked with higher ORR however, no difference in PFS or OS was seen [38]. These results are also different from those of the meta-analysis conducted by Tagliamento et al. They found PD-L1 positive tumors to be more responsive to single-agent immunotherapy [50]. The reason for this difference could be as we conducted meta-regression to derive a linear relationship, while they performed subgroup analysis. Regardless, trials are highly heterogenous when accounting for PD-L1 assessment as inconsistent immunohistochemical clones and cut-offs were applied. Moreover, a recent study found that the neoantigenic potential of MM could be attributed to structural chromosomal rearrangements [54]. Due to lack of specific data, meta-regression could not be performed based on the histology of the tumor.
This study highlights PD-1/PD-L1 inhibitors as promising second-line treatments for advanced-stage malignant mesothelioma (MM), demonstrating efficacy and safety profiles that may improve patient outcomes. For clinicians, these results offer evidence-based support for integrating ICIs into treatment strategies for patients with prior therapies. Significant gaps remain, particularly around biomarkers like PD-L1 expression as predictors of response. Inconsistent study designs and PD-L1 assessment methods underscore the need for standardized biomarker evaluation. Future multi-institutional studies should address these gaps, focusing on MM’s diverse subtypes and molecular characteristics. Over the next five years, we anticipate advances in combination IO therapies and personalization based on molecular profiling. These developments could transform the treatment landscape of MM, offering new, more effective options and a better quality of life for patients.
Our meta-analysis has some limitations. Firstly, we were restricted to a single-arm meta-analysis without any comparator as phase III trials are underway and results are yet to be posted. However, this study provides landmark results for pretreated asMM patients, which can provide a basis for future clinical trials. Secondly, there was moderate heterogeneity in several outcomes which was due to the retrospective nature of the studies. In this review, we could only search three major electronic databases/ EMBASE and PsycINFO could not be searched due to lack of access. Moreover, most of the studies were retrospective cohort studies which could potentially add biases to our results.
Conclusion
In this meta-analysis we found that anti-PD1/PD-L1 agents could be useful in pretreated asMM patients regardless of the current known predictive factors of treatment. Our study found relatively lower incidence of severe adverse events, greater ORR and DCR as compared to other second-line agents for MM and at least comparable, if not better, mOS and MPFS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- MM
Malignant mesothelioma
- ICIs
Immune checkpoint inhibitors
- CTLA-4
cytotoxic T lymphocyte antigen-4
- PD-1
programmed cell death protein-1
- asMM
advanced-stage malignant mesothelioma
- PD-L1
programmed death ligand 1
- mPFS
median progression-free survival
- mOS
median overall survival
- RCTs
randomized controlled trials
- ORR
objective response rate
- DCR
disease control rate
- mPFS
median progression free survival
Author contributions
AZ, AAR, and AM conceived the idea; A.M, AZ, AAR, AA, and AJK did write up of the manuscript; and finally, A.A, M.J.T, O.N.S, and AZ reviewed and revised the manuscript for intellectual content critically. All authors approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
PROSPERO registration
This systematic review has been registered with PROSPERO under the registration number (CRD42023442350).
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pass HI, Vogelzang NJ, Hahn S, Carbone M. Malignant pleural mesothelioma. Curr Probl Cancer. 2004;28(3):93–174. [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol. 2008;9(2–3):147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekido Y. Molecular pathogenesis of malignant mesothelioma. Carcinogenesis. 2013;34(7):1413–9. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncology: Official J Am Soc Clin Oncol. 2003;21(14):2636–44. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Naghavi M, Allen C, Barber RM, Carter A, Casey DC, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of Disease Study 2015. Lancet (London England). 2016;388(10053):1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driscoll T, Nelson DI, Steenland K, Leigh J, Concha-Barrientos M, Fingerhut M, et al. The global burden of non malignant respiratory disease due to occupational airborne exposures. Am J Ind Med. 2005;48(6):432–45. [DOI] [PubMed] [Google Scholar]
- 7.Chimed-Ochir O, Arachi D, Driscoll T, Lin RT, Takala J, Takahashi K. Burden of mesothelioma deaths by national income category: current status and future implications. Int J Environ Res Public Health. 2020;17(18):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. 2011;89(10). [DOI] [PMC free article] [PubMed]
- 9.Park EK, Takahashi K, Hoshuyama T, Cheng TJ, Delgermaa V, Le GV, et al. Global magnitude of reported and unreported mesothelioma. Environ Health Perspect. 2011;119(4):514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diandini R, Takahashi K, Park EK, Jiang Y, Movahed M, Le GV, et al. Potential years of life lost (PYLL) caused by asbestos-related diseases in the world. Am J Ind Med. 2013;56(9):993–1000. [DOI] [PubMed] [Google Scholar]
- 11.Krug LM, Kindler HL, Calvert H, Manegold C, Tsao AS, Fennell D, et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2015;16(4):447–56. [DOI] [PubMed] [Google Scholar]
- 12.Maio M, Scherpereel A, Calabrò L, Aerts J, Perez SC, Bearz A, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18(9):1261–73. [DOI] [PubMed] [Google Scholar]
- 13.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387(10026):1405–14. [DOI] [PubMed] [Google Scholar]
- 14.Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet (London England). 2021;397(10272):375–86. [DOI] [PubMed] [Google Scholar]
- 15.Sahin TK, Rizzo A, Aksoy S, Guven DC. Prognostic Significance of the Royal Marsden Hospital (RMH) Score in Patients with Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel) [Internet]. 2024 May 1 [cited 2024 Oct 16];16(10). https://pubmed.ncbi.nlm.nih.gov/38791914/ [DOI] [PMC free article] [PubMed]
- 16.Guven DC, Erul E, Kaygusuz Y, Akagunduz B, Kilickap S, De Luca R et al. Immune checkpoint inhibitor-related hearing loss: a systematic review and analysis of individual patient data. Support Care Cancer [Internet]. 2023 Dec 1 [cited 2024 Oct 16];31(12). https://pubmed.ncbi.nlm.nih.gov/37819422/ [DOI] [PubMed]
- 17.Rizzo A, Santoni M, Mollica V, Logullo F, Rosellini M, Marchetti A et al. Peripheral neuropathy and headache in cancer patients treated with immunotherapy and immuno-oncology combinations: the MOUSEION-02 study. Expert Opin Drug Metab Toxicol [Internet]. 2021 [cited 2024 Oct 16];17(12):1455–66. https://pubmed.ncbi.nlm.nih.gov/35029519/ [DOI] [PubMed]
- 18.Rizzo A. Identifying optimal first-line treatment for advanced non-small cell lung carcinoma with high PD-L1 expression: a matter of debate. Br J Cancer [Internet]. 2022 Nov 1 [cited 2024 Oct 16];127(8):1381–2. https://pubmed.ncbi.nlm.nih.gov/36064585/ [DOI] [PMC free article] [PubMed]
- 19.Fujimoto N, Okada M, Kijima T, Aoe K, Kato T, Nakagawa K et al. Clinical efficacy and safety of Nivolumab in Japanese patients with malignant pleural mesothelioma: 3-Year results of the MERIT Study. JTO Clin Res Rep. 2020;2(3). [DOI] [PMC free article] [PubMed]
- 20.Fennell DA, Ewings S, Ottensmeier C, Califano R, Hanna GG, Hill K, et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021;22(11):1530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scherpereel A, Mazieres J, Greillier L, Lantuejoul S, Dô P, Bylicki O, et al. Nivolumab or Nivolumab plus Ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20(2):239–53. [DOI] [PubMed] [Google Scholar]
- 22.Quispel-Janssen J, van der Noort V, de Vries JF, Zimmerman M, Lalezari F, Thunnissen E, et al. Programmed death 1 Blockade with Nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2018;13(10):1569–76. [DOI] [PubMed] [Google Scholar]
- 23.Popat S, Curioni-Fontecedro A, Dafni U, Shah R, O’Brien M, Pope A, et al. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European thoracic oncology platform (ETOP 9–15) PROMISE-meso trial. Annals Oncology: Official J Eur Soc Med Oncol. 2020;31(12):1734–45. [DOI] [PubMed] [Google Scholar]
- 24.Yap TA, Nakagawa K, Fujimoto N, Kuribayashi K, Guren TK, Calabrò L, et al. Efficacy and safety of pembrolizumab in patients with advanced mesothelioma in the open-label, single-arm, phase 2 KEYNOTE-158 study. Lancet Respir Med. 2021;9(6):613–21. [DOI] [PubMed] [Google Scholar]
- 25.Desai A, Karrison T, Rose B, Pemberton E, Hill B, Straus CM et al. Phase II trial of pembrolizumab (P) in patients (pts) with previously-treated mesothelioma (MM). https://doi.org/101200/JCO20183615_suppl8565. 2018;36(15_suppl):8565–8565.
- 26.Hassan R, Thomas A, Nemunaitis JJ, Patel MR, Bennouna J, Chen FL, et al. Efficacy and Safety of Avelumab Treatment in patients with Advanced Unresectable Mesothelioma: phase 1b results from the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019;5(3):351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Reviews. 2021;10(1). [DOI] [PMC free article] [PubMed]
- 28.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M et al. Cochrane. 2022. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). www.training.cochrane.org/handbook
- 29.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clinical Res ed). 2016;355. [DOI] [PMC free article] [PubMed]
- 30.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). 2019;366. [DOI] [PubMed]
- 31.Okada M, Kijima T, Aoe K, Kato T, Fujimoto N, Nakagawa K, et al. Clinical efficacy and safety of Nivolumab: results of a M ulticenter, op e n-label, Single-a r m, Japanese phase II study in Mal i gnant Pleural Meso t helioma (MERIT). Clin Cancer Res. 2019;25(18):5485–92. [DOI] [PubMed] [Google Scholar]
- 32.Canova S, Ceresoli GL, Grosso F, Zucali PA, Gelsomino F, Pasello G et al. Final results of DIADEM, a phase II study to investigate the efficacy and safety of durvalumab in advanced pretreated malignant pleural mesothelioma. ESMO Open [Internet]. 2022 Dec 1 [cited 2024 Oct 16];7(6). https://pubmed.ncbi.nlm.nih.gov/36463732/ [DOI] [PMC free article] [PubMed]
- 33.Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):623–30. [DOI] [PubMed] [Google Scholar]
- 34.Ahmadzada T, Cooper WA, Holmes M, Mahar A, Westman H, Gill AJ et al. Retrospective evaluation of the Use of Pembrolizumab in Malignant Mesothelioma in a Real-World Australian Population. JTO Clin Res Rep. 2020;1(4). [DOI] [PMC free article] [PubMed]
- 35.Marmarelis ME, Wang X, Roshkovan L, Grady CB, Miura JT, Ginsberg MS, et al. Clinical outcomes Associated with Pembrolizumab Monotherapy among adults with diffuse malignant peritoneal mesothelioma. JAMA Netw open. 2023;6(3):E232526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metaxas Y, Rivalland G, Mauti LA, Klingbiel D, Kao S, Schmid S, et al. Pembrolizumab as Palliative Immunotherapy in Malignant Pleural Mesothelioma. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2018;13(11):1784–91. [DOI] [PubMed] [Google Scholar]
- 37.Assié JB, Crépin F, Grolleau E, Canellas A, Geier M, Grébert-Manuardi A et al. Immune-Checkpoint inhibitors for malignant pleural mesothelioma: a French, Multicenter, Retrospective Real-World Study. Cancers. 2022;14(6). [DOI] [PMC free article] [PubMed]
- 38.Cantini L, Belderbos RA, Gooijer CJ, Dumoulin DW, Cornelissen R, Baart S, et al. Nivolumab in pre-treated malignant pleural mesothelioma: real-world data from the Dutch expanded access program. Translational lung cancer Res. 2020;9(4):1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura A, Kondo N, Nakamichi T, Kuroda A, Hashimoto M, Matsumoto S, et al. Initial evaluation of nivolumab in patients with post-operative recurrence of malignant pleural mesothelioma. Jpn J Clin Oncol. 2020;50(8):920–5. [DOI] [PubMed] [Google Scholar]
- 40.Yoneda H, Nokihara H, Mitsuhashi A, Ozaki R, Yabuki Y, Ogino H et al. Correlation between immune-related adverse events and therapeutic effects of nivolumab in patients with malignant pleural mesothelioma. BMC Pulm Med. 2021;21(1). [DOI] [PMC free article] [PubMed]
- 41.Arbour KC, Riely GJ. Systemic therapy for locally Advanced and Metastatic Non-small Cell Lung Cancer: a review. JAMA. 2019;322(8):764–74. [DOI] [PubMed] [Google Scholar]
- 42.Manca P, Raez LE, Salzberg M, Sanchez J, Hunis B, Rolfo C. The value of immunotherapy in head and neck cancer. Expert Opin Biol Ther. 2019;19(1):35–43. [DOI] [PubMed] [Google Scholar]
- 43.Hudson AL, Weir C, Moon E, Harvie R, Klebe S, Clarke SJ et al. Establishing a panel of chemo-resistant mesothelioma models for investigating chemo-resistance and identifying new treatments for mesothelioma. Sci Rep. 2014;4. [DOI] [PMC free article] [PubMed]
- 44.Novello S, Pinto C, Torri V, Porcu L, Di Maio M, Tiseo M et al. The Third Italian Consensus Conference for Malignant Pleural Mesothelioma: State of the art and recommendations. Critical reviews in oncology/hematology. 2016;104:9–20. [DOI] [PubMed]
- 45.Stebbing J, Powles T, McPherson K, Shamash J, Wells P, Sheaff MT, et al. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung cancer (Amsterdam Netherlands). 2009;63(1):94–7. [DOI] [PubMed] [Google Scholar]
- 46.Van Meerbeeck JP, Baas P, Debruyne C, Groen HJ, Manegold C, Ardizzoni A et al. A Phase II Study of Gemcitabine in Patients with Malignant Pleural Mesothelioma. 1999. [DOI] [PubMed]
- 47.Fennell DA, Casbard AC, Porter C, Rudd R, Lester JF, Nicolson M et al. A randomized phase II trial of oral vinorelbine as second-line therapy for patients with malignant pleural mesothelioma. https://doi.org/101200/JCO20213915_suppl8507. 2021;39(15_suppl):8507–8507.
- 48.Petrelli F, Ardito R, Conti B, Coinu A, Cabiddu M, Ghilardi M, et al. A systematic review and meta-analysis of second-line therapies for treatment of mesothelioma. Respir Med. 2018;141:72–80. [DOI] [PubMed] [Google Scholar]
- 49.Popat S, Baas P, Faivre-Finn C, Girard N, Nicholson AG, Nowak AK, et al. Malignant pleural mesothelioma: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2022;33(2):129–42. [DOI] [PubMed] [Google Scholar]
- 50.Tagliamento M, Bironzo P, Curcio H, De Luca E, Pignataro D, Rapetti SG et al. A systematic review and meta-analysis of trials assessing PD-1/PD-L1 immune checkpoint inhibitors activity in pre-treated advanced stage malignant mesothelioma. Crit Rev Oncol/Hematol. 2022;172. [DOI] [PubMed]
- 51.Pinto C, Zucali PA, Pagano M, Grosso F, Pasello G, Garassino MC, et al. Gemcitabine with or without ramucirumab as second-line treatment for malignant pleural mesothelioma (RAMES): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2021;22(10):1438–47. [DOI] [PubMed] [Google Scholar]
- 52.American Cancer Society. Malignant Mesothelioma. 2020 [cited 2023 Jul 7]. Survival Rates for Mesothelioma. https://www.cancer.org/cancer/types/malignant-mesothelioma/detection-diagnosis-staging/survival-statistics.html
- 53.Mesothelioma Prognosis | Understanding Survival. and Cures | MesotheliomaHelp.org [Internet]. [cited 2023 Jul 7]. https://www.mesotheliomahelp.org/mesothelioma/prognosis/
- 54.Mansfield AS, Peikert T, Smadbeck JB, Udell JBM, Garcia-Rivera E, Elsbernd L, et al. Neoantigenic potential of Complex chromosomal rearrangements in Mesothelioma. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2019;14(2):276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.