Abstract
Background
Endometriosis is a chronic, estrogen-dependent, benign condition, affecting 10–15% of women of reproductive age. It is associated with a prevalence of sexual dysfunction that is nearly twice as high as that seen in women with other benign gynecological conditions. Our study aimed to assess the effect of surgical intervention on sexual function, as measured by the FSFI (Female Sexual Function Index) score, in women with endometriosis compared to those with other benign gynecological conditions, both before and after surgery.
Methods
A comparative analysis was conducted at the Medical University of Vienna from 2015 to 2020. The study included patients suspected of having endometriosis, fibroids, adnexal cysts, and/or infertility. Based on findings during surgery, patients were categorized into two groups: those with endometriosis (n = 64) and control patients (n = 38). All participants completed the FSFI questionnaire before surgery and again 8 to 18 weeks after the operation.
Results
No significant differences were observed in the preoperative FSFI scores between the endometriosis patients and the control group. Similarly, no significant differences were found between the two groups in postoperative scores. However, in women diagnosed with endometriosis, surgical removal of endometriotic lesions significantly increased their full-scale FSFI score, and resulted in a significant improvement in the areas “desire” and “satisfaction”. Improvements were noted in all other areas as well, though they were not statistically significant.
Conclusions
Our research indicates that the surgical removal of endometriotic lesions can lead to an improvement in sexual function, as measured by the FSFI, within 8 to 18 weeks post-surgery. This improvement was not observed in the control group, which underwent surgery for other benign gynecological issues.
Keywords: Sexual function, FSFI, Endometriosis, Surgical excision
Background
Endometriosis is a chronic, benign, estrogen-dependent disease that affects 10–15% of women of reproductive age, resulting in a number of about 176 million women worldwide [1]. It is characterized by the implantation of endometrium-like tissue outside the uterus, which can cause a wide range of symptoms [2]. These include severe dysmenorrhea, dyspareunia, dyschezia, dysuria, chronic pelvic pain, infertility, and/or an impairment of the women’s sexual function. These symptoms can have a negative impact on the social, mental, and physical well-being of affected women which can severely reduce their quality of life [1, 3–5]. It has been described that in women suffering from endometriosis, the prevalence of sexual dysfunction is almost double compared to women with other benign gynecologic diseases (i.e., 61% vs. 35%) [6–8]. Furthermore, endometriosis is one of the leading causes of deep dyspareunia. The risk of suffering from dyspareunia was described nine times higher in patients with endometriosis than in women without endometriosis [9]. Particularly deep-infiltrating endometriosis (DIE) seems to have a negative impact on sexual function including desire, the ability to reach orgasm, the frequency of sexual intercourse, and the global sexual satisfaction [10, 11].
In addition, hormonal medications such as combined contraceptive pills, progesterone-only pills, GnRH (gonadotropin-releasing hormone) analogues or hormonal intrauterine devices (IUDs) which are used as a first- and second-line therapeutic options for endometriosis-associated symptoms [12] can also affect female sexuality, potentially limiting desire, sexual arousal, lubrication, and/or the ability to reach an orgasm [13]. Furthermore, possible side effects of these medications include anxiety disorders and mood swings, which can consequently also lead to an impairment of the patient’s sex life [14].
While some studies have shown that surgical excision of endometriotic lesions can result in a significant improvement of dyspareunia [15, 16], most trials that assessed the sexual function in women with endometriosis before and after surgery [17, 18] were limited by their small sample size and retrospective design. To our knowledge, there are no studies comparing pre- and postoperative sexual function in patients with endometriosis compared to controls.
In this prospective study, we compared the impact of surgical therapy on the sexual function measured by the Female Sexual Function Index (FSFI) score, in women suffering from endometriosis compared to controls. Furthermore, we aimed to compare pre- and postoperative sexual functions within each group.
Methods
Our study was conducted at the tertiary referral Endometriosis Centre of the Medical University of Vienna, certified by EuroEndoCert [19]. The study protocol was approved by the local Ethics Committee (EC no. 1907/2019). All patients gave their written and verbal informed consent at the time of inclusion. All patients had undergone laparoscopic surgery at our department between 2015 and 2020. Indications for surgery included suspected endometriosis, fibroids, adnexal cysts, or infertility. In our collective, patients with adnexal cysts had undergone cystectomy, patients with fibroids had undergone myomectomy, and patients suffering from infertility had undergone a diagnostic laparoscopy and in cases of endometriosis findings, excision of endometriotic lesions. These patients were otherwise healthy women without internal or autoimmune diseases. Depending on the intraoperative findings, patients were either classified as endometriosis patients (n, number = 64) or controls (n = 38). Endometriosis was staged intraoperatively, using the revised rASRM score (revised American Society of Reproductive Medicine Score) [20, 21] as well as the ENZIAN classification [20–22]. None of the patients included in this study suffered from intra- and/or postoperative complications that might have changed their postoperative course.
Exclusion criteria were defined as history of breast cancer in the past 10 years or other malignant diseases in the past 5 years, a history of infectious diseases such as HIV, hepatitis, tuberculosis, or systemic autoimmune diseases. All patients included in our study had regular menstrual cycles and had not taken any hormonal medication in the 3 months preceding study inclusion nor in the 5 months following the surgical intervention.
Preoperatively, an exact patient history was taken regarding demographic and clinical data, age, body mass index (BMI), gravidity, parity, menarche as well as the pain intensity of dysmenorrhea, dyspareunia, and dysuria, measured using the visual analogue scale (VAS) and the extent of the influence of dyspareunia on the patient’s sex life. Patients’ sexual function was assessed using the German-validated version of the FSFI questionnaire [23], which was filled in preoperatively, as well as 8 to 18 weeks postoperatively. This whole procedure took 25 min per patient. Regarding postoperative advice, depending on the extent of the surgical intervention, patients were recommended to abstain from sexual intercourse for a period of 2 up to 4 weeks after surgery.
While several validated questionnaires have been developed to evaluate the quality of female sexuality [24–27], the most commonly used is the Female Sexual Function Index (FSFI). This 19-item questionnaire records the sexual function based on the subjective perception of affected women [28]. It has already been in use for two decades in women suffering from a wide range of different diseases and enables the comparison of different groups [27]. Reliability and validity were confirmed by Rosen et al. in 2000 [25].
The FSFI questionnaire is split into six domains: desire (two items), arousal (four items), lubrication (four items), orgasm (three items), satisfaction (three items), and pain (three items). Each item in the questionnaire is scored from 0 to 5. The sum of each domain is then multiplied by a factor that takes into account the amount of influence each domain potentially has on the overall score. A total score of ≤ 26.55 seems to represent a higher risk of sexual dysfunction [25].
Statistics and power analysis
Our data is reported via median and interquartile range (numerical variables). Statistical tests were done with the R software package (version 4.2.1) [29]. Data were plotted with the ggplot2 package (version 3.3.6) [30]; power analysis was performed with the package pwr (version 1.3–0) [31].
The difference in parameters between groups was examined via Wilcoxon rank-sum tests, respectively via Wilcoxon signed rank test with continuity correction in the case of paired groups.
Since it is not possible to directly conduct a power analysis for a non-parametric test, it is necessary to employ Pitman asymptotic relative efficiency (ARE) to at least estimate the effect sizes one is able to detect [32]. Since, at worst, the ARE of the Wilcoxon rank-sum test was 0.864 [33] in comparison to the t-test, we performed a power analysis for the two-sided unpaired t-test under the assumptions of: sample sizes n1 = 33 (~ 38*0.864), n2 = 55 (~ 64*0.864), significance level = 0.05, power = 0.8. We calculated a detectable effect size of at least ~ 0.62 which corresponds to effects of size medium to large [34].
For our comparisons of the paired groups, an analogue approach revealed that we were able to observe effects of sizes 0.38 respectively 0.72, which corresponds to medium respectively large effects [34]. A p-value of < 0.05 was considered as statistically significant.
Results
Our study collective comprised 102 premenopausal women (n, number = 102); 64 women had laparoscopically and histologically confirmed endometriosis. Endometriosis was surgically excluded in 38 women who were therefore included in our control group.
Patient characteristics are presented in Table 1.
Table 1.
Patient characteristics
| Patient characteristics | All (n = 102) | Control group (n = 38) | Endometriosis group (n = 64) | p-value |
|---|---|---|---|---|
| General informationa | ||||
| Age (years) | 34 (31—40) | 35 (31—41.5) | 33 (30.75—39.25) | 0.2556 |
| BMI (kg/m2) | 22.54 (20.70—26.85) | 21.96 (20.70—26.19) | 22.60 (20.71—26.96) | 0.9228 |
| Gravidity | 0.5 (0—2) | 1 (0—2) | 0 (0—1) | 0.3486 |
| Parity | 0 (0—0) | 0 (0—0) | 0 (0—1) | 0.4614 |
| Menarche | 13 (11—14) | 13 (11.25—14) | 12 (11—14) | 0.9972 |
| Smokingb | 0.2348 | |||
| No | 82 | 33 | 49 | |
| Yes | 20 | 5 | 15 | |
| In a relationshipb | 0.1186 | |||
| No | 22 | 5 | 17 | |
| Yes | 80 | 33 | 47 | |
| Completed family planningb | 0.2852 | |||
| No | 66 | 22 | 44 | |
| Yes | 36 | 16 | 20 | |
| Desire to have childrenb | 0.7904 | |||
| No | 64 | 23 | 41 | |
| Yes | 38 | 15 | 23 | |
| Preoperative pain symptoms | ||||
| Overall non-menstrual pain score | 6.5 (3—9) | 6 (3—9) | 7 (3.75—9) | 0.5860 |
| Dysmenorrhea intensity (VAS) | 8 (5—9) | 6 (2—8) | 8 (5—10) | 0.0158 |
| Dyspareunia intensity (VAS) | 1 (0—6) | 0 (0—5) | 3 (0—6) | 0.1197 |
| Influence on sex life (VAS) | 0 (0—6) | 0 (0—4) | 0 (0—7) | 0.0877 |
| Dysuria intensity (VAS) | 0 (0—0) | 0 (0—0) | 0 (0—0) | 0.3442 |
| rASRM score (n) | ||||
| I | NA | 13 | ||
| II | NA | 14 | ||
| III | NA | 14 | ||
| IV | NA | 16 | ||
| NA | NA | 7 | ||
| ENZIAN score (n) | ||||
| A 1–3 | NA | 9 | ||
| B 1–3 | NA | 16 | ||
| C 1–3 | NA | 7 | ||
| FA | NA | 11 | ||
| FB | NA | 3 | ||
| FI | NA | 2 | ||
| FO | NA | 5 | ||
Values are expressed in median and interquartile range. Categories are presented in absolute values
aWilcoxon rank-sum test with continuity correction
bBarnard’s unconditional test
There were no statistically significant differences between the two groups in median age, BMI, gravidity, parity, and menarche, nor in the pain categories “overall pain score” and “dyspareunia”. However, patients suffering from endometriosis had a significantly higher intensity of “dysmenorrhea” than controls (p = 0.0158). There were no significant differences between FSFI full-scale score of controls and patients with endometriosis in preoperatively assessed total FSFI score and in the six domains (Table 2).
Table 2.
Preoperative FSFI scores: controls vs. endometriosis patients
| Variable | Controls (n = 38) | Endometriosis (n = 64) | p-value |
|---|---|---|---|
| Desire | 3.0 (2.4–3.6) | 3.0 (2.4–3.8) | 0.4599 |
| Arousal | 3.9 (2.5–5.0) | 3.9 (2.0–4.8) | 0.6346 |
| Lubrication | 5.1 (3.2–5.9) | 4.4 (3.3–5.7) | 0.5742 |
| Orgasm | 4.8 (2.1–5.9) | 4.4 (2.8–5.6) | 0.8366 |
| Satisfaction | 4.8 (3.4–6.0) | 4.4 (2.8–5.3) | 0.3937 |
| Pain | 4.8 (2.5–6.0) | 3.8 (1.6–5.7) | 0.2234 |
| Full-scale score | 27.7 (17.5–30.7) | 23.8 (15.4–29.3) | 0.3395 |
Values are expressed in median (interquartile range)
In addition, no significant differences between the groups were found 8 to 18 weeks postoperatively (Table 3).
Table 3.
Postoperative FSFI scores: controls vs. endometriosis patients
| Variable | Controls (n = 38) | Endometriosis (n = 64) | p-value |
|---|---|---|---|
| Desire | 3.6 (3.0–4.7) | 3.6 (2.4–4.8) | 0.5157 |
| Arousal | 4.5 (3.1–5.3) | 4.5 (3.0–5.4) | 0.9889 |
| Lubrication | 5.6 (3.7–6.0) | 5.4 (3.5–6.0) | 0.5860 |
| Orgasm | 4.8 (3.3–6.0) | 4.6 (2.4–5.6) | 0.3810 |
| Satisfaction | 5.2 (4.0–6.0) | 5.2 (4.0–6.0) | 0.8374 |
| Pain | 5.6 (2.8–6.0) | 5.2 (0.0–6.0) | 0.5542 |
| Full-scale score | 28.6 (22.0–31.4) | 27.9 (18.0–32.4) | 0.8059 |
Values are expressed in median (interquartile range)
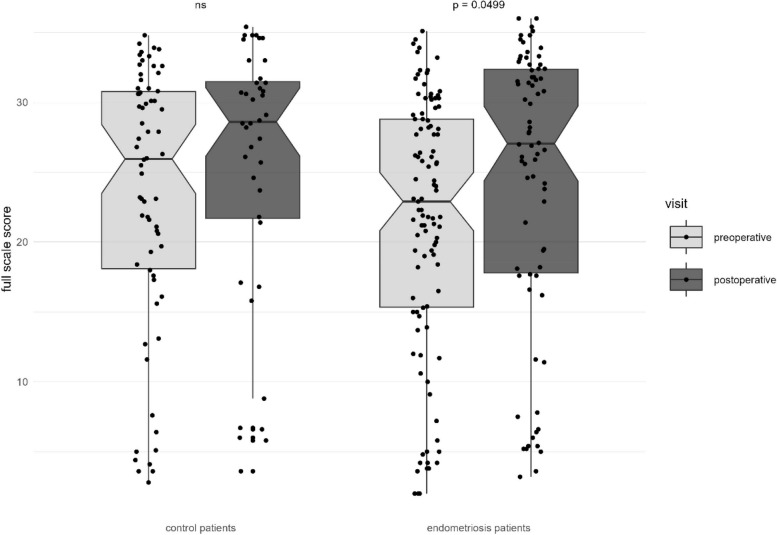
Furthermore, we compared pre- and postoperative sexual function within the groups. In women suffering from endometriosis, surgical excision of endometriotic lesions resulted in a significant improvement of the FSFI full-scale score (23.8 vs. 27.9, p = 0.0499) as well as of the domains “desire” (3.0 vs. 3.6, p = 0.0008), and “satisfaction” (4.4 vs. 5.2, p = 0.0024). Although an improvement was found in all other domains, this showed no statistical significance (Table 4).
Table 4.
FSFI scores of patients with endometriosis: preoperative vs. postoperative values
| Variable | Preoperative (n = 64) | Postoperative (n = 64) | p-value |
|---|---|---|---|
| Desire | 3.0 (2.4–3.8) | 3.6 (2.4–4.8) | 0.0008 |
| Arousal | 3.9 (2.0–4.8) | 4.5 (3.0–5.4) | 0.0542 |
| Lubrication | 4.4 (3.3–5.7) | 5.4 (3.5–6) | 0.2641 |
| Orgasm | 4.4 (2.8–5.6) | 4.6 (2.4–5.6) | 0.7904 |
| Satisfaction | 4.4 (2.8–5.3) | 5.2 (4.0–6.0) | 0.0024 |
| Pain | 3.8 (1.6–5.7) | 5.2 (0.0–6.0) | 0.2880 |
| Full-scale score | 23.8 (15.4–29.3) | 27.9 (18.0–32.4) | 0.0499 |
Values are expressed in median (interquartile range), Wilcoxon signed rank test with continuity correction. Significant p-values are set in italics
Comparing pre- and postsurgical assessment, women included in the control group showed a statistically significant improvement in the domain “desire” (3.0 vs. 3.6, p = 0.0254). All other domains as well as the full-scale score showed no statistically significant improvement after surgery. Details are presented in Table 5.
Table 5.
FSFI scores of controls: preoperative vs. postoperative values
| Variable | Preoperative (n = 38) | Postoperative (n = 38) | p-value |
|---|---|---|---|
| Desire | 3.0 (2.4–3.6) | 3.6 (3.0–4.7) | 0.0254 |
| Arousal | 3.9 (2.5–5.0) | 4.5 (3.1–5.3) | 0.1990 |
| Lubrication | 5.1 (3.25–6.0) | 5.6 (3.7–6.0) | 0.2271 |
| Orgasm | 4.8 (2.1–5.9) | 4.8 (3.3–6.0) | 0.2635 |
| Satisfaction | 4.8 (3.4–6.0) | 5.2 (4.0–6.0) | 0.2693 |
| Pain | 4.8 (2.5–6.0) | 5.6 (2.8–6.0) | 0.3359 |
| Full-scale score | 27.7 (17.5–30.7) | 28.6 (22.0–31.4) | 0.1944 |
Values are expressed in median (interquartile range), Wilcoxon signed rank test with continuity correction. Significant p-values are set in italics
The changes of the full-scale score between the groups and between the two different time points within each group are shown in Fig. 1.
Fig. 1.
Changes of the FSFI full-scale score pre- and postoperatively, control patients vs. endometriosis patients
Discussion
Endometriosis is often associated with dyspareunia, which can strongly affect the patient’s overall sexual function.
Pain in itself is a very complex phenomenon influenced by a multitude of factors. The data regarding endometriosis-associated pain is therefore not consistent throughout the literature. While our findings confirmed results from some previous studies [35–37] regarding dysmenorrhea (VAS 8 in the endometriosis vs. VAS 6 in controls, p = 0.0158), one other study showed no difference in intensity of menstrual pain between patients with endometriosis and controls [28]. Regarding dyspareunia however, the systematic review of Shi et al. (2022) described a significant difference in dyspareunia intensity [38], whereas other previous papers [39, 40] and our current study showed no difference between the groups.
These results are mirrored in our data regarding the FSFI: while the domain “pain” was increased in endometriosis patients compared to controls, the difference between the two groups, in contrast to other studies [27, 28, 36, 41], showed no statistical significance.
When examining the effect of surgical therapy on dyspareunia, we found no significant improvement. Another cause for dyspareunia has been found in the pelvic floor muscles. Recently published data show the importance of a pelvic floor examination in patients with dyspareunia in order to evaluate myofascial components, which are known to play a potentially important role in this type of chronic pelvic pain [42]. This examination is however lacking in our and other previously mentioned endometriosis studies [38].
Regarding the full-scale score of FSFI, we noticed a lower preoperative score (median 23.8) in the endometriosis group compared to controls (median 27.7). Although this difference was not statistically significant, patients suffering from endometriosis had a higher potential to benefit from surgery, confirmed by a statistically significant post-surgical increase of the full-scale score in this group (from 23.8 to 27.9, p < 0.05). While many gynecological disorders seen in our patient collectives can lead to a reduction in the FSFI score, endometriosis patients seem to benefit more from surgical therapy than our controls. The same can be said for the domain “satisfaction” which improved in both groups, yet significantly only in patients with endometriosis (p = 0.0024).
While no difference was found here between the two groups, sexual desire was the only parameter that significantly improved post surgically in both the endometriosis and the control group. However, it has to be underlined that sexual desire can be influenced by a multitude of psychological factors (e.g., performance anxiety, depression) [43], which leads us to question whether this improvement might be due to a mere positive psychological reaction caused by the beneficial effects of surgery in women affected by gynecological diseases, underlining the importance of further studies in this collective with a longer follow-up period.
Strength and limitations
A strength of our study is the prospective cohort design of well-characterized study groups [39, 44]. While laparoscopy has been replaced as the gold-standard for the diagnosis of DIE, adenomyosis, and endometriomas, it allows the confirmation or exclusion of peritoneal lesions [45] and a more precise classification of the disease. In all endometriosis patients, the disease was intraoperatively scored by a member of our certified Endometriosis Centre according to rASRM and ENZIAN [20–22] classifications and all visible endometriotic lesions were resected. In contrast to other studies [28, 46], our collective comprised all endometriosis subtypes (peritoneal, ovarian, DIE). While this allowed the analysis of the changes of the FSFI in all endometriosis subtypes, representing the whole entity of this disease, the lack of stratification according to endometriosis subtype, in particular to the occurrence of DIE lesions, can be seen as a limitation of our study, as the results do therefore not reflect the possible influence of certain lesions on particular aspects of sexual function, such as dyspareunia. Furthermore, all patients who had used hormonal contraceptives within 3 months prior to the study [28, 41] were not included in our study, hereby eliminating the potential bias of hormonal contraceptives regarding sexual function [14].
In addition, we have not found previous studies that describe the effect of surgery on FSFI changes in patients with endometriosis compared to controls.
We find a key factor of conducting endometriosis studies to be the selection of the control group [17, 18]. In contrast to some other studies that simply chose asymptomatic women, we strived to define a control group in which the presence of endometriosis had been surgically excluded. All our patients however suffered from benign gynecological diseases that affected their pelvic organs and potentially by extension their sexuality. Finding an “ideal control group” is in our opinion a difficulty that needs to be solved through further studies.
Another limitation of our study is the short time of follow-up. Our follow-up time was eight to 18 weeks postoperatively. In order to truly evaluate a long-term effect of surgical excision of endometriotic lesions, further data with a longer follow-up period should follow.
Conclusions
This study demonstrates that the surgical removal of endometriotic lesions can lead to significant improvements in sexual function, as measured by the FSFI within 8 to 18 weeks’ post-surgery. Unlike surgeries performed for other benign gynecological conditions, the benefits observed in women with endometriosis suggest that impaired sexual function could become an increasingly important factor in the decision-making process for surgical intervention in these patients. This is particularly relevant for those suffering from deep dyspareunia and other forms of sexual dysfunction, which are prevalent in endometriosis.
In a clinical context, these results underscore the importance of addressing sexual health as a key component of patient care in endometriosis management. By highlighting the positive outcomes of surgical treatment on sexual function, this research supports the consideration of surgery not only for alleviating pain but also for improving the overall quality of life for women with endometriosis. This evidence may guide clinicians in recommending surgery earlier in the treatment process for patients experiencing significant sexual dysfunction, thereby potentially altering the current therapeutic landscape. Future studies with larger sample sizes and longer follow-up periods are necessary to further validate these findings and solidify the role of surgical intervention in the management of sexual dysfunction associated with endometriosis.
Acknowledgements
We would like to thank all our patients who participated in this study.
Abbreviations
- BMI
Body mass index
- DIE
Deep-infiltrating endometriosis
- FSFI
Female Sexual Function Index
- GnRH
Gonadotropin-releasing hormone
- IUD
Intrauterine device
- n
Number
- rASRM
Revised American Society of Reproductive Medicine
- VAS
Visual analogue scale
Authors’ contributions
A.P. and M.G. contributed equally to this study and share first authorship. All authors contributed equally to conception and design of study as well as manuscript finalization. AP, MG, SI, FH, LS, JH, HH, LK, CB, and RW were involved in the conception, design, and interpretation of the results. AP, MG, SI, LS, JH, HH, LK, and CB acquired the data. AP, MG, and RW conducted the analyses and wrote the first draft of the manuscript. HH, LK, and RW were the responsible surgeons. AP, MG, SI, and FH did statistical analysis. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Medical University of Vienna (EC no. 1907/2019). All patients gave their verbal and written informed consent at the time of study inclusion.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alexandra Perricos and Manuela Gstoettner contributed equally to this work.
References
- 1.Marinho MCP, Magalhaes TF, Fernandes LFC, Augusto KL, Brilhante AVM, Bezerra LRPS. Quality of life in women with endometriosis: an integrative review. J Womens Heal. 2018;27(3):399–408. [DOI] [PubMed] [Google Scholar]
- 2.Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–9. [DOI] [PubMed] [Google Scholar]
- 3.Johnson NP, Hummelshoj L. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552–68. [DOI] [PubMed] [Google Scholar]
- 4.Jaeger M, Gstoettner M, Fleischanderl I. “A little monster inside me that comes out now and again”: endometriosis and pain in Austria. Cad Saude Publica. 2022;38(2):e00226320. [DOI] [PubMed] [Google Scholar]
- 5.Gstoettner M, Wenzl R, Radler I, Jaeger M. “I think to myself ‘why now?’” – a qualitative study about endometriosis and pain in Austria. BMC Womens Health. 2023;23(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Donato N, Montanari G, Benfenati A, Monti G, Leonardi D, Bertoldo V, et al. Sexual function in women undergoing surgery for deep infiltrating endometriosis: a comparison with healthy women. J Fam Plan Reprod Heal Care. 2015;41(4):278–83. [PubMed] [Google Scholar]
- 7.Vercellini P, Somigliana E, Buggio L, Barbara G, Frattaruolo MP, Fedele L. “I can’t get no satisfaction”: deep dyspareunia and sexual functioning in women with rectovaginal endometriosis. Fertil Steril. 2012;98(6):1503. [DOI] [PubMed] [Google Scholar]
- 8.Ferrero S, Esposito F, Abbamonte LH, Anserini P, Remorgida V, Ragni N. Quality of sex life in women with endometriosis and deep dyspareunia. Fertil Steril. 2005;83(3):573–9. [DOI] [PubMed] [Google Scholar]
- 9.Vercellini P, Frattaruolo MP, Somigliana E, Jones GL, Consonni D, Alberico D, et al. Surgical versus low-dose progestin treatment for endometriosis-associated severe deep dyspareunia II: effect on sexual functioning, psychological status and health-related quality of life. Hum Reprod. 2013;28(5):1221–30. [DOI] [PubMed] [Google Scholar]
- 10.Barabara G, Facchin F, Buggio L, Somigliana E, Berlanda N, Kustermann A, et al. What is known and unknown about the association between endometriosis and sexual functioning: a systematic review of the literature. Reprod Sci. 2017;24(12):1566–76. [DOI] [PubMed] [Google Scholar]
- 11.Dior UP, Reddington C, Cheng C, Levin G, Healey M. Sexual function of women with deep endometriosis before and after surgery: a prospective study. J Sex Med. 2022;19(2):280–9. [DOI] [PubMed] [Google Scholar]
- 12.Zondervan KT, Becker CM, Missmer SA. Endometriosis. Longo DL, editor. N Engl J Med. 2020;382(13):1244–56. [DOI] [PubMed] [Google Scholar]
- 13.Smith NK, Jozkowski KN, Sanders SA. Hormonal contraception and female pain, orgasm and sexual pleasure. J Sex Med. 2014;11(2):462–70. [DOI] [PubMed] [Google Scholar]
- 14.Buggio L, Barbara G, Facchin F, Ghezzi L, Dridi D, Vercellini P. The influence of hormonal contraception on depression and female sexuality: a narrative review of the literature. Gynecol Endocrinol. 2022;38:193–201. [DOI] [PubMed] [Google Scholar]
- 15.Fritzer N, Haas D, Oppelt P, Renner S, Hornung D, Wölfler M, et al. More than just bad sex: sexual dysfunction and distress in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):392–6. [DOI] [PubMed] [Google Scholar]
- 16.Fritzer N, Hudelist G. Love is a pain? Quality of sex life after surgical resection of endometriosis: a review. Eur J Obstet Gynecol Reprod Biol. 2017;209:72–6. [DOI] [PubMed] [Google Scholar]
- 17.Lima RV, Pereira AMG, Beraldo FB, Gazzo C, Martins JA, Lopes RGC. Female sexual function in women with suspected deep infiltrating endometriosis. Rev Bras Ginecol Obstet. 2018;40(3):115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lermann J, Topal N, Renner SP, Beckmann MW, Burghaus S, Adler W, et al. Comparison of preoperative and postoperative sexual function in patients with deeply infiltrating endometriosis with and without bowel resection. Eur J Obstet Gynecol Reprod Biol. 2019;239:21–9. [DOI] [PubMed] [Google Scholar]
- 19.EuroEndoCert - Certification by EuroEndoCert. Available from: https://www.euroendocert.de/en/.
- 20.Haas D, Shebl O, Shamiyeh A, Oppelt P. The rASRM score and the Enzian classification for endometriosis: their strengths and weaknesses. Acta Obstet Gynecol Scand. 2012;92(1):3–7. [DOI] [PubMed] [Google Scholar]
- 21.Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, et al. World endometriosis society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315–24. [DOI] [PubMed] [Google Scholar]
- 22.Keckstein J, Saridogan E, Ulrich UA, Sillem M, Oppelt P, Schweppe KW, et al. The #Enzian classification: a comprehensive non-invasive and surgical description system for endometriosis. Acta Obstet Gynecol Scand. 2021;100(7):1165–75. [DOI] [PubMed] [Google Scholar]
- 23.Berner MM, Kriston L, Zahradnik HP, Härter M, Rohde A. Überprüfung der Gültigkeit und Zuverlässigkeit des Deutschen Female Sexual Function Index (FSFI-d). Geburtshilfe Frauenheilkd. 2004;64(3):293–303. [Google Scholar]
- 24.Symonds T, Boolell M, Quirk F. Development of a questionnaire on sexual quality of life in women. J Sex Marital Ther. 2005;31(5):385–97. [DOI] [PubMed] [Google Scholar]
- 25.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. [DOI] [PubMed] [Google Scholar]
- 26.Rust J, Derogatis L, Rodenberg C, Koochaki P, Schmitt S, Golombok S. Development and validation of a new screening tool for hypoactive sexual desire disorder: the Brief Profile of Female Sexual Function © (B-PFSF © ). Gynecol Endocrinol. 2007;23(11):638–44. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-López FR, Ornat L, Pérez-Roncero GR, López-Baena MT, Sánchez-Prieto M, Chedraui P. The effect of endometriosis on sexual function as assessed with the female sexual function index: systematic review and meta-analysis. Gynecol Endocrinol. 2020;36(11):1015–23. [DOI] [PubMed] [Google Scholar]
- 28.Evangelista A, Dantas T, Zendron C, Soares T, Vaz G, Oliveira MAP. Sexual function in patients with deep infiltrating endometriosis. J Sex Med. 2014;11(1):140–5. [DOI] [PubMed] [Google Scholar]
- 29.R Core Team. R: A language and environment for statistical computing. 2022. https://www.R-project.org.
- 30.Wickham H. ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag; 2016. Available from: https://www.r-project.org/.
- 31.Champely S. _pwr: basic functions for power analysis_. R package version 1.3–0. 2020. Available from: https://cran.r-project.org/package=pwr.
- 32.Simon S. Sample size for the Mann-Whitney U test. 2000. Available from: http://new.pmean.com/mann-whitney-sample-size/.
- 33.Hollander M, Wolfe DA, Chicken E. Nonparametric statistical methods. 1. Hoboken, New Jersey: John Wiley & Sons Inc; 1973.
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 35.Somigliana E, Facchin F, Busnelli A, Benaglia L, Biancardi R, Catavorello A, et al. Natural pregnancy seeking in subfertile women with endometriosis. Reprod Sci. 2020;27(1):389–94. [DOI] [PubMed] [Google Scholar]
- 36.Melis I, Litta P, Nappi L, Agus M, Melis GB, Angioni S. Sexual function in women with deep endometriosis: correlation with quality of life, intensity of pain, depression, anxiety, and body image. Int J Sex Heal. 2015;27(2):175–85. [Google Scholar]
- 37.De Graaff AA, Van Lankveld J, Smits LJ, Van Beek JJ, Dunselman GAJ. Dyspareunia and depressive symptoms are associated with impaired sexual functioning in women with endometriosis, whereas sexual functioning in their male partners is not affected. Hum Reprod. 2016;31(11):2577–86. [DOI] [PubMed] [Google Scholar]
- 38.Shi C, Xu H, Zhang T, Gao Y. Endometriosis decreases female sexual function and increases pain severity: a meta-analysis. Arch Gynecol Obstet. 2022;307(1):195–204. [DOI] [PubMed] [Google Scholar]
- 39.Perricos A, Wenzl R, Husslein H, Eiwegger T, Gstoettner M, Weinhaeusel A, et al. Does the use of the “proseek® multiplex oncology i panel” on peritoneal fluid allow a better insight in the pathophysiology of endometriosis, and in particular deep-infiltrating endometriosis? J Clin Med. 2020;9(6):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perricos A, Husslein H, Kuessel L, Gstoettner M, Weinhaeusel A, Eiwegger T, et al. Does the use of the “Proseek® multiplex inflammation I panel” demonstrate a difference in local and systemic immune responses in endometriosis patients with or without deep-infiltrating lesions? Int J Mol Sci. 2023;24(5):5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghajarzadeh M, Tanha FD, Akrami M, Mohseni M, Askari F, Farsi L. Do Iranian women with endometriosis suffer from sexual dysfunction? Sex Disabil. 2014;32(2):189–95. [Google Scholar]
- 42.Ross V, Detterman C, Hallisey A. Myofascial pelvic pain: an overlooked and treatable cause of chronic pelvic pain. Journal of Midwifery and Women’s Health. J Midwifery Womens Health. 2021;66:148–60. [DOI] [PubMed] [Google Scholar]
- 43.Kingsberg SA, Simon JA. Female hypoactive sexual desire disorder: a practical guide to causes, clinical diagnosis, and treatment. J Womens Health (Larchmt). 2020;29(8):1101–12. [DOI] [PubMed] [Google Scholar]
- 44.Tiringer D, Pedrini AS, Gstoettner M, Husslein H, Kuessel L, Perricos A, et al. Evaluation of quality of life in endometriosis patients before and after surgical treatment using the EHP30 questionnaire. BMC Womens Health. 2022;22(1):538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ESHRE Endometriosis Guideline Development Group. Endometriosis: guideline of ESHRE. 2022. Available from: https://www.eshre.eu/guidelines.
- 46.Yang X, Xu X, Lin L, Xu K, Xu M, Ye J, et al. Sexual function in patients with endometriosis: a prospective case–control study in China. J Int Med Res. 2021;49(4):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.