Abstract
Purpose
GATA2 deficiency is an autosomal dominant disease that manifests with a range of clinical symptoms, including increased susceptibility to viral, bacterial, and fungal infections. Furthermore, the increased susceptibility to infections in GATA2 deficiency can trigger hemophagocytic lymphohistiocytosis (HLH) in these patients. Our systematic review evaluates reported cases of GATA2 deficiency and HLH in the literature.
Methods
A systematic review of case reports was conducted following PRISMA 2020 guidelines, encompassing studies retrieved from Ovid MEDLINE ALL, Embase via Ovid SP, Scopus, Web of Science, and Google Scholar from inception until June 14, 2024. This review included studies reporting patients diagnosed with GATA2 deficiency or having a documented history of the condition, who subsequently developed or were concurrently diagnosed with HLH. Various study types were considered, such as case reports, case series, letters to editors, original articles, correspondences, and commentaries, without any restrictions on language.
Results
In our systematic review, 15 studies from 2016 to 2024 were analyzed, encompassing 23 patients with GATA2 deficiency and HLH. the mean (SD) age of patients was 23.48 (10.54) years, ranging from 7 to 57 years. These patients exhibited diverse genetic mutations and a spectrum of infections, particularly Mycobacterium avium (M. avium), Mycobacterium kansasii (M. kansasii), Epstein-Barr virus (EBV), cytomegalovirus (CMV), varicella-zoster virus (VZV), herpes simplex virus (HSV), and influenza A, often leading to HLH. Family histories of GATA2-deficient patients with HLH occasionally reveal confirmed GATA2 mutations or suspicious cases among first-degree relatives. Hematopoietic stem cell transplantation (HSCT) was performed in 8 patients with GATA2 deficiency and HLH. Among them, 6 patients survived post-therapy, while 2 patients died following HSCT. Currently, 1 patient is being considered for HSCT. The overall mortality rate among GATA2 deficiency patients who experienced HLH was 39.13%.
Conclusions
This systematic review highlights GATA2 deficiency’s association with diverse infections triggering HLH, emphasizing early infection management to mitigate mortality risks. This comprehensive analysis contributes to scientific knowledge, offering important insights for clinicians and researchers in diagnosing and managing this rare condition.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10145-1.
Keywords: GATA2 deficiency, Hemophagocytic lymphohistiocytosis, HLH, NK-cell
Introduction
Hemophagocytic lymphohistiocytosis (HLH), first defined by Scott and Robb-Smith in 1939, is an uncommon and severe immunological disorder that affects both children and adults, with high mortality rates [1–3]. HLH, characterized by the overactivation of CD8 + cells and macrophages, which induce local and systemic activation of inflammatory cytokines, including interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6, IL-10, and IL-18, ultimately leading to cytolysis, tissue damage, and end-organ injury [1, 4]. Additionally, inflammatory cytokines contribute significantly to clinical features, with elevated levels correlating with worse prognosis [5]. Timely identification of HLH is crucial as patients can deteriorate rapidly, leading to multiorgan failure and mortality [1].
Genetic predisposition to HLH centers on genes linked to cell-mediated cytotoxicity and lymphocyte function. Currently, over 100 HLH-associated genes have been identified, with clear evidence supporting the involvement of 17 of these genes in HLH [5, 6]. HLH has been classified into a primary/familial and secondary type, first described by Farquhar and Claireaux in 1952, and by Risdall et al. in 1979, respectively [1, 7]. Primary HLH, characterized by autosomal recessive mutations in genes such as PRF1, UNC13D, STX11, and STXBP2, represents about a quarter of all cases and typically presents in infants during their first year of life [1, 7–9]. Secondary HLH, driven by acquired factors like chronic inflammation, infection, or malignancy, typically affects adolescents and adults and does not demonstrate known genetic associations [7, 9]. Interestingly, a small proportion of adult HLH cases arise from delayed onset primary HLH, lacking any identifiable cause [4]. In recent times, there has been a growing diagnosis of primary HLH in adults [10].
GATA-binding protein 2 (GATA2), a member of the GATA family of transcription factors, plays a critical role in hematopoiesis [11]. Numerous mutations have been identified in GATA2 gene, predominantly germline mutations fundamental to GATA2 deficiency, while somatic mutations are observed in leukemia patients. To date, over 500 cases have been reported, with nearly 180 different mutations identified [12–14]. GATA2 deficiency, caused by germline GATA2 mutations that are thought to be loss-of-function and result in haploinsufficiency, is a rare autosomal dominant genetic disease that leads to various clinical manifestations, including myeloid malignancies like leukemia and non-malignant presentations such as infections and bone marrow failure [11, 13, 15].
Multiple studies worldwide have reported associations between GATA2 mutations and HLH in both pediatric and adult patients [6, 16–18]. Therefore, considering genetic testing for GATA2 mutations is advisable for individuals diagnosed with HLH [19]. Our systematic review evaluates reported cases of GATA2 deficiency and HLH in the literature. This comprehensive analysis enriches scientific discourse, providing valuable perspectives for clinicians and researchers in this rare condition.
Materials and methods
Search strategy and study selection
The review was reported utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [20]. Ovid MEDLINE ALL, Embase via Ovid SP, Scopus, Web of Science, and Google Scholar from inception until June 14, 2024 were systematically searched, encompassing articles published until that date. The search strategy utilized a combination of keywords and medical subject headings (MeSH) related to “Hemophagocytic lymphohistiocytosis” and “GATA2 deficiency.” Detailed search terms and strategies are outlined in Supplementary Table 1. A reference list of relevant review articles was reviewed to identify other relevant articles, using backward and forward searches through June 14, 2024. Two authors (MRZR and SDA) separately contributed to title and abstract screening and after that these two authors conducted full-text screening based on our inclusion criteria and any discrepancies reviewed by third author (HM).
Eligibility criteria
All studies reporting patients currently diagnosed with GATA2 deficiency or with a history of documented GATA2 deficiency, who experienced HLH (as described by the authors) occurring concurrently or after the diagnosis of GATA2 deficiency, were considered, encompassing case reports, case series, letters to editors, original articles, correspondences, and commentaries. Review articles and papers lacking adequate data or deemed low-quality based on quality assessment methods were excluded from the study. This systematic review was conducted without restrictions on patient age, publication time, or language, encompassing articles written in English or any language with an English abstract.
Data extraction
All data extraction was independently conducted by two authors (MRZR and HM) using a standardized template. The data were entered into a predefined Excel sheet, capturing the first author’s name, year of publication, country of study, patient age and sex, past medical history, initial presentation, genetic findings, hematological and paraclinical results, HLH diagnostic methods, infection sources, treatments, follow-up details, and outcomes using Microsoft Excel 2019 version (Microsoft Corporation, Redmond, WA, USA). The corresponding author (SDA) verified the accuracy of data extraction, addressed any contradictions, and resolved discrepancies in the data extraction process.
Data analysis
We used descriptive statistics to analyze the extracted data, reporting frequencies, percentages, and proportions. Continuous data are presented as median (IQR) or mean (± SD), while categorical variables and outcomes are expressed as numbers and percentages. Statistical analyses were conducted utilizing SPSS software (version 22).
Risk of bias assessment
The selected papers were assessed for risk of bias based on Murad et al., 2018, by two authors (MRZR and SDA) independently [21]. Study quality was evaluated according to four domains: selection, ascertainment, causality, and reporting. Any discrepancies reviewed by a third author (HM).
Results
Methodology of literature review and findings
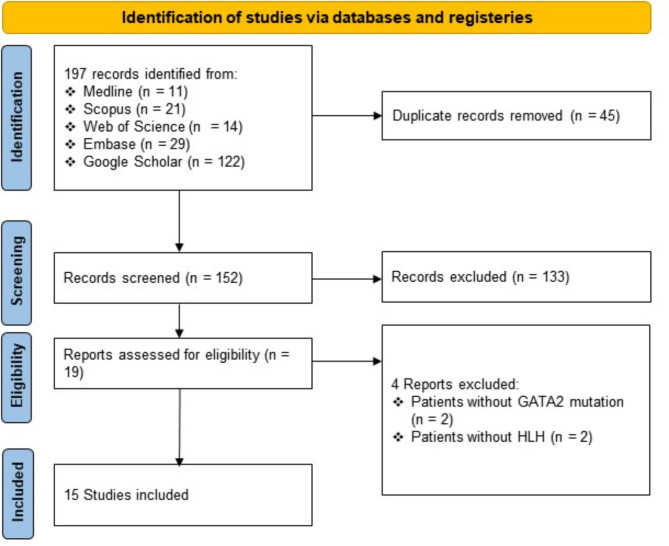
In total, 197 records were initially obtained. After eliminating duplicates, 152 distinct records underwent screening. Subsequently, 15 studies met the inclusion criteria following a comprehensive assessment of eligibility [6, 11, 16–18, 22–31]. The review process is illustrated, as the flowchart, in Fig. 1.
Fig. 1.
The PRISMA statement flowchart: visual representation of study selection process
Characteristics of the included studies
In the final analysis of 15 studies conducted from 2016 to 2024, a total of 23 patients with GATA2 deficiency and HLH were evaluated. The characteristics of these patients, including the year and country of study, age, sex, past medical history, initial presentation, genetic findings, HLH diagnostic methods, infection sources, treatments, follow-up, and outcomes, are detailed in Table 1. Additionally, hematological laboratory findings and paraclinical findings (such as bone marrow (BM) evaluation, positron emission tomography/computed tomography (PET/CT), abdominal ultrasonography, lymph node biopsy, etc.) are shown for each patient in Table 2, if available.
Table 1.
The basic characteristics features, treatment, and outcome of the patients with GATA2 mutation and HLH
| Study | Country | Age, Sex | PMH | Presentation | Genetic findings | HLH diagnosis | Infection | Treatment | Follow-up and outcome | Note |
|---|---|---|---|---|---|---|---|---|---|---|
| Wu et al., 2024 | China | 28, M | NA |
Recurrent fever (Tmax 39.5 °C), night sweats, and afternoon/evening spikes temperature spikes for > 4 months prior to presentation; weight lost approximately 5 kg since onset. |
Heterozygous missense variant in GATA2 gene (Gene location: chr3.128200691; Variant: c.1072G > A: p.A358T; Source: De novo). |
Based on HLH-2004 diagnostic index (7/8). | M. avium, EBV. |
HLH treatment: Ruxolitinib combined with doxorubicin-etoposide-methylprednisolone regimen; M. avium treatment: azithromycin, clofazimine, and amikacin. |
1-year follow-up: Smaller lymph nodes, intermittent pulmonary infections, thrombocytopenia, enlarged spleen, negative for M. avium (NGS) and EBV-DNA. |
The patient’s 3-year-old daughter had the same pathogenic GATA2 variant, but his parents and older brother did not; Patient unable to undergo allo-HSCT due to economic constraints. |
| 22, F | NA | Intermittent fever (Tmax 41 °C) without obvious cause, since 4 months prior to presentation. |
Heterozygous missense variant in GATA2 gene (Gene location: chr3.128200155; Variant: c.1108 A > G: p.R370G; Source: De novo). |
Based on HLH-2004 diagnostic index (5/8). | EBV | HLH treatment: Ruxolitinib combined with doxorubicin-etoposide-methylprednisolone regimen; patient proposed for allo-HSCT. | Condition stabilized, intermittently positive for EBV-DNA post-treatment, diagnosed with MDS three years later. | No GATA2 gene variant found in her parents; | ||
| 26, M | Fever, fatigue, and leukopenia for over 10 years; BM cytology suggests MDS not excluded. | Chest tightness and fever without obvious triggers, since 1 month prior to presentation. |
Heterozygous missense variant in GATA2 gene (Gene location: chr3.128200118; Variant: c.1145G > A: p.R382Q; Source: Unverified). |
Based on HLH-2004 diagnostic index (6/8). | M. kansasii, EBV. | Rifampicin, doxycycline, and gentamicin with a poor anti-infection effects. | Patient experienced sustained pancytopenia and repeated infections. |
His father died of acute leukemia; Patient unable to undergo allo-HSCT due to economic constraints. |
||
|
Huang et al., 2023 |
china | 28, M | Intermittent fever (for 3 years) and erythema nodosa (for 7 months) that treated with various antibiotics, but fever persisted mostly in the afternoon and at night. |
Recurrent fever; lymphadenopathy; pancytopenia; splenomegaly; erythema nodosa |
Heterozygous nonsense variant in GATA2 gene (Variant: c.599dupG, p.Ser201*) |
Based on HLH-2004 diagnostic index. | M. avium; Klebsiella pneumonia |
Cyclosporin, dexamethasone, ruxolitinib, and 4-drug antituberculosis therapy. |
Died from severe pulmonary infection one month later. | The GATA2 variant in this patient creates stop codons, leading to the absence of both zinc finger domains; his father and sister carried the wild type of the GATA2 gene. The absence of this variant in the proband’s father and sister suggesting it might be a de novo mutation. |
| Burak et al., 2021 | USA | 22, F | Congenital absence of the right kidney, right-sided hearing loss, and leukopenia (negative BM biopsy); history of infectious mononucleosis at age 13 years; frequent upper respiratory viral infections, vaginal candida infections, and severe acne. |
Generalized malaise, fever, chest pain, cough, and shortness of breath for 3 weeks; At admission: headaches, weakness, fevers, chills, lack of appetite, palpitations, severe anxiety, sinus tachycardia, tachypnea, and fever (Tmax 39.4 °C), splenomegaly. |
Heterozygous mutation in the GATA2 gene (Variant: c.1009 C > T, p.Arg337*) | Based on HLH-2004 diagnostic index (5/8). | CMV | Ganciclovir, intravenous Ig, dexamethasone 10 mg/m², followed by high dosage corticosteroids (IV methylprednisolone, 40 mg/d); | After 18 days in hospital, discharged for acute rehabilitation on a steroid taper and valganciclovir, 900 mg every 12 h. Finally, haploidentical human leukocyte antigen bone marrow transplant approximately 11 months after being diagnosed with GATA2 deficiency. | Her father had benign leukopenia, but no other pertinent family history; this patient exhibits loss of C-terminal zinc-finger domain. |
| Mika et al., 2021 | Germany | 29, F | None. | Recurrent fever (Tmax 40 °C) for more than 1 year, admitted after fever persisted for over two weeks. |
Heterozygous nonsense variant in GATA2 gene (Variant: c.177 C > G, p.Tyr59Ter; Source: De novo) |
Based on HLH-2004 diagnostic index (7/8); HScore of 239 points (98–99% probability of HLH). |
M. avium. | Treatment with amikacin, azithromycin, ethambutol, rifampicin, high-dose dexamethasone, ciclosporin A, IVIG, and repetitive courses of etoposide (75 mg/m², twice weekly) was initiated promptly. Prior to allo-HSCT, the antimycobacterial treatment was switched to azithromycin, clofazimine, and ethambutol. Finally, allo-HSCT therapy following fludarabine (150 mg/m²) and treosulfan (30 g/m²); GVHD prophylaxis: post-transplant cyclophosphamide (100 mg/kg), mycophenolate-mofetil, and ciclosporin. | Complete remission of all inflammatory manifestations was achieved on day + 240 post-allo-HSCT, following 12 months of tuberculostatic therapy. | Family history and GATA2 genetic analysis of first-degree relatives revealed no additional affected individuals; this patient exhibits loss of both zinc finger domains in GATA2. |
|
Sun et al., 2021 |
China | 17, F | At age 6, recurrent fever was observed. At age 10, she had severe pneumonia, pleural effusion, and an erythematous and vesicular skin rash. Anti-VZV antibodies and DNA were detected in her peripheral blood. Three months later, she was diagnosed with HLH. Two years later, erythema nodosa on her lower limbs, accompanied by panniculitis. | Recurrent fever; pancytopenia; splenomegaly; erythema nodosa; panniculitis | Heterozygous nonsense mutation in the GATA2 gene (Variant: c.610 C > T, p.Arg204*) | Based on HLH-2004 diagnostic index | VZV at age 10. | Dexamethasone 10 mg/12 h and GCSF. | Death. | Her father had GATA2 mosaicism, while her 32-year-old sister carried the same mutation as the patient without manifestations; this patient exhibits loss of both zinc finger domains in GATA2; |
| Oleaga-Quintas et al., 2021 | Spain | 27?/M | NA | Inferior vena cava thrombosis; deafness | Mutation in the GATA2 gene (Variant: c.1035_1036insTC TGGCC/WT) | NA. |
At the age of 24 years, M. kansasii infection of the lymph nodes, followed by a disseminated infection three years later; other: C. freundii (bacteremia), Staphylococcus spp., and Candida spp (esophagitis). |
Long-term antibiotic therapy. | Death. | Patient didn’t undergo allo-HSCT. |
| Barber et al., 2021 | USA | 21, F | Multiple ear infections within the first few years of life; multiple episodes of pneumonia, bronchitis, and sinusitis; osteomyelitis of her finger occurred after a fracture at two years of age; probable PCD. | Intermittent lower respiratory tract infections. | Heterozygous mutation in the GATA2 gene (Variant: c.1009 C > T, p.Arg337*) | Based on BM biopsy. | EBV | NA. | Over the three-month hospitalization she developed progressive respiratory failure secondary to HLH and died. | Patient didn’t undergo allo-HSCT. |
| Suzuki et al., 2020 | Japan | 27, F | History of pancytopenia with refractory fever of unknown origin at ages 18 and 23; history of cervical dysplasia, and urticarial rash induced by physical stimulus since age 21; | Eight-day history of persistent fever and pancytopenia; at admission: fever (Tmax 40.2 °C), hepatosplenomegaly, and urticarial rash on her right forearm; | Heterozygous mutation in the GATA2 gene (Variant: c.1061 C > T, p. Thr354Met) | Based on HLH-2004 diagnostic index. | CMV; MRSA |
HLH treatment: methylprednisolone pulse therapy (1000 mg for 5 days) was followed by maintenance therapy with prednisolone (1 mg/kg/day); MRSA treatment: CT-guided drainage for femoral abscess, vancomycin, daptomycin, teicoplanin, trimethoprim/sulfamethoxazole, doxycycline, minocycline. |
NA | There was no similar family history; |
| Eguchi et al., 2018 | Japan | 14, M | Diagnosed with MDS at age 11. | Prolonged fever and weight loss. | Heterozygous frameshift mutation in the GATA2 gene (Variant: c.1077_1078insA) | Based on HLH-2004 diagnostic index. | M. kansasii | HLH treatment: etoposide, cyclosporine, and prednisolone; Antituberculosis therapy: isoniazid, rifampin, and ethambutol; Six months later, he underwent unrelated BM transplantation with conditioning using total body irradiation, etoposide, fludarabine, and melphalan. | He remains well with full donor chimerism 16 months post-transplant. | The patient’s healthy mother had the same mutation with reduced B cells but not monocytes. |
| Prader et al., 2018 | Switzerland | 8, F | In the preceding month, persistent warts and recurrent furuncles on the hands and feet. | Abdominal pain, erythematous vesicular skin rash, and subfebrile body temperatures over 4 days. | Heterozygous mutation in the GATA2 gene (Variant: c.1172_1175del, p.E391Gfs∗85) | Based on HLH-2004 diagnostic index (5/8) | VZV |
VZV treatment: acyclovir, intravenous Ig (0.4 g/kg); HLH treatment: corticosteroids (1.5 mg/kg/d). |
Relapsed after one year, recovered with corticosteroids and broadspectrum antibiotic | The patient’s spleen was not enlarged; |
| 7, M | Known case of GATA2 mutation for three years, manifesting as recurrent fever, oral aphthosis, recurrent furunculosis, and lymphopenia (affecting CD4 T cells, B cells, and NK cells). | Mild fever (Tmax 38.5 °C); erythematous vesicular skin rash; cough; splenomegaly. | Mutation in the GATA2 gene (Variant: c.(16 bp tandem repeat in exon 4), p.T347fs) | Based on HLH-2004 diagnostic index (4/6) | VZV |
VZV treatment: acyclovir, VZIG (22 IU/kg), IVIG (0.4 g/kg); HLH treatment: corticosteroids (2 mg/kg/d). |
NA. | The patient’s mother had treatment-resistant warts on her hands with an identical genotype (p.T347fs). | ||
| Parta et al., 2018 | USA | 17, F | Known case of GATA2 mutation for five years, | NA. | Karyotype: trisomy 8; mutation in the GATA2 gene (Variant: p.R396Q) | NA. | CMV; parvovirus | HSCT therapy from haploidentical related donor recipients with BM donor source. | The patient experienced grade 2 upper gastrointestinal acute GVHD despite post-transplantation cyclophosphamide treatment; After 12 months post-transplant, the patient is alive. | Negative family history. |
| Donadieu et al., 2018 |
France And Belgium |
26, F | Known case of GATA2 mutation since age 19. | NA. | Karyotype: Trisomy 1q Der9 t(1;9)(q12;q1 2), r(9)(q12 ;q ?3 4), 11q23(2); nonsense mutation in the GATA2 gene (Variant: c.610 C > T, p.R204*) | NA. | CMV; EBV; M. avium; VZV; HPV (cutaneous warts); | GCSF; EPO; interferon; chemotherapy. | Death. | CMV infection caused HLH. |
| 57, ? | Known case of GATA2 mutation since age 21. | NA. | Karyotype: Del5 Del7 T8 Add 10 Del12 Monosomy 18, Monosomy 21; missense mutation in the GATA2 gene (Variant: c.937 C > T, p.H313Y) | NA. | EBV, HSV. | HSCT. | Death. | EBV-related hemophagocytic syndrome and lymphoproliferative disease occurred post-HSCT. | ||
| 24, F | Known case of GATA2 mutation since age 21. | NA. | Karyotype: 46,XX [20] / 92,XXXX [2]; missense mutation in the GATA2 gene (Variant: c.1061 C > G, p.T354R) | NA. | M. avium; pulmonary aspergillosis; mucormycosis; HPV (genital warts. | NA. | Death. | M. avium was the cause of HLH and death; the patient also experienced sarcoidosis; | ||
| 28, ? | Known case of GATA2 mutation since age 14. | NA. | Karyotype: normal; frameshift mutation in the GATA2 gene (Variant: c.1142del, p.Asn381Metfs*6) | NA. | Influenza A; aspergillosis; candidiasis; streptococcus sepsis; HPV (cutaneous warts); | NA. | Alive. | Influenza A virus infection caused HLH. | ||
| 10, ? | Known case of GATA2 mutation since age 9. | NA. | Karyotype: monosomy 7; missense mutation in the GATA2 gene (Variant: c.1186 C > T, p.R396W) | NA. | Aspergillosis post-HSCT. | HSCT | Death. | The patient also experienced atypical Kawasaki disease. | ||
| 40, ? | Known case of GATA2 mutation since age 19. | NA. | Karyotype: normal; missense mutation in the GATA2 gene (Variant: c.1193G > A, p.R398Q) | NA. | Mycobacteriosis. | HSCT | Alive. | The patient developed splenic vein thrombosis and had a rectal adenoma (low-grade dysplasia). | ||
| Yamamoto et al., 2018 | Japan | 20, M | Treated EBV-associated HLH with prednisolone 2 years ago. | NA. | Karyotype: normal; mutation in the GATA2 gene (Variant: p.R230Hfs*44); acquired STAG2 mutation (splicing site change, c.820–2 A > G) | NA. | EBV | allo-HSCT from an unrelated donor; | Alive without relapse. | The patient’s father was diagnosed with MDS in his youth and died from leukoencephalopathy around the age of 50; acquired STAG2 mutation could contribute to MDS progression. |
| Cohen et al., 2016 | USA | 24, F | At age 10: vesicular eruption; at age 20: necrotizing lymphadentitis, urinary Histoplasmosis; at age 21: small and large intestine perforation, panniculitis, Weber-Christian disease, two cerebral ischemic infarcts, E. faecium bacteremia, disseminated M. avium infection, DVT, PE, vertebral compression fracture, multiple deep skin ulcers, and osteomyelitis | Fever, diffuse ulcerations in the small intestine and skin. | Unialleleic expression; heterozygous for genomic SNPs exon 2 bp 55 A > C and exon 3 c.15 C > G, hemizygous by cDNA sequence | Based on HLH-2004 diagnostic index (6/8) | EBV | Dexamethasone; etoposide; alemtuzumab; allo-HSCT. | HSCT complicated by liver GVHD, no lymphoma detected, low EBV DNA in blood (low thousands/ml), virus mainly in B cells. | She was finally treated with haploidentical HSCT from her unaffected sister. |
| Spinner et al., 2016 | USA | 18, F | At age 9: severe bilateral lower extremity lymphedema; at age 12: necrotizing pneumonia with pulmonary abscess, lung biopsy identified thick-walled budding yeasts identified as Blastomyces dermatitidis on culture. | Fever; headache | frameshift mutation in the GATA2 gene (Variant: c.871 + 2_3insT) | NA. | HSV-1; Blastomyces dermatitidis | Dexamethasone; vancomycin; meropenem; levofloxacin; acyclovir; liposomal amphotericin B | Death. | None. |
PMH, past medical history; HLH, hemophagocytic lymphohistiocytosis; M, male; NA, not applicable/available; Tmax, maximum temperature; M. avium, mycobacterium avium; EBV, epstein-barr virus; NGS, next-generation sequencing; allo-HSCT, allogeneic hematopoietic stem cell transplantation; F, female; MDS, myelodysplastic syndrome; BM, bone marrow; M. kansasii, mycobacterium kansasii; USA, united states of america; CMV, cytomegalovirus; IV, intravenous; IVIG, intravenous immunoglobulin; GVHD, graft-versus-host disease; VZV, varicella-zoster virus; GCSF, granulocyte colony-stimulating factor; C. freundii, citrobacter freundii; PCD, primary ciliary dyskinesia; MRSA, methicillin-resistant staphylococcus aureus; VZIG, varicella zoster immune globulin; IU/kg, international unit per kilogram; HPV, human papillomavirus; EPO, erythropoietin; HSV, herpes simplex virus; E. faecium, enterococcus faecium; DVT, deep vein thrombosis; PE, pulmonary embolism
Table 2.
The laboratory, biopsy/autopsy, and imaging findings of patients with GATA2 mutation and HLH
| Study | Age, Sex | Hematological laboratory findings | Paraclinical findings |
|---|---|---|---|
| Wu et al., 2024 | 28, M | WBC: 1.5 × 109/L; HGB: 103 g/L; PLT: 63 × 109/L; decreased monocytes: 0.01 × 109/L; TG: 2.82 mmol/L; ESR: 101 mm/h; ferritin: 3897 ng/mL.; low NK activity: 11.48% (normal range > 15.11%); high sCD25 level: 25,825 pg/mL (normal range < 6400 pg/mL); decreased NK ΔCD107a: 4.97%; decreased CTL ΔCD107a (%): 7.6%; EBV-DNA: positive; anti-tuberculosis test: negative for tuberculosis; NGS in peripheral blood: M. avium detected; increases in MIP-1α, MIP-1β, IP-10, IL-6, IL-8, IL-10, IFN-γ, TNF- α, and MCP-1. |
BM cytology: active hyperplasia, granulation, and red line hyperplasia, with no typical progenitor cells; BM flow cytometry: low mature lymphocytes, no abnormal cells; PET/CT: Multiple active lymph nodes (bilateral neck, mediastinum, retroperitoneum), large spleen with slightly active metabolism. Lung shows multiple nodules and patchy high-density shadows with slight metabolic activity; Cervical lymph node pathology: suppurative granulomatous inflammation, no lymphoma, acid-fast staining positive, negative PCR for tuberculosis and EBER, positive PCR for M. avium. |
| 22, F | WBC: 1.27 × 109/L; HGB: 101 g/L; PLT: 96 × 109/L; decreased monocytes: 0.01 × 109/L; ALT: 51.2 U/L; LDH: 610 U/L; fibrinogen: 3.13 g/L; ESR: 65 mm/h; ferritin: 2630.8 ng/mL; low NK activity: 7.89% (normal range > 15.11%); high sCD25 level: 2923 pg/mL (normal range < 6400 pg/mL); decreased NK ΔCD107a: 5.16%; decreased CTL ΔCD107a (%): 6.0%; EBV-DNA: positive; perforin, granzyme, and MUNC13-4 expression levels were within normal ranges. |
BM cytology: active bone marrow hyperplasia and granulation, increased metamyelocytes, active erythroid hyperplasia with normal metarubricytes and polychromatic erythroblasts, nucleated red cells observed, and 189 megakaryocytes; BM flow cytometry: No abnormal cells, no myeloid tumor-related genes (JAK-2, BCR/ABL) found by bone marrow fusion genes, no variants in ASXL1, SF3B1, TP53, etc.; Abdominal ultrasound: splenomegaly; PET/CT: increased metabolism in right tonsil, multiple nodules with elevated metabolism in right submaxillary neck, right clavicular region, posterior neck muscle tissue, enlarged spleen with elevated metabolism, slight and uneven increase in osteoblast metabolism in sternum and pelvis; Cervical lymph node pathology: histiocytic necrotizing lymphadenitis. |
|
| 26, M | WBC: 2.32 × 109/L; HGB: 116 g/L; PLT: 94 × 109/L; decreased monocytes: 0.02 × 109/L; ALT: 195 U/L; LDH: 704 U/L; ferritin: 2370 ng/mL; low NK activity: 14.28% (normal range > 15.11%); high sCD25 level: 16,604 pg/mL (normal range < 6400 pg/mL); NGS detected M. kansasii and EBV. |
BM cytology: impaired granulocyte maturation, active erythroid hyperplasia, and polymorphic megakaryocytes; Abdominal ultrasound: splenomegaly; CT: interstitial pneumonia in right upper and lower lobes, multiple enlarged mediastinal lymph nodes, splenomegaly, and slightly enlarged lymph nodes in hepatic portal area; Cervical lymph node pathology: positive for EBER. |
|
| Huang et al., 2023 | 28, M | WBC: 0.6–1.3 × 109/L; HGB: 72–90 g/L; PLT: 43–64 × 109/L; decreased monocytes: 0.00 × 109/L; ESR: 75 mm/h; ferritin: 895 ng/mL; low NK activity: 1.36% (normal range > 15.11%); high sCD25 level: 6696 pg/mL (normal range < 6400 pg/mL); normal NK ΔCD107a and CTL ΔCD107a, low NK cell ΔPerforin: 72.56%; increases in IL-15, IFN-γ, and CRP; blood culture three weeks after admission: M. avium detected. |
BM cytology: proliferation of erythroid series, predominantly intermediate or late erythroblasts; PET/CT: multiple lesions in bilateral lung fields, hypermetabolic lymphadenopathies in hilum, mediastinum, supraclavicular area, and enlarged spleen; Mediastinal lymph node biopsy: necrotic background with plasma cells, eosinophils, and lymphocyte infiltration; NGS in BAL detected Klebsiella pneumonia. |
| Burak et al., 2021 | 22, F |
WBC: 0.85 × 109/L; HGB: 116 g/L; PLT: 120 × 109/L; absolute monocytes: 9.00 × 1011/L; AST: 159 U/L; ALT: 65 U/L; LDH: 701 U/L; ferritin: 27,514 ng/mL; TG: 380 mg/dL; fibrinogen: 509 mg/dL; flow cytometry of lymphocyte subsets: low levels of B, CD4, and NK cells; PBS: moderate anisocytosis, poikilocytosis, Döhle bodies, ovalocytes, and toxic granulation; CMV IgM > 240 IU/mL (normal range < 30 IU/mL); CMV IgG 0.80 IU/mL (normal range < 0.60 IU/mL); CMV DNA by PCR: 1,890,000 IU/mL; EBV serology: highly suggestive of reactivation; the patient’s Ig studies: normal levels of IgA and IgG, with elevated IgM; elevated sIL-2 receptor: 2452 pg/mL. NK-cell function test was inconclusive due to negligible NK cells. |
BM cytology: normocellular marrow with relative erythroid atypia, reactive plasmacytosis, and hemophagocytosis; BM flow cytometry and myelodysplastic syndromes panel were negative; Abdominal ultrasound: increased echogenicity of portal triads, reflecting acute hepatitis, mild splenomegaly, and an absent right kidney. CTA was negative for pulmonary embolism but revealed diffuse reticulonodular infiltrates in the lungs with numerous nodules, mediastinal lymphadenopathy, and splenomegaly; A bronchoscopy with BAL and transbronchial biopsy: mild airway hypervascularity without significant secretions, with a predominance of neutrophils, no malignant cell, and negative culture. |
| Mika et al., 2021 | 29, F | WBC: 1.5 × 109/L; HGB: 76 g/L; PLT: 78 × 109/L; decreased monocytes: 0.01 × 109/L; B-lymphopenia (9 cell/ml); NK lymphopenia (50 cell/ml); AST: 79 U/L; LDH: 626 U/L; increased ferritin: 3860 g/L; increased TG: 4.56 mmol/L; normal fibrinogen: 5.1 g/L; sIL-2 receptor: 2710 U/L; low NK ΔCD107a and CTL ΔCD107a. |
BM cytology: no evidence of myelodysplasia. BM histopathology: no evidence of myelodysplasia. BM and BAL culture: M. avium detected. Abdominal ultrasound: enlarged abdominal lymph nodes and hepatosplenomegaly; PET/CT: disseminated lymphadenopathy, bone marrow activation, and hepatosplenomegaly at diagnosis. Retroperitoneal lymph node pathology: granulomatous lymphadenitis with acid-fast bacteria, classified as M. avium. Control PET/CT: Following allo-HSCT transplantation (day + 240) and after 12 months of tuberculostatic therapy, showed complete remission of NTM-related lymphadenopathy and remission of HLH-associated hematopoietic activation. |
| Sun et al., 2021 | 17, F |
At age 10: pancytopenia, increased ferritin, hypertriglyceridemia, hypofibrinogenemia, decreased NK cell activity, elevated sIL-2 receptor, anti-VZV antibodies and DNA: detected in peripheral blood. Ate age 17: pancytopenia; monocytopenia; B cell, NK cell, and dendritic cell cytopenias, with relative sparing of T cell counts. |
At age 10: Brain MRI indicated inflammation or white matter demyelination; At age 12: Skin biopsy from erythema nodosa on the lower limbs showed panniculitis. At age 17: BM cytology: myeloid hyperplasia; chest CT: lung infection; patient had not received HSCT due to financial problems and concern about the risk of post-transplant complications. |
| Oleaga-Quintas et al., 2021 | 27?/M | Neutrophils: normal; T cells: normal; B cells: absence; NK cells: decreased; monocytes: decreased; Dendritic cells: NA. | BM cytology: hypoplasia, MDS. |
| Barber et al., 2021 | 11, F | WBC: 7.2 × 109/L (82% neutrophils, 17% lymphocytes, 1% monocytes); CFTR genotyping: negative; IgG: 1596 mg/dL; IgA: 179 mg/dL; IgM: 96 mg/dL; IgE 74 kU/L. | Non-diagnostic nasal ciliary biopsy: normal ultrastructure on TEM; Chest CT: bronchiectasis in multiple lobes; Sinus CT: mucosal thickening consistent with pansinusitis; Low nNO level: 9.8 nL/min. |
| Suzuki et al., 2020 | 27, F | WBC: 1.61 × 109/L; HGB: 119 g/L; PLT: 72 × 109/L; monocytopenia; B and NK lymphocytopenia; ferritin 4746 ng/mL; TG: 172 mg/dL; fibrinogen: 119 mg/dL; sIL-2 receptor 1889 U/mL. CMV IgM: positive; CMV IgG: negative; CMV antigenemia: detected 37/37 positive cells. |
BM cytology: phagocytic findings by macrophages without any malignant findings; MRI: intramuscular abscess formation within the thigh; Chest CT: multiple bilateral nodules on the lung, compatible with septic emboli; transthoracic echography: no vegetations. |
| Eguchi et al., 2018 | 14, M | WBC: 1.58 × 109/L (83% neutrophils, 16% lymphocytes, and 0% monocytes); HGB: 92 g/L; PLT: 10.8 × 109/L; ferritin: 2,206 ng/ml; sIL-2 receptor: 2,349 U/ml. |
BM cytology: hemophagocytosis without evidence of malignancy. BM flow cytometry: No cytogenetic abnormality; FDG/PET: intraperitoneal abscesses. |
| Prader et al., 2018 | 8, F | Normal blood counts at admission; bicytopenia on day 13: absolute neutrophil count: 710 cells/µL; HGB: 73 g/L; AST: 490 U/L; ALT: 374 U/L; LDH: 1619 U/L; increased ferritin 7090 µg/L; increased TG: 5.8 mmol/L; elevated fibrinogen; decreased NK ΔCD107a: 9.4%; VZV DNA in the patient’s blood: peaking at 537,000 copies/mL; sIL-2 receptor: 1622 pg/ml; Perforin expression: within the lower range compared to an adult control and within the range of a control individual with a known heterozygous A91V-perforin mutation; flow cytometry in peripheral blood: normal proportion of TCRγδ + cells. |
BM cytology: evidence of hemophagocytosis; Skin biopsy from the rash: nonspecific changes with epidermal spongiosis, perivascular lymphocytic infiltrates, and rare eosinophilic granulocytes; VZV DNA detected in the fluid from skin vesicles; CXR: miliary pattern of pulmonary opacities. |
| 7, M |
Pancytopenia on day 2: absolute neutrophil count: 380 cells/µL, HGB: 95 g/L, PLT: 53 × 109/L; increased ferritin (from 820 to 4,510 µg/L within 3 days); increased TG (from 0.6 to 2.2 mmol/l within 3 days); mild hypogammaglobulinemia: IgG 6.1 g/l; normal range: 6.7–12.1); decreased NK ΔCD107a: 8.8%; VZV DNA in the patient’s blood: peaking at 183,572 copies/mL; EBV DNA: slightly increased during VZV infection (max 424 copies/ml, normal < 100) and became negative 2 months later; Perforin expression: within the lower range compared to an adult control and within the range of a control individual with a known heterozygous A91V-perforin mutation; flow cytometry in peripheral blood: high proportion of TCRγδ + cells. |
VZV DNA detected in the fluid from skin vesicles. | |
| Parta et al., 2018 | 17, F | NA. | BM cytology: MDS. |
| Donadieu et al., 2018 | 26, F | NA. | BM cytology: MDS, AML4. |
| 57, ? | NA. | BM cytology: MDS, undifferentiated acute leukemia. | |
| 24, F | NA. | BM cytology: MDS. | |
| 28, ? | NA. | BM cytology: MDS. | |
| 10, ? | NA. | BM cytology: MDS. | |
| 40, ? | NA. | BM cytology: MDS. | |
| Yamamoto et al., 2018 | 18, M | NA. | BM cytology: MDS-RCMD with IPSS: Int-1. |
| Cohen et al., 2016 | 24, F | Neutropenia; lymphopenia; anemia; thrombocytopenia; reduced numbers of B cells and NK cells; hypogammaglobulinemia; increased ferritin; increased TG; EBV DNA in peripheral blood T cells: positive (2,022,000 copies/106 T cells); EBV DNA in peripheral B cells: positive (18,410,000 copies/106 B cells); Evidence of T-cell clones in the blood and CSF, along with an EBV-positive T-cell lymphoma of the lung. |
BM cytology: hypocellular with EBV-positive T-cells and B-cell lymphopenia, evidence of hemophagocytosis and a T-cell clone; Skin and intestinal biopsy: EBV-positive hydroa vacciniforme–like lymphoma involving the colon and skin with a clonal T-cell population; Abdominal CT: splenic infarcts and a perisplenic abscess, which was drained. Echocardiography: vegetations on the mitral valve, indicative of presumed marantic endocarditis. |
| Spinner et al., 2016 | 18, F |
At age 12: neutropenia (ANC: 1400 cells/mL), lymphopenia (ALC: 1570 cells/mL, B lymphopenia (absolute CD19+: 23 cells/mL), NK lymphopenia (absolute CD56 + CD16+:<12 cells/mL) with relative T-cell sparing, monocytopenia (AMC: 20 cells/mL), NK-cell function: reduced with minimal response to exogenous IL-2, CD56bright subset: nearly absent; At age 18: WBC: 0.7 × 109/L; HGB: 70 g/L; PLT: 14 × 109/L; AST: 6821 U/L; ALT: 3081 U/L; fibrinogen: <60 mg/dL; ferritin: 49,900 ng/mL; HSV-1 DNA in the blood: positive. |
BM cytology: hypocellular with decreased trilineage hematopoiesis and abundant hemophagocytosis; Autopsy: disseminated HSV-1 infection involving the lungs, liver, and vagina. |
M, male; WBC, white blood cell; HGB, hemoglobin; PLT, platelet; TG, triglycerides; ESR, erythrocyte sedimentation rate; NK, natural killer; sCD25, soluble CD25; CTL, cytotoxic T lymphocyte; EBV, epstein-barr virus; NGS, next-generation sequencing; M. avium, mycobacterium avium; MIP-1α, macrophage inflammatory protein-1 alpha; MIP-1β, macrophage inflammatory protein-1 beta; IP-10, interferon gamma-induced protein 10; IL, interleukin; IFN-γ, interferon gamma; TNF-α, tumor necrosis factor alpha; MCP-1, monocyte chemoattractant protein-1; BM, bone marrow; PET/CT, positron emission tomography/computed tomography; PCR, polymerase chain reaction; EBER, epstein-barr virus-encoded RNA; F, famle; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; M. kansasii, mycobacterium kansasii; CT, computed tomography; CRP, c-reactive protein; BAL, bronchoalveolar lavage fluid; AST, aspartate aminotransferase; PBS, peripheral blood smear
These studies comprised 7 males, 12 females, and 4 not mentioned (NM), resulting in a male-to-female ratio of 0.6:1. The patients’ ages ranged from 7 to 57 years, with a mean (SD) age of 23.48 (10.54) years. Evaluation of family histories based on reports in GATA2-deficient patients revealed that their first-degree relatives often have a confirmed GATA2 mutation. Affected individuals, including father [24], mothers [18, 26], sister [24], and daughter [6] have been identified in these cases. Additionally, there are reports of suspicious cases within families, such as evidence of AML [6], benign leukopenia [22], and MDS [29] in fathers. Evaluation of genetic findings shows that GATA2 mutations can be missense, nonsense, or frameshift mutations in various variants. Additionally, patients may encounter karyotype abnormalities [27, 28].
Among the hematological laboratory findings, 12 patients were diagnosed with pancytopenia [6, 11, 17, 18, 22–24, 26, 30, 31], one with bicytopenia [18], while complete complete blood count (CBC) results were not reported for the remaining 10 patients. Additionally, among the reported patients, NK ΔCD107a was low in 5/6 cases, and CTL ΔCD107a was low in 3/4 cases [6, 11, 18, 23].
These GATA2 deficient patients can be infected previously or newly by a wide range of bacterial infections, such as Mycobacterium (particularly Mycobacterium avium (M. avium) and Mycobacterium kansasii (M. kansasii)), Citrobacter freundii (C. freundii), Klebsiella species (Klebsiella spp.), methicillin-resistant Staphylococcus aureus (MRSA), and Streptococcus species (Streptococcus spp.). Viral infections such as Epstein-Barr virus (EBV), cytomegalovirus (CMV), varicella-zoster virus (VZV), herpes simplex virus (HSV), parvovirus, influenza A, and human papillomavirus (HPV) can also occur. Additionally, fungal infections such as aspergillosis, mucormycosis, Candida species (Candida spp.), and Blastomyces dermatitidis may be present.
Among these GATA2 deficient patients, 8 (34.78%) underwent hematopoietic stem cell transplantation (HSCT). Out of these patients, 6 are currently alive, while 2 died post-therapy—one infected with Aspergillosis and the other with EBV following HSCT. Furthermore, 1 patient (4.34%) is currently being considered for HSCT.
Mortality was observed in 9 GATA2 deficiency patients who experienced HLH at least once (39.13%). The first reported case of mortality occurred in an 18-year-old female in 2016, and the most recent case involved a 28-year-old male in 2023. The youngest patient who died was 10 years old, and the oldest was 57.
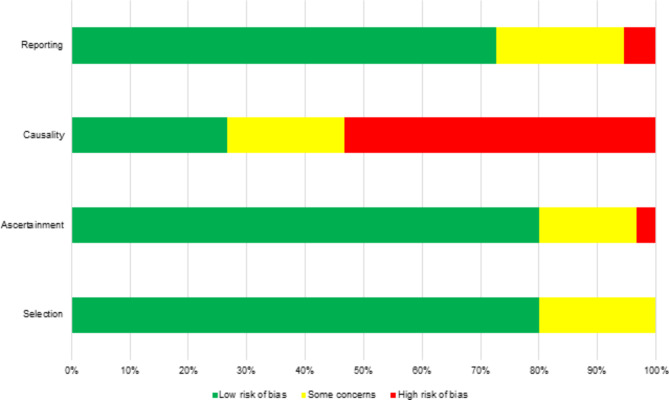
Risk of bias assessment
The selected papers were assessed for bias risk using Murad et al., 2018 criteria across four domains: selection, ascertainment, causality, and reporting. Studies were categorized as having low, some concern, or high risk of bias within each domain. The detailed assessment is shown in Fig. 2.
Fig. 2.
The risk of bias assessment of enrolled studies based on Murad et al., 2018 that explain four domains
Discussion
In vertebrates, six GATA family transcription factors (GATA1–GATA6) have been identified. These factors possess highly conserved dual zinc finger domains (ZF1 and ZF2) in their central regions [30, 32, 33]. ZF1 regulates interactions between proteins, while ZF2 binds to GATA sites on DNA to regulate transcription [34, 35]. Clinically, mutations associated with disease most frequently occur in ZF-1 and ZF-2 [30].
GATA1 and GATA2 are crucial for hematopoiesis, GATA3 for T cell development, and GATA4-GATA6 for cardiac embryogenesis [30]. The GATA2 gene, positioned on cytoband 21.3 of the long arm of human chromosome 3, comprises seven exons, of which five are translated. GATA2 plays a pivotal role in modulating the expression of key target genes involved in hematopoietic differentiation and vascular development, including RUNX, SCL/TAL1, PU.1 (SPI1), FLI1, and LMO2 [12, 36–39]. So, GATA2 is a transcriptional regulator in hematopoiesis and lymphatic angiogenesis, specifically in hematopoietic stem cell activity and self-renewal, maintenance of erythroid precursor cells, and importantly, production of megakaryocytes, mast cells, NK cells, and monocytes. Moreover, GATA2 can be expressed in endothelial cells, the central nervous system, placenta, and fetal liver and heart [12, 13, 40, 41].
GATA2 mutations include null mutations (such as nonsense and frameshift mutations, etc.), missense mutations, and mutations in the GATA2 intron 5 enhancer element, accounting for 60%, 30%, and 5–10% of cases, respectively [39]. Overall, GATA2 somatic mutations are often described as gain-of-function mutations, whereas germline GATA2 mutations are typically characterized as loss-of-function, resulting in haploinsufficiency [15]. In 2008, the initial documentation of hematopoietic abnormalities linked to GATA2 involved two gain-of-function mutations within the GATA2 gene associated with blast crisis in chronic myeloid leukemia [42, 43]. In 2011, it was discovered that loss-of-function heterozygous mutations in GATA2 genes contribute to a spectrum of immunodeficiency disorders. These include MonoMAC syndrome (characterized by monocytopenia with infection by Mycobacterium avium complex), various myeloid malignancies, familial and primary pediatric myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), DCML deficiency (dendritic cell, monocyte, B, and NK cell involvment), and Emberger syndrome (MDS with lymphedema) [12, 44–47]. These diverse clinical conditions now fall under the umbrella term of GATA2 deficiency [39]. As of now, despite the frequent overlap and significant variation in clinical features observed over the course of the disease, there is no universally recognized GATA2 phenotype that serves as a definitive marker [13].
GATA2 deficiency, an autosomal dominant disease with high but incomplete penetrance, presents with a spectrum of clinical manifestations that typically develop in the second and third decades of life, including hematologic and non-hematologic malignancies, cardiovascular, dermatologic, and respiratory complications, immunological abnormalities, and susceptibility to infectious diseases (viral, bacterial, and fungal) [6, 12, 30, 37, 39]. GATA2 deficiency is one of the most frequent genetic causes of BM failure, particularly in children and young adults [12, 39, 48]. Following uneventful pregnancies, GATA2 deficient patients are born with normal BM cellularity and peripheral blood cell counts, but in childhood, the BM typically becomes hypocellular, a hallmark of childhood MDS [12, 34, 39, 49]. The likelihood of developing hematologic malignancies rises with advancing age [39]. Patients with this deficiency face a significant risk of developing MDS or AML by the age of forty [50]. Peripheral blood features include deficiencies of monocytes, DCs, NK cells, and B cells, with neutropenia being less common [51].
The deficiencies in both humoral and cell-mediated immunity directly attributable to high rates of infections [12]. A reduced number and function of NK cells in GATA2 deficiency contribute to impaired viral clearance and inadequate monitoring of malignant transformations. Additionally, the lack of DCs hinders the recognition of viruses and intracellular pathogens, specifically, leading to increased mycobacterial infections susceptibility. Additionally, mycobacterial infection resistance is compromised by monocytopenia and failure of tissue macrophages to form proper inflammatory granulomas [41, 52]. Disseminated mycobacteriosis is infrequent during childhood due to normal blood counts, but its occurrence rises as BM function deteriorates with age [12]. So, prophylaxis with azithromycin for non-tuberculous mycobacteria is suggested once blood counts decrease [53]. Viral infections, including HPV, HSV, VZV, CMV, EBV, and molluscum contagiosum, are highly common in patients with GATA2 deficiency, occurring in up to 70% of cases [30, 54]. HPV is particularly prevalent, affecting between 50% and 63% of these patients, and often manifests as extensive, recurrent, or treatment-resistant warts, condylomas, and/or dysplasia. Consequently, the presence of persistent warts in patients with cytopenia is a strong indicator of GATA2 deficiency [39, 54]. HPV vaccination is crucial due to the heightened susceptibility of GATA2 deficiency patients and the risk of severe oncogenic lesions [55]. Based on Spinner et al.‘s study of 57 patients with GATA2 deficiency, severe HSV infections occurred in 35%, severe VZV in 11%, persistent EBV viremia in 11%, CMV pneumonia or dissemination in 4%, and severe cutaneous molluscum contagiosum in 3.5%. Additionally, about 16% of patients experienced severe fungal infections, including invasive aspergillosis, disseminated histoplasmosis, and recurrent candidiasis [54]. Moreover, the profound immunodeficiency in GATA2 deficiency, with susceptibility to bacterial (especially mycobacterial), viral, and fungal infections, highlights the critical context for HLH cases in these patients. Based on our results, a total of 23 patients with GATA2 deficiency experienced HLH triggered by various infections, including M. avium, M. kansasii, EBV, CMV, VZV, HSV, and influenza A.
NK cell cytotoxicity is reduced in GATA2 deficiency syndrome, accompanied by a specific loss of the CD56bright NK cell subset, which indicates impaired differentiation of cytotoxic NK cells [56]. Degranulation and damage to cytotoxic T lymphocytes (CTLs) and NK cells identify through ΔCD107a analysis [6]. Detection of ΔCD107a on CTL surfaces is highly sensitive and specific for diagnosing HLH associated with genetic disorders [6]. It was hypothesized that the majority of GATA2 mutations in HLH patients disrupted the function of the zinc finger domain or led to its loss, with the onset of HLH triggered by infection [11]; Based on our evaluation, three patients reported an absence of both zinc finger domains [11, 23, 24], and one patient reported a loss of the C-terminal zinc finger domain [22]. GATA2 deficiency causes loss of CD56bright NK cells and the impaired NK cell activity in this patient may be due to missing zinc finger domains reducing perforin release [11, 57]. Low NK cell counts are also a diagnostic criterion for HLH, indicating a high risk for aggressive HLH [22, 58].
Given the familial pattern of GATA2 mutations and the potential for hematologic disorders in first-degree relatives, early detection and monitoring are crucial for preventing disease progression. So, all first-degree relatives of a patient with GATA2 deficiency should be screened. Following the identification of healthy carriers, a BM aspirate with cytogenetics and a baseline biopsy, as recommended by most international societies, should be performed at diagnosis [12, 55].
HLH therapy aims to reduce inflammation and immune cell overactivation. If the cause is unknown, diagnostics should explore potential infections, malignancies, or autoimmune disorders [59]. Currently, the only treatment that can cure patients with GATA2 deficiency is allogeneic hematopoietic stem cell transplantation (allo-HSCT), which addresses the compromised hematopoietic and lymphoid systems such as restoring normal hematopoiesis, resolving MDS, and potentially eliminating longstanding infections [12, 55]. Indications for HSCT primarily include MDS, recurrent infections, declining pulmonary function, and secondary organ damage. Studies indicate a more favorable outcome when HSCT is performed early in the disease course, prior to cytogenetic abnormalities or progression to AML. Symptomatic patients not undergoing HSCT are at heightened risk of neoplastic transformation [12, 28, 54]. Our findings highlight that despite its curative potential, HSCT in GATA2 deficiency still carries significant risks, including infection-related mortality.
The study’s strengths include its broad inclusion criteria across various publication types without restrictions on study design, country, or language. A thorough search strategy adhering to PRISMA 2020 guidelines enhanced the review’s comprehensiveness. The quality of included studies was rigorously assessed for reliability. However, limitations include the inability to access full-text articles from one study, potentially impacting completeness. Publication and language biases in study selection and heterogeneity among studies may affect generalizability. Nonetheless, this study offers valuable insights into GATA2 deficiency among HLH patients.
Conclusions
GATA2 deficiency presents with a wide range of clinical manifestations, including hematologic malignancies, immunodeficiency states, and heightened susceptibility to severe infections, which can precipitate HLH. Our systematic review highlights the crucial role of genetic testing in diagnosing GATA2 mutations among individuals who have experienced HLH, especially those affected by various infections like M. avium. Given the likelihood of first-degree relatives harboring GATA2 mutations, comprehensive genetic evaluations in affected families are imperative. Urgent intervention is critical due to the high mortality rate observed in patients with GATA2 deficiency who experience HLH. HSCT emerges as a pivotal therapeutic approach for managing GATA2-deficient patients, offering potential curative benefits by addressing underlying hematologic and immune deficiencies, though it carries significant risks such as infection-related mortality.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
M.R.Z.R contributed to conceptualization, methodology, and data extraction and drafted the manuscript. H.M was involved in data extraction and level of evidence and drafted the manuscript. M.N wrote the manuscript. S.D.A contributed to data extraction and finalizing the manuscript.
Funding
There is no funding involved in the current study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohammad Rezaei Zadeh Rukerd and Hanieh Mirkamali contributed equally to this work.
References
- 1.Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. [DOI] [PubMed] [Google Scholar]
- 2.Chinnici A, Beneforti L, Pegoraro F, Trambusti I, Tondo A, Favre C, et al. Approaching hemophagocytic lymphohistiocytosis. Front Immunol. 2023;14:1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. J Am Soc Hematol. 2011;118:4041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JC, Logan AC. Diagnosis and management of Adult Malignancy-Associated Hemophagocytic Lymphohistiocytosis. Cancers (Basel). 2023;15:1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Samkari H, Berliner N. Hemophagocytic lymphohistiocytosis. Annu Rev Pathol. 2018;13:27–49. [DOI] [PubMed] [Google Scholar]
- 6.Wu L, Wang J, Song D, You Y, Wang Z. Haemophagocytic lymphohistiocytosis caused by GATA2 deficiency: a report on three patients. BMC Infect Dis. 2024;24:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan MB, Allen CE, Greenberg J, Henry M, Hermiston ML, Kumar A, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:e27929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosado FG, Kim AS. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139:713–27. [DOI] [PubMed] [Google Scholar]
- 9.Hayden A, Park S, Giustini D, Lee AYY, Chen LYC. Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: a systematic scoping review. Blood Rev. 2016;30:411–20. [DOI] [PubMed] [Google Scholar]
- 10.Sieni E, Cetica V, Piccin A, Gherlinzoni F, Sasso FC, Rabusin M et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. 2012. [DOI] [PMC free article] [PubMed]
- 11.Huang X, Wu B, Wu D, Huang X, Shen M. Case Report: missing zinc finger domains: hemophagocytic lymphohistiocytosis in a GATA2 deficiency patient triggered by non-tuberculous mycobacteriosis. Front. 2023;14:1191757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabozzi F, Strocchio L, Mastronuzzi A, Merli P. GATA2 and marrow failure. Best Pract Res Clin Haematol. 2021;34:101278. [DOI] [PubMed] [Google Scholar]
- 13.Kotmayer L, Romero-Moya D, Marin‐Bejar O, Kozyra E, Català A, Bigas A, et al. GATA2 deficiency and MDS/AML: experimental strategies for disease modelling and future therapeutic prospects. Br J Haematol. 2022;199:482–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mir MA, Kochuparambil ST, Abraham RS, Rodriguez V, Howard M, Hsu AP, et al. Spectrum of myeloid neoplasms and immune deficiency associated with germline GATA2 mutations. Cancer Med. 2015;4:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bresnick EH, Jung MM, Katsumura KR. Human GATA2 mutations and hematologic disease: how many paths to pathogenesis? Blood Adv. 2020;4:4584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber AT, Davis SD, Boutros H, Zariwala M, Knowles MR, Leigh MW. Use caution interpreting nasal nitric oxide: overlap in primary ciliary dyskinesia and primary immunodeficiency. Pediatr Pulmonol. 2021;56:4045–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki T, Takaya S, Kunimatsu J, Kutsuna S, Hayakawa K, Shibata H, et al. GATA2 mutation underlies hemophagocytic lymphohistiocytosis in an adult with primary cytomegalovirus infection. J Infect Chemother. 2020;26:252–6. [DOI] [PubMed] [Google Scholar]
- 18.Prader S, Felber M, Volkmer B, Truck J, Schwieger-Briel A, Theiler M, et al. Life-threatening primary varicella zoster virus infection with Hemophagocytic Lymphohistiocytosis-Like Disease in GATA2 haploinsufficiency accompanied by expansion of double negative T-Lymphocytes. Front. 2018;9:2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spinner MA, Odio C, Calvo KR, Hsu AP, Zerbe CS, Cuellar-Rodriguez J, et al. Hemophagocytic Lymphohistiocytosis Associated with NK Cell Dysfunction and disseminated Herpesvirus infection in GATA2 Deficiency/Monomac Syndrome. Blood. 2014;124:3. [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burak N, Jan N, Kessler J, Oei E, Patel P, Feldman S. Diagnosis of GATA2 Deficiency in a Young Woman with Hemophagocytic Lymphohistiocytosis triggered by Acute systemic cytomegalovirus infection. Am J Case Rep. 2021;22:e927087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mika T, Vangala D, Eckhardt M, La Rosee P, Lange C, Lehmberg K, et al. Case Report: Hemophagocytic Lymphohistiocytosis and Non-tuberculous Mycobacteriosis caused by a Novel GATA2 variant. Front. 2021;12:682934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Xu N, Shen M, Wang R, Sun Y, Zhuang J, et al. GATA2 mutation with recurrent haemophagocytic lymphohistiocytosis and panniculitis: a case report. Rheumatology (Oxford). 2021;60:e229–31. [DOI] [PubMed] [Google Scholar]
- 25.Oleaga-Quintas C, de Oliveira-Junior EB, Rosain J, Rapaport F, Deswarte C, Guérin A, et al. Inherited GATA2 deficiency is dominant by haploinsufficiency and displays incomplete clinical penetrance. J Clin Immunol. 2021;41:639–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eguchi K, Ishimura M, Sonoda M, Ono H, Shiraishi A, Kanno S, et al. Nontuberculous mycobacteria-associated hemophagocytic lymphohistiocytosis in MonoMAC syndrome. Pediatr Blood Cancer. 2018;65:e27017. [DOI] [PubMed] [Google Scholar]
- 27.Parta M, Shah NN, Baird K, Rafei H, Calvo KR, Hughes T, et al. Allogeneic hematopoietic stem cell transplantation for GATA2 Deficiency using a Busulfan-based regimen. Biol Blood Marrow Transpl. 2018;24:1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donadieu J, Lamant M, Fieschi C, de Fontbrune FS, Caye A, Ouachee M, et al. Natural history of GATA2 deficiency in a survey of 79 French and Belgian patients. Haematologica. 2018;103:1278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto H, Hattori H, Takagi E, Morishita T, Ishikawa Y, Terakura S, et al. [MonoMAC syndrome patient developing myelodysplastic syndrome following persistent EBV infection]. Rinsho Ketsueki. 2018;59:315–22. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JI. GATA2 Deficiency and Epstein-Barr Virus Disease. Front Immunol. 2017;8:1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinner MA, Ker JP, Stoudenmire CJ, Fadare O, Mace EM, Orange JS, et al. GATA2 deficiency underlying severe blastomycosis and fatal herpes simplex virus-associated hemophagocytic lymphohistiocytosis. J Allergy Clin Immunol. 2016;137:638–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimizu R, Yamamoto M. GATA-related hematologic disorders. Exp Hematol. 2016;44:696–705. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu R, Yamamoto M. Gene expression regulation and domain function of hematopoietic GATA factors. Semin Cell Dev Biol. 2005;16:129–36. [DOI] [PubMed] [Google Scholar]
- 34.Hirabayashi S, Wlodarski MW, Kozyra E, Niemeyer CM. Heterogeneity of GATA2-related myeloid neoplasms. Int J Hematol. 2017;106:175–82. [DOI] [PubMed] [Google Scholar]
- 35.Katsumura KR, Bresnick EH, Group GFM. The GATA factor revolution in hematology. Blood J Am Soc Hematol. 2017;129:2092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crispino JD, Horwitz MS. GATA factor mutations in hematologic disease. Blood J Am Soc Hematol. 2017;129:2103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aktar A, Heit B. Role of the pioneer transcription factor GATA2 in health and disease. J Mol Med (Berl). 2023;101:1191–208. [DOI] [PubMed] [Google Scholar]
- 38.Vicente C, Conchillo A, García-Sánchez MA, Odero MD. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit Rev Oncol/Hematol. 2012;82:1–17. [DOI] [PubMed] [Google Scholar]
- 39.Rajput RV, Arnold DE. GATA2 Deficiency: predisposition to myeloid malignancy and hematopoietic cell transplantation. Curr Hematol Malig Rep. 2023;18:89–97. [DOI] [PubMed] [Google Scholar]
- 40.Peters IJA, de Pater E, Zhang W. The role of GATA2 in adult hematopoiesis and cell fate determination. Front Cell Dev Biol. 2023;11:1250827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collin M, Dickinson R, Bigley V. Haematopoietic and immune defects associated with GATA2 mutation. Br J Haematol. 2015;169:173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu R, Yamamoto M. Quantitative and qualitative impairments in GATA2 and myeloid neoplasms. IUBMB Life. 2020;72:142–50. [DOI] [PubMed] [Google Scholar]
- 43.Zhang S-J, Ma L-Y, Huang Q-H, Li G, Gu B-W, Gao X-D, et al. Gain-of-function mutation of GATA-2 in acute myeloid transformation of chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2008;105:2076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahn CN, Chong C-E, Carmichael CL, Wilkins EJ, Brautigan PJ, Li X-C, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. 2011;43:929–31. [DOI] [PubMed] [Google Scholar]
- 48.Wlodarski MW, Hirabayashi S, Pastor V, Starý J, Hasle H, Masetti R, et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127:1387–97. quiz 1518. [DOI] [PubMed] [Google Scholar]
- 49.Ganapathi KA, Townsley DM, Hsu AP, Arthur DC, Zerbe CS, Cuellar-Rodriguez J, et al. GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood J Am Soc Hematol. 2015;125:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koyunlar C, Gioacchino E, Vadgama D, de Looper H, Zink J, Ter Borg MND, et al. Gata2-regulated Gfi1b expression controls endothelial programming during endothelial-to-hematopoietic transition. Blood Adv. 2023;7:2082–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McReynolds LJ, Calvo KR, Holland SM. Germline GATA2 mutation and bone marrow failure. Hematology/Oncology Clin. 2018;32:713–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruzzese A, Leardini D, Masetti R, Strocchio L, Girardi K, Algeri M, et al. GATA2 related conditions and predisposition to pediatric myelodysplastic syndromes. Cancers. 2020;12:2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu AP, McReynolds LJ, Holland SM. GATA2 deficiency. Curr Opin Allergy Clin Immunol. 2015;15:104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood J Am Soc Hematol. 2014;123:809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santiago M, Liquori A, Such E, Zúñiga Á, Cervera J. The clinical spectrum, diagnosis, and Management of GATA2 Deficiency. Cancers. 2023;15:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. 2013;121:2669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang D, Uyemura B, Hashemi E, Bjorgaard S, Riese M, Verbsky J, et al. Role of GATA2 in human NK Cell Development. Crit Rev Immunol. 2021;41:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henter J-I, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31. [DOI] [PubMed] [Google Scholar]
- 59.Atim-Oluk M. Cytomegalovirus associated haemophagocytic lymphohistiocytosis in the immunocompetent adult managed according to HLH-2004 diagnostic using clinical and serological means only. Eur J Microbiol Immunol (Bp). 2013;3:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.