Abstract
Background
The adoption of robotic pancreaticoduodenectomy (PD) has increased in recent years for the treatment of pancreatic head tumors and periampullary lesions. Some potential benefits seem to be demonstrated; however, obtaining specimens through this method can potentially compromise the diagnosis depending on the timing of the specimen retrieval, and the impact of longer perioperative time on ischemia and autolysis of the surgical specimen has not been analyzed. The aim of this study is to evaluate the histological changes associated with timing of specimen retrieval during robotic PD.
Methods
A review of histopathology files was performed for all pancreatic specimens collected at our hospital from January 2022 to March 2024. Both warm ischemia time (WIT) and cold ischemia time (CIT) were collected. Histological features related to ischemic damage were evaluated in normal duodenal and pancreatic parenchyma as well as pancreatic tumor, and were graded as: absent, mild, moderate and severe. Univariate and multivariate analyses were performed to determine which variables were associated with moderate and severe ischemic changes.
Results
Sixty surgical specimens were analyzed: 20 open PD, 17 robotic PD with cold ischemia, and 23 robotic PD. Median total WIT was 182 min (open PD 57 min vs. RPD 190 min vs. RPD-CI 198 min; p < 0.001). Median CIT was 760 min (740–835) in samples stored at 4ºC. Univariate analysis showed that longer intraoperative time, male gender and cold ischemia were associated with pancreatic tissue ischemic changes. In multivariate analysis, cold ischemia was the only independent factor associated with normal pancreatic tissue and tumor tissue moderate and severe ischemic changes.
Conclusions
Prolonged ischemia time, especially in the case of cold storage, has a strong effect on the degradation of normal and tumor tissue without affecting pathological evaluation. Operative teams should aim to optimize both the duration and efficiency of the surgical procedure, ensuring minimal ischemic time. Simultaneously, pathology departments must be equipped to process pancreatic specimens promptly, with protocols in place to minimize the time between resection and analysis.
Keywords: Robotic pancreaticoduodenectomy, Cold ischemia, Specimen damage, Warm ischemia
Introduction
Pancreatic cancer remains one of the most challenging and devastating oncological diseases. Its incidence is increasing worldwide, estimating that by the year 2030, pancreatic cancer could become the second most common cause of cancer-related death in the United States, surpassing both breast cancer and colorectal cancer [1]. Due to its late detection, biological aggressiveness, and resistance to conventional therapies, this disease presents several clinical and therapeutic challenges. Despite advances in medical care, the overall 5-year survival rate for pancreatic cancer remains alarmingly low, as only about 10% of patients survive beyond this period [2].
Pancreaticoduodenectomy (PD), also known as the Whipple procedure, is established as the gold standard for the surgical treatment of resectable tumors in the head of the pancreas and duodenum [3]. However, this complex surgical procedure carries significant risks. These include a high rate of perioperative complications and a long postoperative recovery [4, 5]. The development of minimally invasive surgical techniques is a major focus in the field of pancreatic surgery, with the aim of improving postoperative outcomes and quality of life. In this context, robotic pancreaticoduodenectomy (RPD) has emerged as a promising alternative to conventional open pancreaticoduodenectomy (OPD) [6]. RPD makes use of advanced robotic technology to perform complex resections of the pancreas with greater precision and with less trauma to the tissue [7]. Robotic surgical systems allow for more precise dissection and meticulous reconstruction with significant advantages such as three-dimensional visualization, optical magnification, and improved instrument articulation.
These features have led to increasing adoption of RPD in surgical centers around the world, with studies indicating comparable or even superior surgical outcomes compared to OPD. In addition to the technical advantages, RPD has also been associated with lower rates of post-operative complications [8]. These include reduced perioperative morbidity, a lower incidence of pancreatic fistulas and faster patient recovery. Also, some studies have shown a higher number of yielded lymph nodes and higher rates of R0 [9]. In the context of pancreatic cancer, where reducing perioperative complications can have a significant impact on patient survival and quality of life, these findings are particularly encouraging. However, despite these potential benefits, RPD remains a complex procedure. It requires a significant learning curve and technical expertise on the part of the surgeon.
However, when PD is performed using a minimally invasive approach, the final specimen containing the tumor is sometimes removed following the reconstruction phase (including 3 anastomoses).
In this context, the surgical specimen goes through the phenomenon of ischemia and autolysis, which may lead to microscopic degradation of the tissue. This issue may be particularly relevant as it may have an impact on the final quality and oncological evaluation of the histological and oncological assessment.
Therefore, although robotic surgery offers a variety of benefits for patient recovery post-surgery, obtaining specimens through this method can potentially compromise the diagnosis depending on the timing of the specimen retrieval.
As there is no previous literature on this topic, the aim of this study is to evaluate the impact of timing of specimen retrieval on the histological changes following robotic PD.
Methods
After approval by the institutional review board of our Institution with identification number HCB/2022/0095, a search of the archival histopathology files was conducted for all pancreatic specimens collected at our hospital from January 2022 to March 2024.
All consecutive patients undergoing robotic PD for pancreatic, ampullary, and biliary malignancies at our institution were included in this study. Patients with preoperative pancreatitis were excluded. Patients undergoing neoadjuvant treatment were also excluded. Patients undergoing RPD receiving conversion to open surgery were also excluded: in these cases, the specimens were sent to the pathologist for fresh frozen section, therefore without additional ischemia time. No specimens obtained during open surgery were preserved in cold conditions: this was due to the shorter duration of surgery and therefore no need for cold storage.
In our Center, pancreatic specimens are examined according to the protocols provided by the College of American Pathologists. The guidelines from the College of American Pathologists and final reports are issued following the cancer protocol templates [10, 11].
Surgical procedure
Surgical procedure is performed in all patients according to center protocols, and mitigation strategies are used in each case based on the surgeon’s experience, as previously described [5, 12]. Pancreatic neck is divided by ultrasonic energy devices, in all cases.
In open procedures, once the specimen is completely detached from the superior mesenteric vein (SMV), it is sent to the pathology Department. Contrary, in minimally invasive approach, once the specimen is completely detached from the SMV, it is kept in the abdominal cavity until the reconstruction is completed.
Surgical time is collected in all cases. All surgeries were performed by the senior author (FA) using the same method of specimen retrieval by plastic bag.
Specimen storage
All pancreatic specimens arriving before 5 PM at the Pathology Department undergo fresh examination, including orientation, measuring, and identification of the relevant structures and margins. Pancreatic neck margin, uncinate margin, vascular groove area, and common bile duct margin are then inked with different colors [11]. Anterior and posterior free surfaces are also inked. Fixation and harvesting are performed by letting the specimen be properly fixed in formalin for 24 h. Nontumoral tissue sections from the pancreas and the adjacent organs are also included. Several images of the specimen in both fresh (before and after inking, bivalving, and sectioning) and fixed states are also taken.
If the specimen is extracted after 5 PM, to mitigate the activation of enzymes producing autolysis, samples are stored dry at 4 °C to control cold ischemia until they can be examined the following day before undergoing formalin fixing. PD surgery is not performed on Friday to avoid long-term issues with degradation.
Ischemia time measurement
Both warm (WIT) and cold (CIT) ischemia times were collected. Intraoperative warm ischemia was defined as the time from complete dissection of the posterior lamina to specimen extraction; this was determined by reviewing the surgical videos of each case included. Additionally, time to arrival at the pathology department for fresh examination and inking, up to formalin storage was added as warm ischemic time: this time was calculated by reviewing the electronic records.
In case of specimen extraction after 5 PM, time of cold ischemia was added. It was defined as the time between cold storage of the specimen following extraction and the formalin storage following the inking at the pathology department on the next day.
Histopathological analysis
Quality of the pathological assessment was reviewed by two dedicated pathologists with large experience in pancreatic assessment who were blinded to the surgical approach. For the purpose of this study, only the pancreatic tumor, normal pancreatic parenchyma, and normal duodenum were evaluated.
All slides of every case were assessed under light microscopy. Histologic features related to ischemic damage such as cytoplasmic eosinophilia, nuclear degeneration or nuclear absence with ghost outlines of cells, karyolisis, and karyorrhexis were evaluated in neoplastic cells, pancreatic parenchyma including acinar and ductal cells, and duodenal epithelium. Ischemic changes were then semi-quantitatively graded as absent (0), mild (1), moderate (2) and severe (3) depending on the extent of the changes [13].
Additionally, the acinar/collagen/fat score was also determined as previously described. Briefly, the acinar cell, collagen, and fat content were evaluated as a proportion of the total surface area of tissue on the slides comprising the pancreatic neck margin [14].
A final evaluation of histological assessment was provided on tumor grade, perineural invasion, lymphovascular invasion, number of total lymph nodes, metastatic lymph nodes, resection margin status, and final pTNM.
Statistical analysis
Consistency and accuracy of the data were ensured by cross-verification among two observers (CGA and FA) reviewing each patient’s record about: date of surgery, operating theatre, and final pathology report.
All categorical data are presented as the number of cases and percentages. Chi-square and Fisher’s exact tests, when appropriate, were used to compare proportional data. Continuous nonparametric data were expressed as the median with interquartile range (IQR), while parametric data were expressed as the mean with standard deviation (SD). The Mann–Whitney U test was used for comparing nonparametric variables, and the t test was used for parametric continuous variables. For continuous variables without a standardized risk cut-off, receiver operating characteristics (ROC) curves were constructed, and the best cut-off values were determined as those showing the highest Youden’s index. Binary and linear regression analyses were performed to control for the effects of covariates on the clinical outcomes by including in the multivariate analysis all the variables that reached p values < 0.05 in the univariate analyses.
All the tests were 2-sided, and the threshold of significance was set at p < 0.05. Statistical analyses were performed using Statistical Package for Social Sciences software (IBM SPSS Statistics, version 27 for Macintosh; IBM Corp., Armonk, NY, USA).
Results
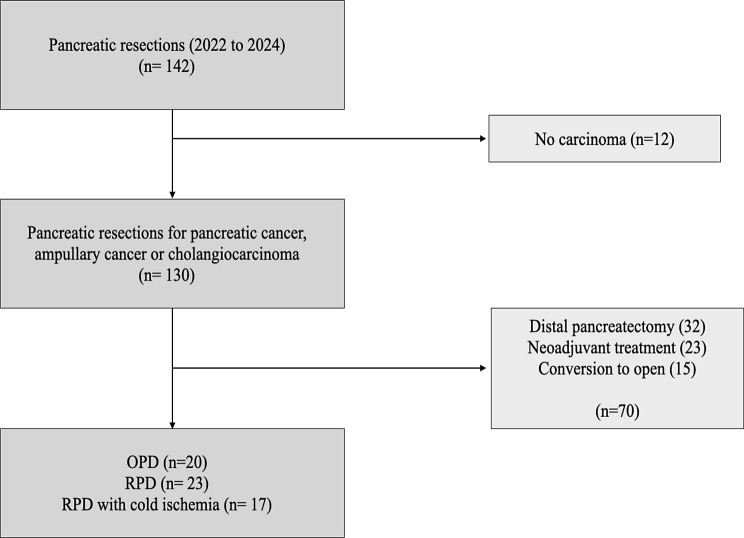
Out of 140 pancreatic resections, 60 patients fulfilled the inclusion criteria: 20 patients undergoing open PD without intraoperative warm ischemia and no cold ischemia, 17 patients undergoing robotic PD with cold ischemia, and 23 patients undergoing robotic PD with warm ischemia only. Specimen extraction was always performed following the reconstruction phase in all robotic PD, therefore prolonged warm ischemia was reported in all these cases.
The final histologic diagnoses were pancreatic adenocarcinoma (31), distal cholangiocarcinoma (8), ampullary adenocarcinoma (8), G2-G3 pancreatic neuroendocrine tumor (9), carcinoma arising on intraductal papillary mucinous neoplasm IPMN (3) and duodenal adenocarcinoma (1).
The baseline features of the patients included in this study are shown in Table 1.
Table 1.
Baseline features of patients
| Open PD (n = 20) |
Robotic PD (n = 23) |
Robotic PD with cold ischemia (n = 17) | P value | |
|---|---|---|---|---|
| Age, years (IQR) | 68 (49–73) | 71 (59–78) | 69 (66–76) | 0.837 |
| Gender, Male, n (%) | 12 (60.0) | 4 (17.4) | 12 (70.6) | < 0.001 |
| ASA score ≥3, n (%) | 8 (40.0) | 3 (13.0) | 3 (17.6) | 0.086 |
| BMI, kg/m2 (IQR) | 24 (21–27) | 23 (20–26) | 26 (22–34) | 0.038 |
| > 1 comorbidity, n (%) | 16 (80.0) | 19 (82.6) | 10 (58.8) | 0.187 |
| Indication (PDAC), n (%) | 9 (45.0) | 10 (43.5) | 6 (35.3) | 0.816 |
| Operating time, minutes, (IQR) | 276 (225–327) | 393 (353–433) | 516 (416–615) | < 0.001 |
PD: pancreaticoduodenectomy; IQR, interquartile range
Ischemia time analysis
In all patients, both warm (WIT) and cold (CIT) ischemia times were collected. WIT included intraoperative warm ischemia and postoperative warm ischemia (from specimen extraction to formalin storage). Median intraoperative WIT was 140 (120–155) minutes (all RPD patients). Median postoperative WI was 60 (52–65) minutes (all patients). Median total WIT was 182 min (OPD 57 min vs. RPD 190 min vs. RPD-CI 198 min; p < 0.001). Median CIT was 760 min (740–835), in specimens that were stored at 4ºC. There was no cold ischemia in patients undergoing open PD.
Pathological assessment
Ischemic damage in the pancreatic tissue and in the tumor was assessed (Table 2). Most of the specimens with moderate or severe pancreatic or tumor ischemic damage belonged to the RPD group undergoing cold storage. No difference was observed in terms of acinar score and pancreatic gland assessment.
Table 2.
Ischemic damage of the surgical specimen. PD, pancreaticoduodenectomy; CI, cold ischemia
| Open PD (n = 20) |
Robotic PD (n = 23) |
Robotic PD with CI (n = 17) | P value | |
|---|---|---|---|---|
| Pancreatic Ischemic damage | ||||
| · Absent | 17 (85.0) | 15 (65.2) | 2 (11.8) | |
|
· Mild · Moderate · Severe |
3 (15.0) 0 (0) 0 (0) |
7 (30.4) 1 (4.3) 0 (0) |
6 (35.3) 3 (17.6) 6 (35.3) |
< 0.001 |
| Tumor Ischemic damage | ||||
| · Absent | 18 (90.0) | 15 (62.5) | 6 (35.3) | |
|
· Mild · Moderate · Severe |
2 (10.0) 0 (0) 0 (0) |
7 (30.4) 0 (0) 1 (4.3) |
3 (17.6) 4 (23.5) 4 (23.5) |
< 0.001 |
| Pancreatic assessment | ||||
| · Acinar score | 72 (35–93) | 75 (65–90) | 65 (57–87) | 0.551 |
|
· Collagen score · Fat score |
7.5 10 (5–25) |
10 10 (5–20) |
5.0 20 (10–30) |
0.503 0.197 |
Pathological features related to tumor stage are shown in Table 3.
Table 3.
Pathological outcomes of the extracted specimens. CI, cold ischemia; T, tumor stage; N, nodal stage
| Open PD (n = 20) |
Robotic PD (n = 23) |
Robotic PD with CI (n = 17) | P value | |
|---|---|---|---|---|
| · perineural invasion | 15 (75.0) | 12 (52.2) | 10 (58.8) | 0.295 |
| · lymphovascular invasion | 14 (70.0) | 14 (60.9) | 10 (58.8) | 0.744 |
| · number of total lymph nodes | 23 (15–31) | 12 (9–26) | 17 (10–25) | 0.235 |
| · metastatic lymph nodes | 2 (0–4) | 1 (0–2) | 1 (0–2) | 0.567 |
| · resection margin status (R0) | 15 (75.0) | 17 (73.9) | 14 (82.4) | 0.680 |
| · Tumor | ||||
|
NA pT1 pT2 pT3 pT4 |
2 (10.0) 3 (15.0) 8 (40.0) 5 (25.0) 2 (10.0) |
2 (8.7) 10 (43.5) 9 (39.1) 2 (8.7) 0 (0) |
3 (17.6) 5 (29.4) 6 (35.3) 2 (11.8) 1 (5.9) |
0.440 |
| · pN+ | ||||
| NA | 2 (10.0) | 2 (8.7) | 3 (17.6) | |
| PN0 | 6 (30.0) | 11 (47.8) | 6 (35.3) | 0.258 |
| pN1 | 6 (30.0) | 9 (39.1) | 4 (23.5) | |
| pT2 | 6 (30.0) | 1 (4.3) | 4 (23.5) |
Assessment of surgical specimen histological quality was also analyzed. There was no difference between groups when analyzing tumor size, perineurial invasion, lymphovascular invasion, and lymph node assessment. Other features were poorly assessed in the group of RPD with CI without reaching statistical significance (Table 4). Macroscopic and microscopic differences are shown in Figs. 1, 2 and 3 respectively.
Table 4.
Surgical specimen assessment quality. Specimens with good quality for assessment of different histological features are noted
| Open PD (n = 20) |
Robotic PD (n = 23) |
Robotic PD with CI (n = 17) | P value | |
|---|---|---|---|---|
| Histology, good quality, n (%) | 20 (100) | 23 (100) | 16 (94.1) | 0.276 |
| Resection margin, good quality, n (%) | 20 (100) | 23 (100) | 15 (88.2) | 0.198 |
| TNM assessment, good quality, n (%) | 20 (100) | 23 (100) | 15 (88.2) | 0.198 |
| R category, good quality, n (%) | 20 (100) | 23 (100) | 16 (94.1) | 0.276 |
Fig. 1.
Flowchart diagram of patient inclusion. OPD; open pancreaticoduodenectomy, RPD; robotic pancreaticoduodenectomy
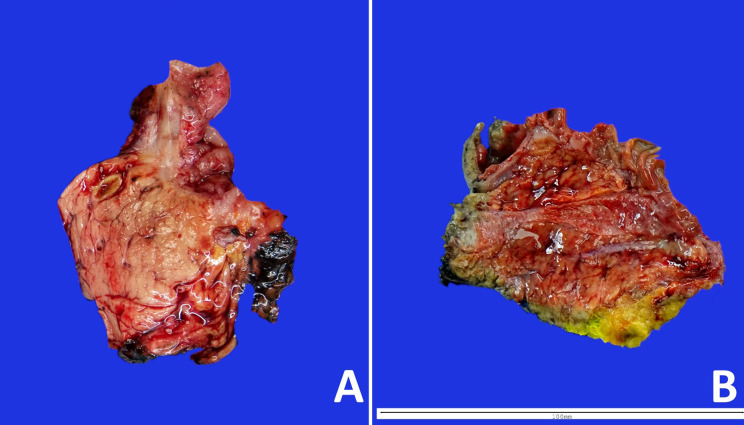
Fig. 2.
Macroscopic appearance of robotic pancreaticoduodenectomy arrived at the Pathology Department right after surgery (A) showing firm and solid pancreatic parenchyma, and surgical specimen arrived 12 h after surgery (B), with gelatinous and friable parenchyma
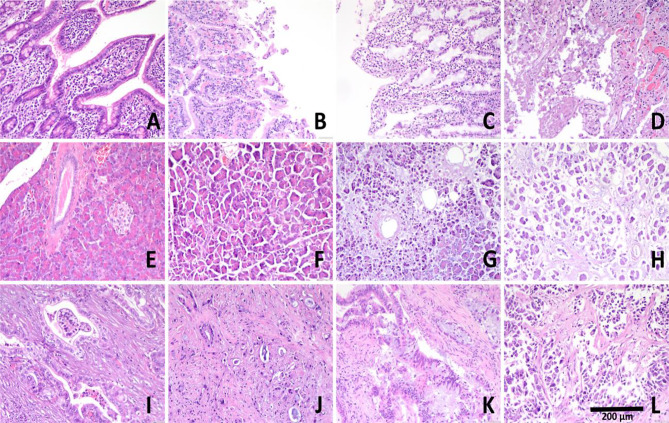
Fig. 3.
Microscopic appearance of surgical specimens. Representative images of normal duodenum (A), duodenum with mild (B) moderate (C) and severe (D) ischemic changes; normal pancreatic parenchyma (E) and pancreatic parenchyma with mild (F) moderate (G) and severe (H) ischemic changes; and adenocarcinoma without ischemic changes (I), and with mild (J) moderate (K) and severe (L) ischemic changes (x 20)
Univariate and multivariate analysis
Ischemic damage was grouped as follows: none and mild vs. moderate and severe. Since cold ischemic time had very little difference between patients, it was analyzed as a categorical variable (cold storage). ROC curves were constructed to determine cut-off values for BMI and total warm ischemia time.
The ROC curve showed an optimal BMI at 27 (AUC 80.5%, p = 0.022; sensitivity and specificity of 71% and 79% respectively) and total WIT at 150 min (AUC 76.1%, p = 0.023; sensitivity and specificity of 80% and 77%, respectively).
Factors associated with ischemic changes in the normal pancreatic tissue and in the tumor were assessed by univariate and multivariate analysis. Cold storage was the only factor independently associated with ischemic damage. Results are shown in Tables 5 and 6.
Table 5.
Factors associated with pancreatic ischemic damage. Univariate and multivariate analysis for normal pancreatic tissue ischemia is shown. OR, odds ratio; CI, confidence interval; ASA, American Society of Anesthesiologists Classification; BMI, body mass index, PDAC, pancreatic ductal adenocarcinoma
| Pancreatic tissue ischemic damage | ||||||
|---|---|---|---|---|---|---|
| Variables | Univariate analysis | Multivariate analysis | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Male gender | 14.684 | 1.722-125.239 | 0.014 | 15.702 | 0.847-290.968 | 0.064 |
| ASA ≥ III | 3.162 | 0.365–27.432 | 0.296 | |||
| BMI ≥ 27 | 3.000 | 0.341–26.427 | 0.091 | |||
| Cold storage | 47.250 | 5.236-426.425 | < 0.001 | 57.848 | 3.358–996.610 | 0.005 |
| PDA | 0.545 | 0.126–2.356 | 0.417 | |||
| Intraoperative ischemia > 150 min | 9.000 | 1.918–42.236 | 0.005 | 2.077 | 0.150-28.759 | 0.584 |
Table 6.
Factors associated with tumor ischemic damage. Univariate and multivariate analysis for humoral tissue ischemia is shown. Results from hierarchical logistic regression analyses on the association between the variables of interest and ischemic damage of pancreatic tissue. OR, odds ratio; CI, confidence interval; ASA, American Society of Anesthesiologists Classification; BMI, body mass index, PDAC, pancreatic ductal adenocarcinoma
| Tumor tissue ischemic damage | ||||||
|---|---|---|---|---|---|---|
| Variables | Univariate analysis | Multivariate analysis | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Male gender | 10.33 | 1.183–90.256 | 0.035 | 5.920 | 0.577–60.711 | 0.134 |
| ASA ≥ III | 2.33 | 0.262–20.792 | 0.448 | |||
| BMI ≥ 27 | 2.200 | 0.243–19.897 | 0.483 | |||
| Cold storage | 29.4 | 3.23-266.89 | 0.003 | 21.318 | 2.244-202.544 | 0.008 |
| PDAC | 0.818 | 0.177–3.792 | 0.798 | |||
| Intraoperative ischemia > 150 min | 3.857 | 0.750-19.844 | 0.106 | |||
Discussion
This is the first study to describe the effects of warm and cold ischemia on pancreaticoduodenectomy specimens after minimally invasive surgery. We were able to demonstrate cold ischemia can have a detrimental effect on the microscopic features of pancreatic and peripancreatic tissue. Although we have not been able to show that the evaluation of the pancreatic specimen is really compromised, the macroscopic and especially the microscopic features of the specimen can certainly be damaged. This clearly affects the quality of the pathological evaluation of the tumor, especially in samples that have been stored at low temperatures. It is essential to emphasize that while extended ischemia duration significantly impacts the deterioration of both normal and tumor tissues, our findings indicate that high-quality histological evaluations were preserved in the majority of instances. This suggests that despite nearly 50% of the specimens subjected to cold ischemia exhibiting moderate to severe damage, the assessment of these specimens proved to be considerably more complex and necessitated a substantial investment of time and expertise.
Minimally invasive surgery for PD is increasing and may offer some potential benefits to patients compared to open surgery [6]. However, there is no previous literature analyzing the effect of warm ischemia time on the pancreatic specimen in minimally invasive procedures.
However, an accurate histopathological analysis of the specimen is crucial for determining the best postoperative treatment and assessing the patient’s prognosis, as high-quality human tissue samples are the basis for diagnosing diseases and identifying therapies [10]. For the pancreas, ischemia is an almost inevitable factor in sampling due to its deep location and extensive anatomical structure [15]. In addition, the abundance of digestive enzymes in pancreatic tissue poses a major challenge to sample handling.
To date, the effect of cold ischemia time (CIT) is well known in several organs. In breast cancer, hormone receptor expression decreases with increasing CIT, making it difficult to assess subtype classification [16]. In ovarian cancer, a CIT greater than 2 h leads to poorer sample quality [17]. When analysing the effects of CIT at the cellular level, significant morphological changes occur during tissue degradation, including altered intensity of nuclear staining and loss of cell border definition. In addition, some cell surface receptors, such as epidermal growth factor receptor (EGFR), are highly sensitive to CIT [18]. There are no reports in the pancreatic tissue. We showed that prolonged cold storage has a direct effect on developing moderate to severe ischemic changes in the pancreatic parenchyma by reducing cell viability. This important effect in pancreatic tissue compared to other organs is probably due to the presence of digestive enzymes. Some authors hypothesize that the timing also has negative effects on molecular characteristics, including nucleotide integrity, global gene expression, protein abundance, and post-translational modifications [15]. On the other hand, ischemia also affects the viability of tumor cells in pancreatic neoplasms: this does not seem to prevent obtaining a definitive histopathological diagnosis, although it does reduce the possibility of performing more detailed analyses, such as translational studies.
Some authors have suggested that tissue handling methods may also influence the quality of pancreatic specimens [15, 19]. In our study, all samples were handled according to the same protocol, so this is unlikely to have a direct effect on our sample.
According to the literature and our results, avoiding cold storage would be necessary to improve the quality of surgical specimens, and if not possible, at least a reduction of cold ischemia should be attempted. An earlier start of surgery should be promoted, especially during the learning curve or when high difficulty is expected.
Otherwise, the effect of WIT has not been well studied in the context of pathological specimen analysis. Previous reports on head and neck squamous cell carcinoma specimens have shown that ex vivo warm ischemia time is an important determinant of tissue quality, which may explain the inconsistent results of biomarkers [20]. Otherwise, extensive studies have analyzed the effect of WIT in solid organ transplantation, showing inferior outcomes when the organ is exposed to higher WIT [21]. Regarding WIT, our data suggest that intraoperative ischemia may also have an effect on normal pancreatic tissue and tumor tissue. Some organisational efforts could be made to ensure the handling of the specimen once it has been removed from the patient. To date, some surgical groups harvest the specimen immediately after retroportal lamina dissection [22], but many groups harvest the specimen after the reconstruction phase, as doing so earlier could prolong the surgical procedure, force pneumoperitoneum exsufflation, and robot redocking. According to our results, retrieval of the surgical specimen after complete detachment should be recommended in order to avoid an increase in warm ischemia. Some groups recommend the use of a Pfannenstiel incision with gel port placement for using it as an assistance port and for specimen retrieval. In fact, following the results of our study, we changed our policy to immediate specimen extraction following the dissection phase.
Organisational issues are usually behind the cold ischemia time, such as the time at which the surgical specimen is taken or the availability of staff in the pathology team. Therefore, CIT is usually long because it is associated with the collection of the specimen from the operating theatre after office hours. Therefore, the goal should be to eliminate cold ischemia in surgical specimens by improving circulation. Our study shows that by avoiding cold ischemia and making some changes to the surgical technique, we could significantly improve the quality of the specimen obtained by minimally invasive surgery. Also, these procedures may not be scheduled if the pathologist is unavailable to collect and store the specimen. The logistical aspect of this is crucial, as PD should not be performed in the afternoon unless a proper pathological assessment is available. It is interesting to note that male sex was associated with ischemic damage in the univariate analysis. We can speculate that this is probably due to increased difficulty and therefore longer operative time. As previously published, the difficulty associated with BMI in robotic pancreaticoduodenectomy differs between men and women: RPD is considered difficult when men have a BMI > 25, whereas for women it is difficult when the BMI is > 30 [23].
We observed that an extended duration of warm ischemia, primarily associated with the robotic procedure, did not demonstrate independent statistically significant association with microscopic damage. It is plausible to suggest that this lack of significance may be attributed to the considerably greater influence of prolonged cold ischemia, coupled with the relatively brief warm ischemia time, which may not be sufficient to inflict significant damage on the specimen.
We emphasize the critical importance of reducing operative time during robotic pancreaticoduodenectomy, particularly to mitigate the risk of prolonged ischemia, which can have multiple deleterious effects. Prolonged warm ischemia, defined as the time when tissues are deprived of oxygen during surgery, has a well-documented negative impact on pancreatic parenchyma. Another critical aspect relates to the specimen handling after resection. Delays in processing the pancreatic tissue for pathological evaluation, especially when the specimen is preserved under cold storage overnight, can induce significant histological changes. Cold ischemia can exacerbate autolysis, a process that occurs rapidly in pancreatic tissue due to the gland’s high enzymatic activity. Autolysis can cause premature degradation of both normal and malignant cells, resulting in distorted histopathological features. This degradation is especially problematic in the assessment of tumor margins, lymph node involvement, and grading of pancreatic tumors. Inaccurate histopathological analysis due to tissue changes from ischemia or autolysis can lead to erroneous conclusions regarding the extent of the disease and impact the decisions on adjuvant therapies. Underestimation of final staging is not the only potential issue. Prolonged ischemia, even at cold temperatures, can result in alterations in tissue architecture that may mimic tumor necrosis therefore leading to overtreatments in the adjuvant setting.
There are several limitations to the study. It is a retrospective study, therefore the data used were originally measured for other purposes and may be inconsistent. Also, not all relevant factors may have been recorded. The sample size of the study is small, so we do not have evidence of the reproducibility of our findings, which would need to be validated in a larger cohort of patients. In addition, we were not able to assess whether poor histological assessment has an oncological impact on the patient; however, this would need to be studied for each histological subtype. Furthermore, we could not evaluate the effects of specimen handling during surgical procedures, which may have adverse consequences if conducted by robotic arms. Consequently, future research should investigate suitable logistical strategies that facilitate prompt specimen extraction and prevent both warm and cold ischemia. It may also be beneficial for precise pathological evaluations to incorporate a detailed account of ischemic and autolytic damage in the official reports. In conclusion, prolonged ischemia time, especially in cases of cold storage, has a severe effect on the degradation of normal and tumor tissue. Although this does not affect tumor evaluation, it could clearly impair the possibility of performing molecular analyses.
Further studies are needed to validate our results and the oncologic impact of our findings.
Author contributions
A.F conceptualized the work; A.F, C.G and M.C defined the methodology; C.G and I.A prepares the original draft; K.L, N.V and C.G curated the data, C.G and F.A did the formal analysis; A.F and M.C reviewed the draft writing; A.F supervised all the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data availability
General data is provided within the manuscript. The datasets analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
The study was approved by the Comité de Ética de la Investigación con medicamentos (CEIm), the institutional review board (IRB) of our Institution. Protocol identification ID was HCB/2022/0095.
Consent for publication
Not Applicable.
Informed consent
Patient consent was waived by the IRB (Comité de Ética de la Investigación con medicamentos, CEIm) due to the retrospective nature of the study and the non-interference of the study with clinical management.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pancreas cancer. World Health Organization. [on-line: https://platform.who.int/mortality/themes/theme-details/topics/indicator-groups/indicator-group-details/MDB/pancreas-cancer]
- 2.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–705. 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mihaljevic A, Al-Saeedi M, Hackert T. Pancreatic surgery: we need clear definitions. Langenbeck’s Archives Surg. 2019;404(2):159–65. 10.1007/s00423-018-1725-7. [DOI] [PubMed] [Google Scholar]
- 4.Ausania F, Gonzalez-Abós C, Martinez-Perez A, Arrocha C, Pineda-Garcés C, Landi F, Fillat C, Garcia-Valdecasas JC. Postoperative day one systemic inflammatory response syndrome is a powerful early biomarker of clinically relevant pancreatic fistula. HPB: Official J Int Hepato Pancreato Biliary Association. 2023;25(1):73–80. 10.1016/j.hpb.2022.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Ausania F, Martínez-Pérez A, Rio SD, Borin P, Melendez A, R., Casal-Nuñez JE. Multifactorial mitigation strategy to reduce clinically relevant pancreatic fistula in high-risk pancreatojejunostomy following pancreaticoduodenectomy. Pancreatology: Official J Int Association Pancreatology (IAP) … et al ]. 2021;21(2):466–72. 10.1016/j.pan.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Zwart MJW, van den Broek B, de Graaf N, Suurmeijer JA, Augustinus S, Riele T, van Santvoort WW, Dutch Pancreatic Cancer Group, et al. The feasibility, proficiency, and Mastery Learning curves in 635 robotic pancreatoduodenectomies following a Multicenter Training Program: standing on the Shoulders of Giants. Ann Surg. 2023;278(6):e1232–41. 10.1097/SLA.0000000000005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Dong X, Felsenreich DM, Gogna S, Rojas A, Zhang E, Dong M, Azim A, Gachabayov M. Robotic pancreaticoduodenectomy provides better histopathological outcomes as compared to its open counterpart: a meta-analysis. Sci Rep. 2021;11(1):3774. 10.1038/s41598-021-83391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyyappan T, Wilson GC, Zeh HJ, Hogg ME, Lee KK, Zureikat AH, Paniccia A. Robotic approach mitigates the effect of major complications on survival after pancreaticoduodenectomy for periampullary cancer. Surg Endosc. 2023;37(2):1181–7. 10.1007/s00464-022-09638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao W, Liu C, Li S, Geng D, Feng Y, Sun M. Safety and efficacy for robot-assisted versus open pancreaticoduodenectomy and distal pancreatectomy: a systematic review and meta-analysis. Surg Oncol. 2018;27(3):468–78. 10.1016/j.suronc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Burgart LJ, Chopp WV, Jain D. Protocol for the Examination of Specimens From Patients With Carcinoma of the Pancreas. College of American Pathologists (CAP); 2021 [Internet]. [Revised 5 June 2023]. https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates
- 11.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer staging Manual: continuing to build a bridge from a population-based to a more personalized approach to cancer staging. Cancer J Clin. 2017;67(2):93–9. 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 12.Ausania F, Sanchez-Cabus S, Del Rio S, Borin P, Ayuso A, Bodenlle JR, Espinoza P, Cuatrecasas S, Conill M, Saurí C, Ferrer T, Fuster J, García-Valdecasas J, Melendez JC, R., Fondevila C. Clinical impact of preoperative tumour contact with superior mesenteric-portal vein in patients with resectable pancreatic head cancer. Langenbeck’s Archives Surg. 2021;406(5):1443–52. 10.1007/s00423-020-02065-w. [DOI] [PubMed] [Google Scholar]
- 13.Alwelaie Y, Point du Jour KS, Pandya S, Goodman AL, Centeno BA, Adsay V, Reid MD. Acinar cell induced autolysis is a frequent occurrence in CytoLyt-fixed pancreatic fine needle aspiration specimens: an analysis of 157 cytology samples. Cancer Cytopathol. 2021;129(4):283–90. 10.1002/cncy.22378. [DOI] [PubMed] [Google Scholar]
- 14.Nahm CB, Brown KM, Townend PJ, Colvin E, Howell VM, Gill AJ, Connor S, Samra JS, Mittal A. Acinar cell density at the pancreatic resection margin is associated with post-pancreatectomy pancreatitis and the development of postoperative pancreatic fistula. HPB (Oxford). 2018;20(5):432–40. 10.1016/j.hpb.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Peng J, Liu L, Huang D, Chen H, Dai M, Guo J, Zhang T, Liao Q, Jiang J, Wang W, Guo D, Cao D, Xuan Z, Li D, Zhao Y, Wu W, et al. Impact of ischemia on sample quality of human pancreatic tissues. Pancreatology: Official J Int Association Pancreatology(IAP). 2020;20(2):265–77. 10.1016/j.pan.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Deavers MT, Guo M, Liu P, Gong Y, Albarracin CT, Middleton LP, Huo L. The effect of prolonged cold ischemia time on estrogen receptor immunohistochemistry in breast cancer. Mod Pathology: Official J United States Can Acad Pathol Inc. 2013;26(1):71–8. 10.1038/modpathol.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricci A, Dugo M, Pisanu ME, De Cecco L, Raspagliesi F, Valeri B, Veneroni S, Chirico M, Palombelli G, Daidone MG, Podo F, Canese R, Mezzanzanica D, Bagnoli M, Iorio E. Impact of Cold Ischemia on the Stability of 1H-MRS-Detected metabolic profiles of ovarian Cancer specimens. J Proteome Res. 2024;23(1):483–93. 10.1021/acs.jproteome.3c00665. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Kil C, Considine K, Smarkucki B, Stankewich MC, Balgley B, Vortmeyer AO. Intrinsic indicators for specimen degradation. Lab Invest. 2013;93(2):242–53. 10.1038/labinvest.2012.164. [DOI] [PubMed] [Google Scholar]
- 19.Hatzis C, Sun H, Yao H, et al. Effects of tissue handling on RNA integrity and microarray measurements from resected breast cancers. J Natl Cancer Inst Dec. 2011;21(24):1871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tower JI, Lingen MW, Seiwert TY, Langerman A. Impact of warm ischemia on phosphorylated biomarkers in head and neck squamous cell carcinoma. Am J Translational Res. 2014;6(5):548–57. [PMC free article] [PubMed] [Google Scholar]
- 21.Kalisvaart M, Croome K, Hernandez-Alejandro R, Pirenne J, Cortés-Cerisuelo M, Miñambres E, Abt PL. Donor warm ischemia time in DCD Liver Transplantation-Working Group Report from the ILTS DCD, Liver Preservation, and machine perfusion Consensus Conference. Transplantation. 2021;105(6):1156–64. 10.1097/TP.0000000000003819. [DOI] [PubMed] [Google Scholar]
- 22.Xu DB, Zhao ZM, Xu Y, Liu R. Hybrid pancreatoduodenectomy in laparoscopic and robotic surgery: a single-center experience in China. Surg Endosc. 2021;35(4):1703–12. 10.1007/s00464-020-07557-w. [DOI] [PubMed] [Google Scholar]
- 23.Napoli N, Cacace C, Kauffmann EF, Jones L, Ginesini M, Gianfaldoni C, International Consortium on Minimally Invasive Pancreatic Surgery (I-MIPS), et al. The PD-ROBOSCORE: a difficulty score for robotic pancreatoduodenectomy. Surgery. 2023;173(6):1438–46. Epub 2023 Mar 25. PMID: 36973127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
General data is provided within the manuscript. The datasets analysed during the current study are available from the corresponding author on reasonable request.