Abstract
Background
. CD8+ Cytotoxic T lymphocytes play a key role in the pathogenesis of autoimmune diseases and clinical conditions such as graft versus host disease and graft rejection. Mesenchymal Stromal Cells (MSCs) are multipotent cells with tissue repair and immunomodulatory capabilities. Since they are able to suppress multiple pathogenic immune responses, MSCs have been proposed as a cellular therapy for the treatment of immune-mediated diseases. However, the mechanisms underlying their immunosuppressive properties are not yet fully understood. MSCs have the remarkable ability to sense tissue injury and inflammation and respond by donating their own mitochondria to neighboring cells. Whether mitochondrial transfer has any role in the repression of CD8+ responses is unknown.
Methods and results
. We have utilized CD8+ T cells from Clone 4 TCR transgenic mice that differentiate into effector cells upon activation in vitro and in vivo to address this question. Allogeneic bone marrow derived MSCs, co-cultured with activated Clone 4 CD8+ T cells, decreased their expansion, the production of the effector cytokine IFNγ and their diabetogenic potential in vivo. Notably, we found that during this interaction leading to suppression, MSCs transferred mitochondria to CD8+ T cells as evidenced by FACS and confocal microscopy. Transfer of MSC mitochondria to Clone 4 CD8+ T cells also resulted in decreased expansion and production of IFNγ upon activation. These effects overlapped and were additive with those of prostaglandin E2 secreted by MSCs. Furthermore, preventing mitochondrial transfer in co-cultures diminished the ability of MSCs to inhibit IFNγ production. Finally, we demonstrated that both MSCs and MSC mitochondria downregulated T-bet and Eomes expression, key transcription factors for CTL differentiation, on activated CD8+ T cells.
Conclusion
. In this report we showed that MSCs are able to interact with CD8+ T cells and transfer them their mitochondria. Mitochondrial transfer contributed to the global suppressive effect of MSCs on CD8+ T cell activation by downregulating T-bet and Eomes expression resulting in impaired IFNγ production of activated CD8+ T cells.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-024-03980-1.
Keywords: Mesenchymal stem/stromal cells, CD8+ T cells, Mitochondrial transfer, Immunotherapy, Autoimmunity
Background
The main role of CD8+ T lymphocytes is arguably to defend the organism against infections. Naïve CD8+ T cells become activated by cognate antigen. T cell receptor (TCR) triggering along with co-stimulation and cytokine signaling lead to their expansion and differentiation into effector CD8+ cytotoxic T lymphocytes (CTL), also termed Tc1 cells [1]. This differentiation process is highly dependent on CD4+ T helper cells that can activate antigen presenting dendritic cells and secrete the helper IL-2 and IL-21 cytokines [1, 2]. Effector CD8+ T cell differentiation is orchestrated by the upregulation of key transcription factors such as T-bet, Blimp-1, Id2, and STAT4, and the repression of Eomesodermin (Eomes), Bcl-6, Id3 and STAT3, which drive the expression of effector molecules [1, 2]. Thus, effector CD8+ T cells can directly kill target cells and secrete high amounts of pro-inflammatory cytokines such as IFNγ and TNFα [1, 2]. T cell responses are tightly regulated and modulated by the mechanisms of self-tolerance to ensure an efficient pathogen clearance and to preserve the integrity of the organism [2]. However, deregulation of T cell responses is not infrequent giving rise to the onset of T cell-mediated autoimmune disorders. Autoreactive CD8+ T cells are known to contribute to the pathogenesis of a number of autoimmune diseases such as type 1 diabetes, multiple sclerosis, Crohn disease, vitiligo and rheumatoid arthritis [2, 3]. CD8+ T cells are also involved in clinical conditions such as graft versus host disease and graft rejection [4]. Cellular therapies able to restrain unwanted CTL cell responses, including the use of mesenchymal stromal cells (MSCs), have gained increasing attention in recent years because they do not induce general immunosuppression and present less adverse effects than classical immunosuppressive drugs [5].
MSCs are characterized by the expression of CD73, CD90 and CD105 as well as by the lack of expression of hematopoietic and endothelial markers. They are multipotent cells able to differentiate into adipocytes, osteoblasts and chondrocytes. They also secrete numerous trophic factors and cytokines. MSCs are abundant in the bone marrow but they are also present in adipose tissue, umbilical cord, placenta and dental pulp. However, phenotypic and functional differences have been found depending on their origin. Apart from their tissue repair capabilities, MSCs also present strong immunomodulatory properties [6–9]. Whereas under physiological conditions they may have a homeostatic role promoting the survival of components of the immune system, under inflammatory conditions, in the presence of IFNγ or TNFα, they convert into immunosuppressive cells [10, 11]. Interestingly, bone marrow-derived MSCs present the highest immunosuppressive potential compared to MSCs from other tissues [12]. For these reasons, and because of their poor immunogenicity that allows their use in allogenic settings, they have attracted an enormous interest for their therapeutic use to treat autoimmune diseases, graft versus host disease and other immune-mediated disorders [8].
MSCs have been shown to modulate the function of immune cell types towards an immunosuppressive state. They promote the expansion or differentiation of suppressor cells such as Tregs, Tr1, Th3, CD8+ CD28− regulatory cells, Bregs and MDSCs [7, 10, 13]. MSCs promote the differentiation of tissue-protecting M2 macrophages, Th2 cells, ILC2 and ILC3. On the other hand, MSCs inhibit the differentiation and activation of inflammatory innate immune cells such as NK cells, M1 macrophages, dendritic cells, neutrophils and NKT cells. They also suppress pathogenic B and T cell responses in vitro and in vivo [7, 10, 13, 14]. MSCs inhibit the expansion and activation in response to antigen of both CD4+ and CD8+ T cells [15–17]. Furthermore, they suppress the differentiation and inhibit the function of effector CD4+ Th1, Th17 and CD8+ cytotoxic T cells [18–24]. The mechanisms via which MSCs exert their immunosuppressive effects on T cells are multiple and may vary depending on the experimental system. Under inflammatory conditions, MSCs upregulate PD-L1, which can attenuate pathogenic T cell responses by engaging PD-1 expressed on effector T cells [25–29]. MSCs also express FasL that can mediate direct killing of activated T cells expressing Fas [30, 31]. MSCs secrete anti-inflammatory cytokines and molecules such as IL-10, TGFβ, IL-1Ra, HGF, Prostaglandin E2 (PGE2) that block T cell proliferation, prevent Th1 and Th17 differentiation and induce Tregs [19, 32, 33]. Pro-inflammatory cytokine-activated human MSCs express high levels of indoleamine 2 3-dioxygenase (IDO) that degrades tryptophane into kynurenine. Tryptophan deprivation and toxic kynurenine derivatives suppress T cell proliferation [18, 34]. Unlike human MSCs, murine MSCs do not express IDO. They do express iNOS instead and release nitric oxide that in turn can suppress T cell differentiation [35]. Interestingly, it has been recently shown that extracellular vesicles secreted by MSCs can recapitulate many of their immunosuppressive effects on T cell responses [36].
It is now well established that MSCs are able to transfer their mitochondria to multiple cell types, mostly through tunneling nanotubes. Additionally, MSCs release extracellular vesicles that may shuttle mitochondria from one cell to another [37–39]. In cancer cells, MSC mitochondrial transfer resulted in enhanced proliferation and resistance to cytotoxic drugs via a metabolic shift, notably enhancing oxidative phosphorylation [37, 39, 40]. MSCs have also been shown to transfer mitochondria to non-malignant cells from different tissues in vivo and in vitro [38, 39]. MSCs can sense tissue injury and react transferring their mitochondria to damaged cells protecting them. Mitochondrial transfer modulate and restore the metabolism of recipient damaged cells [38, 39]. In the immune system, macrophages that had received MSC mitochondria displayed increased phagocytosis of pathogenic bacteria [41]. Additionally, MSC mitochondrial transfer promoted the induction of M2 macrophages that were able to decrease inflammation in a model of lung injury [42]. We have recently shown that MSCs transfer mitochondria to human Th17 cells resulting in their conversion into Tregs [43]. MSC mitochondrial transfer induced human Tregs by stabilizing the expression of FoxP3 [44]. Induction of Tregs by MSC mitochondria has also been shown in a mouse model of GVHD [45]. Additionally, transfer of MSC mitochondria to natural Tregs enhanced their immunosuppressive activity [46]. These results demonstrate that MSC mitochondrial transfer is an important mechanism involved in promoting CD4+ Foxp3+ Treg activity.
In a transgenic mouse model of type 1 diabetes we have shown that MSCs delayed disease onset and decreased severity [47]. This model consists of three mouse lines, Clone 4 TCR transgenic (Clone 4) mice expressing a MHC class I-restricted influenza hemagglutinin (HA)-specific TCR, HNT TCR transgenic (HNT) mice expressing a MHC class II-restricted influenza hemagglutinin (HA)-specific TCR and InsHA mice that express HA in the beta cells of the pancreas. Co-transfer of Clone 4 CD8+ T cells and HNT CD4+ T cells into InsHA mice results in their activation and the onset of autoimmune diabetes under inflammatory conditions [48–52]. MSCs suppressed the differentiation of HNT CD4+ T cells into Th1 effector cells and impaired their diabetogenic potential [47]. In vitro studies demonstrated that MSC mitochondrial transfer to HNT CD4+ T cells critically contributed to the repression of Th1 responses by downregulating T-bet expression, the Th1 master transcription factor [47]. These results revealed that MSC mitochondrial transfer plays also an important role in inhibiting CD4+ T cell effector responses contributing to the immunosuppressive effects of MSCs. MSCs also have a direct suppressive effect on effector cytotoxic CD8+ T cells. However, whether mitochondrial transfer from MSCs to CD8+ T cells plays a role remained unknown. Here, we sought to investigate this question utilizing our transgenic mouse model.
Methods
Mice
C57BL/6mice were purchased from Charles River Laboratories. Balb/c Clone 4 TCR transgenic mice express a MHC class I H2-Kd restricted TCR specific for the influenza virus A/PR8/1934 hemagglutinin (HA) epitope 533–541 [48, 51]. Balb/c HNT TCR transgenic mice express a MHC class II I-Ad restricted TCR specific for the influenza virus PR/8 hemagglutinin (HA) epitope 126–138 [48, 51]. Balb/c InsHA transgenic mice express the influenza virus HA under the control of rat insulin promoter, driving its expression to pancreatic beta cells [48, 51]. Both males and females 8 to 16 weeks of age were used in all experiments. Mice were euthanized by carbon dioxide asphyxiation. Mice were bred and maintained under specific pathogen free conditions in an enriched environment at the animal facility of the Institute for Neurosciences of Montpellier Saint Eloi. The work has been reported in line with the ARRIVE guidelines 2.0.
MSCs
C57BL/6 MSCs have been previously described [53]. Briefly, bone marrow cell suspensions were seeded at a concentration of 1 × 106 cells/cm2 in modified minimum essential Eagle’s medium (MEM) supplemented with 10% fetal bovine serum (Hyclone, Thermo Fisher Scientific), 2 mM glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin (Lonza, Levallois-Perret, France) and 2 ng/mL human basic fibroblast growth factor (bFGF) (R&D Systems, Lille, France). MSCs were CD29+, Sca1+, CD73+. After several passages, cells were cryopreserved and assessed functionally. MSCs had capacity to differentiate into adipocytes, chondrocytes and osteoblasts under specific conditions [53].
T cell isolation, activation and culture
CD8+ T cells were purified from single cell suspensions of lymph nodes and spleen of Clone 4 TCR transgenic mice using the Dynal® CD8+ negative isolation kit according to the manufacturer’s instructions (Thermo Fisher Scientific). Purified CD8+ T cells were activated in anti-CD3 (Clone 145-2C11, BioXcell) and anti-CD28 (Clone 37.51, BioXcell) coated 24-well plates at a density of 106 cells per well in RPMI Media 1640 1X + Glutamax (Gibco, Life Technologies, UK) containing 10% heat-inactivated FBS (Gibco, Life Technologies, Germany), 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin (Gibco, Life Technologies, USA), 50 µM beta-mercaptoethanol (Gibco, Life Technologies, USA) in the presence of mouse recombinant IL-2, 12 U/ml, (PeproTech, USA) and cultured at 37 °C, 5% CO2 for up to 3 days as indicated. To study proliferation, isolated T cells were labeled with 5 µM CFSE (CFSE Cell Proliferation Kit, Invitrogen, Thermo Fisher, USA) in PBS for 10 min at 37 °C and plated after washing. In some experiments, PGE2 (PeproTech, USA) was added to T cell media at the indicated concentrations on day 0. Experiments were performed in triplicate wells.
For co-culture experiments, MSCs were either pre-treated for 48 h with 10 ng/ml recombinant murine TNFα (PeproTech, USA) and 20 ng/ml recombinant murine IFNγ (PeproTech, USA) in MSC media (DMEM 1X + Glutamax + 4.5 g/L D-Glucose + Pyruvate) or left untreated in MSC media. Cells were then harvested and added to activated T cells in T cell media on day 0 at a 1:25 ratio (MSC: T cell). Non treated, in the absence of MSCs, activated T cells served as controls (Non-Tr). For trans-well experiments, 24-well plates with 1 μm pore size inserts were used. 5 × 105 T cells were seeded in antibody coated wells and 2 × 104 MSCs were seeded in the insert. To label mitochondria, TNFα and IFNγ-treated MSCs (MSC-A) were labeled with 250 nM MitoTracker Deep Red FM (Molecular Probes, Invitrogen, USA) fluorescent mitochondrial dye according to manufacturer’s instructions. After labeling, MSCs were further cultured for 24 h, harvested, washed again extensively and added to Clone 4 CD8+ T cells. Experiments were performed in duplicate or triplicate wells.
Adoptive transfer experiments
Day 3 activated Clone 4 CD8+ T cells or day 3 activated Clone 4 CD8+ T cells in the presence of MSC-A (3 × 106 cells/mouse) and purified naïve HNT CD4+ T cells (3 × 106 cells/mouse) were co-injected i.v. into InsHA mice that had been sublethally irradiated (3 Gy) 24 h before in a RS2000 irradiator (RadSource, USA). Some mice also received 106 MSCs i.v. the day of T cell transfer and 5 days later. Gender and age-matched individuals were randomly assigned to control and experimental groups. Blood glucose levels were monitored using a glucometer (AccuCheck). Mice were considered diabetic when blood glucose levels were > 300 mg/dl for 2 consecutive time points. Measurements were performed at the same time of the day and in the same order. Diabetic mice were monitored daily and euthanized at first signs of distress. All treated animals were included in the analysis.
MSC mitochondria isolation and transfer to CD8 + T cells
This procedure, termed mitoception, was performed as previously described [47, 54]. 5 × 105 TNFα and IFNγ treated MSCs, trypsinized without EDTA, were lysed in ice-cold mannitol buffer (mannitol 210 mM, saccharose 70 mM, EDTA 1mM, HEPES 10 mM) in the presence of a protease/phosphatase inhibitor cocktail. Lysates were first centrifuged at 800 g at 4 °C for 10 min, to eliminate nuclei, and then at 8000 g at 4 °C for 10 min. The pellet containing mitochondria was resuspended in mannitol buffer and kept on ice. Isolated mitochondria were then diluted in RPMI T cell media for immediate transfer to purified CD8+ T cells in 96-well plates, 106 cells per well. Culture plates were centrifuged at 3000 g at RT for 15 min and incubated at 37 °C, 5% CO2. After 12 h, T cells were harvested and plated in 24-well plates for activation and culture as described above. Control mock mitocepted CD8+ T cells underwent the same procedure without the addition of mitochondria. In some experiments, mitocepted T cells were also treated with PGE2 at the indicated concentrations during the activation/culture period. To verify MSC mitochondrial transfer efficiency, MitoTracker Deep Red FM-labeled TNFα and IFNγ-treated MSCs were used as a source of mitochondria and labeled mitochondria were transferred to T cells. Mitochondria uptake was verified by flow cytometry and confocal microscopy analysis of T cells 12 h later.
Flow cytometry analysis
Activated Clone 4 CD8+ T cells were harvested and stained in PBS containing 2% FCS and 0.02% sodium azide at 4 °C for 20 min with the following mAbs: anti-CD8-APC-eF 780, anti-CD62L-PerCP-Cy5.5 (eBioscience, USA), anti-PD-1-PE (Biolegend, USA), anti-CD25-PE-Cy7 and anti-LAG-3-PerCP-Cy5.5 (BD Pharmingen™, USA). To assess transcription factors expression staining was performed using the Fixation and Permeabilization Kit (eBioscience, USA) according to manufacturer’s instructions with anti-Eomes-PE (eBioscience, USA) and anti-Tbet-eFlour660 (BD Pharmingen™, USA). Isotype-matched conjugated antibodies were used as controls. To assess activated CD8+ T cells IFNγ production, harvested cells were stimulated for 4 h with 50 ng/mL phorbolmyristate acetate (PMA) (Sigma-Aldrich) and 1 µg/mL ionomycin (Sigma-Aldrich) in T cell media in the presence of 10 µg/mL brefeldin A (Sigma-Aldrich, USA). Then, intracellular cytokine staining was performed using the Cytofix/Cytoperm Kit (BD PharMingen) with anti-IFNγ-APC (BD Pharmingen™, USA). To assess mitochondrial transfer, CD8+ T cells that had been co-cultured with MitoTracker Deep Red FM-labeled TNFα and IFNγ-treated MSCs or that had received isolated mitochondria from labeled MSCs were harvested after 12 h, washed and immediately analyzed in a FACSCanto II apparatus (BDB). Files were analyzed using Diva or FlowJo software (BDB).
Confocal microscopy
Clone 4 CD8+ T cells were labeled with green cell tracker CMFDA (Molecular Probes, Invitrogen, USA) and cultured with MitoTracker Deep Red FM-labeled TNFα and IFNγ-treated MSCs. After 12 h, T cells were harvested, labeled with DAPI and seeded on glass slides. Cells were then fixed with paraformaldehyde 3.7% and mounted with Prolong Gold. 1024 × 1024 pixel images were acquired with a Leica TCS SP8-X laser scanning confocal microscope using a Leica-HC PL APO CS2 63x/1.40 oil lens and a 2x numerical zoom. Line average of 8 was applied to each channel. For z-stack imaging, a total of 35 slices were acquired with a 0.3 μm z-step size. 3D reconstruction was done using LAS X 3D Visualization advanced module software (Leica) and maximum intensity projection was performed using ImageJ.
Statistical analysis
Values are represented as means ± SEM. Statistical tests were performed using GraphPad Prism. Comparisons were made using two-tailed Mann–Whitney U-test. Multiple comparisons were made using one-way ANOVA. P values were considered significant at *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Results
MSCs transfer mitochondria to CD8 + T cells and inhibit their differentiation into effectors
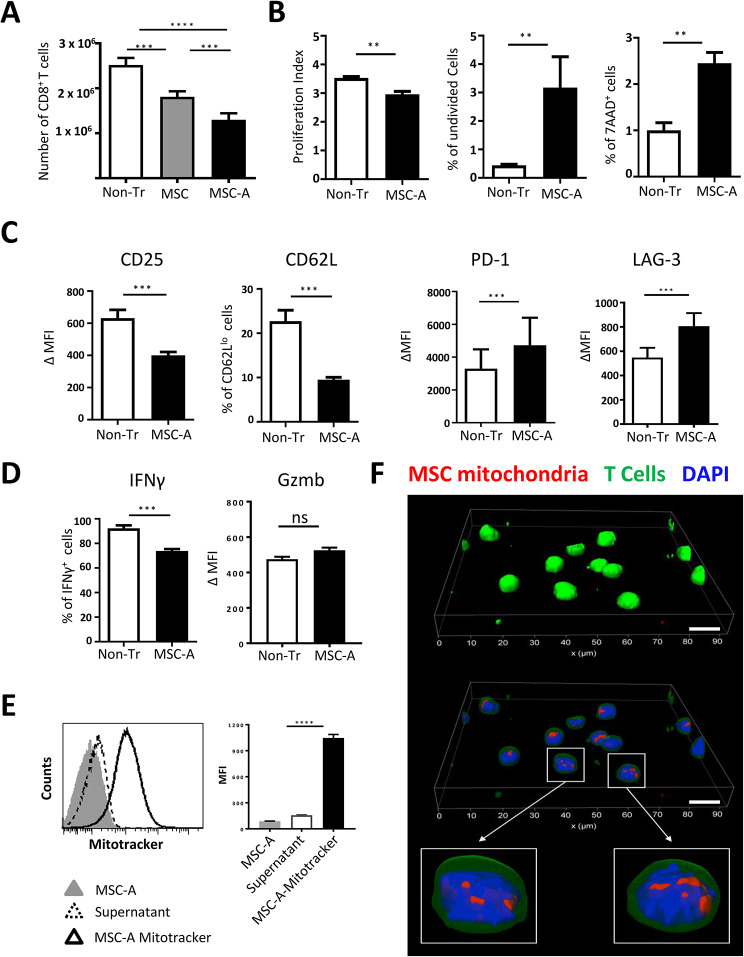
We have previously shown in a transgenic mouse model of type 1 diabetes that MSCs delayed disease onset [47]. Adoptively transferred Clone 4 CD8+ T cells are diabetogenic in InsHA mice with the help of HNT CD4+ T cells in InsHA under inflammatory conditions [48–52]. To study the suppressive effect of MSCs on Clone 4 CD8+ T cell responses, we utilized an allogenic setting, which is currently favored in the clinic. Allogeneic bone marrow-derived MSCs from C57Bl/6 mice, already described [47, 53], were used in co-culture experiments. When activated in vivo, or in vitro, Clone 4 CD8+ T cells differentiate into effector CTL [48–52]. Isolated naïve Clone 4 CD8+ T cells were activated with anti-CD3 and anti-CD28 mAbs and co-cultured with MSCs, at a 1:25 ratio (106 MSC: 4 × 104 T cells), or left untreated without MSCs (Non-Tr). In parallel, CD8+ T cells were cultured with activated MSCs (MSC-A) that had previously been cultured for 48 h with TNFα and IFNγ, again at a 1:25 ratio. It is well established that proinflammatory cytokines “license” quiescent MSCs as immunosuppressive cells [11, 55]. After three days, CD8+ T cells had proliferated extensively (Fig. 1A). We found that MSC-A were significantly more efficient than MSCs at suppressing the expansion of Clone 4 CD8+ T cells, reducing the number of harvested cells by 48% (Fig. 1A). Thus, we only utilized MSC-A in the next experiments. CFSE labeling was used to assess activated Clone 4 CD8+ T cell proliferation. CFSE profiles revealed that CD8+ T cells underwent up to 7 rounds of division (Fig. S1A). Co-culture with MSC-A did not change the number of rounds of division detected. However, the proliferation index was significantly lower in the presence of MSC-A (Fig. 1B and Fig. S1A). This reduction reflects that there was a 7-fold increase of undivided cells and less cells that underwent more rounds of division in the presence of MSC-A (Fig. 1B and Fig. S1A). Additionally, we assessed with the vital dye 7-AAD whether MSC-A could affect the viability of proliferating cells. We found a 2.4-fold increase in the number of dying Clone 4 CD8+ T cells in the presence of MSC-A (Fig. 1B). Next, we investigated the activation status of CD8+ T cells by assessing the expression of key activation markers. As expected, activated Clone 4 CD8+ T cells upregulated CD25 and downregulated CD62L (Fig. S1B and C and Fig. 1C). MSC-A inhibited CD25 expression upregulation and decreased the proportion of CD62Llo Clone 4 CD8+ T cells (Fig. 1C and Fig. S1C). MSC-A significantly increased the expression of the negative regulators of T cell activation PD-1 and LAG-3 (Fig. 1C and Fig. S1C). Activated Clone 4 CD8+ T cells differentiated into effector cells that acquired the potential to secrete the proinflammatory cytokine IFNγ and expressed granzyme B (Fig. 1D and Fig. S1D and E). Whereas MSC-A significantly reduced the percentage of IFNγ-producing cells (Fig. 1D and Fig. S1D and E), no significant differences in granzyme B expression were found in activated Clone 4 CD8+ T cells (Fig. 1D). To assess the diabetogenic potential of suppressed cells, we transferred Clone 4 CD8+ T cells activated in the presence or absence of MSC-A along with naïve HNT CD4+ T cells into irradiated InsHA hosts. Co-culture of Clone 4 CD8+ T cells with MSC-A delayed the onset of autoimmune diabetes in InsHA (Fig. S2). Notably, this effect was enhanced by the injection of MSC-A in vivo (Fig. S2). Taken together, these results demonstrate that allogeneic MSC-A repress Clone 4 CD8+ T cell responses by decreasing expansion, increasing apoptosis of proliferating cells and inhibiting their differentiation into effector CTL upon TCR activation.
Fig. 1.
Allogeneic bone marrow-derived MSCs suppress CD8 + T cell responses and transfer mitochondria to them. A. Purified Clone 4 CD8+ T cells were activated with anti-CD3 and anti-CD28 mAbs and cultured with C57Bl/6 bone marrow-derived MSCs (MSC), TNFα and IFNγ-treated MSCs (MSC-A) or left untreated in the absence of MSCs (Non-Tr). After 3 days, CD8+ T cells were harvested and enumerated. Absolute numbers of Clone 4 CD8+ T cells are shown. B. CFSE-labeled Clone 4 CD8+ T cells were activated and cultured with MSC-A or left untreated. After 3 days, T cells were harvested and CFSE fluorescence analyzed by FACS. Viability was assessed by 7-AAD uptake. Proliferation index was calculated using FlowJo software. C. Day 3 activated Clone 4 CD8+ T cells cultured in the presence or absence of MSC-A were harvested and CD25, CD62L, PD-1 and LAG-3 expression were assessed by FACS. Mean fluoresce intensity (MFI) minus that of the isotype controls is indicated. D. Day 3 activated Clone 4 CD8+ T cells cultured with or without MSC-A were restimulated with PMA and ionomycin in the presence of brefeldin A and intracellular IFNγ was assessed by FACS. Percentage of cytokine producing CD8+ T cells is indicated. Expression of intracellular Granzyme B (Gzmb) was assessed by FACS without restimulation. E. Clone 4 CD8+ T cells were activated and cultured with MSC-A or Mitotracker Deep Red labeled MSC-A. As control for dye leakage, Clone 4 CD8+ T cells were activated and cultured with the supernatant of Mitotracker Deep Red labeled MSC-A that underwent the same process of labeling, washing and culturing time. After 12 h, T cells were analyzed by FACS. Data from 3 independent experiments is presented in panels A to D and values are represented as mean ± SEM. F. Clone 4 CD8+ T cells were stained with green cell tracker CMFDA, activated and cultured with Mitotracker Deep Red-labeled MSC-A. 12 h later T cells were harvested and processed for microscopy. Three-dimensional reconstruction of Clone 4 CD8+ T cells. Cell membrane and cytoplasm appear in green, nuclei in blue and mitochondria in red. In middle and lower panels green channel transparency was increased to confirm localization of labeled mitochondria inside the cells. Scale bar 10 μm
Finally, we tested whether during the co-culture there was transfer of mitochondria from MSC-A to activated Clone 4 CD8+ T cells. We labeled MSC-A with Mitotracker, which specifically stains mitochondria, and, after extensive washing, co-cultured them with activated Clone 4 CD8+ T cells for 12 h. To rule out the possibility that CD8+ T cells became stained by the uptake of dye released to the media from labeled MSC-A rather than by mitochondrial transfer, activated Clone 4 CD8+ T cells cultured for 12 h with supernatants from Mitotracker labeled MSC-A were used as controls (Fig. 1E). Fluorescence acquired by control T cells was negligible compared to that observed after co-culture, illustrating mitochondrial transfer from MSC-A to CD8+ T cells (Fig. 1E). To confirm this result, Clone 4 CD8+ T cells were analyzed by confocal microscopy. Experiments reveled that all analyzed cells had labeled mitochondria in their cytoplasm (Fig. 1F and Fig. S3A). We assessed whether the ability to transfer mitochondria is maintained in different MSC preparations. For this purpose, we utilized MSC-2-A, an independent bone marrow-derived MSC preparation from a different C57Bl/6 mouse that is able to inhibit Clone 4 CD8+ T cell expansion and cytokine secretion as MSC-A do (Fig. S4A and B). MSC-2-A were as efficient as MSC-A in transferring mitochondria to T cells Fig. S4C). Our results demonstrate that activated Clone 4 CD8+ T cells took up mitochondria from neighboring MSCs during their interaction leading to immunosuppression.
MSC mitochondria suppress CD8 + T cell responses
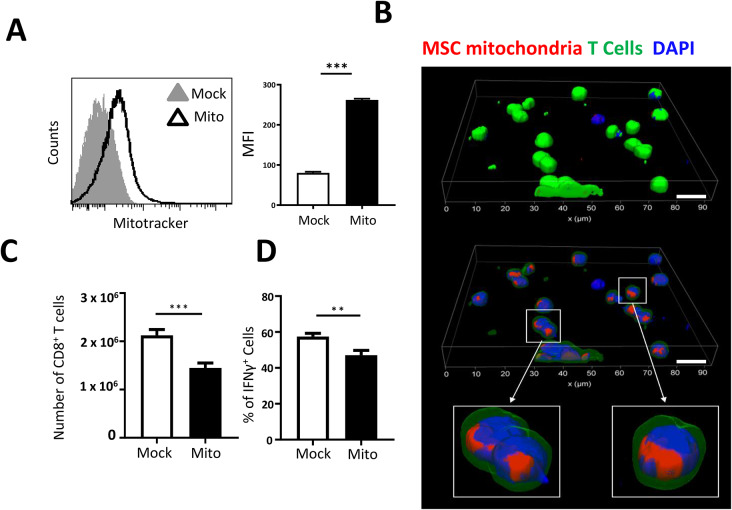
To analyze the effects of MSC mitochondrial transfer, we have previously conceived a method, termed mitoception, to transfer isolated mitochondria from MSCs into target cells [54]. Importantly, mitoception mimicked some of the effects that MSCs had on tumor cells and CD4+ T cells after co-culture [43, 47, 54]. Thus, we evaluated the effect of isolated MSC-A mitochondria on Clone 4 CD8+ T cells. First, we verified that Clone 4 CD8+ T cells had acquired mitochondria after mitoception. Mitochondria from 4 × 104 Mitotracker-labeled MSC-A were transferred to 106 Clone 4 CD8+ T cells, keeping the same ratio used in co-culture experiments (1:25, MSC: T cells). Mock mitocepted Clone 4 CD8+ T cells were used as controls. After 12 h, Clone 4 CD8+ T cells were analyzed by FACS and consistently found to have acquired Mitotracker-labeled mitochondria (Fig. 2A). Confocal microscopy experiments confirmed that mitochondria were present in the cytoplasm of Clone 4 cells (Fig. 2B and Fig. S3B). After validation, we assessed the effect of mitoception, using unlabeled MSC-A mitochondria, on Clone 4 CD8+ T cell expansion and functionality. Twelve hours after mitoception, CD8+ T cells were harvested, activated with anti-CD3 and anti-CD28 mAbs and cultured for 3 days. Then, CD8+ T cells were enumerated and their functionality analyzed by FACS. We found that MSC mitochondria significantly reduced Clone 4 CD8+ T cell expansion and the percentage of IFNγ-producing cells (Fig. 2C and D, and Fig. S5A). Our results demonstrate that MSC mitochondria are able to repress Clone 4 CD8+ T cell activation by decreasing expansion and gain of effector function.
Fig. 2.
MSC mitochondria inhibit expansion and gain of effector function of CD8 + T cells. A. Clone 4 CD8+ T cells were mitocepted with isolated mitochondria from Mitotracker Deep Red labeled MSC-A or mock mitocepted. 12 h later, Clone 4 CD8+ T cells were analyzed by FACS. Data from 3 independent experiments is presented. Values represent MFI ± SEM. B. Clone 4 CD8+ T cells were stained with green cell tracker CMFDA and mitocepted with isolated mitochondria from Mitotracker Deep Red-labeled MSC-A or mock mitocepted. 12 h later T cells were harvested and processed for microscopy. Three-dimensional reconstruction of Clone 4 CD8+ T cells. Cell membrane and cytoplasm appear in green, nuclei in blue and mitochondria in red. Scale bar 10 μm. C. Clone 4 CD8+ T cells were mitocepted with isolated mitochondria from MSC-A or mock mitocepted. 12 h later CD8+ T cells were activated with anti-CD3 and anti-CD28 mAbs and cultured during 3 days. Absolute numbers of harvested T cells are presented. D. Day 3 activated Clone 4 CD8+ T cells were restimulated with PMA and ionomycin in the presence of brefeldin A and intracellular IFNγ was assessed by FACS. Percentage of cytokine-producing T cells is indicated. Data from 4 independent experiments is presented. Values are represented as mean ± SEM
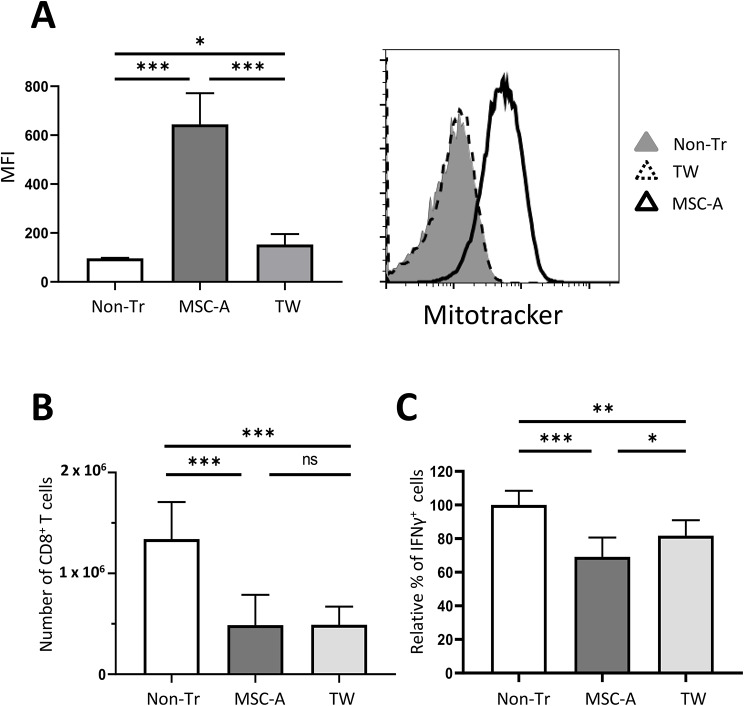
Tunneling nanotubes are the most common mechanism utilized to transfer mitochondria and it requires cell contact. Thus, in order to prevent mitochondrial exchange we set up co-cultures in a trans-well setting. Indeed, in the trans-well condition MSC-A mitochondrial transfer to Clone 4 CD8+ T cells was highly reduced (Fig. 3A). Then, we used trans-well co-cultures to assess the effect of MSC-A on T cell activation under conditions in which mitochondrial transfer is hindered. Surprisingly, we found that inhibition of Clone CD8+ T cell expansion by MSC-A was as effective in trans-well as in regular co-cultures (Fig. 3B). These results indicated that soluble factors have a dominant suppressive effect on CD8+ T cell expansion. On the other hand, trans-well partly impaired the inhibition of IFNγ production by MSC-A (Fig. 3C and Fig. S5B). Taken together, our results indicate that mitochondrial transfer contributes to MSC-A suppression of CD8+ T cell activation and is critical for IFNγ repression.
Fig. 3.
Trans-well co-cultures prevent mitochondrial transfer and reverse IFNγ inhibition. A. Clone 4 CD8+ T cells were activated and cultured with Mitotracker Deep Red-labeled MSC-A in regular wells (MSC-A), in trans-wells (Tw), or left untreated (Non-Tr). After 12 h, T cells were analyzed by FACS. Histogram depicts data from one representative experiment. Data from 3 independent experiments is presented and values are represented as mean ± SEM. B. Clone 4 CD8+ T cells were activated and cultured with MSC-A (MSC-A), in trans-wells (Tw), or left untreated (Non-Tr). After 3 days, T cells were enumerated, absolute numbers of harvested T cells are indicated. Data from 7 independent experiments is presented and values are represented as mean ± SEM. C. Day 3 activated Clone 4 CD8+ T cells were restimulated with PMA and ionomycin in the presence of brefeldin A and production of intracellular IFNγ was assessed by FACS. Percentage of cytokine producing T cells is indicated. Data from 5 independent experiments is presented and values are represented as mean ± SEM
MSC mitochondria cooperate with PGE2 in the suppression of CD8 + T cell responses
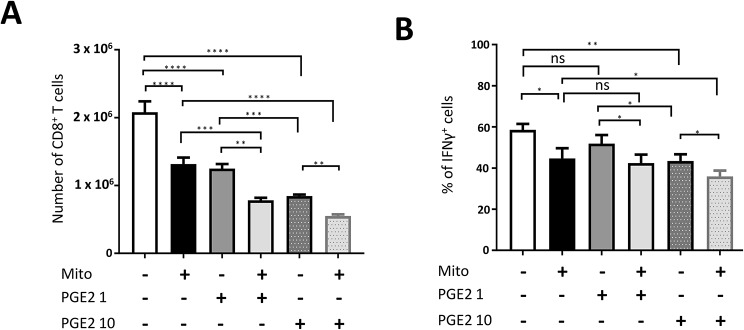
MSCs are known to exert their immunomodulatory effects through a vast array of secreted molecules. Proinflammatory cytokine-activated MSCs secrete high amounts of PGE2, which has been shown to play a key role in the suppression of T cell responses [10, 13, 47]. Thus, we analyzed whether mitochondrial transfer and PGE2 could cooperate in suppression of Clone 4 CD8+ T cell activation. Mitocepted and mock-mitocepted Clone 4 CD8+ T cells were activated and cultured with PGE2 at 1 ng/ml and 10 ng/ml. The lower concentration, 1 ng/ml, is close to the amount found in culture supernatants of murine MSCs [56]. PGE2 at 1 ng/ml inhibited Clone 4 CD8+ T cell expansion by 37%, an effect that was similar to that induced by mitoception (40% decrease). Inhibition by PGE2 was much higher at 10 ng/ml, where a 60% reduction was observed (Fig. 4A). On the other hand, we could only find a significant reduction in IFNγ production at 10 ng/ml PGE2 (Fig. 4B and Fig. S5C). This effect was equivalent to that induced by mitoception. Although we did not find any synergistic effects between mitochondria and PGE2, we did find an additive effect on the suppression of Clone 4 CD8+ T cell expansion and IFNγ production (Fig. 4 and Fig. S5C).
Fig. 4.
MSC mitochondria and PGE2 cooperate in the suppression of CD8 + T cell responses. Clone 4 CD8+ T cells were mitocepted with isolated mitochondria from MSC-A or mock mitocepted. 12 h later, Clone 4 CD8+ T cells were activated with anti-CD3 and anti-CD28 mAbs and cultured for 3 days in the presence or absence of PGE2 at the indicated concentrations (1 or 10 ng/ml). Data from 3 independent experiments is presented and values are represented as mean ± SEM. A. Absolute numbers of cultured Clone 4 CD8+ T cells. B. Activated Clone 4 CD8+ T cells were restimulated with PMA and ionomycin in the presence of brefeldin A and production of intracellular IFNγ was assessed by FACS. Percentage of cytokine producing T cells is indicated
MSCs and MSC mitochondria modulate the balance T-bet/Eomes in activated CD8 + T cells
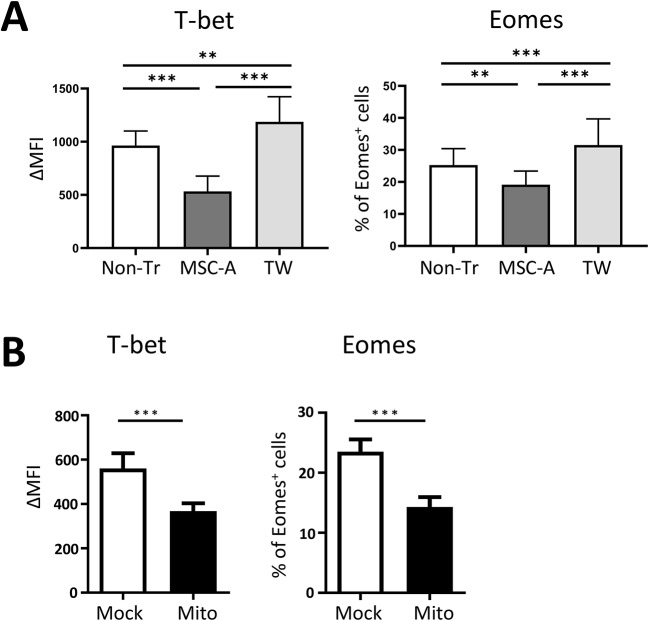
CD8+ T cell differentiation is orchestrated by the induction of a battery of transcription factors that trigger a well characterized transcriptional program leading to the generation of effector and memory cells [1, 57]. T-bet and Eomes play key roles in this process. T-bet is strongly upregulated early upon TCR signaling and Eomes is expressed subsequently. They drive the expression of genes involved in CTL effector function and in particular IFNγ. The balance between them is essential in the differentiation process. Whereas Eomes is expressed at lower levels in effector cells, its expression is increased in memory precursors with little effector function [1, 57]. We hypothesized that one mechanism involved in suppression of Clone 4 CD8+ T cell responses could be by modulating T-bet and Eomes expression. Thus, we compared their expression in activated Clone 4 CD8+ T cells cultured in the presence or absence of MSC-A by intracellular staining. As expected, T-bet expression was strongly upregulated in most Clone 4 CD8+ T cells 24 h after activation (Fig. 5A and Fig. S6A and B). On the other hand, Eomes was only upregulated in an average of 26% of activated T cells (Fig. 5A and Fig. S6D and E). Remarkably, we observed that MSC-A significantly downregulated both T-bet and Eomes expression on activated Clone 4 CD8+ T cells (Fig. 5A and Fig. S6B and E), suggesting that regulation of T-bet and Eomes expression is, at least in part, underlying the immunosuppressive effect of MSC-A.
Fig. 5.
MSCs and MSC mitochondria downregulate T-bet and Eomes expression in activated CD8 + T cells. A. Clone 4 CD8+ T cells were activated with anti-CD3 and anti-CD28 mAbs and co-cultured with MSC-A in regular wells (MSC-A), in trans-wells (TW) or left untreated with no MSCs (Non-Tr). After 24 h, Clone 4 CD8+ T cells were harvested and the expression of intracellular T-bet and Eomes were analyzed by FACS. Mean fluoresce intensity (MFI) minus that of the isotype controls is indicated. Data from 6 independent experiments is presented and values are represented as mean ± SEM. B. Clone 4 CD8+ T cells were mitocepted with isolated mitochondria from MSC-A or mock mitocepted. 12 h later, CD8+ T cells were activated with anti-CD3 and anti-CD28 mAbs and cultured during 24 h. Expression of intracellular T-bet and Eomes were analyzed by FACS. Mean fluoresce intensity (MFI) minus that of the isotype controls is indicated. Values are represented as mean ± SEM. Data from 3 independent experiments is presented
Next, we assessed whether MSC-A mitochondria could modulate T-bet and Eomes expression. To this end, mitocepted Clone 4 CD8 + T cells were activated and analyzed after 24 h. We found similar effects of MSC mitochondria to that observed upon MSC-A co-culture. MSC-A mitochondria also downregulated T-bet and Eomes expression in activated Clone 4 CD8+ T cells compared to mock mitocepted cells (Fig. 5B and Fig. S6C and F). Notably, when activated Clone 4 CD8+ T cells where co-cultured with MSC-A in trans-wells, conditions in which mitochondrial transfer was impaired, T-bet and Eomes downregulation was reversed (Fig. 5A and Fig. S6B and E). Finally, we assessed the role of PGE2 in the modulation of these transcription factors and found that it did not have any effect on T-bet and only modestly reduced Eomes expression (Fig. S7). Taken together, our results indicate that T-bet downregulation is likely at the basis of suppression induced by isolated MSC mitochondria and that it contributes to the overall effect of MSC-A on CD8+ T cell activation and in particular in the production of IFNγ.
Discussion
CD8+ T cells become activated when they encounter antigen presenting cells bearing their cognate antigen. They receive signals through their TCR via antigen recognition, co-stimulatory molecules engaged by their ligands and cytokine receptors recognizing IL-2, IL-12 and type 1 Interferons. These signals are integrated through the MAPK, JNK, PI3K and IKK pathways and promote an epigenetic remodeling that permits the initiation of a complex transcriptional program. This program is mainly mediated by the transcription factors T-bet, RUNX3, Eomes, BLIMP-1, ID2, STAT4 and BATF. The result is extensive proliferation, acquisition of effector functions, such as cytokine production and killing potential, as well as a particular phenotype that allows egress of effector cells to tissues [1, 57]. In this report we have shown that bone marrow-derived MSCs downregulated the expression of the key transcription factors T-bet and Eomes and mediated suppression of CD8+ T cell responses. MSCs repressed the expansion of CD8+ T cells and inhibited their differentiation into effector CTL in response to TCR and co-stimulatory signals. Furthermore, during the interaction MSCs transferred their own mitochondria to CD8+ T cells. MSC mitochondrial transfer contributed to immunosuppression mainly by downregulating T-bet expression that in turns inhibited IFNγ secretion by CD8+ T cells. We have previously shown that MSCs transfer mitochondria preferentially to non-activated CD4+ T cells rather than to non-activated CD8+ T cells [43]. Here, our results demonstrate that MSCs efficiently transfer mitochondria to activated CD8+ T cells, those likely to be present in pathological conditions, and are able to interfere with their activation.
Both, co-culture of CD8+ T cells with MSCs and mitoception with isolated MSC mitochondria resulted in compromised IFNγ secretion and expansion of activated CD8+ T cells. However, when co-cultures were performed under conditions in which mitochondrial transfer was hampered, inhibition of CD8+ T cell expansion was equally efficient. Soluble factors and in particular PGE2 secreted by MSCs appear to have a dominant suppressive effect on T cell expansion. On the other hand, mitochondrial transfer have a critical role in IFNγ inhibition since this effect was partly reversed in trans-well co-cultures and PGE2 had no effect at physiological concentrations. Because trans-well co-cultures prevent T cell - MSC contacts, we cannot rule out the possibility that additional intercellular interactions other than mitochondrial transfer are implicated in our system. These results illustrate the complexity of MSC mediated immunosuppression, the multiple pathways involved and give a key role for secreted PGE2 and mitochondrial transfer in the inhibition of CD8+ T cell responses.
MSCs are able to suppress expansion of human and murine CD8+ T cells in response to alloantigens and peptide antigens [16, 17, 21, 58]. Here we have shown that both MSCs and MSC mitochondria can suppress the expansion of activated CD8+ T cells. In agreement with previous reports, we showed that MSCs decreased proliferation but also enhanced apoptosis of activated CD8+ T cells [59]. Both human and murine MSCs can directly induce T cell apoptosis mediated by the expression of Fas and interaction with FasL expressed on activated T cells [30, 31]. Expression of IDO can also promote apoptosis of CD8+ T cells via the secretion of kynurenine toxic derivatives and tryptophan deprivation [59]. On the other hand, MSCs can induce cell cycle arrest in activated mouse and human CD8+ T cells [60]. PGE2 and TGFβ secreted by MSCs have also been shown to prevent proliferation of human CD8+ T cells [58]. IDO expression in MSCs is also required to prevent the expansion of human T cells and induce cell cycle arrest [58]. IDO-mediated tryptophan deprivation inhibits mTORC1 signaling on activated T cells that in turn prevents aerobic glycolysis supportive of proliferation [61]. We have previously shown that MSC mitochondrial transfer decreased proliferation of human Th17 and murine Th1 cells and increased oxidative phosphorylation [43, 47]. Furthermore, human MSCs inhibit proliferation and differentiation of human naïve CD8+ T cells by downregulating mTOR phosphorylation [62]. Thus, a likely explanation of how MSC and MSC mitochondria inhibited CD8+ T cell expansion in our studies is by mTOR signaling blockade and inhibition of glycolysis.
Our results demonstrate that MSCs and MSC mitochondria inhibit the differentiation of activated CD8+ T cells into IFNγ-producing effector cells. However, we did not find any difference in the amount of granzyme B produced by activated T cells in the presence or absence of MSCs. This does not necessarily indicate that MSCs do not inhibit cytotoxicity. It has been shown that hyporesponsive CD8+ T cells with defects in cytolysis present a normal content of perforin and granzymes in granules but have defective degranulation [63]. Both MSCs and MSC mitochondria inhibited the expression of two key transcription factors involved in CD8+ T cell differentiation, T-bet and Eomes [1, 57, 64]. Acquisition of effector functions, as well as memory generation, after antigen, co-stimulation and cytokine signaling, is critically dependent on their transcriptional activity. T-bet and Eomes have certain overlapping functions and both can mediate transcription of IFNγ and granzyme b genes. T-bet is rapidly upregulated by TCR signaling in activated CD8+ T cells. Expression of Eomes occurs later and is dependent on RUNX3 [1, 57, 64]. In effector cells, continued TCR and strong IL-12R signaling further increase T-bet expression and repress Eomes. Thus, low Eomes levels and a high level of T-bet expression promote the differentiation of short-lived terminally differentiated effector cells. On the other hand, lower T-bet levels and high Eomes levels are found in memory precursors with no effector functions [1, 57, 64]. In our experiments, activated Clone 4 CD8+ T cells engaged TCR, CD28 and IL-2R and although there is no IL-12, they differentiated into IFNγ+ Gzmb+ T-bethi Eomeslo effector cells. MSC co-culture and MSC mitochondrial transfer downregulated both T-bet and Eomes in activated CD8+ T cells and this is likely underlying decreased IFNγ production. These results are in agreement with our studies on CD4+ Th1 cells [47]. Indeed, we found that both MSC co-culture and MSC mitochondrial transfer also inhibited IFNγ production and T-bet upregulation in activated CD4+ Th1 cells [47]. Since T-bet expression is triggered by TCR signaling and also dependent on mTORC1 activity [64], it is likely that mitochondrial uptake by CD8+ and Th1 cells is interfering with the IP3-AKT-mTOR pathway. Additionally, MSCs and MSC mitochondria downregulated CD25, IL2Ra, a component of the high affinity IL-2 receptor. Low IL-2 signaling may also contribute to T-bet downregulation [62]. Thus, T-bet downregulation by mitochondrial transfer represents a general mechanism via which MSC repress type 1 adaptive immune responses.
Activated CD8+ T cells in the presence of MSCs acquire a distinctive phenotype, IFNγlo PD-1hi LAG-3hi, that resembles that of exhausted CD8+ T cells [63, 65–67]. Viral infections that cannot be resolved after primary T cell responses and remain with a chronic antigen production fail to generate memory CD8+ T cells. Instead, dysfunctional CD8+ T cells with reduced effector functions appear in response to sustained TCR stimulation and low inflammatory levels. Terminally differentiated exhausted CD8+ T cells progressively downregulate IL-2, TNFα and IFNγ production and strongly upregulate the expression of inhibitory receptors [63, 65–67]. These cells are generated from exhausted T cell precursors by the reciprocal expression of TCF1 and TOX. Exhausted T cell precursors downregulate TCF1 and upregulate TOX, which establish a terminally differentiated exhausted T cell program [66–70]. We did not find increased levels of TOX in our activated Clone 4 CD8+ T cells in the presence of MSCs. It is possible that as observed in exhausted T cells TOX is only expressed after chronic TCR engagement [63]. Exhausted T cells also appear in the context of developing tumors where they are repetitively stimulated by antigen and in the presence of suppressive cells such as Tregs, Tr1 cells and MSDCs [63]. Interestingly, these cells that are present in the tumor microenvironment share with MSCs the production of immunosuppressive molecules, including TGFβ, IDO, iNOS, IL-10, PGE2, likely involved in the establishment of exhaustion.
The efficacy of MSCs to inhibit pathological immune responses in pre-clinical models has promoted their use in numerous clinical trials for the treatment of GVHD, graft rejection and autoimmune diseases in the last years. MSCs have demonstrated excellent safety in most settings. For GVHD, condition for which the first trials were initiated, significant therapeutic efficacy has been shown. In the case of autoimmune diseases, reports from early phase trials have started to show that efficacy may be highly dependent on the particular disease with better results for inflammatory bowel disease [71]. Progressive understanding of MSC immunosuppressive mechanisms has recently permitted the formulation of cell-free therapeutic strategies. MSC derived extracellular vesicles (EVs) have attracted a great attention in the last few years because they exert similar immunomodulatory effects that the parental MSCs [72]. EVs contain proteins, lipids, metabolites, nucleic acids and, large EVs may also contain functional mitochondria. Since EVs are able to deliver their cargo to target cells, they are currently under study to shuttle mitochondria to cells or tissues where a pathological condition affects mitochondrial metabolism [73]. Although there are still some limitations to a broad clinical application such as yield, functional mitochondrial content and target specificity, donor cells chemical and genetic engineering methods are being developed to implement EVs therapeutic potential [73, 74]. In light of data presented here and our previous results [45, 47], it is interesting to postulate mitochondria and PGE2 enriched MSC-A derived EVs as a potential mitotherapy for T cell mediated diseases.
Conclusion
Our results support the therapeutic use of MSCs or MSCs derived EVs to treat conditions where pathogenic CD8+ T cell responses are present. We have demonstrated that MSCs inhibit CD8+ T cell proliferation and differentiation into effectors in response to antigenic stimulation, by modulating the transcriptional program orchestrated by the key transcription factors T-bet and Eomes. We have also unveiled that MSCs transfer mitochondria to activated CD8+ T cells when in contact. This transfer contributes to immunosuppression mainly by inhibiting IFNγ production.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Additional file 1: Fig. S1. Flow cytometry data of CD8+ T cell suppression by MSCs. Fig. S2. In vivo immunosuppressive potential of MSCs in an autoimmune diabetes model. Fig. S3. Confocal microscopy data of mitochondrial transfer to CD8+ T cells. Fig. S4. Comparison of suppression and mitochondrial transfer ability of different MSCs. Fig. S5. Flow cytometry data of IFNγ production by CD8+ T cells after mitochondrial transfer. Fig. S6. Flow cytometry data of T-bet and Eomes expression. Fig. S7. Modulation of T-bet and Eomes expression by PGE2.
Acknowledgements
The authors would like to thank the animal facility staff at the RAM-Saint Eloi platform at the Institute of Neuroscience of Montpellier. We acknowledge the flow cytometry facility MRI at the IRMB, member of the France-BioImaging national infrastructure supported by the French National Research Agency (ANR-10-INBS-04, Investments for the future). We would like to thank the tissue engineering facility CARTIGEN at the IRMB for imaging. The authors declare that they have not used Artificial Intelligence in this study.
Abbreviations
- CTL
Cytotoxic T lymphocyte
- EV
Extracellular vesicle
- GVHD
Graft versus host disease
- HA
Hemagglutinin
- IDO
Indoleamine 2 3-dioxygenase
- MSC
Mesenchymal stromal cell
- MSC‑A
Activated by TNFα and IFNγ MSC
- PGE2
Prostaglandin E 2
- TCR
T cell receptor
Author contributions
JH conceived the project with the input of CJ and M-LV. JH and M-LV designed the experiments. LV, WA, JN and MS performed the experiments. LV, WA, JN, MS, M-LV MV and JH analyzed the results. JH wrote the paper. All authors read and approved the final manuscript.
Funding
Work at the IRMB was supported by Inserm, University of Montpellier and Région Occitanie. We acknowledge the Agence Nationale pour la Recherche (ANR) for the financial support to the project “MITOSTEM” (ANR-14-CE12-0002). LV was supported by the Labex Mabimprove. WA was supported by Campus France Pakistan.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
Animal studies were conducted according to the European guidelines for animal welfare. Protocols of the project entitled “Evaluation of new biotherapies targeting autoreactive T cells in autoimmunity” were approved by the French ethics committee number 036 on November the 1st 2022 under the approval number APAFIS #38007-2022071516301186 v3.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Loic Vaillant and Waseem Akhter Co-first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Martin Villalba, Christian Jorgensen and Marie-Luce Vignais contributed equally.
References
- 1.Chung HK, McDonald B, Kaech SM. The architectural design of CD8 + T cell responses in acute and chronic infection: parallel structures with divergent fates. J Exp Med. 2021;218:e20201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun L, Su Y, Jiao A, Wang X, Zhang B. T cells in health and disease. Sig Transduct Target Ther. 2023;8:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter U, Santamaria P. CD8 + T cells in autoimmunity. Curr Opin Immunol. 2005;17:624–31. [DOI] [PubMed] [Google Scholar]
- 4.Passerini L, Gregori S. Induction of Antigen-specific tolerance in T cell mediated diseases. Front Immunol. 2020;11:2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Liu X, Cao F, Bellanti JA, Zhou J, Zheng SG. Prospects of the Use of Cell Therapy to Induce Immune Tolerance. Front Immunol. 2020;11:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djouad F, Bouffi C, Ghannam S, Noël D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5:392–9. [DOI] [PubMed] [Google Scholar]
- 7.Gao F, Chiu SM, Motan DAL, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leyendecker A Jr, Pinheiro CCG, Amano MT, Bueno DF. The Use of Human mesenchymal stem cells as therapeutic agents for the in vivo treatment of Immune-Related diseases: a systematic review. Front Immunol. 2018;9:2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35:e00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller L, Tunger A, Wobus M, et al. Immunomodulatory properties of mesenchymal stromal cells: an update. Front Cell Dev Biol. 2021;9:637725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krampera M. Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia. 2011;25:1408–14. [DOI] [PubMed] [Google Scholar]
- 12.Petrenko Y, Vackova I, Kekulova K, et al. A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Sci Rep. 2020;10:4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Liu Q, Huang W, et al. Stanniocalcin-2 contributes to mesenchymal stromal cells attenuating murine contact hypersensitivity mainly via reducing CD8 + Tc1 cells. Cell Death Dis. 2018;9:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. [DOI] [PubMed] [Google Scholar]
- 16.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. [DOI] [PubMed] [Google Scholar]
- 17.Krampera M, Cosmi L, Angeli R, et al. Role for Interferon-γ in the Immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–98. [DOI] [PubMed] [Google Scholar]
- 18.Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90:1312–20. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q-H, Wu F, Liu L, et al. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res Ther. 2020;11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghannam S, Pène J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T Regulatory Cell phenotype. JI. 2010;185:302–12. [DOI] [PubMed] [Google Scholar]
- 21.Malcherek G, Jin N, Hückelhoven AG, et al. Mesenchymal stromal cells inhibit proliferation of virus-specific CD8(+) T cells. Leukemia. 2014;28:2388–94. [DOI] [PubMed] [Google Scholar]
- 22.Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4 + T-cell subsets expressing a regulatory/suppressive phenotype. Haematol. 2005;90:516–25. [PubMed] [Google Scholar]
- 23.Rozenberg A, Rezk A, Boivin M-N, et al. Human mesenchymal stem cells impact Th17 and Th1 responses through a prostaglandin E2 and myeloid-dependent mechanism: hMSC reciprocal modulation of Th1/Th17 responses. STEM CELLS Translational Med. 2016;5:1506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Sun B, Wang D, et al. Murine bone marrow mesenchymal stem cells cause mature dendritic cells to promote T-cell tolerance. Scand J Immunol. 2008;68:607–15. [DOI] [PubMed] [Google Scholar]
- 25.Augello A, Tasso R, Negrini S, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–90. [DOI] [PubMed] [Google Scholar]
- 26.Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO-Independent suppression of T cell effector function by IFN-γ–Licensed human mesenchymal stromal cells. JI. 2014;192:1491–501. [DOI] [PubMed] [Google Scholar]
- 27.Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cell secretion of programmed Death-1 Ligands regulates T cell mediated Immunosuppression. Stem Cells. 2017;35:766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luz-Crawford P, Noël D, Fernandez X, et al. Mesenchymal Stem Cells Repress Th17 Molecular Program through the PD-1 pathway. Unutmaz D, Ed. PLoS ONE. 2012;7:e45272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheng H, Wang Y, Jin Y, et al. A critical role of IFNγ in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–57. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Wang L, Jin Y, Shi S. Fas ligand regulates the Immunomodulatory properties of Dental Pulp Stem cells. J Dent Res. 2012;91:948–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-Induced Immunoregulation involves FAS-Ligand-/FAS-Mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4 + CD25(high) forkhead box P3 + regulatory T cells. Clin Exp Immunol. 2009;156:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouffi C, Bony C, Courties G, Jorgensen C, Noël D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE. 2010;5:e14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang F, Chen M, Chen W, et al. Human gingiva-derived mesenchymal stem cells inhibit Xeno-Graft-versus-host disease via CD39-CD73-Adenosine and IDO signals. Front Immunol. 2017;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su J, Chen X, Huang Y, et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo L, Lai P, Wang Y, et al. Extracellular vesicles from mesenchymal stem cells prevent contact hypersensitivity through the suppression of Tc1 and Th1 cells and expansion of regulatory T cells. Int Immunopharmacol. 2019;74:105663. [DOI] [PubMed] [Google Scholar]
- 37.Hekmatshoar Y, Nakhle J, Galloni M, Vignais M-L. The role of metabolism and tunneling nanotube-mediated intercellular mitochondria exchange in cancer drug resistance. Biochem J. 2018;475:2305–28. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez A-M, Nakhle J, Griessinger E, Vignais M-L. Intercellular mitochondria trafficking highlighting the dual role of mesenchymal stem cells as both sensors and rescuers of tissue injury. Cell Cycle. 2018;17:712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammadalipour A, Dumbali SP, Wenzel PL. Mitochondrial transfer and regulators of mesenchymal stromal cell function and therapeutic efficacy. Front Cell Dev Biol. 2020;8:603292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakhle J, Khattar K, Özkan T, et al. Mitochondria transfer from mesenchymal stem cells confers Chemoresistance to Glioblastoma Stem Cells through metabolic rewiring. Cancer Res Commun. 2023;3:1041–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson MV, Morrison TJ, Doherty DF, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34:2210–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant Lung Injury models by Extracellular Vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luz-Crawford P, Hernandez J, Djouad F, et al. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res Ther. 2019;10:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Do J-S, Zwick D, Kenyon JD, et al. Mesenchymal stromal cell mitochondrial transfer to human induced T-regulatory cells mediates FOXP3 stability. Sci Rep. 2021;11:10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Court AC, Le-Gatt A, Luz‐Crawford P, et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21:e48052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piekarska K, Urban-Wójciuk Z, Kurkowiak M, et al. Mesenchymal stem cells transfer mitochondria to allogeneic Tregs in an HLA-dependent manner improving their immunosuppressive activity. Nat Commun. 2022;13:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akhter W, Nakhle J, Vaillant L, et al. Transfer of mesenchymal stem cell mitochondria to CD4 + T cells contributes to repress Th1 differentiation by downregulating T-bet expression. Stem Cell Res Ther. 2023;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Saout C, Mennechet S, Taylor N, Hernandez J. Memory-like CD8 + and CD4 + T cells cooperate to break peripheral tolerance under lymphopenic conditions. Proc Natl Acad Sci U S A. 2008;105:19414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Saout C, Villard M, Cabasse C, Jacquet C, Taylor N, Hernandez J. IL-2 mediates CD4 + T cell help in the breakdown of memory-like CD8 + T cell tolerance under lymphopenic conditions. PLoS ONE. 2010;5:e12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espinosa-Carrasco G, Villard M, Le Saout C, Louis-Plence P, Vicente R, Hernandez J. Systemic LPS translocation activates cross-presenting dendritic cells but is dispensable for the Breakdown of CD8 + T cell peripheral tolerance in irradiated mice. PLoS ONE. 2015;10:e0130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Espinosa-Carrasco G, Le Saout C, Fontanaud P, et al. CD4 + T helper cells play a key role in maintaining Diabetogenic CD8 + T cell function in the pancreas. Front Immunol. 2018;8:2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espinosa-Carrasco G, Le Saout C, Fontanaud P, et al. Integrin β1 optimizes Diabetogenic T Cell Migration and function in the pancreas. Front Immunol. 2018;9:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luz-Crawford P, Espinosa-Carrasco G, Ipseiz N, et al. Gilz-activin A as a Novel Signaling Axis orchestrating mesenchymal stem cell and Th17 cell interplay. Theranostics. 2018;8:846–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caicedo A, Fritz V, Brondello J-M, et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci Rep. 2015;5:9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.English K, Barry FP, Field-Corbett CP, Mahon BP. IFN-γ and TNF-α differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007;110:91–100. [DOI] [PubMed] [Google Scholar]
- 56.Djouad F, Charbonnier L-M, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–32. [DOI] [PubMed] [Google Scholar]
- 57.Kaech SM, Cui W. Transcriptional control of effector and memory CD8 + T cell differentiation. Nat Rev Immunol. 2012;12:749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M, Sun X, Kuang X, Liao Y, Li H, Luo D. Mesenchymal stem cells suppress CD8 + T cell-mediated activation by suppressing natural killer group 2, member D protein receptor expression and secretion of prostaglandin E 2, indoleamine 2, 3-dioxygenase and transforming growth factor-β: mesenchymal stem cells suppress CD8 + T cells. Clin Exp Immunol. 2014;178:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Xu Z, Bai J et al. Umbilical Cord Tissue-Derived Mesenchymal Stem Cells Induce T Lymphocyte Apoptosis and Cell Cycle Arrest by Expression of Indoleamine 2, 3-Dioxygenase. Stem Cells International. 2016; 2016: 1–11. [DOI] [PMC free article] [PubMed]
- 60.Lee S, Kim S, Chung H, Moon JH, Kang SJ, Park C-G. Mesenchymal stem cell-derived exosomes suppress proliferation of T cells by inducing cell cycle arrest through p27kip1/Cdk2 signaling. Immunol Lett. 2020;225:16–22. [DOI] [PubMed] [Google Scholar]
- 61.Böttcher M, Hofmann D, Bruns A. Mesenchymal stromal cells disrupt mTOR-Signaling and aerobic glycolysis during T-Cell activation: MSCs Act as a metabolic checkpoint for T-Cells. Stem Cells. 2016;34:516–21. [DOI] [PubMed] [Google Scholar]
- 62.Papait A, Vertua E, Signoroni PB, et al. Amniotic MSC affect CD8 naive polarization toward SLEC/MPEC subsets by down-modulating IL-12Rβ1 and IL-2Rα signaling pathways. iScience. 2023;26:108483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Philip M, Schietinger A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr Opin Immunol. 2019;58:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paley MA, Kroy DC, Odorizzi PM, et al. Progenitor and terminal subsets of CD8 + T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He R, Hou S, Liu C, et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412–28. [DOI] [PubMed] [Google Scholar]
- 67.Im SJ, Hashimoto M, Gerner MY, et al. Defining CD8 + T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scott AC, Dündar F, Zumbo P, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alfei F, Kanev K, Hofmann M, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019;571:265–9. [DOI] [PubMed] [Google Scholar]
- 70.Khan O, Giles JR, McDonald S, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L-T, Liu K-J, Sytwu H-K, Yen M-L, Yen BL. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. STEM CELLS Translational Med. 2021;10:1288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomzikova MO, James V, Rizvanov AA. Therapeutic application of mesenchymal stem cells derived Extracellular vesicles for Immunomodulation. Front Immunol. 2019;10:2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu S, Yang T, Ma M, et al. Extracellular vesicles meet mitochondria: potential roles in regenerative medicine. Pharmacol Res. 2024;206:107307. [DOI] [PubMed] [Google Scholar]
- 74.Manickam DS. Delivery of mitochondria via extracellular vesicles – a new horizon in drug delivery. J Controlled Release. 2022;343:400–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Additional file 1: Fig. S1. Flow cytometry data of CD8+ T cell suppression by MSCs. Fig. S2. In vivo immunosuppressive potential of MSCs in an autoimmune diabetes model. Fig. S3. Confocal microscopy data of mitochondrial transfer to CD8+ T cells. Fig. S4. Comparison of suppression and mitochondrial transfer ability of different MSCs. Fig. S5. Flow cytometry data of IFNγ production by CD8+ T cells after mitochondrial transfer. Fig. S6. Flow cytometry data of T-bet and Eomes expression. Fig. S7. Modulation of T-bet and Eomes expression by PGE2.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.