Abstract
Background
Post-stroke cognitive impairment (PSCI) severely reduces quality of life of patients with stroke. This study aimed to assess the effects of electroacupuncture (EA) on PSCI and the role of the mTOR/NLRP3-mediated autophagy-inflammatory pathway in this process.
Methods
The rat focal cerebral ischemia model was established using middle cerebral artery occlusion (MCAO). Following successful induction of the model, EA was applied to the bilateral Fengchi, Fengfu, and Dazhui acupoints, and brain tissue samples were collected on day 15. Cognitive function was assessed using the Morris water maze test. Cerebral infarct volume was quantified by Triphenyltetrazolium chloride (TTC) staining. Hematoxylin–eosin and TUNEL staining were performed to evaluate pathological changes and apoptosis rates. Apoptosis-, inflammation-, and autophagy-related biomarkers were measured, and autophagosomes were visualized using transmission electron microscopy.
Results
MCAO rats exhibited slower weight gain, reduced mobility, increased infarct size, pathological damage, and apoptosis, confirming successful establishment of the MCAO rat model. Following EA treatment, MCAO rats displayed faster weight gain, improved mobility, and shorter escape latency. EA also reduced the area of cerebral infarction and alleviated pathological damage and apoptosis in MCAO rats. Furthermore, EA downregulated IL-1β, IL-18, NLRP3, and LC3 II/LC3 I expression and upregulated p62, mTOR, and Beclin-1 expression in MCAO rats. EA treatment also decreased the number of autophagosomes in these rats.
Conclusions
EA effectively mitigates post-stroke cognitive impairment by reducing apoptosis, inflammation, and autophagy through the regulation of the mTOR/NLRP3-mediated autophagy-inflammatory pathway, offering valuable therapeutic insights for stroke rehabilitation.
Keywords: Post-stroke cognitive impairment, mTOR/NLRP3 pathway, Apoptosis, Inflammation, Autophagy
Background
Stroke is a common cerebrovascular disease characterized by multiple complex pathophysiological mechanisms [1]. Post-stroke cognitive impairment (PSCI) is a significant complication for stroke survivors, with research demonstrating that the morbidity of PSCI in young patients with stroke is 62.33% [2]. PSCI affects various cognitive domains, including language, attention, executive function, memory, praxis, and number processing, severely affecting patients’ quality of life [3]. Current Western medicines such as cholinesterase inhibitors and memantine nootropics, are often ineffective and costly for managing PSCI [4]. Acupuncture demonstrates effectiveness in treating post-stroke cognitive dysfunction, and several clinical studies indicate that combining acupuncture and cognitive rehabilitation further improves PSCI compared to that of the cognitive rehabilitation alone [5]. However, the role and mechanism of electroacupuncture (EA) in improving PSCI remain unclear.
Despite the high morbidity associated with cognitive impairment worldwide, no gold standard approach to cognitive rehabilitation has been established. EA, a traditional Chinese medicine therapy, has been used to treat Alzheimer's disease and stroke to prevent cognitive impairment [6]. EA encompasses a range of interventions that operate through somatosensory autonomic reflexes [7]. Liu et al. demonstrated that EA stimulates immune-related neural pathways, and that adjusting the site, intensity, and duration of treatment can result in different effects on the inflammatory response and survival status of mice, this suggests that stimulating different acupoints activates distinct autonomic pathways [8]. Additionally, the selection of acupoints determines the efficacy of EA therapy, with combined acupoint therapy generally proving more effective than single acupoint therapy [9, 10]. Previous studies have demonstrated that EA performed on Baihui (GV 20), Sishencong (EX-HN1), Fengchi (GB 20), and Shenting (GV 24), when combined with computer-based cognitive rehabilitation, can restore cognitive function of patients with mild cognitive impairment [11]. Furthermore, EA stimulation at Shangxing (GV23) and Fengfu (GV16) improves cognitive impairment in rats by inhibiting oxidative stress and neuroinflammation [12]. Furthermore, EA stimulation at Baihui (DU 20) and Dazhui (DU 14) ameliorates cognitive deficits in rats with Alzheimer's disease by regulating GABAergic interneurons [13]. Fengchi (GB 20), Fengfu (GV16), and Dazhui (DU 14) have been identified as commonly used acupoints for treating ischemic stroke [14]. However, the effects of EA stimulation at these points on PSCI remain unclear. Additionally, the overall effectiveness of EA is still debated. Therefore, further research is needed to explore the underlying mechanisms of EA’s role in treating cognitive dysfunction.
Neuronal death resulting from cerebral ischemia is a key factor in the mortality and disability rates of stroke. EA has been shown to inhibit neuronal apoptosis in ischemic stroke [15, 16]. Another study demonstrated that EA ameliorates neuronal injury in cerebral ischemia by regulating NLRP3 [17]. Additionally, previous studies have reported that EA inhibits neuronal autophagy to promote neuronal repair [18]. Autophagy is closely associated with anti-apoptotic and anti-inflammatory pathways, and the activation of inflammatory vesicles by NLRP3 and the inflammatory response can be suppressed by autophagy through mTOR pathway activation [19–21]. However, the role of the mTOR-NLRP3-mediated autophagy-inflammatory pathway in the EA treatment of PSCI has not been explored.
Based on previous research, EA interventions were performed on rats with focal cerebral ischemia in this study. Although previous studies have identified effective acupoints for cognitive impairment, this study specifically focuses on the combination of Fengchi (GB 20), Fengfu (GV 16), and Dazhi (DU 14) for PSCI treatment, offering new guideline for acupoint selection in EA therapy. This study evaluated the efficacy of EA on mobility, cerebral infarct size, pathological tissue damage, apoptosis, inflammation, and autophagy in rats, providing evidence for PSCI treatment. Additionally, the role of the mTOR/NLRP3-mediated autophagy-inflammation pathway in EA treatment of PSCI was investigated, offering new insights into the underlying biological processes.
Methods
Middle cerebral artery occlusion (MCAO) rat model establishment and treatment
Twenty specific pathogen-free healthy Sprague Dawley rats (male, 250 ± 20 g) were obtained from Beijing Vital River Laboratories (Beijing, China). This study was approved by the Ethics Committee of Xiamen University (XMULAC20220034-23). The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation. Rats were raised in a room at 23 ± 2 °C with 60 ± 10% humidity and a 12-h light/dark cycle and were given a standard rodent diet. Rats were randomly divided into four groups. (i) For the Sham group (n = 5), an incision was made at the same location as in the MCAO group, and the common, external, internal carotid arteries were separated without blockage. The incision was then sterilized and sutured; (ii) For the MCAO group (n = 5), the left middle cerebral artery of rats was blocked, and this was followed by 2-h reperfusion as performed in previous studies [22]; (iii) For the MCAO + sham EA group (n = 5), the left middle cerebral artery of rats was blocked, and this was followed by 2-h reperfusion. The EA therapeutic apparatus (HANS-200, Nanjing Jisheng Medical) was used to stimulate the tail root at the end of the proximal trunk of rats [23]. The parameter settings of the EA therapeutic apparatus were consistent with those in MCAO + EA group; (iv) For the MCAO + EA group (n = 5), the left middle cerebral artery of rats was blocked, and this was followed by 2-h reperfusion. The rats were raised for 10 days for wound healing before EA treatment. Rats were subjected to bilateral EA stimulation at four acupoints that included Bilateral Fengchi (GB 20) located 3 mm lateral to the midpoint of a line joining the two ears at the back of the head, Fengfu (GV 16) located in the dorsal depression of occipitoatlantal joint behind the crest of occipital bone, and Dazhui (GV 14) located between the 7th cervical vertebra and the 1st thoracic vertebra at the median back. These acupoints were selected based on previous research [14, 24]. Two needles (diameter: 0.3 mm, length: 25 mm) were inserted approximately 2–3 mm depth into the acupoints. The intervention parameters were set to 20 Hz continuous wave and 1 mA. Treatment was performed once daily for 30 min for a total of 14 days. The body weights of rats were measured on days 7 and 14 after treatment.
Assessment of neurological deficits
The Zea-Longa neurobehavioral score [25] was assessed on days 7 and 14 after treatment.
Morris water maze experiment
The water maze was performed on day 14 after treatment. The rats were placed in the water at four randomly selected starting positions (east, west, south, and north) and facing the wall of the pool. The spent time of rats from starting position to underwater platform was recorded. Rats that spent > 60 s were guided to the underwater platform. Rats were allowed to remain on the platform for 10 s. Rats were then dried under a 150 W incandescent lamp for 5 min and placed back in the cage. Rats were trained 4 times each day for 20 min each time over five consecutive days. The escape latency, platform crossing time, searching distance, and time spent in target were recorded.
Triphenyltetrazolium chloride (TTC) staining
Rats were anesthetized with 5% isoflurane, perfused, and fixed with saline and paraformaldehyde (P0099, Beyotime). The brains were removed by severing the heads on ice. Fresh brain tissue was chilled at -20℃ for 20 min and then cut into five thin slices with a razor blade. Subsequently, the brain tissue was stained with 2,3,5-TTC solution (G3005, Solarbio). The infarcted areas were stained white, while the non-infarcted areas were stained red. The stained brain sections were imaged, and the infarct volume of each sample was calculated using the Image Pro Plus software.
Hematoxylin–eosin (HE) staining
Hippocampal neuron tissues in brains were isolated from rats. The brain tissues were fixed with 4% paraformaldehyde solution and routinely dehydrated to obtain paraffin sections. The sections were stained with hematoxylin solution (C0105S, Beyotime), and fractionated with 1% hydrochloric acid alcohol. Then, sections were treated with 0.6% ammonia and stained with 0.5% eosin solution. Finally, sections were observed under a microscope (BX53, Olympus).
TUNEL staining
Paraffin-embedded tissue sections were routinely dewaxed, rinsed twice with PBS (C0221A, Beyotime), and incubated in a proteinase K working solution (ST532, Beyotime) for 15 min at 37℃. TdT enzyme reaction solution (50 µL, C1086, Beyotime) was added to each sample, and coverslips were wetted at 37℃ for 60 min. After washing with PBS, cell nuclei were stained with DAPI. Finally, the sections were sealed and imaged using a Leica fluorescence microscope (DM2500, Leica). Ten high-magnification fields were randomly selected for imaging and counting.
Enzyme-linked immunosorbent assay (ELISA)
Interleukin (IL)-1β and IL-18 levels in serum of rats were tested using ELISA kits (Mlbio, China) according to manufacturer’s instructions. A microplate reader (Wuxi Hiwell Diatek Instruments Co., Ltd, China) was used to measure the absorbance at 450 nm.
RT-qPCR
Total RNA from brain tissues was extracted using TRIzol reagent (Invitrogen, CA, USA). RT-qPCR was performed as previously described [26]. Primers are listed in Table 1.
Table 1.
Primer sequences for RT-qPCR
| Gene | Primer sequence | |
|---|---|---|
| GAPDH | Forward | 5′-GGA GAT TAC TGC CCT GGC TCC TA-3′ |
| Reverse | 5′-GAC TCA TCG TAC TCC TGC TTG CTG-3′ | |
| mTOR | Forward | 5′-GCT TAT CAA GCA AGC GAC ATC TCA-3′ |
| Reverse | 5′-TCC ACT GGA AGC ACA GAC CAA G-3′ | |
| Beclin-1 | Forward | 5′-GAA ACT GGA CAC GAG CTT CAA GA-3′ |
| Reverse | 5′-ACC ATC CTG GCG AGT TTC AAT A-3′ | |
| NLRP3 | Forward | 5′-CCA TCG GCA AGA CCA AGA-3′ |
| Reverse | 5′-ACA GGC TCA GAA TGC TCA TC-3′ | |
| Caspase-1 | Forward | 5′-TCA CCT CTT TCA CCA TCT CC-3′ |
| Reverse | 5′-TCTTTCTGACAGACGGATATGCT-3′ | |
Western blot
Brain tissue (100 mg) was added to tissue lysate (P0013B, Beyotime) and homogenized. The supernatant was then centrifuged to obtain the total protein. Protein concentration was determined using the BCA kit (P0012S, Beyotime). Proteins were separated in 12% polyacrylamide gel by electrophoresis and transferred to PVDF membranes (FFP24, Beyotime). The membranes were blocked with 5% skimmed milk (P0216, Beyotime) for 1 h and washed with TBST (ST677, Beyotime). Primary antibodies against Bax (1:1,000; ab32503, Abcam), Bcl-2 (1:1,000; #AF6139, Affinity, CA, USA), caspase-1 (1:1,000; ab207802, Abcam), cleaved caspase-3 (1:1,000; #AF7022, Affinity, CA, USA), NLRP3 (1:1,000; ab263899, Abcam), LC3 (1:1,000; #12,741, CST, MA, USA), p62 (1:1,000; #39,749, CST, MA, USA), mTor (1:1,000; ab32028, Abcam), Beclin-1 (1:1,000; ab210498, Abcam), and β-actin (1:1,000; ab8227, Abcam) were incubated with membranes at 4 °C overnight. The membranes were then incubated with a secondary antibody (1:3,000; ab205718, Abcam) for 1 h at room temperature. The optical density values of the protein bands were analyzed using QuantityOne (BioRad, CA., USA).
Transmission electron microscopy
Rats were anesthetized, and the brains were severed. The ischemic brain tissue was separated on ice, rinsed with pre-chilled saline, removed, and aspirated before being quickly placed in light-proof glutaraldehyde solution (30,092,436, Sinopharm). The tissue was washed twice with double-distilled water, dehydrated step by step in acetone (10,000,418, Sinopharm), permeabilized, embedded, trimmed, and sectioned to a thickness of 60 nm using an ultra-thin sectioning machine. The sections were then stained with lead citrate (39,476,466, Sinopharm) and uranyl acetate. Neuronal structures were observed by transmission electron microscopy and imaged. Autophagosomes in brain tissue were observed in 10 randomly selected fields.
Statistical analyses
Statistical analysis was performed using SPSS 27.0 (IBM, IL, USA). Data are presented as mean ± standard deviation and were tested for normality using the Shapiro-Wilks test, while Levene’s test was used to assess homogeneity of variance. One-way ANOVA and t-test were applied to compare differences between groups. P < 0.05 was considered statistically significant. Each experiment was repeated three times.
Results
EA treatment increased body weight and improved mobility in MCAO rats
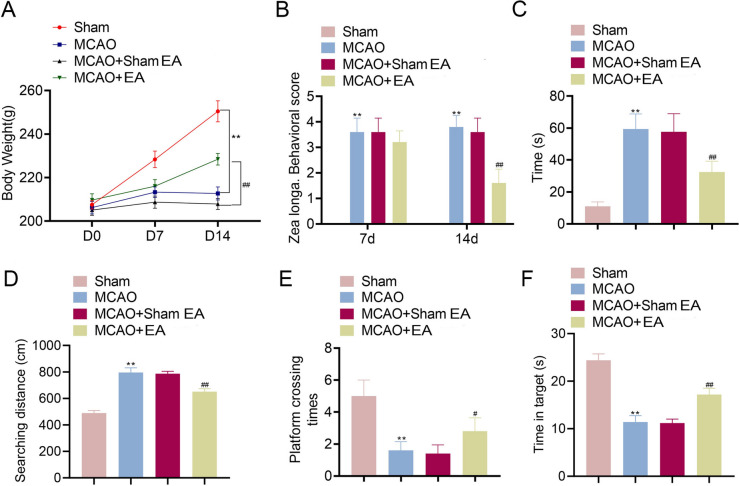
The effects of EA on body weight and mobility in MCAO rats were assessed. The results demonstrated that the body weight of MCAO rats increased slowly compared to that of the Sham group on day 7 and 14 (Fig. 1A). Rats in the EA treatment group exhibited faster weight gain compared to that of the model group (p < 0.01). The Zea Longa neurobehavioral scores were higher in the model group compared to that of the Sham group but decreased after 14 days of EA treatment (p < 0.01, Fig. 1B). Additionally, the Morris water maze experiment revealed that the MCAO group had longer to evade, with greater latency and searching distance than that of the Sham group (p < 0.01), both of which were reduced in the MCAO + EA group (p < 0.01, Fig. 1C and D). Similarly, platform crossing frequency and time spent in target zone were notably lower in the MCAO group compared to those of the Sham group, but both improved after EA treatment (p < 0.05, Fig. 1E and F).
Fig. 1.
EA treatment increased body weight and improved mobility in MCAO rats. A Body weight of rats. B Zea longa neurobehavioral scores were assessed on day 7 and 14 after treatment. C–E Escape latency, searching distance, the times of platform crossing, and time spent in target were measured using water maze experiment. **p < 0.01 compared to Sham group. #p < 0.05 and ##p < 0.01 compared to MCAO + Sham EA
EA treatment reduced cerebral infarct size in MCAO rats
The cerebral infarct volumes of MCAO rats were evaluated after EA treatment. Results revealed that, compared to that of the Sham group, the cerebral infarct volume was increased in the MCAO group, and was significantly reduced by EA treatment (p < 0.01, Fig. 2).
Fig. 2.
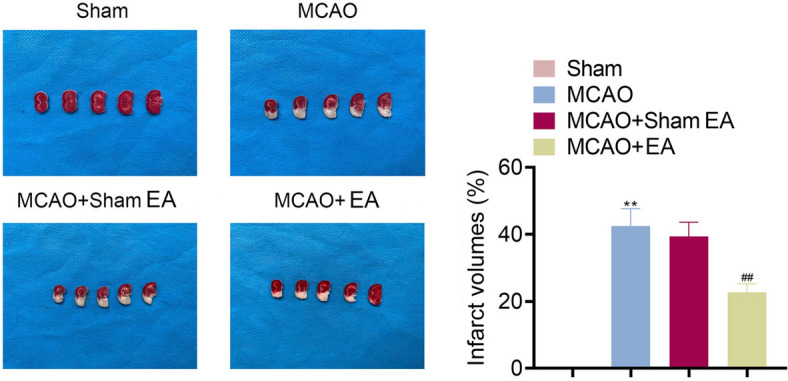
EA treatment reduced cerebral infarct size in MCAO rats. TTC staining was used to assess the cerebral infarct volume. **p < 0.01 compared to Sham group. ##p < 0.01 compared to the MCAO + Sham EA group
EA treatment alleviated the pathological tissue damage and apoptosis in MCAO rats
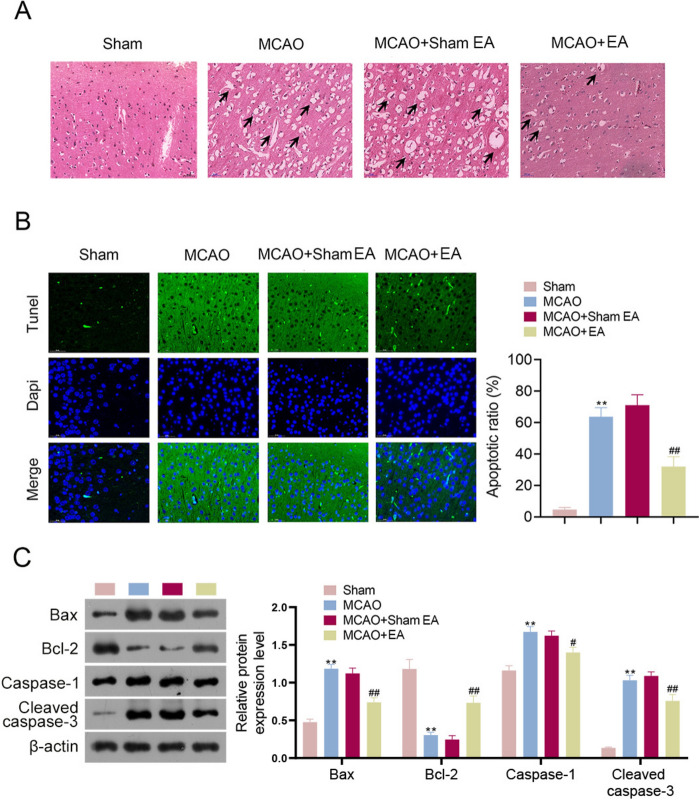
The effect of EA treatment on pathological tissue damage in MCAO rats was evaluated. MCAO rats exhibited an increase in vacuolated degenerative necrotic cells in glial cells of brain tissues compared to that of the Sham group (Fig. 3A). EA treatment reduced both the number and severity of vacuolated necrotic cells in MCAO rats. Moreover, TUNEL staining revealed increased apoptotic cells in MCAO rats compared to that in sham rats (p < 0.01, Fig. 3B). EA treatment significantly reduced the number of apoptotic cells in MCAO rats (p < 0.01). Additionally, Bcl-2 and caspase families are critical regulators of apoptosis in ischemic stroke [27]. Western blotting results indicated that the expression of Bax, caspase-1, and cleaved caspase-3 was upregulated in MCAO rats, while Bcl-2 expression was downregulated (p < 0.01, Fig. 3C). However, EA treatment reversed this phenomenon (p < 0.05, p < 0.01).
Fig. 3.
EA treatment alleviated the pathological tissue damage and apoptosis in MCAO rats. A HE staining revealed the histopathological damage of brains (Scale bar = 50 μm). B Detection of tissue apoptosis using a TUNEL assay kit (Scale bar = 50 μm). C Western blotting was used to assess the expression of Bax, Bcl-2, caspase-1, and cleaved caspase-3 in rats. **p < 0.01 compared to the Sham group. #p < 0.05 and ##p < 0.01 compared to the MCAO + Sham EA group
EA treatment inhibited inflammation and autophagy in MCAO rats
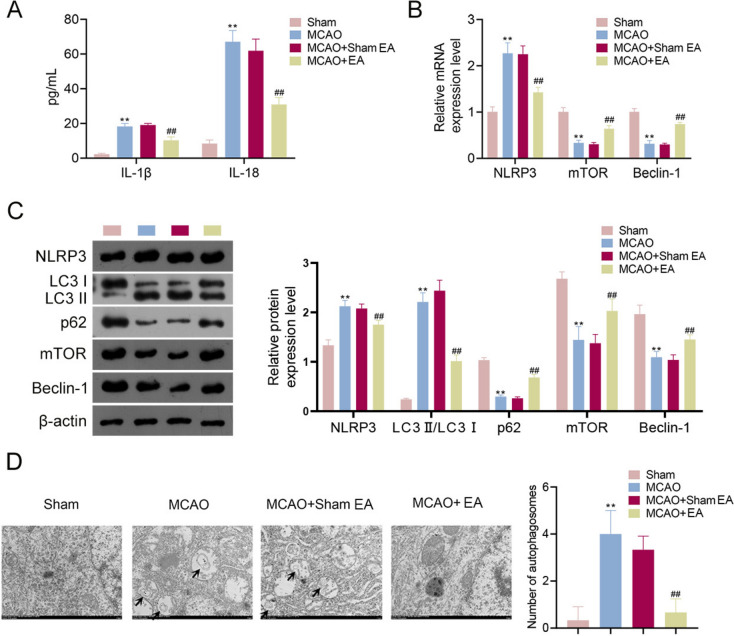
The effects of EA on inflammation and autophagy were explored in MCAO rats. As presented in Fig. 4A, the levels of pro-inflammatory cytokines (IL-1β and IL-18) were significantly increased in MCAO rats compared to levels in the Sham group, and these increases were reversed by EA treatment (p < 0.01). RT-qPCR confirmed that NLRP3 (an immune-inflammatory target) levels were higher in MCAO rats than that in controls, and EA treatment decreased NLRP3 expression in MCAO rats (p < 0.01, Fig. 4B). Previous research has reported that EA inhibits neuronal autophagy to repair neuronal damage by activating the expression of mTOR and Beclin-1 (autophagy-related proteins) [28]. Consistently, the results revealed that mTOR and Beclin-1 mRNA expression declined in MCAO rats compared to that of the Sham group, and this was reversed by EA treatment (p < 0.01). Western blotting demonstrated that EA decreased NLRP3 and LC3 II/LC3 I levels while increasing p62, mTOR, and Beclin-1 levels in MCAO rats (p < 0.01, Fig. 4C), further confirming the mitigative effects of EA on inflammation and autophagy. Additionally, transmission electron microscopy indicated that compared to the Sham group, the nuclei of brain tissue were consolidated, the cytoplasm was unevenly distributed or vacuolated, and autophagosomes were significantly increased in MCAO rats. These changes were reversed following EA treatment ((p < 0.01, Fig. 4D).
Fig. 4.
EA treatment alleviated inflammation and autophagy in MCAO rats. A ELISA measured levels of pro-inflammatory cytokines (IL-1β and IL-18) in rats. B RT-qPCR detected NLRP3, mTOR, and Beclin-1 levels. C Western blotting detected NLRP3, LC3 II/LC3 I, p62, mTOR, and Beclin-1 levels. D Transmission electron microscopy observed the number of autophagosomes in brain tissue cells (Scale bar = 1 μm). **p < 0.01 compared to the Sham group. ##p < 0.01 compared to the MCAO + Sham EA group
Discussion
PSCI is a common and persistent complication in patients with stroke that often leads to disability [29, 30]. Cognitive impairments in areas such as memory, spatial structure, calculation, attention, and orientation often occur after a stroke [31]. Research has shown that EA can alleviate post-stroke cognitive dysfunction resulting from ischemic stroke [23]. However, the underlying mechanism of EA in treating PSCI remains unclear. This study demonstrated that EA alleviates PSCI through the mTOR-NLRP3 autophagy-inflammation pathway based on an MCAO rat model.
EA significantly improves mobility of rats with ischemic injury, and previous studies have demonstrated that Fengchi (GB 20), Fengfu (GV 16), and Dazhui (GV 14) are effective acupoints for EA treatment of ischemic stroke [32–34]. In this study, these combined acupoints: bilateral Fengchi (GB 20), Fengfu (GV 16), and Dazhui (GV 14) were used for EA treatment. The results indicated that MCAO rats exhibited increased body weight, reduced Zea Longa neurobehavioral scores, and decreased cerebral infarct volume after EA treatment, suggesting that EA treatment can alleviate the pathological damage and improve mobility impairment in MCAO rats. These findings are consistent with the previous study reported by Liu et al. [35].
During the development of ischemic stroke, excessive apoptosis contributes to impaired cognitive nerve function, ultimately leading to PSCI [36]. Previous studies have demonstrated that the neuronal apoptosis rate of MCAO rats was significantly higher than that in Sham group, with increased expression of pro-apoptotic proteins and decreased expression of anti-apoptotic proteins [37]. Consistently, our results demonstrated that Bax, caspase-1, and cleaved caspase-3 were upregulated in the brain tissues of MCAO rats, while Bcl-2 expression was downregulated. These changes were reversed by EA, suggesting that EA may inhibit apoptosis to improve learning memory impairment of rats. Neuronal apoptosis is induced by inflammatory mediators released by neuroinflammation [38]. Hou et al. demonstrated that drug treatment decreased the elevated levels of inflammatory cytokine and inflammasome-related protein in MCAO rats [39]. Similarly, we observed that EA treatment downregulated the elevated levels of NLRP3, IL-1β, and IL-18 induced by MCAO. Moreover, basal autophagy is responsible for clearing and recycling intracellular components, while the induction of microglial protective autophagy may phagocytose apoptotic debris to prevent neuronal damage and inflammation. However, excessive autophagy may lead to abnormal self-digestion and degradation of critical cellular components, accelerating cell death [40]. Research has demonstrated that BAG3 overexpression can promote autophagy and inhibit apoptosis, thereby alleviating cerebral ischemic injury [41]. However, another study reported that bilobalide reduced autophagy levels in MCAO rats, enhancing self-repair after ischemic stroke [42]. In this study, EA treatment downregulated the LC3 II/LC3 I and autophagosome and upregulated the expression of p62, mTOR, and Beclin-1 in MCAO rats. These findings suggest that the repair of neuronal cells is promoted through the regulation of the apoptotic process in conjunction with the autophagic network system.
This study has several limitations. First, the sample size of animals used for research was limited. Second, this study assessed the effect of EA on PSCI without comparing its therapeutic efficacy of EA to that of the other common therapies. Furthermore, signaling pathways do not act in isolation; they also interact with each other. Therefore, exploring the horizontal interactions between signaling pathways may be a promising research direction for the future. Overall, this study offers a new insights into the pathological mechanisms of PSCI and identifies novel targets for its treatment. Additionally, this study presents a non-drug treatment approach for the clinical management of PSCI, avoiding the side effects and adverse reactions associated with pharmaceuticals. The combined acupoint therapy using bilateral Fengchi, Fengfu, and Dazhui provides a novel strategy for EA treatment of PSCI.
Conclusion
This study demonstrated that EA treatment improved the mobility of MCAO rats, reduced cerebral infarct size, and mitigated pathological tissue damage. Moreover, EA alleviated MCAO-induced apoptosis, inflammation, and autophagy. Additionally, the mTOR/NLRP3 autophagy-inflammation pathway mediates the effect of EA in treating PSCI. In summary, EA alleviates PSCI by activating the mTOR pathway to inhibit NLRP3 activation. These findings may provide novel molecular targets and directions for the treatment of PSCI.
Acknowledgements
Not applicable.
Abbreviations
- Beclin-1
B-cell lymphoma-1
- ELISA
Enzyme-linked immunosorbent assay
- HE
Hematoxylin–eosin
- IL
Interleukin
- MCAO
Middle cerebral artery occlusion
- PSCI
Post-stroke cognitive impairment
- TTC
Triphenyltetrazolium chloride
Author contributions
Conception and design of the research: Jiawang Lang, Jianchang Luo, Boxu Lang; Acquisition of data: Luodan Wang, Wenbin Xu; Analysis and interpretation of data: Jie Jia, Zhipeng Zhao; Statistical analysis: Jiachen Lang; Obtaining funding: Jianchang Luo, Boxu Lang; Drafting the manuscript: Jiawang Lang, Jianchang Luo; Revision of manuscript for important intellectual content: Boxu Lang. Jiawang Lang and Jianchang Luo are contributed equally. All authors have read and approved the final manuscript.
Funding
This work was supported by [2022 Zhejiang provincial public welfare Technology Application Research Funded Project] [number LGF22H270001] and [Construction project of inheritance studio of famous and old TCM experts in Zhejiang Province in 2020] [number GZS2020049].
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was obtained from the Ethics Committee of XIAMEN UNIVERSITY (XMULAC20220034-23).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiawang Lang and Jianchang Luo have contributed equally to this work.
References
- 1.Xiang W, Wei H, Liang Z, Zhang M, Sun Z, Lv Y, et al. FLAIR vascular hyperintensity combined with asymmetrical prominent veins in acute anterior circulation ischemic stroke: prediction of collateral circulation and clinical outcome. Eur J Med Res. 2023;28(1):446. 10.1186/s40001-023-01445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji Y, Wang X, Zheng K, Jiang Y, Zhu H, Li S, et al. Incidence and influencing factors of post-stroke cognitive impairment in convalescent young patients with first-ever stroke. J Stroke Cerebrovasc Dis. 2024;33(1): 107511. 10.1016/j.jstrokecerebrovasdis.2023.107511. [DOI] [PubMed] [Google Scholar]
- 3.Milosevich ET, Moore MJ, Pendlebury ST, Demeyere N. Domain-specific cognitive impairment 6 months after stroke: the value of early cognitive screening. Int J Stroke. 2024;19(3):331–41. 10.1177/17474930231205787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn TJ, Richard E, Teuschl Y, Gattringer T, Hafdi M, O’Brien JT, et al. European stroke organisation and European academy of neurology joint guidelines on post-stroke cognitive impairment. Eur J Neurol. 2021;28(12):3883–920. 10.1111/ene.15068. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Chen F, Qin P, Zhao L, Li X, Han J, et al. Acupuncture treatment vs. cognitive rehabilitation for post-stroke cognitive impairment. A systematic review and meta-analysis of randomized controlled trials. Front Neurol. 2023;14:1035125. 10.3389/fneur.2023.1035125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xin Y, Zhou S, Chu T, Zhou Y, Xu A. Protective role of electroacupuncture against cognitive impairment in neurological diseases. Curr Neuropharmacol. 2024. 10.2174/1570159x22999240209102116. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Wang Z, Su Y, Qi L, Yang W, Fu M, et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 2021;598(7882):641–5. 10.1038/s41586-021-04001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Wang ZF, Su YS, Ray RS, Jing XH, Wang YQ, et al. Somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by electroacupuncture. Neuron. 2020;108(3):436-450.e437. 10.1016/j.neuron.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lina Q, Yinan S, Lianhong T, Yanshu J, Yongsheng Y. Efficacy of electroacupuncture stimulating Shenmen (HT7), Baihui (GV20), Sanyinjiao (SP6) on spatial learning and memory deficits in rats with insomnia induced by para-chlorophenylalanine: a single acupoint combined acupoints. J Tradit Chin Med. 2023;43(4):704–14. 10.19852/j.cnki.jtcm.20230308.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, Guo X, Zhang R, Cheng Y, Liu Y, Zeng D, et al. Discovering different acupoint combinations of manual or electro-acupuncture to treat chemotherapy-induced nausea and vomiting based on the complex networks analysis. Support Care Cancer. 2024;32(1):78. 10.1007/s00520-023-08289-y. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Han JY, Park GC, Lee JS. Effects of electroacupuncture combined with computer-based cognitive rehabilitation on mild cognitive impairment: study protocol for a pilot randomized controlled trial. Trials. 2019;20(1):478. 10.1186/s13063-019-3599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong T, Hao C, Shen J, Liu S, Yan S, Aslam MS, et al. Electroacupuncture ameliorates chronic unpredictable mild stress-induced depression-like behavior and cognitive impairment through suppressing oxidative stress and neuroinflammation in rats. Brain Res Bull. 2024;206:110838. 10.1016/j.brainresbull.2023.110838. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Lai L, Li X, Wang R, Fang X, Xu N, et al. Electroacupuncture ameliorates cognitive impairment by regulating γ-amino butyric acidergic interneurons in the hippocampus of 5 familial Alzheimer’s disease mice. Neuromodulation. 2024;27(4):730–41. 10.1016/j.neurom.2022.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Chavez LM, Huang SS, MacDonald I, Lin JG, Lee YC, Chen YH. Mechanisms of acupuncture therapy in ischemic stroke rehabilitation: a literature review of basic studies. Int J Mol Sci. 2017. 10.3390/ijms18112270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao R, Zong N, Hu Y, Chen Y, Xu Y. Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci Bull. 2022;38(10):1229–47. 10.1007/s12264-022-00859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing Y, Yang SD, Wang MM, Dong F, Feng YS, Zhang F. Electroacupuncture alleviated neuronal apoptosis following ischemic stroke in rats via midkine and ERK/JNK/p38 signaling pathway. J Mol Neurosci. 2018;66(1):26–36. 10.1007/s12031-018-1142-y. [DOI] [PubMed] [Google Scholar]
- 17.Tang B, Li Y, Xu X, Du G, Wang H. Electroacupuncture ameliorates neuronal injury by NLRP3/ASC/caspase-1 mediated pyroptosis in cerebral ischemia-reperfusion. Mol Neurobiol. 2024;61(4):2357–66. 10.1007/s12035-023-03712-1. [DOI] [PubMed] [Google Scholar]
- 18.Wang MM, Zhang M, Feng YS, Xing Y, Tan ZX, Li WB, et al. Electroacupuncture inhibits neuronal autophagy and apoptosis via the PI3K/AKT pathway following ischemic stroke. Front Cell Neurosci. 2020;14:134. 10.3389/fncel.2020.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang VW, Liu Y, Kim J, Shroyer KR, Bialkowska AB. Increased genetic instability and accelerated progression of colitis-associated colorectal cancer through intestinal epithelium–specific deletion of Klf4. Mol Cancer Res. 2019;17(1):165–76. 10.1158/1541-7786.Mcr-18-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuo X, Wu Y, Yang Y, Gao L, Qiao X, Chen T. Knockdown of LSD1 meliorates Ox-LDL-stimulated NLRP3 activation and inflammation by promoting autophagy via SESN2-mesiated PI3K/Akt/mTOR signaling pathway. Life Sci. 2019;233:116696. 10.1016/j.lfs.2019.116696. [DOI] [PubMed] [Google Scholar]
- 21.Dong B, Sun Y, Cheng B, Xue Y, Li W, Sun X. Activating transcription factor (ATF) 6 upregulates cystathionine β synthetase (CBS) expression and hydrogen sulfide (H(2)S) synthesis to ameliorate liver metabolic damage. Eur J Med Res. 2023;28(1):540. 10.1186/s40001-023-01520-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z, Xu N, Matei N, McBride DW, Ding Y, Liang H, et al. Sodium butyrate attenuated neuronal apoptosis via GPR41/Gβγ/PI3K/Akt pathway after MCAO in rats. J Cereb Blood Flow Metab. 2021;41(2):267–81. 10.1177/0271678x20910533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Chen S, Bai Y, Zhang Y, Li X, Wang Y, et al. Electroacupuncture improves cognitive impairment after ischemic stroke based on regulation of mitochondrial dynamics through SIRT1/PGC-1α pathway. Brain Res. 2024;1844:149139. 10.1016/j.brainres.2024.149139. [DOI] [PubMed] [Google Scholar]
- 24.Luo J, Lang J, Xu W, Wang L, Zhao Z, Jia J, et al. Electroacupuncture alleviates post-stroke cognitive impairment through inhibiting miR-135a-5p/mTOR/NLRP3 axis-mediated autophagy. Neuroscience. 2024;545:185–95. 10.1016/j.neuroscience.2024.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Ren W, Wang L, Mao L, Mazhar M, Zhou C, et al. Exploring the ferroptosis mechanism of Zhilong Huoxue Tongyu capsule for the treatment of intracerebral hemorrhage based on network pharmacology and in vivo validation. Evid Based Complement Alternat Med. 2022;2022:5033135. 10.1155/2022/5033135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sindhuja S, Amuthalakshmi S, Nalini CN. A review on PCR and POC-PCR—a boon in the diagnosis of covid 19. Curr Pharm Anal. 2022. 10.2174/1573412918666220509032754. [Google Scholar]
- 27.Kang JB, Koh PO. Retinoic acid alleviates the reduction of Akt and bad phosphorylation and regulates Bcl-2 family protein interactions in animal models of ischemic stroke. PLoS ONE. 2024;19(5): e0303213. 10.1371/journal.pone.0303213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li ZF, Zhang R, Zhao GR, Kuang TY. Electroacupuncture in the treatment of neurogenic urine retention through autophagy mediated by AMPK/mTOR pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47(4):488–96. 10.3390/antiox10101593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaywant A, Keenan A. Pathophysiology, assessment, and management of post-stroke cognitive impairment, depression, and fatigue. Phys Med Rehabil Clin N Am. 2024;35(2):463–78. 10.1016/j.pmr.2023.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Gallucci L, Sperber C, Guggisberg AG, Kaller CP, Heldner MR, Monsch AU, et al. Post-stroke cognitive impairment remains highly prevalent and disabling despite state-of-the-art stroke treatment. Int J Stroke. 2024;19(8):888–97. 10.1177/17474930241238637. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Li S, Pan Y, Wang M, Liao X, Shi J, et al. The effects of blood pressure on post stroke cognitive impairment: BP and PSCI. J Clin Hypertens (Greenwich). 2021;23(12):2100–5. 10.1111/jch.14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen D, Xiang Y, Wu D, Wang H, Huang Y, Xiao H. Electroacupuncture ameliorates neuronal damage and neurological deficits after cerebral ischemia-reperfusion injury via restoring telomerase reverse transcriptase. Cell Biochem Biophys. 2024. 10.1007/s12013-024-01504-5. [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ, Shin HK, Kim JH, Choi BT. Transcriptome analysis of the striatum of electroacupuncture-treated naïve and ischemic stroke mice. J Pharmacopunct. 2024;27(2):162–71. 10.3831/kpi.2024.27.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu XQ, Wang Y, Han W, Zhang LD, Zhang JY, Zhang GQ, et al. Effect of electroacupuncture pretreatment on ferroptosis in neurons of rats with cerebral ischemia-reperfusion injury. Zhen Ci Yan Jiu. 2023;48(8):754–63. 10.13702/j.1000-0607.20230148. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Zhang Q, Li M, Wang N, Li C, Song D, et al. Early post-stroke electroacupuncture promotes motor function recovery in post-ischemic rats by increasing the blood and brain irisin. Neuropsychiatr Dis Treat. 2021;17:695–702. 10.2147/NDT.S290148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chi X, Wang L, Liu H, Zhang Y, Shen W. Post-stroke cognitive impairment and synaptic plasticity: a review about the mechanisms and Chinese herbal drugs strategies. Front Neurosci. 2023;17:1123817. 10.3389/fnins.2023.1123817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang G, Tang X, Zhao F, Qin X, Wang F, Yang D, et al. Total saponins from Trilliumtschonoskii Maxim promote neurological recovery in model rats with post-stroke cognitive impairment. Front Pharmacol. 2023;14:1255560. 10.3389/fphar.2023.1255560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alsbrook DL, Di Napoli M, Bhatia K, Biller J, Andalib S, Hinduja A, et al. Neuroinflammation in acute ischemic and hemorrhagic stroke. Curr Neurol Neurosci Rep. 2023;23(8):407–31. 10.1007/s11910-023-01282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou Y, Yan Z, Wan H, Yang J, Ding Z, He Y. A combination of astragaloside IV and hydroxysafflor yellow A attenuates cerebral ischemia-reperfusion injury via NF-κB/NLRP3/caspase-1/GSDMD pathway. Brain Sci. 2024. 10.3390/brainsci14080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beccari S, Sierra-Torre V, Valero J, Pereira-Iglesias M, García-Zaballa M, Soria FN, et al. Microglial phagocytosis dysfunction in stroke is driven by energy depletion and induction of autophagy. Autophagy. 2023;19(7):1952–81. 10.1080/15548627.2023.2165313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Ye Q, Huang Z, Li X, Zhang L, Liu X, et al. BAG3 overexpression attenuates ischemic stroke injury by activating autophagy and inhibiting apoptosis. Stroke. 2023;54(8):2114–25. 10.1161/strokeaha.123.041783. [DOI] [PubMed] [Google Scholar]
- 42.Zheng Y, Wu Z, Yi F, Orange M, Yao M, Yang B, et al. By activating Akt/eNOS bilobalide B inhibits autophagy and promotes angiogenesis following focal cerebral ischemia reperfusion. Cell Physiol Biochem. 2018;47(2):604–16. 10.1159/000490016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.