Abstract
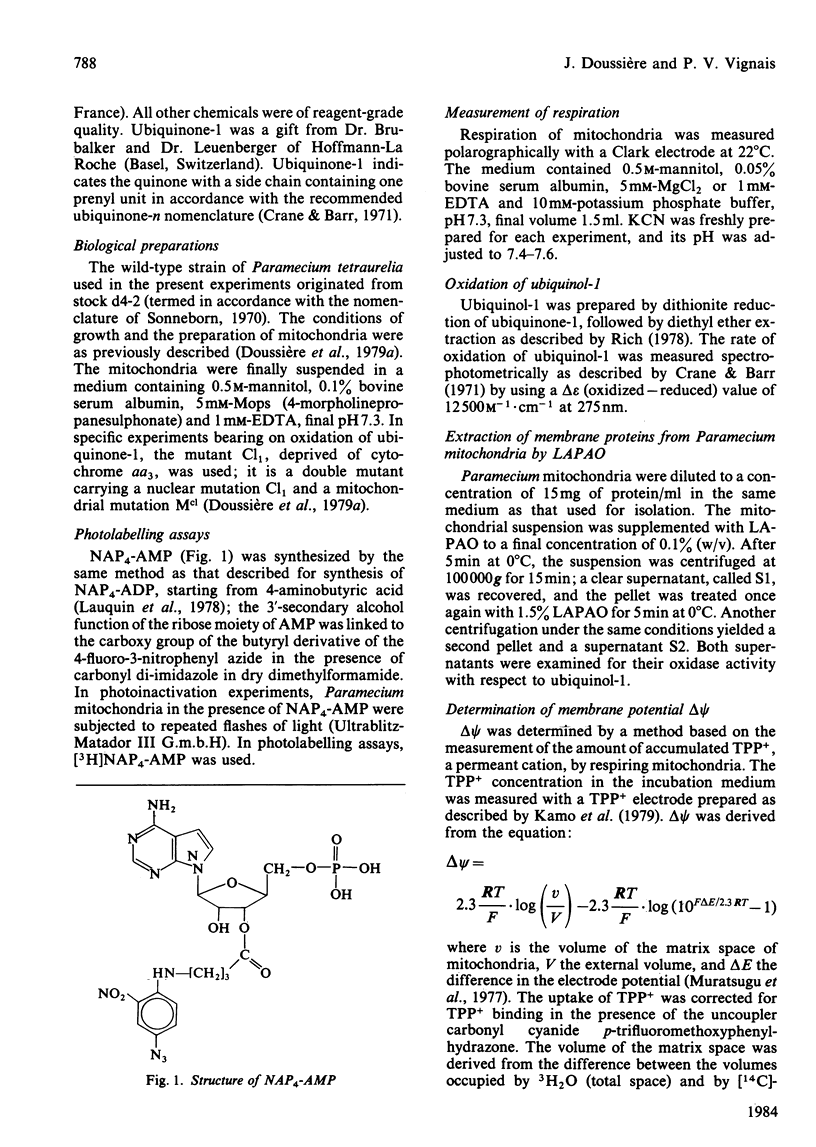
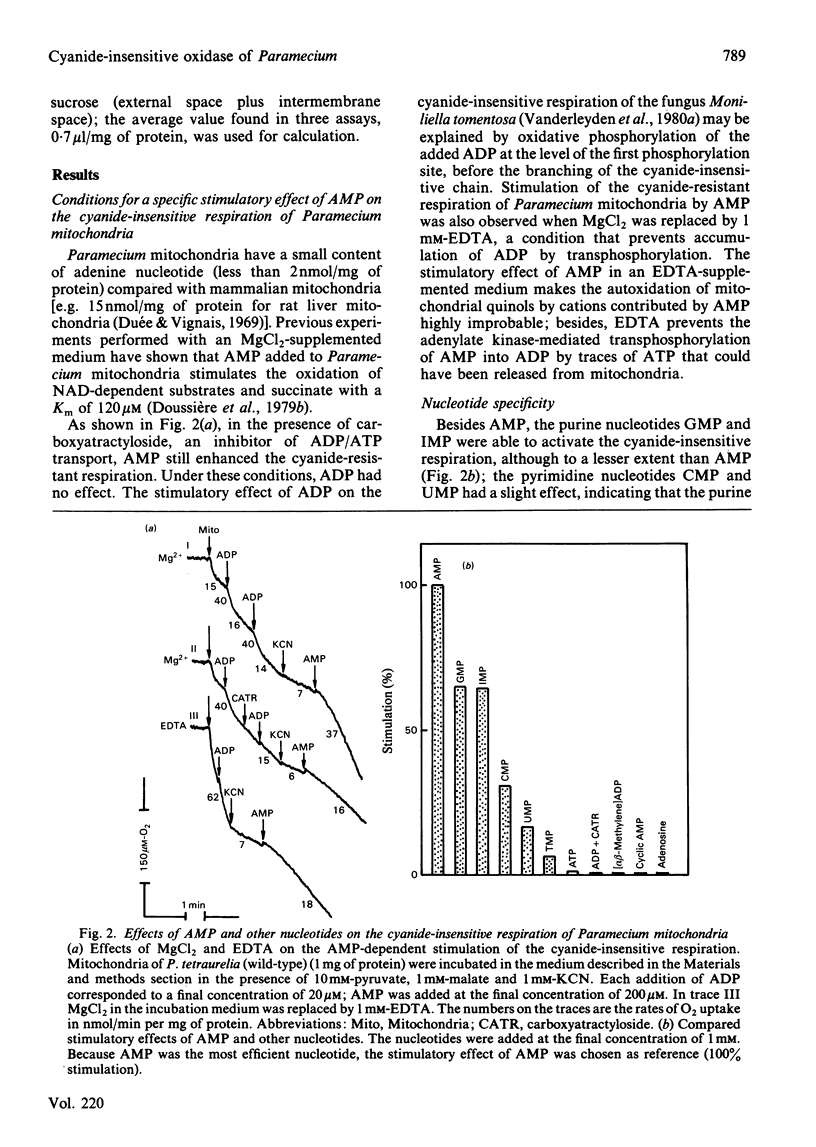
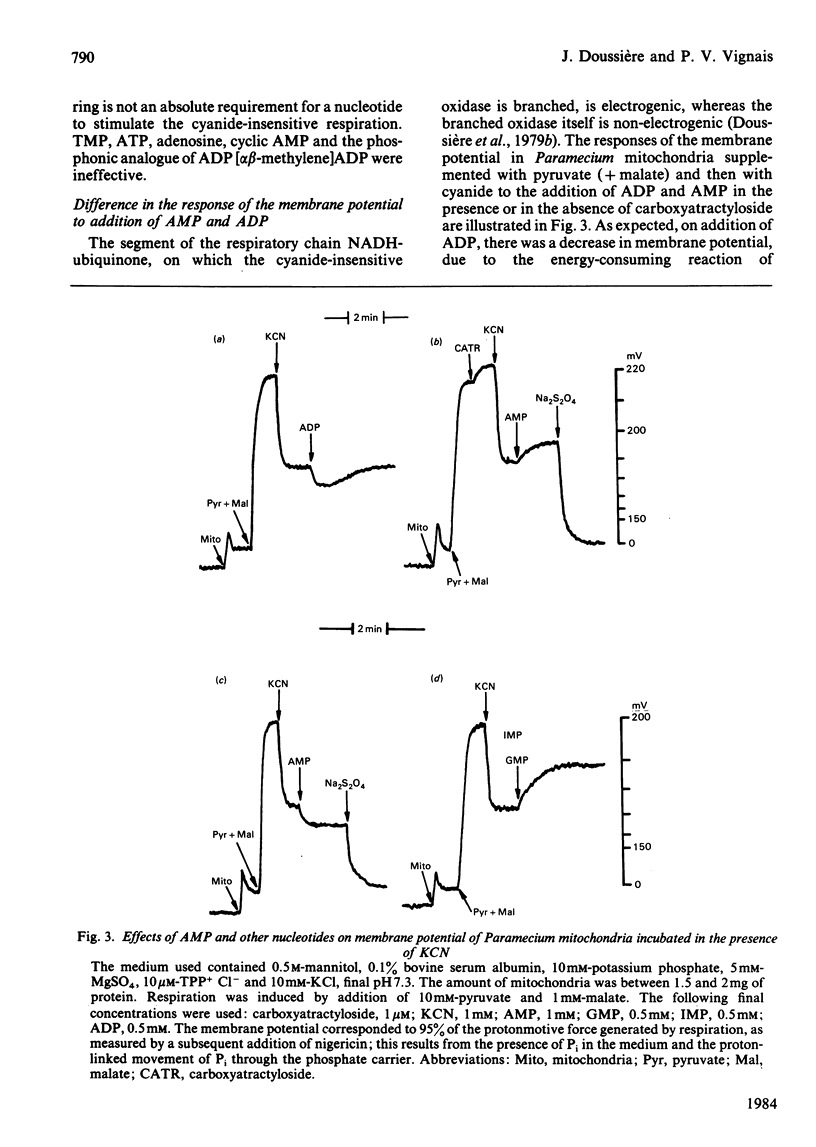
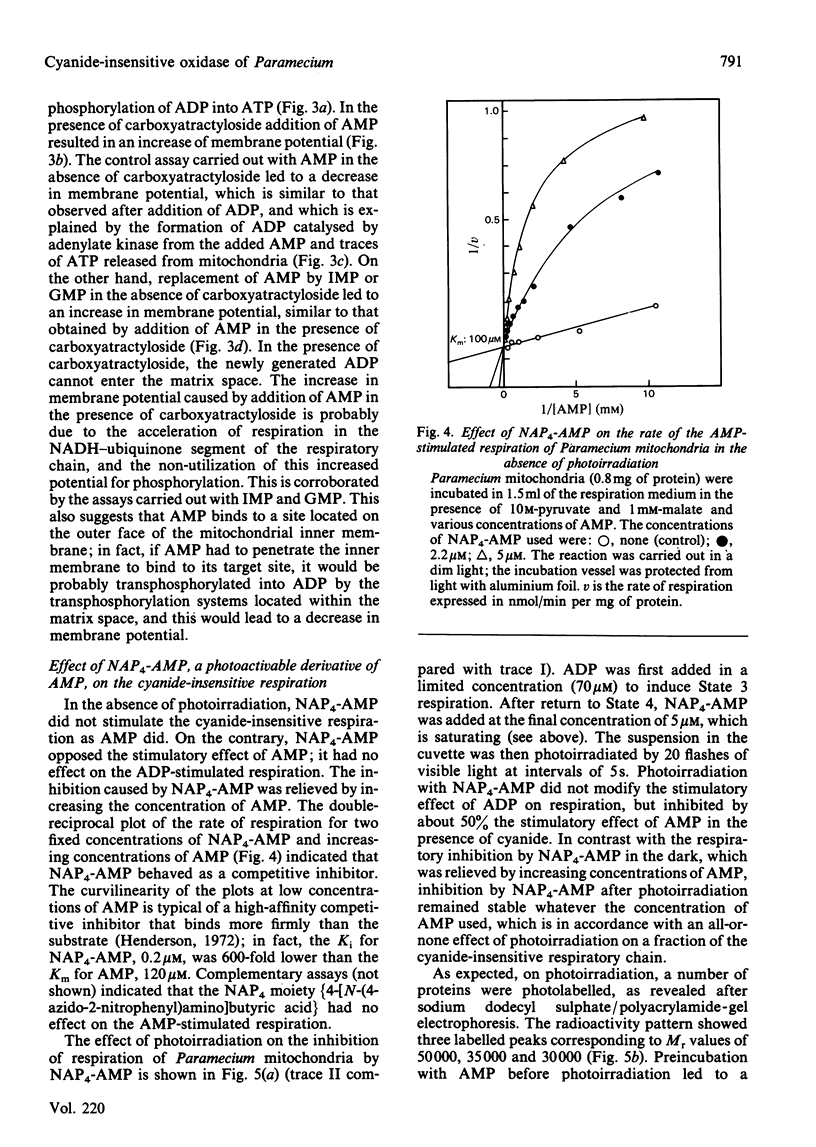
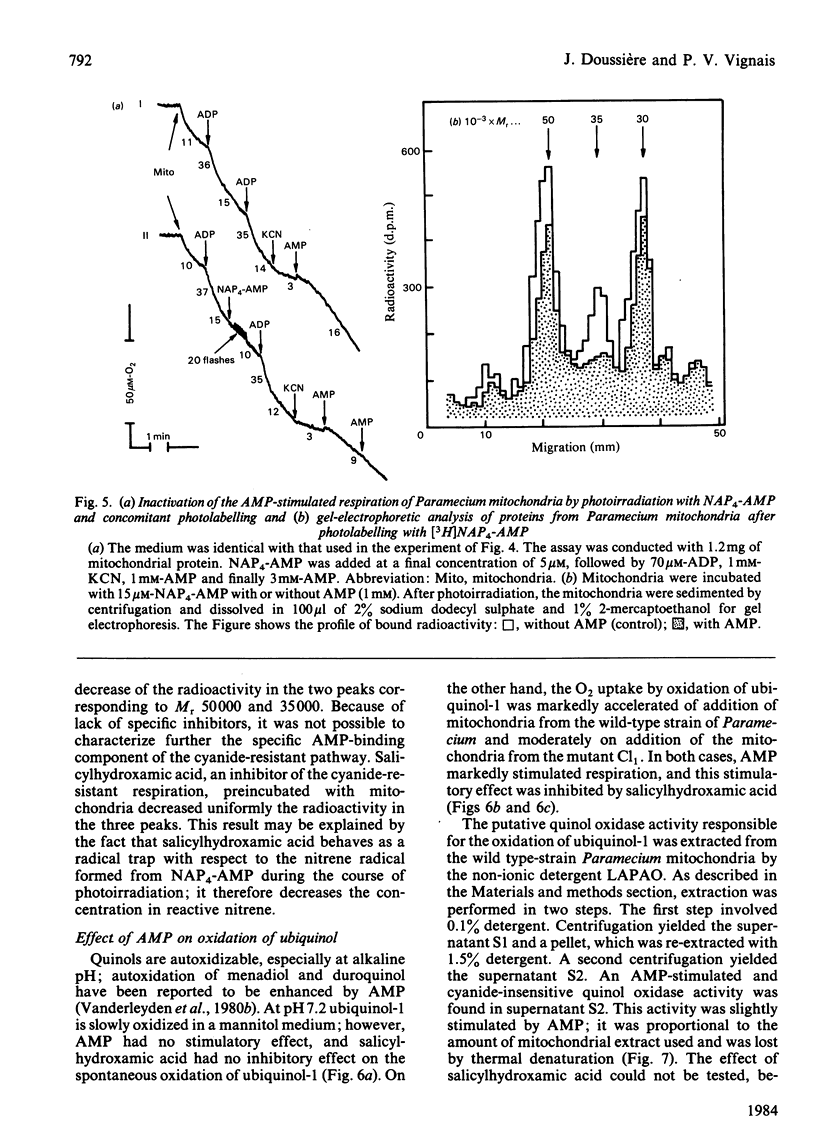
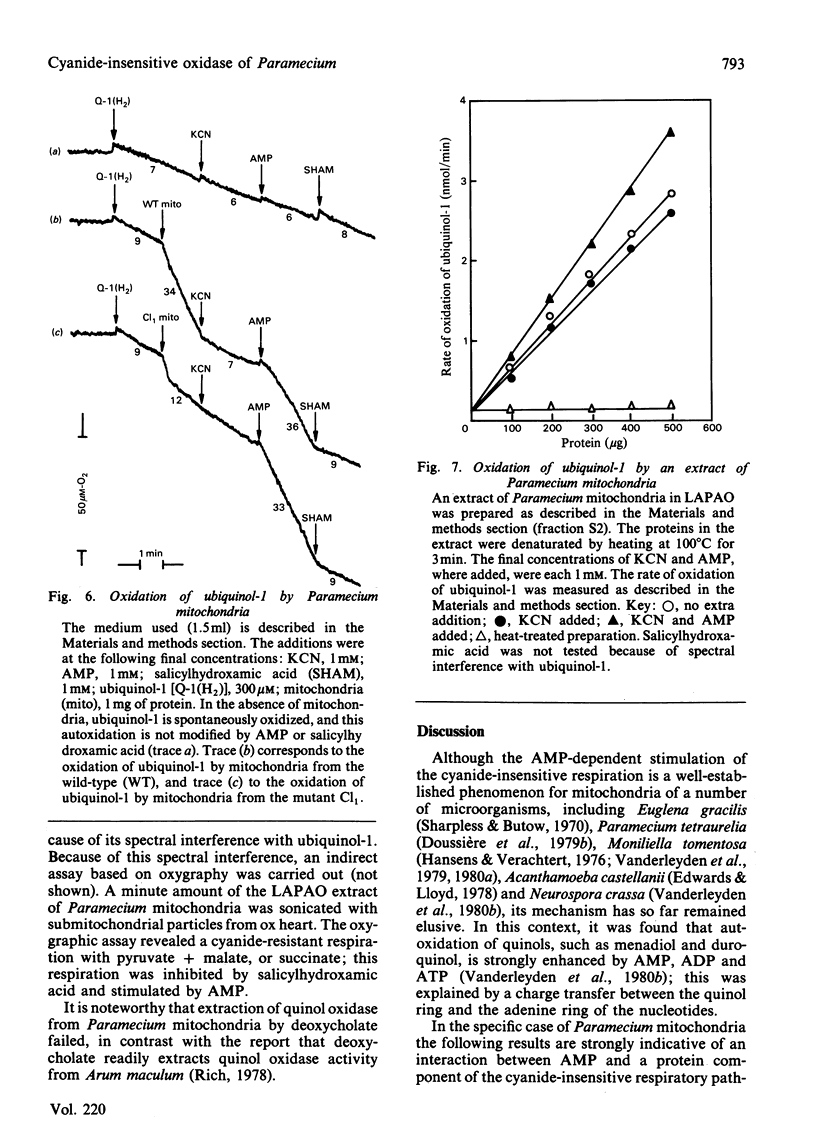
The AMP-dependent stimulation of the cyanide-insensitive respiration of Paramecium mitochondria was investigated. The nucleotides exhibiting a stimulatory effect on the cyanide-insensitive oxidation of pyruvate (+ malate) in a medium supplemented with EDTA or carboxyatractyloside were, in decreasing order of efficiency, AMP, GMP, IMP, UMP and TMP. On the other hand, ADP, ATP and cyclic AMP were ineffective. In the presence of carboxyatractyloside, addition of AMP to Paramecium mitochondria incubated with pyruvate (+malate) led to an increase in membrane potential. In the absence of light, the photoactivable derivative of AMP, 3'-[4-[N-(4-azido-2-nitrophenyl)amino]butyryl]-AMP (NAP4-AMP) added to Paramecium mitochondria opposed the stimulatory effect of AMP on the cyanide-insensitive respiration; the Ki for NAP4-AMP was much lower than the Km for AMP, 0.2 microM compared with 120 microM. The ADP-stimulated respiration was not affected. Photoirradiation of Paramecium mitochondria in the presence of NAP4-AMP resulted in irreversible inhibition of the AMP-stimulated cyanide-insensitive respiration. No effect on the ADP-stimulated respiration was observed. A heatlabile cyanide-insensitive ubiquinol oxidase was extracted from Paramecium mitochondria with the detergent NN-dimethyl-N-(3-laurylamidopropyl)amine oxide. The quinol oxidase activity was slightly stimulated by AMP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doussière J., Sainsard-Chanet A., Vignais P. V. The respiratory chain of Paramecium tetraurelia in wild type and the mutant Cl1. I. Spectral properties and redox potentials. Biochim Biophys Acta. 1979 Nov 8;548(2):224–235. doi: 10.1016/0005-2728(79)90131-2. [DOI] [PubMed] [Google Scholar]
- Doussière J., Sainsard-Chanet A., Vignais P. V. The respiratory chain of Paramecium tetraurelia in wild type and the mutant Cl1. II. Cyanide-insensitive respiration. Function and regulation. Biochim Biophys Acta. 1979 Nov 8;548(2):236–252. doi: 10.1016/0005-2728(79)90132-4. [DOI] [PubMed] [Google Scholar]
- Duée E. D., Vignais P. V. Kinetics and specificity of the adenine nucleotide translocation in rat liver mitochondria. J Biol Chem. 1969 Jul 25;244(14):3920–3931. [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. Properties of mitochondria isolated from cyanide-sensitive and cyanide-stimulated cultures of Acanthamoeba castellanii. Biochem J. 1978 Jul 15;174(1):203–211. doi: 10.1042/bj1740203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssens L., Verachtert H. Adenosine 5'-monophosphate-stimulated cyanide-insensitive respiration in mitochondria of Moniliella tomentosa. J Bacteriol. 1976 Mar;125(3):829–836. doi: 10.1128/jb.125.3.829-836.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J. A linear equation that describes the steady-state kinetics of enzymes and subcellular particles interacting with tightly bound inhibitors. Biochem J. 1972 Apr;127(2):321–333. doi: 10.1042/bj1270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo N., Muratsugu M., Hongoh R., Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979 Aug;49(2):105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Lauquin G. J., Brandolin G., Lunardi J., Vignais P. V. Photoaffinity labeling of the adenine nucleotide carrier in heart and yeast mitochondria by an arylazido ADP analog. Biochim Biophys Acta. 1978 Jan 11;501(1):10–19. doi: 10.1016/0005-2728(78)90091-9. [DOI] [PubMed] [Google Scholar]
- Muratsugu M., Kamo N., Kurihara K., Kobatake Y. Selective electrode for dibenzyl dimethyl ammonium cation as indicator of the membrane potential in biological systems. Biochim Biophys Acta. 1977 Feb 4;464(3):613–619. doi: 10.1016/0005-2736(77)90035-9. [DOI] [PubMed] [Google Scholar]
- Sharpless T. K., Butow R. A. An inducible alternate terminal oxidase in Euglena gracilis mitochondria. J Biol Chem. 1970 Jan 10;245(1):58–70. [PubMed] [Google Scholar]
- Vanderleyden J., Kurth J., Verachtert H. Characterization of cyanide-insensitive respiration in mitochondria and submitochondrial particles of Moniliella tomentosa. Biochem J. 1979 Aug 15;182(2):437–443. doi: 10.1042/bj1820437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderleyden J., Peeters C., Verachtert H., Bertrand H. Stimulation of the alternative oxidase of Neurospora crassa by Nucleoside phosphates. Biochem J. 1980 Apr 15;188(1):141–144. doi: 10.1042/bj1880141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderleyden J., Van Den Eynde E., Verachtert H. Nature of the effect of adenosine 5'-monophosphate on the cyanide-insensitive respiration in mitochondria of Moniliella tomentosa. Biochem J. 1980 Jan 15;186(1):309–316. doi: 10.1042/bj1860309. [DOI] [PMC free article] [PubMed] [Google Scholar]



