ABSTRACT
The endophyte is closely related to medicinal plant growth and development, stress resistance, and active ingredients’ accumulation. However, a seasonal succession of endophytes and the association with active ingredients is still unclear. In this study, we used high-throughput sequencing methods to compare the endophyte diversity of Rheum palmatum under different seasons and analyze the association between endophytes and five active ingredients. The results show that the diversity of endophytic fungi increased and then decreased, while bacterial diversity increased with the change of season. Community composition showed that the dominant genera of endophytic fungi were different under the different seasons, while the dominant genera of endophytic bacteria were Delftia. Analysis of co-occurrence network maps showed that the connectivity and complexity of endophytic fungi and bacterial networks decreased with the change of season. Spearman analysis indicated that the active ingredients of R. palmatum were significantly positive correlation with genera of endophytic fungi (Chalara). FUNGuild and PICRUSt predictive analysis indicated that the function of endophytic fungi and bacteria, respectively, were symbiotroph and metabolism, and relative abundances were different under the different seasons. Our results help elucidate the mechanism of medicinal plant–endophyte interaction.
IMPORTANCE
Through the investigation of the seasonal succession of endophytes and the association with active ingredients in Rheum palmatum, we found that the diversity and composition of endophytes in R. palmatum exhibited seasonal dynamics, and the active ingredients of R. palmatum showed a significantly positive correlation with the genus of endophytic fungi (Chalara). Our results may lay a foundation for understanding the interaction mechanism of endophyte and medicinal plant, and can also provide a theoretical basis for sustainable production of medicinal plants.
KEYWORDS: endophyte, seasonal succession, Rheum palmatum, correlation analysis, microbial network
INTRODUCTION
Endophytes inhabited the tissues of almost all plants without causing any disease symptoms (1). Endophytes play an important role in promoting plant growth and development, and stress tolerance, especially stimulating active ingredients of medicinal plants (2–5). Tao et al. isolated four strains of endophytic bacteria from Pairs polyphylla, which significantly increased the host’s biomass (6). Gao et al. reported that endophytic Paenibacillus polymyxa can promote P. ginseng growth, improve ginsenoside content, and decrease plant disease (7). Eid et al. found that endophytes also help to promote host plant growth, increase the absorption of nutrients, reduce the debilitating effects of diseases, and improve host resistance against environmental stresses via active ingredients’ accumulation (8). Therefore, analysis of the diversity and composition of endophytes could provide valuable resources for regulating the synthesis and accumulation of active ingredients in medicinal plants.
Many reports that the synthesis and accumulation of active ingredients of medicinal plants exist in seasonal dynamics (9, 10). Silva et al. found that the active ingredients of Vitex negundo are β-caryophyllene and cineole, which have the highest content in November and January, respectively (11). Wang et al. found that the best harvest season for the three Sedum medicinal species should be the full-bloom period between the end of April and the beginning of May (12). Liao et al. concluded that the shoots of Oxalis corymbosa may have good oxidation resistance during winter (13). Meanwhile, many previous works highlighted that seasonality is a key factor driving the environment, composition, and diversity of microorganisms (14–16). Such as temperature, light, and rainfall, all show obvious synergistic changes with the seasons, and directly or indirectly affect the structure, diversity, and function of microbial communities (17). However, studies on the seasonal succession of endophytes are still limited in medicinal plants.
Rheum palmatum is a perennial herb of the genus Rheum in the Polygonaceae family, which is a famous Traditional Chinese Medicines (TCM), mainly using dried roots as medicine. The active ingredients were mainly anthraquinones, including aloe-emodin, rhein, emodin, chrysophanol, and physcion (17). It can treat many diseases such as constipation, jaundice, and ulcers because of its excellent bioactivities (18). In previous work, we found that there were differences in endophyte diversity and community composition in different regions, ages, and tissue types of R. palmatum (19, 20) and that the active ingredients were positively correlated with the diversity of endophytic fungi (19). Meanwhile, we have isolated endophytic Trichoderma citrinoviride HT-1 from R. palmatum root, which can promote the growth, development, and accumulation of active ingredients (21). However, the seasonal succession of endophytes and the association with active ingredients of R. palmatum are still unknown. Therefore, we used amplicon sequencing to compare the endophyte diversity of R. palmatum under the different seasons and to analyze the association between endophyte and five active ingredients. These results may lay a foundation for understanding the interaction mechanism of endophyte and medicinal plant, and can also provide a theoretical basis for sustainable production of medicinal plants.
MATERIALS AND METHODS
Experimental materials
Three-year-old roots of R. palmatum were collected from Xuanshui, Lixian county, Gansu Province, China (104°77′89″, 32°85′26″,736 m) in different seasons (spring, Ar; summer, Br; and autumn, Cr). Three biological replicates were selected for uniformity based on size and weight. 0.5 g of root (principal root) of R. palmatum was collected from each season as a sample, which kept a 10–15 cm distance from the stem base. The samples were separated and washed with running tap water and then rinsed thrice with distilled water. To sterilize the surface of the plant parts, the root samples were successively immersed in 70% ethanol for 5 min, 2.5% sodium hypochlorite for 1–2 min, and 70% ethanol for 1 min, and then rinsed five times with sterile Millipore water. The last portion of the washing water was inoculated in potato dextrose agar (PDA) at 28°C for 10 days and nutrient agar (NA) at 37°C for 3 days to validate sterilization efficiency. All samples were stored at −80°C until DNA extraction.
DNA extraction, polymerase chain reaction (PCR) amplification, and sequence processing
The total genomic DNA was extracted from all samples by using the MOBIO Power-Soil Kit (MOBIO Laboratories, Inc., Carlsbad, CA, USA), according to the manufacturer’s instructions. The DNA extracts were analyzed for their concentration using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Model 2000, Waltham, MA, USA) and stored at −20°C for further PCR amplification. The PCR assays were performed in 20 µL mixture containing 4 µL of 5 × Fast Pfu buffer, 2 µL of 2.5 mM dNTPs, 0.8 µL of each primer 5 µM, 0.4 µL of FastPfu Polymerase, 10 ng of template DNA, and ddH2O. Bacterial 16S gene was amplified with primers 799F (5′-AACMGGATTAGATACCCKG-3′) and 1193R (5′-ACGTCATCCCCACCTTCC-3′). Amplification was performed under the following conditions: initial denaturation at 95°C for 3 min, 30 cycles at 95°C for 30 s, 52°C for 30 s, and 72°C for 45 s, and a final extension at 72°C for 5 min. The fungal ITS genes were amplified using the primers fITS7 (5′-GTGARTCATCGAATCTTTG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The PCR reactions were conducted using the following program: 3 min of denaturation at 95°C, 35 cycles of 30 s at 95°C, 30 s for annealing at 55°C, and 45 s for elongation at 72°C, and a final extension at 72°C for 10 min. The PCR products were analyzed by agarose gel electrophoresis. For each sample, three successful PCR products were pooled and purified using EasyPureTM PCR Clean up/Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions. Purified amplicons were sequenced on an Illumina NovaSeq platform for paired ends according to the standard protocols (22).
Active ingredients of R. palmatum quantitative analysis
Standard aloe-emodi, rhein, emodin, chrysophanol, and physcion were obtained from the Shanghai R&D Center for Standardization of TCM. High-performance liquid chromatography (HPLC)-ultrapure water, analytical-grade methanol, NaHCO3, and phosphoric acid were purchased from Sangon Biotech, Ltd. (Shanghai, China).
According to the Chinese Pharmacopoeia (Edition 2015) (23), the dried root of each season (spring, summer, and autumn) was pulverized and sieved through a 300 µm mesh. A total of 1.5 g of powder of each sample was precisely weighed, added to 10 mL 0.1% NaHCO3 aqueous solution, and treated with ultrasound (30–40°C, 700 W) for 20 min. Then, 40 mL methanol was added for ultrasonic treatment for 50 min. Filtrate was obtained by filtration of 0.22 µm Millipore filter unit, and 10 µL of sample solution was injected into HPLC for determination.
According to the method of Chen et al. (24), the samples were analyzed by HPLC (Waters) using C18 (4.6 × 250 mm, 5.0 µm, Waters E2695, Milford, MA, USA) at 30°C, and the content of metabolites was determined: The mobile phase was methanol −0.1% phosphoric acid (80:20). The flow rate was 1 mL·min−1. The detection wavelength was 254 nm.
Analysis of amplicon sequencing data
The data were analyzed by utilizing the QIIME pipeline, as previously performed in (25). Fungal and bacterial sequences were trimmed and assigned to each sample based on their barcodes. Operational taxonomic units (OTUs) at the species level by searching all sequences against the Silva bacterial 16S database (26). OTUs were classified at the species level by searching against the UNITE fungal database (27). Sequences were binned into OTUs at a 97% similarity level by using USEARCH software (http://drive5.com/uparse/, accessed on November 14, 2020) (26).
Statistical analysis
With QIIME software (Version 1.9.1), blast method (25) (http://qiime.org/scripts/assign_taxonomy.html) in the UNITE (version) database (https://unite.ut.ee/. PHP) (28) annotated the OTUs sequences of samples and analyzed the community composition of endophytes of R. palmatum under different seasons. QIIME (Version 1.9.1) was used to analyze the community diversity index of endophytes of R. palmatum in different seasons, including good coverage, Chao 1, Shannon, and other indicators (19). The stacked bar chart of species composition was used to represent the composition of various species. The visual distribution of the composition of each sample in phylum and genus classification levels was realized through statistical analysis of the feature table after the removal of singleton, and the analysis results were presented in the bar chart. Nonmetric multidimensional permutations analysis (NMDS) was used to analyze the discrepancies between the samples at the level of OTUs based on the Bray–Curtis distance. The correlation and visual analysis were carried out using packages such as vegan, ggplot2, psych, and WGCNA in R 3.6.3. Metabolic and ecologically relevant functions were annotated by PICRUSt for the 16S rDNA OTU and FUNGuild v1.0 for the ITS OUT (29).
Co-occurrence network analysis
OTUs with 50% samples were screened to construct the correlation matrix. The coexisting network was constructed with correlation coefficient R > 0.6 or <−0.6 and significance P < 0.05 as the threshold. The core flora was defined according to the degree, intermediate centrality, and compact centrality. Force Atlas2 layout algorithm of GePhi 0.9.2 (https://gephi.org/) was used to visualize the community network structure of endophytes in R. palmatum in different seasons based on a species-level matrix.
RESULTS
Surface-sterilization efficiency
The results showed that no colonies were found in the PDA and NA medium after a period of cultivation. It indicated that surface sterilization was effective and ready for subsequent experiments.
Analysis of sequencing data and alpha diversity
The results of the rarefaction curve can reveal the changes in species diversity and richness of samples with sequencing amount (28, 30). As shown in Fig. 1, the rarefaction curves of the samples based on the number of observed species tended to be stable with the increase in the amount of sequencing effort, which proved that sequencing data were gradually becoming reasonable.
Fig 1.
Rarefaction curves based on pyrosequencing of endophytic fungi (a) and bacteria (b) of root for each sample.
A total of 299,252 and 293,298 effective tags were obtained for the fungal and bacterial samples, respectively, after filtering and removing chimeric particles, with the library coverage of the samples being higher than 0.988, which indicates that the sequencing data confidently reflected the structure of the endophytic fungi and bacteria community of the samples. Alpha diversity indices (Chao1 and Shannon’s diversity index) presented differences among all samples of R. palmatum. Chao1 indicated a fungal and bacterial community richness trend of Br >Cr > Ar and Ar >Br > Cr, respectively. Shannon’s indicated the fungal and bacterial community richness trend of Br >Ar > Cr and Ar >Br > Cr (Table 1).
TABLE 1.
Community diversity of endophytes under different seasons samples
| Sample | Endophytic fungi | Endophytic bacteria | ||||||
|---|---|---|---|---|---|---|---|---|
| Effective tags | Shannon | Chao1 | Good-coverage | Effective tags | Shannon | Chao1 | Good-coverage | |
| Ar | 100,462 | 3.19 ± 0.09b | 121.03 ± 3.57b | 0.999 | 93,107 | 2.44 ± 0.78 a | 588.31 ± 59.89 a | 0.994 |
| Br | 99,537 | 3.51 ± 0.07 a | 193.33 ± 4.22 a | 0.999 | 103,018 | 1.23 ± 0.27b | 402.16 ± 56.76b | 0.995 |
| Cr | 99,253 | 2.91 ± 0.057 c | 124.07 ± 7.03b | 0.999 | 97,173 | 0.83 ± 0.14 c | 147.6 ± 42.67 c | 0.998 |
In all libraries, 10 fungal OTUs and 38 bacterial OTUs were shared under different seasons samples. The numbers of fungal OTUs that occurred only in spring roots, summer roots, and autumn roots samples were 216, 459, and 261, respectively, while the numbers of bacterial OTUs that occurred only in spring roots, summer roots, and autumn roots samples were 948, 509, and 137, respectively (Fig. 2).
Fig 2.
Venn diagram showing the fungal OTUs of different seasons samples (a) and bacterial OTUs of different seasons samples (b).
Community composition
The fungal OTUs were assigned into 10 phyla and 190 genera. The dominant fungal phylum across all of the samples was Ascomycota, with relative abundances ranging from 62.54% to 83.85% (Fig. 3a). At the genus level, Phialophora was the dominant genus in the sample of Ar and Br (28.29% and 34.24%, respectively), Nothodactylaria was the dominant genus in the sample of Cr (15.51%). (Fig. 3b).
Fig 3.
Relative abundances of the endophytic fungi at the phylum level (a), endophytic fungi at the genus level (b), endophytic bacteria at the phylum level (c), and endophytic bacteria at the genus level (d) for each sample. Relative abundances are based on the proportional frequencies of the DNA sequences that could be classified. “Other” represents the total relative abundance outside the top 10 maximum relative abundance levels.
The bacterial OTUs were assigned to 30 phyla and 307 genera. The dominant bacterial phylum across all of the samples was Proteobacteria, with relative abundances ranging from 49.89% to 69.94% (Fig. 3c). At the genus level, Delftia was the dominant genus in all samples, with relative abundances ranging from 8.94% to 36.20%. (Fig. 3d).
Each point in the NMDS plot represents a sample, and different colored points indicate different samples (groups). Since NMDS uses rank ordering, it can be approximated that the closer (far) the distance between two points, the smaller (greater) the difference in the microbial communities in the two samples.
NMDS analysis showed that the fungal and bacterial community composition was generally separated when comparing roots from different seasons (Fig. 4). This implied that the community structure of fungi and bacteria exists difference under different seasons.
Fig 4.
NMDS results of fungal (a) and bacterial (b) community compositions. The digital number represented three biological replicates for each sample.
Co-occurrence network analysis
To explore whether changes in microbial community assemblies were accompanied by changes in microbial interactions, we performed a co-occurrence network analysis and estimated the topological properties to uncover the complexity of potential associations and connections between endophytic microorganisms of the root under different seasons.
To compare how different seasons affect the complexity of endophytic fungi and bacteria of the root co-occurrence networks, six networks were constructed across the seasonal succession. Co-occurrence network analysis revealed that endophytic fungal and bacterial co-occurrence network connectivity and complexity decreased gradually with seasonal variation (Fig. 5). The endophytic fungi and bacteria of the root co-occurrence networks under different seasons followed different changing trajectories based on the network topological parameters. In addition, the network topology parameters also change significantly with the seasons. A total number of nodes (network size) strongly decreased with the succession of seasons, as did the total number of links and average degree (average links per node). Relative modularity (RM) is highest in autumn. Map density is highest in summer (Table 2).
Fig 5.
Co-occurrence networks under different seasons. Note: Co-occurrence networks of endophytic fungal in spring (a), co-occurrence networks of endophytic fungal in summer (b), co-occurrence networks of endophytic fungal in autumn (c), co-occurrence networks of endophytic bacterial in spring (d), co-occurrence networks of endophytic bacterial in summer (e), and co-occurrence networks of endophytic bacterial in autumn (f).
TABLE 2.
Topological properties of microbial networks
| Network topology parameter | Endophytic fungi | Endophytic bacteria | ||||
|---|---|---|---|---|---|---|
| Ar | Br | Cr | Ar | Br | Cr | |
| Total number of nodes | 63 | 54 | 43 | 162 | 71 | 34 |
| Total number of links | 759 | 628 | 287 | 4570 | 880 | 190 |
| Positive edges (>0.7 with P < 0.05) | 474 | 471 | 176 | 2420 | 464 | 113 |
| Negative edges (<−0.7 with P < 0.05) | 285 | 157 | 111 | 2150 | 416 | 77 |
| RM (relative modularity) | 0.418 | 0.504 | 0.665 | 0.543 | 0.541 | 0.59 |
| Average degree (average links per node) | 24.059 | 23.259 | 13.349 | 56.42 | 24.789 | 11.176 |
| Map density | 0.389 | 0.439 | 0.318 | 0.35 | 0.354 | 0.339 |
Correlation analysis between the endophyte and active ingredients of R. palmatum
Correlation analysis was performed based on the relative abundance of 10 dominant fungal and bacterial genera with the active ingredients of R. palmatum under different seasons. Correlation analysis showed that the contents of aloe-emodin, rhein, emodin, and chrysophanol were significantly positively correlated with the dominant endophytic fungal genus Chalara (Fig. 6a), while the contents of aloe-emodin, rhein, and emodin were significantly negatively correlated with the dominant endophytic genus bacterial Vibrio (Fig. 6b).
Fig 6.
Correlation analysis between metabolites and the top ten dominant genera of endophytic fungi (a), Correlation analysis between metabolites and the top ten dominant genera of endophytic bacteria (b). Note: A is aloe-emodin, B is rhein, C is emodin, D is chrysophanol, and E is physcion. “*”indicates the differences are significant at P < 0.05 and “**”indicates the differences are significant at P < 0.01.
Function prediction of PICRUSt and FUNGuild
FUNGuild was used to predict the nutritional and functional groups of the fungal communities with different samples. The prediction results of endophytes functional fungi relative abundance of R. palmatum based on FUNGuild database showed that seven trophic mode groups could be classified, including Pathotroph, Symbiotroph, Saprotroph, Pathotroph-Saprotroph–Symbiotroph, Pathotroph–Symbiotroph, Pathotroph–Saprotroph, and Saprotroph–Symbiotroph. The trophic mode of all samples was mainly symbiotroph, and their relative abundance first increased and then decreased with the change of season (Fig. 7a).
Fig 7.
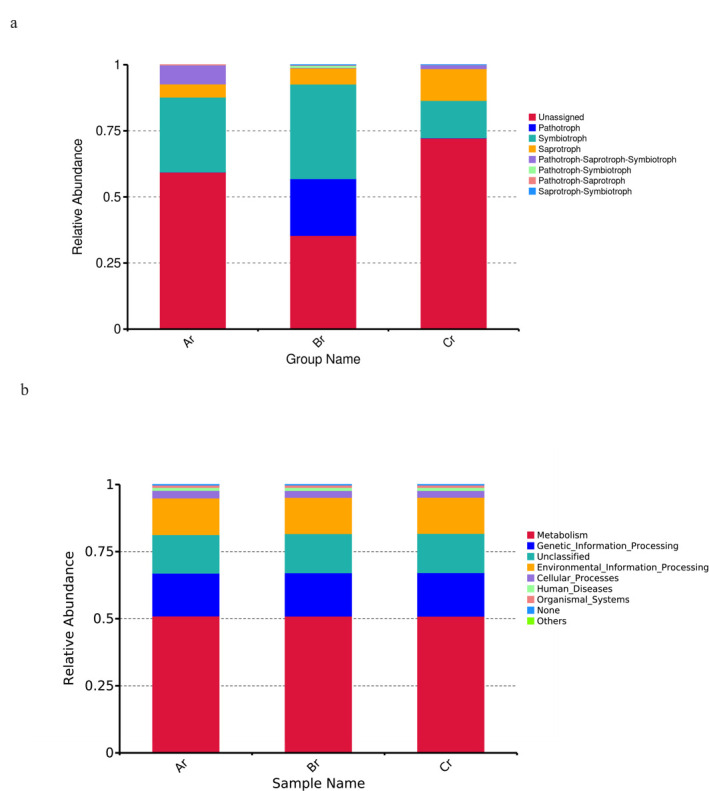
Relative abundance of predicted trophic mode of fungi (a) and relative abundance of predicted KEGG Orthologs functional profiles (KEGG level 1) of bacteria (b).
To study bacterial function, we used PICRUSt to perform bacterial function prediction analysis. Through comparisons with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, six categories of biological metabolic pathways (primary functional level) were obtained, which included Metabolism, Genetic_Information_Processing, Environmental_Information_Processing, Cellular_Processes, Human_Diseases, and Organismal_Systems. The metabolism pathway was the primary component in all samples, accounting for 50.95%–51.04% (Fig. 7b).
DISCUSSION
In this study, the results of alpha diversity showed that the Shannon index and Chao1 index of R. palmatum were different under different seasons. On the seasonal scale, the abundance and diversity of the bacterial community decreased gradually with the seasonal changes of R. palmatum, while the abundance and diversity of the fungal community increased first and then decreased. The results of community composition showed that the dominant fungal and bacterial phyla were Ascomycota and Proteobacteria, respectively. The relative abundance of dominant fungal phylum decreased with the change of seasons, while the relative abundance of dominant bacterial phylum increased with the change of seasons. Many studies also have shown that Ascomycota and Proteobacteria were the dominant phyla of fungal and bacterial endophytes in many plants (31–33). Both Proteobacteria and Ascomycota contain many functional strains related to denitrification and carbon and nitrogen cycling (34, 35), which indicates that the endophyte community of R. palmatum is mainly composed of beneficial microorganisms. At the genus level, the dominant genera of endophytes and their relative richness were different, which may be caused by differences in sunshine duration, average annual precipitation, and average annual temperature under different seasons (36, 37). In order to investigate the effects of seasonal changes on the differences between endophytic fungal and bacterial communities in R. palmatum, a co-occurrence network of microbial communities was constructed, and we found that endophytic fungal and bacterial co-occurrence network connectivity and complexity decreased gradually with seasonal variation.
To explore the correlation between endophytes and the active ingredients of R. palmatum, correlation analysis was performed based on the relative abundance of 10 dominant fungal and bacterial genera with the active ingredients of R. palmatum under different seasons. We found that active ingredients were significantly positively correlated with the dominant endophytic fungal genera (Chalara) and negatively correlated with the dominant endophytic bacterial genera (Vibrio), which was consistent with the results of previous studies. Chen et al. showed that active ingredients of R. palmatum were positively correlated with the diversity and abundance of endophytic fungi (20). Plant-related bioactive substances and plant-based medicines have been a part of traditional health care in most parts of the world. In the past, research on medicinal plants has mainly focused on their composition and pharmacological effects. However, with the rapid development of molecular technology, the microbiome composition and function of medicinal plants have received more and more attention. A large number of studies believe that the microbiome directly or indirectly affects the synthesis of pharmacologically active ingredients, which provides a new idea for a sustainable production of drug resources (38, 39).
FUNGuild is often applied to compare functions and to identify specific functional classifications in the study of fungi; it also can be used in research about fungal community (40). In this study, we found that the trophic mode of all samples was mainly symbiotroph, and their relative abundance increased first and then decreased with the change of season through function prediction of FUNGuild, which indicated that seasons had a great influence on the functional fungal groups of endophytes in R. palmatum. Although FUNGuild has been employed to analyze the function of fungi to a certain extent, the approach has certain limitations as it is based on existing literature and data. Hence, to study the function of endophytic fungi comprehensively, we ought to further investigate the classification and functional groups of soil fungi. PICRUSt analysis is capable of predicting the metabolic function of bacterial communities with high reliability, and it has been employed to investigate bacterial functions in numerous plants (41). We used high-throughput sequencing results for PICRUSt function prediction analysis, and the results show that metabolism was the major component in all samples. This result is similar to that obtained in a study of the function of the water-level fluctuation zone of the Danjiangkou reservoir in China during the dry period of rhizosphere bacteria by Chen et al. (42). Pepe-Ranney et al. (43) found that endophytes originate from the rhizosphere microbiome, resulting in similar outcomes. Due to the limitations of PICRUSt functional prediction analysis, we were unable to reveal how endophytes affect plant metabolism through functional prediction and so on. Although the functions of certain endophytic bacterial taxa remain unclear, they demonstrate significant potential and merit further investigation. In our works, the function of the relevant endophytic bacteria was only preliminarily predicted. In the next studies, further validation should be conducted using other methods such as metagenomics, which can help to better understand the endophytic bacterial function.
Conclusion
Our data suggest that endophytes diversity and composition of R. palmatum existed in seasonal dynamics, and the connectivity and complexity of endophyte co-occurrence network decreased with the change of seasons. The active ingredients of R. palmatum were significantly positively correlated with genera of endophytic fungi (Chalara), which may play an important role in the accumulation of active ingredients. Our results may lay a foundation for understanding the interaction mechanism of endophyte and medicinal plant, and can also provide a theoretical basis for sustainable production of medicinal plants.
ACKNOWLEDGMENTS
National Natural Science Foundation of China (82360745), Gansu Province Science and Technology Plan Project (23JRRA694), Gansu Province Science and Technology Plan project—Technology innovation guidance plan (23CXGA0031), Research Ability Improvement Program for Young Teachers of Northwest Normal University (grant no.NWNU-LKQN2023-20), Key Laboratory of Strategic Mineral Resources of the Upper Yellow River, Ministry of Natural Resources (No.YSMRKF202308), Gansu science and technology plan (23CXGA0045).
K.S. and D.C.: developed the concept, designed the lab experiments, and got financial support; N.L. and Y.M.: conducted lab experiments and wrote the article; and Y.H., L.Z., L.H., and X.L., Y.D.: modified paper.
Contributor Information
DaWei Chen, Email: gansudaweichen@126.com.
Kun Sun, Email: kunsun@nwnu.edu.cn.
Florian M. Freimoser, Agroscope, Nyon, Switzerland
DATA AVAILABILITY
All raw sequencing data have been submitted to the NCBI Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra) under the accession number (PRJNA1105668).
ETHICS APPROVAL
All authors agreed to participate in the study. All authors agreed to publish this article.
REFERENCES
- 1. Wani ZA, Mirza DN, Arora P, Riyaz-Ul-Hassan S. 2016. Molecular phylogeny, diversity, community structure, and plant growth promoting properties of fungal endophytes associated with the corms of saffron plant: an insight into the microbiome of Crocus sativus Linn. Fungal Biol 120:1509–1524. doi: 10.1016/j.funbio.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 2. Matsumoto H, Fan X, Wang Y, Kusstatscher P, Duan J, Wu S, Chen S, Qiao K, Wang Y, Ma B, Zhu G, Hashidoko Y, Berg G, Cernava T, Wang M. 2021. Bacterial seed endophyte shapes disease resistance in rice. Nat Plants 7:60–72. doi: 10.1038/s41477-020-00826-5 [DOI] [PubMed] [Google Scholar]
- 3. Lata R, Chowdhury S, Gond SK, White JF. 2018. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol 66:268–276. doi: 10.1111/lam.12855 [DOI] [PubMed] [Google Scholar]
- 4. Compant S, Saikkonen K, Mitter B, Campisano A, Mercado-Blanco J. 2016. Editorial special issue: soil, plants and endophytes. Plant Soil 405:1–11. doi: 10.1007/s11104-016-2927-9 [DOI] [Google Scholar]
- 5. Hou QZ, Chen DW, Wang YP, Ehmet N, Ma J, Sun K. 2022. Analysis of endophyte diversity of two Gentiana plants species and the association with secondary metabolite. BMC Microbiol 22:90. doi: 10.1186/s12866-022-02510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tao L, Qiuhong L, Fuqiang Y, Shuhui Z, Suohui T, Linyuan F. 2022. Plant growth-promoting activities of bacterial endophytes isolated from the medicinal plant Pairs polyphylla var. yunnanensis. World J Microbiol Biotechnol 38:15. doi: 10.1007/s11274-021-03194-0 [DOI] [PubMed] [Google Scholar]
- 7. Gao Y, Liu Q, Zang P, Li X, Ji Q, He Z, Zhao Y, Yang H, Zhao X, Zhang L. 2015. An endophytic bacterium isolated from Panax ginseng C.A. Meyer enhances growth, reduces morbidity, and stimulates ginsenoside biosynthesis. Phytochem Lett 11:132–138. doi: 10.1016/j.phytol.2014.12.007 [DOI] [Google Scholar]
- 8. Eid AM, Fouda A, Abdel-Rahman MA, Salem SS, Elsaied A, Oelmüller R, Hijri M, Bhowmik A, Elkelish A, Hassan S-D. 2021. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plants (Basel) 10:935. doi: 10.3390/plants10050935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Compant S, Samad A, Faist H, Sessitsch A. 2019. A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J Adv Res 19:29–37. doi: 10.1016/j.jare.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel PR, Patel ND, Patel SG, Kanaki NS, Patel AJ. 2020. Fingerprint analysis of vitex negundo by HPLC coupled with multi-components analysis. CPA 16:743–751. doi: 10.2174/1573412915666190312161325 [DOI] [Google Scholar]
- 11. Silva PT, Santos HS, Teixeira AMR, Bandeira PN, Holanda CL, Vale JPC, Menezes J, Pereira EJP, Rodrigues THS, Souza EB, Silva HC, Santiago GMP. 2019. Seasonal variation in the chemical composition and larvicidal activity against Aedes aegypti of essential oils from Vitex gardneriana Schauer. S Afr J Bot 124. doi: 10.1016/j.exppara.2022.108405 [DOI] [Google Scholar]
- 12. Wang L, Mei Q, Wan D. 2014. Simultaneous determination by HPLC of quercetin and kaempferol in three Sedum medicinal plants harvested in different seasons. J Chromatogr Sci 52:334–338. doi: 10.1093/chromsci/bmt035 [DOI] [PubMed] [Google Scholar]
- 13. Xian-Yan L, Hui-Min X, Peng F, Ya-Xi W, Jun-Yi H. 2019. Evaluation of Oxalis corymbosa extracts from different plant parts and seasons as a potential source of antioxidants. CTNR 17:50–55. doi: 10.37290/ctnr2641-452X.17:50-55 [DOI] [Google Scholar]
- 14. Kumar V, Prasher IB. 2022. Seasonal variation and tissues specificity of endophytic fungi of Dillenia indica L. and their extracellular enzymatic activity. Arch Microbiol 204:341–341. doi: 10.1007/s00203-022-02933-7 [DOI] [PubMed] [Google Scholar]
- 15. Sadeghi F, Samsampour D, Seyahooei MA, Bagheri A, Soltani J. 2019. Diversity and spatiotemporal distribution of fungal endophytes associated with Citrus reticulata cv. Siyahoo. Curr Microbiol 76:279–289. doi: 10.1007/s00284-019-01632-9 [DOI] [PubMed] [Google Scholar]
- 16. Shen SY, Fulthorpe R. 2015. Seasonal variation of bacterial endophytes in urban trees. Front Microbiol 6:427. doi: 10.3389/fmicb.2015.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu YC, Wu P, Li YB, Liu TC, Zhang L, Zhou YH. 2018. Natural deep eutectic solvents as new green solvents to extract anthraquinones from Rheum palmatum L. RSC Adv 8:15069–15077. doi: 10.1039/c7ra13581e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shang X, Dai L, He J, Yang X, Wang Y, Li B, Zhang J, Pan H, Gulnaz I. 2022. A high-value-added application of the stems of Rheum palmatum L. as a healthy food: the nutritional value, chemical composition, and anti-inflammatory and antioxidant activities. Food Funct 13:4901–4913. doi: 10.1039/d1fo04214a [DOI] [PubMed] [Google Scholar]
- 19. Usano-Alemany J, Herraiz-Peñalver D, Cuadrado J, Díaz S, Santa-Cruz M, Palá-Paúl J. 2012. Seasonal variation of the essential oils of Salvia lavandulifolia: antibacterial activity. J Essent Oil Bear Plants 15:195–203. doi: 10.1080/0972060X.2012.10644036 [DOI] [Google Scholar]
- 20. Chen DW, Jia LY, Hou QZ, Zhao X, Sun K. 2021. Analysis of endophyte diversity of Rheum palmatum from different production areas in Gansu province of China and the association with secondary metabolite. Microorganisms 9:978. doi: 10.3390/microorganisms9050978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hou QZ, Chen DW, Wang YP, Ehmet N, Ma J, Sun K. 2022. Analysis of endophyte diversity of Gentiana officinalis among different tissue types and ages and their association with four medicinal secondary metabolites. PeerJ 10:e13949. doi: 10.7717/peerj.13949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. doi: 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pharmacopoeia Committee of China (Ed.) . 2015. Chinese pharmacopoeia, p 23–25. Chemical Industry Publishing House, Beijing, China. [Google Scholar]
- 24. Chen C, Fu Z, Zhou W, Chen Q, Wang C, Xu L, Wang Z, Zhang H. 2020. Ionic liquid-immobilized NaY zeolite-based matrix solid phase dispersion for the extraction of active constituents in Rheum palmatum L. Microchem J 152:104245. doi: 10.1016/j.microc.2019.104245 [DOI] [Google Scholar]
- 25. Penton CR, Gupta VVSR, Yu J, Tiedje JM. 2016. Size matters: assessing optimum soil sample size for fungal and bacterial community structure analyses using high throughput sequencing of rRNA gene amplicons. Front Microbiol 7:824. doi: 10.3389/fmicb.2016.00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magoč T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mejía LC, Rojas EI, Maynard Z, Bael SV, Arnold AE, Hebbar P, Samuels GJ, Robbins N, Herre EA. 2008. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol Control 46:4–14. doi: 10.1016/j.biocontrol.2008.01.012 [DOI] [Google Scholar]
- 28. Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, et al. 2013. Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277. doi: 10.1111/mec.12481 [DOI] [PubMed] [Google Scholar]
- 29. Chen Y, Tian W, Shao Y, Li YJ, Lin LA, Zhang YJ, Han H, Chen ZJ. 2020. Miscanthus cultivation shapes rhizosphere microbial community structure and function as assessed by Illumina MiSeq sequencing combined with PICRUSt and FUNGUIld analyses. Arch Microbiol 202:1157–1171. doi: 10.1007/s00203-020-01830-1 [DOI] [PubMed] [Google Scholar]
- 30. Chi WC, Chen W, He CC, Guo SY, Cha HJ, Tsang LM, Ho TW, Pang KL. 2019. A highly diverse fungal community associated with leaves of the mangrove plant Acanthus ilicifolius var. xiamenensis revealed by isolation and metabarcoding analyses. PeerJ 7:e7293. doi: 10.7717/peerj.7293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dong L, Cheng R, Xiao L, Wei F, Wei G, Xu J, Wang Y, Guo X, Chen Z, Chen S. 2018. Diversity and composition of bacterial endophytes among plant parts of Panax notoginseng. Chin Med 13:41. doi: 10.1186/s13020-018-0198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Urumbil SK, Kumar MA. 2020. Diversity analysis of endophytic bacterial microflora in Emilia sonchifolia (linn.) DC on Illumina Mi Seq platforms. J Pure Appl Microbiol 14:679–687. doi: 10.22207/JPAM.14.1.70 [DOI] [Google Scholar]
- 33. Varanda CMR, Oliveira M, Materatski P, Landum M, Clara MIE, Félix M do R. 2016. Fungal endophytic communities associated to the phyllosphere of grapevine cultivars under different types of management. Fungal Biol 120:1525–1536. doi: 10.1016/j.funbio.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 34. Fierer N, Hamady M, Lauber CL, Knight R. 2008. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A 105:17994–17999. doi: 10.1073/pnas.0807920105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mothapo N, Chen HH, Cubeta MA, Grossman JM, Fuller F, Shi W. 2015. Phylogenetic, taxonomic and functional diversity of fungal denitrifiers and associated N2O production efficacy. Soil Biol Biochem 83:160–175. doi: 10.1016/j.soilbio.2015.02.001 [DOI] [Google Scholar]
- 36. Oita S, Ibáñez A, Lutzoni F, Miadlikowska J, Geml J, Lewis LA, Hom EFY, Carbone I, U’Ren JM, Arnold AE. 2021. Climate and seasonality drive the richness and composition of tropical fungal endophytes at a landscape scale. Commun Biol 4:313. doi: 10.1038/s42003-021-01826-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adhikary R, Mandal S, Mandal V. 2022. Seasonal variation imparts the shift in endophytic bacterial community between mango and its hemiparasites. Curr Microbiol 79:287–287. doi: 10.1007/s00284-022-02987-2 [DOI] [PubMed] [Google Scholar]
- 38. Pang Z, Chen J, Wang T, Gao C, Li Z, Guo L, Xu J, Cheng Y. 2021. Linking plant secondary metabolites and plant microbiomes: a review. Front Plant Sci 12:621276. doi: 10.3389/fpls.2021.621276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu M, Bai HY, Fu WQ, Sun K, Wang HW, Xu DL, Dai CC, Jia Y. 2021. Endophytic bacteria promote the quality of Lyophyllum decastes by improving non-volatile taste components of mycelia. Food Chem 336:127672. doi: 10.1016/j.foodchem.2020.127672 [DOI] [PubMed] [Google Scholar]
- 40. Martínez-Diz M del P, Andrés-Sodupe M, Bujanda R, Díaz-Losada E, Eichmeier A, Gramaje D. 2019. Soil-plant compartments affect fungal microbiome diversity and composition in grapevine. Fungal Ecol 41:234–244. doi: 10.1016/j.funeco.2019.07.003 [DOI] [Google Scholar]
- 41. Luo J, Tao Q, Wu K, Li J, Qian J, Liang Y, Yang X, Li T. 2017. Structural and functional variability in root-associated bacterial microbiomes of Cd/Zn hyperaccumulator Sedum alfredii. Appl Microbiol Biotechnol 101:7961–7976. doi: 10.1007/s00253-017-8469-0 [DOI] [PubMed] [Google Scholar]
- 42. Chen ZJ, Shao Y, Li YJ, Lin LA, Chen Y, Tian W, Li BL, Li YY. 2020. Rhizosphere bacterial community structure and predicted functional analysis in the water-level fluctuation zone of the Danjiangkou reservoir in China during the dry period. Int J Environ Res Public Health 17:1266. doi: 10.3390/ijerph17041266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pepe-Ranney C, Keyser C, Trimble JK, Bissinger B. 2020. Surveying the Sweetpotato rhizosphere, endophyte, and surrounding soil microbiomes at two North Carolina farms reveals underpinnings of Sweetpotato microbiome community assembly. Phytobiomes J 4:75–89. doi: 10.1094/PBIOMES-07-19-0038-R [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw sequencing data have been submitted to the NCBI Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra) under the accession number (PRJNA1105668).








