Abstract
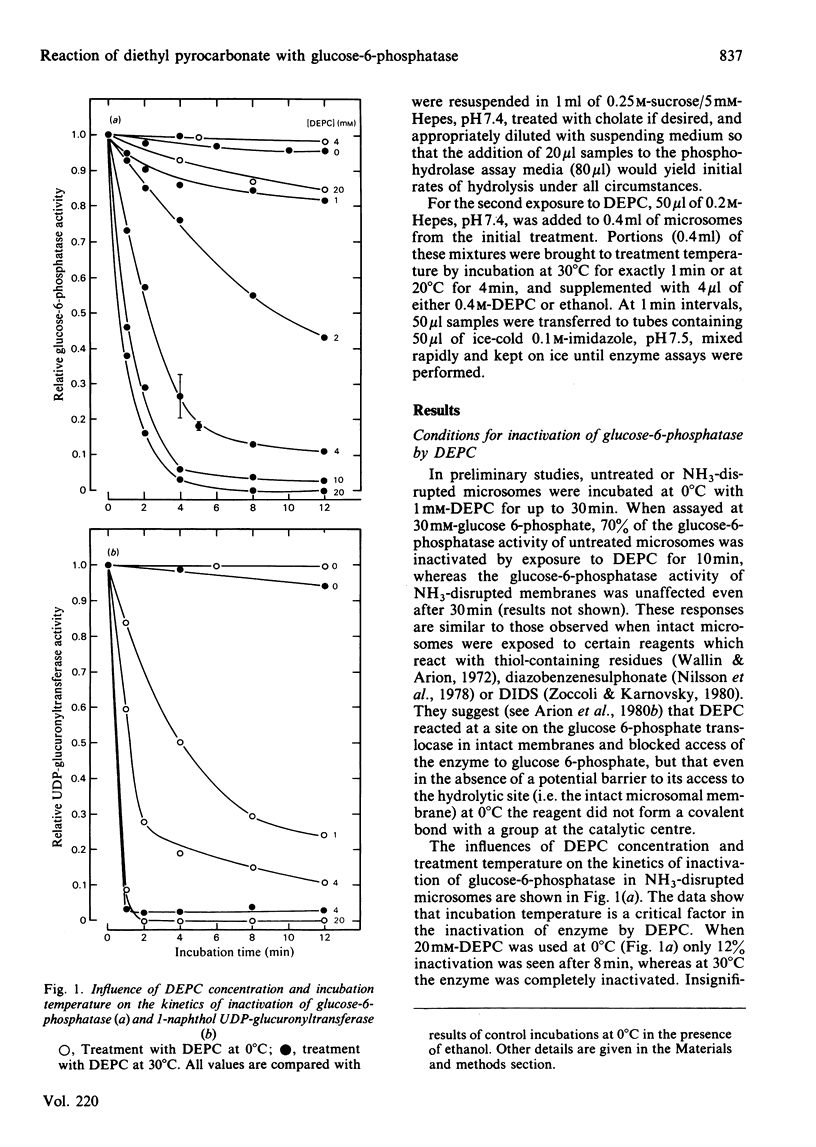
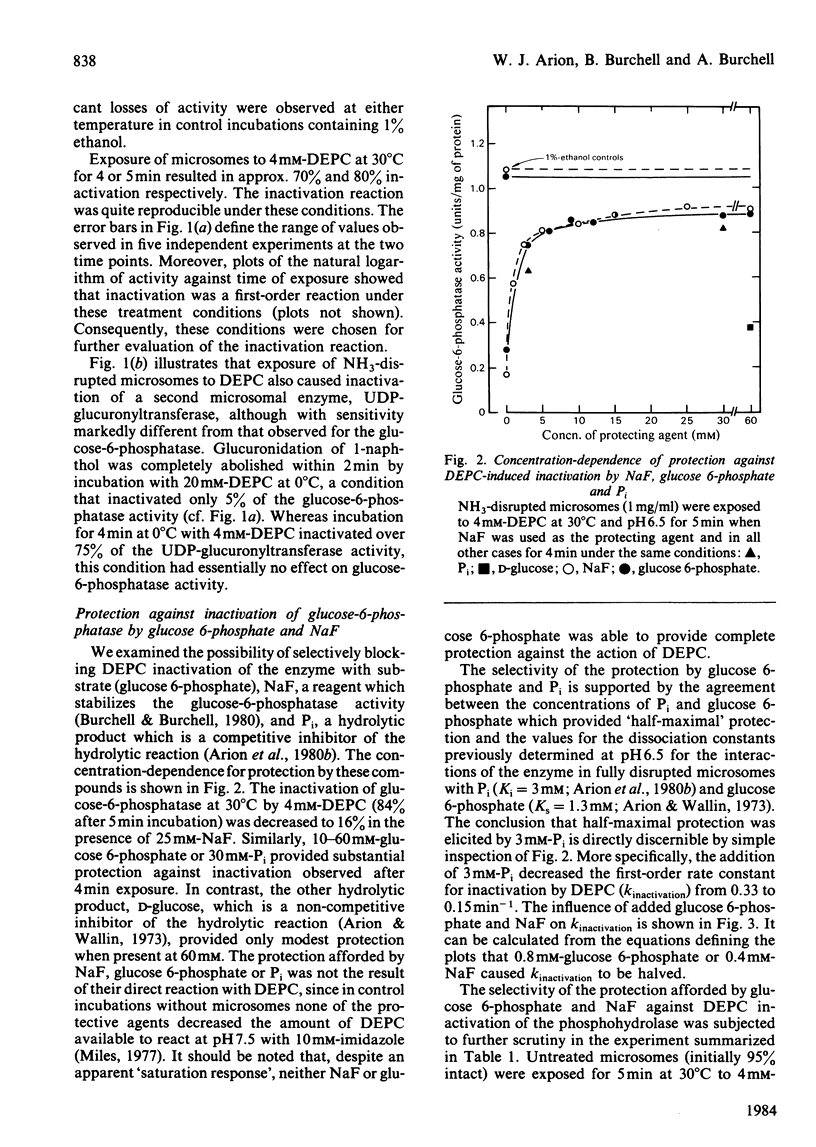
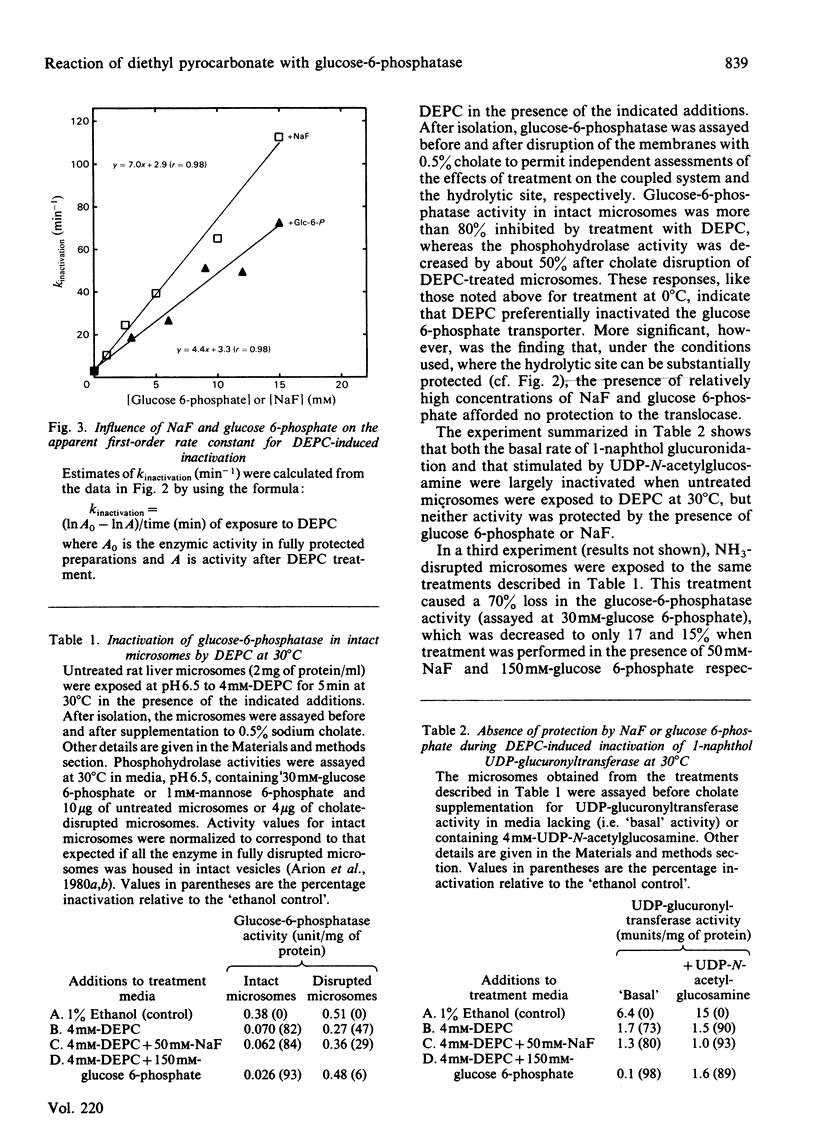
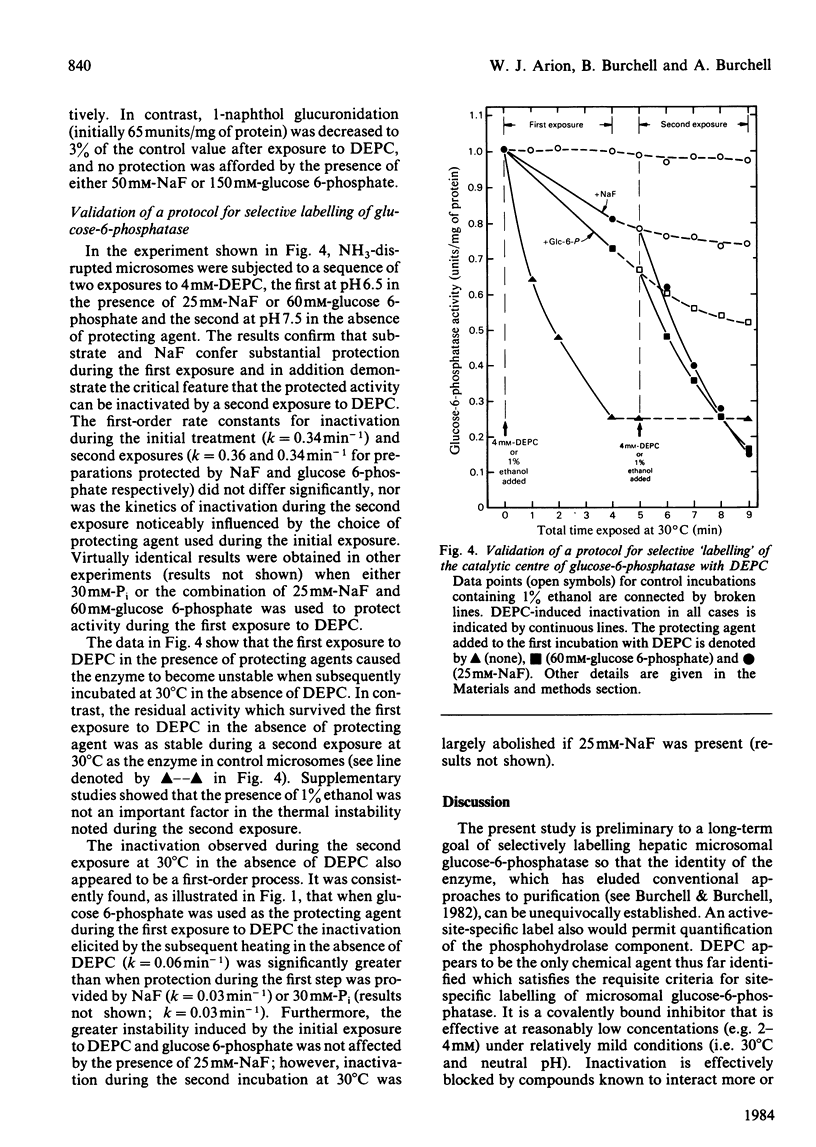
We have examined the interactions of the histidine-specific reagent diethyl pyrocarbonate (DEPC) with the components of the rat hepatic glucose-6-phosphatase system (EC 3.1.3.9). DEPC is the first known reagent that satisfies the criteria of an active-site-specific label for the phosphohydrolase component. (a) It inactivates through formation of a stable covalent bond. (b) It is effective at reasonably low concentrations (2-4 mM) under relatively mild conditions (e.g. 30 degrees C at neutral pH). (c) Inactivation is substantially blocked by glucose 6-phosphate, Pi and NaF, compounds which are known to interact quite specifically with the phosphohydrolase. (d) Under conditions where glucose 6-phosphate and NaF protect the enzyme, no protection is provided against DEPC-mediated inactivation of two other functional components of the membrane, the glucose 6-phosphate translocase and UDP-glucuronyltransferase. DEPC also shows potential for use at 0 degree C as a label for UDP-glucuronyltransferase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion W. J., Carlson P. W., Wallin B. K., Lange A. J. Modifications of hydrolytic and synthetic activities of liver microsomal glucose 6-phosphatase. J Biol Chem. 1972 Apr 25;247(8):2551–2557. [PubMed] [Google Scholar]
- Arion W. J., Lange A. J., Ballas L. M. Quantitative aspects of relationship between glucose 6-phosphate transport and hydrolysis for liver microsomal glucose-6-phosphatase system. Selective thermal inactivation of catalytic component in situ at acid pH. J Biol Chem. 1976 Nov 10;251(21):6784–6790. [PubMed] [Google Scholar]
- Arion W. J., Lange A. J., Walls H. E., Ballas L. M. Evidence for the participation of independent translocation for phosphate and glucose 6-phosphate in the microsomal glucose-6-phosphatase system. Interactions of the system with orthophosphate, inorganic pyrophosphate, and carbamyl phosphate. J Biol Chem. 1980 Nov 10;255(21):10396–10406. [PubMed] [Google Scholar]
- Arion W. J., Lange A. J., Walls H. E. Microsomal membrane integrity and the interactions of phlorizin with the glucose-6-phosphatase system. J Biol Chem. 1980 Nov 10;255(21):10387–10395. [PubMed] [Google Scholar]
- Arion W. J., Wallin B. K., Carlson P. W., Lange A. J. The specificity of glucose 6-phosphatase of intact liver microsomes. J Biol Chem. 1972 Apr 25;247(8):2558–2565. [PubMed] [Google Scholar]
- Arion W. J., Wallin B. K. Kinetics of the glucose 6-phosphate-glucose exchange activity and glucose inhibition of glucose 6-phosphatase of intact and disrupted rat liver microsomes. J Biol Chem. 1973 Apr 10;248(7):2372–2379. [PubMed] [Google Scholar]
- Arion W. J., Wallin B. K., Lange A. J., Ballas L. M. On the involvement of a glucose 6-phosphate transport system in the function of microsomal glucose 6-phosphatase. Mol Cell Biochem. 1975 Feb 28;6(2):75–83. doi: 10.1007/BF01732001. [DOI] [PubMed] [Google Scholar]
- Arion W. J., Walls H. E. The importance of membrane integrity in kinetic characterizations of the microsomal glucose-6-phosphatase system. J Biol Chem. 1982 Oct 10;257(19):11217–11220. [PubMed] [Google Scholar]
- BEAUFAY H., HERS H. G., BERTHET J., DE DUVE C. Le sustème hexose-phosphatasique. V. Influence de divers agents sur l'activité et la stabilité de la glucose-6-phosphatase. Bull Soc Chim Biol (Paris) 1954;36(11-12):1539–1550. [PubMed] [Google Scholar]
- Ballas L. M., Arion W. J. Measurement of glucose 6-phosphate penetration into liver microsomes. Confirmation of substrate transport in the glucose-6-phosphatase system. J Biol Chem. 1977 Dec 10;252(23):8512–8518. [PubMed] [Google Scholar]
- Bickerstaff G. F., Burchell B. Studies on the purification of glucose 6-phosphatase from rabbit liver microsomal fraction [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):389–390. doi: 10.1042/bst0080389. [DOI] [PubMed] [Google Scholar]
- Burchell A., Burchell B. Identification and purification of a liver microsomal glucose 6-phosphatase. Biochem J. 1982 Sep 1;205(3):567–573. doi: 10.1042/bj2050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell A., Burchell B. Stabilization of partially-purified glucose 6-phosphatase by fluoride. Is enzyme inactivation caused by dephosphorylation? FEBS Lett. 1980 Sep 8;118(2):180–184. doi: 10.1016/0014-5793(80)80214-6. [DOI] [PubMed] [Google Scholar]
- Feldman F., Butler L. G. Detection and characterization of the phosphorylated form of microsomal glucose-6-phosphatase. Biochem Biophys Res Commun. 1969 Jul 7;36(1):119–125. doi: 10.1016/0006-291x(69)90657-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange A. J., Arion W. J., Beaudet A. L. Type Ib glycogen storage disease is caused by a defect in the glucose-6-phosphate translocase of the microsomal glucose-6-phosphatase system. J Biol Chem. 1980 Sep 25;255(18):8381–8384. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Nilsson O. S., Arion W. J., Depierre J. W., Dallner G., Ernster L. Evidence for the involvement of a glucose-6-phosphate carrier in microsomal glucose-6-phosphatase activity. Eur J Biochem. 1978 Jan 16;82(2):627–634. doi: 10.1111/j.1432-1033.1978.tb12059.x. [DOI] [PubMed] [Google Scholar]
- Nilsson R., Peterson E., Dallner G. Permeability of microsomal membranes isolated from rat liver. J Cell Biol. 1973 Mar;56(3):762–776. doi: 10.1083/jcb.56.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlie R. C., Lygre D. G. The inhibition by citrate of inorganic pyrophosphate-glucose phosphotransferase and glucose 6-phosphatase. J Biol Chem. 1966 Jul 10;241(13):3136–3141. [PubMed] [Google Scholar]
- Nordlie R. C., Sukalski K. A., Muñoz J. M., Baldwin J. J. Type Ic, a novel glycogenosis. Underlying mechanism. J Biol Chem. 1983 Aug 25;258(16):9739–9744. [PubMed] [Google Scholar]
- Otani G., Abou-El-Makarem M. M., Bock K. W. UDP-glucuronyltransferase in perfused rat liver and in microsomes - III. Effects of galactosamine and carbon tetrachloride on the glucuronidation of 1-naphthol and bilirubin. Biochem Pharmacol. 1976 Jun 1;25(11):1293–1297. doi: 10.1016/0006-2952(76)90092-7. [DOI] [PubMed] [Google Scholar]
- Stetten M. R., Burnett F. F. Some properties of variously activated microsomal glucose-6-phosphatase, inorganic pyrophosphatase and inorganic pyrophosphate-glucose phosphotransferase. Shift in pH optimum. Biochim Biophys Acta. 1967 May 16;139(1):138–147. doi: 10.1016/0005-2744(67)90120-9. [DOI] [PubMed] [Google Scholar]
- Wallin B. K., Arion W. J. The requirement for membrane integrity in the inhibition of hepatic glucose 6-phosphatase by sulfhydryl reagents and taurocholate. Biochem Biophys Res Commun. 1972 Aug 7;48(3):694–699. doi: 10.1016/0006-291x(72)90404-4. [DOI] [PubMed] [Google Scholar]
- Zoccoli M. A., Hoopes R. R., Karnovsky M. L. Identification of a rat liver microsomal polypeptide involved in the transport of glucose 6-phosphate. Labeling with 4,4'-diisothiocyano-1,2-diphenyl[3H]ethane-2,2'-disulfonic acid. J Biol Chem. 1982 Apr 10;257(7):3919–3924. [PubMed] [Google Scholar]
- Zoccoli M. A., Karnovsky M. L. Effect of two inhibitors of anion transport on the hydrolysis of glucose 6-phosphate by rat liver microsomes. Covalent modification of the glucose 6-P transport component. J Biol Chem. 1980 Feb 10;255(3):1113–1119. [PubMed] [Google Scholar]


