Abstract
Objective
The hippocampus plays a critical role in cognitive networks. The anterior hippocampus is vulnerable to early‐life stress and socioeconomic status (SES) with alterations persisting beyond childhood. How SES modifies the relationship between early hippocampal development and cognition remains poorly understood. This study examined associations between SES, structural and functional development of neonatal hippocampus, and 18‐month cognition in very preterm neonates.
Methods
In total, 179 preterm neonates were followed prospectively. Structural and resting‐state functional MRI were obtained early‐in‐life and at term‐equivalent age (median 32.9 and 41.1 weeks post‐menstrual age) to calculate anterior and posterior hippocampal volumes and hippocampal functional connectivity strength. Eighteen‐month cognition was assessed via Bayley‐III. Longitudinal statistical analysis using generalized estimating equations, accounting for birth gestational age, post‐menstrual age at scan, sex, and motion, was performed.
Results
SES, measured as maternal education level, modified associations between anterior but not posterior hippocampal volumes and 18‐month cognition (interaction term p = 0.005), and between hippocampal connectivity and cognition (interaction term p = 0.05). Greater anterior hippocampal volumes and hippocampal connectivity were associated with higher cognitive scores only in the lowest SES group. Maternal education alone did not predict neonatal hippocampal volume from early‐in‐life and term.
Interpretation
SES modified the relationship between neonatal hippocampal development and 18‐month cognition in very preterm neonates. The lack of direct association between maternal education and neonatal hippocampal volumes indicates that socio‐environmental factors beyond the neonatal period contribute to modifying the relationship between hippocampal development and cognition. These findings point toward opportunities to more equitably promote optimal neurodevelopmental outcomes in very preterm infants.
Introduction
Impaired cognitive function of children are linked to social disparities.1, 2 Families of lower socioeconomic status (SES) experience multifaceted challenges and confront numerous stressors encompassing employment, safety, finances, and health.3, 4, 5 Heightened deprivation correlates with greater adverse health effects and more pronounced alterations in brain development.6, 7, 8, 9 Children with higher educated parents exhibit fewer mental health problems during stressful circumstances, highlighting the role of parental education in mitigating the effects of chronic stress on children.4, 10
The hippocampus is a key brain structure for cognition and is pivotal in chronic stress processing. 11 Early‐life adversities, including stress and environmental factors, affect the hippocampus in animal models and human studies. Children exposed to poverty, abuse, and low SES measured with job attainment and parental education exhibit smaller hippocampal volumes and altered functional networks.1, 9, 11, 12, 13, 14, 15, 16, 17 Clinical factors including younger gestational age (GA), bronchopulmonary dysplasia (BDP), as well as adverse events such as stress, neonatal pain, midazolam exposure, or brain injury were further linked to hippocampal development across ages.1, 11, 13, 17, 18 Hippocampal volumetric or functional alterations in response to early‐life adversities were accompanied by impaired neurodevelopmental outcomes.1, 9, 11, 12, 13, 14, 15, 16, 17
Healthy infants exhibit robust local connectivity between hippocampus and adjacent brain regions as early as 3 weeks of age. 19 Maturation involves the development of key functional networks resembling adult‐like networks within the first year of life. 19 Structural and functional alterations in the hippocampus of very preterm neonates emerging in childhood persist into adulthood, and are related to neurodevelopmental outcomes.17, 20, 21 Literature in children, adults, and animal models, describe a distinct vulnerability of the anterior hippocampus to stress.22, 23, 24 Regional specificity along the hippocampal long axis may stem from differences in microstructure, gene expression, and maturation processes.15, 22, 23, 24
Preterm neonates experience numerous procedures and stressors during neonatal intensive care, coinciding with rapid brain development.12, 25 Maturation of higher order networks including those for attention, emotion, and cognition, occurs during the third trimester, making preterm brains uniquely susceptible to early extrauterine exposures.26, 27, 28 For example, neonatal midazolam exposure is associated with altered neonatal hippocampal growth, impacting hippocampal volumes and working memory at 8 years of age. 17 Likewise, elevated prenatal maternal inflammatory response was recently shown to correlate with lower SES and to link immune dysfunction, pre/perinatal stress, SES, and brain development. 28 Finally, the complex interplay of genetic and environmental factors influencing SES–brain relations need to be acknowleged. 6
Few studies have assessed the development of hippocampal functional connectivity in preterm neonates. A study comparing very preterm neonates to in vivo fetuses found generalized alterations in brain functional connectivity and stronger connectivity in sensory and stress‐related areas including the hippocampus. 14 These findings are clinically relevant as in healthy term‐born neonates, hippocampal functional connectivity in the first year of life is linked to cognitive function in childhood. 19
Despite improved intensive care, preterm birth is linked to early and lifelong alterations in cognition, unveiling a particularly intricate interplay between SES and GA at birth, and their implications on alterations in neuroimaging and behavioral outcomes.29, 30, 31, 32, 33 Greater maternal social disadvantage and lower gestational age were shown to be associated with less favorable cognitive outcome at 15 years age. 32 A recent study examining alterations in brain structure in a neonatal cohort reported a stronger association between low birth GA and neonatal brain development, relative to SES. 33
Given the significant role of the hippocampus in cognitive brain networks, its vulnerability to early‐life adversity and stress‐sensitivity, and the high amount of stress exposures experienced by very preterm neonates, we sought to investigate how socioeconomic status, reflected by maternal education, (1) predicts anterior and posterior hippocampal volumes in preterm neonates, and (2) modifies the relationship between neonatal hippocampal volumes as well as functional connectivity with cognition at 18 months of age. Maternal education emerges as a robust indicator of SES within individual families and serves as a reliable SES proxy in preterm and other normative cohorts.2, 34, 35, 36, 37 In post hoc analysis, additional neighborhood‐level data, such as neighborhood environment and income, was examined complementing individual‐level SES assessments. Finally, we addressed whether biological sex, an important determinant of developmental outcomes in preterm neonates,29, 30 modifies the relationship between hippocampal volumes and functional connectivity with cognition.
Methods
Study population and clinical characteristics
This prospective cohort study enrolled 179 very preterm neonates (24–32 weeks' gestation). Between 2015 and 2019, 167 neonates were enrolled at The Hospital for Sick Children (SickKids) and Mount Sinai Hospital, both tertiary‐level neonatal intensive care units (NICU) in Toronto, Canada. Twelve neonates who were prospectively studied at SickKids (2013–2014) in the pilot phase of this project were also included. Neonates with TORCH infection, congenital malformation or genetic syndrome were excluded. The Research Ethics Boards of both hospitals reviewed and approved this study. Written informed consent was obtained from the parents/guardians.
Pregnancy, delivery, and neonatal course data were collected through daily chart review. Clinical characteristics were defined as previously described and have been shown to be associated with neurodevelopmental outcomes in very preterm neonates.2, 25, 29, 30
Socioeconomic status
Maternal education is considered a significant indicator of socioeconomic status,2, 4, 6, 34, 35, 38, 39 and is related to income, access to resources such as health care, educational environment, health, and nutrition.5, 40 Based on mother's highest level of education, preterm neonates were categorized into three groups: (1) high school, (2) college or undergraduate degree, and (3) postgraduate degree. 2 Maternal and paternal levels of education did not significantly differ in this cohort.
While maternal education was our primary measure for SES, the role of neighborhood environment was examined in post hoc analyses. Using the most detailed neighborhood information available in our cohort—the first three digits of postal code—we categorized families into rural versus urban population areas per Statistics Canada definition. Rural was defined as population areas <30,000 inhabitants (Statistics Canada definition for rural and small population centers), urban with ≥30,000 inhabitants (medium and large urban population centers). 41 Further, we investigated how neighborhood‐level income, as an additional SES dimension, influences the relationship between hippocampal development and cognitive outcome. Analyzing neighborhood‐based average income derived from families' postal codes using Statistics Canada 2016 Census data, 136 families were categorized relative to the provincial median household income ($74,287 in Ontario in 2015), with 48% above and 52% below this threshold. 42 Statistical analysis utilized GEE models as described below.
Magnetic resonance imaging studies
Preterm neonates underwent early‐in‐life (EIL) MRI scans as soon as they were clinically stable and again at term‐equivalent age (TEA). The MRIs were acquired with a designated neonatal head coil on a Siemens 3T scanner without sedation: 156 EIL (median post‐menstrual age [PMA] 32.9 weeks, IQR 31.8–34.3) and 150 term scans (median PMA 41.1 weeks, IQR 39.3–43.6) (Supplementary Material). During the study, a scanner upgrade from Tim Trio to Prisma Fit resulted in scans conducted with the Tim Trio system before January 2017 and with Prisma Fit thereafter.
Hippocampus segmentation and quantification
Segmentations of the left and right hippocampal volumes of each neonate were performed on the native 3D T1‐weighted image early‐in‐life and/or at term‐equivalent age using a well‐established method: In an initial step, the MAGeT–brain pipeline was used to obtain the hippocampal segmentations automatically. 43 To achieve the most accurate measurement of the hippocampal volumes, all automatically obtained hippocampal segmentations were manually reviewed and—in case of minor areas missing or being incorrected labeled—revised by an experienced rater (J.K.). For the revision process, we followed similar manual segmentation protocols that were described in our previous study.12, 43 The mean hippocampal volume of both hemispheres was calculated for each neonate and scan. Data from 154 neonates (140 EIL and 139 TEA scans) were included in subsequent volumetric analysis, excluding those without SES information or with severe motion artifacts (6 EIL and 2 TEA scans) (Fig. 1).
Figure 1.
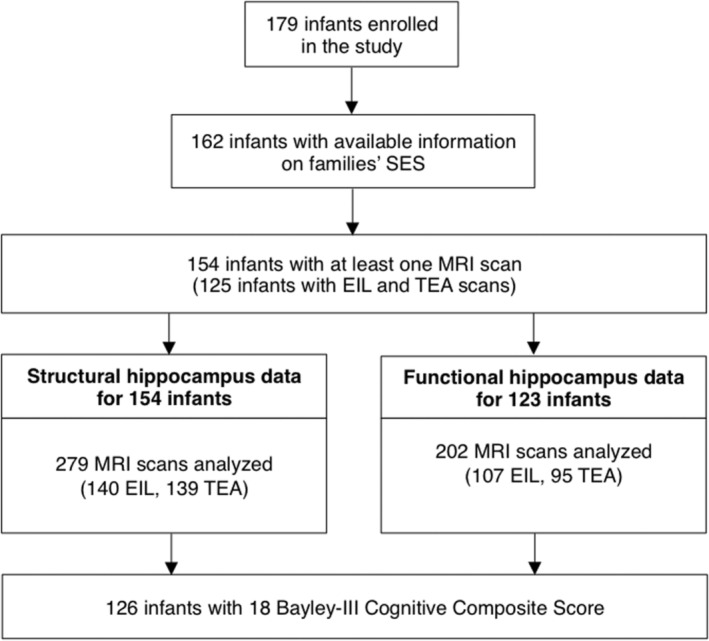
Cohort flowchart. Cohort flowchart—162 neonates with SES data were included in the final analysis; 126 infants had at least one MRI scan as well as an 18‐month Bayley‐III Cognitive Composite score.
Given the regional sensitivity of the anterior hippocampus to stress, 23 the hippocampus was further divided into anterior and posterior subregions, using the uncal apex of the parahippocampal gyrus as the landmark (Fig. 2), previously adopted in studies of adults and children (Supplementary Material). 23 For statistical analyses, each scan and patient was assigned one single value for mean anterior and one value for mean posterior hippocampal volumes.
Figure 2.
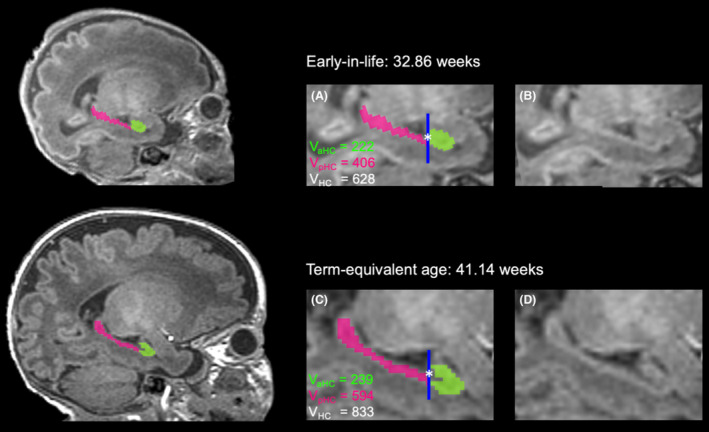
Subregional hippocampus segmentation at two time points. Sagittal T1‐weighted MR images showing hippocampus segmentation early‐in‐life (top row) and at term‐equivalent age (bottom row) for the same neonate. Manual segmentation of the anterior (green) and posterior (pink) hippocampus is shown in the full brain images (left), and in more detail with (A and C) and without (B and D) segmentation labels (right). The dark blue line marks the slice (*) where the hippocampus is divided (landmark structure: uncal apex), which is counted as the most posterior slice of the anterior hippocampus. Anterior, posterior, and total hippocampal volumes are calculated (VaHC, VpHC, and VHC). VaHC, volume anterior hippocampus (in mm3); VpHC, volume posterior hippocampus (in mm3); VHC, volume total hippocampus (in mm3).
Functional connectivity analyses
Functional connectivity data were preprocessed using the FMRIB Software Library (FSL; version 6.0.0) as outlined in the Supplement.44, 45 Each fMRI series was decomposed into components, consisting of a spatial map (Fig. 3) and a corresponding time course. Each independent component represents neuronal signal, structured noise, or a mixture of both. Identifying and removing structured noise components minimizes signal contributions from head motion, cerebral blood flow, cerebrospinal fluid, and white matter. Following established classification rules,26, 46 independent components were manually labeled as signal or noise. A conservative approach was followed to remove highly probable noise components while preserving maximum signal. Noise‐labeled components were regressed out.
Figure 3.
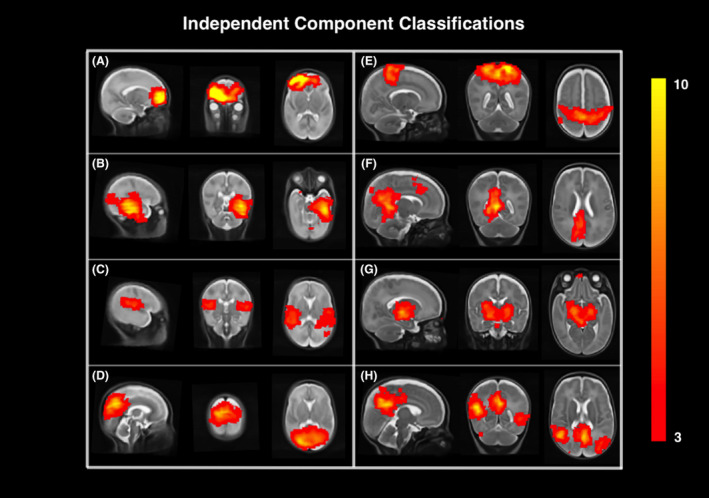
Spatial maps of independent component analysis. Representative spatial maps showing the independent component analysis results of resting‐state functional MRI data on sagittal, coronal, and axial slices of the early‐in‐life (A–D) and term‐equivalent age (E–H) T2‐weighted brain template. Labeled signal of neuronal activity networks are displayed in red to yellow colors, with the color bar demonstrating the image intensity threshold (z‐scored) on the right. (A) Frontal lobe, (B) temporal lobe, (C) auricular network, (D) parietooccipital area, (E) sensorimotor area, (F) posterior cingular cortex, (G) basal ganglia, (H) default mode network.
In a next step, anatomical labels were mapped to each subject's native fMRI space, serving as the foundation for functional connectivity analysis as elaborated in the Supplementary Material.47, 48, 49 Functional connectivity was defined as the absolute value of the Pearson correlation coefficient between two BOLD signals and was computed between each pair of anatomical regions for each fMRI scan. The resulting connection matrices or functional connectomes consisted of 90 cortical and subcortical regions and 4005 connections between them. Mean functional connectivity between the hippocampus (average of left and right) and every other brain region (mean whole brain), and that between the hippocampus and 16 important regions in cognitive networks (superior frontal gyrus, inferior frontal gyrus, insula, anterior cingulate gyrus, middle cingulate gyrus, posterior cingulate gyrus, parahippocampal gyrus, amygdala, superior parietal gyrus, inferior parietal lobule, precuneus, caudate, thalamus, superior temporal gyrus, middle/inferior temporal gyrus, and temporal pole) were assessed.14, 50 To account for residual effects of head motion (both translational and rotational) not removed during preprocessing, the average relative root‐mean‐squared motion between consecutive frames for each scan was included as a nuisance variable in statistical analyses. The mean relative motion values were 0.50 mm (EIL) and 0.51 mm (TEA), indicating minimal head movement throughout the scans. 51
Neurodevelopmental outcomes
Cognitive outcome at 18 months' corrected age were assessed by experienced staff at the Neonatal Follow‐up Programs using the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley‐III; normative mean of 100, SD 15). 52
Statistical analyses
Clinical characteristics and demographic variables across SES groups were compared using Fisher's exact test and Kruskal–Wallis test. Linear nonparametric test for trends was used to assess linear trends across ordered groups for hippocampal volumes by SES. Subregional hippocampal volumes of both hemispheres were not significantly different, thus the means of bilateral anterior and posterior hippocampal volumes were used in subsequent statistical analyses.
Associations between SES and neonatal anterior and posterior hippocampal volumes as well as hippocampal functional connectivity from EIL and TEA were longitudinally assessed using generalized estimating equations (GEE). These equations effectively account for repeated measures and potential correlations in longitudinal analysis within the cohort while controlling for covariates. Associations between cognitive outcome and (1) anterior/posterior hippocampal volumes with an interaction term of SES, and (2) mean hippocampus to whole brain functional connectivity with an interaction term of SES were analyzed with GEE as well. Taking into account key covariates for cognitive development in preterm neonates,29, 53 models were adjusted for birth GA, PMA at scan, and sex. For connectivity analysis, a motion metric was included. For analysis involving SES, the reference measure was SES group 1 (high school degree). Similar models were used to assess the role of neighborhood environment and neighborhood income in modifying associations between hippocampal development and cognitive outcome.
To assess whether sex modified the relationship between hippocampal development and neurodevelopmental outcomes, GEE was used to examine the associations between cognitive scores with (1) anterior/posterior hippocampal volumes, and with (2) mean hippocampal to whole brain functional connectivity. Both models included an interaction term of sex to test effect modification, and adjusted GA at birth, SES, PMA at scan, and motion metrics (for functional connectivity analysis). The main indicator for SES was maternal level of education. For volumetric hippocampal analysis, primary metrics included anterior and posterior hippocampal volumes. Functional analysis focused on examining the functional connectivity between the hippocampus and the whole brain. In the analyses of anterior/posterior hippocampal volumes, p < 0.025 (p < 0.05 for interaction term) was considered significant, while in functional connectivity analyses p < 0.05 (p < 0.1 for interaction term) denoted significance.
R statistical software package (version 2021.09.0) and Stata software (version 17.0, StataCorp, College Station, TX) were used for statistical analysis. GEE models and Stata codes will be provided on request.
Results
Clinical characteristics
Clinical neonatal and maternal antenatal characteristics, neonatal hippocampal volumes, and cognitive outcome at 18 months by maternal level of education are presented in Table 1. Birth GA, birth weight, maternal age at birth, and BPD differed between SES groups (p = 0.02, p = 0.03, p = 0.03, and p = 0.04). Including BPD as a covariate did not meaningfully change results. Table 2 outlines characteristics for infants with and without 18‐month cognitive scores.
Table 1.
Clinical neonatal and maternal antenatal characteristics, neonatal hippocampal volumes and 18‐month cognitive outcome by maternal level of education.
| High school | College/undergraduate | Postgraduate | p Value | |
|---|---|---|---|---|
| Clinical characteristics | n = 32 | n = 114 | n = 16 | – |
| Gestational age at birth (weeks), median (IQR) | 26.9 (25.9–28.8) | 26.7 (25.0–28.9) | 29.7 (27.4–30.4) | 0.016* |
| Birth weight (g), median (IQR) | 865 (660–1140) | 890 (700–1200) | 1200 (915–1555) | 0.026* |
| Male, no (%) | 12 (37.5) | 66 (57.9) | 11 (68.8) | 0.06 |
| Small for gestational age, no (%) | 4 (12.5) | 13 (11.4) | 3 (18.8) | 0.63 |
| APGAR score (5 min), median (IQR) | 7.5 (6.5–9) | 8 (6–9) | 8 (5–9) | 0.95 |
| Culture positive infection, no (%) | 8 (25) | 36 (31.6) | 4 (25) | 0.79 |
| Bronchopulmonary dysplasia, no (%) | 5 (15.6) | 45 (39.5) | 5 (31.3) | 0.038* |
| Major surgeries, no (%) | 7 (21.9) | 19 (16.7) | 0 | 0.13 |
| Retinopathy of prematurity, no (%) | 5 (15.6) | 5 (4.4) | 0 | 0.07 |
| Cumulative midazolam dose, mean (min–max) | 0 | 0.007 (0–0.18) | 0 | 0.87 |
| Maternal characteristics | ||||
| Maternal age, median (IQR) | 33 (25.5–36) | 33 (29–36) | 35 (33–40) | 0.026* |
| Maternal BMI, median (IQR) | 31.0 (27.7–35.9) | 26.9 (23.7–32.7) | 32.4 (26.3–33.4) | 0.16 |
| Maternal hypertension, no (%) | 3/29 (10.3) | 25/111 (22.5) | 5/16 (31.3) | 0.20 |
| Maternal diabetes, no (%) | 2 /31 (6.5) | 10/111 (9.0) | 1/16 (6.3) | 1.0 |
| Hippocampal volumes at early‐in‐life | n = 28 | n = 96 | n = 15 | – |
| Post‐menstrual age at scan (weeks), median (IQR) | 32.4 (31.4–33.9) | 33.0 (31.9–34.4) | 33.0 (31.9–34.7) | 0.23 |
| Mean hippocampal volume anterior (mm3) | 226.5 (192.5–269) | 226.5 (186.5–262.5) | 234.8 (185–298) | 0.95 |
| Mean hippocampal volume posterior (mm3) | 345 (309.5–400) | 367.5 (321.5–424) | 367.5 (314–401.5) | 0.76 |
| Mean hippocampal volume total (mm3) | 582 (525–633) | 592 (528–656) | 598.8 (493.5–686) | 0.91 |
| Hippocampal volumes at term‐equivalent age | n = 28 | n = 96 | n = 15 | – |
| Post‐menstrual age at scan (weeks), median (IQR) | 41.7 (39.1–43.7) | 40.9 (39.4–43.6) | 41.4 (39.3–42.9) | 0.98 |
| Mean hippocampal volume anterior (mm3) | 323.8 (276.8–397.5) | 318.8 (277.3–404.3) | 370.5 (305–436.5) | 0.36 |
| Mean hippocampal volume posterior (mm3) | 562.3 (514.5–608.8) | 583.8 (498.8–642) | 613.5 (524–673.5) | 0.15 |
| Mean hippocampal volume total (mm3) | 875.5 (802.8–996.8) | 917.3 (767.3–1032.3) | 977 (848–1087) | 0.16 |
| 18‐month cognitive outcome | n = 24 | n = 93 | n = 15 | – |
| Bayley‐III Cognitive Composite score, median (IQR) | 90.0 (82.5–97.5) | 95.0 (90.0–100.0) | 105.0 (95.0–115.0) | 0.011* |
Kruskal–Wallis test for continuous variables, Fisher's exact test for categorical variables, and linear nonparametric test for trends for hippocampal volumes.
Abbreviations: BMI, body mass index; IQR, interquartile range.
p value <0.05.
Table 2.
Comparison of characteristics between infants with available and missing Bayley‐III Cognitive Composite scores at 18 months and available information on maternal level of education.
| Available cognitive score at 18 months | Missing cognitive score at 18 months | p Value | |
|---|---|---|---|
| Clinical characteristics | n = 133 | n = 29 | – |
| Gestational age at birth (weeks), median (IQR) | 27.0 (25.43–29.29) | 26.86 (25.0–29.86) | 0.96 |
| Birth weight (g), median (IQR) | 930 (700–1200) | 920 (670–1290) | 0.96 |
| Male, no (%) | 74 (55.6) | 15 (51.7) | 0.43 |
| Small for gestational age, no (%) | 17 (12.8) | 3 (10.3) | 0.50 |
| APGAR Score (5 min), median (IQR) | 8 (6–9) | 7 (5.5–8) | 0.10 |
| Maternal age, median (IQR) | 34 (30–36) | 29 (24–33) | 0.0002* |
| Culture positive infection, no (%) | 41 (30.8) | 7 (24.1) | 0.32 |
| Bronchopulmonary dysplasia, no (%) | 44 (33.1) | 11 (37.9) | 0.38 |
| Major surgeries, no (%) | 21 (15.8) | 5 (17.2) | 0.52 |
| Retinopathy of prematurity, no (%) | 8 (6.0) | 2 (6.9) | 0.56 |
Kruskal–Wallis test. Infants with and without Bayley‐III Cognitive Composite scores at 18 months only differed in maternal age at birth (p = 0.0002).
Abbreviation: IQR, interquartile range.
p value <0.05.
SES and hippocampal volumes
In GEE models (Table 3), neither anterior (SES 2: p = 0.47, SES 3: p = 0.82) nor posterior (SES 2: p = 0.87, SES 3: p = 0.84) hippocampal volumes from EIL and TEA were associated with maternal level of education, adjusting for sex, birth GA, and PMA at scan. In post hoc analysis, when conducting a two‐group analysis (comparing SES group 1 to SES groups 2 and 3) results did not meaningfully differ (Table S1). Of note, male sex was associated with larger anterior hippocampal volume (p = 0.015; posterior: p = 0.058) and increasing PMA at scan was associated with larger anterior and posterior hippocampal volumes (p < 0.001).
Table 3.
Associations between maternal level of education and anterior/posterior hippocampal volumes.
| Mean hippocampal volume anterior | Mean hippocampal volume posterior | |||||
|---|---|---|---|---|---|---|
| ß | 95% CI | p Value | ß | 95% CI | p Value | |
| Maternal level of education | ||||||
| College/undergraduate (reference = high school) | −7.56 | −28.03 to 12.92 | 0.47 | −2.05 | −27.00 to 22.90 | 0.87 |
| Postgraduate (reference = high school) | 3.89 | −29.72 to 37.50 | 0.82 | 3.76 | −33.33 to 40.85 | 0.84 |
| Post‐menstrual age at scan (weeks) | 13.33 | 11.74 to 14.93 | <0.001* | 22.75 | 20.23 to 25.26 | <0.001* |
| Male sex | 22.13 | 4.34 to 39.92 | 0.015* | 23.01 | −0.79 to 46.80 | 0.058 |
| Gestational age at birth (weeks) | −0.57 | −4.13 to 3.00 | 0.76 | 2.64 | −2.35 to 7.62 | 0.30 |
Abbreviations: CI, confidence interval; β, beta coefficient.
p value <0.025.
SES modified the association between anterior hippocampal volumes as well as hippocampal functional connectivity and cognitive outcome
In the 126 infants with Bayley‐III Cognitive Composite scores, the interaction of anterior hippocampal volume and maternal level of education was significantly associated with cognition (p = 0.005). Specifically, greater anterior hippocampal volumes were associated with higher cognitive scores in SES group 1 (high school degree), but not in groups 2 (college or undergraduate degree) or 3 (postgraduate degree) (Fig. 4). This association was not observed for the posterior hippocampus (p = 0.12). Birth GA was associated with 18 months cognitive scores (anterior p = 0.011; posterior p = 0.010). When including scanner (pre and post software upgrade) into the GEE models or accounting for total cerebral volume, results did not meaningfully differ. Post hoc GEE analysis (Table S2) compared SES group 1 to combined SES group of 2 and 3, showing no meaningful differences, indicating consistency of findings for SES group 1. This suggests stable association between anterior hippocampal volume and cognitive scores for infants with mothers attaining a high school degree, regardless of SES grouping.
Figure 4.
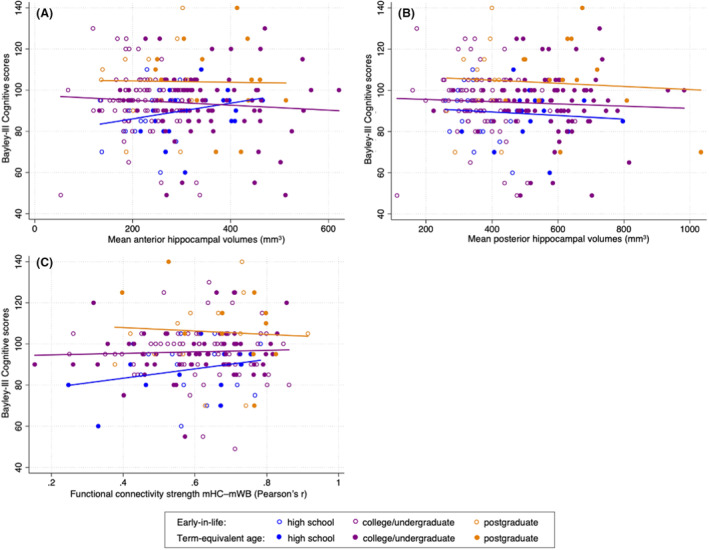
Hippocampal subregional volumes (A and B) as well as hippocampal functional connectivity (C) and 18‐month cognitive outcome by maternal level of education (high school, college/undergraduate degree, and postgraduate degree). Maternal level of education modifies the association between anterior hippocampal volumes (A) and 18‐month cognitive outcome, and that between hippocampal functional connectivity (C) and 18‐month cognitive outcome. mHC, mean total hippocampus; mWB, mean whole brain.
The interaction term between hippocampal functional connectivity (mean total hippocampus [mHC] – mean whole brain [mWB]) and maternal level of education was significantly associated with cognitive outcome at 18 months (p = 0.050) (Table S3). Birth GA was also significantly associated with 18‐month cognition (p = 0.01).
Consistent with the results for volumetric data, higher hippocampal functional connectivity strength was associated with higher cognitive scores in neonates born to mothers with a high school diploma. Again, this relationship was not evident for neonates whose mothers possessed college/undergraduate or postgraduate degrees (Fig. 4).
In exploratory analyses, SES also significantly modifies the relationship between functional connectivity of the hippocampus to 16 specific regions that are important in cognitive networks and cognitive outcome at 18 months (Table 4).
Table 4.
Associations between Bayley‐III Cognitive Composite scores at 18 months and hippocampus functional connectivity (mean hippocampus—mean whole brain and mean hippocampus—16 brain regions involved in cognition) with an interaction term of SES (maternal level of education) and sex.
| Functional connectivity mean hippocampus | Cognitive scores at 18 months (with SES as interaction) | Cognitive scores at 18 months (with sex as interaction) |
|---|---|---|
| p Value global interaction term | p Value global interaction term | |
| Mean whole brain | 0.050* | 0.94 |
| Superior frontal gyrus (dorsal and medial) | 0.046* | 0.55 |
| Inferior frontal gyrus (opercular) and inferior frontal gyrus (triangular) | 0.071* | 0.91 |
| Insula | 0.008* | 0.60 |
| Anterior cingulate gyrus | 0.021* | 0.48 |
| Middle cingulate gyrus | 0.009* | 0.78 |
| Posterior cingulate gyrus | 0.014* | 0.96 |
| Parahippocampal gyrus | 0.019* | 0.92 |
| Amygdala | 0.048* | 0.98 |
| Superior parietal gyrus | 0.001* | 0.98 |
| Inferior parietal lobule | 0.012* | 0.93 |
| Precuneus | 0.047* | 0.62 |
| Caudate | 0.021* | 0.74 |
| Thalamus | 0.008* | 0.73 |
| Superior temporal gyrus | 0.005* | 0.99 |
| Middle temporal gyrus + inferior temporal gyrus | 0.001* | 0.49 |
| Temporal pole (superior) + temporal pole (middle) | 0.019* | 0.96 |
All models adjusted for gestational age at birth, sex, post‐menstrual age at scan, and motion. SES, but not sex, is a modifier of hippocampal functional connectivity with cognitive outcome.
Abbreviation: SES, socioeconomic status.
p value <0.1.
Post hoc analysis exploring the role of neighborhood environment and neighborhood income on the relationship of hippocampal development to cognition
In post hoc analyses, maternal level of education was unrelated to neighborhood environment using the rural versus urban population area definition mentioned above (p = 0.21). Neighborhood environment neither predicted hippocampal volumes nor significantly modified the relationship between hippocampal development and 18‐month cognition. Of note, clinical characteristics between rural and urban groups did not significantly differ, except for birth GA and birth weight (Table S4). For income‐related analysis, 52% of families (N = 71) were categorized as below the median province‐wide household income based on neighborhood‐based average income from the first three digits of postal code. Using individual neighborhood‐based income in the previously described GEE models, no significant associations were observed. In this provincial framework, low‐income neighborhoods were defined as having an income less than 50% of the province's median household income; notably, no family in this cohort resided in a low‐income neighborhood.
Sex did not modify associations between hippocampal development and cognitive outcome
Sex did not modify the relationship between hippocampal volumes and cognitive outcome at 18 months, when accounting for birth GA, PMA at scan and SES. Sex also did not modify the relationship between functional connectivity of the hippocampus networks and cognition (Table 4).
Discussion
In this prospective longitudinal cohort study of very preterm neonates, SES, indicated by maternal level of education, significantly modified the relationship between anterior but not posterior hippocampal volumes and 18‐month cognitive outcome. Similarly, the interaction of SES and hippocampal functional connectivity was significantly associated with cognitive outcome. Importantly, greater anterior hippocampal volumes and whole hippocampus functional connectivity were associated with higher cognitive scores in the lowest SES group only. However, maternal level of education did not predict hippocampal volumes from EIL and TEA, suggesting that SES‐related differences in hippocampal development might evolve over time beyond the NICU period. Of note, biological sex, an important factor for cognitive and motor development in preterm neonates,29, 30 did not significantly modify these relationships.
Prior studies demonstrated that structural and functional maturation of the hippocampus is related to neurodevelopment in children.11, 13, 17, 19, 27, 50 Hippocampal volumes were associated with cognitive performances in healthy children and children with congenital heart disease. 54 Hippocampal functional connectivity from term age to 1 year was shown to be predictive of working memory at preschool age in healthy infants. 19 Adverse events such as stress exposure, pain or brain injury were associated with alterations in the developmental trajectories of the hippocampus across different ages; these brain changes were accompanied by poorer neurodevelopmental outcomes.1, 11, 13, 15, 17, 18 Furthermore, alterations in cognitive functional networks involving the hippocampus were identified in preterm neonates at TEA relative to healthy term‐born infants. 55 However, literature focusing on SES as an effect modifier of hippocampal developmental trajectories regarding cognitive outcome, especially in very preterm neonates, is scarce.2, 13, 16 A recent study in a large normative cohort of children, adolescents, and young adults demonstrated that SES was associated with cognitive function and that this association was mediated by anterior, but not posterior, hippocampal volumes. 23 We found that maternal education modified associations between neonatal hippocampal volumes and 18‐month cognitive outcome with specificity for the anterior hippocampus. Similarly, maternal education was an important modifier of the relationship between hippocampal functional connectivity and cognition.
Greater anterior hippocampal volumes and hippocampal functional connectivity correlated with higher cognitive scores in the lower SES group only. This relationship was attenuated in higher SES groups and is consistent with prior observations in independent cohorts using different methods.2, 23 Socioeconomic disparities in hippocampal development were most apparent among children of parents with lower education attainment. 9 Similarly, high SES attenuated the association between cognitive outcome and brain injury in very preterm neonates. 2 These findings highlight the importance of maternal education as a modifier of volumetric and functional development of the hippocampus in relation to cognition. Acknowledging the significance of education and a stimulating environment for neurodevelopment in preterm infants will prompt health policy to expand its focus, strengthening additional areas of support for caregivers beyond the medical domain in order to more equitably promoting optimal neurodevelopment.
The subregion specificity of the hippocampus in relation to SES and cognition merits further attention. Following the principle of long‐axis differentiation in the hippocampus, emotion, anxiety‐related behaviors, and encoding memory were attributed to the anterior, whereas spatial memory was related to the posterior hippocampus.22, 24 Underlying mechanisms for different developmental trajectories of hippocampal subregions are microstructural, metabolic and cellular in nature.15, 22, 24 Data from animal models suggested subregional microstructural changes such as apical dendrite atrophy and suppression of neurogenesis or cell proliferation in CA3 of cornu ammonis and dentate gyrus in response to chronic stress, which were found to coincide with hippocampal‐dependent learning and memory deficits. 56 Anterior but not posterior hippocampal volumes correlated positively with annual family income in children and adolescents. 23 These income‐related anterior hippocampal volumetric differences predicted neurodevelopment measured by the strength of the gaps in memory and language. 23 These findings are consistent with those in the present study relating SES and cognition with the anterior hippocampus. We assessed functional connectivity of the whole hippocampus for two reasons. First, technical limitations in measuring very small regions of interest made assessing subregional functional connectivity challenging. Second, neonatal functional connectivity of the hippocampus showed a lack of long axis specialization with less differentiated network patterns. 57 The earliest age when functional anterior–posterior specialization and an association between subregional hippocampus networks and episodic memory function were demonstrated was early childhood. 50 These findings suggest that the subregional specificity and stress sensitivity of the anterior hippocampus to environmental and socioeconomic factors evolve as early as preterm age for structural maturation.
Multiple studies linked lower SES with reduced hippocampal volumes in children 3 years and older.1, 9, 11, 23, 36 SES was associated with brain development and altered hippocampal volumes in healthy term born neonates. 58 Likewise, an association between parental education and altered in vivo fetal brain development has been described, 59 suggesting that SES may influence structural brain development even before birth. While evidence strongly suggests a correlation between SES and brain volumes in early infancy,1, 9, 23, 37 few studies did not observe this association with whole brain or white matter volume.33, 60 Recently, a study in preterm neonates examined the impact of birth GA and SES on brain development and found, that lower birth GA was associated with widely distributed differences in brain structure and cortical morphology at TEA, whereas changes associated with maternal education were less widely distributed. 33 One study in healthy term‐born infants even reported an opposite finding with lower SES being associated with increased regional brain volumes. 61
Our study is the first to examine subregional hippocampal volumes from the early‐life period to term and their associations with SES in very preterm neonates. We did not find that maternal education directly predicts hippocampal subregional volumes. Reasons for this might be multifactorial. First, the distribution of maternal education in our cohort appeared quite homogeneous, with a substantial proportion of mothers having obtained a college or undergraduate degree. This observation aligns with data from the Canadian census, despite a slight underrepresentation of mothers with a high school diploma. 62 The relatively low number of infants born to mothers with either a high school or postgraduate degree may account for the absence of direct relationships between SES and hippocampal volumes in our analysis. Moreover, direct relationships between SES and hippocampal volumes may emerge over time, given that equal access to specialized health care was provided by a publicly funded health care system in contrast to other studies. More equitable NICU treatment may mitigate the association of differences in family support with brain development. Finally, additional factors beyond maternal education may need to be further explored to better elucidate the absence of a direct association between SES and hippocampal volumes. In this study, we used maternal level of education as a reliable measure for SES based on our prior work and existing literature.2, 40 Different measures of SES in other studies may have contributed to differences in findings related to neurodevelopmental outcomes and brain development.1, 2, 9, 23, 59 Further, individual differences in health status in our cohort of very preterm neonates need to be acknowledged when comparing the relationship between SES and hippocampal volumes with normative cohorts.23, 58, 59
Sex is recognized as an important predictor of volumetric and microstructural brain development.29, 63 Studies reported sex‐related volumetric differences in the developing hippocampus with boys having larger hippocampal volumes than girls. 64 How these sex‐specific volumetric hippocampal trajectories are related to neurodevelopmental outcomes in neonates remain unclear. In a recent study examining functional connectivity between very preterm and full‐term neonates, very preterm boys were shown to have greater alterations in resting neurophysiological network communication and significant group differences from their full‐term peers compared to girls. 65 In our study, we did not find sex to modify the relationship between hippocampal structural and functional development with 18‐month cognition in very preterm neonates. Our findings reinforce the need for more attention to environmental factors reflected in SES as they relate to hippocampal development and cognition in preterm children. Given the distinct functional contributions of anterior and posterior hippocampus in memory processes and representations, it is important to look at the relationship of specific memory functions in later childhood and adolescence and regional specific hippocampal volumes.
Given the COVID‐19 pandemic, our in‐person follow‐up rate was 74%. Yet, those attending follow‐up were representative of the entire cohort (Table 2). Due to technical limitations, functional connectivity analysis was only achieved on the whole hippocampus. However, this second neuroimaging modality allowed us to address structural and functional development of the hippocampus more comprehensively. Direct clinical measures of stress, such as blood cortisol levels or maternal cytokine exposure, could permit deeper insights into stress‐induced hypothalamic–pituitary–adrenal (HPA) axis hippocampal metabolism.10, 11, 28 Given that maternal education is a robust SES indicator within individual families,33, 38 it served as a reliable proxy for SES in our study, as well as in other preterm and normative cohorts.2, 34, 35, 36, 37 Maternal education correlates highly with other measures of SES. 66 Of note, in additional post hoc analyses we found that SES relationships were unrelated to neighborhood environment and neighborhood income. It is crucial to note that neighborhood data utilized were not deeply phenotyped as we were able to access rural and urban local and neighborhood‐based income metrics using the first three digits of families' postal codes. No neighborhood met the criteria for classification as a “low‐income neighborhood,” potentially further affecting the generalizability of results. We acknowledge that other socioeconomic indicators besides maternal education, like the Area Deprivation Index or family income, likely play a crucial role shaping children's brain development and cognition. There is increasing evidence supporting the importance of family income in children's cognitive development.9, 23, 33, 67 A recent study investigating the role of family‐level versus neighborhood‐level SES measures on brain development in preterm infants found, that family‐level SES measures were more closely associated with brain structure, underscoring the role of genetic determinants of brain anatomy, exposure to stress, nutrition and other factors that influence brain development, which are not fully captured by neighborhood deprivation. 33 In another study, neighborhood poverty, as measured by Area Deprivation Index, correlated with lower cognitive scores and decreased regional brain volumes including the right hippocampus, even after adjusting for individual family income. 68 Ongoing research is dedicated to identify these SES‐related factors, revealing its multifaceted impacts on brain development and children's cognitive abilities through various pathways.5, 9, 23, 33, 39, 40, 68 Our findings, along with others, emphasize the need for political interventions addressing not only education, but also income and neighborhood support.
In conclusion, this study highlights the anatomical specificity of the anterior hippocampus in relation to SES‐linked differences in cognition starting as early as preterm age. These findings suggest that the pathways linking hippocampal structural and functional development and cognition are related to aspects of SES beyond the time frame of neonatal care. Identifying key factors mediating the complex relationship of the regionally specific association between anterior hippocampal volumes, hippocampal functional connectivity, cognition, and SES is an important next step to mitigate SES‐related gaps in altered brain development and to attenuate disparities for preterm neonates. These steps will ultimately help achieve optimal care for neonates born very preterm and promote their neurodevelopment more equitably.
Author Contributions
JK, TG, and SPM conceptualized and designed the study. JK, TG, SU, TS, MS, and EA contributed to the acquisition of data; JK, TG, TS, and SU carried out analysis. HB, LL, EK, REG, VC, and SPM supervised data acquisition. JK, TG, TS, SU, and SPM contributed to drafting the manuscript, figures, and tables. All authors critically reviewed and revised the manuscript and approved submitting the article for publication.
Conflict of Interest
The authors do not have any relevant conflicts of interest to declare.
Supporting information
Supplementary Methods and Tables S1–S4
Acknowledgments
This study was funded by Canadian Institutes of Health Research (MOP‐136966 and PJT‐168894), CP Alliance (PG‐016817), Brain Canada, and Ontario Brain Institute (OBI; CP‐NET). TS is supported by CIHR Canada Graduate Scholarships – Master's and Doctoral Awards; Ontario Ministry of Health—University of Toronto Clinician Investigator Program, and SickKids Research Institute Clinician Scientist Training Program. JK was supported by Children's Hospital Dritter Orden, Munich, Germany and the B. Braun Foundation. SPM received support from the Bloorview Children's Hospital Foundation Chair in Pediatric Neuroscience and is currently supported by the Hudson Family Hospital Chair in Pediatric Medicine (BCCH) and the James & Annabel McCreary Chair in Pediatrics (UBC). The authors would like to acknowledge Stephanie Au‐Young, Giselle DaRocha, Mark LePine, Diane Wilson, and the Neonatal Follow‐up teams at Mount Sinai Hospital and the Hospital for Sick Children. We would also like to thank the children and families who participated in this research study.
Funding Statement
This work was funded by Canadian Institutes of Health Research grants MOP‐136966 and PJT‐168894; Cerebral Palsy Alliance grant PG‐016817; CIHR Canada Graduate Scholarships; Ontario Ministry of Health—University of Toronto Clinician Investigator Program; SickKids Research Institute Clinician Scientist Training Program; Children's Hospital Dritter Orden; B. Braun Foundation ; Bloorview Children’s Hospital Foundation Chair in Pediatric Neuroscience; Hudson Family Hospital Chair in Pediatric Medicine; James & Annabel McCreary Chair in Pediatrics; Brain Canada ; Ontario Brain Insitute (OBI).
Data Availability Statement
Data are available from authors upon reasonable request with appropriate data sharing agreements in place.
References
- 1. Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain: neural correlates of socioeconomic status. Dev Sci. 2012;15(4):516‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benavente‐Fernández I, Synnes A, Grunau RE, et al. Association of Socioeconomic Status and Brain Injury with Neurodevelopmental Outcomes of very preterm children. JAMA Netw Open. 2019;2(5):e192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Senn TE, Walsh JL, Carey MP. The mediating roles of perceived stress and health behaviors in the relation between objective, subjective, and neighborhood socioeconomic status and perceived health. Ann Behav Med. 2014;48(2):215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reiss F, Meyrose A‐K, Otto C, Lampert T, Klasen F, Ravens‐Sieberer U. Socioeconomic status, stressful life situations and mental health problems in children and adolescents: results of the German BELLA cohort‐study. PLoS One. 2019;14(3):e0213700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker SP, Wachs TD, Grantham‐McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378(9799):1325‐1338. [DOI] [PubMed] [Google Scholar]
- 6. Kweon H, Aydogan G, Dagher A, et al. Human brain anatomy reflects separable genetic and environmental components of socioeconomic status. Sci Adv. 2022;8:eabm2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we Don't. Ann N Y Acad Sci. 1999;896(1):3‐15. [DOI] [PubMed] [Google Scholar]
- 8. Schnittker J. Education and the changing shape of the income gradient in health. J Health Soc Behav. 2004;45(3):286‐305. [DOI] [PubMed] [Google Scholar]
- 9. Noble KG, Houston SM, Brito NH, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tarullo AR, Tuladhar CT, Kao K, Drury EB, Meyer J. Cortisol and socioeconomic status in early childhood: a multidimensional assessment. Dev Psychopathol. 2020;32(5):1876‐1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hanson JL, Nacewicz BM, Sutterer MJ, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77(4):314‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duerden EG, Guo T, Dodbiba L, et al. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants: midazolam in neonates. Ann Neurol. 2016;79(4):548‐559. [DOI] [PubMed] [Google Scholar]
- 13. Strahle JM, Triplett RL, Alexopoulos D, et al. Impaired hippocampal development and outcomes in very preterm infants with perinatal brain injury. NeuroImage Clin. 2019;22:101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Asis‐Cruz J, Kapse K, Basu SK, et al. Functional brain connectivity in ex utero premature infants compared to in utero fetuses. NeuroImage. 2020;219:117043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dahmen B, Puetz VB, Scharke W, von Polier GG, Herpertz‐Dahlmann B, Konrad K. Effects of early‐life adversity on hippocampal structures and associated HPA Axis functions. Dev Neurosci. 2018;40(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 16. Sripada RK, Swain JE, Evans GW, Welsh RC, Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology. 2014;39(9):2244‐2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duerden EG, Guo T, Chau C, et al. Association of Neonatal Midazolam Exposure with Hippocampal Growth and Working Memory Performance in children born preterm. Neurology. 2023;101:e1863‐e1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chau CMY, Ranger M, Bichin M, et al. Hippocampus, amygdala, and thalamus volumes in very preterm children at 8 years: neonatal pain and genetic variation. Front Behav Neurosci. 2019;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu J, Chen Y, Stephens R, et al. Hippocampal functional connectivity development during the first two years indexes 4‐year working memory performance. Cortex. 2021;138:165‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aanes S, Bjuland KJ, Skranes J, Løhaugen GCC. Memory function and hippocampal volumes in preterm born very‐low‐birth‐weight (VLBW) young adults. NeuroImage. 2015;105:76‐83. [DOI] [PubMed] [Google Scholar]
- 21. Nosarti C, Froudist‐Walsh S. Alterations in development of hippocampal and cortical memory mechanisms following very preterm birth. Develop Med Child Neuro. 2016;58(S4):35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016;17(3):173‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Decker AL, Duncan K, Finn AS, Mabbott DJ. Children's family income is associated with cognitive function and volume of anterior not posterior hippocampus. Nat Commun. 2020;11(1):4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655‐669. [DOI] [PubMed] [Google Scholar]
- 25. Selvanathan T, Zaki P, McLean MA, et al. Early‐life exposure to analgesia and 18‐month neurodevelopmental outcomes in very preterm infants. Pediatr Res. 2023;94:738‐746. doi: 10.1038/s41390-023-02536-y [DOI] [PubMed] [Google Scholar]
- 26. Doria V, Beckmann CF, Arichi T, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A. 2010;107(46):20015‐20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smyser CD, Wheelock MD, Limbrick DD, Neil JJ. Neonatal brain injury and aberrant connectivity. NeuroImage. 2019;185:609‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanders AFP, Tirado B, Seider NA, et al. Prenatal exposure to maternal disadvantage‐related inflammatory biomarkers: associations with neonatal white matter microstructure. Transl Psychiatry. 2024;14(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr. 2015;169(12):1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Synnes A, Luu TM, Moddemann D, et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child Fetal Neonatal Ed. 2016;F1‐F9. doi: 10.1136/archdischil-d-2016-311228 [DOI] [PubMed] [Google Scholar]
- 31. Ene D, Der G, Fletcher‐Watson S, et al. Associations of socioeconomic deprivation and preterm birth with speech, language, and communication concerns among children aged 27 to 30 months. JAMA Netw Open. 2019;2(9):e1911027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joseph RM, Hooper SR, Heeren T, et al. Maternal social risk, gestational age at delivery, and cognitive outcomes among adolescents born extremely preterm. Paediatric Perinatal Epid. 2022;36(5):654‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mckinnon K, Galdi P, Blesa‐Cábez M, et al. Association of Preterm Birth and Socioeconomic Status with Neonatal Brain Structure. JAMA Netw Open. 2023;6(5):e2316067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Twilhaar ES, Pierrat V, Marchand‐Martin L, Benhammou V, Kaminski M, Ancel PY. Profiles of functioning in 5.5‐year‐old very preterm born children in France: the EPIPAGE‐2 study. J Am Acad Child Adolesc Psychiatry. 2022;61(7):881‐891. [DOI] [PubMed] [Google Scholar]
- 35. Bergelson E, Soderstrom M, Schwarz I‐C, et al. Everyday language input and production in 1,001 children from six continents. Proc Natl Acad Sci USA. 2023;120(52):e2300671120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rakesh D, Whittle S. Socioeconomic status and the developing brain – a systematic review of neuroimaging findings in youth. Neurosci Biobehav Rev. 2021;130:379‐407. [DOI] [PubMed] [Google Scholar]
- 37. Olson L, Chen B, Fishman I. Neural correlates of socioeconomic status in early childhood: a systematic review of the literature. Child Neuropsychol. 2021;27(3):390‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hollingshead AB. Four factor index of social status. https://sociology.yale.edu/sites/default/files/files/yjs_fall_2011.pdf
- 39. Long K, Renbarger R. Persistence of poverty: how measures of socioeconomic status have changed over time. Educ Res. 2023;52(3):144‐154. [Google Scholar]
- 40. Joseph RM, O'Shea TM, Allred EN, et al. Maternal educational status at birth, maternal educational advancement, and neurocognitive outcomes at age 10 years among children born extremely preterm. Pediatr Res. 2018;83(4):767‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Statistics Canada . Population Centre and Rural Area Classification. 2016. https://www.statcan.gc.ca/en/subjects/standard/pcrac/2016/introduction
- 42. Ontario . 2016 census highlights. https://www.ontario.ca/document/2016‐census‐highlights/fact‐sheet‐7‐income#:~:text=The%20median%20household%20income%20was,incomes%20among%20provinces%20in%202015
- 43. Guo T, Winterburn JL, Pipitone J, et al. Automatic segmentation of the hippocampus for preterm neonates from early‐in‐life to term‐equivalent age. NeuroImage Clin. 2015;9:176‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825‐841. [DOI] [PubMed] [Google Scholar]
- 45. Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137‐152. [DOI] [PubMed] [Google Scholar]
- 46. Griffanti L, Douaud G, Bijsterbosch J, et al. Hand classification of fMRI ICA noise components. NeuroImage. 2017;154:188‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shi F, Yap P‐T, Wu G, et al. Infant brain atlases from neonates to 1‐ and 2‐year‐olds. PLoS One. 2011;6(4):e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical Parcellation of the MNI MRI single‐subject brain. NeuroImage. 2002;15(1):273‐289. [DOI] [PubMed] [Google Scholar]
- 49. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54:2033‐2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Riggins T, Geng F, Blankenship SL, Redcay E. Hippocampal functional connectivity and episodic memory in early childhood. Dev Cogn Neurosci. 2016;19:58‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bayley N. Bayley Scales of Infant and Toddler Development(R)‐3rd Edition. NCS Pearson, Inc; 2006. [Google Scholar]
- 53. Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school‐aged children who were born preterm: a meta‐analysis. JAMA. 2002;288(6):728‐737. [DOI] [PubMed] [Google Scholar]
- 54. Naef N, Ciernik A, Latal B, Liamlahi R, For the Children's Heart and Development Research Group . Hippocampal volume and cognitive performance in children with congenital heart disease. Pediatr Res. 2023;94:99‐102. doi: 10.1038/s41390-022-02457-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gozdas E, Parikh NA, Merhar SL, Tkach JA, He L, Holland SK. Altered functional network connectivity in preterm infants: antecedents of cognitive and motor impairments? Brain Struct Funct. 2018;223(8):3665‐3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fenoglio K, Brunson K, Baram T. Hippocampal neuroplasticity induced by early‐life stress: functional and molecular aspects. Front Neuroendocrinol. 2006;27(2):180‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Howell AL, Osher DE, Li J, Saygin ZM. The intrinsic neonatal hippocampal network: rsfMRI findings. J Neurophysiol. 2020;124(5):1458‐1468. [DOI] [PubMed] [Google Scholar]
- 58. Triplett RL, Lean RE, Parikh A, et al. Association of Prenatal Exposure to early‐life adversity with neonatal brain volumes at birth. JAMA Netw Open. 2022;5(4):e227045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu Y‐C, Kapse K, Andersen N, et al. Association between socioeconomic status and in utero fetal brain development. JAMA Netw Open. 2021;4(3):e213526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hanson JL, Hair N, Shen DG, et al. Family poverty affects the rate of human infant brain growth. PLoS One. 2013;8(12):e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Spann MN, Bansal R, Hao X, Rosen TS, Peterson BS. Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child Neuropsychol. 2020;26(2):170‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Statistics Canada . Highest level of education by census year: Canada, provinces and territories, census metropolitan areas and census agglomerations. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=9810038401
- 63. Ball G, Aljabar P, Nongena P, et al. Multimodal image analysis of clinical influences on preterm brain development: clinical factors and preterm brain development. Ann Neurol. 2017;82(2):233‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Herting MM, Johnson C, Mills KL, et al. Development of subcortical volumes across adolescence in males and females: a multisample study of longitudinal changes. NeuroImage. 2018;172:194‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kozhemiako N, Nunes AS, Vakorin VA, et al. Sex differences in brain connectivity and male vulnerability in very preterm children. Hum Brain Mapp. 2020;41(2):388‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rowland C, Krajewski G, Meints K, et al. The (Null) Effect of Socio‐Economic Status on the Language and Gestures of Infants Learning Ten Languages. CDI Demographics; 2022. https://osf.io/7ufrx [Google Scholar]
- 67. Duncan GJ, Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. WIRES Cognitive Science. 2012;3(3):377‐386. [DOI] [PubMed] [Google Scholar]
- 68. Taylor RL, Cooper SR, Jackson JJ, Barch DM. Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw Open. 2020;3(11):e2023774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods and Tables S1–S4
Data Availability Statement
Data are available from authors upon reasonable request with appropriate data sharing agreements in place.


