Abstract
The increasing prevalence of obesity and type 2 diabetes has led to a greater interest in adipose tissue physiology. Adipose tissue is now understood as an organ with endocrine and thermogenic capacities in addition to its role in fat storage. It plays a critical role in systemic metabolism and energy regulation, and its activity is tightly regulated by the nervous system. Fat is now recognized to receive sympathetic innervation, which transmits information from the brain, as well as sensory innervation, which sends information into the brain. The role of sympathetic innervation in adipose tissue has been extensively studied. However, the extent and the functional significance of sensory innervation have long been unclear. Recent studies have started to reveal that sensory neurons robustly innervate adipose tissue and play an important role in regulating fat activity. This brief review will discuss both historical evidence and recent advances, as well as important remaining questions about the sensory innervation of adipose tissue.
1. Introduction
The increasing prevalence of obesity and type 2 diabetes in recent years has led to heightened interest in studying adipose tissue physiology. Adipose tissue (fat) was first identified as a lipid droplet comprising connective tissue and has long been regarded as an organ that passively stores excess energy. Growing data suggest, however, that, in addition to lipid storage, adipose tissues also have thermogenic capacity and endocrine functions and that they play critical roles in systemic metabolism and energy regulation [1–5].
The activity of adipose tissues is tightly regulated by the nervous system. Adipose tissues receive bidirectional innervation: sympathetic efferent that transmits signal from central nervous system to the fat, and sensory afferent that sends information from the fat to the brain. To date, however, sympathetic innervation has been explored in much greater depth than sensory innervation. In the traditional paradigm of adipose-brain crosstalk, adipose tissues secrete hormones that are transmitted to the brain via circulation, while the brain regulates adipose tissue activities via sympathetic innervation. Indeed, sympathetic innervation is now well recognized for its important roles in regulating adipose lipolysis, thermogenesis, and adipogenesis [6–11]. By contrast, although sensory innervation has been documented, the evidence is still preliminary, and the extent and roles of sensory innervation remain inconclusive. Here, we review historical evidence as well as recent advances in the characterization and functional understanding of adipose sensory innervation and discuss key open questions related to this fat-to-brain communication pathway.
2. Key roles of adipose tissue in homeostasis
Adipose tissues are metabolically active organs that control whole-body energy homeostasis, glucose and lipid metabolism, and thermogenesis. Historically, mammals have been considered to have two types of functionally different adipose tissues – white and brown fat. Brown adipose tissue (BAT) and white adipose tissue (WAT) are comprised of morphologically and functionally distinct adipocytes that engage in various aspects of energy homeostasis. [1,2].
BAT is the main source of non-shivering thermogenesis. Brown adipocytes in BAT have small multilocular lipid droplets and large numbers of mitochondria highly expressing uncoupling protein 1 (Ucp1), which uncouples substrate oxidation and electron transport, leading to the dissipation of chemical energy as heat. BAT thermogenesis can be mediated by UCP1-dependent and independent molecular pathways, and it can be stimulated by cold exposure or sympathetic activation [5,12].
WAT contributes to energy balance by storing energy as triglycerides in white adipocytes, which feature huge unilocular lipid droplets and few mitochondria, and then mobilizing free fatty acids through lipolysis when energy is needed (i.e., under fasting conditions) [9,10]. In addition to being a calorie reservoir, storing lipids within adipocytes removes excess free fatty acids from circulation, protecting peripheral tissues such as the heart, muscle, and liver from lipotoxicity, which disrupts the action of insulin on metabolism. Indeed, elevated fatty acids during chronic obesity can lead to ectopic lipid accumulation and contribute to insulin resistance [13–15].
Some adipocytes, which ordinarily resemble white adipocytes, can be altered to have brown adipocyte-like thermogenic capacity and elevated UCP1 expression in response to cold exposure or sympathetic activation, a process known as beiging or browning [5,12,16]. WAT containing these beige adipocytes is also known as beige adipose tissue.
Thermogenic adipose tissues (BAT and beige adipose tissues) are also important in glucose metabolism [5]. Insulin and norepinephrine-stimulated glucose uptake in BAT contributes significantly to glucose removal from circulation [17]. Genetic ablation of UCP1+ cells in mice resulted in obesity and hyperphagia [18]. The significance of beige fat in metabolism has recently attracted interest following the identification of PRDM16, the key transcriptional regulator of beige adipocytes that promotes beiging [19]. Transgenic mice models overexpressing PRDM16 had more beige adipocytes and improved glucose and insulin tolerance [20,21], whereas adipocyte-specific deletion of PRDM16 led to beige adipocyte loss and impaired insulin resistance [22].
The anatomical locations of different types of adipose tissues in human and rodents are extensively documented [23]. Most rodent studies focus on these representative depots: interscapular BAT (iBAT) for BAT, perigonadal WAT (pgWAT, epididymal WAT (eWAT) for male and periovarian WAT for female) for WAT and inguinal WAT (iWAT) for beige adipose tissues.
Adipose tissues also regulate whole-body homeostasis through endocrine functions [24,25]. Following the cloning of adiposin and leptin [26,27], a number of adipokines secreted from adipose tissues have been identified, including tumor growth factor alpha (TNFα), adiponectin, resistin, and RBP4 [1]. It has been generally assumed that fat informs the brain about the metabolic states through different adipokines. For example, circulating leptin levels are highly correlated with body fat percentage [28,29]. Furthermore, many adipokines have significant impacts on other major tissues such as skeletal muscle, liver, and brain and regulate feeding behavior and energy homeostasis [30]. Recent evidence suggests, however, that communication between fat and the brain occurs via other pathways as well, notably through neural regulation.
3. Neural regulation of fat: overview
Adipose tissues are known to be heavily innervated, and an increasing body of research underscores the importance of neural modulation of adipose activity. In 1898, Dogiel noticed visible nerves of unknown types and origin enter WAT near the heart, which was the first histological evidence of nerves in adipose tissues [31]. Early data suggested that neural regulation of adipose tissues may be important, as patients undergoing regional denervation experienced a significant increase in adipose mass in the denervated regions [32]; reviewed in [6,8]. Since these early findings, histology, denervation, and neural tracing have been the main tools used to study neural regulation of fat. By the early 2000s, through application of these methods, it was known that adipose tissues are innervated by both the sensory and autonomic nervous systems. For the autonomic nervous system, adipose tissues have extensive sympathetic but little parasympathetic innervation [6,8,10,33–35]. The sympathetic innervation of adipose tissue received more attention than other neuronal components, and for a long time, it was widely believed that the neural control of fat came nearly entirely from sympathetic innervation.
3.1. Sympathetic nervous system
3.1.1. Anatomy
The sympathetic nervous system is characterized by catecholamine-producing tyrosine hydroxylase (TH)-positive neurons. They have postganglionic neuronal bodies in both paravertebral (i.e., sympathetic chain ganglia) and prevertebral (i.e., celiac ganglia). Sympathetic innervation influences thermogenesis, lipolysis, and adipogenesis in adipose tissues, and its anatomy and functions have been extensively reviewed [10,11,34,36,37].
Sympathetic innervation of BAT was first described by histofluorescence [38,39]. Despite initial confusion regarding which parts of adipose tissues received innervation, the presence of both sympathetic parenchymal and vascular innervation was soon confirmed [34]. Although histofluorescence evidence suggests that sympathetic fibers innervate WAT vasculature [39], its functional significance has long been debated. Owing to the low concentration of norepinephrine (NE) in WAT, circulating catecholamine was thought to be the primary effector on WAT. Later studies revealed that NE turnover in WAT was accelerated in response to cold exposure and was unaffected by adrenal demedullation [40], indicating that sympathetic innervation is the primary driver of WAT activity. Furthermore, Bartness et al. performed tracing studies in Siberian hamsters by injecting the retrograde fluorescent tracer Fluoro-Gold in iWAT or eWAT and the anterograde fluorescent tracer DiI in postganglionic sympathetic ganglia, enabling them to conclusively demonstrate sympathetic innervation of WAT [41]. The development of pseudorabies virus (PRV) tracing has provided more anatomical evidence for adipose tissue sympathetic innervation. It has been shown that iBAT receives postganglionic sympathetic projection from sympathetic chain ganglia (SChG) from upper thoracic levels (stellate ganglion (T1) and T2-5) [42], whereas iWAT and pgWAT receive projection from thoracic-lumbar level SChG (T13-L2). [41,43,44]. It is generally agreed that BAT has more sympathetic innervation than WAT.
3.1.2. Neurotransmitters
Sympathetic innervation releases the neurotransmitter norepinephrine, and its effects on adipose metabolic pathways have been extensively studied [45,46]. Norepinephrine binds to adrenergic receptors and acts on cAMP-PKA pathways. It promotes lipolysis and thermogenesis, induces beiging, and impacts adipose remodeling. Mice lacking β-adrenergic receptors, which are required for norepinephrine activities, have dysregulated metabolism and are prone to obesity [47,48]. However, it should be noted that norepinephrine is not the only neurotransmitter released from the SNS; it has also been reported that the sympathetic fibers co-releases neuropeptide Y (NPY) and ATP.
NPY is co-released from sympathetic terminal with norepinephrine to act on NPY receptor subtypes 1 and 2, which inhibit cAMP-PKA pathways and antagonize adrenergic signaling [49]. NPY inhibits lipolysis and stimulates adipogenesis, and the release of NPY can be triggered by cold exposure or overfeeding [50–52]. Knockout of the NPY2 receptor in adipose tissue reduced obesity [51]. Thus, it has been hypothesized that NPY serves as a feedback system, fine-tuning norepinephrine actions.
ATP is also a co-transmitter released by sympathetic nerve endings. ATP binds to ionotropic purinergic receptors (P2XRs) and metabotropic purinergic receptors (P2YRs). Although the functional roles of these receptors in adipose tissues have not been extensively investigated, accumulating evidence shows purinergic signaling plays a role in multiple processes, including lipid metabolism and adipogenesis [53,54].
3.1.3. Neural plasticity and physiological and pathological adaptations
Sympathetic innervation of adipose tissues is highly dynamic and changes in response to various physiological and pathological conditions. Recent advances in immunolabeling-compatible 3D tissue clearing, which allows direct visualization of fluorescently labeled structures within intact tissues, have enabled more thorough examination of sympathetic innervation patterns in adipose tissues. Tissue clearing has demonstrated the presence of TH+ fibers in iBAT, iWAT, and pgWAT, with iWAT having a higher density of TH+ fibers than pgWAT [42,55], which appears to correlate with the thermogenic capacity of different fat depots. TH+ fibers are regionally heterogeneous [55] and can make putative contact with adipocytes in iWAT, which may be related to the unique beiging ability of iWAT [56,57]. Interestingly, transgenic expression of PRDM16 promotes sympathetic innervation [20], whereas deletion of PRDM16 in adipocytes decreases sympathetic innervation density [55].
Metabolic disorders such as obesity have been linked to sympathetic dysregulation and have been shown to disrupt catecholamine-mediated adipocyte functions such as lipolysis and adipose remodeling [58,59]. Aside from changes in central drive to sympathetic neurons, it was recently found that sympathetic arborization decreases in leptin-deficient (ob/ob) obese mice, which can be compensated for by chronic leptin treatment [60]. These findings suggest that sympathetic plasticity is critical for proper adipose functions.
Multiple neurotrophic factors, including nerve growth factor (NGF), neurotrophin-3, S100b, and brain-derived neurotrophic factor (BDNF), have been found to be capable of regulating sympathetic arborization and fat activity [60–64]. However, further research is needed to determine the mechanisms through which sympathetic neuroplasticity adapts to physiological and pathological conditions. It should be highlighted that TH immunolabeling or transgenic labeling is used in all morphological research on sympathetic innervation. Because TH is known to be expressed in some sensory neuronal subtypes [65,66], the conclusion of sympathetic arborization may be complicated by the sensory component (see below).
3.2. Parasympathetic nervous system
In contrast to the majority of visceral organs that receive dual innervation from the autonomic system, adipose tissues receive little parasympathetic innervation. Immunohistochemical studies demonstrated that BAT receives sparse parasympathetic innervation and not in the main iBAT depots [34]. PRV tracing and immunohistochemical studies demonstrated that WAT also lacks significant parasympathetic innervation [67,68]. Consistent with these observations, recent tissue clearing studies of adipose tissues with immunolabeling against choline acetyltransferase (ChAT), a marker for parasympathetic neurons, also detected few ChAT+ fibers in iWAT [57].
4. Sensory system and fat
4.1. Overview
Afferent and efferent.
The nerve fibers in the peripheral nervous system are generally divided into efferents and afferents. Motor and autonomic nerves (i.e., sympathetic and parasympathetic) are considered efferents carry signals away from the central nervous system (i.e., the spinal cord and brain) to the peripheral organs. The sensory nerves, on the other hand, are afferents which convey sensory information from peripheral to the central nervous system. The mammalian sensory system consists of somatosensory neurons with cell bodies primarily in the dorsal root ganglia (DRG) and vagal sensory neurons with cell bodies in the nodose ganglia (NG). Somatosensory DRG neurons are primarily studied in skin and muscle innervation, whereas vagal sensory neurons innervate the majority of visceral organs [70]. The sensory system detects and transmits various chemical and mechanical stimuli from the external world as well as from internal organs to the central nervous system. In addition to afferent functions, some sensory neurons have efferent functions, releasing neuropeptides locally to act on end tissues. However, tracing investigations revealed that adipose tissues receive very little vagal sensory innervation and predominantly have DRG innervation [66,71,72]. It should be noted that WAT has more sensory innervation compared to BAT [66], and hence we mainly focus here on the sensory innervation of WAT.
Although the presence of sensory nerve-associated neuropeptides by immunohistochemistry in both BAT and WAT suggests that adipose tissues receive sensory innervation [6,69], sensory innervation has received far less attention than sympathetic innervation. The series of studies performed by Bartness and colleagues provides the foundation of our current understanding of sensory innervation of adipose tissues; nonetheless, the extent and functional consequences of sensory innervation remain largely ambiguous due to limitations in available tools at the time.
4.2. Genetic evidence
Early evidence for potential sensory neural regulation of adipose phenotypes and whole-body homeostasis was provided by genetic knockout models of ion channels and neuropeptides that are predominantly expressed by sensory neurons, such as transient receptor potential (TRP) channels, including TRPV1, TRPV2, TRPV4, and TRPM8, calcitonin gene-related peptide (CGRP), and Substance P (SP).
TRP channels have long been linked to energy homeostasis [73]. For example, TRPV1, an ion channel important for pain and thermal sensation, plays an important role in metabolism [74]. TRPV1 knockout mice were protected from diet-induced obesity and diabetes [75,76], and exhibited increased longevity due to improved energy expenditure profiles during ageing [77]. Moreover, the human TRPV1 variant (Val585Ile) correlates significantly with abdominal adiposity [78]. Capsaicin, a natural TRPV1 ligand found in chili peppers, has been demonstrated to suppress food intake and enhance energy expenditure [79]. Adipose phenotypes are also altered by global knockout of other TRP channels. Knocking out TRPM8, the ion channel for noxious cold sensation, or TRPV4, an ion channel that responds to multiple stimuli, also protects mice from obesity [80–82]. Mice deficient in TRPV2, an ion channel responsive to noxious heat, however, grow more white adipose tissues and gain more weight when fed on high-fat diet [83].
CGRP and SP are neuropeptides that are predominantly released by a subset of sensory neurons and can be detected in the fat of obese mice [84]. Mice with whole-body or sensory neuron-specific knockout of CGRP are protected against diet-induced obesity [85–87]. Pharmacological inhibition or genetic deletion of SP receptors (neurokinin-1 receptor) improved glucose metabolism in mice fed on high-fat diet [88,89].
Despite the fact that these whole-body knockout models are not specific to sensory neurons and cannot conclusively demonstrate which end organs are causing the effects, they collectively imply that sensory innervation may play an important role in adipose activities and whole-body metabolism.
4.3. Anatomical evidence of sensory innervation in adipose tissues
The identification of CGRP and SP in WAT by immunohistochemistry in rats and hamsters was the first indication of the presence of sensory innervation in fat [6,36]. However, DRG neurons are highly heterogeneous, and CGRP and SP mark less than half of the total DRG population [90,91]. More recently, different attempts have been made to determine the neuroanatomy of sensory nerves in fat, with retrograde tracing and tissue clearing being the most used approaches.
Retrograde tracing studies have provided more direct evidence of sensory innervation. The first tracing study found labeling in T13-L3 DRGs in rats after injecting the retrograde tracer True Blue into iWAT [71]. Later investigations in hamsters utilizing the transsynaptic tracer herpes simplex virus (HSV) demonstrated labeling in T13 DRG as well as higher brain circuits [72]. Previous research using chlora toxin subunit B (CTB) in iWAT reported few neurons being labeled in DRG in mice, calling the existence of sensory innervation into question [57]. Recently, we observed significant CTB labeling in mice in thoracic-lumbar DRGs (T12-L3) for iWAT and thoracic-lumbar DRGs (T13/L1 and L6) for eWAT [66]. This inconsistency in retrograde labeling could be due to inherent differences in the animal species, where the tracer was injected in the tissues, or different tracer affinity to terminals.
Tissue clearing is also widely utilized to study adipose innervation. Traditional histological studies, in which sections of fixed tissue are cut into thin slices, are inadequate for defining innervation patterns in the whole tissue and have been shown to be notoriously difficult to apply to adipose tissue due to the high lipid content of fat. The development of modern clearing methods, such as iDISCO and AdipoClear [55,92], which remove lipids and render the entire tissue optically transparent, has substantially advanced our understanding of adipose innervation. However, both iDISCO and AdipoClear quench endogenous fluorescent signals and require immunolabeling, which can lead to considerable background staining. Moreover, due to the lack of precise markers for all sensory neurons and the fact that the sympathetic marker TH is also expressed in a subset of DRG neurons [65], it has been difficult to interpret the results from the available research.
For example, a recent study co-stained the pan-neuronal marker synaptophysin with TH in iDISCO-cleared iWAT and found that 98.8% of synaptophysin+ fibers express TH [57], indicating the vast majority of nerve fibers in fat are sympathetic. Later research, however, performing CGRP staining in AdipoClear-cleared iWAT indicated the considerable presence of sensory fibers in iWAT [93]. Additionally, another study employing Nav1.8-TdTomato mice, in which TdTomato is driven by the sensory neuron-specific sodium channel Nav1.8, also found many TdTomato+ fibers in iWAT [94]. To further complicate the issue, it has been recently reported that many transgenic lines that are thought to have sensory neuron specific transgene expression, such as Advillin-Cre, Nav1.8-Cre, and Pirt-Cre, also have expression in sympathetic neurons [66,95]. Thus, the extent of sensory innervation of fat still remains to be defined.
We recently developed several tools to overcome the aforementioned limitations and to establish the anatomical basis of sensory innervation in fat. To circumvent the potential bias induced by retrograde tracers, we developed an anterograde tracing procedure by intraganglionic injection, in which adeno-associated virus (AAV) encoding a fluorescent protein is injected directly into DRGs without infecting sympathetic neurons [66]. In addition, our novel tissue clearing approach named HYBRiD preserves endogenous fluorescent proteins while being compatible with immunolabeling and is optimized for peripheral tissues [96]. By directly labeling T13/L1 DRGs, we resolved sensory fibers in iWAT and eWAT. Moreover, we were able to clear the adult torso from these labeled mice and directly visualize axonal projections from DRG soma to iWAT, unequivocally demonstrating that WAT receives robust sensory innervation [66].
This specific DRG labeling also allowed us to examine sensory fiber morphology. DRG fibers were found in both big bundles traveling along the vasculature and smaller parenchymal fibers in apposition to adipocytes [66]. Tight vasculature-wrapping patterns, similar to those reported for sympathetic fibers, were not seen in these DRG fibers. [55]. Surprisingly, by immunostaining against TH, we identified a substantial amount of thin parenchymal DRG fibers colocalized with TH signals [66], suggesting that previous observations of sympathetic arborization can be confounded by this sensory component.
4.4. Approaches for manipulating fat innervation.
For many years, denervation was the primary approach for studying innervation. High doses of capsaicin or the capsaicin analog resiniferatoxin were commonly used for chemical denervation of sensory nerves, as overactivation of the TRPV1 receptor results in massive ion influx and leads to cell death. Early research suggested that systemic capsaicin administration impacts adiposity and recruitment of thermogenic adipocytes in the cold [97,98]. However, as with the whole-body genetic knockout mice described above, it is unclear whether these effects are specifically mediated by sensory fibers innervating fat. Thus, organ-specific interventions are necessary to examine the roles of adipose sensory innervation. WAT-specific surgical and chemical denervation approaches were pioneered by Bartness and colleagues. However, the specificity of both strategies needs to be reassessed. Surgical denervation involves the removal of nerves entering the tissue and cannot distinguish between sensory and sympathetic components. Chemical denervation involves local delivery of high doses of capsaicin in WAT, which is biased towards TRPV1-expressing sensory neurons [99,100], and can also act on other fat-resident TRPV1+ cells [101–103].
In recent years, several genetic methods, including optogenetics and chemogenetics, have been developed to manipulate neuronal activity without changing total nerve supply. Optogenetics provides precise temporal and spatial control through the implantation of optical fibers or LEDs. Channelrhodopsin-2 (ChR2), a blue light-sensitive cation channel, has been widely employed in research studying DRGs and pain sensation as well as fat, where it is used to stimulate adipocytes or sympathetic fibers [56,104]. However, it is challenging to apply to fat-innervating DRGs due to the need for fiber implantation. Fiber implantation on multiple DRG soma remains technically difficult, and direct implantation in fat must be carefully controlled, as recent research has shown that blue light can activate photoreceptor opsin3 in white and brown adipocytes to induce lipolysis and thermogenesis [105,106]. Chemogenetics, or designer receptors exclusively activated by designer drugs (DREADD) technologies, allow for the excitation or inhibition of neural activity over a prolonged period of time. The efficacy of DREADD in sympathetic fibers and adipocytes in fat has been recently demonstrated [62,107–109]. DREADD has been utilized less frequently in DRGs than optogenetics, but it is a viable route to pursue. However, because DREADD is activated by systemic administration of the ligand clozapine-N-oxide (CNO), improved genetic methods are required to confine its expression to fat-innervating sensory neurons. Despite these challenges, both optogenetic and chemogenetic strategies offer substantial potential and are worth investigating; nonetheless, rigorous electrophysiological characterization of optogenetics and/or chemogenetics evoked neural activity in peripheral neurons is necessary to demonstrate the usability.
We used AAV to achieve target-specific manipulation. Traditional viral tracing vectors, including PRV and HSV, have neurotoxicity and transsynaptic transmission, limiting their use for functional manipulation. Over the years, AAV has become a better choice for in vivo gene delivery as it is non-toxic and solicits minimal immune responses [110]. Since the discovery that AAV can be retrogradely transported, numerous efforts have been made to engineer AAV for retrograde labeling in select brain circuits. However, the currently available AAV serotypes are neither efficient in nor selective to fat-specific DRG neurons. As a result, we adapted an established in vivo AAV directed evolution platform [111,112]. We injected a library of AAV capsids with random peptides displayed on AAV9 capsid surface into iWAT. By recovering DNA from thoracic-lumbar DRGs, we identified a capsid variant that can efficiently and specifically transduce fat-innervating DRGs with little labeling in sympathetic neurons as well as minimal systemic leakage, which we named retrograde vector optimized for organ tracing (ROOT). We were therefore able to perform fat-targeted sensory ablation by combining the ROOT vector with the previously described intraganglionic surgical procedure. We injected Cre-dependent diphtheria toxin subunit A (DTA) in T13/L1 DRGs, while injecting ROOT carrying Cre recombinase in iWAT. This allowed us to perform unilateral and bilateral sensory ablation with superior specificity: we showed only DRGs innervating iWAT were affected, while sympathetic innervation of fat and sensory innervation of other tissues such as skin remain intact. The main caveat of this strategy, however, is its efficiency. When we quantified CTB-labeled DRG neurons after sensory ablation, we found only a ~50% decrease in the ablated side compared to the control side [66]. Thus, a more efficient retrograde AAV vector or better genetic Cre lines are needed to further improve the efficiency. Regardless, the retrograde AAV vector is a valuable tool that can be used in conjunction with the optogenetic and chemogenetic tools described above to achieve more comprehensive manipulation.
4.5. Understanding the role of sensory innervation.
Genetic knockout of sensory channels and neuropeptides indicates sensory innervation is important for adipose physiology; however, the role of fat-innervating sensory neurons has remained obscure. Several attempts have been made to dissect the functions of these sensory neurons in an organ-specific way. Surgical denervation of WAT in rats and hamsters enhanced fat mass as well as white adipocyte proliferation and differentiation [113]. Capsaicin-mediated chemical denervation in hamsters had no effect on the denervated fat pad but increased fat mass in other fat depots [114,115]. Although this evidence is inconclusive, it suggests that sensory innervation plays a role in body fat regulation. A more recent study discovered that iWAT chemical denervation leads to decreased norepinephrine turnover (NETO) in iWAT and iBAT but an increase in mesenteric WAT in the cold. It should be noted that this result could be confounded by capsaicin-induced changes in systemic sympathetic tone. Moreover, NETO is measured by pharmacological blockade of TH, which may affect the central dopaminergic transmission of certain DRG neurons [116], thus influencing NE release from sympathetic neurons. Regardless of these limitations, it implies a potential involvement of sensory innervation in communication with the sympathetic system and feedback in whole-body energy homeostasis.
We set out to investigate the role of sensory innervation of fat with the newly developed surgical and viral strategy described above. We found sensory ablation in iWAT upregulated thermogenic and lipogenic gene programs only in the manipulated fat but not other fat depots, and that this upregulation could be attenuated by sympathetic denervation. This implies that sensory innervation operates upstream of sympathetic innervation, and sensory ablation is similar to the outcome of sympathetic overactivation. Furthermore, mice with bilateral sensory ablation showed no changes in temperature sensation or systemic sympathetic drive, but they had more beige adipocytes in iWAT. These mice exhibited a mild increase in core body temperature in thermoneutrality but not room temperature, indicating enhanced thermogenesis but no change in temperature setting point. When fed a high-fat diet, these mice also showed improved glucose metabolism despite no significant changes in body weight compared to controls, which is consistent with previous reports on the involvement of beige adipocytes in glucose metabolism [66]. The physiological changes after sensory ablation in fat is striking given the partial ablation efficiency. Based on these results, we proposed that sensory innervation acts as a brake on the local sympathetic functions in fat.
The observation that the sensory system provides negative feedback to the sympathetic system is intriguing. In most visceral organs, the parasympathetic nervous system counteracts the sympathetic nervous system. Fat lacks parasympathetic innervation; therefore, a potential sensory feedback mechanism may provide this function instead. Other evidence also implies that the negative sensory feedback is not uncommon: vagal baroreceptors and DRG neurons regulate blood pressure [117–119], and CGRP knockout mice have increased sympathetic drive [120,121]. Although sensory modulation of sympathetic activity has been documented at the spinal or supraspinal level, the mechanisms of sensory-sympathetic interactions need further investigation.
5. Outstanding challenges and future directions
It has been assumed that fat informs the brain about metabolic states through circulating hormones. Many recent findings, including ours, have indicated that fat receives robust sensory innervation, which can be a rapid and spatially encoded neural transmission mechanism. Although we demonstrated that sensory innervation functionally suppresses sympathetic activities, several directions related to sensory innervation of adipose tissues remain understudied.
We showed that DRG neurons extensively innervate iWAT and pgWAT, but only sparsely innervate iBAT. So far, functional studies on sensory innervation have been largely focused on iWAT, while its role(s) for other fat depots remains unexplored. It’s important to note that subcutaneous WAT and visceral WAT are functionally different, and that some fat depots have additional roles beyond energy storage. For example, pgWAT regulates reproductive states in addition to lipid storage [122,123]. Moreover, adipose activities vary between the genders [124]. Thus, it would be interesting to examine the functions of sensory innervation in different fat depots and sexes.
Despite reports of sensory impairment in non-diabetic obese patients [125], it is unclear if sensory fibers remodel similarly to sympathetic neurons in response to metabolic challenges such as cold or obesity. It was recently discovered that adipose mTORC2 deletion reduces fat sensory innervation [93], implying neuroplasticity of sensory fibers. It should be noted that many neurotrophic factors that impact sympathetic arborization, such as NGF and BDNF, can also affect sensory axon growth. Thus, it is possible sensory fibers undergo adaptations and remodeling in physiological and pathological conditions, but the extent and mechanisms need to be investigated.
We demonstrated that the sensory system serves as a feedback mechanism on sympathetic functions. However, the mechanisms underlying these sensory-sympathetic interactions remain unclear. Previous research indicates the sensory system may interact with the sympathetic system in the central nervous system at the spinal or supraspinal levels [126–128]. The lack of significant changes in NE amount in bulk iWAT tissues after sensory ablation, however, implies that sensory regulation may also happen downstream of NE release, which is supported by the upregulation of adipocyte-specific β3 adrenergic receptor (Adrb3) in iWAT after sensory ablation [66]. The involvement of sensory efferent function is also corroborated by the upregulation of Adrb3 in WAT of CGRP knockout mice [86]. Thus, the contribution of both sensory afferent and efferent components to sympathetic activity control needs to be further investigated.
The most important remaining question is what is being sensed. DRG neurons are known to be heterogenous and express multiple receptors as revealed by recent single-cell RNA sequencing experiments [90,91]. It is unclear which DRG subtypes innervate fat and whether different subtypes have varied effects on adipose activity. Thus, single-cell RNA sequencing analysis on fat-innervating DRGs would be valuable for establishing the molecular profiles of these sensory neurons as well as revealing endogenous stimuli. There have been several attempts to address the potential endogenous stimuli. DRG neurons have been reported to express leptin receptor [129]. Local infusion of leptin or free fatty acids increased spontaneous spiking frequency in fat-associated nerves [129,130], indicating that leptin and lipolytic products can activate sensory neurons. However, local infusion of high doses of ligand may not be physiologically relevant, and electrophysiological recording cannot be linked to genetic identities.
Recent advances in in vivo calcium imaging allow real-time monitoring of neural activity and have been coupled with in situ hybridization to register activity patterns to molecular profiles [131,132]. In vivo calcium imaging has been successfully performed in nodose ganglia and DRG in anesthetized mice to monitor the activity of sensory neurons innervating lung, gut, and bladder [133–136]. With the emerging long-term DRG imaging techniques [137,138], the ability to track neural activity over time will be incredibly valuable in determining the triggering signals.
6. Summary
To summarize, neural regulation of adipose tissues is essential for tissue activity and whole-body homeostasis. For a long time, it was thought that sympathetic neurons mediated the majority of neural regulation, and the sensory components were largely disregarded. Here we reviewed recent progress in understanding the roles of fat sensory innervation. Our recent findings revealed that adipose tissues, particularly WAT, are endowed with robust sensory innervation that acts as a brake on local sympathetic activities. It hints to a rapid and spatially resolved feedback regulation of fat physiology, in addition to systemic hormonal control. Furthermore, these findings have implications beyond the realm of adipose tissues. In addition to the well-studied vagal system, we demonstrated that DRG neurons are also critical mediators of internal organ senses, which could serve as proof-of-principle for investigating the role of DRG neurons innervating a variety of other internal organs and their contribution to internal homeostasis.
Figure 1.
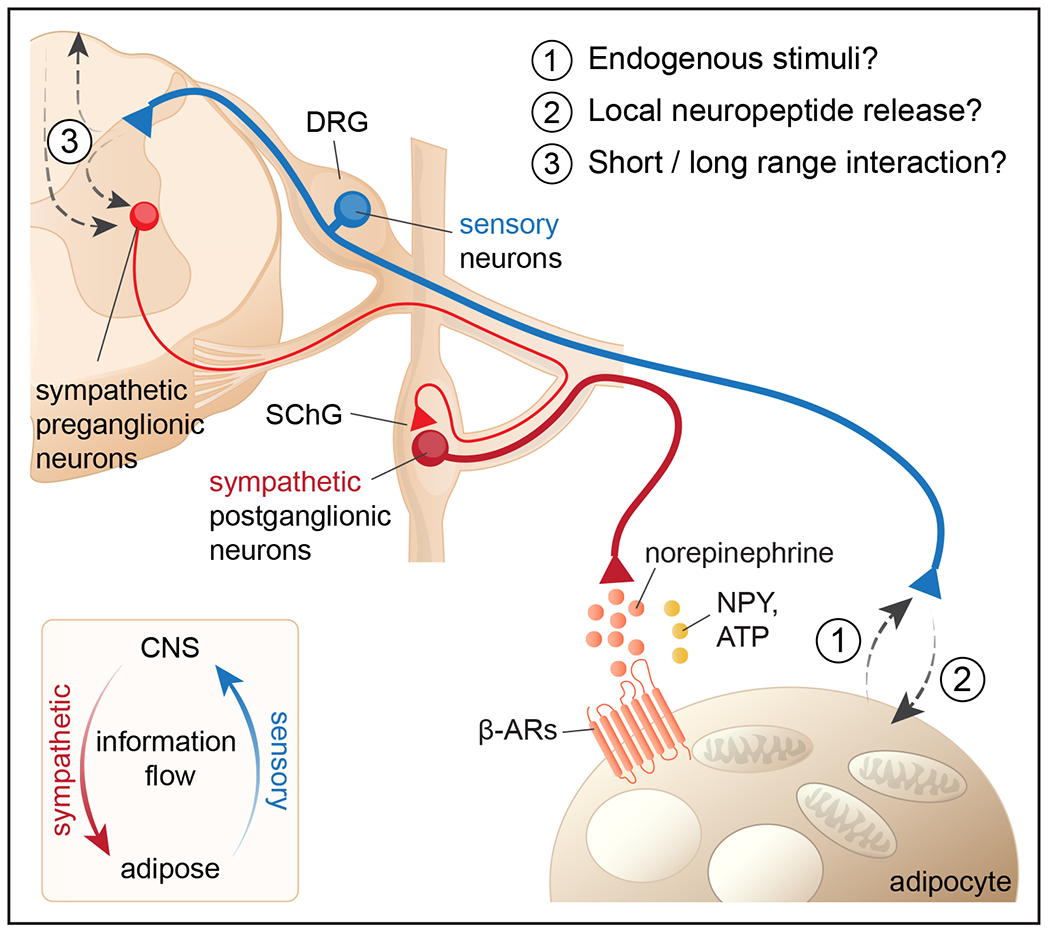
A schematic overview of white adipose tissue innervation. White adipose tissue receives afferent sensory nerves (blue) and efferent sympathetic nerves (red). Sympathetic postganglionic neurons are located in SChG and release norepinephrine, which acts on adipocytes via beta-adrenergic receptors together with other neurotransmitters. Sensory neurons located in DRG project from fat to the dorsal horn of the spinal cord. Several aspects of sensory innervation of adipose tissues still remain unclear, including: (1) what are endogenous stimuli of sensory neurons? (2) do sensory neurons locally release neuropeptide, and if so, how? (3) where and how do sensory neurons interact with sympathetic neurons?
SChG: sympathetic chain ganglia; DRG: dorsal root ganglia; NPY: neuropeptide Y
Acknowledgements
This work was supported by NIH Director’s New Innovator Award DP2DK128800, NIDDK K01DK114165, Whitehall Foundation and Baxter Foundation; Y.W. was supported by the Dorris Scholars fellowship. This manuscript is based on work presented during the 2022 Annual Meeting of the Society for the Study of Ingestive Behavior, July 12–16, 2022 in Porto, Portugal.
Footnotes
Declaration of Competing Interest
The authors of this manuscript declare no conflict of interest.
Reference
- [1].Rosen ED, Spiegelman BM, What we talk about when we talk about fat, Cell. 156 (2014) 20–44. 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kajimura S, Spiegelman BM, Seale P, Brown and Beige Fat: Physiological Roles beyond Heat Generation, Cell Metab. 22 (2015) 546–559. 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cohen P, Spiegelman BM, Brown and Beige Fat: Molecular Parts of a Thermogenic Machine, Diabetes. 64 (2015) 2346–2351. 10.2337/db15-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cohen P, Spiegelman BM, Cell biology of fat storage, Molecular Biology of the Cell. 27 (2016) 2523–2527. 10.1091/mbc.e15-10-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cohen P, Kajimura S, The cellular and functional complexity of thermogenic fat, Nat. Rev. Mol. Cell Biol 22 (2021) 393–409. 10.1038/s41580-021-00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fredholm BB, Chapter 3 - Nervous control of circulation and metabolism in white adipose tissue, in: Cryer A, Van RLR (Eds.), New Perspectives in Adipose Tissue, Butterworth-Heinemann, 1985: pp. 45–64. 10.1016/B978-0-408-10857-7.50008-7. [DOI] [Google Scholar]
- [7].Trayhurn P, Ashwell M, Control of white and brown adipose tissues by the autonomic nervous system, Proc. Nutr. Soc 46 (1987) 135–142. 10.1079/pns19870017. [DOI] [PubMed] [Google Scholar]
- [8].Bartness TJ, Bamshad M, Innervation of mammalian white adipose tissue: implications for the regulation of total body fat, Am. J. Physiol 275 (1998) R1399–411. 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- [9].Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK, Sensory and sympathetic nervous system control of white adipose tissue lipolysis, Mol. Cell. Endocrinol 318 (2010) 34–43. 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guilherme A, Henriques F, Bedard AH, Czech MP, Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus, Nat. Rev. Endocrinol 15 (2019) 207–225. 10.1038/s41574-019-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Martinez-Sanchez N, Sweeney O, Sidarta-Oliveira D, Caron A, Stanley SA, Domingos AI, The sympathetic nervous system in the 21st century: Neuroimmune interactions in metabolic homeostasis and obesity, Neuron. 110 (2022) 3597–3626. 10.1016/j.neuron.2022.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harms M, Seale P, Brown and beige fat: development, function and therapeutic potential, Nat. Med 19 (2013) 1252–1263. 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- [13].Unger RH, Clark GO, Scherer PE, Orci L, Lipid homeostasis, lipotoxicity and the metabolic syndrome, Biochim. Biophys. Acta 1801 (2010) 209–214. 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- [14].Engin AB, What Is Lipotoxicity?, Adv. Exp. Med. Biol 960 (2017) 197–220. 10.1007/978-3-319-48382-5_8. [DOI] [PubMed] [Google Scholar]
- [15].Yazıcı D, Sezer H, Insulin Resistance, Obesity and Lipotoxicity, Adv. Exp. Med. Biol 960 (2017) 277–304. 10.1007/978-3-319-48382-5_12. [DOI] [PubMed] [Google Scholar]
- [16].Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang A-H, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM, Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human, Cell. 150 (2012) 366–376. 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cooney GJ, Caterson ID, Newsholme EA, The effect of insulin and noradrenaline on the uptake of 2-[1–14C]deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse, FEBS Lett. 188 (1985) 257–261. 10.1016/0014-5793(85)80383-5. [DOI] [PubMed] [Google Scholar]
- [18].Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS, Development of obesity in transgenic mice after genetic ablation of brown adipose tissue, Nature. 366 (1993) 740–742. 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- [19].Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM, Transcriptional control of brown fat determination by PRDM16, Cell Metab. 6 (2007) 38–54. 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM, Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice, Journal of Clinical Investigation. 121 (2011) 96–105. 10.1172/jci44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P, Tajima K, Ajuwon KM, Soga T, Kajimura S, UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis, Nat. Med 23 (2017) 1454–1465. 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JPG, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM, Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch, Cell. 156 (2014) 304–316. 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL, Mechanisms and metabolic implications of regional differences among fat depots, Cell Metab. 17 (2013) 644–656. 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kershaw EE, Flier JS, Adipose tissue as an endocrine organ, J. Clin. Endocrinol. Metab 89 (2004) 2548–2556. 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- [25].Scheja L, Heeren J, The endocrine function of adipose tissues in health and cardiometabolic disease, Nat. Rev. Endocrinol 15 (2019) 507–524. 10.1038/s41574-019-0230-6. [DOI] [PubMed] [Google Scholar]
- [26].Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, Spiegelman BM, Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve, Science. 237 (1987) 402–405. 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- [27].Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM, Positional cloning of the mouse obese gene and its human homologue, Nature. 372 (1994) 425–432. 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- [28].Dua A, Hennes MI, Hoffmann RG, Maas DL, Krakower GR, Sonnenberg GE, Kissebah AH, Leptin: a significant indicator of total body fat but not of visceral fat and insulin insensitivity in African-American women, Diabetes. 45 (1996) 1635–1637. 10.2337/diab.45.11.1635. [DOI] [PubMed] [Google Scholar]
- [29].Ostlund RE Jr, Yang JW, Klein S, Gingerich R, Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates, The Journal of Clinical Endocrinology & Metabolism. 81 (1996) 3909–3913. https://doi.org/2016092613304400266. [DOI] [PubMed] [Google Scholar]
- [30].Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V, Adipokine dysregulation and adipose tissue inflammation in human obesity, Eur. J. Clin. Invest 48 (2018) e12997. 10.1111/eci.12997. [DOI] [PubMed] [Google Scholar]
- [31].Dogiel AS, Die sensiblen Nervenendigungen im Herzen und in den Blutgefässen der Säugethiere, Archiv Für Mikroskopische Anatomie. 52 (1898) 44–70. 10.1007/BF02976209. [DOI] [Google Scholar]
- [32].Mansfeld G, Müller F, Der Einfluss des Nervensystems auf die Mobilisierung von Fett: Ein Beitrag zur Physiologie der Fettwanderung, Pflüger’s Arch. 152 (1913) 61–67. 10.1007/BF01680895. [DOI] [Google Scholar]
- [33].Romijn JA, Fliers E, Sympathetic and parasympathetic innervation of adipose tissue: metabolic implications, Curr. Opin. Clin. Nutr. Metab. Care. 8 (2005) 440–444. 10.1097/01.mco.0000172586.09762.55. [DOI] [PubMed] [Google Scholar]
- [34].Bartness TJ, Vaughan CH, Song CK, Sympathetic and sensory innervation of brown adipose tissue, Int. J. Obes . 34 Suppl 1 (2010) S36–42. 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Blaszkiewicz M, Willows JW, Johnson CP, Townsend KL, The Importance of Peripheral Nerves in Adipose Tissue for the Regulation of Energy Balance, Biology . 8 (2019) 10. 10.3390/biology8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bartness TJ, Liu Y, Shrestha YB, Ryu V, Neural innervation of white adipose tissue and the control of lipolysis, Front. Neuroendocrinol 35 (2014) 473–493. 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Münzberg H, Floyd E, Chang JS, Sympathetic Innervation of White Adipose Tissue: to Beige or Not to Beige?, Physiology . 36 (2021) 246–255. 10.1152/physiol.00038.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wirsén C, Adrenergic Innervation of Adipose Tissue examined by Fluorescence Microscopy, Nature. 202 (1964) 913–913. 10.1038/202913a0. [DOI] [PubMed] [Google Scholar]
- [39].Wirsén C, Distribution of adrenergic nerve fibers in brown and white adipose tissue, in: Terjung R (Ed.), Comprehensive Physiology, 1st ed., Wiley, 1965: pp. 197–199. https://onlinelibrary.wiley.com/doi/10.1002/cphy.cp050119. [Google Scholar]
- [40].Garofalo MA, Kettelhut IC, Roselino JE, Migliorini RH, Effect of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue, J. Auton. Nerv. Syst 60 (1996) 206–208. 10.1016/0165-1838(96)00037-9. [DOI] [PubMed] [Google Scholar]
- [41].Youngstrom TG, Bartness TJ, Catecholaminergic innervation of white adipose tissue in Siberian hamsters, Am. J. Physiol 268 (1995) R744–51. 10.1152/ajpregu.1995.268.3.R744. [DOI] [PubMed] [Google Scholar]
- [42].Francois M, Torres H, Huesing C, Zhang R, Saurage C, Lee N, Qualls-Creekmore E, Yu S, Morrison CD, Burk D, Berthoud HR, Munzberg H, Sympathetic innervation of the interscapular brown adipose tissue in mouse, Ann N Y Acad Sci. 1454 (2019) 3–13,. 10.1111/nyas.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wiedmann NM, Stefanidis A, Oldfield BJ, Characterization of the central neural projections to brown, white, and beige adipose tissue, FASEB J. 31 (2017) 4879–4890. 10.1096/fj.201700433R. [DOI] [PubMed] [Google Scholar]
- [44].Huesing C, Qualls‐Creekmore E, Lee N, François M, Torres H, Zhang R, Burk DH, Yu S, Morrison CD, Berthoud H, Neuhuber W, Münzberg H, Sympathetic innervation of inguinal white adipose tissue in the mouse, Journal of Comparative Neurology. 529 (2021) 1465–1485. 10.1002/cne.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Collins S, β-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure, Front. Endocrinol . 2 (2011) 102. 10.3389/fendo.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ramseyer VD, Granneman JG, Adrenergic regulation of cellular plasticity in brown, beige/brite and white adipose tissues, Adipocyte. 5 (2016) 119–129. 10.1080/21623945.2016.1145846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bachman ES, Dhillon H, Zhang C-Y, Cinti S, Bianco AC, Kobilka BK, Lowell BB, betaAR signaling required for diet-induced thermogenesis and obesity resistance, Science. 297 (2002) 843–845. 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- [48].Douris N, Desai BN, Fisher FM, Cisu T, Fowler AJ, Zarebidaki E, Nguyen NLT, Morgan DA, Bartness TJ, Rahmouni K, Flier JS, Maratos-Flier E, Beta-adrenergic receptors are critical for weight loss but not for other metabolic adaptations to the consumption of a ketogenic diet in male mice, Mol Metab. 6 (2017) 854–862. 10.1016/j.molmet.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang W, Cline MA, Gilbert ER, Hypothalamus-adipose tissue crosstalk: neuropeptide Y and the regulation of energy metabolism, Nutr. Metab . 11 (2014) 27. 10.1186/1743-7075-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bradley RL, Mansfield JPR, Maratos-Flier E, Neuropeptides, including neuropeptide Y and melanocortins, mediate lipolysis in murine adipocytes, Obes. Res 13 (2005) 653–661. 10.1038/oby.2005.73. [DOI] [PubMed] [Google Scholar]
- [51].Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z, Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome, Nat. Med 13 (2007) 803–811. 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- [52].Yang K, Guan H, Arany E, Hill DJ, Cao X, Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor, FASEB J. 22 (2008) 2452–2464. 10.1096/fj.07-100735. [DOI] [PubMed] [Google Scholar]
- [53].Tozzi M, Novak I, Purinergic Receptors in Adipose Tissue As Potential Targets in Metabolic Disorders, Front. Pharmacol 8 (2017) 878. 10.3389/fphar.2017.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jain S, Jacobson KA, Purinergic signaling in diabetes and metabolism, Biochem. Pharmacol 187 (2021) 114393. 10.1016/j.bcp.2020.114393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chi J, Wu Z, Choi CHJ, Nguyen L, Tegegne S, Ackerman SE, Crane A, Marchildon F, Tessier-Lavigne M, Cohen P, Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density, Cell Metab. 27 (2018) 226–236.e3. 10.1016/j.cmet.2017.12.011. [DOI] [PubMed] [Google Scholar]
- [56].Zeng W, Pirzgalska RM, Pereira MMA, Kubasova N, Barateiro A, Seixas E, Lu Y-H, Kozlova A, Voss H, Martins GG, Friedman JM, Domingos AI, Sympathetic neuro-adipose connections mediate leptin-driven lipolysis, Cell. 163 (2015) 84–94. 10.1016/j.cell.2015.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jiang H, Ding X, Cao Y, Wang H, Zeng W, Dense Intra-adipose Sympathetic Arborizations Are Essential for Cold-Induced Beiging of Mouse White Adipose Tissue, Cell Metab. 26 (2017) 686–692.e3. 10.1016/j.cmet.2017.08.016. [DOI] [PubMed] [Google Scholar]
- [58].Thorp AA, Schlaich MP, Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome, J Diabetes Res. 2015 (2015) 341583. 10.1155/2015/341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Reilly SM, Saltiel AR, Adapting to obesity with adipose tissue inflammation, Nat. Rev. Endocrinol 13 (2017) 633–643. 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- [60].Wang P, Loh KH, Wu M, Morgan DA, Schneeberger M, Yu X, Chi J, Kosse C, Kim D, Rahmouni K, Cohen P, Friedman J, A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue, Nature. 583 (2020) 839–844. 10.1038/s41586-020-2527-y. [DOI] [PubMed] [Google Scholar]
- [61].Hu B, Jin C, Zeng X, Resch JM, Jedrychowski MP, Yang Z, Desai BN, Banks AS, Lowell BB, Mathis D, Spiegelman BM, gammadelta T cells and adipocyte IL-17RC control fat innervation and thermogenesis, Nature. 578 (2020) 610–614. 10.1038/s41586-020-2028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Meng X, Qian X, Ding X, Wang W, Yin X, Zhuang G, Zeng W, Eosinophils regulate intra-adipose axonal plasticity, Proc. Natl. Acad. Sci. U. S. A 119 (2022) e2112281119. 10.1073/pnas.2112281119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cui X, Jing J, Wu R, Cao Q, Li F, Li K, Wang S, Yu L, Schwartz G, Shi H, Xue B, Shi H, Adipose tissue-derived neurotrophic factor 3 regulates sympathetic innervation and thermogenesis in adipose tissue, Nat. Commun 12 (2021) 5362. 10.1038/s41467-021-25766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zeng X, Ye M, Resch JM, Jedrychowski MP, Hu B, Lowell BB, Ginty DD, Spiegelman BM, Innervation of thermogenic adipose tissue via a calsyntenin 3β–S100b axis, Nature. 569 (2019) 229–235. 10.1038/s41586-019-1156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD, The functional organization of cutaneous low-threshold mechanosensory neurons, Cell. 147 (2011) 1615–1627. 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang Y, Leung VH, Zhang Y, Nudell VS, Loud M, Servin-Vences MR, Yang D, Wang K, Moya-Garzon MD, Li VL, Long JZ, Patapoutian A, Ye L, The role of somatosensory innervation of adipose tissues, Nature. 609 (2022) 569–574. 10.1038/s41586-022-05137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ, White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation, Am. J. Physiol. Regul. Integr. Comp. Physiol 291 (2006) R1243–55. 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- [68].Berthoud H-R, Fox EA, Neuhuber WL, Vagaries of adipose tissue innervation, Am. J. Physiol. Regul. Integr. Comp. Physiol 291 (2006) R1240–2. 10.1152/ajpregu.00428.2006. [DOI] [PubMed] [Google Scholar]
- [69].Norman D, Mukherjee S, Symons D, Jung RT, Lever JD, Neuropeptides in interscapular and perirenal brown adipose tissue in the rat: a plurality of innervation, J. Neurocytol 17 (1988) 305–311. 10.1007/BF01187853. [DOI] [PubMed] [Google Scholar]
- [70].Prescott SL, Liberles SD, Internal senses of the vagus nerve, Neuron. 110 (2022) 579–599. 10.1016/j.neuron.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fishman RB, Dark J, Sensory innervation of white adipose tissue, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 253 (1987) R942–R944. 10.1152/ajpregu.1987.253.6.r942. [DOI] [PubMed] [Google Scholar]
- [72].Song CK, Schwartz GJ, Bartness TJ, Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue, Am. J. Physiol. Regul. Integr. Comp. Physiol 296 (2009) R501–11. 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Uchida K, Dezaki K, Yoneshiro T, Watanabe T, Yamazaki J, Saito M, Yada T, Tominaga M, Iwasaki Y, Involvement of thermosensitive TRP channels in energy metabolism, J. Physiol. Sci 67 (2017) 549–560. 10.1007/s12576-017-0552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ahern GP, Transient receptor potential channels and energy homeostasis, Trends Endocrinol. Metab 24 (2013) 554–560. 10.1016/j.tem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch H-M, TRPV1+ Sensory Neurons Control β Cell Stress and Islet Inflammation in Autoimmune Diabetes, Cell. 127 (2006) 1123–1135. 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- [76].Motter AL, Ahern GP, TRPV1-null mice are protected from diet-induced obesity, FEBS Lett. 582 (2008) 2257–2262. 10.1016/j.febslet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, de Magalhaes Filho CD, Merkwirth C, Dillin A, TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling, Cell. 157 (2014) 1023–1036. 10.1016/j.cell.2014.03.051. [DOI] [PubMed] [Google Scholar]
- [78].Snitker S, Fujishima Y, Shen H, Ott S, Pi-Sunyer X, Furuhata Y, Sato H, Takahashi M, Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications, Am. J. Clin. Nutr 89 (2009) 45–50. 10.3945/ajcn.2008.26561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Westerterp-Plantenga MS, Smeets A, Lejeune MPG, Sensory and gastrointestinal satiety effects of capsaicin on food intake, Int. J. Obes . 29 (2005) 682–688. 10.1038/sj.ijo.0802862. [DOI] [PubMed] [Google Scholar]
- [80].Ma S, Yu H, Zhao Z, Luo Z, Chen J, Ni Y, Jin R, Ma L, Wang P, Zhu Z, Li L, Zhong J, Liu D, Nilius B, Zhu Z, Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity, J. Mol. Cell Biol 4 (2012) 88–96. 10.1093/jmcb/mjs001. [DOI] [PubMed] [Google Scholar]
- [81].Reimúndez A, Fernández-Peña C, García G, Fernández R, Ordás P, Gallego R, Pardo-Vazquez JL, Arce V, Viana F, Señarís R, Deletion of the Cold Thermoreceptor TRPM8 Increases Heat Loss and Food Intake Leading to Reduced Body Temperature and Obesity in Mice, J. Neurosci 38 (2018) 3643–3656. 10.1523/JNEUROSCI.3002-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MJ, Boström P, Mepani RJ, Laznik D, Kamenecka TM, Song X, Liedtke W, Mootha VK, Puigserver P, Griffin PR, Clapham DE, Spiegelman BM, TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis, Cell. 151 (2012) 96–110. 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sun W, Uchida K, Suzuki Y, Zhou Y, Kim M, Takayama Y, Takahashi N, Goto T, Wakabayashi S, Kawada T, Iwata Y, Tominaga M, Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue, EMBO Rep. 17 (2016) 383–399. 10.15252/embr.201540819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhu Q, Glazier BJ, Hinkel BC, Cao J, Liu L, Liang C, Shi H, Neuroendocrine Regulation of Energy Metabolism Involving Different Types of Adipose Tissues, Int. J. Mol. Sci 20 (2019) 2707. 10.3390/ijms20112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Walker CS, Li X, Whiting L, Glyn-Jones S, Zhang S, Hickey AJ, Sewell MA, Ruggiero K, Phillips ARJ, Kraegen EW, Hay DL, Cooper GJS, Loomes KM, Mice Lacking the Neuropeptide α-Calcitonin Gene-Related Peptide Are Protected Against Diet-Induced Obesity, Endocrinology. 151 (2010) 4257–4269. https://doi.org/2016092613364900079. [DOI] [PubMed] [Google Scholar]
- [86].Liu T, Kamiyoshi A, Sakurai T, Ichikawa-Shindo Y, Kawate H, Yang L, Tanaka M, Xian X, Imai A, Zhai L, Hirabayashi K, Dai K, Tanimura K, Liu T, Cui N, Igarashi K, Yamauchi A, Shindo T, Endogenous Calcitonin Gene-Related Peptide Regulates Lipid Metabolism and Energy Homeostasis in Male Mice, Endocrinology. 158 (2017) 1194–1206. 10.1210/en.2016-1510. [DOI] [PubMed] [Google Scholar]
- [87].Makwana K, Chodavarapu H, Morones N, Chi J, Barr W, Novinbakht E, Wang Y, Nguyen PT, Jovanovic P, Cohen P, Riera CE, Sensory neurons expressing calcitonin gene-related peptide α regulate adaptive thermogenesis and diet-induced obesity, Mol Metab. 45 (2021) 101161. 10.1016/j.molmet.2021.101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Karagiannides I, Torres D, Tseng Y-H, Bowe C, Carvalho E, Espinoza D, Pothoulakis C, Kokkotou E, Substance P as a novel anti-obesity target, Gastroenterology. 134 (2008) 747–755. 10.1053/j.gastro.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Karagiannides I, Stavrakis D, Bakirtzi K, Kokkotou E, Pirtskhalava T, Nayeb-Hashemi H, Bowe C, Bugni JM, Nuño M, Lu B, Gerard NP, Leeman SE, Kirkland JL, Pothoulakis C, Substance P (SP)-neurokinin-1 receptor (NK-1R) alters adipose tissue responses to high-fat diet and insulin action, Endocrinology. 152 (2011) 2197–2205. 10.1210/en.2010-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S, Molecular Architecture of the Mouse Nervous System, Cell. 174 (2018) 999–1014.e22. 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, Ginty DD, The emergence of transcriptional identity in somatosensory neurons, Nature. 577 (2020) 392–398. 10.1038/s41586-019-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M, iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging, Cell. 159 (2014) 896–910. 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- [93].Frei IC, Weissenberger D, Ritz D, Heusermann W, Colombi M, Shimobayashi M, Hall MN, Adipose mTORC2 is essential for sensory innervation in white adipose tissue and whole-body energy homeostasis, Mol Metab. 65 (2022) 101580. 10.1016/j.molmet.2022.101580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Willows JW, Blaszkiewicz M, Lamore A, Borer S, Dubois AL, Garner E, Breeding WP, Tilbury KB, Khalil A, Townsend KL, Visualization and analysis of whole depot adipose tissue neural innervation, IScience. 24 (2021) 103127. 10.1016/j.isci.2021.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, Pellegrino K, del Mármol J, Castro TBR, Furuichi M, Perkins M, Han W, Rao A, Pickard AJ, Cross JR, Honda K, de Araujo I, Mucida D, Microbiota modulate sympathetic neurons via a gut–brain circuit, Nature. 583 (2020) 441–446. 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Nudell V, Wang Y, Pang Z, Lal NK, Huang M, Shaabani N, Kanim W, Teijaro J, Maximov A, Ye L, HYBRiD: hydrogel-reinforced DISCO for clearing mammalian bodies, Nat. Methods 19 (2022) 479–485. 10.1038/s41592-022-01427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cui J, Himms-Hagen J, Long-term decrease in body fat and in brown adipose tissue in capsaicin-desensitized rats, Am. J. Physiol 262 (1992) R568–73. 10.1152/ajpregu.1992.262.4.R568. [DOI] [PubMed] [Google Scholar]
- [98].Giordano A, Morroni M, Carle F, Gesuita R, Marchesi GF, Cinti S, Sensory nerves affect the recruitment and differentiation of rat periovarian brown adipocytes during cold acclimation, J. Cell Sci 111 ( Pt 17) (1998) 2587–2594. 10.1242/jcs.111.17.2587. [DOI] [PubMed] [Google Scholar]
- [99].Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D, A capsaicin-receptor homologue with a high threshold for noxious heat, Nature. 398 (1999) 436–441. 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- [100].Julius D, TRP channels and pain, Annu. Rev. Cell Dev. Biol 29 (2013) 355–384. 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- [101].Baboota RK, Singh DP, Sarma SM, Kaur J, Sandhir R, Boparai RK, Kondepudi KK, Bishnoi M, Capsaicin Induces “Brite” Phenotype in Differentiating 3T3-L1 Preadipocytes, PLoS ONE. 9 (2014) e103093. 10.1371/journal.pone.0103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Chen J, Li L, Li Y, Liang X, Sun Q, Yu H, Zhong J, Ni Y, Chen J, Zhao Z, Gao P, Wang B, Liu D, Zhu Z, Yan Z, Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx, Cardiovasc. Diabetol. 14 (2015) 22. 10.1186/s12933-015-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Baskaran P, Krishnan V, Ren J, Thyagarajan B, Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel‐dependent mechanisms, British Journal of Pharmacology. 173 (2016) 2369–2389. 10.1111/bph.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Tajima K, Ikeda K, Tanabe Y, Thomson EA, Yoneshiro T, Oguri Y, Ferro MD, Poon ASY, Kajimura S, Wireless optogenetics protects against obesity via stimulation of non-canonical fat thermogenesis, Nat. Commun 11 (2020) 1730. 10.1038/s41467-020-15589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Nayak G, Zhang KX, Vemaraju S, Odaka Y, Buhr ED, Holt-Jones A, Kernodle S, Smith AN, Upton BA, D’Souza S, Zhan JJ, Diaz N, Nguyen M-T, Mukherjee R, Gordon SA, Wu G, Schmidt R, Mei X, Petts NT, Batie M, Rao S, Hogenesch JB, Nakamura T, Sweeney A, Seeley RJ, Van Gelder RN, Sanchez-Gurmaches J, Lang RA, Adaptive Thermogenesis in Mice Is Enhanced by Opsin 3-Dependent Adipocyte Light Sensing, Cell Rep. 30 (2020) 672–686.e8. 10.1016/j.celrep.2019.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Sato M, Tsuji T, Yang K, Ren X, Dreyfuss JM, Huang TL, Wang C-H, Shamsi F, Leiria LO, Lynes MD, Yau K-W, Tseng Y-H, Cell-autonomous light sensitivity via Opsin3 regulates fuel utilization in brown adipocytes, PLoS Biol. 18 (2020) e3000630. 10.1371/journal.pbio.3000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Caron A, Reynolds RP, Castorena CM, Michael NJ, Lee CE, Lee S, Berdeaux R, Scherer PE, Elmquist JK, Adipocyte Gs but not Gi signaling regulates whole-body glucose homeostasis, Mol Metab. 27 (2019) 11–21. 10.1016/j.molmet.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang L, Pydi SP, Zhu L, Barella LF, Cui Y, Gavrilova O, Bence KK, Vernochet C, Wess J, Adipocyte Gi signaling is essential for maintaining whole-body glucose homeostasis and insulin sensitivity, Nat. Commun 11 (2020) 2995. 10.1038/s41467-020-16756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kimura T, Pydi SP, Wang L, Haspula D, Cui Y, Lu H, König GM, Kostenis E, Steinberg GR, Gavrilova O, Wess J, Adipocyte Gq signaling is a regulator of glucose and lipid homeostasis in mice, Nat. Commun 13 (2022) 1652. 10.1038/s41467-022-29231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bedbrook CN, Deverman BE, Gradinaru V, Viral Strategies for Targeting the Central and Peripheral Nervous Systems, Annu. Rev. Neurosci 41 (2018) 323–348. 10.1146/annurev-neuro-080317-062048. [DOI] [PubMed] [Google Scholar]
- [111].Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu W-L, Yang B, Huber N, Pasca SP, Gradinaru V, Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain, Nat. Biotechnol 34 (2016) 204–209. 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu W-L, Sánchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradinaru V, Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems, Nat. Neurosci 20 (2017) 1172–1179. 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Foster MT, Bartness TJ, Sympathetic but not sensory denervation stimulates white adipocyte proliferation, Am. J. Physiol. Regul. Integr. Comp. Physiol 291 (2006) R1630–7. 10.1152/ajpregu.00197.2006. [DOI] [PubMed] [Google Scholar]
- [114].Shi H, Bartness TJ, White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity, Am. J. Physiol. Regul. Integr. Comp. Physiol 289 (2005) R514–R520. 10.1152/ajpregu.00036.2005. [DOI] [PubMed] [Google Scholar]
- [115].Nguyen NLT, Xue B, Bartness TJ, Sensory denervation of inguinal white fat modifies sympathetic outflow to white and brown fat in Siberian hamsters, Physiol. Behav 190 (2018) 28–33. 10.1016/j.physbeh.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Brumovsky PR, Dorsal root ganglion neurons and tyrosine hydroxylase – An intriguing association with implications for sensation and pain, Pain. 157 (2016) 314–320. 10.1097/j.pain.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wehrwein EA, Joyner MJ, Chapter 8 - Regulation of blood pressure by the arterial baroreflex and autonomic nervous system, in: Buijs RM, Swaab DF (Eds.), Handbook of Clinical Neurology, Elsevier, 2013: pp. 89–102. https://www.sciencedirect.com/science/article/pii/B9780444534910000080 (accessed November 28, 2022). [DOI] [PubMed] [Google Scholar]
- [118].Zeng W-Z, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, Patapoutian A, PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex, Science. 362 (2018) 464–467. 10.1126/science.aau6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Morelli C, Castaldi L, Brown SJ, Streich LL, Websdale A, Taberner FJ, Cerreti B, Barenghi A, Blum KM, Sawitzke J, Frank T, Steffens LK, Doleschall B, Serrao J, Ferrarini D, Lechner SG, Prevedel R, Heppenstall PA, Identification of a population of peripheral sensory neurons that regulates blood pressure, Cell Rep. 35 (2021) 109191. 10.1016/j.celrep.2021.109191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Gangula PRR, Zhao H, Supowit SC, Wimalawansa SJ, Dipette DJ, Westlund KN, Gagel RF, Yallampalli C, Increased Blood Pressure in α-Calcitonin Gene–Related Peptide/Calcitonin Gene Knockout Mice, Hypertension. 35 (2000) 470–475. 10.1161/01.HYP.35.1.470. [DOI] [PubMed] [Google Scholar]
- [121].Mai T, Wu J, Diedrich A, Garland EM, Robertson D, Calcitonin Gene Related Peptide (CGRP) in Autonomic Cardiovascular Regulation and Vascular Structure, J Am Soc Hypertens. 8 (2014) 286–296. 10.1016/j.jash.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Chu Y, Huddleston GG, Clancy AN, Harris RBS, Bartness TJ, Epididymal Fat Is Necessary for Spermatogenesis, but not Testosterone Production or Copulatory Behavior, Endocrinology. 151 (2010) 5669–5679. https://doi.org/2020071612321521300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Yang L, Chen L, Lu X, Tan A, Chen Y, Li Y, Peng X, Yuan S, Cai D, Yu Y, Peri-ovarian adipose tissue contributes to intraovarian control during folliculogenesis in mice, Reproduction. 156 (2018) 133–144. 10.1530/REP-18-0120. [DOI] [PubMed] [Google Scholar]
- [124].Chang E, Varghese M, Singer K, Gender and Sex Differences in Adipose Tissue, Curr. Diab. Rep 18 (2018) 69. 10.1007/s11892-018-1031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Yadav RL, Sharma D, Yadav PK, Shah DK, Agrawal K, Khadka R, Islam MN, Somatic neural alterations in non-diabetic obesity: a cross-sectional study, BMC Obes. 3 (2016) 50. 10.1186/s40608-016-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ja W¨nig, Central organization of somatosympathetic reflexes in vasoconstrictor neurones, Brain Research. 87 (1975) 305–312. 10.1016/0006-8993(75)90427-8. [DOI] [PubMed] [Google Scholar]
- [127].Liu S, Wang Z-F, Su Y-S, Ray RS, Jing X-H, Wang Y-Q, Ma Q, Somatotopic Organization and Intensity Dependence in Driving Distinct NPY-Expressing Sympathetic Pathways by Electroacupuncture, Neuron. 108 (2020) 436–450.e7. 10.1016/j.neuron.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Liu S, Wang Z, Su Y, Qi L, Yang W, Fu M, Jing X, Wang Y, Ma Q, A neuroanatomical basis for electroacupuncture to drive the vagal–adrenal axis, Nature. 598 (2021) 641–645. 10.1038/s41586-021-04001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Murphy KT, Schwartz GJ, Nguyen NLT, Mendez JM, Ryu V, Bartness TJ, Leptin-sensitive sensory nerves innervate white fat, Am. J. Physiol. Endocrinol. Metab 304 (2013) E1338–47. 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Garretson JT, Szymanski LA, Schwartz GJ, Xue B, Ryu V, Bartness TJ, Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis, Mol Metab. 5 (2016) 626–634. 10.1016/j.molmet.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Xu S, Yang H, Menon V, Lemire AL, Wang L, Henry FE, Turaga SC, Sternson SM, Behavioral state coding by molecularly defined paraventricular hypothalamic cell type ensembles, Science. 370 (2020) eabb2494. 10.1126/science.abb2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].von Buchholtz LJ, Ghitani N, Lam RM, Licholai JA, Chesler AT, Ryba NJP, Decoding Cellular Mechanisms for Mechanosensory Discrimination, Neuron. 109 (2021) 285–298.e5. 10.1016/j.neuron.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD, Vagal Sensory Neuron Subtypes that Differentially Control Breathing, Cell. 161 (2015) 622–633. 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Prescott SL, Umans BD, Williams EK, Brust RD, Liberles SD, An Airway Protection Program Revealed by Sweeping Genetic Control of Vagal Afferents, Cell. 181 (2020) 574–589.e14. 10.1016/j.cell.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD, Sensory Neurons that Detect Stretch and Nutrients in the Digestive System, Cell. 166 (2016) 209–221. 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Marshall KL, Saade D, Ghitani N, Coombs AM, Szczot M, Keller J, Ogata T, Daou I, Stowers LT, Bönnemann CG, Chesler AT, Patapoutian A, PIEZO2 in sensory neurons and urothelial cells coordinates urination, Nature. 588 (2020) 290–295. 10.1038/s41586-020-2830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chen C, Zhang J, Sun L, Zhang Y, Gan W-B, Tang P, Yang G, Long-term imaging of dorsal root ganglia in awake behaving mice, Nat. Commun 10 (2019) 3087. 10.1038/s41467-019-11158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ju F, Jian W, Han Y, Huang T, Ke J, Liu Z, Cai S, Liu N, Wang L, Wei P, Long-term two-photon imaging of spinal cord in freely behaving mice, (2022). 10.1101/2022.01.09.475306. [DOI] [Google Scholar]


