Abstract
Background:
Astigmatism is the most prevalent refractive error among children and adults, and it can lead to visual impairment if left uncorrected. The management of compound hyperopic astigmatism is more challenging. This study presents the 12-month outcomes of toric implantable collamer lens (ICL) implantation in eyes with hyperopic astigmatism.
Methods:
This interventional case series included patients with simple or compound hyperopic astigmatism who underwent toric ICL implantation. All eligible individuals underwent a detailed ocular examination. Uncorrected and corrected distance visual acuities (UDVA and CDVA, respectively), intraocular pressure, and manifest and cycloplegic refraction results were documented. Pentacam corneal tomography was performed to assess the central corneal thickness, iridocorneal angle width, and anterior chamber depth. Endothelioscopy was performed to determine endothelial cell density. The ICL V4b model was implanted in all the included eyes. Safety and efficacy indices were calculated as postoperative CDVA/preoperative CDVA and postoperative UDVA/preoperative CDVA, respectively.
Results:
Twenty-six eyes with low-grade simple or compound hyperopic astigmatism were included. All eyes experienced a significant improvement of four lines in postoperative UDVA (P < 0.001), and their postoperative CDVA remained stable at the 12-month follow-up (P > 0.05). The safety and efficacy indices were 1.0. None of the eyes lost two or more lines of CDVA; in 81% of the eyes, CDVA was unchanged, and the proportion of eyes with 20/30 or better postoperative UDVA was identical to that with 20/30 or better preoperative CDVA (81% for each). The mean manifest spherical equivalent at the 12-month postoperative visit had significantly improved (P < 0.001). The percentages of eyes with postoperative spherical equivalent within ± 0.50 D and ± 1.00 D were 81% and 96%, respectively. The postoperative refractive cylinder improved significantly (P < 0.05), and the percentage of eyes with refractive cylinder within ± 0.50 DC and ± 1.00 DC were 50% and 77%, respectively.
Conclusions:
Our outcomes indicate that toric ICL implantation is safe and effective for managing low-grade simple or compound hyperopic astigmatism. The proportion of eyes with 20/30 or better postoperative UDVA was identical to that with a 20/30 or better preoperative CDVA. The manifest spherical equivalent and refractive cylinder were significantly reduced. No serious safety concerns were observed. Further prospective large-scale studies with a wide range of ages and grades of hyperopic astigmatism are required to verify these preliminary outcomes.
Key Words: astigmatism, hyperopic astigmatism, phakic intraocular lenses, intraocular collamer lens, compound hyperopic astigmatism, ocular refraction
INTRODUCTION
Complications related to uncorrected refractive errors are responsible for a global financial burden, annually [1]. Astigmatism is the most prevalent refractive error among children and adults [2] and may lead to visual impairment if uncorrected [3]. The management of compound myopic or hyperopic astigmatism is more challenging [1-3]. Although corrective lenses are a feasible treatment option for myopic and hyperopic astigmatism, they are associated with several complications. Therefore, alternative treatment modalities, such as keratorefractive surgery or intraocular lens implantation, have been introduced [4-9].
Keratorefractive surgery using an excimer laser is a common procedure for correcting refractive errors. However, corneal thickness and a high degree of astigmatism restrict its use, and it has limitations in correcting a high degree of ametropia [4-6]. Phakic intraocular lens implantation for refractive correction has several advantages over keratorefractive procedures, and the advantages are more pronounced for high degrees of ametropia. These include better contrast sensitivity, retinal image magnification, and a lower rate of surgically induced optical aberrations [7-9]. Phakic intraocular implants are safe and effective in correcting hyperopia. Preservation of accommodation is a distinct advantage over other treatment modalities such as clear lens extraction [10]. An implantable collamer lens (ICL) is commonly used to correct refractive errors because its material is safe and well tolerated. Its long-term safety, efficacy, and predictability were established in a 10-year follow-up study [11].
Although many studies have evaluated the efficacy, safety, and predictability of intraocular lenses in correcting myopia [12-14], hyperopia [10, 11, 15, 16], and myopic astigmatism [17-20], to our knowledge, only one study has reported the results of surgical correction of hyperopic astigmatism using the toric ICL. The findings of that study indicated that hyperopic toric ICL implantation is safe and yields stable refractive outcomes in patients with high hyperopia and astigmatism [21].
Our study aimed to present one-year outcomes of toric ICL implantation in eyes with simple or compound hyperopic astigmatism.
METHODS
This interventional case series analyzed the medical records of patients with simple or compound hyperopic astigmatism who underwent refractive surgery with toric ICL implantation at a tertiary eye hospital in the eastern province of Saudi Arabia from January 2017 to March 2018. This study was approved by the Institutional Research and Ethics Committee of Dhahran Eye Specialist Hospital, Aljamiah District, Dhahran, Saudi Arabia. The study protocol complied with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants prior to the surgical procedure.
Patients aged ≥ 21 years with stable refraction for at least one year, an endothelial count > 2200 cells/mm2, iridocorneal angle width of ≥ 30°, normal pupillary reflexes without iris anomalies, mesopic pupil size of ≤ 6 mm, and anterior chamber depth (ACD) of ≥ 3 mm were included. Eyes with unstable refraction, irregular corneal topography, active corneal disease, cataract, chronic or recurrent uveitis, ocular hypertension, glaucoma, active or old retinal entities, previous intraocular or corneal surgery, history of ocular trauma, and those with active systemic diseases such as collagen vascular disease, autoimmune disorders, or allergies were excluded.
The detailed medical histories and current medical statuses of all participants were recorded. All eligible individuals underwent detailed ocular examinations, including orthoptic assessment, uncorrected and corrected distance visual acuities (UDVA and CDVA, respectively) measured using a Snellen chart (Good-Lite Company, Elgin, Illinois, USA), anterior and posterior segment slit-lamp (Haag-Streit Photo-Slit Lamp BX 900, Haag-Streit, Koeniz, Switzerland) examinations, and intraocular pressure (IOP) measurement using Goldmann applanation tonometry (Haag-Streit).
The decision to perform additional imaging studies such as fluorescein angiography and visual field tests was left to the discretion of the ophthalmologist after the initial detailed ocular examination. Objective manifest refraction with spherical equivalent and refractive cylinder using autorefractometry (Topcon KR-800, Topcon Medical Systems, Oakland, NJ, USA) with subjective refinement, cycloplegic refraction using cyclopentolate hydrochloride 1% (Cyclogyl, Alcon Laboratories, Fort Worth, TX, USA), and measurement of best spectacle-corrected visual acuity with a vertex distance of 12.0 mm (soft contact lens of known power and curvature was used in some cases) were performed.
Automated keratometry (Topcon KR-800, Topcon Medical Systems), ultrasound corneal pachymetry, photopic and scotopic pupil diameter measurement, Orbscan (Orbscan IIz, Bausch & Lomb, Rochester, NY, USA), and Pentacam corneal tomography (Oculus Pentacam HR, Oculus Optikgeräte GmbH, Wetzlar, Germany) to assess the central corneal thickness (CCT), iridocorneal angle width, ACD, and distance from the corneal apex to the limbus were performed. Endothelioscopy (Konan Noncon Robo SP8000, Konan Medical, Irvine, CA, USA) was performed to determine the endothelial cell density (ECD) and morphology of the inner corneal layer. ACD was also measured using optical coherence tomography (OCT) (Cirrus OCT, Carl Zeiss Meditec, Dublin, CA, USA).
The ICL V4b model (Visian ICL™, STAAR Surgical Inc., Monrovia, CA, USA) was implanted in all included eyes. The ICL power was calculated using the STAAR Surgical Customer Service Department formula, which uses ACD, central corneal pachymetry, mean corneal keratometry reading or simulated keratometry values, horizontal white-to-white (WTW) diameter from caliper measurements, and refraction results 12.0 mm from the corneal vertex or with a contact lens.
On the day of surgery, patients were administered dilating and cycloplegic drops. The horizontal axis of each eye was marked with the patient in sitting position; using a surgical marker, two dots were placed on the corneal-limbal area indicating 0 and 180 meridians as a reference for later toric ICL alignment. All surgeries were performed under topical anesthesia using a standard surgical technique with standard surgical precautions. Two paracentesis incisions were made, and the anterior segment was filled with an ophthalmic viscosurgical device. A 3.2-mm clear corneal temporal incision was made. The ICL V4b was inserted using a cartridge, and the footplates were placed in the retropupillary position, followed by lens rotation to the desired axis position. The viscoelastic material was removed by irrigation cannula, and intracameral 1% acetylcholine chloride (Miochol®-E, Bausch & Lomb) was injected to induce miosis. The corneal wounds were secured via stromal hydration. Peripheral iridectomy was undertaken using a neodymium:yttrium–aluminum–garnet (Nd:YAG) laser [21] one week before surgery, or surgically during the operation. Acetazolamide (250 mg, Diamox) was administered two hours postoperatively. All eyes received 1% prednisolone acetate (Pred-Forte®; Allergan, Irvine, CA, USA) and 0.5% moxifloxacin hydrochloride (Vigamox; Alcon Laboratories) eye drops four times daily for two weeks. The eyes were evaluated regularly according to the postoperative schedule. The baseline and 12-month postoperative records were retrieved for analysis.
If UDVA at the 12-month follow-up was 20/40 or better, the outcome was considered successful. If intra- or postoperative complications could not be managed, resulting in explantation of the implant, the outcome was considered a complete failure. If the complications were minor and could be managed without surgery, the outcome was considered partially safe. If there were no complications except a residual refractive error of ± 0.5 D, without symptoms of asthenia, the procedure was considered safe. At the 12-month follow-up examination, the safety and efficacy indices were calculated as postoperative CDVA/preoperative CDVA and postoperative UDVA/preoperative CDVA, respectively [12, 21].
Statistical analyses were performed using IBM SPSS for Windows (version 22; IBM Corp., Armonk, NY, USA). Normality of the data distribution was assessed using the Shapiro–Wilk test. A paired t-test was used to compare preoperative and postoperative measurements, and the means (standard deviations [SDs]) of the data are reported. Pearson’s correlation was used to determine correlations between parameters. Non-normally distributed data were compared using the Wilcoxon signed-rank test for the preoperative versus postoperative visits, and the median and range of data are reported. Spearman’s rho correlation was used to assess correlation between parameters. The IOPs at different follow-up visits were compared using Friedman’s test and post-hoc analysis. P-values < 0.05 were considered statistically significant.
RESULTS
Twenty-six eyes of 13 consecutive patients (6 men [46.2%] and 7 women [53.8%]) with a median (range) age of 21.0 (21.0–25.3) years were included. Table 1 summarizes the preoperative and 12-month postoperative parameters of the included eyes. The postoperative UDVA demonstrated a significant improvement of four lines (P < 0.001) compared with the preoperative UDVA, and the postoperative CDVA remained stable (P > 0.05) compared with the preoperative value (Table 1). The safety and efficacy indices were 1.0 (1.0 – 1.0) and 1.0 (0.74 – 1.0), respectively.
Table 1.
Preoperative and 12-month postoperative parameters in included eyes
| Parameter | Preoperative | Postoperative | P -value |
|---|---|---|---|
| UDVA (logMAR), Median (Range) | 0.52 (0.30 to 0.70) | 0.13 (0.00 to 0.22) | < 0.001 |
| CDVA (logMAR), Median (Range) | 0.00 (0.00 to 0.15) | 0.00 (0.00 to 0.22) | 0.115 |
| Spherical equivalent (D), Mean ± SD | + 5.14 ± 2.21 | - 0.20 ± 0.54 | < 0.001 |
| Refractive cylinder (DC), Median (Range) | - 1.38 (- 2.00 to - 0.75) | - 0.63 (-1.13 to - 0.25) | 0.001 |
| IOP (mmHg), Mean ± SD | 16.38 ± 3.16 | 13.21 ± 2.67 | < 0.001 |
| ECD (cell/mm 2 ), Median (Range) | 2870 (2656 to 2942) | 2624 (2528 to 2846) | < 0.001 |
| Angle wide using Pentacam (degree), Mean ± SD | 39.74 ± 8.67 | 28.72 ± 4.08 | < 0.001 |
| ACD using Pentacam (mm), Mean ± SD | 3.28 ± 0.28 | 3.02 ± 0.33 | 0.002 |
Abbreviations: UDVA, uncorrected distance visual acuity; logMAR, logarithm of the minimum angle of resolution; CDVA, corrected distance visual acuity; D, diopter; DC, diopters cylinder; IOP, intraocular pressure; mmHg, millimeter of mercury; ECD, endothelial cell density; cell/mm 2 , cells per millimeter square; ACD, anterior chamber depth; mm, millimeter. Note: P -values < 0.05 are shown in bold; Spherical equivalent was calculated by adding the sum of the spherical component of manifest refraction with half of the cylindrical component of manifest refraction.
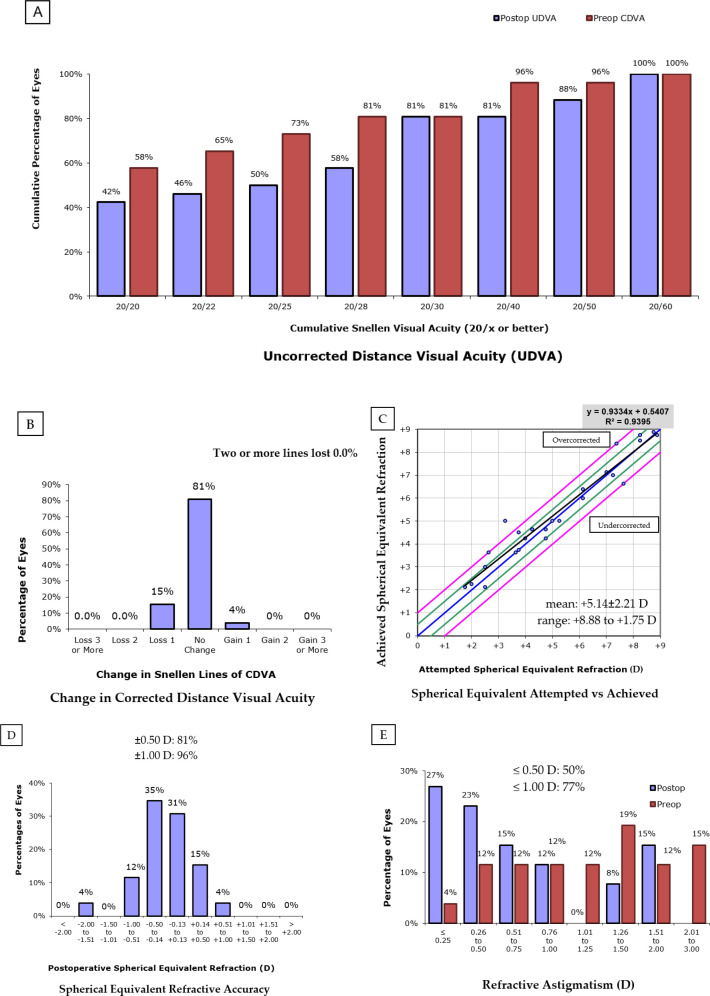
Figure 1A shows the cumulative preoperative CDVA and the UDVA at the 12-month postoperative visit. When comparing the proportion of eyes with a visual acuity of 20/20, 58% of eyes had this level of CDVA before ICL implantation, compared to 42% of eyes with this level of UDVA at the 12-month postoperative visit. Similarly, at the 12-month postoperative examination, 46% of the eyes had 20/22 or better UDVA compared with 65% CDVA at baseline, 50% had 20/25 or better UDVA compared with 73% CDVA at baseline, and 58% had 20/28 or better UDVA compared with 81% CDVA at baseline. The proportion of eyes with 20/30 or better UDVA at the 12-month visit was identical to that with 20/30 or better CDVA at baseline (81% for each). Figure 1B illustrates the CDVA change in Snellen lines. As shown in this figure, at the 12-month postoperative visit, none of the eyes had lost two or more lines of CDVA, and in 81% of the eyes, the CDVA was unchanged.
Figure 1 (A-E).
Changes between preoperative (red color) and 12-month postoperative (blue color) uncorrected distance visual acuity (UDVA)/corrected distance visual acuity (CDVA), spherical equivalent, and refractive cylinder in 26 included eyes.
The mean (SD) preoperative manifest and cycloplegic spherical equivalents were + 5.14 (2.21) D and + 5.88 (2.50) D, respectively, with a mean (SD) latent hyperopia of + 0.74 (1.03) D. The mean (SD) manifest spherical equivalent at the 12-month postoperative visit was - 0.20 (0.54) D (Table 1), indicating that the ICL implantation caused a myopic shift of 5.34 (2.13) D. The predictability of the ICL implantation procedure was analyzed as shown in Figure 1C. The horizontal axis represents the attempted change, and the vertical axis represents the achieved change in the spherical equivalent of refraction. The diagonal line indicates the optimum cases for such attempted and achieved corrections, with green and red diagonal lines indicating the - 0.50 to + 0.50 D and - 1.00 to + 1.00 D residual refraction bands, respectively. As shown in this figure, the precision of the spherical equivalent according to the linear correlation of the attempted versus achieved spherical equivalents had an R2 of 0.9395, with a small undercorrection according to the linear equation factor of 0.9334. The percentages of eyes with postoperative spherical equivalent within ± 0.50 D and ± 1.00 D were 81% and 96%, respectively (Figure 1D).
The median (range) preoperative and postoperative refractive cylinders were -1.38 (- 2.00 to - 0.75) DC and - 0.63 (- 1.13 to - 0.25) DC, respectively, indicating a significant improvement (P = 0.001). Figure 1E illustrates that the percentages of eyes with refractive cylinder within ± 0.50 DC and ± 1.00 DC were 50% and 77%, respectively.
The mean (SD) angle width measured using the Pentacam decreased significantly from 39.74° (8.67°) to 28.72° (4.08°) (P < 0.001). The mean postoperative ACD measured by Pentacam decreased significantly by a value of 0.26 mm compared with the preoperative value (P < 0.05) (Table 1). The mean (SD) 12-month postoperative ACD measured by OCT was 2.15 (0.23) mm, which was shallower than that measured by Pentacam (3.02 [0.33] mm). The mean (SD) WTW distance measured using calipers was 12.34 (0.49) mm. Postoperative ECD was significantly decreased compared with the preoperative value (P < 0.001) (Table 1), with a 6% cell loss. The mean (SD) CCT at the 12-month postoperative visit was 561.7 (42.8) µm. There was a 3.2 mmHg decrease in IOP at the 12-month postoperative visit compared to baseline (P < 0.001) (Table 1). Compared with baseline, the mean (SD) IOP was unchanged at the one-day postoperative visit (P > 0.99), then decreased insignificantly to 15.20 mmHg at six months (P = 0.519), and further significantly decreased to 13.21 mmHg at 12 months (P < 0.001). There was no significant difference in the mean IOP between the one-day and six-month postoperative visits (P > 0.99); however, at 12 months, it was significantly lower than at day 1 (P = 0.004) and six months (P = 0.003).
The mean (SD) vault using OCT at 12 months after ICL implantation was 0.35 (0.10) mm, with no significant correlation with the vault measured using a slit-lamp (r = - 0.28; P = 0.163). Significant correlations were detected between baseline WTW and ECD loss (r = - 0.51; P = 0.008); between final vault and baseline manifest spherical equivalent (r = - 0.50; P = 0.009); between age and baseline WTW (r = + 0.50; P = 0.009), baseline ECD (r = - 0.58; P = 0.002), and baseline CCT (r = - 0.55; P = 0.003); and between baseline manifest spherical equivalent and baseline ACD (r = + 0.43; P = 0.027). The other parameters were not significantly correlated.
Slit-lamp examination revealed two eyes (7.7%) with subepithelial infiltrates, which resolved at the last follow-up, and one eye with a high vault (3.8%). Dilated fundus examination revealed choroidal nevus in one eye (3.8%). All ICL implants were in position, and no cataracts were detected throughout the 12-month follow-up. The peripheral iridotomy was not patent in one eye (3.8%), with a high IOP detected, was partially patent in three eyes (11.5%), and was patent in 22 eyes (84.6%). Augmentation was performed using an Nd:YAG laser in four eyes with non-patent or partially patent peripheral iridotomy.
DISCUSSION
Our participants with low-grade simple or compound hyperopic astigmatism experienced a significant improvement of four lines in postoperative UDVA, and their postoperative CDVA remained stable at the 12-month follow-up. The safety and efficacy indices were 1.0. At the 12-month postoperative visit, none of the eyes had lost two or more lines of CDVA; in 81% of eyes, CDVA was unchanged, and the proportion of eyes with 20/30 or better postoperative UDVA was identical to the proportion with 20/30 or better preoperative CDVA. The mean manifest spherical equivalent at the 12-month postoperative visit revealing a significant improvement and indicating that the ICL implantation caused a mean myopic shift of 5.34 D. The predictability of the ICL implantation procedure was analyzed, and the precision of the spherical equivalent according to the linear correlation of the attempted versus achieved spherical equivalents had an R2 of 0.9395, with a small undercorrection according to the linear equation factor of 0.9334. The percentage of eyes with postoperative spherical equivalents within ± 0.50 D and ± 1.00 D were 81% and 96%, respectively. The postoperative refractive cylinder improved significantly, and the percentages of eyes with refractive cylinder within ± 0.50 DC and ± 1.00 DC were 50% and 77%, respectively. The mean angle width and ACD by Pentacam decreased significantly, and the mean 12-month postoperative ACD by OCT was shallower than that by Pentacam. However, compared with the mean IOP at baseline, IOP was unchanged at the one-day postoperative visit, then decreased insignificantly to 15.20 mmHg at six months, and further decreased significantly to 13.21 mmHg at 12 months. There was no significant difference in the mean IOP between the one-day and six-month postoperative visits; however, at 12 months, it was significantly lower than at the one-day and six-month postoperative visits. Postoperative ECD showed a significant reduction, with a 6% cell loss. A significant moderate inverse correlation was detected between baseline WTW and ECD loss, between baseline ECD or CCT and age, and between final vault and baseline manifest spherical equivalent, whereas a significant moderate direct correlation was found between age and baseline WTW and between baseline manifest spherical equivalent and baseline ACD. All ICLs were in position, and no cataracts were detected at the one-year follow-up. Augmentation was performed using an Nd:YAG laser in four eyes with non-patent or partially patent peripheral iridotomy.
Bartels et al. [16] found that the Artisan toric phakic intraocular lens could effectively correct moderate to high degrees of hyperopia combined with astigmatism in a mean (range) follow-up of 11.1 (6–36) months. No serious complications were observed during the study [16]. Sanders et al. [17] conducted a prospective nonrandomized clinical trial on the efficacy of toric ICLs for treating moderate to high myopic astigmatism and proved the safety, efficacy, and predictability of this phakic IOL over a 12-month period. The proportion of eyes with postoperative UDVA of 20/20 or better was equal to the proportion of eyes with preoperative best-corrected spherical visual acuity of 20/20 or better (83.1% for each) [17]. Guo et al. [18] investigated the efficacy of toric posterior chamber intraocular lens implantation after phacoemulsification in eyes with high myopia versus those with emmetropia or low myopia. The study revealed equal efficacy in astigmatism correction and similar rotational stability between the two eye groups. Visual acuity improved significantly in both groups during a short-term follow-up of three months [18]. The outcomes of these studies [16-18], along with our findings, may indicate the safety and efficacy of toric intraocular lens implantation in managing astigmatism.
Coskunseven et al. [21] followed 20 eyes with hyperopic astigmatism with a mean (SD, range) astigmatism of - 1.44 (0.88, - 3.25 to 0.00) DC who were treated using Visian toric ICL implantation. At the 12-month postoperative visit, none of the eyes lost two or more lines of CDVA; in 50% of the eyes, CDVA was unchanged, and the proportion of eyes with 20/33 or better postoperative UDVA was identical to that with 20/33 or better preoperative CDVA (70% for each). No cataract formation or complications related to the IOP were observed at the 12-month postoperative visit [21]. The precision of spherical equivalent according to the linear correlation of the attempted versus achieved spherical equivalents had an R2 of 0.75912, with a small undercorrection according to the linear equation factor of 0.8236. The percentage of eyes with postoperative spherical equivalent between ± 0.50 D and ± 1.00 D were 35% and 70%, respectively. The postoperative refractive cylinder improved significantly, and the percentage of eyes with refractive cylinder between ± 0.50 DC and ±1.00 DC were 70% and 85%, respectively. This study [21] is one of the few that are similar to ours. However, the outcomes differed to some extent. Our study demonstrated that toric ICL implantation was safe at the 12-month postoperative follow-up and was an effective method for providing stable refractive outcomes in patients with hyperopia and astigmatism, with the added value of finding a significant inverse correlation between baseline WTW and ECD loss.
In a review by Kohnen et al. on phakic intraocular lenses, this treatment modality was reported to have good outcomes if the eyes had proper anatomical characteristics [22]. Therefore, we elected to implant ICLs in eyes with low grade simple or compound hyperopic astigmatism with stable refraction for at least one year, an endothelial count > 2200 cells/mm2, iridocorneal angle width of ≥ 30°, normal pupillary reflexes without iris anomalies, mesopic pupil size of ≤ 6 mm, and ACD of ≥ 3 mm, and the one-year outcomes of ICL implantation revealed safety and effectiveness in these individuals.
Saxena et al. [23] found a significant negative correlation between ACD and ECD loss during long-term follow-up after implantation of an Artisan phakic intraocular lens; however, the patient age was not significantly correlated with ECD loss [23]. In the current study, the baseline ACD had no significant correlation with ECD loss (r = - 0.27; P = 0.184) at the 12-month follow-up. This observed difference could be due to the different follow-up periods, as Saxena et al. [23] reported an initial non-significant increase in ECD at the one-year postoperative visit and a subsequent significant ECD loss from three years onward. Furthermore, they included myopic eyes with ACD > 2.6 mm and ECD > 2000 cells/mm2 [23], which differs from our inclusion criteria.
The safety and efficacy index values in our study were equal to 1, indicating that this procedure is safe and effective for managing low-grade simple or compound hyperopic astigmatism. Cano-Ortiz et al. [24] conducted a retrospective study of 126 eyes with low- or high-grade astigmatism according to the implanted ICL power. The safety and efficacy indices for low astigmatism were 1.09 ± 0.16 and 1.05 ± 0.17, respectively, and for high astigmatism were 1.11 ± 0.17 and 1.06 ± 0.16, respectively. This indicates safety and efficacy of toric ICL implantation regardless of the degree of astigmatism. Cano-Ortiz et al. [24] obtained R2 values of 0.9998 and 0.998 for low- and high-grade astigmatism, respectively. Similarly, in the current study, eyes with low-grade hyperopic astigmatism had an R2 value of 0.9395. These findings indicate the predictability of ICL implantation in both studies.
In this study, at the 12-month postoperative refraction, the percentage of eyes with postoperative spherical equivalent between ± 0.50 D and ± 1.00 D were 81% and 96%, respectively. At the six-month postoperative refraction, Cano-Ortiz et al. [24] found that the percentage of eyes with postoperative spherical equivalent within ± 0.50 D and ± 1.00 D were 98% and 100% in all included eyes with low- and high-grade astigmatism [24]. None of the eyes lost two or more lines of CDVA, 15% lost one line of CDVA, 4% gained one line of CDVA, and in 81% of the eyes, CDVA was unchanged. Cano-Ortiz et al. [24] observed no loss in lines of CDVA, and in 65.9% of eyes, CDVA was unchanged; however, they detected one line and two or more lines of gain in CDVA in 27.0% and 7.1% of eyes, respectively.
The novelty of the current study, which focused on eyes with low-grade simple or compound hyperopic astigmatism, is considerable; however, it has certain limitations. The limited number of eyes included, retrospective design, short-term follow-up, and narrow age range may limit the generalizability of our outcomes. Although the toric ICL implantation procedure was safe in eyes with low-grade hyperopic astigmatism, as seen at the 12-month follow-up, its long-term safety should be ascertained through additional studies with longer follow-up. By introducing artificial intelligence and big data analytics, the ICL vault and sizing prediction has significantly improved. In addition, artificial intelligence-based data were found to have significantly higher predictability than conventional manufacturer’s nomograms [25, 26]. Further studies using big data on preoperative and postoperative ECD in ICL-implanted eyes may enable clinicians to predict the loss of ECD in their practice.
CONCLUSIONS
Our safety and efficacy indices indicate that toric ICL implantation is safe and effective for managing low-grade simple or compound hyperopic astigmatism. None of the eyes lost two or more lines of CDVA; in 81% of the eyes, CDVA was unchanged, and the proportion of eyes with 20/30 or better postoperative UDVA was identical to the proportion with 20/30 or better preoperative CDVA at baseline (81% for each). The manifest spherical equivalent and refractive cylinder were significantly reduced. Despite the significantly narrow anterior chamber angle and shallow ACD, a significant reduction in IOP was observed during the one-year follow-up period. Further prospective large-scale studies with a wide range of ages and grades of hyperopic astigmatism are required to verify these preliminary outcomes.
ETHICAL DECLARATIONS
Ethical approval:
This study was approved by the Institutional Research and Ethics Committee of Dhahran Eye Specialist Hospital, Aljamiah District, Dhahran, Saudi Arabia. The study protocol complied with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants prior to the surgical procedure.
Conflict of interest:
None.
FUNDING
None.
ACKNOWLEDGMENTS
None.
References
- 1.Smith TS, Frick KD, Holden BA, Fricke TR, Naidoo KS. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull World Health Organ. 2009 Jun;87(6):431–7. doi: 10.2471/BLT.08.055673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashemi H, Fotouhi A, Yekta A, Pakzad R, Ostadimoghaddam H, Khabazkhoob M. Global and regional estimates of prevalence of refractive errors: Systematic review and meta-analysis. J Curr Ophthalmol. 2017 Sep;30(1):3–22. doi: 10.1016/j.joco.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naidoo KS, Leasher J, Bourne RR, Flaxman SR, Jonas JB, Keeffe J, Limburg H, Pesudovs K, Price H, White RA, Wong TY, Taylor HR, Resnikoff S. Vision Loss Expert Group of the Global Burden of Disease Study Global Vision Impairment and Blindness Due to Uncorrected Refractive Error 1990-2010. Optom Vis Sci. 2016 Mar;93(3):227–34. doi: 10.1097/OPX.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 4.Kim TI, Alió Del Barrio JL, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. 2019 May;393(10185):2085–2098. doi: 10.1016/S0140-6736(18)33209-4. [DOI] [PubMed] [Google Scholar]
- 5.Gyldenkerne A, Ivarsen AR, Hjortdal JØ. Factors affecting the decision for refractive surgery in patients with high degrees of ametropia. J Cataract Refract Surg. 2014 Aug;40(8):1371–6. doi: 10.1016/j.jcrs.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 6.Marino GK, Santhiago MR, Wilson SE. Femtosecond Lasers and Corneal Surgical Procedures. Asia Pac J Ophthalmol (Phila). 2017 Sep-Oct;6(5):456–464. doi: 10.22608/APO.2017163. [DOI] [PubMed] [Google Scholar]
- 7.El Danasoury MA, El Maghraby A, Gamali TO. Comparison of iris-fixed Artisan lens implantation with excimer laser in situ keratomileusis in correcting myopia between -9 00 and -19 50 diopters: a randomized study. Ophthalmology. 2002 May;109(5):955–64. doi: 10.1016/s0161-6420(02)00964-8. [DOI] [PubMed] [Google Scholar]
- 8.Sanders DR. Matched population comparison of the Visian Implantable Collamer Lens and standard LASIK for myopia of -3 00 to -7 88 diopters. J Refract Surg. 2007 Jun;23(6):537–53. doi: 10.3928/1081-597X-20070601-02. [DOI] [PubMed] [Google Scholar]
- 9.Schallhorn S, Tanzer D, Sanders DR, Sanders ML. Randomized prospective comparison of visian toric implantable collamer lens and conventional photorefractive keratectomy for moderate to high myopic astigmatism. J Refract Surg. 2007 Nov;23(9):853–67. doi: 10.3928/1081-597X-20071101-01. [DOI] [PubMed] [Google Scholar]
- 10.Alshamrani AA, Alharbi SS. Phakic intraocular lens implantation for the correction of hyperopia. J Cataract Refract Surg. 2019 Oct;45(10):1503–1511. doi: 10.1016/j.jcrs.2019.05.051. [DOI] [PubMed] [Google Scholar]
- 11.Pesando PM, Ghiringhello MP, Di Meglio G, Fanton G. Posterior chamber phakic intraocular lens (ICL) for hyperopia: ten-year follow-up. J Cataract Refract Surg. 2007 Sep;33(9):1579–84. doi: 10.1016/j.jcrs.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Huang D, Schallhorn SC, Sugar A, Farjo AA, Majmudar PA, Trattler WB, Tanzer DJ. Phakic intraocular lens implantation for the correction of myopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2009 Nov;116(11):2244–58. doi: 10.1016/j.ophtha.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Silva RA, Jain A, Manche EE. Prospective long-term evaluation of the efficacy, safety, and stability of the phakic intraocular lens for high myopia. Arch Ophthalmol. 2008 Jun;126(6):775–81. doi: 10.1001/archopht.126.6.775. [DOI] [PubMed] [Google Scholar]
- 14.Leccisotti A, Fields SV. Clinical results of ZSAL-4 angle-supported phakic intraocular lenses in 190 myopic eyes. J Cataract Refract Surg. 2005 Feb;31(2):318–23. doi: 10.1016/j.jcrs.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 15.Koivula A, Zetterström C. Phakic intraocular lens for the correction of hyperopia. J Cataract Refract Surg. 2009 Feb;35(2):248–55. doi: 10.1016/j.jcrs.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Bartels MC, Santana NT, Budo C, van Rij G, Mulder PG, Luyten GP. Toric phakic intraocular lens for the correction of hyperopia and astigmatism. J Cataract Refract Surg. 2006 Feb;32(2):243–9. doi: 10.1016/j.jcrs.2005.12.083. [DOI] [PubMed] [Google Scholar]
- 17.Sanders DR, Schneider D, Martin R, Brown D, Dulaney D, Vukich J, Slade S, Schallhorn S. Toric Implantable Collamer Lens for moderate to high myopic astigmatism. Ophthalmology. 2007 Jan;114(1):54–61. doi: 10.1016/j.ophtha.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Guo T, Gao P, Fang L, Guo L, Fan Y, Liu C. Efficacy of Toric intraocular lens implantation in eyes with high myopia: A prospective, case-controlled observational study. Exp Ther Med. 2018 Jun;15(6):5288–5294. doi: 10.3892/etm.2018.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimbel HV, Ziémba SL. Management of myopic astigmatism with phakic intraocular lens implantation. J Cataract Refract Surg. 2002 May;28(5):883–6. doi: 10.1016/s0886-3350(01)01098-7. [DOI] [PubMed] [Google Scholar]
- 20.Zheng LY, Zhu SQ, Su YF, Zou HY, Wang QM, Yu AY. Comparison between toric and spherical phakic intraocular lenses combined with astigmatic keratotomy for high myopic astigmatism. Eye Vis (Lond). 2017 Aug;:4–20. doi: 10.1186/s40662-017-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coskunseven E, Kavadarli I, Sahin O, Kayhan B, Pallikaris I. Refractive Outcomes of 20 Eyes Undergoing ICL Implantation for Correction of Hyperopic Astigmatism. J Refract Surg. 2017 Sep;33(9):604–609. doi: 10.3928/1081597X-20170504-06. [DOI] [PubMed] [Google Scholar]
- 22.Kohnen T, Kook D, Morral M, Güell JL. Phakic intraocular lenses: part 2: results and complications. J Cataract Refract Surg. 2010 Dec;36(12):2168–94. doi: 10.1016/j.jcrs.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Saxena R, Boekhoorn SS, Mulder PG, Noordzij B, van Rij G, Luyten GP. Long-term follow-up of endothelial cell change after Artisan phakic intraocular lens implantation. Ophthalmology. 2008 Apr;115(4):608–613. doi: 10.1016/j.ophtha.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 24.Cano-Ortiz A, Sánchez-Ventosa Á, Membrillo A, Castillo R, Gomera A, López-Pérez MD, Villarrubia A. Astigmatism correction with toric implantable collamer lens in low and high astigmatism groups. Eur J Ophthalmol. 2022 Jan;32(1):183–192. doi: 10.1177/1120672121999991. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya K, Ryu IH, Yoo TK, Kim JS, Lee IS, Kim JK, Ando W, Shoji N, Yamauchi T, Tabuchi H. Prediction of Phakic Intraocular Lens Vault Using Machine Learning of Anterior Segment Optical Coherence Tomography Metrics. Am J Ophthalmol. 2021 Jun;:226:90–99. doi: 10.1016/j.ajo.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Shen Y, Wang L, Jian W, Shang J, Wang X, Ju L, Li M, Zhao J, Chen X, Ge Z, Wang X, Zhou X. Big-data and artificial-intelligence-assisted vault prediction and EVO-ICL size selection for myopia correction. Br J Ophthalmol. 2023 Feb;107(2):201–206. doi: 10.1136/bjophthalmol-2021-319618. [DOI] [PMC free article] [PubMed] [Google Scholar]