Abstract
Background
Shoulder dysfunction is a common problem in patients treated for head and neck cancer. Both neck dissections and radiotherapy can cause morbidity to the shoulder joint. Exercise interventions have been suggested as a treatment option for this population.
Objectives
To evaluate the effectiveness and safety of exercise interventions for the treatment of shoulder dysfunction caused by the treatment of head and neck cancer.
Search methods
We searched the Cochrane ENT Group Trials Register; CENTRAL; PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; ISRCTN and additional sources for published and unpublished trials. The date of the search was 7 July 2011.
Selection criteria
Randomized controlled trials (RCTs) comparing any type of exercise therapy compared with any other intervention in patients with shoulder dysfunction due to treatment of head and neck cancer.
Data collection and analysis
Two review authors independently selected trials, assessed risk of bias and extracted data from studies. We contacted study authors for information not provided in the published articles.
Main results
Three trials involving 104 people were included. We classified one study as having low risk of bias; the others had some limitations and we classified them as having high risk of bias.
Two studies (one with low risk of bias and the other with high risk of bias) applied progressive resistance training (PRT) combined with range of motion exercises and stretching; the comparison group received standard care. Pooled data demonstrated that PRT can improve shoulder pain (mean difference (MD) ‐6.26; 95% confidence interval (CI) ‐12.20 to ‐0.31) and shoulder disability (MD ‐8.48; 95% CI ‐15.07 to ‐1.88), both measured using the Shoulder Pain and Disability Index (SPADI) (range 0 to 100). Similarly, secondary outcomes were also improved: active range of motion for external rotation (MD 14.51 degrees; 95% CI 7.87 to 21.14), passive range of motion for abduction (MD 7.65 degrees; 95% CI 0.64 to 14.66), forward flexion (MD 6.20 degrees; 95% CI 0.69 to 11.71), external rotation (MD 7.17 degrees; 95% CI 2.20 to 12.14) and horizontal abduction (MD 7.34 degrees; 95% CI 2.86 to 11.83). Strength and resistance of scapular muscles was assessed in one study and the results showed a statistically significant benefit of PRT. The studies did not demonstrate a statistically significant difference in quality of life. Only two non‐serious adverse events were described in the PRT group compared with none in the standard care group.
One study with high risk of bias used a broad spectrum of techniques including free active exercises, stretching and postural care for a period of three months following surgery. This study did not demonstrate a difference between the exercise group and routine postoperative physiotherapy care in shoulder function and quality of life, but serious methodological limitations could explain this. No serious adverse events were reported.
Authors' conclusions
Limited evidence from two RCTs demonstrated that PRT is more effective than standard physiotherapy treatment for shoulder dysfunction in patients treated for head and neck cancer, improving pain, disability and range of motion of the shoulder joint, but it does not improve quality of life. However, although statistically significant the measured benefits of the intervention may be small. Other exercise regimes were not shown to be effective compared to routine postoperative physiotherapy. Further studies which apply other exercise interventions in head and neck cancer patients in the early postoperative and radiotherapy period are needed, with long‐term follow‐up.
Keywords: Humans; Carcinoma, Squamous Cell; Carcinoma, Squamous Cell/therapy; Exercise Therapy; Exercise Therapy/methods; Head and Neck Neoplasms; Head and Neck Neoplasms/therapy; Joint Diseases; Joint Diseases/etiology; Joint Diseases/rehabilitation; Muscle Stretching Exercises; Muscle Stretching Exercises/methods; Neck Dissection; Neck Dissection/adverse effects; Neck Dissection/methods; Radiotherapy; Radiotherapy/adverse effects; Randomized Controlled Trials as Topic; Resistance Training; Resistance Training/methods; Shoulder Joint; Shoulder Joint/radiation effects; Shoulder Pain; Shoulder Pain/etiology; Shoulder Pain/rehabilitation
Plain language summary
Exercise interventions for shoulder dysfunction in patients treated for head and neck cancer
Both neck dissection and radiotherapy can cause morbidity to the shoulder joint. ‘Neck dissection’ is often used to prevent the spread of cancer to the lymph nodes of the neck, however this surgery can cause ‘shoulder syndrome’. This is defined as shoulder droop, ‘winged scapula’ (abnormal protruding of the shoulder blades), an inability to shrug and a dull, non‐localized pain that is made worse by movement. Shoulder problems can be present in as many as 50% to 100% of patients who have had a radical neck dissection.
Physiotherapy interventions are used to reduce the impact of surgery on the shoulder and include a wide range of rehabilitative techniques. These include passive, active or active‐assisted range of motion exercises (the patient’s joint is moved either by an external force (e.g. device or person) or active muscle contraction or a combination); progressive resistance training (the patient exercises the muscle against an external force); and proprioceptive neuromuscular facilitation (PNF) exercises (a method used to improve strength, endurance and stretch of muscles).
This review identified three randomized controlled trials involving 104 patients. Two studies compared progressive resistance training with standard care (usual treatment process). When we combined their results we found that progressive resistance training improved shoulder pain, shoulder disability, active range of motion for external rotation, passive range of motion for abduction, forward flexion, external rotation and horizontal abduction. The size of this improvement was small. The studies did not demonstrate a statistically significant difference in quality of life. Two non‐serious adverse events were reported in the progressive resistance training group and none in the standard care group.
Another study compared a broad spectrum of techniques, including free active exercises, stretching, postural care, re‐education of scapulothoracic postural muscles, and strength of shoulder muscles, with routine postoperative physiotherapy care for three months following surgery. This study did not demonstrate a difference between the exercise group and the routine physiotherapy care group in shoulder function or quality of life. No adverse effects were reported.
Further studies which apply other exercise interventions in head and neck cancer patients in the early postoperative period and after radiotherapy are needed, with long‐term follow‐up.
Background
Description of the condition
Each year more than 600,000 new cases of head and neck cancer are diagnosed worldwide, accounting for 5% of all malignant tumors. Unfortunately most cases are diagnosed at advanced stages of the disease (60% are stages III and IV) and are squamous cell carcinoma (De Boer 1999; Herchenhorn 2004; Leitzmann 2008). It is therefore necessary to treat these patients with multimodal interventions. Spread of the tumor to the cervical lymph nodes is common in this type of cancer and neck dissections are a valuable method of treating the neck, with the objective of controlling this spread (Güldiken 2005; Bessell 2011). However, the surgical procedures cause what is called 'shoulder syndrome', defined as a shoulder droop, winged scapula, inability to shrug and a dull, non‐localized pain that is present in all patients and exacerbated by movement, particularly abduction (Nahum 1961). Most of shoulder morbidities are caused principally by spinal accessory nerve damage that occurs in neck dissections or radiotherapy. A group of patients report shoulder pain even if they have normal nerve function after head and neck cancer treatment; the causes could be explained by deafferentation pain, myofascial pain or neuromas (van Wilgen 2003). The prevalence of shoulder complaints after radical neck dissection has been reported to be between 50% and 100% (Saunders 1985; Short 1984). Following other types of surgery that preserve the accessory nerve, such as functional or modified neck dissections and selective neck dissections, shoulder complaints have been reported by 29% to 60% of patients (Dijkstra 2001). Radiotherapy treatments can also cause shoulder morbidities and are considered a negative factor for long‐term shoulder function (Chepeha 2002; van Wouwe 2009).
Description of the intervention
Several researchers have demonstrated the importance of physiotherapy interventions in reducing the impact of postoperative morbidities related to the shoulder after the treatment of head and neck cancer (Herring 1987; Johnson 1978; Laska 2001; McNeely 2004; McNeely 2008). There are a variety of rehabilitative techniques that can be used for shoulder dysfunction:
Passive range of motion exercises, whereby the patient’s joint is moved entirely by an external force, with little or no voluntary contraction of his or her muscles. The external force may be due to gravity, a device, a person or another body part of the individual.
Active range of motion movements are produced by an active contraction of the muscles that cross that joint.
Active‐assisted range of motion exercise is a form of active exercise in which an external force provides assistance because muscles need help to complete the movement (Kisner 2005).
Progressive resistance training (PRT) is a method by which the participant exercises their muscle against an external force, and this resistance is adjusted throughout the training. The resistance can be applied by elastic bands or tubing (i.e. therabands), cuff weights, free weights, isokinetic machines or other weight machines (Fialka 1989; Liu 2009; McNeely 2004; McNeely 2008; Salerno 2002; Saunders 1975).
Proprioceptive neuromuscular facilitation (PNF) exercises are designed to enhance the response of neuromuscular mechanisms by stimulating proprioceptors. The patterns of PNF exercises have a spiral, diagonal direction and the performance of these patterns is in line with the topographic arrangement of the muscles being used (Voss 1985).
How the intervention might work
Therapeutic exercises for shoulder dysfunction in patients with head and neck cancer are generally applied with the objective of reducing or preventing shoulder pain by reducing shoulder load and increasing other scapula stabilizing muscles to compensate for the loss of function of the trapezius muscle. Patients seem to benefit from these physical therapy programs in terms of improved pain, shoulder disability, range of motion and quality of life (Fialka 1989; McNeely 2004; McNeely 2008; Salerno 2002; Saunders 1975). Rehabilitative treatment also aims for early recovery of the passive motion of the scapulohumeral girdle, to facilitate the recovery of the active range of motion and to avoid the occurrence of joint fibrosis and secondary adhesive capsulitis, thus reducing shoulder complaints and promoting a better quality of life for these patients (Salerno 2002).
Why it is important to do this review
This review seeks to clarify the role of physical therapy in the treatment of shoulder dysfunction caused by head and neck cancer treatments. Although some studies have been published on the topic, there is currently no systematic review summarizing them.
Objectives
To evaluate the effectiveness and safety of exercise interventions for shoulder dysfunction caused by the treatment of head and neck cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Adults with a clinical and histological diagnosis of head and neck cancer, at any stage, and with dysfunction of the shoulder due to having received any type of cancer treatment (surgical treatment, associated or not with radiotherapy or chemotherapy, and radiotherapy, associated or not with chemotherapy) of the head and neck region.
Types of interventions
Intervention
Active or active‐assisted range of motion exercises, passive range of motion exercises, stretching exercises, resistance exercises, proprioceptive neuromuscular facilitation or any other exercise with a focus on shoulder dysfunction treatment or prevention, whether combined or not with pharmacological intervention.
Control
Any other intervention, such as no treatment, standard treatment, placebo, sham exercises and pharmacological interventions.
Types of outcome measures
We planned to analyze all outcomes described below if the trial authors described the method of assessing the outcome, or used a validated and recognized instrument that may be replicated.
Primary outcomes
Shoulder pain
Shoulder disability
Secondary outcomes
Quality of life
Active and passive range of motion of the shoulder
Strength of scapular muscles
Endurance of scapular muscles
Adverse effects
Adherence to the exercise interventions
Search methods for identification of studies
We conducted systematic searches for randomized controlled trials. There were no language, publication year or publication status restrictions. The date of the search was 7 July 2011.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 2); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; CNKI; ISRCTN; ClinicalTrials.gov; ICTRP and Google.
We modeled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomized controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase, NHS Evidence ‐ ENT & Audiology and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. We searched for conference abstracts using the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by the electronic search to a reference management database (Endnote) and removed duplicates. Two review authors (Carvalho APV, Vital FMR) independently examined the remaining references. We excluded those studies which clearly did not meet the inclusion criteria and obtained copies of the full text of potentially relevant references. Two review authors (Carvalho APV, Vital FMR) independently assessed the eligibility of the retrieved papers. Disagreements were resolved by discussion between the two authors and where necessary by a third author (Soares BGO). We documented reasons for exclusion of studies.
Data extraction and management
Two review authors (Carvalho APV, Vital FMR) independently extracted all data. We contacted the authors of primary trials if there were doubts regarding missing data or methodological details of the trial. We used a standard form to extract the following data: characteristics of the study (design, risk of bias); participants (inclusion criteria, age, gender, stage of the disease and stage of cancer treatment, previous cancer and shoulder treatments received, number enrolled in each group); intervention (type of physiotherapy, frequency and duration of therapy, physiotherapy co‐interventions, drugs and others multidisciplinary interventions); outcomes (types of outcome measures, timing of outcomes, adverse events) and duration of follow‐up. The primary author (Carvalho APV) entered these data into Review Manager 5 (RevMan 2011) and also identified and resolved discrepancies in the data extraction forms.
For dichotomous outcomes (e.g. adverse events) we extracted the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at endpoint, in order to estimate a risk ratio.
For continuous outcomes (e.g. quality of life or pain measures), we extracted the final value and standard deviation of the outcome of interest and the number of patients assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms and its standard error.
Where possible, all data extracted were those relevant for an intention‐to‐treat analysis, in which participants were analyzed in the groups to which they were assigned.
We noted the time points at which outcomes were collected and reported.
Assessment of risk of bias in included studies
To evaluate the methodological quality of selected studies, we independently assessed the methods section of the RCTs, considering the following items associated with risk of bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other source of bias. We judged each of these criteria as 'high risk of bias', 'low risk of bias' or 'unclear risk of bias', according to The Cochrane Collaboration 'Risk of bias' tool (Handbook 2011). For details please see Appendix 2.
Two review authors (Carvalho APV, Vital FMR) independently applied the 'Risk of bias' tool and resolve differences by discussion or by appeal to a third author (Soares BGO). We summarized results in both a 'Risk of bias' graph and a 'Risk of bias' summary. We interpreted the results of meta‐analyses in the light of the findings with respect to risk of bias.
Unit of analysis issues
Cluster‐randomized and cross‐over trials were to be included in the review and investigated as potential sources of heterogeneity. However, we identified no such studies.
Dealing with missing data
We proposed to carry out available case analysis and intention‐to‐treat analysis. If necessary we contacted original investigators to request any missing data. For continuous data, we performed available data analysis. If in future updates we find new trials with dichotomous data, we will use the imputation of data by assuming poor outcomes.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots and by estimation of the percentage of heterogeneity between trials which cannot be ascribed to sampling variation (I² statistic) (Higgins 2003) and using a formal statistical test of the significance of the heterogeneity (Chi² test) (Deeks 2001). We considered P values < 0.10 to be statistically significant. Subgroup analyses could not be performed. If we find new studies in future updates we will look for evidence of substantial heterogeneity and investigate and report the possible reasons for this.
Assessment of reporting biases
If we find enough studies in future updates (at least 10), we will examine funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small study effects such as publication bias. If these plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random‐effects model, we will perform further meta‐analyses using fixed‐effect models.
Data synthesis
We implemented meta‐analysis using RevMan 5 if there were two or more randomized trials with comparable populations undergoing similar interventions. When there was clear evidence of poor homogeneity between trials, we produced a narrative summary of the findings.
For any dichotomous outcomes, we planned to calculate the risk ratio with respective 95% confidence interval (CI) for each study, which would then be pooled. For statistically significant results, we also planned to present the number needed to treat/number needed to harm.
For continuous outcomes, we pooled the mean differences between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale, otherwise we planned to pool using standardized mean differences.
If we find in future updates any trial with multiple treatment groups, we will divide the ‘shared’ comparison group into the number of treatment groups and comparisons between each treatment group and treat the split comparison group as independent comparisons.
We used random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
If further studies are added in future updates of this review, we will use subgroup analysis to explore possible sources of heterogeneity. This will be based on the following.
-
Type of surgical procedure performed (radical neck dissection, modified radical neck dissection and selective neck dissection) and its association with radiotherapy.
Radical neck dissection versus modified neck dissection.
Modified neck dissection versus selective neck dissection.
Selective neck dissection versus radical neck dissection.
Patients that received surgery associated with radiotherapy versus surgery alone.
Types of intervention: isolated techniques versus combined interventions (only one type of exercise, such as progressive resistance exercise training (PRT) versus PRT combined with passive range of motion exercises and stretching or other types of physical therapy).
Duration of the intervention, grouping studies as short‐term (up to six months) and long‐term (more than six months).
Range in time from surgery to the beginning of intervention, grouping studies with a time range shorter than six months and those with a time range longer than six months.
Sensitivity analysis
Sensitivity analysis could not be carried out, but if we include a greater number of studies in future updates we will perform sensitivity analyses excluding studies at high risk of bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Wee retrieved a total of 91 references from the searches (26 from CENTRAL, 22 from EMBASE, 18 from PubMed, 10 from the Cochrane ENT Group Trials Register, eight from BIOSIS Previews, six from Web of Science and one from Clinicaltrials.gov). We excluded 71 of these in first‐level screening (i.e. removal of duplicates and clearly irrelevant references), leaving 20 references for further consideration. Two review authors (Carvalho APV, Vital FMR) independently selected seven references. Disagreements were discussed with the third author (Soares BGO). We finally included three studies in this review. We identified one ongoing study that is described in detail in the table Characteristics of ongoing studies. See Figure 1 for a PRISMA flow diagram detailing the search process.
1.

Study flow diagram.
Included studies
We included three studies that met the inclusion criteria for this review: McNeely 2004; McNeely 2008 and Lauchlan 2011 (see Characteristics of included studies). All the included studies were published in English. We contacted McNeely to confirm if they had included data from the first study in the second; we received a clear response that they are two different sets of data.
Design
All the three studies were randomized controlled trials and evaluated the effectiveness of an exercise intervention for the treatment of shoulder dysfunction in patients treated for head and neck cancer.
Samples sizes
McNeely 2004 included 20, McNeely 2008 included 52 and Lauchlan 2011 included 32 participants.
Settings
McNeely 2004 and McNeely 2008 were performed in Edmonton, Canada and Lauchlan 2011 did not describe where it was conducted.
Participants
In McNeely 2004 all patients were diagnosed with head and neck cancer and all had histologically confirmed squamous cell carcinoma. Included participants were divided into two groups: early group (within eight weeks of neck dissection) and late group (eight or more weeks after neck dissection). They had a mean age of 61 years and 82% were male. The majority were in the advanced stages of disease (65% stage IV). The most prevalent primary location was oropharynx (41%). Almost all received radiotherapy associated with surgery. Only 25% received radical neck dissection and the most prevalent type was selective neck dissection (36%); other participants received modified neck dissection. In McNeely 2008 the mean age was 52 years, 71% were male and 20 were on disability (38%). The median time from surgery to the beginning of the intervention was 15 months, ranging from 2 to 180 months. The most prevalent primary site was oral cavity/oropharynx and the majority were diagnosed in the advanced stages of the disease (58% stage IV). Modified neck dissection was the predominant type of neck dissection (48%); 71% received bilateral neck radiation and 27% received chemotherapy. In Lauchlan 2011 patients were selected if they had radical neck dissection or selective neck dissection: they excluded participants with previous significant injury to the arm, shoulder, neck or chest and an existing clinical presentation of adhesive capsulitis of the glenohumeral joint. The baseline characteristics of participants were not described in this study.
Interventions
Two studies (McNeely 2004; McNeely 2008) used one exercise protocol, mostly progressive resistance training (PRT). Some other exercises were implemented together with PRT, such as range of motion and stretching. The study authors compared progressive resistance training with standard care. One difference between the interventions given in these two studies is that in the first study the intensity of the exercise began with 1 to 2 kg and in the other the intensity applied at the beginning of the treatment was 25% to 30% of the patient 1‐repetition maximum (1‐RM). Lauchlan 2011 used a broad spectrum of techniques, including free active exercises, stretching, postural care, re‐education of scapulothoracic postural muscles and strength of shoulder muscles, for a period of three months following surgery. This was compared with a control group that received only routine physiotherapy inpatient care and advice in the postoperative period.
Outcomes
McNeely 2004 analyzed the following outcomes: active and passive range of motion, shoulder pain and disability, quality of life, recruitment rate and completion rate. McNeely 2008 assessed the following: shoulder pain and disability, muscular strength of the upper extremity, muscular endurance, active and passive range of motion and quality of life. In Lauchlan 2011 shoulder function and quality of life were the outcomes presented.
Excluded studies
We excluded three studies from the review (Hou 2002; Pfister 2008; van Wilgen 2007). The reasons were principally that they did not meet our inclusion criteria. Excluded studies either did not apply exercise interventions or the participants were not head and neck cancer survivors. One further reference was linked to an included study (Lauchlan 2003, protocol for Lauchlan 2011).
Risk of bias in included studies
See Figure 2 for a 'Risk of bias' graph which shows our judgments about each risk of bias item presented as percentages across all included studies, and Figure 3 for a 'Risk of bias' summary of our judgments about each risk of bias item for each included study.
2.

'Risk of bias' graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgments about each risk of bias item for each included study.
Allocation
All included studies described an appropriate method of randomization, however description of allocation concealment varied.
McNeely 2008 had an adequate description of allocation concealment and was classified as having low risk of bias. Lauchlan 2011 did not described how allocation concealment was performed; we classified this domain as unclear risk of bias. In McNeely 2004 the method of concealment was not described in the study; we contacted the author and were informed that randomization was computer‐generated by their statistician. We therefore classified it as unclear risk of bias.
Blinding
In McNeely 2008, outcome assessors were blinded for the range of motion, strength and endurance tests (classified as low risk of bias). For McNeely 2004, we contacted the author and found that there were no independent blinded assessors because they were looking for feasibility. We therefore classified this domain as high risk of bias. Lauchlan 2011 has a low risk of bias: outcomes were measured by a blinded evaluator at one year post‐surgery.
Incomplete outcome data
McNeely 2008 conducted an intention to‐treat analysis, so we classified it as low risk of bias.
Although McNeely 2004 and Lauchlan 2011 do not describe an intention to‐treat analysis, we also classified them as low risk of bias because both described the reasons for missing outcome data, which were balanced in numbers across interventions groups.
Selective reporting
One study did not publish data for five outcomes pre‐specified in the protocol: shoulder range of motion (ROM) of active forward flexion, abduction and the passive measurements of forward flexion, external rotation and horizontal abduction (McNeely 2008). Only two statistically significant outcomes that favored the intervention group were published (active external rotation and passive abduction). Only the outcome of passive external rotation that was sent had positive effects in favor of the intervention tested. We contacted the author and were provided with a complete analysis of measured outcomes which are presented in this review. We classified this study as low risk of bias for this domain.
We classified the other two studies as unclear risk of bias for this domain (Lauchlan 2011; McNeely 2004) as the protocols were not available and there was insufficient information to permit judgment.
Other potential sources of bias
We classified two studies as low risk of bias (McNeely 2004; McNeely 2008), where no additional issues were identified.
Lauchlan 2011 had some others problems that could put it at a high risk of bias, including the instrument used to measure the outcome quality of life. They used an insensitive instrument which can lead to underestimation of both beneficial and harmful effects. As an effectiveness/exploratory trial, Lauchlan 2011 also had some inconsistencies in the implementation of interventions, which were described by the author as expected due to the design of the study. We classified this study as high risk of bias.
Effects of interventions
We were able to pool data from two studies (McNeely 2004; McNeely 2008) as the intervention analyzed was progressive resistance training (PRT) and the control was standard care that was similar. The studies pooled had different risks of bias; we judged one as low risk of bias (McNeely 2008) and the other as high risk of bias (McNeely 2004). We carried out meta‐analysis and observed no significant changes in the results in comparison to the individual study results. No evidence of heterogeneity was detected in the pooled studies (McNeely 2004; McNeely, 2008) using the I² statistic and Chi² test.
Data from the third study (Lauchlan 2011) could not be pooled because of significant differences in protocols and measured outcomes. This study did not demonstrate a statistically significant difference between the interventions analyzed. However, the time points of outcome measurement were different, they compared patients preoperatively with one year postoperatively, the protocol for exercises applied was different, and the study presented some methodological limitations that could explain why it did not show statistically significant results.
Shoulder pain
Shoulder pain was measured in two studies (McNeely 2004; McNeely 2008) at baseline and after 12 weeks of physical therapy. It was assessed by the Shoulder Pain and Disability Index (SPADI) which contains a pain subscale. The scores range from 0 to 100 with higher scores indicating greater impairment. Analysis included 69 participants; statistical significance favored PRT (mean difference (MD) ‐6.26; 95% confidence interval (CI) ‐12.20 to ‐0.31) (Analysis 1.1).
1.1. Analysis.

Comparison 1 PRT versus standard care, Outcome 1 Shoulder Pain and Disability Index (pain score) 12 weeks.
Shoulder disability
This outcome was assessed by the SPADI in two studies (McNeely 2004; McNeely 2008), with higher scores indicating greater impairment. There is evidence that PRT is superior to standard care (MD ‐8.48; 95% CI ‐15.07 to ‐1.88) (Analysis 1.2).
1.2. Analysis.

Comparison 1 PRT versus standard care, Outcome 2 Shoulder Pain and Disability Index (disability subscale) 12 weeks.
Shoulder Pain and Disability Index (total score)
This total score is obtained by averaging the pain and disability subscales scores, with higher scores indicating greater impairment. There was no statistical difference between interventions (MD ‐5.77; 95% CI ‐14.00 to 2.46) (Analysis 1.3).
1.3. Analysis.

Comparison 1 PRT versus standard care, Outcome 3 Shoulder Pain and Disability Index (total score) 12 weeks.
Shoulder function assessed by functional component score of American Shoulder and Elbow Surgeons Shoulder Assessment (ASESSA)
One study analyzed shoulder function after one year of intervention (Lauchlan 2011). This study did not demonstrate a statistically significant difference between the group that performed the exercise protocol for three months after hospital discharge and the group that performed only postoperative physiotherapy care while in hospital. The non‐significant result could be explained by limitations of the study, for example the tool used was not a disease‐specific questionnaire, there was large loss to follow‐up (8 from 32) and there were difficulties in maintaining protocol compliance.
Shoulder function assessed by the Constant Shoulder Assessment
Lauchlan 2011 assessed disability using the Constant Shoulder Assessment and no statistical significant difference was shown between the group that performed exercises for three months and the control group that was treated with routine postoperative physiotherapy care (P = 0.74).
Shoulder active range of motion
Abduction
Pooled data from McNeely 2004 and McNeely 2008 failed to differentiate PRT from standard care for shoulder abduction after 12 weeks of intervention (MD 9.45 degrees; 95% CI ‐6.26 to 25.17) (Analysis 1.4).
1.4. Analysis.

Comparison 1 PRT versus standard care, Outcome 4 Active range of motion (abduction).
Forward flexion
Pooled data from McNeely 2004 and McNeely 2008 showed no significant difference between PRT and standard care (MD 7.01 degrees; 95% CI ‐1.93 to 15.95) (Analysis 1.5).
1.5. Analysis.

Comparison 1 PRT versus standard care, Outcome 5 Active range of motion (forward flexion).
External rotation
Pooled data from McNeely 2004 and McNeely 2008 showed a statistically significant benefit in favor of the PRT group compared to the standard care group (MD 14.51 degrees; 95% CI 7.87 to 21.14) (Analysis 1.6).
1.6. Analysis.

Comparison 1 PRT versus standard care, Outcome 6 Active range of motion (external rotation).
Shoulder passive range of motion
Two studies examined the effect of progressive resistance training on the passive range of motion of the shoulder joint (McNeely 2004; McNeely 2008). Pooled data from these studies included 69 participants and showed a statistically significant difference in favor of PRT for the following movements:
abduction (MD 7.65 degrees; 95% CI 0.64 to 14.66 (Analysis 1.7);
forward flexion: (MD 6.20 degrees; 95% CI 0.69 to 11.71 (Analysis 1.8);
external rotation: (MD 7.17 degrees; 95% CI 2.20 to 12.14 (Analysis 1.9); and
horizontal abduction: (MD 7.34 degrees; 95% CI 2.86 to 11.83 (Analysis 1.10).
1.7. Analysis.

Comparison 1 PRT versus standard care, Outcome 7 Passive range of motion (abduction).
1.8. Analysis.

Comparison 1 PRT versus standard care, Outcome 8 Passive range of motion (forward flexion).
1.9. Analysis.

Comparison 1 PRT versus standard care, Outcome 9 Passive range of motion (external rotation).
1.10. Analysis.

Comparison 1 PRT versus standard care, Outcome 10 Passive range of motion (horizontal abduction).
Quality of life
All studies included in this systematic review analyzed this outcome, but we could only pool the data from McNeely 2004 and McNeely 2008. Quality of life was measured by the Functional Assessment of Cancer Therapy ‐ General (FACT‐G) scale and failed to show differences between PRT and standard care (MD 5.05; 95% CI ‐3.01 to 13.12) (Analysis 1.11).
1.11. Analysis.

Comparison 1 PRT versus standard care, Outcome 11 Quality of life (FACT‐G).
One study (McNeely 2008) measured quality of life with the FACT ‐ Anemia and Fatigue (FACT‐An) scale that assessed quality of life and fatigue together (range from 0 to 188), with higher values representing better quality of life. No statistically significant difference was found between the two groups (MD 8.00; 95% CI ‐8.77 to 24.77) (Analysis 1.13).
1.13. Analysis.

Comparison 1 PRT versus standard care, Outcome 13 Quality of life measured by FACT‐An scale.
McNeely 2004 used the questionnaire FACT ‐ Head and Neck (FACT‐H&N), with scores ranging from 0 to 152. There was no statistically significant difference between PRT and standard care (MD 3.90; 95% CI ‐16.30 to 24.10) (Analysis 1.14).
1.14. Analysis.

Comparison 1 PRT versus standard care, Outcome 14 Quality of life measured by FACT‐H&N questionnaire.
Lauchlan 2011 assessed quality of life with the SF‐12 questionnaire, and no statistically significant differences were shown between the group that received physical therapy after discharge from hospital and the group that received only routine care in the hospital. SF‐12 is considered to have limited sensitivity to evaluate this outcome.
McNeely 2008 used the Neck Dissection Impairment Index (NDII) to assess treatment‐specific quality of life and no statistically significant difference was shown between interventions (MD 8.40; 95% CI ‐3.54 to 20.34) (Analysis 1.15).
1.15. Analysis.

Comparison 1 PRT versus standard care, Outcome 15 Quality of life assessed by NDII questionnaire.
Endurance of scapular muscles
Only one study examined endurance of scapular muscles (McNeely 2008) as measured by repetitions per kg. A highly significant difference favored PRT in comparison to standard care (MD 320 repetitions x kg; 95% CI 89.75 to 550.25; Analysis 1.16)
1.16. Analysis.
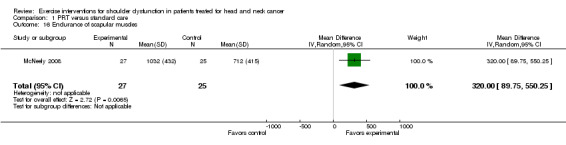
Comparison 1 PRT versus standard care, Outcome 16 Endurance of scapular muscles.
Strength of scapular muscles
Only McNeely 2008 described this outcome and statistically significant differences were shown in favor of the PRT group for:
seated row with both extremities tested (two‐arm test: MD 18.90 kg; 95% CI 6.84 to 30.96 (Analysis 1.17)) and one extremity tested (one‐arm test: MD 7.00 kg; 95% CI 1.17 to 12.83 (Analysis 1.18)); and
chest press two‐arm test (MD 14.40 kg; 95% CI 3.05 to 25.75) (Analysis 1.19) and one‐arm test (MD 6.50 kg; 95% CI 0.93 to 12.07) (Analysis 1.20).
1.17. Analysis.
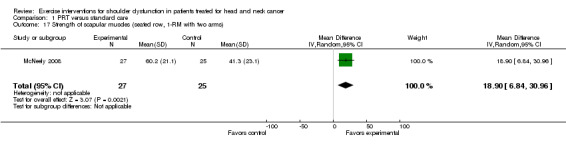
Comparison 1 PRT versus standard care, Outcome 17 Strength of scapular muscles (seated row, 1‐RM with two arms).
1.18. Analysis.

Comparison 1 PRT versus standard care, Outcome 18 Strength of scapular muscles (seated row, 1‐RM affected shoulder).
1.19. Analysis.
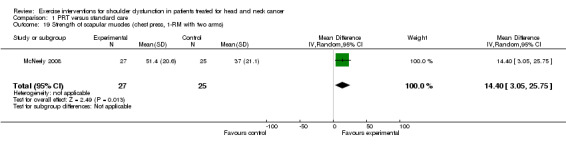
Comparison 1 PRT versus standard care, Outcome 19 Strength of scapular muscles (chest press, 1‐RM with two arms).
1.20. Analysis.

Comparison 1 PRT versus standard care, Outcome 20 Strength of scapular muscles (chest press, 1‐RM affected shoulder).
Adverse effects
Two studies described adverse events (McNeely 2004; McNeely 2008). The former described one case of nausea, where the patient was in the early exercise group and was in the final stages of radiation therapy. This was related to minimal nutritional intake before exercise. McNeely 2008 described one case of increased pain as a result of soft‐tissue injury to the scapular region.
Lauchlan 2011 did not describe adverse effects in the methods, results or discussion sections.
Adherence to the treatment
One study (McNeely 2004) showed 93% adherence in the PRT group, but the results for the control group were not shown.
In McNeely 2008 adherence to PRT was superior to the control group: 95% versus 87% respectively.
In Lauchlan 2011 no comments on adherence were provided.
Discussion
Summary of main results
Results pooled from two studies conducted by the same authors show that progressive resistance training (PRT) is better than standard care for patients treated for head and neck cancer. The third study included in this review, classified as high risk of bias, failed to differentiate a physical intervention based on a broad spectrum of techniques, in comparison to standard physiotherapy.
Pooled data from McNeely 2004 and McNeely 2008 demonstrated that PRT improves shoulder pain, shoulder disability, active range of motion (external rotation) and passive range of motion (abduction, forward flexion, external rotation and horizontal abduction), in comparison to standard care. However, there is no strong evidence that an exercise program with a focus on shoulder dysfunction can improve quality of life in these patients. Possible reasons include: an active control group, patient characteristics (various co‐morbidities that impact quality of life) or type 2 error. Also, there may be an interpretation related to a minor impact of exercise interventions on the quality of life of these patients. To clarify these possibilities, further studies with strict methodological criteria and larger sample sizes are needed to demonstrate the impact on quality of life. In one study (McNeely 2008), a PRT program significantly improved strength and endurance of the scapular muscles. It is important to emphasize that the participants included in the two trials pooled in the meta‐analysis had a wide range of time between neck dissection and the start of the intervention, therefore the effectiveness of exercise interventions immediately after oncological treatments remains questionable.
When adverse events were evaluated in McNeely 2004 and McNeely 2008, it was demonstrated that it is safe to apply their exercise protocol. The PRT program described appeared to have better adherence compared with standard care, but these findings must be interpreted cautiously because adherence to treatment in randomized trials may not translate into similar levels of adherence in normal practice. Lauchlan 2011 failed to demonstrate statistically significant differences in quality of life and shoulder function between the group that performed physical therapy for three months immediately after surgery and the group that received only routine care in the hospital (respiratory care and verbal advice on early active movement of the neck and affected shoulder). We classified this study as high risk of bias, which also limits its value.
All primary outcomes preselected for this review and some secondary ones were statistically significant, however in general the results presented in the meta‐analysis had an effect size very close to the null hypothesis. This suggests that although statistically significant, the benefits of the intervention may be small compared to the standard intervention. Although the scores obtained for shoulder pain and shoulder disability from the SPADI questionnaire were low in both studies, the results still favored the group treated with PRT. The low score observed can be explained by the inclusion of participants within a wide range of time from surgery to the beginning of the intervention, and a majority of less radical surgeries performed. On the other hand, some studies with head and neck cancer patients demonstrated that it is also possible to have signs of shoulder dysfunction without presenting pain, probably because patients managed to cope with the dysfunction (van Wilgen 2003).
Overall completeness and applicability of evidence
Studies included in this systematic review partially answer our research question with some limitations, mostly related to the limited number of randomized controlled trials published with a focus on the treatment of shoulder dysfunction in patients treated for head and neck cancer. Data from only two studies could be pooled in a meta‐analysis and it was not possible to perform a subgroup analysis since these studies were from the same group of authors and had similar designs and interventions. The significant question of whether exercise interventions have a better effect in patients who have had a less radical neck dissection in comparison to patients with radical neck dissections could not be answered, nor could we answer the question of whether early exercises are better than delayed.
We identified only two different types of protocols designed for this population, one with a focus on PRT and another that applied various techniques. Other physical therapies must be investigated, since a wide range of exercise techniques are described in the literature for the treatment of shoulder dysfunction due to head and neck cancer treatments.
Quality of the evidence
Only McNeely 2008 was classified as low risk of bias for all six domains of The Cochrane Collaboration 'Risk of bias' tool. We classified the other two studies as high risk of bias, due to limitations such as lack of blinded assessors, uncertainties about allocation concealment and other bias that could significantly impact the effect of the interventions being tested.
Based on the GRADE system, we have classified the quality of the evidence as moderate, due to the limited number of trials and poor quality of two of the three identified studies.
Potential biases in the review process
We used a sensitive search strategy to identify studies relevant to this systematic review. We also contacted the first authors from the included trials to identify further studies. Selection, data extraction and assessment of the risk of bias were independently performed by the first and second authors, and there were no disagreements between the authors. We have presented and discussed all outcomes described in the protocol for this review that were available for analysis, whether statistically significant or not.
Agreements and disagreements with other studies or reviews
This is the first systematic review on this topic. Other studies have been published in the literature which applied exercise interventions in patients treated for head and neck cancer, but these are not randomized studies. One controlled but non‐randomized study (Salerno 2002) used range of motion exercises combined with stretching, and demonstrated that the group that performed exercise after neck dissection was superior to the group that received no treatment. A statistically significant difference was shown for passive forward elevation, active forward elevation, abduction, external rotation, internal rotation, pain, daily activities, recreational activities, nocturnal rest and ability to elevate the arm. These findings are consistent with our results that demonstrate that an exercise intervention was capable of improving range of motion of the shoulder joint, pain and disability. One case series and one retrospective study (Chida 2002; Shimada, 2007) used the same exercise protocol consisting of active and passive range of motion exercises, sanding and wiping exercises and isometric strengthening combined with other techniques. They demonstrated that their protocol had a positive effect on active and passive range of motion of shoulder joint for abduction and flexion, but pain was not improved. There is one ongoing randomized controlled trial that may be included in this review when results are presented (McGarvey 2011).
Authors' conclusions
Implications for practice.
Limited evidence from two randomized controlled trials demonstrates that a program of exercises with a main focus on progressive resistance training (PRT) for shoulder dysfunction in patients treated for head and neck cancer, applied for 12 weeks, three times per week, is more effective than standard care in relation to shoulder pain, shoulder disability and range of motion of the glenohumeral joint. However, although pooled data were statistically significant, the effect sizes presented were small. This exercise protocol appears to be safe, with only two non‐serious adverse events reported by the authors of these trials. It is possible that PRT can improve strength and resistance of the scapular muscle, but this outcome was analyzed in only one study. It is notable that patients recruited in the trials started the physical treatment within a wide range of time from surgery.
Implications for research.
Although many studies have been published on the subject of dysfunction caused by neck dissection performed in patients with head and neck cancer, few controlled studies are available. Randomized controlled trials with more strict methods need to be developed, following the Consolidated Standards of Reporting Trials (CONSORT) statement. Critical processes must be followed: reliable randomization, adequate allocation concealment, outcome assessor blinding, reporting of all outcomes related in the protocol of the study and efforts made to minimize incomplete outcome data. The use of a sensitive instrument to assess quality of life in these patients is also important.
Other types of exercise interventions could also be investigated for these patients, including proprioceptive neuromuscular facilitation and shoulder mobilization. It is important to have studies that apply the intervention in the postoperative period and which have a long follow‐up. Patients treated with radical neck dissection must be compared to those with other types of neck dissection, and patients with neck dissection associated with radiotherapy should be compared to those receiving neck dissection alone.
Acknowledgements
We especially thank Dr McNeely for providing a significant amount of relevant clinical data from her studies. We also thank Dr Lauchlan for answering our enquiries.
We thank the Cochrane Ear, Nose and Throat Disorders group, the Gynaecological Cancer and Orphan Review Group and the Brazilian Cochrane Center for help and support during the preparation of this review.
Appendices
Appendix 1. Search strategies
| CENTRAL | Cochrane ENT Group's Trials Register | PubMed | EMBASE (Ovid) |
| #1 MeSH descriptor Head and Neck Neoplasms explode all trees #2 MeSH descriptor Neck Dissection explode all trees #3 ((head or neck or upper aerodigestive tract or uadt) and (cancer* or neoplas* or tumor* or tumour* or malignan* or carcinom*)) #4 (neck AND dissect*) OR hnscc #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Shoulder Pain explode all trees #7 MeSH descriptor Shoulder explode all trees with qualifier: IN #8 MeSH descriptor Shoulder Joint explode all trees with qualifier: IN #9 (shoulder* OR scapul* OR trapezius OR glenohumeral OR "adhesive capsulitis" OR "accessory nerve*" OR ((11th or eleventh) and nerve*)) AND (morbidit* OR disabilit* OR function* OR dysfunction* OR pain* OR syndrome OR droop* OR lesion* OR impair* OR injur*) #10 (#6 OR #7 OR #8 OR #9) #11 (#5 AND #10) #12 MeSH descriptor Neck Dissection explode all trees with qualifier: AE #13 (#11 OR #12) #14 MeSH descriptor Rehabilitation explode all trees #15 MeSH descriptor Physical Therapy Modalities explode all trees #16 MeSH descriptor Exercise explode all trees #17 MeSH descriptor Yoga explode all trees #18 MeSH descriptor Tai Ji explode all trees #19 MeSH descriptor Therapeutic Touch explode all trees #20 "physical therap*" or physio* OR exercise* OR movement* OR aerobic* OR pilates OR stretch* OR "tai chi" or "tai ji" OR yoga OR (resistance AND training) OR rehab* #21 (#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20) #22 (#13 AND #21) | (shoulder* OR scapul* OR trapezius OR glenohumeral OR "adhesive capsulitis" OR "accessory nerve*" OR 11th or eleventh) AND (physical OR physio* OR exercise* OR movement* OR aerobic* OR pilates OR stretch* OR tai OR yoga OR resistance OR rehab*) | #1 “Head and Neck Neoplasms” [Mesh] OR “Neck Dissection” [Mesh] OR ((head [tiab] OR neck [tiab] OR “upper aerodigestive tract” [tiab] OR uadt [tiab]) and (cancer* [tiab] OR neoplas* [tiab] OR tumOR* [tiab] OR tumour* [tiab] OR malignan* [tiab] OR carcinom* [tiab])) OR (neck [tiab] AND dissect* [tiab]) OR hnscc [tiab] #2 “Shoulder Pain” [Mesh] OR “Shoulder” [Mesh] OR ((shoulder* [tiab] OR scapul* [tiab] OR trapezius [tiab] OR glenohumeral [tiab] OR "adhesive capsulitis" [tiab] OR "accessory nerve*" [tiab] OR ((11th [tiab] OR eleventh [tiab]) and nerve* [tiab])) AND (morbidit* [tiab] OR disabilit* [tiab] OR function* [tiab] OR dysfunction* [tiab] OR pain* [tiab] OR syndrome [tiab] OR droop* [tiab] OR lesion* [tiab] OR impair* [tiab] OR injur* [tiab]) #3 "Shoulder joint" [Mesh] #4 #2 OR #3 #5 #1 AND #4 #6 "Neck Dissection/adverse effects"[Mesh] #7 #5 OR #6 #8 “Rehabilitation” [Mesh] OR “Physical Therapy Modalities” [Mesh] OR “Exercise” [Mesh] OR “Yoga” [Mesh] OR “Tai Ji” [Mesh] OR “Therapeutic Touch” [Mesh] OR "physical therap*" [tiab] OR physio* [tiab] OR exercis* [tiab] OR movement* [tiab] OR aerobic* [tiab] OR pilates [tiab] OR stretch* [tiab] OR "tai chi" [tiab] OR "tai ji" [tiab] OR yoga [tiab] OR (resistance [tiab] AND training [tiab]) OR rehab* [tiab] #9 #7 AND #8 | 1 exp "head and neck cancer"/ 2 exp neck dissection/ 3 ((head or neck or (upper and aerodigestive and tract) or uadt) and (cancer* or neoplas* or tumor* or tumour* or malignan* or carcinom*)).tw. 4 ((neck and dissect*) or hnscc).tw. 5 1 or 2 or 3 or 4 6 exp SHOULDER/ 7 shoulder injury/ 8 shoulder pain/ 9 (shoulder* or scapul* or trapezius or glenohumeral or (adhesive and capsulitis) or (accessory and nerve*) or ((11th or eleventh) and nerve*)).tw. 10 (morbidit* or disabilit* or function* or dysfunction* or pain* or syndrome or droop* or lesion* or impair* or injur*).tw. 11 9 and 10 12 6 or 7 or 8 or 11 13 neck dissection/rh [Rehabilitation] 14 exp REHABILITATION/ 15 exp physiotherapy/ 16 exp MANIPULATIVE MEDICINE/ 17 exp exercise/ 18 exp kinesiotherapy/ 19 ((physical and therap*) or physio* or exercise* or movement* or aerobic* or pilates or stretch* or tai or yoga or (resistance and training) or rehab*).tw. 20 13 or 14 or 15 or 16 or 17 or 18 or 19 21 5 and 12 and 20 22 13 AND 20 23 21 OR 22 |
| CINAHL (EBSCO) | Web of Science | BIOSIS Previews (Web of Knowledge) | Clinicaltrials.gov |
| S1 (MH "Head and Neck Neoplasms+") S2 (MH "Radical Neck Dissection") S3 TX ((head or neck or (upper and aerodigestive and tract) or uadt) and (cancer* or neoplas* or tumor* or tumour* or malignan* or carcinom*)) S4 TX (neck and dissect*) or hnscc S5 S1 or S2 or S3 or S4 S6 (MH "Shoulder") OR (MH "Shoulder Injuries+") OR (MH "Shoulder Joint+") OR (MH "Shoulder Pain") S7 TX (shoulder* or scapul* or trapezius or glenohumeral or (adhesive and capsulitis) or (accessory and nerve*) or ((11th or eleventh) and nerve*)) S8 TX (morbidit* or disabilit* or function* or dysfunction* or pain* or syndrome or droop* or lesion* or impair* or injur*) S9 S7 and S8 S10(MH "Rehabilitation+") S11 (MH "Physical Therapy+") S12(MH "Exercise+") S13 (MH "Therapeutic Touch") S14 (MH "Yoga") OR (MH "Tai Chi") S15 TX ((physical and therap*) or physio* or exercise* or movement* or aerobic* or pilates or stretch* or tai or yoga or (resistance and training) or rehab*) S16 s10 or S11 or S12 or S13 or S14 or S15 S17 S6 or S9 S18 s5 AND s16 AND s17 | #1 TS=((head or neck or (upper and aerodigestive and tract) or uadt) and (cancer* or neoplas* or tumor* or tumour* or malignan* or carcinom*)) #2 TS=((neck and dissect*) or hnscc) #3 #2 OR #1 #4 TS=(shoulder* or scapul* or trapezius or glenohumeral or (adhesive and capsulitis) or (accessory and nerve*) or ((11th or eleventh) and nerve*)) #5 TS=(morbidit* or disabilit* or function* or dysfunction* or pain* or syndrome or droop* or lesion* or impair* or injur*) #6 TS=((physical and therap*) or physio* or exercise* or movement* or aerobic* or pilates or stretch* or tai or yoga or (resistance and training) or rehab*) #7 #6 AND #5 AND #4 AND #3 | #1 TS=((head or neck or (upper and aerodigestive and tract) or uadt) and (cancer* or neoplas* or tumor* or tumour* or malignan* or carcinom*)) #2 TS=((neck and dissect*) or hnscc) #3 #2 OR #1 #4 TS=(shoulder* or scapul* or trapezius or glenohumeral or (adhesive and capsulitis) or (accessory and nerve*) or ((11th or eleventh) and nerve*)) #5 TS=(morbidit* or disabilit* or function* or dysfunction* or pain* or syndrome or droop* or lesion* or impair* or injur*) #6 TS=((physical and therap*) or physio* or exercise* or movement* or aerobic* or pilates or stretch* or tai or yoga or (resistance and training) or rehab*) #7 #6 AND #5 AND #4 AND #3 | neck AND dissection AND shoulder (cancer OR carcinoma OR neoplasm) AND shoulder |
Appendix 2. 'Risk of bias' assessment
Random sequence generation
Low risk of bias (e.g. participants were assigned to treatments on the basis of a computer‐generated random sequence or a table of random numbers).
High risk of bias (e.g. participants were assigned to treatments on the basis of date of birth, clinic ID number or surname, or no attempt was made to randomize participants).
Unclear risk of bias (e.g. not reported, information not available).
Allocation concealment
Low risk of bias (e.g. where the allocation sequence could not be foretold).
High risk of bias (e.g. allocation sequence could be foretold by patients, investigators or treatment providers).
Unclear risk of bias (e.g. not reported).
Blinding of participants and personnel
Low risk of bias
High risk of bias
Unclear risk of bias
Blinding of outcome assessment
Low risk of bias
High risk of bias
Unclear risk of bias
Incomplete outcome data
Was loss to follow‐up less than 20% and were the reasons for loss to follow‐up similar in both arms?
Low risk of bias
High risk of bias
Unclear risk of bias
Selective reporting
Low risk of bias
High risk of bias
Unclear risk of bias
Other sources of bias
Low risk of bias
High risk of bias
Unclear risk of bias
Data and analyses
Comparison 1. PRT versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shoulder Pain and Disability Index (pain score) 12 weeks | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐6.26 [‐12.20, ‐0.31] |
| 2 Shoulder Pain and Disability Index (disability subscale) 12 weeks | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐8.48 [‐15.07, ‐1.88] |
| 3 Shoulder Pain and Disability Index (total score) 12 weeks | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐12.00, 2.46] |
| 4 Active range of motion (abduction) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 9.45 [‐6.26, 25.17] |
| 5 Active range of motion (forward flexion) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 7.01 [‐1.93, 15.95] |
| 6 Active range of motion (external rotation) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 14.51 [7.87, 21.14] |
| 7 Passive range of motion (abduction) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 7.65 [0.64, 14.66] |
| 8 Passive range of motion (forward flexion) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 6.20 [0.69, 11.71] |
| 9 Passive range of motion (external rotation) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 7.17 [2.20, 12.14] |
| 10 Passive range of motion (horizontal abduction) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 7.34 [2.86, 11.83] |
| 11 Quality of life (FACT‐G) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 5.05 [‐3.01, 13.12] |
| 12 Adverse event | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.1 Pain increase | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Nausea | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Quality of life measured by FACT‐An scale | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 8.0 [‐8.77, 24.77] |
| 14 Quality of life measured by FACT‐H&N questionnaire | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐16.30, 24.10] |
| 15 Quality of life assessed by NDII questionnaire | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 8.40 [‐3.54, 20.34] |
| 16 Endurance of scapular muscles | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 320.0 [89.75, 550.25] |
| 17 Strength of scapular muscles (seated row, 1‐RM with two arms) | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 18.90 [6.84, 30.96] |
| 18 Strength of scapular muscles (seated row, 1‐RM affected shoulder) | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 7.0 [1.17, 12.83] |
| 19 Strength of scapular muscles (chest press, 1‐RM with two arms) | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 14.40 [3.05, 25.75] |
| 20 Strength of scapular muscles (chest press, 1‐RM affected shoulder) | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 6.5 [0.93, 12.07] |
1.12. Analysis.

Comparison 1 PRT versus standard care, Outcome 12 Adverse event.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lauchlan 2011.
| Methods | Randomized controlled trial Described as an exploratory trial | |
| Participants |
Inclusion criteria: patients were selected if they had selective neck dissection or radical neck dissection Relevant details for the participants (such as sex, age, stage of the disease) were not described in the article Exclusion criteria: patients who had severe cardiac or respiratory disease, severe cognitive impairment/unable to consent, previous significant injury to the arm, shoulder, neck or chest; an existing clinical presentation of adhesive capsulitis of the glenohumeral joint |
|
| Interventions |
Intervention group: free active exercises of all physiological movements of the glenohumeral joint (GHJ), passive stretching of the GHJ, postural care, re‐education of scapulothoracic postural muscles, strengthening of the shoulder muscles with graded elastic tension bands to provide resistance to all muscle groups involved in all physiological movements of the GHJ and patient advice and instruction leaflet. This was associated with routine postoperative care (respiratory care, verbal advice on early active movement of the neck and affected shoulder associated with free active exercises of all physiological movements of the GHJ). The intervention consisted of 3 months of outpatient physiotherapy care, immediately after discharge from hospital. Frequency: 3 times a week in the first month, twice a week in the second month and once a week in the third month Control group: routine postoperative physiotherapy care in the hospital (respiratory care and verbal advice on early active movement of the neck and affected shoulder) |
|
| Outcomes |
All measurements were done pre‐operatively and 1 year postoperatively |
|
| Notes | Some information about the participants and intervention, such as intensity and duration of the exercise, was not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was carried out electronically through a web‐based randomisation programme in the Newcastle Otolaryngology Trials Office at Newcastle University." |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is insufficient information to judge this item |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "A blinded independent rater (S. P.) was used to record values for all outcome measures both pre‐operatively and at 1 year post‐operatively" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: reasons for missing outcome data were described and were balanced in numbers across interventions groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: we have insufficient information to judge this item |
| Other bias | High risk | Comment: they use a insensitive instrument to measure the outcome quality of life and the study had some inconsistencies in the implementation of the interventions |
McNeely 2004.
| Methods | Randomized controlled trial | |
| Participants | The patients were divided into 2 stratas: early (within 8 weeks of neck dissection) and late (8 or more weeks after neck dissection) Eligibility criteria: all patients were diagnosed with head and neck cancer and squamous cell carcinoma was histologically confirmed. Surgical treatment (radical neck dissection, modified radical neck dissection and other variants of selective neck dissection) patients were required to have a medical diagnosis of shoulder dysfunction (defined as winging of the scapula with shoulder abduction in the coronal plane and limitation of shoulder range of motion); Karnofsky performance status of 60% or higher; no evidence of residual cancer in the neck; and no distant metastasis. Setting: Cross Cancer Institute and University of Alberta in Edmonton, Canada Range in time from surgery to initiation of intervention: the early group had a mean and standard deviation of 6.8 (1.3) weeks after neck dissection, and the late group had 97 (116.7) weeks Age: mean age 61 years Sex: 14 (82%) male Stage of disease: 3 (18%) of the patients were stage I, 3 (18%) stage III and 11 (65%) stage IV Site of the tumor: 7 oropharynx (41%), 5 larynx/hypopharynx (29%), 2 oral cavity (12%), 1 nasopharynx (6%), 1 parotid and 1 unknown primary site Type of neck dissection: 7 (25%) participants had radical neck dissection, 8 (29%) had modified neck dissection to level 5, 3 (11%) had modified neck dissection to level 4 and 10 (36%) had selective neck dissection to level 3 Adjuvant treatments: radiation therapy was not performed in 1 patient only and the type of radiation was similar between the groups analyzed Other variables: pain medication was monitored before and during treatment; past exercise was checked and no differences between the groups was shown |
|
| Interventions |
Intervention group: patients performed 12 weeks of progressive resistance exercise training, with a frequency of 3 times per week Warm‐up exercises: participants did 5 to 10 minutes of range of motion exercises before the beginning of the strengthening for the glenohumeral joint in supine position Muscle groups strengthened: rhomboids (scapular retraction); lavator scapulae (scapular elevation); biceps (elbow flexion); triceps (elbow extension); infraspinatus, posterior deltoid (external rotation); and middle deltoid and supraspinatus and subscapularis (abduction in the plane of the scapula) Intensity: started with resistance of 1 kg to 2 kg weights and progress within guidelines. The patient must be able to maintain posture and scapular stability (no winging of scapula). A rate of perceived exertion on the Borg scale of no greater than 13 of 20 (described as "somewhat hard"). Repetitions: 15 to 20 progressing to maximum of 25 repetitions initially when the participant was performing only one set Sets: initially 1 set and progress to 2 sets; when the patient was able to do 2 sets of 20 the weight was increased Rest interval: 1 to 2 minutes between exercise stations and up to 4 minutes between sets. The concentric tempo was about 2 to 4 seconds with the patient exhaling, and the eccentric tempo was about 4 seconds with the patient inhaling. Cool‐down exercise: after the PRT, stretching exercises with focus on pectoralis major and minor and serratus anterior were done for 5 to 10 minutes Criteria for workload reduction: excessive fatigue after exercise, muscle soreness for more than 48 hours and increased pain after exercise Criteria for terminate exercise: pain, dizziness and general malaise Control group: this group had standard care consisting of active and passive range of motion exercises and stretching exercises. At the 6th week of treatment the participants progressed to scapular retraction and elevation strengthening exercises with a elastic resistance band, but the strengthening was not done in a progressive way. |
|
| Outcomes |
|
|
| Notes | This was described as a pilot study and some limitations were described: the need of stratification on the basis of performed neck dissection, a more stringent control of confounders and the lack of long‐term follow‐up of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to exercise and standard care intervention by means of a computer‐generated code" |
| Allocation concealment (selection bias) | Unclear risk | Quote (from correspondence): "computer‐generated by our statistician" Comment: it is not clear if allocation concealment was performed appropriately. Data provided by author did not clarify. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote (from correspondence): "We did not have independent blinded assessors for the pilot study as we were looking at feasibility" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the trial described the reasons for withdrawals and it was balanced in numbers across the groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: there is insufficient information to judge this item |
| Other bias | Low risk | Comment: the study is free of other bias that could risk its validity |
McNeely 2008.
| Methods | Randomized controlled trial | |
| Participants | Participants were stratified by tumor location (oral/oropharynx versus hypopharynx/larynx versus thyroid) and type of neck dissection (radical neck dissection versus modified radical neck dissection/variants of selective neck dissection) Setting: Cross Cancer Institute and University of Alberta in Edmonton, Canada Age: mean age 52 years Sex: 15 (29%) women On disability: 20 (38%) Range in time from surgery to initiation of intervention: 15 months ranging from 2 to 180 months. 22 (42%) participants had less than 9 months until exercise intervention, 8 (15%) had 8 to 17 months, and 23 (44%) had more than 18 months. Diagnosis: 32 (62%) oral/oropharynx cancer, 12 (23%) larynx/hypopharynx, 2 (4%) thyroid, 2 (4%) parotid, 2 (4%) sarcoma in mandible and 2 (4%) unknown primary site Disease stage: 30 (58%) were stage IV, 12 (23%) stage III, 6 (12%) stage II and 3 (6%) stage I Type of neck dissections: 40 (77%) had bilateral neck dissection, 9 (17%) had radical neck dissection, 5 (10%) modified neck dissection with sacrifice of sternocleidomastoid muscle, 20 (38%) modified neck dissection to level 5 and 18 (35%) selective neck dissection with the level 5 spared Radiation therapy: 37 (71%) bilateral neck radiation, 5 (10%) intensity‐modulated radiotherapy and 2 (4%) unilateral neck radiotherapy Chemotherapy protocol: 9 (17%) cisplatin, 3 (6%) carboplatin and 2 (4%) carboplatin/cisplatin plus 5‐fluorouracil Pain medication: 12 (23%) took daily narcotic medication All the characteristics of the participants were balanced across the 2 groups and no differences were seen between the groups for any of the criteria described above |
|
| Interventions |
Intervention group: PRT group, 12 weeks of intervention, 2 sessions per week with the option of a third session at the center or at home Active and passive range of motion, stretching exercises and postural exercises Muscles strengthened: rhomboids/middle trapezius, levator scapula/upper trapezius, biceps and triceps, deltoid and pectoralis major Intensity: based in a 1‐repetition maximum (1‐RM); patients began the exercise with 25% to 30% of the 1‐RM and slowly progressed to 60% to 70% of their 1‐RM by the end of the 12 weeks. The patient must be able to maintain posture and scapular stability (e.g. no winging of scapula). A rate of perceived exertion on the Borg scale of no greater than 13 to 15 of 20 (described as "somewhat hard" to "hard") Resistance weight was increased 1 kg to 2.5 kg if participant was able to complete 2 sets of 15 repetitions with proper form Sets: 2 Repetitions: 10 to 15 Rest interval: 1 to 2 minutes between exercise stations and up to 4 minutes between sets. The concentric tempo was about 2 to 4 seconds with the patient exhaling, and the eccentric tempo was about 4 seconds with the patient inhaling. Cool‐down exercise: after the PRT, stretching exercises with focus on pectoralis major and minor and serratus anterior were done for 5 to 10 minutes The workload was reduced if the participants had excessive fatigue after exercise, muscle soreness for more than 48 hours and increased pain after exercise. The criteria for terminating exercise were pain, dizziness and general malaise. Control group: standardized therapeutic exercise group, consisted of supervised active and passive range of motion and stretching exercises, postural exercises and basic strengthening exercises with light weights (1 to 5 kg) and elastic resistance bands Muscles trained: rhomboids/middle trapezius, levator scapula/upper trapezius, biceps and triceps, deltoid and pectoralis major. For participants with recovery of active trapezius muscle function, specific exercises focused in the trapezius were introduced in week 6 and 8 of the intervention. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent researcher generated the allocation sequence by using a computer‐generated code" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence and contents of the envelopes were enclosed in sequentially numbered and sealed (opaque) envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Independent assessors who were blinded to group assignment performed the ROM and strength and endurance tests" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analyses were conducted |
| Selective reporting (reporting bias) | Low risk | Comment: although some outcomes described in the protocol and methods section of the trial were not described in the results, we entered into contact with the author and obtained the missing outcomes |
| Other bias | Low risk | Comment: the study is free of other bias that could risk its validity |
1‐RM: 1‐repetition maximum; GHJ: glenohumeral joint; PRT: progressive resistance training; ROM: range of motion; SPADI: Shoulder Pain and Disability Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hou 2002 | ALLOCATION Randomized controlled trial PARTICIPANTS Did not include patients with head and neck cancer |
| Pfister 2008 | ALLOCATION Randomized controlled trial PARTICIPANTS Patients with cancer with a history of neck dissection INTERVENTION Patients received acupuncture versus usual care |
| van Wilgen 2007 | ALLOCATION Not a randomized controlled trial |
Characteristics of ongoing studies [ordered by study ID]
McGarvey 2011.
| Trial name or title | Effect of a progressive resisted exercise program on shoulder pain and function following accessory nerve neurapraxia after neck dissection surgery for cancer |
| Methods | Randomized controlled trial |
| Participants | Patients that have undergone neck dissection surgery for cancer within 8 weeks, that present with clinical signs of accessory nerve injury: dropped scapula, winged scapula and reduced active abduction on the operated side. Participants of both genders, 18 years or over, with accessory nerve preservation and fully healed scar will be included. |
| Interventions |
Intervention group: patients will perform progressive strengthening exercises using hand weights, active‐assisted range of movement exercises of the shoulder, active range of movement exercises of the neck, and stretches of the shoulder and neck. The supervising physiotherapist will ensure that no worsening of pain or fatigue occurs during sessions. Duration of section: half an hour Frequency and duration of intervention: 12 weeks. Supervised exercise program in the physiotherapy department once a week and the same exercises at home twice a week. Exercise diaries will be utilized to ensure compliance and no self upgrading of exercises. Control group: patients will receive current usual care, which is referral to physiotherapy only if they report pain or problems in their shoulder after surgery. If they report shoulder pain and problems with movement, they will receive a handout of generalized shoulder and neck exercises and advice about scar care. Participants may receive physiotherapy elsewhere and what they receive will be up to the treating physiotherapist. |
| Outcomes |
|
| Starting date | 4 January 2009 |
| Contact information | aoife.mcgarvey@uon.edu.au |
| Notes | We contacted the author and they reported that they are still collecting data for this study |
Differences between protocol and review
We changed the Cochrane 'Risk of bias' tool used during the preparation of the review to the new version in RevMan 5.1.
Contributions of authors
Conceiving the review: Alan Pedrosa Viegas de Carvalho (AC), Flávia MR Vital (FV), Bernardo Soares (BS) Co‐ordinating the review: BS Undertaking manual searches: AC, FV Screening search results: AC, FV Organizing retrieval of papers: AC, FV Screening retrieved papers against inclusion criteria: AC, FV Appraising quality of papers: AC, FV Abstracting data from papers: AC, FV Writing to authors of papers for additional information: AC, FV, BS Providing additional data about papers: AC, FV, BS Obtaining and screening data on unpublished studies: AC, FV, BS Data management for the review: AC, FV, BS Entering data into Review Manager (RevMan 5): AC RevMan 5 statistical data: AC, FV Other statistical analysis not using RevMan 5: FV, BS Double entry of data: (data entered by person one: AC; data entered by person two: AC, FV) Interpretation of data: AC, FV Statistical inferences: FV, BS Writing the review: AC Securing funding for the review: AC Performing previous work that was the foundation of the present study: AC Guarantor for the review (one author): AC Person responsible for reading and checking review before submission: AC, FV
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
There are no known conflicts of interest.
New
References
References to studies included in this review
Lauchlan 2011 {published data only}
- Lauchlan DT, McCaul JA, McCarron T, Patil S, McManners J, McGarva J. An exploratory trial of preventative rehabilitation on shoulder disability and quality of life in patients following neck dissection surgery. European Journal of Cancer Care 2011;20(1):113‐22. [DOI] [PubMed] [Google Scholar]
- Lauchlan DT, McCaul JA, Patil S, McGarva G, Mackay T, McCarron T, et al. A randomized controlled trial of early, out‐patient physiotherapy for shoulder disability in patients following neck dissection surgery. British Journal of Oral and Maxillofacial Surgery 2003;41:283‐304. [Google Scholar]
McNeely 2004 {published data only}
- McNeely ML, Parliament M, Courneya KS, Seikaly H, Jha N, Scrimger R, et al. A pilot study of a randomized controlled trial to evaluate the effects of progressive resistance exercise training on shoulder dysfunction caused by spinal accessory neurapraxia/neurectomy in head and neck cancer survivors. Head and Neck 2004;26(6):518‐30. [DOI] [PubMed] [Google Scholar]
McNeely 2008 {published data only}
- Randomized controlled trial of progressive resistance exercise training for spinal accessory neurapraxia/neurectomy in head and neck cancer survivors. http://clinicaltrials.gov/ct2/show/NCT00248235. [NCT00248235]
- McNeely ML, Parliament MB, Seikaly H, Jha N, Magee DJ, Haykowsky MJ, et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer 2008;113(1):214‐22. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Hou 2002 {published data only}
- Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger‐point sensitivity. Archives of Physical Medicine and Rehabilitation 2002;83(10):1406‐14. [DOI] [PubMed] [Google Scholar]
Pfister 2008 {published data only}
- Pfister DG, Cassileth BR, Deng GE, Yeung KS, Lee JS, Garrity D, et al. Acupuncture for pain and dysfunction after neck dissection: results of a randomized controlled trial. Journal of Clinical Oncology 2010;28(15):2565‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Wilgen 2007 {published data only}
- Wilgen P. Morbidity after neck dissection in cancer. Dutch Journal of Physical Therapy 2007;117(3):116‐7. [Google Scholar]
References to ongoing studies
McGarvey 2011 {unpublished data only}
- Effect of a progressive resisted exercise program on shoulder pain and function following accessory nerve neurapraxia after neck dissection surgery for cancer. http://apps.who.int/trialsearch/trial.aspx?trialid=ACTRN12611000066987. [ACTRN12611000066987]
Additional references
Bessell 2011
- Bessell A, Glenny AM, Furness S, Clarkson JE, Oliver R, Conway DI, et al. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD006205.pub3] [DOI] [PubMed] [Google Scholar]
Chepeha 2002
- Chepeha DB, Taylor RJ, Chepeha JC, Teknos TN, Bradford CR, Sharma PK, et al. Functional assessment using Constant's Shoulder Scale after modified radical and selective neck dissection. Head and Neck 2002;24(5):432‐6. [DOI] [PubMed] [Google Scholar]
Chida 2002
- Chida S, Shimada Y, Matsunaga T, Sato M, Hatakeyama K, Mizoi K. Occupational therapy for accessory nerve palsy after radical neck dissection. Tohoku Journal of Experimental Medicine 2002;196(3):157‐65. [DOI] [PubMed] [Google Scholar]
De Boer 1999
- Boer MF, McCormick LK, Pruyn JF, Ryckman RM, Borne BW. Physical and psychosocial correlates of head and neck cancer: a review of the literature. Otolaryngology ‐ Head and Neck Surgery 1999;120(3):427‐36. [PUBMED: 10064650] [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London (UK): BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Dijkstra 2001
- Dijkstra PU, Wilgen PC, Buijs RP, Brendeke W, Goede CJ, Kerst A, et al. Incidence of shoulder pain after neck dissection: a clinical explorative study for risk factors. Head and Neck 2001;23(11):947‐53. [PUBMED: 11754498] [DOI] [PubMed] [Google Scholar]
Fialka 1989
- Fialka V, Vinzenz K. Physiotherapy and diagnosis of shoulder lesions after radical neck dissection. Deutsche Zeitschrift für Mund‐, Kiefer‐ und Gesichts‐Chirurgie 1989;13:220‐5. [PUBMED: 2625014] [PubMed] [Google Scholar]
Güldiken 2005
- Güldiken Y, Orhan KS, Demirel T, Ural HI, Yücel EA, Deger K. Assessment of shoulder impairment after functional neck dissection: long term results. Auris, Nasus, Larynx 2005;32(4):387‐91. [PUBMED: 16076539] [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Herchenhorn 2004
- Herchenhorn D, Dias FL. Advances in radiochemotherapy in the treatment of head and neck cancer. Revista do Hospital das Clínicas 2004;59(1):39‐46. [PUBMED: 15029284] [DOI] [PubMed] [Google Scholar]
Herring 1987
- Herring D, King AI, Connelly M. New rehabilitation concepts in management of radical neck dissection syndrome. A clinical report. Physical Therapy 1987;67(7):1095‐9. [PUBMED: 3602104] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327:557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1978
- Johnson EW, Aseff JN, Saunders W. Physical treatment of pain and weakness following radical neck dissection. Ohio State Medical Journal 1978;74(11):711‐4. [PUBMED: 704002] [PubMed] [Google Scholar]
Kisner 2005
- Kisner C, Colby LA. Therapeutic Exercise: Foundations and Techniques. 4th Edition. Barueri, São Paulo: Manole, 2005. [Google Scholar]
Laska 2001
- Laska T, Hannig K. Physical therapy for spinal accessory nerve injury complicated by adhesive capsulitis. Physical Therapy 2001;81(3):936‐44. [PUBMED: 11268158] [PubMed] [Google Scholar]
Leitzmann 2008
- Leitzmann MF, Koebnick C, Freedman ND, Park Y, Ballard‐Barbash R, Hollenbeck AR, et al. Physical activity and head and neck cancer risk. Cancer Causes and Control 2008;19(10):1391‐9. [PUBMED: 18704714] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2009
- Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002759.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nahum 1961
- Nahum AM, Mullally W, Marmor L. A syndrome resulting from radical neck dissection. Archives of Otolaryngology 1961;74:424‐8. [PUBMED: 14477989] [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Salerno 2002
- Salerno G, Cavaliere M, Foglia A, Pellicoro DP, Mottola G, Nardone M, et al. The 11th nerve syndrome in functional neck dissection. Laryngoscope 2002;112:1299‐307. [PUBMED: 12169917] [DOI] [PubMed] [Google Scholar]
Saunders 1975
- Saunders WH, Johnson EW. Rehabilitation of the shoulder after radical neck dissection. Annals of Otology, Rhinology, and Laryngology 1975;84(6):812‐6. [PUBMED: 1200571] [DOI] [PubMed] [Google Scholar]
Saunders 1985
- Saunders JR Jr, Hirata RM, Jaques DA. Considering the spinal accessory nerve in head and neck surgery. American Journal of Surgery 1985;150(4):491‐4. [PUBMED: 4051114] [DOI] [PubMed] [Google Scholar]
Shimada, 2007
- Shimada Y, Chida S, Matsunaga T, Sato M, Hatakeyama K, Itoi E. Clinical results of rehabilitation for accessory nerve palsy after radical neck dissection. Acta Oto‐Laryngologica 2007;127(5):491‐7. [DOI] [PubMed] [Google Scholar]
Short 1984
- Short SO, Kaplan JN, Laramore GE, Cummings CW. Shoulder pain and function after neck dissection with or without preservation of the spinal accessory nerve. American Journal of Surgery 1984;148(4):478‐82. [PUBMED: 6486316] [DOI] [PubMed] [Google Scholar]
van Wilgen 2003
- Wilgen CP, Dijkstra PU, Laan BF, Plukker JT, Roodenburg JL. Shoulder complaints after neck dissection; is the spinal accessory nerve involved?. British Journal of Oral and Maxillofacial Surgery 2003;41(1):7‐11. [DOI] [PubMed] [Google Scholar]
van Wouwe 2009
- Wouwe M, Bree R, Kuik DJ, Goede CJ, Verdonck‐de Leeuw IM, Doornaert P, et al. Shoulder morbidity after non‐surgical treatment of the neck. Radiotherapy and Oncology 2009;90(2):196‐201. [PUBMED: 19054587] [DOI] [PubMed] [Google Scholar]
Voss 1985
- Voss DE, Ionta MK, Myers BJ. Proprioceptive Neuromuscular Facilitation Patterns and Techniques. Philadelphia: Harper & Row, 1985. [Google Scholar]


