Abstract
Background
A reduction in salt intake lowers blood pressure (BP) and, thereby, reduces cardiovascular risk. A recent meta‐analysis by Graudal implied that salt reduction had adverse effects on hormones and lipids which might mitigate any benefit that occurs with BP reduction. However, Graudal's meta‐analysis included a large number of very short‐term trials with a large change in salt intake, and such studies are irrelevant to the public health recommendations for a longer‐term modest reduction in salt intake. We have updated our Cochrane meta‐analysis.
Objectives
To assess (1) the effect of a longer‐term modest reduction in salt intake (i.e. of public health relevance) on BP and whether there was a dose‐response relationship; (2) the effect on BP by sex and ethnic group; (3) the effect on plasma renin activity, aldosterone, noradrenaline, adrenaline, cholesterol, low‐density lipoprotein (LDL), high‐density lipoprotein (HDL) and triglycerides.
Search methods
We searched MEDLINE, EMBASE, Cochrane Hypertension Group Specialised Register, Cochrane Central Register of Controlled Trials, and reference list of relevant articles.
Selection criteria
We included randomised trials with a modest reduction in salt intake and duration of at least 4 weeks.
Data collection and analysis
Data were extracted independently by two reviewers. Random effects meta‐analyses, subgroup analyses and meta‐regression were performed.
Main results
Thirty‐four trials (3230 participants) were included. Meta‐analysis showed that the mean change in urinary sodium (reduced salt vs usual salt) was ‐75 mmol/24‐h (equivalent to a reduction of 4.4 g/d salt), the mean change in BP was ‐4.18 mmHg (95% CI: ‐5.18 to ‐3.18, I2=75%) for systolic and ‐2.06 mmHg (95% CI: ‐2.67 to ‐1.45, I2=68%) for diastolic BP. Meta‐regression showed that age, ethnic group, BP status (hypertensive or normotensive) and the change in 24‐h urinary sodium were all significantly associated with the fall in systolic BP, explaining 68% of the variance between studies. A 100 mmol reduction in 24 hour urinary sodium (6 g/day salt) was associated with a fall in systolic BP of 5.8 mmHg (95%CI: 2.5 to 9.2, P=0.001) after adjusting for age, ethnic group and BP status. For diastolic BP, age, ethnic group, BP status and the change in 24‐h urinary sodium explained 41% of the variance between studies. Meta‐analysis by subgroup showed that, in hypertensives, the mean effect was ‐5.39 mmHg (95% CI: ‐6.62 to ‐4.15, I2=61%) for systolic and ‐2.82 mmHg (95% CI: ‐3.54 to ‐2.11, I2=52%) for diastolic BP. In normotensives, the mean effect was ‐2.42 mmHg (95% CI: ‐3.56 to ‐1.29, I2=66%) for systolic and ‐1.00 mmHg (95% CI: ‐1.85 to ‐0.15, I2=66%) for diastolic BP. Further subgroup analysis showed that the decrease in systolic BP was significant in both whites and blacks, men and women. Meta‐analysis of hormone and lipid data showed that the mean effect was 0.26 ng/ml/hr (95% CI: 0.17 to 0.36, I2=70%) for plasma renin activity, 73.20 pmol/l (95% CI: 44.92 to 101.48, I2=62%) for aldosterone, 31.67 pg/ml (95% CI: 6.57 to 56.77, I2=5%) for noradrenaline, 6.70 pg/ml (95% CI: ‐0.25 to 13.64, I2=12%) for adrenaline, 0.05 mmol/l (95% CI: ‐0.02 to 0.11, I2=0%) for cholesterol, 0.05 mmol/l (95% CI: ‐0.01 to 0.12, I2=0%) for LDL, ‐0.02 mmol/l (95% CI: ‐0.06 to 0.01, I2=16%) for HDL, and 0.04 mmol/l (95% CI: ‐0.02 to 0.09, I2=0%) for triglycerides.
Authors' conclusions
A modest reduction in salt intake for 4 or more weeks causes significant and, from a population viewpoint, important falls in BP in both hypertensive and normotensive individuals, irrespective of sex and ethnic group. With salt reduction, there is a small physiological increase in plasma renin activity, aldosterone and noradrenaline. There is no significant change in lipid levels. These results provide further strong support for a reduction in population salt intake. This will likely lower population BP and, thereby, reduce cardiovascular disease. Additionally, our analysis demonstrates a significant association between the reduction in 24‐h urinary sodium and the fall in systolic BP, indicating the greater the reduction in salt intake, the greater the fall in systolic BP. The current recommendations to reduce salt intake from 9‐12 to 5‐6 g/d will have a major effect on BP, but are not ideal. A further reduction to 3 g/d will have a greater effect and should become the long term target for population salt intake.
Keywords: Humans; Age Factors; Aldosterone; Aldosterone/blood; Blood Pressure; Blood Pressure/physiology; Hypertension; Hypertension/blood; Hypertension/diet therapy; Hypertension/ethnology; Lipids; Lipids/blood; Norepinephrine; Norepinephrine/blood; Randomized Controlled Trials as Topic; Renin; Renin/blood; Sodium; Sodium/urine; Sodium Chloride, Dietary; Sodium Chloride, Dietary/administration & dosage; Time Factors
Plain language summary
Modest salt reduction lowers blood pressure in all ethnic groups at all levels of blood pressure without adverse consequences
The public health recommendations in most countries are to reduce salt intake from the current levels of approximately 9‐12 grams per day to less than 5‐6 grams per day. Our pooled analysis of randomised trials of 4 weeks or more in duration shows that such a reduction in salt intake lowers blood pressure both in individuals with raised blood pressure and in those with normal blood pressure. The fall in blood pressure is shown in both whites and blacks, men and women. Additionally, our results show that a longer‐term modest reduction in salt intake has no adverse effect on hormone and lipid levels. These findings provide further strong support for a reduction in population salt intake. This will likely lower population blood pressure and reduce strokes, heart attacks and heart failure. Furthermore, our results are consistent with the fact that the lower the salt intake, the lower the blood pressure. The current recommendations to reduce salt intake to 5‐6 grams per day will lower blood pressure, but a further reduction to 3 grams per day will lower blood pressure more. Therefore, 3 grams per day should become the long‐term target for population salt intake.
Summary of findings
Summary of findings for the main comparison. Change in systolic and diastolic blood pressure (SBP, DBP) from usual to reduced salt intake in hypertensive and normotensive individuals.
| Outcomes |
Number of Trials |
Number of Participants |
Median BP on usual salt (mmHg) |
Mean change in BP* (mmHg) with salt reduction [95% CI], P |
Quality of the evidence (GRADE) |
|
|
All trials together Duration of salt reduction: Median: 4 weeks (range 4 weeks to 3 years) Mean reduction in UNa: 75 mmol/24h (equivalent to 4.4 g/d salt) |
SBP | 33 | 3206 | 141 | ‐4.18 [‐5.18, ‐3.18], P<0.00001 | ⊕⊕⊕⊕ High |
| DBP | 34 | 3230 | 86 | ‐2.06 [‐2.67, ‐1.45], P<0.00001 | ⊕⊕⊕⊕ High |
|
|
Hypertensives Duration of salt reduction: Median 5 weeks (range 4 weeks to 1 year) Mean reduction in UNa: 75 mmol/24h (equivalent to 4.4 g/d salt) |
SBP | 21 | 966 | 148 | ‐5.39 [‐6.62, ‐4.15], P<0.00001 | ⊕⊕⊕⊕ High |
| DBP | 22 | 990 | 93 | ‐2.82 [‐3.54, ‐2.11], P<0.00001 | ⊕⊕⊕⊕ High |
|
|
Normotensives Duration of salt reduction: Median 4 weeks (range 4 weeks to 3 years) Mean reduction in UNa: 75 mmol/24h (equivalent to 4.4 g/d salt) |
SBP | 12 | 2240 | 127 | ‐2.42 [‐3.56, ‐1.29], P<0.0001 | ⊕⊕⊕⊕ High |
| DBP | 12 | 2240 | 77 | ‐1.00 [‐1.85, ‐0.15], P=0.02 | ⊕⊕⊕⊕ High |
* Negative value indicates that the effect favours reduced salt. UNa: urinary sodium.
Summary of findings 2. Change in 24h urinary sodium (UNa) and blood pressure (BP) in hypertensive and normotensive individuals by ethnic group.
|
Number of Trials |
Number of Participants |
Mean effect [95% CI] mmHg | P value | |
| Hypertensive whites | ||||
| SBP (mmHg) | 16 | 599 | ‐5.12 [‐6.27, ‐3.96] | <0.00001 |
| DBP (mmHg) | 17 | 623 | ‐2.66 [‐3.37, ‐1.95] | <0.00001 |
| 24h UNa (mmol) | 17 | 623 | ‐77.44 [‐85.22, ‐69.66] | <0.00001 |
| Hypertensive blacks | ||||
| SBP (mmHg) | 5 | 171 | ‐7.83 [‐10.96, ‐4.71] | <0.00001 |
| DBP (mmHg) | 5 | 171 | ‐4.08 [‐5.90, ‐2.26] | <0.0001 |
| 24h UNa (mmol) | 5 | 171 | ‐66.87 [‐82.79, ‐50.95] | <0.00001 |
| Hypertensive Asians | ||||
| SBP (mmHg) | 1 | 29 | ‐5.41 [‐9.27, ‐1.56] | 0.008 |
| DBP (mmHg) | 1 | 29 | ‐2.17 [‐4.31, ‐0.03] | 0.047 |
| 24h UNa (mmol) | 1 | 29 | ‐68.42 [‐89.19, ‐47.64] | <0.001 |
| Normotensive whites | ||||
| SBP (mmHg) | 12 | 1901 | ‐2.11 [‐3.03, ‐1.19] | <0.00001 |
| DBP (mmHg) | 12 | 1901 | ‐0.88 [‐1.68, ‐0.08] | 0.03 |
| 24h UNa (mmol) | 12 | 1901 | ‐76.45 [‐89.52, ‐63.38] | <0.00001 |
| Normotensive blacks | ||||
| SBP (mmHg) | 3 | 412 | ‐4.02 [‐7.44, ‐0.61] | 0.02 |
| DBP (mmHg) | 3 | 412 | ‐1.98 [‐4.45, 0.49] | 0.12 |
| 24h UNa (mmol) | 3 | 412 | ‐40.31 [‐97.16, 16.55] | 0.16 |
SBP: Systolic blood pressure. DBP: Diastolic blood pressure. UNa: Urinary sodium.
Summary of findings 3. Change in 24h urinary sodium (UNa) and blood pressure (BP) in hypertensive and normotensive individuals by sex.
|
Number of Trials |
Number of Participants |
Mean effect [95% CI] mmHg | P value | |
| Hypertensive men | ||||
| SBP (mmHg) | 9 | 227 | ‐6.40 [‐8.00, ‐4.80] | <0.00001 |
| DBP (mmHg) | 10 | 239 | ‐3.96 [‐5.47, ‐2.46] | <0.00001 |
| 24h UNa (mmol) | 10 | 239 | ‐86.07 [‐100.17, ‐71.97] | <0.00001 |
| Hypertensive women | ||||
| SBP (mmHg) | 9 | 181 | ‐7.11 [‐8.81, ‐5.41] | <0.00001 |
| DBP (mmHg) | 10 | 193 | ‐3.41 [‐4.29, ‐2.53] | <0.00001 |
| 24h UNa | 10 | 193 | ‐69.56 [‐77.56, ‐61.55] | <0.00001 |
| Normotensive men | ||||
| SBP (mmHg) | 6 | 1391 | ‐3.39 [‐5.63, ‐1.16] | 0.003 |
| DBP (mmHg) | 6 | 1391 | ‐1.78 [‐3.01, ‐0.55] | 0.005 |
| 24h UNa (mmol) | 6 | 1391 | ‐67.26 [‐81.90, ‐52.62] | <0.00001 |
| Normotensive women | ||||
| SBP (mmHg) | 6 | 691 | ‐4.26 [‐6.20, ‐2.31] | <0.0001 |
| DBP (mmHg) | 6 | 691 | ‐2.18 [‐2.95, ‐1.41] | <0.00001 |
| 24h UNa (mmol) | 6 | 691 | ‐62.98 [‐88.59, ‐37.37] | <0.00001 |
SBP: Systolic blood pressure. DBP: Diastolic blood pressure. UNa: Urinary sodium.
Summary of findings 4. Change in plasma renin activity, aldosterone, noradrenaline and adrenaline.
|
Number of Trials |
Number of Participants |
Median value on usual salt |
Mean change with salt reduction [95% CI] |
P value | |
|
Plasma renin activity (ng/ml/hr) |
14 | 455 | 1.07 | 0.26 [0.17, 0.36] | <0.00001 |
|
Aldosterone (pmol/l) |
9 | 340 | 299 | 73.20 [44.92, 101.48] | <0.00001 |
|
Noradrenaline (pg/ml) |
6 | 129 | 351 | 31.67 [6.57, 56.77] | 0.01 |
|
Adrenaline (pg/ml) |
4 | 84 | 64 | 6.70 [‐0.25, 13.64] | 0.06 |
Summary of findings 5. Change in plasma lipids.
|
Number of Trials |
Number of Participants |
Median value on usual salt |
Mean change with salt reduction [95% CI] |
P value | |
|
Cholesterol (mmol/l) |
8 | 365 | 5.3 | 0.05 [‐0.02, 0.11] | 0.18 |
|
Low‐density lipoprotein (LDL) (mmol/l) |
5 | 262 | 3.2 | 0.05 [‐0.01, 0.12] | 0.11 |
|
How‐density lipoprotein (HDL) (mmol/l) |
6 | 278 | 1.3 | ‐0.02 [‐0.06, 0.01] | 0.19 |
|
Triglycerides (mmol/l) |
6 | 309 | 1.3 | 0.04 [‐0.02, 0.09] | 0.22 |
Background
The current public health recommendations in most countries are to reduce salt intake from approximately 9‐12 g/d to 5‐6 g/d (WHO 2003; SACN 2003). There is much evidence demonstrating that such a reduction in salt intake lowers blood pressure (BP). The evidence comes from different types of studies including epidemiological, migration, population‐based intervention, genetic and animal studies, as well as treatment trials (Elliott 1996; Poulter 1990; Forte 1989; Lifton 1996; Denton 1995; He 2002). As raised BP throughout its range is a major cause of cardiovascular disease, a reduction in salt intake lowers BP and, therefore, would reduce cardiovascular risk. Indeed, both prospective cohort studies and outcome trials have demonstrated that a lower salt intake is related to a reduced risk of cardiovascular disease (Strazzullo 2009;He 2011).
Despite the evidence above, a recent meta‐analysis by Graudal et al (Graudal 2011; Graudal 2012) implied that salt reduction had adverse effects on hormones and lipids which might mitigate any benefit that occurs with the reduction in BP. However, Graudal et al's meta‐analysis (Graudal 2011; Graudal 2012) is flawed from a public health perspective, as they included a large number of very short‐term trials with a large change in salt intake, e.g. from 20 to less than 1 g/d for only 4‐5 days, and such metabolic studies are irrelevant to the current public health recommendations for a modest reduction in salt intake for a long period of time. We have updated our Cochrane meta‐analysis to determine the effects of a longer‐term modest reduction in salt intake (i.e. of public health relevance) on BP, plasma renin activity, aldosterone, noradrenaline, adrenaline, cholesterol, low‐density lipoprotein (LDL), high‐density lipoprotein (HDL) and triglycerides, as well as further sub‐group analyses to study the effects of salt reduction on BP by ethnic group and sex.
Objectives
Our systematic review aimed to determine the effect of a longer‐term modest reduction in salt intake on BP in both hypertensive and normotensive individuals and to assess whether there was a dose‐response to salt reduction. We also assessed the effect of salt reduction on BP by ethnic group and sex. Furthermore, we aimed to study the effect of salt reduction on plasma renin activity, aldosterone, noradrenaline, adrenaline, cholesterol, LDL, HDL and triglycerides.
Methods
Criteria for considering studies for this review
Types of studies
For inclusion, trials needed to satisfy the following criteria:
1. Random allocation either to a modestly reduced salt intake or usual salt intake (i.e. control).
2. No concomitant interventions (i.e. nonpharmacologic interventions, antihypertensive or other medications) in either group.
3. The reduction in 24‐h urinary sodium must be within the range of 40 to 120 mmol (i.e. 2.3 to 7.0 g/d salt). The reduction in 24‐h urinary sodium was calculated as UNa (Post) ‐ UNa (Pre) for crossover trials, where UNa (Post) designated to the average 24‐h urinary sodium at the end of the reduced salt intake period and UNa (Pre) designated to the average 24‐h urinary sodium at the end of the usual salt intake period (i.e. control period). In parallel trials the change in urinary sodium was calculated as {[UNa (Post) ‐ UNa (Pre)] reduced salt group} ‐ {[UNa (Post) ‐ UNa (Pre)] usual salt group}, where UNa (Post) designated to the average 24‐h urinary sodium at the end of follow‐up and UNa (Pre) designated to the average 24‐h urinary sodium at baseline.
4. Duration of salt reduction must have been for 4 or more weeks.
Types of participants
Studies of adults (18 years or older) with normal or raised BP, irrespective of gender and ethnicity, were included. Trials in children, pregnant women, or patients with other diseases rather than hypertension, such as diabetes, heart failure, were excluded.
Types of interventions
The intervention included was to reduce salt intake. Studies with concomitant interventions (i.e. nonpharmacologic interventions, antihypertensive or other medications) were excluded. One trial with factorial design (i.e. the Trials of Hypertension Prevention, Phase II) was included (TOHP II 1997), however, in this trial the low salt arm (without weight intervention) was compared to the control group (without salt and without weight intervention).
Types of outcome measures
The main outcome measures extracted from each individual trial were the changes in systolic and diastolic BP, and 24h urinary sodium excretion. These were calculated as the differences between the reduced salt and the usual salt groups for mean change from baseline for parallel trials. For crossover trials, the changes were calculated as the mean differences between the end of reduced salt and the usual salt period. Other outcome measures included plasma renin activity, aldosterone, noradrenaline, adrenaline, cholesterol, LDL, HDL and triglycerides.
Search methods for identification of studies
In our first meta‐analysis (He 2002), we developed a search strategy to search for randomised salt reduction trials. In this current update, our original search strategy was modified by Douglas Salzwedel, Trials Search Coordinator at the Cochrane Hypertension Group. Using the updated strategy, the following electronic databases were searched:
The Cochrane Hypertension Group Specialised Register 1948 to November 2012;
The Cochrane Central Register of Controlled Trials (CENTRAL) Issue 11, 2012;
Ovid MEDLINE(R) 1946 to November 2012;
Ovid Embase 1974 to November 2012
Additionally, we reviewed reference list of relevant original and review articles to search for more trials. There were no language restrictions. Electronic databases were searched using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE with selected MeSH and free text terms for salt and blood pressure. The MEDLINE search strategy (Appendix 1) was translated into Embase (Appendix 2), CENTRAL (Appendix 3), and the Hypertension Group Specialised Register (Appendix 4) using the appropriate controlled vocabulary as applicable.
Data collection and analysis
Data Extraction: Data were extracted independently by two persons (F.J. He and J.F. Li) using a standard form and differences were resolved by discussion with a third reviewer (G.A. MacGregor). Relevant data recorded were characteristics of the study, design (parallel or crossover), type of the study (open, single‐blind or double‐blind), method of randomisation, method of blinding (use of placebo, random‐zero or automated sphygmomanometers, or BP observer‐blind), study duration, pre‐ and post‐intervention results. For the purpose of pooled analyses, statistics that could be used to estimate the variance of the outcome measures were also recorded.
Statistical Analyses: For each trial, we calculated the treatment effect for systolic and diastolic BP, and other outcome measures. For crossover trials, the treatment effect was the difference in outcomes between the end of reduced salt period and the end of usual salt (i.e. control) period. For parallel trials, the treatment effect was the difference between the two treatment groups in the change in outcomes from baseline to the end of follow‐up.
For each trial, we also calculated the variance of the treatment effect for outcomes. This was derived from standard deviations or standard errors of paired differences between baseline and the end of follow‐up for each group in a parallel trial (Cappuccio 1991) or between the two treatment periods in a crossover trial, or if these statistics were not given, from confidence intervals, exact t or P values. If the exact variance of paired difference was not derivable, it was imputed either by inverting a boundary P value (e.g. P<0.05 became P=0.05) or assuming a correlation coefficient of 0.5 between the initial and final measurement (Follmann 1992).
To assess the mean effect sizes, we pooled the data by the inverse variance method in random‐effects meta‐analysis. We used the I2 test to examine heterogeneity, with I2>50% considered to be important (Higgins 2003). To explore the source of heterogeneity, we performed meta‐regression analyses (multiple regression models) weighted by the inverse variance of the change in systolic or diastolic BP. The meta‐regression analysis was also used to examine whether there was a dose‐response relationship between the change in 24‐h urinary sodium and the change in BP. We used funnel plot asymmetry to detect whether there was publication bias and Egger's regression test to measure funnel plot asymmetry (Egger 1997; Sterne 2001).
Prespecified subgroupings included BP status (i.e. hypertensive or normotensive) and further subgroupings by ethnic group and sex. The purpose of the subgroup analysis was to determine whether there was a significant effect of salt reduction on BP in each group itself rather than identifying difference in the effect between groups. For the analysis stratified by ethnic group, trials were included in the group of "white" if ≥85% of participants were white. If the information on ethnic group was not available, the trial was excluded from this subgroup analysis. For hormone and lipid data, subgroup analyses were not performed because of the small number of trials that reported such outcomes.
Statistical analyses were performed using Cochrane Collaboration Review Manager 5.2 software and the Statistical Package for the Social Sciences (SPSS).
Results
Description of studies
The search strategy identified 3252 citations, of which we excluded 2995 on the basis of abstract and title. A detailed assessment was given to 257 papers, of which 227 were excluded and the reasons for exclusion were summarised in PRISMA Flow Diagram (Figure 1).
1.
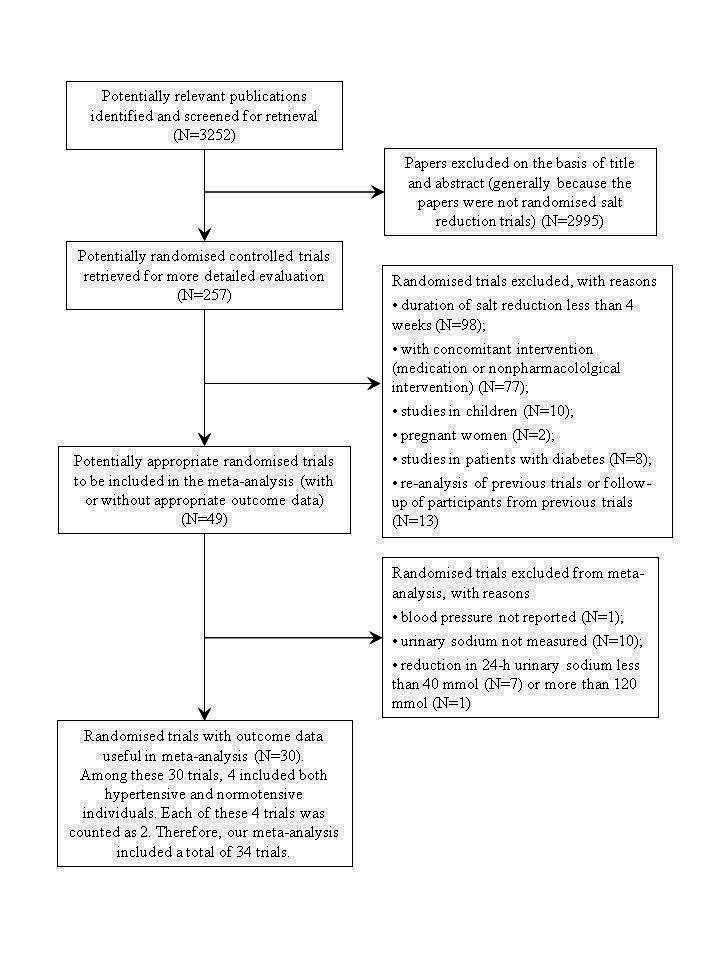
PRISMA Flow Diagram
A total of 30 papers met our inclusion criteria and were included in our meta‐analysis. Among these 30 papers, 4 included both hypertensive and normotensive individuals (Sacks 2001 (H); Sacks 2001 (N); Puska 1983 (H); Puska 1983 (N); Cappuccio 1997 (H); Cappuccio 1997 (N); Melander 2007 (H); Melander 2007 (N)). In our meta‐analysis, the main outcome data (i.e. BP) were recorded for hypertensives and normotensives separately. To avoid confusion in counting the number of trials, each of these 4 papers was counted as 2 trials. Therefore, our meta‐analysis included a total of 34 trials, of which 22 were in hypertensive individuals and 12 in normotensive individuals.
In 3 studies (Morgan 1981 (F); Morgan 1981 (M); Nestel 1993 (F); Nestel 1993 (M); Watt 1985 (HH) offspring of two parents with high BP; Watt 1985 (LL) offspring of two parents with low BP) where subgroup data were reported only, they were entered for subgroups separately. For 3 trials that included both hypertensives and normotensives (Sacks 2001 (H); Sacks 2001 (N); Cappuccio 1997 (H); Cappuccio 1997 (N); Melander 2007 (H); Melander 2007 (N)), each had an additional entry for all participants, i.e. hypertensives and normotensives combined, where the data for lipids or hormones were reported. Therefore, there were a total of 40 entries in the table of Characteristics of included studies.
For 2 papers (MacGregor 1989; Sacks 2001 (H); Sacks 2001 (N)) where 3 levels of salt intakes were studied, we included the high and intermediate levels (i.e. urinary sodium reduced from 190 to 108 mmol/24h) in one trial (MacGregor 1989) and in the other (Sacks 2001 (H); Sacks 2001 (N)) we included the high and low levels (i.e. urinary sodium reduced from 145 to 65 mmol/24h in hypertensive individuals and from 139 to 64 mmol/24h in normotensive individuals on the normal American diet). The characteristics of the trials included in the meta‐analysis are summarised in Table: "Characteristics of included studies".
Risk of bias in included studies
Criteria for the assessment of study quality were as follows.
Allocation concealment
Adequate: The randomisation method described did not allow participants and investigators to foresee assignment, e.g. a prior numbered or coded tablet containers of identical appearance prepared by an independent pharmacy, central randomisation.
Unclear: Randomisation was stated, but no detailed information was provided on the method used to generate the random allocation sequence and the mechanism used to implement the random allocation sequence.
Inadequate: Method of randomisation allowed participants or investigators to foresee assignment, e.g. unsealed envelopes or alternate medical record numbers.
Blinding
Blinding of the investigator: yes/no/not stated.
Blinding of the participant: yes/no/not stated.
Blinding of the outcome assessor: yes/no/not stated.
Incomplete outcome data addressed?
Yes: Intention to treat analysis was undertaken, or all participants who were randomised, completed the study, or detailed information was reported on the number of participants who were lost of follow‐up after randomisation as well as reasons.
Unclear: No information provided.
No: Incomplete outcome data were not adequately addressed.
The risk of bias assessments for the trials included in the meta‐analysis are shown in Table: Characteristics of included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Effect on BP
Trials in all individuals
A total of 34 trials with 3230 participants were included. The characteristics of the trials included in the meta‐analysis are summarised in Table (Characteristics of included studies). The median age was 50 years (ranging from 22 to 73 years). Of the 34 trials, 23 used crossover design and 11 used paralleled comparisons. Twenty‐two of the 34 trials were double blind, 11 were BP observer blind, and 1 did not report any blinding procedure. The study duration varied from 4 weeks to 3 years (median: 4 weeks). The median BP on the usual salt intake was 141/86 mmHg. The median 24‐h urinary sodium on the usual salt intake was 160 mmol (9.4 g/d salt), ranging from 125 to 200 mmol (7.3 to 11.7 g/d salt). The pooled estimate of the change in 24‐h urinary sodium from the usual to the reduced salt intake was ‐75 mmol (range ‐40 to ‐118 mmol), equivalent to a reduction in salt intake of 4.4 g/d (range 2.3 to 6.9 g/d). This average reduction in salt intake is similar to that of the current public health recommendations.
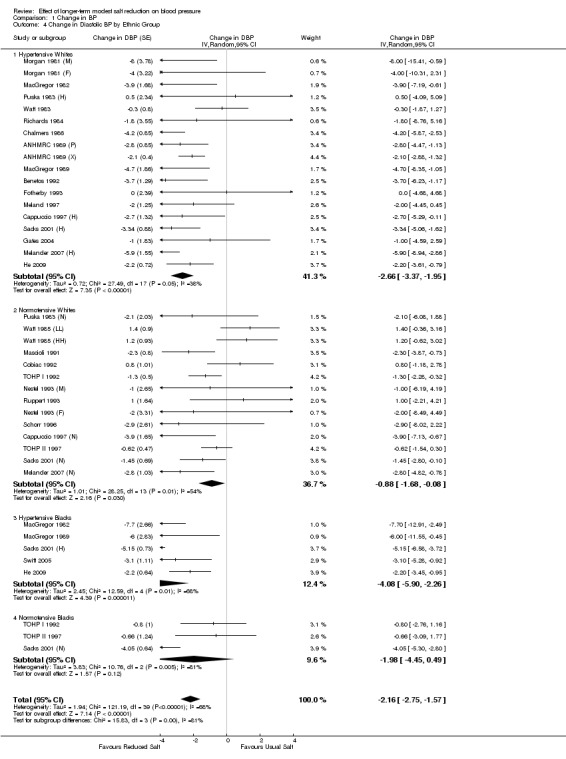
Analysis 1.1; Analysis 1.2 show the change in BP in individual trials included in the meta‐analysis and the mean effect size. The pooled estimates of changes in BP were ‐4.18 mmHg (95% CI: ‐5.18 to ‐3.18, P<0.00001, I2=75%) for systolic and ‐2.06 mmHg (95% CI: ‐2.67 to ‐1.45, P<0.00001, I2=68%) for diastolic BP (Table 1).
1.1. Analysis.
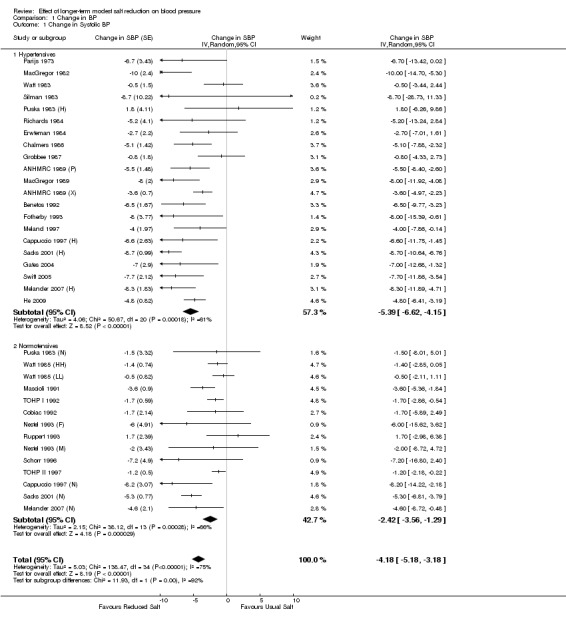
Comparison 1 Change in BP, Outcome 1 Change in Systolic BP.
1.2. Analysis.
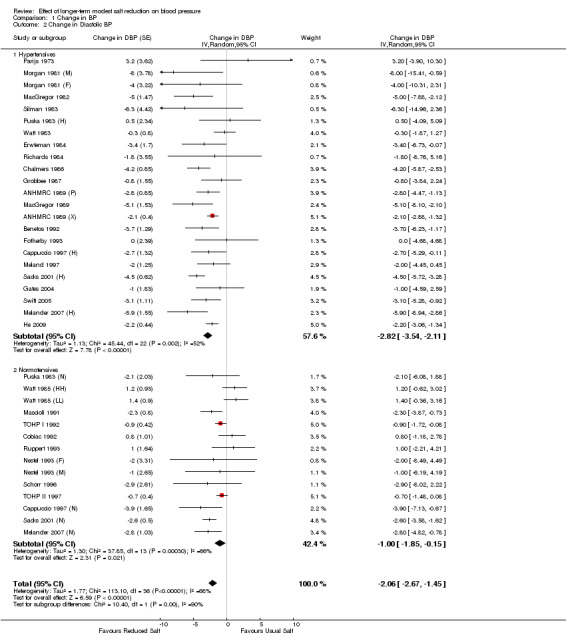
Comparison 1 Change in BP, Outcome 2 Change in Diastolic BP.
To explore the source of heterogeneity, meta‐regression analysis was performed with the change of BP (systolic or diastolic) as dependent variable and the independent variables included age (mean age of the participants in individual trials), BP status (hypertensive=1; normotensive=0), ethnic group (i.e. the proportion of whites as a continuous variable), and the change in 24‐h urinary sodium. The results showed that the change in 24‐h urinary sodium, age, BP status and ethnic group were all significantly associated with the change in systolic BP. The regression coefficients indicated that a 100 mmol reduction in 24 hour urinary sodium (6 g/d salt) was associated with a decrease of 5.8 mmHg (95% CI: 2.5 to 9.2, P=0.001) in systolic BP, a one‐year increase in age was associated with a 0.06 mmHg (95% CI: 0.006 to 0.116, P=0.030) greater decrease in systolic BP with salt reduction, being hypertensive was associated with a greater fall in systolic BP (P=0.042) compared with normotensives, and a larger proportion of whites (or a smaller proportion of blacks) was associated with a smaller fall in systolic BP (P=0.001). These 4 variables together explained 68% of the variance between studies. In a separate regression model, sex (i.e. the proportion of men as a continuous variable) was added to the independent variable list, and there was little change to the adjusted R2 (R2=0.68 without sex in the regression model and R2=0.70 with sex added to the regression model). Sex was not significantly associated with the change in systolic BP. For diastolic BP, age, ethnic group, BP status and 24‐h urinary sodium together explained 41% of the variance between studies. Among these 4 variables, only ethnic group was significant (P=0.021) and the other 3 variables were not significantly associated with the change in diastolic BP. When sex was added to the regression model, there was little change in the adjusted R2 (R2=0.41 and 0.44 for the regression model with and without sex respectively). Sex was not significantly associated with the change in diastolic BP.
Trials in hypertensive individuals
Nine hundred and ninety hypertensive individuals were studied in 22 trials (Table: Characteristics of included studies). Median age was 50 years (ranging from 24 to 73 years). Of the 22 trials, 16 used crossover design and 6 used paralleled comparisons. Fourteen of the 22 trials were double blind, 7 were BP observer blind, and 1 did not report any blinding procedure. The study duration varied from 4 weeks to 1 year (median: 5 weeks). The median BP on usual salt intake was 148/93 mmHg. The median 24‐h urinary sodium on the usual salt intake was 162 mmol (9.5 g/d salt), ranging from 125 to 191 mmol (7.3 to 11.2 g/d salt). The pooled estimate of the change in 24‐h urinary sodium from the usual to the reduced salt intake was ‐75 mmol (range ‐53 to ‐117 mmol), equivalent to a reduction in salt intake of 4.4 g/d (range 3.1 to 6.8 g/d).
Analysis 1.1; Analysis 1.2 show the change in BP in individual trials included in the meta‐analysis and the mean effect size. The pooled estimates of changes in BP were ‐5.39 mmHg (95% CI: ‐6.62 to ‐4.15, P<0.00001, I2=61%) for systolic and ‐2.82 mmHg (95% CI: ‐3.54 to ‐2.11, P<0.00001, I2=52%) for diastolic BP (Table 1).
Meta‐regression with the change in BP as dependent variable and age, ethnic group and the change in 24‐h urinary sodium as independent variables, showed that the change in 24‐h urinary sodium and ethnic group were significantly associated with the fall in systolic BP, whereas age was not significantly associated with the fall in systolic BP. A 100 mmol reduction in 24 hour urinary sodium (6 g/day salt) was associated with a fall in systolic BP of 10.8 mmHg (95CI: 3.5 to 18.2, P<0.01) after adjusting for age and ethnic group. All 3 variables together explained 46% of the variance between studies. For diastolic BP, the 3 variables together explained 11% of the variance between studies and none of the 3 variables was significantly associated with the fall in diastolic BP.
Trials in normotensive individuals
Two thousand two hundred and forty individuals with normal BP were studied in 12 trials (Table: Characteristics of included studies). Median age was 50 years (ranging from 22 to 67 years). Of the 12 trials, 7 used crossover design and 5 used paralleled comparisons. Eight of the 12 trials were double blind and 4 were BP observer blind. The study duration varied from 4 weeks to 3 years (median: 4 weeks). The median BP on usual salt intake was 127/77 mmHg. The median 24‐h urinary sodium on the usual salt intake was 153 mmol (8.9 g/d salt), ranging from 128 to 200 mmol (7.5 to 11.7 g/d salt). The pooled estimate of the change in 24‐h urinary sodium from the usual to the reduced salt intake was ‐75 mmol (range ‐40 to ‐118 mmol), equivalent to a reduction in salt intake of 4.4 g/d (range 2.3 to 6.9 g/d).
Analysis 1.1; Analysis 1.2 show the change in BP in individual trials included in the meta‐analysis and the mean effect size. The pooled estimates of changes in BP were ‐2.42 mmHg (95% CI: ‐3.56 to ‐1.29, P<0.0001, I2=66%) for systolic and ‐1.00 mmHg (95% CI: ‐1.85 to ‐0.15, P=0.02, I2=66%) for diastolic BP (Table 1).
Meta‐regression with the change in BP as dependent variable and age, ethnic group and the change in 24‐h urinary sodium as independent variables, showed that the change in 24‐h urinary sodium was significantly associated with the fall in systolic BP, whereas age and ethnic group were not significantly associated with the fall in systolic BP. A 100 mmol reduction in 24 hour urinary sodium (6 g/day salt) was associated with a fall in systolic BP of 4.3 mmHg (95% CI: 0.1 to 8.5, P<0.05) after adjusting for age and ethnic group. All 3 variables together explained 51% of the variance between studies. For diastolic BP, the 3 variables together explained 43% of the variance between studies. Among these 3 variables, only ethnic group was significant (P=0.042) and the other 2 variables were not significantly associated with the change in diastolic BP.
Further sub‐group analysis
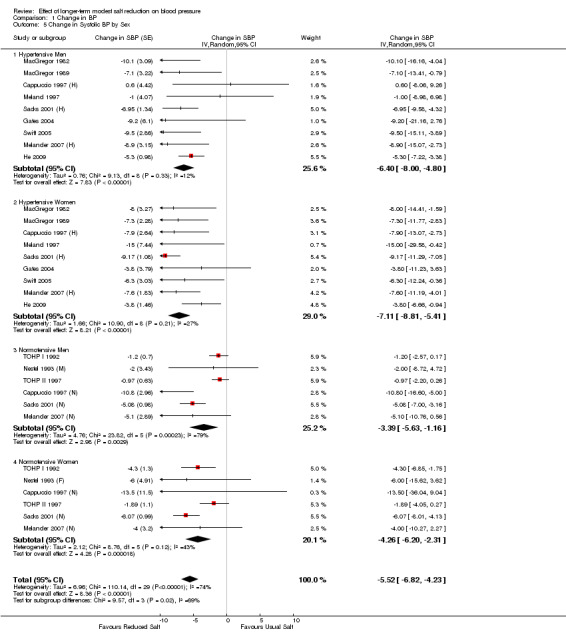
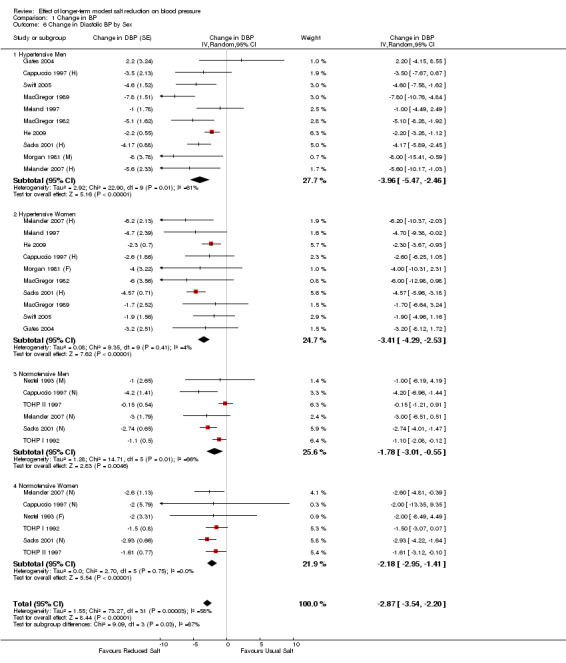
Table 2 and Table 3 show the pooled results of 24‐h urinary sodium and BP by ethnic group and sex for hypertensives and normotensives separately. There was a significant fall in systolic BP in both whites and blacks, men and women. The fall in diastolic BP was significant in most of the subgroups. There was only 1 trial in Asians (He 2009). Most of the participants in this trial were of South Asian origin (i.e. originating from the Indian subcontinent) and all participants had raised BP. The study showed that there was a significant fall in both systolic and diastolic BP with a modest reduction in salt intake (Table 2).
Effect on hormones and lipids
Plasma renin activity
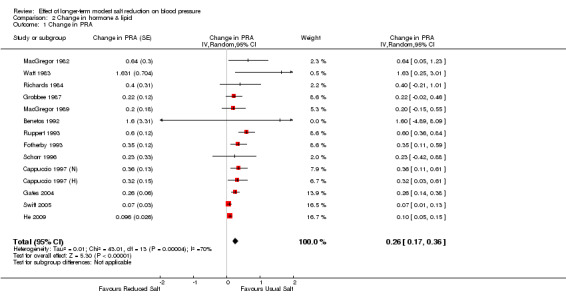
Of the 34 trials, 14 reported the data of plasma renin activity. One study reported plasma renin concentration (Melander 2007) and it was excluded from the analysis for plasma renin activity. The median plasma renin activity was 1.07 ng/ml/hr on the usual salt intake. The pooled estimate of the change in plasma renin activity was 0.26 ng/ml/hr (95% CI: 0.17 to 0.36, P<0.00001, I2=70%) (Table 4).
Aldosterone
Of the 34 trials, 9 had plasma aldosterone measured. One trial (Benetos 1992) was excluded from the aldosterone analysis as the plasma aldosterone was extremely high after the unit conversion (235277.8 pmol/l on the usual salt and 269166.7 pmol/l on the reduced salt intake). The median plasma aldosterone was 299 pmol/l on the usual salt intake. The pooled estimate of the change in aldosterone was 73.20 pmol/l (95% CI: 44.92 to 101.48, P<0.00001, I2=62%) (Table 4).
Noradrenaline
Of the 34 trials, 6 reported the data of plasma noradrenaline. The median plasma noradrenaline was 351 pg/ml on the usual salt intake. The pooled estimate of the change in plasma noradrenaline was 31.67 pg/ml (95% CI: 6.57 to 56.77, P=0.01, I2=5%) (Table 4).
Adrenaline
Of the 34 trials, only 4 trials reported the data of plasma adrenaline. The median plasma adrenaline was 64 pg/ml on the usual salt intake. The pooled estimate of the change in plasma adrenaline was 6.70 pg/ml (95% CI: ‐0.25 to 13.64, P=0.06, I2=12%) (Table 4).
In addition to the above, one other trial reported that "no changes were observed in plasma catecholamines” (Schorr 1996), but data were not provided. Therefore, this trial was excluded from the above pooled analysis for noradrenaline and adrenaline.
Cholesterol
Of the 34 trials, 8 reported the data of plasma cholesterol. The median plasma cholesterol was 5.3 mmol/l on the usual salt intake. The pooled estimate of the change in cholesterol was 0.05 mmol/l (95% CI: ‐0.02 to 0.11, P=0.18, I2=0%) (Table 5). Two other trials reported no significant change in cholesterol (Chalmers 1986; Erwteman 1984), however, no data were provided for pooled analysis.
LDL
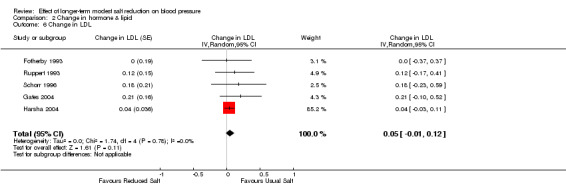
Of the 34 trials, 5 reported the data of plasma LDL. The median plasma LDL was 3.2 mmol/l on the usual salt intake. The pooled estimate of the change in LDL was 0.05 mmol/l (95% CI: ‐0.01 to 0.12, P=0.11, I2=0%) (Table 5).
HDL
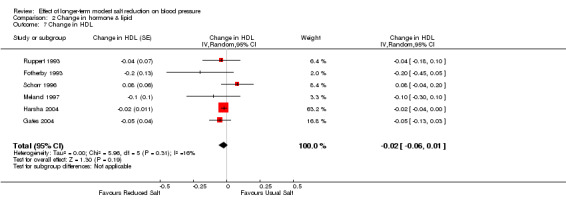
Of the 34 trials, 6 reported the data of plasma HDL. The median plasma HDL was 1.3 mmol/l on the usual salt intake. The pooled estimate of the change in HDL was ‐0.02 mmol/l (95% CI: ‐0.06 to 0.01, P=0.19, 16%) (Table 5). One other trial reported no significant change in HDL, but data were not provided (Erwteman 1984), therefore, this trial was excluded from the pooled analysis.
Triglycerides
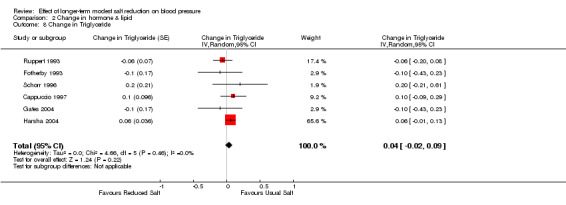
Of the 34 trials, 6 reported the data of plasma triglycerides. The median plasma triglycerides was 1.3 mmol/l on the usual salt intake. The pooled estimate of the change in triglycerides was 0.04 mmol/l (95% CI: ‐0.02 to 0.09, P=0.22, I2=0%) (Table 5).
Study quality
The risk of bias graph is shown in Figure 2. Among the 34 trials included in our meta‐analysis, 26 were judged to have adequate concealment of allocation of treatments (Table:Characteristics of included studies). In 8 trials the information on concealment of allocation was not available. Despite the fact that only 7 out of 34 trials performed intention‐to‐treat analysis, the percentage of participants who were lost of follow‐up after randomisation was small (6.7% on average).
2.
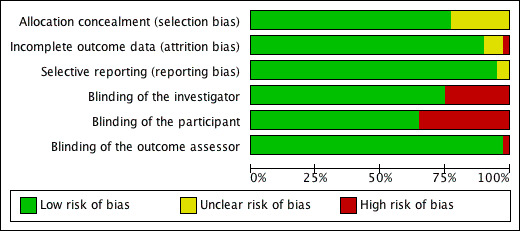
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We included double‐blind, BP observer‐blind, and open studies due to the fact that 1) some trials e.g. the DASH (Dietary Approaches to Stop Hypertension)‐Sodium (Sacks 2001 (H); Sacks 2001 (N)), although non‐double‐blinded, were well conducted with good compliance to different diets; and 2) it is very difficult to make any dietary intervention study double‐blind. In relation to salt, this can only be done by the use of salt tablets (Slow Sodium and placebo). Among the 34 trials included in our meta‐analysis, 22 were double‐blind, 11 were BP observer‐blind and only one small trial in hypertensives was non‐blind. Re‐analysing the data by excluding the non‐blind study (Parijs 1973) showed that the results were unchanged. The mean net change in BP for hypertensive individuals was ‐5.35 mmHg (95%CI: ‐6.62 to ‐4.09) for systolic and ‐2.88 mmHg (95%CI: ‐3.58 to ‐2.18) for diastolic BP after the non‐blinded study was excluded.
Publication bias
We created the funnel plots by plotting the treatment effect against the reciprocal of the standard error of the treatment effect (Figure 3; Figure 4). For diastolic BP the funnel plots were symmetrical around the mean effect size line (asymmetry test: P=0.416) (Egger 1997). For systolic BP, the graphic plot was suggestive of bias (asymmetry test: P=0.025). This asymmetry of funnel plot might be because smaller studies showing no effect were under‐reported in the literature. However, in our meta‐analysis it is more likely to be due to the smaller effects of two larger and longer‐term trials (TOHP I 1992; TOHP II 1997). The smaller effects in these two trials are attributable to the smaller reduction of salt intake achieved in the longer‐term trials. When these two trials were removed from the analysis, the asymmetry test was not significant (P=0.247).
3.
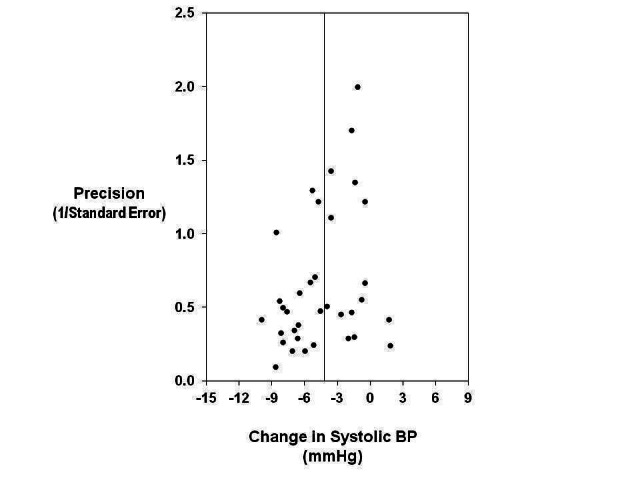
Funnel plot to explore publication bias (systolic BP). The vertical line is at the mean effect size. Precision is the reciprocal of the standard error of the change in systolic BP.
4.
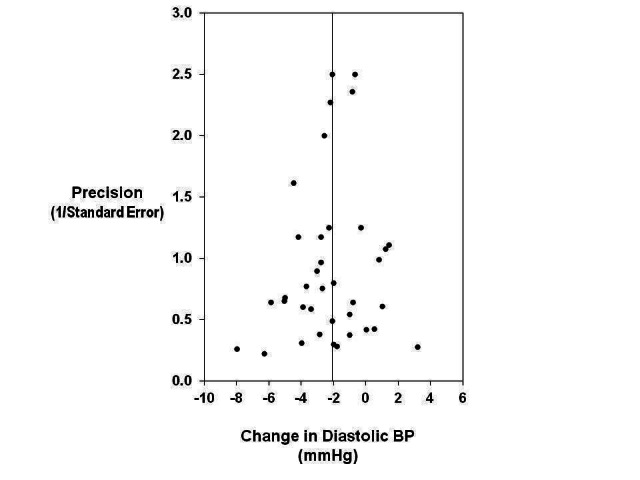
Funnel plot to explore publication bias (diastolic BP). The vertical line is at the mean effect size. Precision is the reciprocal of the standard error of the change in diastolic BP.
Discussion
Our meta‐analysis demonstrates that a longer‐term modest reduction in salt intake of 4.4 g/d on average, causes significant and, from a population viewpoint, important falls in BP in individuals with both raised and normal BP. The BP falls, on average, by 5/3 mmHg in hypertensives and 2/1 mmHg in normotensives. Further subgroup analyses demonstrate that a modest reduction in salt intake leads to a significant fall in systolic BP in both whites and blacks, men and women. These results provide further strong support for a reduction in population salt intake which will result in a lower population BP and, thereby, a reduction in strokes, heart attacks and heart failure.
The effect of a chronic high salt intake is a gradual increase in BP throughout life. The INTERSALT study (International Study of Salt and Blood Pressure) suggested a strong relationship between salt intake and a progressive increase in BP with age, i.e. 0.4 mmHg per year for a 6 g/d salt intake (Elliott 1996). A reduction in salt intake is, therefore, likely to attenuate the rise of BP with aging, in addition to the immediate BP‐lowering effect.
Dose‐response to salt reduction
Our meta‐regression analysis shows a significant dose‐response relationship between the reduction in salt intake and the fall in systolic BP, i.e. the greater the reduction in salt intake, the greater the fall in systolic BP. A reduction of 6 g/d in salt intake predicts a decrease of 5.8 mmHg in systolic BP after adjusting for age, ethnic group and BP status.
It is acknowledged that the does‐response relationship from meta‐regression (i.e. between‐study investigation) should be viewed as exploratory and may be potentially prone to confounding. Meta‐analysis with individual participant data would have an advantage both statistically and clinically (Riley 2010; Berlin 2002) and, if available, should be used in the future to explore the dose‐response relationship further. Nevertheless, the dose‐response relationship found in our study is consistent with that observed from rigorously controlled trials with multi‐levels of salt intake which provided the most persuasive evidence. There have been two trials that studied three levels of salt intake. The first one was the randomised double‐blind cross‐over study in 20 individuals with untreated essential hypertension, where salt intake was reduced from 11.2 to 6.4 and to 2.9 g/d, each for one month (MacGregor 1989). BP was 163/100 mmHg with a salt intake of 11.2 g/d, and reduced to 155/95 mmHg when salt intake was decreased to 6.4 g/d (i.e. a decrease of 8/5 mmHg). BP fell further to 147/91 mmHg when salt intake was reduced to 2.9 g/d (i.e. a further fall of 8/4 mmHg). After the trial was completed, individuals continued the lowest salt intake. Among the 20 participants, 19 were followed up for 1 year. In 16 individuals, BP remained controlled without any antihypertensive medication and the average BP was 142/87 mmHg with a salt intake of 3.2 g/d (MacGregor 1989). The other trial that has studied the dose‐response relationship is the DASH‐Sodium study. Over 400 individuals with normal or mildly raised BP were randomised to receive either the normal American diet (control group) or the DASH diet which is rich in fruits, vegetables, and low‐fat dairy products. Within each group, participants were given 3 levels of salt intake (i.e. 8 to 6 and 4 g/d) in a randomised crossover manner, each for 4 weeks. The results demonstrated a clear dose‐response relationship both on the normal American diet and on the DASH diet. The fall in BP was greater at a lower level of salt intake, i.e. from 6 to 4 g/d compared with that from 8 to 6 g/d (Sacks 2001 (H); Sacks 2001 (N)).
From the evidence above, it is clear that the recommendations to reduce salt from the current levels of approximately 9‐12 g/d to 5‐6 g/d will have a significant effect on BP, but are not ideal. A further reduction to 3 g/d will have a much greater effect on BP. Therefore, 3 g/d should become the long‐term target for population salt intake. Indeed, the UK government's health advisory agency, the National Institute for Health and Clinical Excellence (NICE) has recommended a reduction in the population's salt consumption to 3 g/d by 2025 (NICE 2010). In USA, it is recommended that sodium intake should be reduced to less than 2.3 g/d (i.e. ≈6 g/d salt) for adults, with an even further reduction to 1.5 g/d (i.e. ≈4 g/d salt) for about half the population, including African Americans, all adults 51 and older, and those with hypertension, diabetes or chronic kidney disease (IOM 2010).
Study duration
In spite of including studies of 1 month or more, the median duration of salt reduction in our meta‐analysis was only 5 weeks in the hypertensives and 4 weeks in the normotensives. Whether salt reduction has exerted its maximum effect by 4‐5 weeks is not known, but much evidence would suggest that this is unlikely (Forte 1989). Among the 34 trials included in our meta‐analysis, two had duration of over 1 year (TOHP I 1992; TOHP II 1997) and both trials were in normotensive individuals. These two trials did not show a greater fall in BP compared with other trials in normotensive individuals. However, the reduction in salt intake achieved in these two trials was half that achieved in other trials. On average, salt intake was reduced by 2.4 g/d in these two longer‐term trials, whereas in the other trials in normotensives, salt intake was reduced by 4.8 g/d. These longer‐term studies clearly highlight the difficulty in keeping individuals on a lower salt intake due to the widespread presence of salt in nearly all processed, canteen and restaurant food.
Variations of BP response to salt reduction
Previous studies have shown that, for a given reduction in salt intake, the fall in BP was larger in individuals of African origin, in older people and in those with raised BP compared to whites, young people and individuals with normal BP respectively (Bray 2004; He 1998; He 2001). The results from our meta‐regression analyses are consistent with these observations.
The term "salt sensitivity" has been commonly used to describe the variations of BP response to salt reduction. However, almost all of the studies on "salt sensitivity" have used a protocol of very large and sudden changes in salt intake. Such studies are irrelevant to the public health recommendations of more modest reduction in salt intake for a prolonged period of time. Our meta‐analysis demonstrates that a longer‐term modest reduction in salt intake has a significant effect on BP in both hypertensive and normotensive individuals, men and women, whites and blacks; although there is a variation in the extent of the fall in BP. These results in conjunction with other evidence (He 2010), particularly that a reduction in salt intake also lowers blood pressure in children (He 2006), provide strong support that salt reduction should be carried out in the whole population. A reduction in population salt intake lowers population BP. Even a small reduction of BP across the entire population would have a large impact on reducing the burden of cardiovascular disease (Whelton 2002).
Effect of salt reduction on hormones and lipids
A recent meta‐analysis by Graudal et al (Graudal 2011; Graudal 2012) implied that salt reduction had adverse effects on plasma hormone and lipid levels which might mitigate any benefit that occurs with a long‐term fall in BP. However, Graudal et al’s meta‐analysis included a large number of very short‐term trials with a large change in salt intake, e.g. from 20 to less than 1 g/d for only 4‐5 days, and such metabolic studies are irrelevant to the current public health recommendations for a modest reduction in salt intake for a long period of time. Our meta‐analysis demonstrates that, with a longer‐term modest reduction in salt intake, there is no significant change in plasma cholesterol, LDL, HDL or triglycerides. Indeed, in Graudal et al's own meta‐analysis, the changes in lipids only occurred with short term trials, and a sub‐group analysis including trials with a duration of 4 or more weeks showed no significant change in lipid levels (Graudal 2011; Graudal 2012).
When salt intake is reduced, there is a fall in extracellular volume and physiological stimulation of the renin‐angiotensin‐aldosterone system, as well as the sympathetic nervous system. These compensatory responses are bigger with sudden and large decreases in salt intake, and much smaller or minimal with a longer‐term modest salt reduction. Our meta‐analysis shows that, with a longer‐term modest reduction in salt intake, there is only a small physiological increase in plasma renin activity, aldosterone and noradrenaline. It is worth noting that all of the studies that were included in our meta‐analysis with these hormones measured, had a duration of only 4‐6 weeks (median duration: 4 weeks). It is likely that such effects may attenuate over time. Indeed, a study by Beckmann et al demonstrated that a modest reduction in salt intake, along with a reduction in body weight and saturated fat for one year, significantly reduced arterial plasma noradrenaline and adrenaline in hypertensive individuals (Beckmann 1995).
Salt reduction lowers BP by a similar mechanism to that of thiazide diuretics. Both stimulate the renin‐angiotensin system and, in the short term, the sympathetic nervous system. However, outcome trials have demonstrated that long‐term treatment with thiazide diuretics significantly reduced cardiovascular morbidity and mortality in hypertensive individuals (ALLHAT 2002).
Effect of salt reduction on cardiovascular risk
There is much evidence that raised BP throughout its range starting at 115/75 mmHg is a major cause of cardiovascular disease (PSC 2002). A modest reduction in salt intake lowers BP and, therefore, would reduce cardiovascular risk. It was estimated that a reduction of 6 g/d in salt intake would reduce stroke by 24% and coronary heart disease by 18% (He 2003). This would prevent ≈35,000 stroke and coronary heart disease deaths a year in the UK and ≈2.5 million deaths worldwide.
Both prospective cohort studies and outcome trials have shown that a lower salt intake is related to a reduced risk of cardiovascular disease (Strazzullo 2009;He 2011). Two recent papers in JAMA (Journal of the American Medical Association), however, claimed that a lower salt intake was associated with higher cardiovascular mortality (Stolarz‐Skrzypek 2011) or a J‐shaped association existed between salt intake and cardiovascular risk (O'Donnell 2011). These two papers have many methodological flaws, e.g. measurement error in assessing daily salt intake, confounding factors not controlled for, and reverse causality (i.e. the low salt intake is the result rather than the cause of participants' illness) (He 2011a; He 2012). Therefore, the results from these studies should be interpreted with great caution. A meta‐analysis of 12 cohort studies showed that an increase of 5 g/d in salt intake was associated with a 23% increase in the risk of stroke and a 17% increase in the risk of cardiovascular disease (Strazzullo 2009).
Evidence from outcome trials of long term salt reduction is very limited due to the innate difficulty in conducting such trials. A recent meta‐analysis of 7 randomised trials by Taylor et al, published simultaneously in The Cochrane Library (Taylor 2011a) and the American Journal of Hypertension (Taylor 2011), claimed that "Cutting down on the amount of salt has no clear benefits in terms of likelihood of dying or experiencing cardiovascular disease" and The Cochrane Library’s press release headline stated “Cutting down on salt does not reduce your chance of dying” (Cochrane 2011). Both of these statements are incorrect. Despite this, these headline grabbing statements received very misleading worldwide media publicity.
Among the 7 trials included in Taylor et al’s meta‐analysis, one in heart failure should not have been included as the participants were severely salt and water depleted due to aggressive diuretic therapy (Paterna 2008). Additionally, the findings in patients with severe heart failure on multiple drug treatments are not generalisable to the general population. In the remaining 6 trials, there is a reduction in all clinical outcomes (all‐cause mortality, cardiovascular mortality and events), although none of these are statistically significant. The non‐significant findings are most likely due to a lack of statistical power, particularly as Taylor et al analysed the trials for hypertensives and normotensives separately. A re‐analysis of the data by combining hypertensives and normotensives together shows that there is a significant reduction in cardiovascular events by 20% (P<0.05) (Figure 5) and a non‐significant reduction in all‐cause mortality (5‐7%), in spite of the small reduction in salt intake of 2.0‐2.3 g/d (He 2011). These results add strongly to the evidence that salt reduction has a major impact on reducing strokes, heart attacks and heart failure.
5.
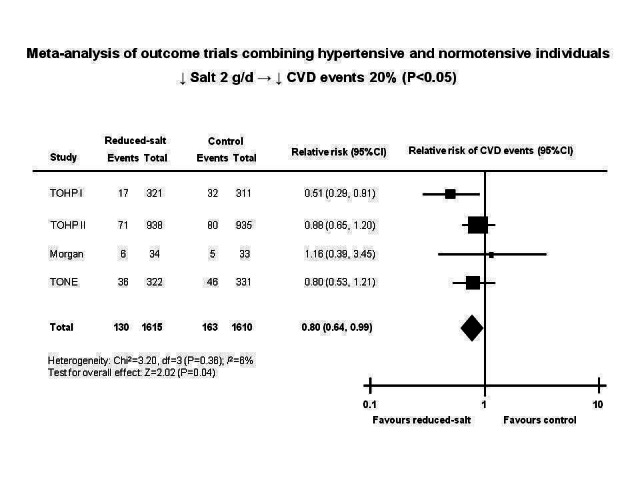
Cardiovascular disease (CVD) events in a meta‐analysis of randomised salt reduction trials using fixed effect model with normotensives and hypertensives combined. TOHP I: Trial of Hypertension Prevention, phase 1. TOHP II: Trial of Hypertension Prevention, phase 2. TONE: Trial of Nonpharmacologic Interventions in Elderly.
Salt reduction is one of the most cost‐effective public health measures to reduce cardiovascular disease
Several studies have shown that a reduction in salt intake is one of the most cost‐effective interventions to reduce cardiovascular disease in both developed and developing countries. For instance, a recent study in the US showed that even a very modest reduction in salt intake of only 10% which could be easily achieved, as demonstrated in the UK, would prevent hundreds of thousands of strokes and heart attacks over the lifetimes of adults aged 40‐85 years who are alive today, and could save more than $32 billion in medical expenses in the US alone (Smith‐Spangler 2010). A larger decrease in salt intake would result in a larger health improvement and greater cost savings (Bibbins‐Domingo 2010).
The UK salt reduction campaigns which started in 2003/2004 have been successful and the average salt intake, as measured by 24‐hour urinary sodium, has fallen gradually from 9.5 to 8.1 g/d by 2011 (i.e. a 15% reduction, P<0.05 for the downward trend) (FSA 2008). A cost‐effective analysis by NICE showed that the UK salt reduction campaigns cost £15 million and a 0.9 g/d reduction in salt intake that was achieved by 2008, led to ≈6000 fewer CVD deaths per year, saving the UK economy ≈£1.5 billion per annum (NICE 2010). Based on NICE’s estimation, the further reduction of 0.5 g/d from 2008 to 2011, would prevent approximately additional 3000 CVD deaths per year and result in even greater cost savings to the UK economy.
Asaria et al estimated the effects and cost of strategies to reduce salt intake and control tobacco use for 23 low‐ and middle‐income countries that account for 80% of chronic disease burden in the developing world. They demonstrated that a 15% reduction in mean population salt intake could avert 8.5 million cardiovascular deaths and a 20% reduction in smoking prevalence could avert 3.1 million cardiovascular deaths over 10 years (Asaria 2007). The modest reduction in salt intake could be achieved by a voluntary reduction in the salt content of processed foods and condiments by manufacturers combined with a sustained mass‐media campaign aimed to encourage dietary change within households and communities. The main costs of the strategy to reduce salt consumption would be awareness campaigns through mass‐media outlets and regulation of food products by public‐health officers, with an average cost estimated to be US$0.09 per person per year. The cost for tobacco control, including both price and non‐price measures, was US$0.26 per person per year. These figures clearly suggest that a reduction in salt intake is more, or at the very least just, as cost‐effective as tobacco control in terms of reducing cardiovascular disease on its own, the leading cause of death and disability worldwide.
Authors' conclusions
Implications for practice.
Our meta‐analysis demonstrates that a modest reduction in salt intake, as currently recommended, has a significant effect on BP both in individuals with raised BP and in those with normal BP. The fall in BP is observed in both whites and blacks, men and women. These findings provide further strong support for a reduction in population salt intake. This will likely lower population BP and, thereby, likely reduce strokes, heart attacks and heart failure. Furthermore, our analysis demonstrates a dose‐response relationship, i.e. the greater the reduction in salt intake, the greater the fall in BP. The current recommendations to reduce salt intake to 5‐6 g/d will have a major effect on BP, but are not ideal. A further reduction to 3 g/d will have a greater effect. Therefore, 3 g/d should become the long‐term target for population salt intake. Indeed, NICE has recommended a reduction in salt intake to 3 g/d by 2025 for UK adult population (NICE 2010).
Many developed countries are now adopting a policy of reducing salt intake, firstly by persuading the food industry to reformulate food with less salt, as is occurring successfully in the UK (FSA 2008) and Finland (Karppanen 2006), and also encouraging people to use less salt in their own cooking and at the table. The major challenge now is to spread this out to all other countries, particularly developing countries where often salt intake is high and ≈80% of the global BP‐related disease burden occurs. All countries should adopt a coherent and workable strategy to reduce salt intake. A reduction in population salt intake will likely have major beneficial effects on health along with major cost savings in all countries around the world.
Implications for research.
The evidence that relates salt intake to BP is very strong. The mechanisms whereby salt raises BP are not fully understood. The existing concepts focus on the tendency for an increase in extracellular fluid volume. Increasing evidence suggests that small increases in plasma sodium may have a direct effect on BP independent of extracellular volume (Friedman 1990; de Wardener 2004; He 2005). Further studies are needed to investigate the mechanisms, in particular, the role of plasma sodium in regulating BP.
What's new
| Date | Event | Description |
|---|---|---|
| 28 February 2013 | New search has been performed |
Summary results: Our updated review confirms that a longer‐term modest reduction in salt intake lowers blood pressure significantly in both hypertensive and normotensive individuals, and the greater the reduction in salt intake, the greater the fall in blood pressure. Compared with our previous review, our current update demonstrates that the effects of salt reduction on systolic blood pressure are significant in both whites and blacks, men and women. Furthermore, our updated review demonstrates that, with a longer‐term modest reduction in salt intake, there is only a small physiological increase in plasma renin activity, aldosterone and noradrenaline. There is no significant change in adrenaline, cholesterol, low‐density lipoprotein (LDL), high‐density lipoprotein (HDL) or triglycerides. These findings provide further strong support for a reduction in population salt intake. This will likely lower population blood pressure and reduce strokes, heart attacks and heart failure. |
| 28 February 2013 | New citation required and conclusions have changed | 2013 update |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 30 March 2011 | Amended | Converted to new review format. |
| 24 May 2006 | New search has been performed | Minor update |
| 9 May 2005 | New citation required but conclusions have not changed | A repeated search using the search strategy developed previously (Journal of Human Hypertension 2002) was carried out in April 2005. Three new trials met the inclusion criteria and have been added to the meta‐analysis. |
Acknowledgements
We would like to thank
the authors who kindly provided the subgroup data and the data necessary for the computation of some of the variables included in our meta‐analysis.
Douglas Salzwedel at the Cochrane Hypertension Group for his help with the development of search strategy and running the search strategy for electronic databases.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 11 December 2012 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 sodium chloride, dietary/ 2 exp sodium, dietary/ 3 diet, sodium‐restricted/ 4 ((sodium or salt) adj3 (restrict$ or curb$ or limit$ or minimi$ or low$ or reduc$ or intake or diet$ or free)).tw. 5 or/1‐4 6 randomized controlled trial.pt. 7 controlled clinical trial.pt. 8 randomized.ab. 9 placebo.ab. 10 clinical trials as topic/ 11 randomly.ab. 12 trial.ti. 13 or/6‐12 14 animals/ not (humans/ and animals/) 15 13 not 14 16 5 and 15
Appendix 2. EMBASE search strategy
Database: Embase <1974 to 2012 Week 49> Search Date: 11 December 2012 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 sodium chloride, dietary/ 2 sodium intake/ 3 sodium restriction/ 4 ((sodium or salt) adj3 (restrict$ or curb$ or limit$ or minimi$ or low$ or reduc$ or intake or diet$ or free)).tw. 5 or/1‐4 6 randomized controlled trial/ 7 crossover procedure/ 8 double‐blind procedure/ 9 random$.tw. 10 (crossover$ or cross‐over$).tw. 11 placebo$.tw. 12 (doubl$ adj blind$).tw. 13 assign$.tw. 14 allocat$.tw. 15 or/6‐14 16 (animal$ not (human$ and animal$)).mp. 17 15 not 16 18 5 and 17
Appendix 3. CENTRAL search strategy
Database: Cochrane Central Register of Controlled Trials on Wiley <Issue 11, 2012> Search Date: 11 December 2012 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MeSH descriptor: [Sodium Chloride, Dietary] this term only #2 MeSH descriptor: [Sodium, Dietary] explode all trees #3 MeSH descriptor: [Diet, Sodium‐Restricted] this term only #4 sodium near/3 (restrict* or curb* or limit* or minimi* or low* or reduc* or intake or diet* or free):ti,ab in Trials #5 salt near/3 (restrict* or curb* or limit* or minimi* or low* or reduc* or intake or diet* or free):ti,ab in Trials #6 #1 or #2 or #3 or #4 or #5 in Trials
Appendix 4. Hypertension Group Specialised Register search strategy
Database: Hypertension Group Specialised Register Search Date: 11 December 2012 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Salt[TI] and (curb* or diet* or free or intake or limit* or low* or minimi* or reduc* or restrict*)[All fields] 2 Salt[TI] and (curb* or diet* or free or intake or limit* or low* or minimi* or reduc* or restrict*)[All fields] 3 1 or 2
Data and analyses
Comparison 1. Change in BP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Systolic BP | 35 | Change in SBP (Random, 95% CI) | ‐4.18 [‐5.18, ‐3.18] | |
| 1.1 Hypertensives | 21 | Change in SBP (Random, 95% CI) | ‐5.39 [‐6.62, ‐4.15] | |
| 1.2 Normotensives | 14 | Change in SBP (Random, 95% CI) | ‐2.42 [‐3.56, ‐1.29] | |
| 2 Change in Diastolic BP | 37 | Change in DBP (Random, 95% CI) | ‐2.06 [‐2.67, ‐1.45] | |
| 2.1 Hypertensives | 23 | Change in DBP (Random, 95% CI) | ‐2.82 [‐3.54, ‐2.11] | |
| 2.2 Normotensives | 14 | Change in DBP (Random, 95% CI) | ‐1.00 [‐1.85, ‐0.15] | |
| 3 Change in Systolic BP by Ethnic Group | 31 | Change in SBP (Random, 95% CI) | ‐4.41 [‐5.39, ‐3.44] | |
| 3.1 Hypertensive Whites | 16 | Change in SBP (Random, 95% CI) | ‐5.12 [‐6.27, ‐3.96] | |
| 3.2 Normotensive Whites | 14 | Change in SBP (Random, 95% CI) | ‐2.11 [‐3.03, ‐1.19] | |
| 3.3 Hypertensive Blacks | 5 | Change in SBP (Random, 95% CI) | ‐7.83 [‐10.96, ‐4.71] | |
| 3.4 Normotensive Blacks | 3 | Change in SBP (Random, 95% CI) | ‐4.02 [‐7.44, ‐0.61] | |
| 4 Change in Diastolic BP by Ethnic Group | 33 | Change in DBP (Random, 95% CI) | ‐2.16 [‐2.75, ‐1.57] | |
| 4.1 Hypertensive Whites | 18 | Change in DBP (Random, 95% CI) | ‐2.66 [‐3.37, ‐1.95] | |
| 4.2 Normotensive Whites | 14 | Change in DBP (Random, 95% CI) | ‐0.88 [‐1.68, ‐0.08] | |
| 4.3 Hypertensive Blacks | 5 | Change in DBP (Random, 95% CI) | ‐4.08 [‐5.90, ‐2.26] | |
| 4.4 Normotensive Blacks | 3 | Change in DBP (Random, 95% CI) | ‐1.98 [‐4.45, 0.49] | |
| 5 Change in Systolic BP by Sex | 16 | Change in SBP (Random, 95% CI) | ‐5.52 [‐6.82, ‐4.23] | |
| 5.1 Hypertensive Men | 9 | Change in SBP (Random, 95% CI) | ‐6.40 [‐6.00, ‐4.80] | |
| 5.2 Hypertensive Women | 9 | Change in SBP (Random, 95% CI) | ‐7.11 [‐8.81, ‐5.41] | |
| 5.3 Normotensive Men | 6 | Change in SBP (Random, 95% CI) | ‐3.39 [‐5.63, ‐1.16] | |
| 5.4 Normotensive Women | 6 | Change in SBP (Random, 95% CI) | ‐4.26 [‐6.20, ‐2.31] | |
| 6 Change in Diastolic BP by Sex | 18 | Change in DBP (Random, 95% CI) | ‐2.87 [‐3.54, ‐2.20] | |
| 6.1 Hypertensive Men | 10 | Change in DBP (Random, 95% CI) | ‐3.96 [‐5.47, ‐2.46] | |
| 6.2 Hypertensive Women | 10 | Change in DBP (Random, 95% CI) | ‐3.41 [‐4.29, ‐2.53] | |
| 6.3 Normotensive Men | 6 | Change in DBP (Random, 95% CI) | ‐1.78 [‐3.01, ‐0.55] | |
| 6.4 Normotensive Women | 6 | Change in DBP (Random, 95% CI) | ‐2.18 [‐2.95, ‐1.41] |
1.3. Analysis.
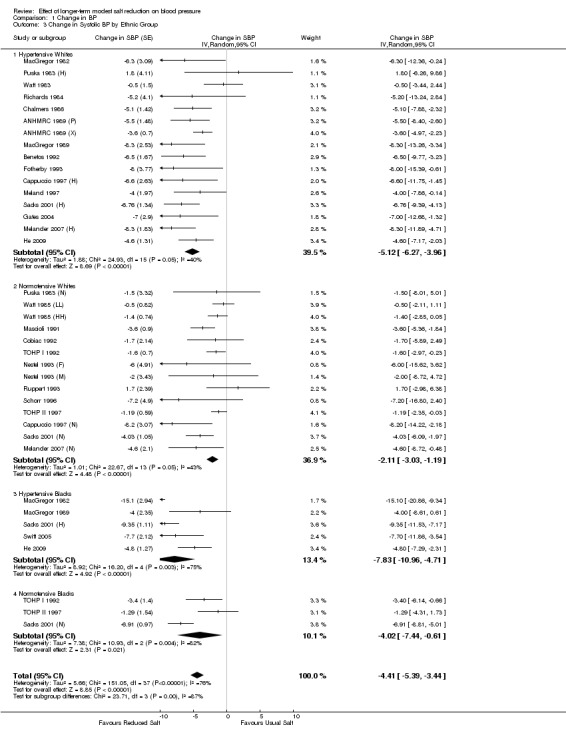
Comparison 1 Change in BP, Outcome 3 Change in Systolic BP by Ethnic Group.
1.4. Analysis.
Comparison 1 Change in BP, Outcome 4 Change in Diastolic BP by Ethnic Group.
1.5. Analysis.
Comparison 1 Change in BP, Outcome 5 Change in Systolic BP by Sex.
1.6. Analysis.
Comparison 1 Change in BP, Outcome 6 Change in Diastolic BP by Sex.
Comparison 2. Change in hormone & lipid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in PRA | 14 | Change in PRA (Random, 95% CI) | 0.26 [0.17, 0.36] | |
| 2 Change in Aldosterone | 9 | Change in Aldosterone (Random, 95% CI) | 73.20 [44.92, 101.48] | |
| 3 Change in Noradrenaline | 6 | Change in Noradrenaline (Random, 95% CI) | 31.67 [6.57, 56.77] | |
| 4 Change in Adrenaline | 4 | Change in Adrenaline (Random, 95% CI) | 6.70 [‐0.25, 13.64] | |
| 5 Change in Cholesterol | 8 | Change in Cholesterol (Random, 95% CI) | 0.05 [‐0.02, 0.11] | |
| 6 Change in LDL | 5 | Change in LDL (Random, 95% CI) | 0.05 [‐0.01, 0.12] | |
| 7 Change in HDL | 6 | Change in HDL (Random, 95% CI) | ‐0.02 [‐0.06, 0.01] | |
| 8 Change in Triglyceride | 6 | Change in Triglyceride (Random, 95% CI) | 0.04 [‐0.02, 0.09] |
2.1. Analysis.
Comparison 2 Change in hormone & lipid, Outcome 1 Change in PRA.
2.2. Analysis.
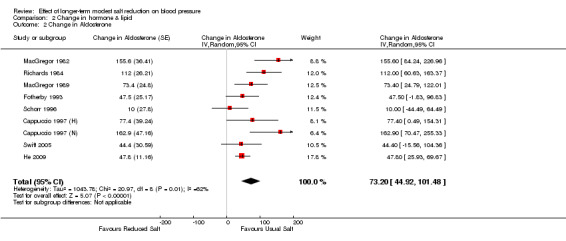
Comparison 2 Change in hormone & lipid, Outcome 2 Change in Aldosterone.
2.3. Analysis.
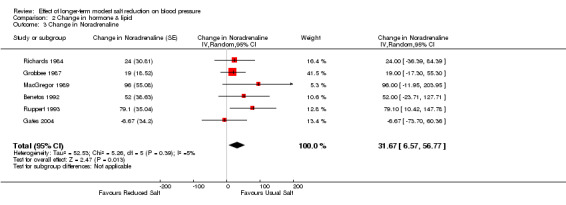
Comparison 2 Change in hormone & lipid, Outcome 3 Change in Noradrenaline.
2.4. Analysis.
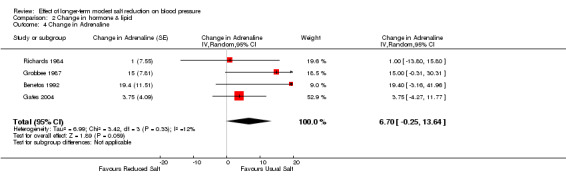
Comparison 2 Change in hormone & lipid, Outcome 4 Change in Adrenaline.
2.5. Analysis.
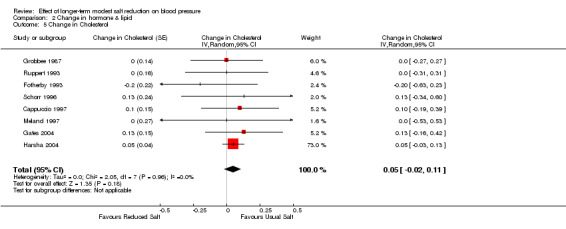
Comparison 2 Change in hormone & lipid, Outcome 5 Change in Cholesterol.
2.6. Analysis.
Comparison 2 Change in hormone & lipid, Outcome 6 Change in LDL.
2.7. Analysis.
Comparison 2 Change in hormone & lipid, Outcome 7 Change in HDL.
2.8. Analysis.
Comparison 2 Change in hormone & lipid, Outcome 8 Change in Triglyceride.
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Parijs 1973.
| Methods | X | |
| Participants | N=15 Age: 41 yr Male: 43% Hypertensive | |
| Interventions | UNa: ‐98 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | High risk | Not blinded to investigator |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | High risk | Not blinded to outcome assessor |
Morgan 1981 (F).
| Methods | BP obs P | |
| Participants | Intervention: N=6 Control: N=6 Age: 38 yr Male: 0% Hypertensive White: 100% |
|
| Interventions | UNa: ‐78 mmol/24h Duration: 8 wks | |
| Outcomes | DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised completed the study. |
| Selective reporting (reporting bias) | Unclear risk | DBP was reported and was included in our meta‐analysis. SBP was also reported, however, it was reported in combination with another group of patients who were on BP treatment. Because our meta‐analysis included participants who were not on any treatment and, in this trial, we could not separate the effect on salt reduction on SBP for individuals who were not on treatment, we did not include SBP in our pooled analysis. |
| Blinding of the investigator | High risk | Not blinded to investigator |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Blood pressure observer blind |
Morgan 1981 (M).
| Methods | BP obs P | |
| Participants | Intervention: N=6 Control: N=6 Age: 40 yr Male: 100% Hypertensive White: 100% |
|
| Interventions | UNa: ‐98 mmol/24h Duration: 8 wks | |
| Outcomes | DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised completed the study. |
| Selective reporting (reporting bias) | Unclear risk | DBP was reported and was included in our meta‐analysis. SBP was also reported, however, it was reported in combination with another group of patients who were on BP treatment. Because our meta‐analysis included participants who were not on any treatment and, in this trial, we could not separate the effect on salt reduction on SBP for individuals who were not on treatment, we did not include SBP in our pooled analysis. |
| Blinding of the investigator | High risk | Not blinded to investigator |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Blood pressure observer blind |
MacGregor 1982.
| Methods | DB X | |
| Participants | N=19 Age: 49 (30‐66) yr Male: 59% Hypertensive White: 63%; Black: 37% |
|
| Interventions | UNa: ‐76 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP PRA Aldo | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised completed the study. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Watt 1983.
| Methods | DB X | |
| Participants | N=18 Age: 52 (31‐64) yr Male: 33% Hypertensive White: 100% |
|
| Interventions | UNa: ‐56 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP PRA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Silman 1983.
| Methods | BP obs (RZ) P | |
| Participants | Intervention: N=10 Control: N=15 Age: 50‐64 yr Hypertensive | |
| Interventions | UNa: ‐53 mmol/24h Duration: 12 months | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | High risk | Not blinded to investigator |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Use of random zero sphygmomanometer |
Puska 1983 (H).
| Methods | BP obs P | |
| Participants | Intervention: N=15 Control: N=19 Age: 30‐50 yr Hypertensive White: 100% |
|
| Interventions | UNa: ‐117 mmol/24h Duration: 6 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised completed the study. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | High risk | Not blinded to investigator |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Blood pressure observer blind |
Puska 1983 (N).
| Methods | BP obs P | |
| Participants | Intervention: N=19 Control: N=19 Age: 30‐50 Normotensive White: 100% |
|
| Interventions | UNa: ‐117 mmol/24h Duration: 6 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised completed the study. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | High risk | Not blinded to investigator |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Blood pressure observer blinded |
Richards 1984.
| Methods | BP obs (A) X | |
| Participants | N=12 Age: 19‐52 yr Male: 67% Hypertensive White: 100% |
|
| Interventions | UNa: ‐105 mmol/24h Duration: 4‐6 wks | |
| Outcomes | SBP DBP PRA Aldo Noradrenaline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not stated |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | High risk | Not blinded to investigator |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Use of an automated version of the London School of Hygiene sphygmomanometer. |
Erwteman 1984.
| Methods | BP obs (RZ) P | |
| Participants | Intervention: N=44 Control: N=50 Age: 46 (20‐70) yr Male: 62% Hypertensive White: 76%; Black: 24% |
|
| Interventions | UNa: ‐58 mmol/24h Duration: 6 months | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Assignment to different salt intakes was single blind. Participants' dietary salt intake was supervised by a dietitian who was asked not to discuss participants' dietary assignment with the physician in charge or the technician. |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Watt 1985 (HH).
| Methods | DB X | |
| Participants | N=35 Age: 22 yr Male: 37% Normotensive White: 100% |
|
| Interventions | UNa: ‐74 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Watt 1985 (LL).
| Methods | DB X | |
| Participants | N=31 Age: 23 yr Male: 45% Normotensive White: 100% |
|
| Interventions | UNa: ‐60 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Chalmers 1986.
| Methods | BP obs (A) P | |
| Participants | Intervention: N=48 Control: N=52 Age: 52 yr Male: 85% Hypertensive All participants were white. |
|
| Interventions | UNa: ‐54 mmol/24h Duration: 12 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis was undertaken |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | High risk | Not blinded to investigator |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Use of automated blood pressure machine |
Grobbee 1987.
| Methods | DB X | |
| Participants | N=40 Age: 18‐28 yr Male: 85% Hypertensive | |
| Interventions | UNa: ‐72 mmol/24h Duration: 6 wks | |
| Outcomes | SBP DBP Noradrenaline Chol | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
ANHMRC 1989 (X).
| Methods | DB X | |
| Participants | N=88 Age: 59 yr Male: 83% Hypertensive All participants were white. |
|
| Interventions | UNa: ‐67 mmol/24h Duration: 8 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis was undertaken |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
ANHMRC 1989 (P).
| Methods | DB P | |
| Participants | Intervention: N=50 Control: N=53 Age: 58 yr Male: 83% Hypertensive All participants were white. |
|
| Interventions | UNa: ‐71 mmol/24h Duration: 8 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis was undertaken |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
MacGregor 1989.
| Methods | DB X | |
| Participants | N=20 Age: 56 (43‐73) yr Male: 55% Hypertensive White: 75%; Black: 25% |
|
| Interventions | UNa: ‐82 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP PRA Aldo Noradrenaline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised completed the study. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Mascioli 1991.
| Methods | DB X | |
| Participants | N=48 Age: 52 yr Male: 79% Normotensive White: 98%; Black: 2% |
|
| Interventions | UNa: ‐20 mmol/8h Duration: 4 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of sodium chloride and placebo capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Benetos 1992.
| Methods | DB X | |
| Participants | N=20 Age: 42 (22‐55) yr Male: 45% Hypertensive White: 100% |
|
| Interventions | UNa: ‐78 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP Noradrenaline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of sodium chloride and placebo capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised completed the study. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Cobiac 1992.
| Methods | DB P | |
| Participants | Intervention: N=26 Control: N=28 Age: 67 (60‐80) yr Male: 67% Normotensive White: 100% |
|
| Interventions | UNa: ‐73 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis was undertaken |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
TOHP I 1992.
| Methods | BP obs (RZ) P | |
| Participants | Intervention: N=327 Control: N=417 Age: 43 (30‐54) yr Male: 71% Normotensive White: 77%; Black: 23% |
|
| Interventions | UNa: ‐44 mmol/24h Duration: 18 months | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, central randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis was undertaken |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | High risk | Not blinded to investigator |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Use of Hawksley random‐zero sphygmomanometer. |
Nestel 1993 (F).
| Methods | DB P | |
| Participants | Intervention: N=15 Control: N=15 Age: 65 (60‐79) yr Male: 0% Normotensive White: 100% |
|
| Interventions | UNa: ‐94 mmol/24h Duration: 6 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Nestel 1993 (M).
| Methods | DB P | |
| Participants | Intervention: N=17 Control: N=19 Age: 66 (60‐79) yr Male: 100% Normotensive White: 100% |
|
| Interventions | UNa: ‐76 mmol/24h Duration: 6 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Fotherby 1993.
| Methods | DB X | |
| Participants | N=17 Age: 73 (66‐79) yr Male: 24% Hypertensive White: 100% |
|
| Interventions | UNa: ‐79 mmol/24h Duration: 5 wks | |
| Outcomes | SBP DBP PRA Aldo | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reason for exclusion was reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Ruppert 1993.
| Methods | DB X | |
| Participants | N=25 Age: 47 (27‐75) yr Male: 40% Normotensive White: 100% |
|
| Interventions | UNa: ‐118 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP PRA Noradrenaline Chol Trig LDL HDL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of salt and placebo capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised completed the study. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Schorr 1996.
| Methods | DB X | |
| Participants | N=16 Age: 64 (60‐72) yr Male: 48% Normotensive White: 100% |
|
| Interventions | UNa: ‐71 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP PRA Aldo Chol Trig LDL HDL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of low sodium and high sodium chloride mineral water. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Cappuccio 1997 (H).
| Methods | DB X | |
| Participants | N=29 Age: 67 (60‐78) yr Male: 51% Hypertensive | |
| Interventions | UNa: ‐87 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP PRA Aldo | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reason for withdrawal was reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Meland 1997.
| Methods | DB X | |
| Participants | N=16 Age: 50 yr Male: 81% Hypertensive White: 100% |
|
| Interventions | UNa: ‐66 mmol/24h Duration: 8 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of salt and placebo capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One patient dropped out during the trial and was replaced by another patient according to the same inclusion and randomisation procedures. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
TOHP II 1997.
| Methods | BP obs (RZ) P | |
| Participants | Intervention: N=515 Control: N=514 Age: 44 (30‐54) yr Male: 67% Normotensive White: 82%; Black: 18% |
|
| Interventions | UNa: ‐40 mmol/24h Duration: 36 months | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, central randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis was undertaken |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | High risk | Not blinded to investigator |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Use of Hawksley random‐zero sphygmomanometer. |
Cappuccio 1997.
| Methods | DB X | |
| Participants | N=47 Age: 67 (60‐78) yr Male: 51% Hypertensive and normotensive White: 89%; Black: 4%; Asian: 7% |
|
| Interventions | UNa: ‐87 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP PRA Aldo Cholesterol |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reason for withdrawal was reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Cappuccio 1997 (N).
| Methods | DB X | |
| Participants | N=18 Age: 67 (60‐78) yr Male: 51% Normotensive | |
| Interventions | UNa: ‐76 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP PRA Aldo | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reason for withdrawal was reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Sacks 2001 (H).
| Methods | BP obs (RZ) X | |
| Participants | N=76
Age: 52 yr
Male: 39%
Hypertensive White: 40%; Black: 60% |
|
| Interventions | UNa: ‐80 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis was undertaken. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | The personnel involved in the collection of the outcome data were unaware of participants' diet assignment. |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Blood pressure observer blinded |
Sacks 2001 (N).
| Methods | BP obs (RZ) X | |
| Participants | N=116 Age: 47 yr Male: 50% Normotensive White: 46%; Black: 54% |
|
| Interventions | UNa: ‐75 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis was undertaken. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | The personnel involved in the collection of the outcome data were unaware of participants' diet assignment. |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | Blood pressure observer blinded |
Gates 2004.
| Methods | DB X | |
| Participants | N=12 Age: 64 yr Male: 50% Hypertensive White: 100% |
|
| Interventions | UNa: ‐89 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP PRA Chol Trig LDL HDL Noradrenaline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised completed the study. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Harsha 2004.
| Methods | BP obs (RZ) X | |
| Participants | N=193 Age: 49 yr Male: 46% Hypertensive and normotensive |
|
| Interventions | UNa: ‐77 mmol/24h Duration: 4 wks |
|
| Outcomes | Cholesterol LDL HDL Triglycerides |
|
| Notes | This paper reported the lipid data from the DASH‐Na trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis was undertaken. |
| Selective reporting (reporting bias) | Low risk | All lipid data from participants who gave fasting blood samples were analysed and reported. |
| Blinding of the investigator | Low risk | The personnel involved in the collection of the outcome data were unaware of participants' diet assignment. |
| Blinding of the participant | High risk | Not blinded to participant |
| Blinding of the outcome assessor | Low risk | The personnel involved in the measurements of lipids were blinded to participants' dietary assignment. |
Swift 2005.
| Methods | DB X | |
| Participants | N=40 Age: 50 yr Male: 43% Hypertensive White: 0%; Black: 100% |
|
| Interventions | UNa: ‐78 mmol/24h Duration: 4 wks | |
| Outcomes | SBP DBP PRA Aldo | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Melander 2007.
| Methods | DB X |
|
| Participants | N=39 Age: 53 (42‐64) yr Male: 51% Hypertensive and normotensive White: 100% |
|
| Interventions | UNa: ‐89 mmol/24h Duration: 4 wks |
|
| Outcomes | SBP DBP |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of salt and placebo capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Melander 2007 (H).
| Methods | DB X |
|
| Participants | N=19 Age: 53 (42‐64) yr Male: 53% Hypertensive White: 100% |
|
| Interventions | UNa: ‐93 mmol/24h Duration: 4 wks |
|
| Outcomes | SBP DBP |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of salt and placebo capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
Melander 2007 (N).
| Methods | DB X |
|
| Participants | N=20 Age: 53 (42‐64) yr Male: 50% Normotensive White: 100% |
|
| Interventions | UNa: ‐85 mmol/24h Duration: 4 wks |
|
| Outcomes | SBP DBP |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of salt and placebo capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed, reasons for withdrawal were reported. |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
He 2009.
| Methods | DB X |
|
| Participants | N=169 Age: 50 yr Male: 67% Hypertensive White: 42%; Black: 41%: Asian: 17% |
|
| Interventions | UNa: ‐55 mmol/24h Duration: 6 wks |
|
| Outcomes | SBP DBP PRA Aldo |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, use of Slow Sodium and placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Properly addressed |
| Selective reporting (reporting bias) | Low risk | Both SBP and DBP were reported. |
| Blinding of the investigator | Low risk | Double blind |
| Blinding of the participant | Low risk | Double blind |
| Blinding of the outcome assessor | Low risk | Outcome assessor blind |
UNa: urinary sodium; BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; X: crossover; P: parallel; DB: Double blind; BP obs: blood pressure observer blinded; RZ: random zero manometer; A: automated sphygmomanometer; F: Female; M: Male; H: Hypertensive; N: Normotensive; HH: offspring of two parents with high blood pressure; LL: offspring of two parents with low blood pressure; PRA: plasma renin activity; Aldo: aldosterone; Chol: cholesterol; Trig: triglyceride; LDL: low‐density lipoprotein; HDL: high‐density lipoprotein.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akita 2003 | Sub‐study of DASH‐Na trial (Sacks 2001) |
| Alam 1999 | Duration of salt reduction less than 4 weeks |
| Alli 1992 | Reduction in 24h urinary sodium less than 40 mmol |
| Ambrosioni 1982 | 24h urinary sodium not measured |
| Ames 2001 | With concomitant intervention |
| Andersson 1984 | With concomitant intervention |
| Andersson 1986 | Duration of salt reduction less than 4 weeks |
| Appel 2001 | Sub‐study of TONE trial (Whelton 1998) |
| Appel 2003 | With concomitant intervention |
| Applegate 1992 | With concomitant intervention |
| Arroll 1995 | With concomitant intervention |
| Barba 2000 | Duration of salt reduction less than 4 weeks |
| Beard 1982 | With concomitant intervention |
| Beckmann 1995 | With concomitant intervention |
| Berglund 1989 | With concomitant intervention |
| Boero 2000 | Duration of salt reduction less than 4 weeks |
| Bompiani 1988 | With concomitant intervention |
| Bray 2004 | Sub‐study of DASH‐Na trial (Sacks 2001) |
| Bruun 1990 | Duration of salt reduction less than 4 weeks |
| Buckley 1994 | Duration of salt reduction less than 4 weeks |
| Bulpitt 1984 | With concomitant intervention |
| Burke 2005 | With concomitant intervention |
| Burke 2007 | With concomitant intervention |
| Burnier 1993 | Duration of salt reduction less than 4 weeks |
| Buyck 2009 | 24h urinary sodium not measured |
| Calabrese 1985 | Studies in children |
| Cappuccio 2006 | Reduction in 24h urinary sodium less than 40 mmol |
| Carney 1991 | With concomitant intervention |
| Charlton 2008 | With concomitant intervention |
| Chen 2008 | Duration of salt reduction less than 4 weeks |
| Chrysant 2000 | With concomitant intervention |
| Cook 2005 | Sub‐study of TOHP II (TOHP II 1997) |
| Cook 2007 | Follow‐up study of TOHP (TOHP 1992, TOHP II 1997) |
| Cook 2009 | Sub‐study of TOHP (TOHP 1992, TOHP II 1997) |
| Cooper 1984 | Studies in children |
| Costa 1981 | 24h urinary sodium not measured |
| CSSS 2007 | 24h urinary sodium not measured |
| Cuzzola 2001 | Duration of salt reduction less than 4 weeks |
| Damasceno 1999 | Duration of salt reduction less than 4 weeks |
| Damasceno 2000 | Duration of salt reduction less than 4 weeks |
| Davis 1994 | With concomitant intervention |
| Davrath 1999 | Duration of salt reduction less than 4 weeks |
| Del Rio 1990 | With concomitant intervention |
| Del Rio 1993 | Duration of salt reduction less than 4 weeks |
| Delemarre 2000 | Studies in pregnant women |
| Dickinson 2009 | Duration of salt reduction less than 4 weeks |
| Dimsdale 1990 | Duration of salt reduction less than 4 weeks |
| Dodson 1984 | Study in patients with diabetes |
| Dodson 1989 | Study in patients with diabetes |
| Donovan 1993 | Duration of salt reduction less than 4 weeks |
| Dubbert 1995 | With concomitant intervention |
| Egan 1991 | Duration of salt reduction less than 4 weeks |
| Egan 1991(b) | Duration of salt reduction less than 4 weeks |
| Ekinci 2009 | Study in patients with diabetes |
| Ekinci 2010 | Study in patients with diabetes |
| el Ashry 1987 | Duration of salt reduction less than 4 weeks |
| Elmer 1995 | With concomitant intervention |
| Elmer 2006 | With concomitant intervention |
| Fagerberg 1984 | With concomitant intervention |
| Fagerberg 1985 | With concomitant intervention |
| Feldman 1996 | Duration of salt reduction less than 4 weeks |
| Ferri 1993 | Duration of salt reduction less than 4 weeks |
| Ferri 1994 | Duration of salt reduction less than 4 weeks |
| Ferri 1996 | Duration of salt reduction less than 4 weeks |
| Fliser 1993(a) | Duration of salt reduction less than 4 weeks |
| Fliser 1993(b) | With concomitant intervention |
| Forrester 2005 | Duration of salt reduction less than 4 weeks |
| Fotherby 1997 | Sub‐study of Fotherby 1993 |
| Friberg 1990 | Duration of salt reduction less than 4 weeks |
| Fuchs 1987 | Duration of salt reduction less than 4 weeks |
| Gillies 1984 | With concomitant intervention |
| Gillum 1981 | Studies in children |
| Gomi 1998 | Duration of salt reduction less than 4 weeks |
| Gow 1992 | Duration of salt reduction less than 4 weeks |
| Grey 1996 | Duration of salt reduction less than 4 weeks |
| Hargreaves 1989 | Duration of salt reduction less than 4 weeks |
| Haythornthwaite 1992 | Duration of salt reduction less than 4 weeks |
| He 2000 | Follow‐up study of TOHP I (TOHP I 1992) |
| He 2005(a) | Sub‐study of previous trials |
| He 2005(b) | Sub‐study of previous trials |
| He, J 2009 | Duration of salt reduction less than 4 weeks |
| Heagerty 1986 | Duration of salt reduction less than 4 weeks |
| Herlitz 1998 | Duration of salt reduction less than 4 weeks |
| Hofman 1983 | Studies in children |
| Houlihan 2002(a) | Study in patients with diabetes |
| Houlihan 2002(b) | Study in patients with diabetes |
| Howe 1991 | Studies in children |
| Howe1994 | With concomitant intervention |
| HPTRG 1990 | Reduction in 24‐h urinary sodium less than 40 mmol |
| Hu 2009 | 24h urinary sodium not measured |
| Inoue 1996 | Duration of salt reduction less than 4 weeks |
| Ireland 2010 | Reduction in 24h urinary sodium less than 40 mmol |
| Ishimitsu 1996 | Duration of salt reduction less than 4 weeks |
| Iso 1996 | With concomitant intervention |
| Iwaoka 1994 | Duration of salt reduction less than 4 weeks |
| Jessani 2008 | Duration of salt reduction less than 4 weeks |
| Johnson 2001 | Duration of salt reduction less than 4 weeks |
| Jula 1990 | With concomitant intervention |
| Jula 1992(a) | With concomitant intervention |
| Jula 1992(b) | With concomitant intervention |
| Kawasaki 1998 | With concomitant intervention |
| Keven 2006 | With concomitant intervention |
| Kirpizidis 2005 | With concomitant intervention |
| Kojuri 2007 | Total 24h urinary sodium excretion not reported. |
| Koolen 1984 (a) | Duration of salt reduction less than 4 weeks |
| Koolen 1984 (b) | Duration of salt reduction less than 4 weeks |
| Koopman 1990 (a) | With concomitant intervention |
| Koopman 1990 (b) | With concomitant intervention |
| Koopman 1997 | With concomitant intervention |
| Kostis 2002 | With concomitant intervention |
| Kristinsson 1988 | With concomitant intervention |
| Kumanyika 1993 | Sub‐study of TOHP I (TOHP I 1992) |
| Kumanyika 2005 | Sub‐study of TOHP II |
| Kurtz 1987 | Duration of salt reduction less than 4 weeks |
| Lawton 1988 | Duration of salt reduction less than 4 weeks |
| Logan 1986 | Reduction in 24‐h urinary sodium less than 40 mmol |
| Luft 1990 | Duration of salt reduction less than 4 weeks |
| Macgregor 1982 | Duration of salt reduction less than 4 weeks |
| MacGregor 1987 | With concomitant intervention |
| Mallamaci 1996 | Duration of salt reduction less than 4 weeks |
| Manunta 2001 | Duration of salt reduction less than 4 weeks |
| Mark 1975 | Duration of salt reduction less than 4 weeks |
| Mattila 2003 | With concomitant intervention |
| Maxwell 1984 | With concomitant intervention |
| McCarron 1997 | With concomitant intervention |
| Meland 2009 | With concomitant intervention |
| Miller 1988 | Studies in children |
| Miller 1997 | Duration of salt reduction less than 4 weeks |
| Morgan 1978 | Reduction in 24‐h urinary sodium less than 40 mmol |
| Morgan 1988 | Duration of salt reduction less than 4 weeks |
| Mtabaji 1990 | Duration of salt reduction less than 4 weeks |
| Mu 2009 | With concomitant intervention |
| Mufunda 1992 | Duration of salt reduction less than 4 weeks |
| Muhlhauser 1996 | With concomitant intervention |
| Myers 1983 | Duration of salt reduction less than 4 weeks |
| Mülhauser 1996 | Study in patients with diabetes |
| Nakamura 2003 | 24h urinary sodium not measured |
| Nowson 1988 | With concomitant intervention |
| Nowson 2003 | With concomitant intervention |
| Nowson 2004 | With concomitant intervention |
| Nowson 2009 | With concomitant intervention |
| Omland 2001 | With concomitant intervention |
| Omvik 1995 | With concomitant intervention |
| Overlack 1993 | Duration of salt reduction less than 4 weeks |
| Overlack 1995 | Duration of salt reduction less than 4 weeks |
| Palacios 2004 | Study in children |
| Palmer 1989 | 24h urinary sodium not measured |
| Parfrey 1981 | 24h urinary sodium not measured |
| Parker 1990 | With concomitant intervention |
| Pechere‐Bertschi 2008 | Duration of salt reduction less than 4 weeks |
| Pedersen 1986 | Duration of salt reduction less than 4 weeks |
| Perry 2003 | Duration of salt reduction less than 4 weeks |
| Petrie 1998 | Duration of salt reduction less than 4 weeks |
| Pimenta 2009 | Duration of salt reduction less than 4 weeks |
| Pogson 2009 | Blood pressure was not measured |
| Pomeranz 2002 | Studies in neonates |
| Redon 1995 | With concomitant intervention |
| Redon‐Mas 1993 | With concomitant intervention |
| Richards 1986 | Duration of salt reduction less than 4 weeks |
| Ruilope 1992 | Duration of salt reduction less than 4 weeks |
| Ruilope 1993 | Duration of salt reduction less than 4 weeks |
| Ruppert 1991 | Duration of salt reduction less than 4 weeks |
| Ruppert 1994 | Duration of salt reduction less than 4 weeks |
| Salvetti 1988 | With concomitant intervention |
| Santello 1997 | Duration of salt reduction less than 4 weeks |
| Saptharishi 2009 | With concomitant intervention |
| Schmid 1990 | Duration of salt reduction less than 4 weeks |
| Schorr 1997(a) | Duration of salt reduction less than 4 weeks |
| Schorr 1997(b) | Duration of salt reduction less than 4 weeks |
| Sciarrone 1990 | With concomitant intervention |
| Sciarrone 1992 | With concomitant intervention |
| Sharma 1990 | Duration of salt reduction less than 4 weeks |
| Sharma 1991 | Duration of salt reduction less than 4 weeks |
| Sharma 1993 | Duration of salt reduction less than 4 weeks |
| Shore 1988 | Duration of salt reduction less than 4 weeks |
| Sinaiko 1993 | Studies in children |
| Singer 1991 | With concomitant intervention |
| Singer 1994 | Duration of salt reduction less than 4 weeks |
| Singer 1995 | With concomitant intervention |
| Skrabal 1980 | Duration of salt reduction less than 4 weeks |
| Skrabal 1981 | Duration of salt reduction less than 4 weeks |
| Skrabal 1984(a) | Duration of salt reduction less than 4 weeks |
| Skrabal 1984(b) | Duration of salt reduction less than 4 weeks |
| Skrabal 1985 | Duration of salt reduction less than 4 weeks |
| Staessen 1988 | Reduction in 24h urinary sodium less than 40 mmol |
| Starmans‐Kool 2011 | Duration of salt reduction less than 4 weeks |
| Steegers 1991 | Studies in pregnant women |
| Sullivan 1980 | Duration of salt reduction less than 4 weeks |
| Suppa 1988 | With concomitant intervention |
| Suzuki 2000 | Duration of salt reduction less than 4 weeks |
| Takahashi 2006 | With concomitant intervention |
| Takashashi 2003 | With concomitant intervention |
| Teow 1985 | Duration of salt reduction less than 4 weeks |
| Thaler 1982 | With concomitant intervention |
| TMHRG 1991 | With concomitant intervention |
| Todd 2010 | With concomitant intervention |
| Todd 2012 | 24h urinary sodium not measured |
| Townsend 2007 | Duration of salt reduction less than 4 weeks |
| Tzemos 2008 | Duration of salt reduction less than 4 weeks |
| Uusitupa 1996 | With concomitant intervention |
| Uzu 1999 | Duration of salt reduction less than 4 weeks |
| Uzu 2009 | With concomitant intervention |
| Vaidya 2009 | Duration of salt reduction less than 4 weeks |
| van BergeLandry 2004 | Extreme change in salt intake, urinary sodium was changed from 309 to 24 mmol/24h |
| van Paassen 1996 | Duration of salt reduction less than 4 weeks |
| Vedovato 2004 | Study in patients with diabetes |
| Visser 2008 | Duration of salt reduction less than 4 weeks |
| Vollmer 2001 | Sub‐study of the DASH‐Na trial |
| Weder 1991 | Duration of salt reduction less than 4 weeks |
| Wedler 1992 | Duration of salt reduction less than 4 weeks |
| Weir 1995 | Duration of salt reduction less than 4 weeks |
| Weir 1997 | With concomitant intervention |
| Weir 1998 | With concomitant intervention |
| Weir 2001 | With concomitant intervention |
| Weir 2010 | With concomitant intervention |
| Whelton 1998 | With concomitant intervention |
| Whitten 1980 | Studies in children |
| Wing 1984 | With concomitant intervention |
| Wing 1998 | With concomitant intervention |
| Yamakoshi 2006 | With concomitant intervention |
| Yamamoto 1997 | With concomitant intervention |
| Yazici 2009 | With concomitant intervention |
| Zhao 2009 | Duration of salt reduction less than 4 weeks |
| Zoccali 1993 | Duration of salt reduction less than 4 weeks |
| Zoccali 1994(a) | Duration of salt reduction less than 4 weeks |
| Zoccali 1994(b) | Duration of salt reduction less than 4 weeks |
| Zoccali 1996 | Duration of salt reduction less than 4 weeks |
Contributions of authors
FH and JL screened the titles and abstracts, assessed trials for inclusion and trial quality, and extracted data. FH performed statistical analyses and wrote the draft manuscript. FH, JL and GM contributed to the revision and final version of the paper.
Sources of support
Internal sources
-
Queen Mary University of London, UK.
While working on this review, Feng He and Graham MacGregor's salaries were paid by Queen Mary University of London
-
China Scholarship Council, China.
While working on this review Jiafu Li was supported by China Scholarship Council.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
ANHMRC 1989 (P) {published data only}
- Australian National Health and Medical Research Council Dietary Salt Study Management Committee. Fall in blood pressure with modest reduction in dietary salt intake in mild hypertension. Lancet 1989;1:399‐402. [PubMed] [Google Scholar]
ANHMRC 1989 (X) {published data only}
- Australian National Health and Medical Rresearch Council Dietary Salt Study Management Committee. Effects of replacing sodium intake in subjects on a low sodium diet: a crossover study. Clin Exp Hypertens 1989;A11:1011‐24. [DOI] [PubMed] [Google Scholar]
Benetos 1992 {published data only}
- Benetos A, Yang Yan X, Cuche JL, Hannaert P, Safar M. Arterial effects of salt restriction in hypertensive patients. A 9‐week, randomized, double‐blind, crossover study. J Hypertens 1992;10:355‐60. [DOI] [PubMed] [Google Scholar]
Cappuccio 1997 {published and unpublished data}
- Cappuccio FP, Markandu ND, Carney C, Sagnella GA, MacGregor GA. Double‐blind randomised trial of modest salt restriction in older people. Lancet 1997;350:850‐4. [DOI] [PubMed] [Google Scholar]
Cappuccio 1997 (H) {published and unpublished data}
- Cappuccio FP, Markandu ND, Carney C, Sagnella GA, MacGregor GA. Double‐blind randomised trial of modest salt restriction in older people. Lancet 1997;350:850‐4. [DOI] [PubMed] [Google Scholar]
Cappuccio 1997 (N) {published and unpublished data}
- Cappuccio FP, Markandu ND, Carney C, Sagnella GA, MacGregor GA. Double‐blind randomised trial of modest salt restriction in older people. Lancet 1997;350:850‐4. [DOI] [PubMed] [Google Scholar]
Chalmers 1986 {published data only}
- Chalmers J, Morgan T, Doyle A, Dickson B, Hopper J, Mathews J, Matthews G, Moulds R, Myers J, Nowson C, Scoggins B, Stebbing M. Australian National Health and Medical Rresearch Council dietary salt study in mild hypertension. J Hypertens 1986;4(suppl 6):S629‐37. [PubMed] [Google Scholar]
Cobiac 1992 {published data only}
- Cobiac L, Nestel PJ, Wing LMH, Howe PRC. A low‐sodium diet supplemented with fish oil lowers blood pressure in the elderly. J Hypertens 1992;10:87‐92. [DOI] [PubMed] [Google Scholar]
Erwteman 1984 {published data only}
- Erwteman TM, Nagelkerke N, Lubsen J, Koster M, Dunning AJ. Beta‐blockade, diuretics, and salt restriction for the management of mild hypertension: a randomised double blind trial. BMJ 1984;289:406‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fotherby 1993 {published data only}
- Fotherby MD, Potter JF. Effects of moderate sodium restriction on clinic and twenty‐four‐hour ambulatory blood pressure in elderly hypertensive subjects. J Hypertens 1993;11:657‐63. [DOI] [PubMed] [Google Scholar]
Gates 2004 {published and unpublished data}
- Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 2004;44:35‐41. [DOI] [PubMed] [Google Scholar]
Grobbee 1987 {published data only}
- Grobbee DE, Hofman A, Roelandt JT, Boomsma F, Schalekamp MA, Valkenburg HA. Sodium restriction and potassium supplementation in young people with mildly elevated blood pressure. J Hypertens 1987;5:115‐9. [DOI] [PubMed] [Google Scholar]
Harsha 2004 {published data only}
- Harsha, D. W.Sacks, F. M.Obarzanek, E.Svetkey, L. P.Lin, P. H.Bray, G. A.Aickin, M.Conlin, P. R.Miller, E. R, 3rd, Appel, L. J. Effect of dietary sodium intake on blood lipids: results from the DASH‐sodium trial. Hypertension 2004;43:393‐8. [DOI] [PubMed] [Google Scholar]
He 2009 {published and unpublished data}
- He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension 2009;54:482‐8. [DOI] [PubMed] [Google Scholar]
MacGregor 1982 {published and unpublished data}
- MacGregor GA, Markandu ND, Best FE, Elder DM, Cam JM, Sagnella GA, Squires M. Double‐blind randomised crossover trial of moderate sodium restriction in essential hypertension. Lancet 1982;1:351‐5. [DOI] [PubMed] [Google Scholar]
MacGregor 1989 {published and unpublished data}
- MacGregor GA, Markandu ND, Sagnella GA, Singer D, Cappuccio FP. Double‐blind study of three sodium intakes and long‐term effects of sodium restriction in essential hypertension. Lancet 1989;2:1244‐7. [DOI] [PubMed] [Google Scholar]
Mascioli 1991 {published data only}
- Mascioli S, Grimm RH, Launer C, Svendsen K, Flack J, Gonzalez N, Elmer P, Neaton J. Sodium chloride raises blood pressure in normotensive subjects: the study of sodium and blood pressure. Hypertension 1991;17(suppl I):I21‐6. [DOI] [PubMed] [Google Scholar]
Meland 1997 {published and unpublished data}
- Meland E, Laerum E, Aakvaag A, Ulvik RJ, Hostmark AT. Salt restriction: effects on lipids and insulin production in hypertensive patients. Scand J Clin Lab Invest 1997;57:501‐5. [DOI] [PubMed] [Google Scholar]
Melander 2007 {published and unpublished data}
- Melander O, Wowern F, Frandsen E, Burri P, Willsteen G, Aurell M, Hulthén UL. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N‐terminal atrial natriuretic peptide in plasma. J Hypertens 2007;25:619‐27. [DOI] [PubMed] [Google Scholar]
Melander 2007 (H) {published and unpublished data}
- Melander O, Wowern F, Frandsen E, Burri P, Willsteen G, Aurell M, Hulthén UL. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N‐terminal atrial natriuretic peptide in plasma. J Hypertens 2007;25:619‐27. [DOI] [PubMed] [Google Scholar]
Melander 2007 (N) {published and unpublished data}
- Melander O, Wowern F, Frandsen E, Burri P, Willsteen G, Aurell M, Hulthén UL. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N‐terminal atrial natriuretic peptide in plasma. J Hypertens 2007;25:619‐27. [DOI] [PubMed] [Google Scholar]
Morgan 1981 (F) {published data only}
- Morgan TO, Myer JB. Hypertension treated by sodium restriction. Med J Aust 1981;2:396‐7. [DOI] [PubMed] [Google Scholar]
Morgan 1981 (M) {published data only}
- Morgan TO, Myer JB. Hypertension treated by sodium restriction. Med J Aust 1981;2:396‐7. [DOI] [PubMed] [Google Scholar]
Nestel 1993 (F) {published data only}
- Nestel PJ, Clifton PM, Noakes M, McArthur R, Howe PR. Enhanced blood pressure response to dietary salt in elderly women, especially those with small waist:hip ratio. J Hypertens 1993;11:1387‐94. [DOI] [PubMed] [Google Scholar]
Nestel 1993 (M) {published data only}
- Nestel PJ, Clifton PM, Noakes M, McArthur R, Howe PR. Enhanced blood pressure response to dietary salt in elderly women, especially those with small waist:hip ratio. J Hypertens 1993;11:1387‐94. [DOI] [PubMed] [Google Scholar]
Parijs 1973 {published data only}
- Parijs J, Joossens JV, Linden LV, Verstreken G, Amery AKPC. Moderate sodium restriction and diuretics in the treatment of hypertension. Am Heart J 1973;85:22‐34. [DOI] [PubMed] [Google Scholar]
Puska 1983 (H) {published data only}
- Puska P, Iacono JM, Nissinen A, Korhonen HJ, Vartiainen E, Pietinen P, Dougherty R, Leino U, Mutanen M, Moisio S, Huttunen J. Controlled, randomised trial of the effect of dietary fat on blood pressure. Lancet 1983;1:1‐5. [DOI] [PubMed] [Google Scholar]
Puska 1983 (N) {published data only}
- Puska P, Iacono JM, Nissinen A, Korhonen HJ, Vartiainen E, Pietinen P, Dougherty R, Leino U, Mutanen M, Moisio S, Huttunen J. Controlled, randomised trial of the effect of dietary fat on blood pressure. Lancet 1983;1:1‐5. [DOI] [PubMed] [Google Scholar]
Richards 1984 {published data only}
- Richards AM, Nicholls MG, Espiner EA, Ikram H, Maslowski AH, Hamilton EJ, Wells JE. Blood‐pressure response to moderate sodium restriction and to potassium supplementation in mild essential hypertension. Lancet 1984;1:757‐61. [DOI] [PubMed] [Google Scholar]
Ruppert 1993 {published data only}
- Ruppert M, Overlack A, Kolloch R, Kraft K, Gobel B, Stumpe KO. Neurohormonal and metabolic effects of severe and moderate salt restriction in non‐obese normotensive adults. J Hypertens 1993;117:743‐9. [DOI] [PubMed] [Google Scholar]
Sacks 2001 (H) {published and unpublished data}
- Sacks FM, Svetkey LR, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons‐Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 2001;344:3‐10. [DOI] [PubMed] [Google Scholar]
Sacks 2001 (N) {published and unpublished data}
- Sacks FM, Svetkey LR, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons‐Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 2001;323:42‐46. [DOI] [PubMed] [Google Scholar]
Schorr 1996 {published data only}
- Schorr U, Distler A, Sharma AM. Effect of sodium chloride‐ and sodium bicarbonate‐rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: a randomized double‐blind crossover trial. J Hypertens 1996;14:131‐5. [PubMed] [Google Scholar]
Silman 1983 {published data only}
- Silman AJ, Locke C, Mitchell P, Humpherson P. Evaluation of the effectiveness of a low sodium diet in the treatment of mild to moderate hypertension. Lancet 1983;1:1179‐82. [DOI] [PubMed] [Google Scholar]
Swift 2005 {published and unpublished data}
- Swift PA, Markandu ND, Sagnella GA, He FJ, MacGregor GA. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives. Hypertension 2005;46:308‐312. [DOI] [PubMed] [Google Scholar]
TOHP I 1992 {published and unpublished data}
- The Trials of Hypertension Prevention Collaborative Research Group. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels: results of the Trials of Hypertension Prevention, phase I. JAMA 1992;267:1213‐20. [DOI] [PubMed] [Google Scholar]
TOHP II 1997 {published data only}
- The Trials of Hypertension Prevention Collaborative Research Group. Effect of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high‐normal blood pressure. The Trials of Hypertension Prevention, Phase II. Arch Intern Med 1997;157:657‐67. [PubMed] [Google Scholar]
Watt 1983 {published data only}
- Watt GCM, Edward C, Hart JT, Heart M, Walton P, Foy CJW. Dietary sodium restriction for mild hypertension in general practice. BMJ 1983;286:432‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watt 1985 (HH) {published data only}
- Watt GC, Foy CJ, Hart JT, Bingham G, Edwards C, Hart M, Thomas E, Walton P. Dietary sodium and arterial blood pressure: evidence against genetic susceptibility. BMJ 1985;291:1525‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watt 1985 (LL) {published data only}
- Watt GC, Foy CJ, Hart JT, Bingham G, Edwards C, Hart M, Thomas E, Walton P. Dietary sodium and arterial blood pressure: evidence against genetic susceptibility. BMJ 1985;291:1525‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akita 2003 {published data only}
- Akita S, Sacks FM, Svetkey LP, Conlin PR, Kimura G, DASH‐Sodium Trial Collaborative Research Group. Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on the pressure‐natriuresis relationship. Hypertension 2003;42(1):8‐13. [DOI] [PubMed] [Google Scholar]
Alam 1999 {published data only}
- Alam S, Purdie DM, Johnson AG. Evaluation of the potential interaction between NaCl and prostaglandin inhibition in elderly individuals with isolated systolic hypertension. Journal of Hypertension 1999;17(8):1195‐202. [DOI] [PubMed] [Google Scholar]
Alli 1992 {published data only}
- Alli C, Avanzini F, Bettelli G, Bonati M, Colombo F, Corso R, Tullio M, Gentile MG, Sangalli L, Taioli E, Tognoni, G. Feasibility of a long‐term low‐sodium diet in mild hypertension. Journal of Human Hypertension 1992;6(4):281‐286. [PubMed] [Google Scholar]
Ambrosioni 1982 {published data only}
- Ambrosioni E, Costa FV, Borghi C, Montebugnoli L, Giordani MF, Magnani B. Effects of moderate salt restriction on intralymphocytic sodium and pressor response to stress in borderline hypertension. Hypertension 1982;4:789‐94. [DOI] [PubMed] [Google Scholar]
Ames 2001 {published data only}
- Ames RP. The effect of sodium supplementation on glucose tolerance and insulin concentrations in patients with hypertension and diabetes mellitus. Am J Hypertens 2001;14:653‐9. [DOI] [PubMed] [Google Scholar]
Andersson 1984 {published data only}
- Andersson OK, Fagerberg B, Hedner T. Importance of dietary salt in the hemodynamic adjustment to weight reduction in obese hypertensive men. Hypertension 1984;6:814‐9. [DOI] [PubMed] [Google Scholar]
Andersson 1986 {published data only}
- Andersson OK, Persson B, Hedner T, Aurell M, Berglund G, Fagerberg B. Central haemodynamics, baroreceptor sensitivity and alpha 1‐adrenoceptor‐mediated vascular reactivity during weight‐stable sodium restriction in obese men with hypertension. Journal of Hypertension 1986;4(1):101‐7. [DOI] [PubMed] [Google Scholar]
Appel 2001 {published data only}
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Archives of Internal Medicine 2001;161(5):685‐93. [DOI] [PubMed] [Google Scholar]
Appel 2003 {published data only}
- Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR, Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003;289:2083‐93. [DOI] [PubMed] [Google Scholar]
Applegate 1992 {published data only}
- Applegate WB, Miller ST, Elam JT, Cushman WC, Derwi D, Brewer A, Graney MJ. Nonpharmacologic intervention to reduce blood pressure in older patients with mild hypertension. Arch Intern Med 1992;152:1162‐6. [PubMed] [Google Scholar]
Arroll 1995 {published data only}
- Arroll B, Beaglehole R. Salt restriction and physical activity in treated hypertensives. N Z Med J 1995;108:266‐8. [PubMed] [Google Scholar]
Barba 2000 {published data only}
- Barba G, Vallance PJ, Strazzullo P, MacAllister RJ. Effects of sodium intake on the pressor and renal responses to nitric oxide synthesis inhibition in normotensive individuals with different sodium sensitivity. J Hypertens 2000;18:615‐21. [DOI] [PubMed] [Google Scholar]
Beard 1982 {published data only}
- Beard TC, Cooke HM, Gray WR, Barge R. Randomised controlled trial of a no‐added‐sodium diet for mild hypertension. Lancet 1982;2:455‐8. [DOI] [PubMed] [Google Scholar]
Beckmann 1995 {published data only}
- Beckmann SL, Os I, Kjeldsen SE, Eide IK, Westheim AS, Hjermann I. Effect of dietary counselling on blood pressure and arterial plasma catecholamines in primary hypertension. Am J Hypertens 1995;8:704‐11. [DOI] [PubMed] [Google Scholar]
Berglund 1989 {published data only}
- Berglund A, Andersson OK, Berglund G, Fagerberg B. Antihypertensive effect of diet compared with drug treatment in obese men with mild hypertension. BMJ 1989;299(6697):480‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boero 2000 {published data only}
- Boero R, Pignataro A, Bancale E, Campo A, Morelli E, Nigra M, Novarese M, Possamai D, Prodi E, Quarello F. Metabolic effects of changes in dietary sodium intake in patients with essential hypertension. Minerva Urologica e Nefrologica 2000;52(1):13‐6. [PubMed] [Google Scholar]
Bompiani 1988 {published data only}
- Bompiani GD, Cerasola G, Morici ML, Condorelli M, Trimarco B, Luca N, Leonetti G, Sampieri L, Cuspidi C, Cottone S, et al. Effects of moderate low sodium/high potassium diet on essential hypertension: results of a comparative study. International Journal of Clinical Pharmacology, Therapy, & Toxicology 1988;26(3):129‐32. [PubMed] [Google Scholar]
Bray 2004 {published data only}
- Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ, DASH Collaborative Research Group. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH‐Sodium Trial. The American journal of cardiology 2004;94(2):222‐7. [DOI] [PubMed] [Google Scholar]
Bruun 1990 {published data only}
- Bruun NE, Skott P, Damkjaer Nielsen M, Rasmussen S, Schutten HJ, Leth A, Pedersen EB, Giese J. Normal renal tubular response to changes of sodium intake in hypertensive man. J Hypertens 1990;8:219‐27. [PubMed] [Google Scholar]
Buckley 1994 {published data only}
- Buckley MG, Markandu ND, Sagnella GA, MacGregor GA. Brain and atrial natriuretic peptides: a dual peptide system of potential importance in sodium balance and blood pressure regulation in patients with essential hypertension. J Hypertens 1994;12:809‐13. [PubMed] [Google Scholar]
Bulpitt 1984 {published data only}
- Bulpitt CJ, Daymond M, Bulpitt PF, Ferrier G, Harrison R, Lewis PJ, Dollery CT. Is low salt dietary advice a useful therapy in hypertensive patients with poorly controlled blood pressure?. Annals of Clinical Research 1984;16(Suppl 43):143‐9. [PubMed] [Google Scholar]
Burke 2005 {published data only}
- Burke V, Beilin LJ, Cutt HE, Mansour J, Wilson A, Mori TA. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens 2005;23(6):1241‐9. [DOI] [PubMed] [Google Scholar]
Burke 2007 {published data only}
- Burke V, Beilin LJ, Cutt HE, Mansour J, Williams A, Mori TA. A lifestyle program for treated hypertensives improved health‐related behaviors and cardiovascular risk factors, a randomized controlled trial. J Clin Epidemiol 2007;60(2):133‐41. [DOI] [PubMed] [Google Scholar]
Burnier 1993 {published data only}
- Burnier M, Rutschmann B, Nussberger J, Versaggi J, Shahinfar S, Waeber B, Brunner HR. Salt‐dependent renal effects of an angiotensin II antagonist in healthy subjects. Hypertension 1993;22:339‐47. [DOI] [PubMed] [Google Scholar]
Buyck 2009 {published data only}
- Buyck JF, Blacher J, Kesse‐Guyot E, Castetbon K, Galan P, Safar M, Hercberg S, Czernichow S. Differential associations of dietary sodium and potassium intake with blood pressure: a focus on pulse pressure. Journal of Hypertension 2009;27(6):1158‐64. [DOI] [PubMed] [Google Scholar]
Calabrese 1985 {published data only}
- Calabrese EJ, Tuthill RW. The Massachusetts Blood Pressure Study, Part 3. Experimental reduction of sodium in drinking water: effects on blood pressure. Toxicology and industrial health 1985;1(1):19‐34. [DOI] [PubMed] [Google Scholar]
Cappuccio 2006 {published data only}
- Cappuccio, F. P.Kerry, S. M.Micah, F. B.Plange, R. J.Eastwood, J. B. A community programme to reduce salt intake and blood pressure in Ghana. BMC Public Health 2006;6:doi:10.1186/1471‐2458‐6‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carney 1991 {published data only}
- Carney SL, Gillies AH, Smith AJ, Smitham S. Increased dietary sodium chloride in patients treated with antihypertensive drugs. Clin Exp Hypertens 1991;13:401‐7. [DOI] [PubMed] [Google Scholar]
Charlton 2008 {published data only}
- Charlton KE, Steyn K, Levitt NS, Peer N, Jonathan D, Gogela T, Rossouw K, Gwebushe N, Lombard CJ. A food‐based dietary strategy lowers blood pressure in a low socio‐economic setting: A randomised study in South Africa. Public Health Nutrition 2008;11(12):1397‐1406. [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen J, Gu D, Jaquish CE, Chen CS, Rao DC, Liu D, Hixson JE, Hamm LL, Gu CC, Whelton PK, He J, GenSalt Collaborative Research Group. Association between blood pressure responses to the cold pressor test and dietary sodium intervention in a Chinese population. Archives of Internal Medicine 2008;168(16):1740‐1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chrysant 2000 {published data only}
- Chrysant SG, Weder AB, McCarron DA, Canossa‐Terris M, Cohen JD, Gunter PA, Hamilton BP, Lewin AJ, Mennella RF, Kirkegaard LW, Weir MR, Weinberger MH. Effects of isradipine or enalapril on blood pressure in salt‐sensitive hypertensives during low and high dietary salt intake. MIST II Trial Investigators. Am J Hypertens 2000;13(11):1180‐8. [DOI] [PubMed] [Google Scholar]
Cook 2005 {published data only}
- Cook NR, Kumanyika SK, Cutler JA, Whelton PK. Trials of Hypertension Prevention Collaborative Research Group. Dose‐response of sodium excretion and blood pressure change among overweight, nonhypertensive adults in a 3‐year dietary intervention study. Journal of Human Hypertension 2005;19(1):47‐54. [DOI] [PubMed] [Google Scholar]
Cook 2007 {published data only}
- Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow‐up of the trials of hypertension prevention (TOHP). British Medical Journal 2007;334(7599):885‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 2009 {published data only}
- Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK, Trials of Hypertension Prevention Collaborative Research Group. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: The trials of hypertension prevention follow‐up study. Archives of Internal Medicine 2009;169(1):32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1984 {published data only}
- Cooper R, Horn L, Liu K, Trevisan M, Nanas S, Ueshima H, Larbi E, Yu CS, Sempos C, LeGrady D, Stamler J. A randomized trial on the effect of decreased dietary sodium intake on blood pressure in adolescents. J Hypertens 1984;2:361‐6. [PubMed] [Google Scholar]
Costa 1981 {published data only}
- Costa FV, Ambrosioni E, Montebugnoli L, Paccaloni L, Vasconi L, Magnani B. Effects of a low‐salt diet and of acute salt loading on blood pressure and intralymphocytic sodium concentration in young subjects with borderline hypertension. Clin Sci 1981;61 (Suppl 7):21s‐23s. [DOI] [PubMed] [Google Scholar]
CSSS 2007 {published data only}
- The China Salt Substitute Study Collaborative Group. Salt substitution: a low‐cost strategy for blood pressure control among rural Chinese. A randomized, controlled trial. Journal of Hypertension 2007;25(10):2011‐8. [DOI] [PubMed] [Google Scholar]
Cuzzola 2001 {published data only}
- Cuzzola F, Mallamaci F, Tripepi G, Parlongo S, Cutrupi S, Cataliotti A, Stancanelli B, Malatino L, Bellanuova I, Ferri C, Galletti F, Filigheddu F, Glorioso N, Strazzullo P, Zoccali C. Urinary adrenomedullin is related to ET‐1 and salt intake in patients with mild essential hypertension. Salt Sensitivity Group of Italian Society of Hypertension. Am J Hypertens 2001;14:224‐30. [DOI] [PubMed] [Google Scholar]
Damasceno 1999 {published data only}
- Damasceno A, Santos A, Serrao P, Caupers P, Soares‐da‐Silva P, Polonia J. Deficiency of renal dopaminergic‐dependent natriuretic response to acute sodium load in black salt‐sensitive subjects in contrast to salt‐resistant subjects. J Hypertens 1999;17:1995‐2001. [DOI] [PubMed] [Google Scholar]
Damasceno 2000 {published data only}
- Damasceno A, Caupers P, Santos A, Lobo E, Sevene E, Bicho M, Polonia J. Influence of salt intake on the daytime‐nighttime blood pressure variation in normotensive and hypertensive black subjects. Revista Portuguesa de Cardiologia 2000;19(3):315‐29. [PubMed] [Google Scholar]
Davis 1994 {published data only}
- Davis BR, Oberman A, Blaufox MD, Wassertheil‐Smoller S, Zimbaldi N, Kirchner K, Wylie‐Rosett J, Langford HG. Lack of effectiveness of a low‐sodium/high‐potassium diet in reducing antihypertensive medication requirements in overweight persons with mild hypertension. Am J Hypertens 1994;7(10 Pt 1):926‐32. [DOI] [PubMed] [Google Scholar]
Davrath 1999 {published data only}
- Davrath LR, Gotshall RW, Tucker A, Sadeh WZ, Luckasen GJ, Downes TR, Coonts CC. Moderate sodium restriction does not alter lower body negative pressure tolerance. Aviat Space Environ Med 1999;70:577‐82. [PubMed] [Google Scholar]
Delemarre 2000 {published data only}
- Delemarre FM, Steegers EA. Dietary sodium restriction in rats and human beings. Am J Obstet Gynecol 2000;182:1647‐8. [DOI] [PubMed] [Google Scholar]
Del Rio 1990 {published data only}
- Rio A, Rodriguez‐Villamil JL, Lopez‐Campos JM, Carrera F. [Effect of moderate salt restriction on the antihypertensive action of nifedipine: a double blind study]. Revista Clinica Espanola 1990;186(1):5‐10. [PubMed] [Google Scholar]
Del Rio 1993 {published data only}
- Rio A, Rodriguez‐Villamil JL. Metabolic effects of strict salt restriction in essential hypertensive patients. J Intern Med 1993;233:409‐14. [DOI] [PubMed] [Google Scholar]
Dickinson 2009 {published data only}
- Dickinson KM, Keogh JB, Clifton PM. Effects of a low‐salt diet on flow‐mediated dilatation in humans. American Journal of Clinical Nutrition 2009;89(2):485‐90. [DOI] [PubMed] [Google Scholar]
Dimsdale 1990 {published data only}
- Dimsdale JE, Ziegler M, Mills P, Berry C. Prediction of salt sensitivity. Am J Hypertens 1990;3:429‐35. [DOI] [PubMed] [Google Scholar]
Dodson 1984 {published data only}
- Dodson PM, Pacy PJ, Bal P, Kubicki AJ, Fletcher RF, Taylor KG. A controlled trial of a high fibre, low fat and low sodium diet for mild hypertension in Type 2 (non‐insulin‐dependent) diabetic patients. Diabetologia 1984;27(5):522‐6. [DOI] [PubMed] [Google Scholar]
Dodson 1989 {published data only}
- Dodson PM, Beevers M, Hallworth R, Webberley MJ, Fletcher RF, Taylor KG. Sodium restriction and blood pressure in hypertensive type II diabetics: randomised blind controlled and crossover studies of moderate sodium restriction and sodium supplementation. BMJ 1989;298:227‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donovan 1993 {published data only}
- Donovan DS, Solomon CG, Seely EW, Williams GH, Simonson DC. Effect of sodium intake on insulin sensitivity. Am J Physiol 1993;264:E730‐4. [DOI] [PubMed] [Google Scholar]
Dubbert 1995 {published data only}
- Dubbert PM, Cushman WC, Meydrech EF, Rowland AK, Maury P. Effects of dietary instruction and sodium excretion feedback in hypertension clinic patients. Behav Ther 1995;26:721‐732. [Google Scholar]
Egan 1991 {published data only}
- Egan BM, Weder AB, Petrin J, Hoffman RG. Neurohumoral and metabolic effects of short‐term dietary NaCl restriction in men. Relationship to salt‐sensitivity status. Am J Hypertens 1991;4:416‐21. [DOI] [PubMed] [Google Scholar]
Egan 1991(b) {published data only}
- Egan BM, Petrin J, Hoffmann RG. NaCl induces differential changes of regional vascular reactivity in salt‐sensitive versus salt‐resistant men. Am J Hypertens 1991;4(12 Pt 1):924‐31. [DOI] [PubMed] [Google Scholar]
Ekinci 2009 {published data only}
- Ekinci EI, Thomas G, Thomas D, Johnson C, Macisaac RJ, Houlihan CA, Finch S, Panagiotopoulos S, O'Callaghan C, Jerums G. Effects of salt supplementation on the albuminuric response to telmisartan with or without hydrochlorothiazide therapy in hypertensive patients with type 2 diabetes are modulated by habitual dietary salt intake. Diabetes Care 2009;32(8):1398‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekinci 2010 {published data only}
- Ekinci EI, Thomas G, MacIsaac RJ, Johnson C, Houlihan C, Panagiotopoulos S, Premaratne E, Hao H, Finch S, O'Callaghan C, Jerums G. Salt supplementation blunts the blood pressure response to telmisartan with or without hydrochlorothiazide in hypertensive patients with type 2 diabetes. Diabetologia 2010;53(7):1295‐303. [DOI] [PubMed] [Google Scholar]
el Ashry 1987 {published data only}
- Ashry A, Heagerty AM, Alton SM, Bing RF, Swales JD, Thurston H. Effects of manipulation of sodium balance on erythrocyte sodium transport. J Hum Hypertens 1987;1:105‐11. [PubMed] [Google Scholar]
Elmer 1995 {published data only}
- Elmer PJ, Grimm R Jr, Laing B, Grandits G, Svendsen K, Heel N, Betz E, Raines J, Link M, Stamler J, et al. Lifestyle intervention: results of the Treatment of Mild Hypertension Study (TOMHS). Prev Med 1995;24(4):378‐88. [DOI] [PubMed] [Google Scholar]
Elmer 2006 {published data only}
- Elmer PJ, Obarzanek E, Vollmer WM, Simons‐Morton D, Stevens VJ, Young DR, Lin PH, Champagne C, Harsha DW, Svetkey LP, Ard J, Brantley PJ, Proschan MA, Erlinger TP, Appel LJ, PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18‐month results of a randomized trial. Annals of Internal Medicine 2006;144(7):485‐95. [DOI] [PubMed] [Google Scholar]
Fagerberg 1984 {published data only}
- Fagerberg B, Andersson OK, Isaksson B, Bjorntorp P. Blood pressure control during weight reduction in obese hypertensive men: separate effects of sodium and energy restriction. Br Med J (Clin Res Ed) 1984;288:11‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagerberg 1985 {published data only}
- Fagerberg B, Andersson OK, Lindstedt G, Waldenstrom J, Aurell M. The sodium intake modifies the renin‐aldosterone and blood pressure changes associated with moderately low energy diets. Acta Medica Scandinavica 1985;218(2):157‐64. [DOI] [PubMed] [Google Scholar]
Feldman 1996 {published data only}
- Feldman RD, Logan AG, Schmidt ND. Dietary salt restriction increases vascular insulin resistance. Clin Pharmacol Ther 1996;60:444‐51. [DOI] [PubMed] [Google Scholar]
Ferri 1993 {published data only}
- Ferri C, Francesco L, Baldoncini R, Bellini C, Desideri G, Carlomagno A, Siati L, Santucci A, Balsano F. [Sodium‐modulating hormones and the pressor response to sodium chloride in essential arterial hypertension]. Ann Ital Med Int 1993;8:89‐94. [PubMed] [Google Scholar]
Ferri 1994 {published data only}
- Ferri C, Bellini C, Carlomagno A, Perrone A, Santucci A. Urinary kallikrein and salt sensitivity in essential hypertensive males. Kidney International 1994;46(3):780‐8. [DOI] [PubMed] [Google Scholar]
Ferri 1996 {published data only}
- Ferri C, Bellini C, Carlomagno A, Desideri G, Santucci A. Active kallikrein response to changes in sodium‐chloride intake in essential hypertensive patients. J Am Soc Nephrol 1996;7:443‐53. [DOI] [PubMed] [Google Scholar]
Fliser 1993(a) {published data only}
- Fliser D, Nowack R, Allendorf‐Ostwald N, Kohl B, Hubinger A, Ritz E. Serum lipid changes on low salt diet. Effects of alpha 1‐adrenergic blockade. Am J Hypertens 1993;6:320‐4. [PubMed] [Google Scholar]
Fliser 1993(b) {published data only}
- Fliser D, Nowack R, Wolf G, Ritz E. Differential effects of ACE inhibitors and vasodilators on renal function curve in patients with primary hypertension. Blood Pressure 1993;2(4):296‐300. [DOI] [PubMed] [Google Scholar]
Forrester 2005 {published data only}
- Forrester T, Adeyemo A, Soarres‐Wynter S, Sargent L, Bennett F, Wilks R, Luke A, Prewitt E, Kramer H, Cooper RS. A randomized trial on sodium reduction in two developing countries. J Hum Hypertens 2005;19:55‐60. [DOI] [PubMed] [Google Scholar]
Fotherby 1997 {published data only}
- Fotherby MD, Potter JF. Metabolic and orthostatic blood pressure responses to a low‐sodium diet in elderly hypertensives. J Hum Hypertens 1997;11(6):361‐6. [DOI] [PubMed] [Google Scholar]
Friberg 1990 {published data only}
- Friberg P, Meredith I, Jennings G, Lambert G, Fazio V, Esler M. Evidence for increased renal norepinephrine overflow during sodium restriction in humans. Hypertension 1990;16:121‐30. [DOI] [PubMed] [Google Scholar]
Fuchs 1987 {published data only}
- Fuchs FD, Wannmacher CM, Wannmacher L, Guimaraes FS, Rosito GA, Gastaldo G, Hoeffel CP, Wagner EM. Effect of sodium intake on blood pressure, serum levels and renal excretion of sodium and potassium in normotensives with and without familial predisposition to hypertension. Braz J Med Biol Res 1987;20:25‐34. [PubMed] [Google Scholar]
Gillies 1984 {published data only}
- Gillies AH, Carney SL, Smith AJ, Waga SM. Adjunctive effect of salt restriction on antihypertensive efficacy. Clin Experiment Pharmacol Physiol 1984;11:395‐398. [DOI] [PubMed] [Google Scholar]
Gillum 1981 {published data only}
- Gillum RF, Elmer PJ, Prineas RJ. Changing sodium intake in children. The Minneapolis Children's Blood Pressure Study. Hypertension 1981;3:698‐703. [DOI] [PubMed] [Google Scholar]
Gomi 1998 {published data only}
- Gomi T, Shibuya Y, Sakurai J, Hirawa N, Hasegawa K, Ikeda T. Strict dietary sodium reduction worsens insulin sensitivity by increasing sympathetic nervous activity in patients with primary hypertension. Am J Hypertens 1998;11:1048‐55. [DOI] [PubMed] [Google Scholar]
Gow 1992 {published data only}
- Gow IF, Dockrell M, Edwards CR, Elder A, Grieve J, Kane G, Padfield PL, Waugh CJ, Williams BC. The sensitivity of human blood platelets to the aggregating agent ADP during different dietary sodium intakes in healthy men. Eur J Clin Pharmacol 1992;43:635‐8. [DOI] [PubMed] [Google Scholar]
Grey 1996 {published data only}
- Grey A, Braatvedt G, Holdaway I. Moderate dietary salt restriction does not alter insulin resistance or serum lipids in normal men. Am J Hypertens 1996;9:317‐22. [DOI] [PubMed] [Google Scholar]
Hargreaves 1989 {published data only}
- Hargreaves M, Morgan TO, Snow R, Guerin M. Exercise tolerance in the heat on low and normal salt intakes. Clin Sci 1989;76:553‐7. [DOI] [PubMed] [Google Scholar]
Haythornthwaite 1992 {published data only}
- Haythornthwaite JA, Pratley RE, Anderson DE. Behavioral stress potentiates the blood pressure effects of a high sodium intake. Psychosom Med 1992;54:231‐9. [DOI] [PubMed] [Google Scholar]
He, J 2009 {published data only}
- He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, Chen JC, Duan X, Huang JF, Chen CS, Kelly TN, Bazzano LA, Whelton PK, GenSalt Collaborative Research Group. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. Journal of Hypertension 2009;27(1):48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2000 {published data only}
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long‐term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000;35(2):544‐550. [DOI] [PubMed] [Google Scholar]
He 2005(a) {published data only}
- He FJ, Markandu ND, MacGregor GA. Modest salt reduction lowers blood pressure in isolated systolic hypertension and combined hypertension. Hypertension 2005;46(1):66‐70. [DOI] [PubMed] [Google Scholar]
He 2005(b) {published data only}
- He FJ, Markandu ND, Sagnella G A, Wardener HE, MacGregor G A. Plasma sodium: ignored and underestimated. Hypertension 2005;45(1):98‐102. [DOI] [PubMed] [Google Scholar]
Heagerty 1986 {published data only}
- Heagerty AM, Alton SM, el‐Ashry A, Bing RF, Thurston H, Swales JD. Effects of changes in sodium balance on leucocyte sodium transport: qualitative differences in normotensive offspring of hypertensives and matched controls. J Hypertens 1986;4:333‐7. [DOI] [PubMed] [Google Scholar]
Herlitz 1998 {published data only}
- Herlitz H, Dahlof B, Jonsson O, Friberg P. Relationship between salt and blood pressure in hypertensive patients on chronic ACE‐inhibition. Blood Pressure 1998;7(1):47‐52. [DOI] [PubMed] [Google Scholar]
Hofman 1983 {published data only}
- Hofman A, Hazebroek A, Valkenburg HA. A randomized trial of sodium intake and blood pressure in newborn infants. JAMA 1983;250:370‐3. [PubMed] [Google Scholar]
Houlihan 2002(a) {published data only}
- Houlihan CA, Akdeniz A, Tsalamandris C, Cooper ME, Jerums G, Gilbert RE. Urinary transforming growth factor‐beta excretion in patients with hypertension, type 2 diabetes, and elevated albumin excretion rate: effects of angiotensin receptor blockade and sodium restriction. Diabetes Care 2002;25(6):1072‐7. [DOI] [PubMed] [Google Scholar]
Houlihan 2002(b) {published data only}
- Houlihan CA, Allen TJ, Baxter AL, Panangiotopoulos S, Casley DJ, Cooper ME, Jerums G. A low‐sodium diet potentiates the effects of losartan in type 2 diabetes. Diabetes Care 2002;25(4):663‐71. [DOI] [PubMed] [Google Scholar]
Howe 1991 {published data only}
- Howe PR, Cobiac L, Smith RM. Lack of effect of short‐term changes in sodium intake on blood pressure in adolescent schoolchildren. J Hypertens 1991;9:181‐6. [DOI] [PubMed] [Google Scholar]
Howe1994 {published data only}
- Howe PR, Lungershausen YK, Cobiac L, Dandy G, Nestel PJ. Effect of sodium restriction and fish oil supplementation on BP and thrombotic risk factors in patients treated with ACE inhibitors. J Hum Hypertens 1994;8(1):43‐9. [PubMed] [Google Scholar]
HPTRG 1990 {published data only}
- Hypertension Prevention Trial Research Group. The Hypertension Prevention Trial: three‐year effects of dietary changes on blood pressure. Arch Intern Med 1990;150:153‐62. [PubMed] [Google Scholar]
Hu 2009 {published data only}
- Hu J, Jiang X, Li N, Yu X, Perkovic V, Chen B, Zhao L, Neal B, Wu Y. Effects of salt substitute on pulse wave analysis among individuals at high cardiovascular risk in rural China: a randomized controlled trial. Hypertension Research 2009;32(4):282‐8. [DOI] [PubMed] [Google Scholar]
Inoue 1996 {published data only}
- Inoue J, Cappuccio FP, Sagnella GA, Markandu ND, Folkerd EJ, Sampson B, Miller MA, Blackwood AM, MacGregor GA. Glucose load and renal sodium handling in mild essential hypertension on different sodium intakes. J Hum Hypertens 1996;10:523‐9. [PubMed] [Google Scholar]
Ireland 2010 {published data only}
- Ireland DM, Clifton PM, Keogh JB. Achieving the salt intake target of 6 g/day in the current food supply in free‐living adults using two dietary education strategies. Journal of the American Dietetic Association 2010;110(5):763‐7. [DOI] [PubMed] [Google Scholar]
Ishimitsu 1996 {published data only}
- Ishimitsu T, Nishikimi T, Matsuoka H, Kangawa K, Kitamura K, Minami J, Matsuo H, Eto T. Behaviour of adrenomedullin during acute and chronic salt loading in normotensive and hypertensive subjects. Clinical Science 1996;91:293‐8. [DOI] [PubMed] [Google Scholar]
Iso 1996 {published data only}
- Iso H, Shimamoto T, Yokota K, Sankai T, Jacobs DR Jr, Komachi Y. Community‐based education classes for hypertension control. A 1.5‐year randomized controlled trial. Hypertension 1996;27(4):968‐74. [DOI] [PubMed] [Google Scholar]
Iwaoka 1994 {published data only}
- Iwaoka T, Umeda T, Inoue J, Naomi S, Sasaki M, Fujimoto Y, Gui C, Ideguchi Y, Sato T. Dietary NaCl restriction deteriorates oral glucose tolerance in hypertensive patients with impairment of glucose tolerance. Am J Hypertens 1994;7:460‐3. [DOI] [PubMed] [Google Scholar]
Jessani 2008 {published data only}
- Jessani S, Hatcher J, Chaturvedi N, Jafar TH. Effect of low vs. high dietary sodium on blood pressure levels in a normotensive Indo‐Asian population. American Journal of Hypertension 2008;21(11):1238‐44. [DOI] [PubMed] [Google Scholar]
Johnson 2001 {published data only}
- Johnson AG, NguyenTV, Davis D. Blood pressure is linked to salt intake and modulated by the angiotensinogen gene in normotensive and hypertensive elderly subjects. Journal of Hypertension 2001;19(6):1053‐60. [DOI] [PubMed] [Google Scholar]
Jula 1990 {published data only}
- Jula A, Ronnemaa T, Rastas M, Karvetti RL, Maki J. Long‐term nopharmacological treatment for mild to moderate hypertension. J Intern Med 1990;227:413‐21. [DOI] [PubMed] [Google Scholar]
Jula 1992(a) {published data only}
- Jula AM, Ronnemaa TE, Piha SJ, Maki JP. Response of diastolic blood pressure to long‐term sodium restriction is posture related. Scand J Clin Lab Invest 1992;52:159‐67. [DOI] [PubMed] [Google Scholar]
Jula 1992(b) {published data only}
- Jula AM, Rönnemaa T, Tikkanen I, Karanko HM. Responses of atrial natriuretic factor to long‐term sodium restriction in mild to moderate hypertension. Journal of Internal Medicine 1992;231(5):521‐529. [DOI] [PubMed] [Google Scholar]
Kawasaki 1998 {published data only}
- Kawasaki T, Itoh K, Kawasaki M. Reduction in blood pressure with a sodium‐reduced, potassium‐ and magnesium‐enriched mineral salt in subjects with mild essential hypertension. Hypertens Res 1998;21(4):235‐43. [DOI] [PubMed] [Google Scholar]
Keven 2006 {published data only}
- Keven K, YalÇin S, Canbakan B, Kutlay S, Sengül S, Erturk S, Erbay B. The impact of daily sodium intake on posttransplant hypertension in kidney allograft recipients. Transplantation Proceedings 2006;38(5):1323‐6. [DOI] [PubMed] [Google Scholar]
Kirpizidis 2005 {published data only}
- Kirpizidis H, Stavrati A, Geleris P. Assessment of quality of life in a randomized clinical trial of candesartan only or in combination with DASH diet for hypertensive patients. Journal of Cardiology 2005;46(5):177‐82. [PubMed] [Google Scholar]
Kojuri 2007 {published data only}
- Kojuri J, Rahimi R. Effect of "no added salt diet" on blood pressure control and 24 hour urinary sodium excretion in mild to moderate hypertension. BMC Cardiovascular Disorders 2007;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koolen 1984 (a) {published data only}
- Koolen MI, Brummelen P. Sodium sensitivity in essential hypertension: role of the renin‐angiotensin‐aldosterone system and predictive value of an intravenous frusemide test. J Hypertens 1984;2:55‐9. [DOI] [PubMed] [Google Scholar]
Koolen 1984 (b) {published data only}
- Koolen MI, Brummelen P. Adrenergic activity and peripheral hemodynamics in relation to sodium sensitivity in patients with essential hypertension. Hypertension 1984;6:820‐5. [DOI] [PubMed] [Google Scholar]
Koopman 1990 (a) {published data only}
- Koopman H, Spreeuwenberg C, Westerman RF, Donker AJ. Dietary treatment of patients with mild to moderate hypertension in a general practice: a pilot intervention study (1). The first three months. J Hum Hypertens 1990;4:368‐71. [PubMed] [Google Scholar]
Koopman 1990 (b) {published data only}
- Koopman H, Spreeuwenberg C, Westerman RF, Donker AJ. Dietary treatment of patients with mild to moderate hypertension in a general practice: a pilot intervention study (2). Beyond three months. J Hum Hypertens 1990;4:372‐4. [PubMed] [Google Scholar]
Koopman 1997 {published data only}
- Koopman H, Devillė W, Eijk JT, Donker AJ, Spreeuwenberg C. Diet or diuretic? Treatment of newly diagnosed mild to moderate hypertension in the elderly. J Hum Hypertens 1997;11(12):807‐12. [DOI] [PubMed] [Google Scholar]
Kostis 2002 {published data only}
- Kostis JB, Wilson AC, Shindler DM, Cosgrove NM, Lacy CR. Persistence of normotension after discontinuation of lifestyle intervention in the trial of TONE. Am J Hypertens 2002;15(8):732‐734. [DOI] [PubMed] [Google Scholar]
Kristinsson 1988 {published data only}
- Kristinsson A, Hardarson T, Palsson K, Petursson MK, Snorrason SP, Thorgeirsson G. Additive effects of moderate dietary salt reduction and captopril in hypertension. Acta Med Scand 1988;223(2):133‐7. [DOI] [PubMed] [Google Scholar]
Kumanyika 1993 {published data only}
- Kumanyika SK, Hebert PR, Cutler JA, Lasser VI, Sugars CP, Steffen‐Batey L, Brewer AA, Cameron M, Shepek LD, Cook NR, et al. Feasibility and efficacy of sodium reduction in the Trials of Hypertension Prevention, phase I. Trials of Hypertension Prevention Collaborative Research Group. Hypertension 1993;22(4):502‐512. [DOI] [PubMed] [Google Scholar]
Kumanyika 2005 {published data only}
- Kumanyika SK, Cook NR, Cutler JA, Belden L, Brewer A, Cohen JD, Hebert PR, Lasser VI, Raines J, Raczynski J, Shepek L, Diller L, Whelton PK, Yamamoto M, Trials of Hypertension Prevention Collaborative Research Group. Sodium reduction for hypertension prevention in overweight adults: further results from the Trials of Hypertension Prevention Phase II. Journal of Human Hypertension 2005;19(1):33‐45. [DOI] [PubMed] [Google Scholar]
Kurtz 1987 {published data only}
- Kurtz TW, Al‐Bander HA, Morris RC Jr. "Salt‐sensitive" essential hypertension in men. Is the sodium ion alone important?. N Engl J Med 1987;317:1043‐8. [DOI] [PubMed] [Google Scholar]
Lawton 1988 {published data only}
- Lawton WJ, Sinkey CA, Fitz AE, Mark AL. Dietary salt produces abnormal renal vasoconstrictor responses to upright posture in borderline hypertensive subjects. Hypertension 1988;11:529‐36. [DOI] [PubMed] [Google Scholar]
Logan 1986 {published data only}
- Logan AG. Sodium manipulation in the management of hypertension. The view against its general use. Can J Physiol Pharmacol 1986;64:793‐802. [DOI] [PubMed] [Google Scholar]
Luft 1990 {published data only}
- Luft FC, Zemel MB, Sowers JA, Fineberg NS, Weinberger MH. Sodium bicarbonate and sodium chloride: effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. J Hypertens 1990;8:663‐70. [DOI] [PubMed] [Google Scholar]
Macgregor 1982 {published data only}
- Macgregor GA, Markandu ND, Sagnella GA. Dietary sodium restriction in normotensive subjects and patients with essential hypertension. Clin‐Sci 1982;63:399s‐402s. [PubMed] [Google Scholar]
MacGregor 1987 {published data only}
- MacGregor GA, Markandu ND, Singer DR, Cappuccio FP, Shore AC, Sagnella GA. Moderate sodium restriction with angiotensin converting enzyme inhibitor in essential hypertension: a double blind study. Br Med J (Clin Res Ed) 1987;294:531‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mallamaci 1996 {published data only}
- Mallamaci F, Leonardis D, Bellizzi V, Zoccali C. Does high salt intake cause hyperfiltration in patients with essential hypertension?. J Hum Hypertens 1996;10:157‐61. [PubMed] [Google Scholar]
Manunta 2001 {published data only}
- Manunta P, Messaggio E, Ballabeni C, Sciarrone MT, Lanzani C, Ferrandi M, Hamlyn JM, Cusi D, Galletti F, Bianchi G. Salt Sensitivity Study Group of the Italian Society of Hypertension. Plasma ouabain‐like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension 2001;38:198‐203. [DOI] [PubMed] [Google Scholar]
Mark 1975 {published data only}
- Mark AL, Lawton WJ, Abboud FM, Fitz AE, Connor WE, Heistad DD. Effects of high and low sodium intake on arterial pressure and forearm vasular resistance in borderline hypertension. A preliminary report. Circ Res 1975;36 (6 Suppl 1):194‐8. [DOI] [PubMed] [Google Scholar]
Mattila 2003 {published data only}
- Mattila R, Malmivaara A, Kastarinen M, Kivela SL, Nissinen A. Effectiveness of multidisciplinary lifestyle intervention for hypertension: a randomised controlled trial. J Hum Hypertens 2003;17:199‐205. [DOI] [PubMed] [Google Scholar]
Maxwell 1984 {published data only}
- Maxwell MH, Kushiro T, Dornfeld LP, Tuck ML, Waks AU. BP changes in obese hypertensive subjects during rapid weight loss. Comparison of restricted v unchanged salt intake. Arch Intern Med 1984;144:1581‐4. [PubMed] [Google Scholar]
McCarron 1997 {published data only}
- McCarron DA, Weder AB, Egan BM, Krishna GG, Morris CD, Cohen M, Oparil S. Blood pressure and metabolic responses to moderate sodium restriction in isradipine‐treated hypertensive patients. Am J Hypertens 1997;10:68‐76. [DOI] [PubMed] [Google Scholar]
Meland 2009 {published data only}
- Meland E, Aamland A. Salt restriction among hypertensive patients: modest blood pressure effect and no adverse effects. Scandinavian Journal of Primary Health Care 2009;27(2):97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1988 {published data only}
- Miller JZ, Weinberger MH, Daugherty SA, Fineberg NS, Christian JC, Grim CE. Blood pressure response to dietary sodium restriction in healthy normotensive children. Am J Clin Nutr 1988;47:113‐9. [DOI] [PubMed] [Google Scholar]
Miller 1997 {published data only}
- Miller JA. Renal responses to sodium restriction in patients with early diabetes mellitus. J Am Soc Nephrol 1997;8:749‐55. [DOI] [PubMed] [Google Scholar]
Morgan 1978 {published data only}
- Morgan T, Adam W, Gillies A, Wilson M, Morgan G, Carney S. Hypertension treated by salt restriction. Lancet 1978;1:227‐30. [DOI] [PubMed] [Google Scholar]
Morgan 1988 {published data only}
- Morgan T, Anderson A. Interaction in hypertensive man between sodium intake, converting enzyme inhibitor (enalapril), plasma renin and blood pressure control. J Hum Hypertens 1988;1:311‐5. [PubMed] [Google Scholar]
Mtabaji 1990 {published data only}
- Mtabaji JP, Nara Y, Yamori Y. The cardiac study in Tanzania: salt intake in the causation and treatment of hypertension. J Hum Hypertens 1990;4:80‐1. [PubMed] [Google Scholar]
Mu 2009 {published data only}
- Mu J, Liu Z, Liu F, Xu X, Liang Y, Zhu D. Family‐based randomized trial to detect effects on blood pressure of a salt substitute containing potassium and calcium in hypertensive adolescents. American Journal of Hypertension 2009;22(9):943‐7. [DOI] [PubMed] [Google Scholar]
Mufunda 1992 {published data only}
- Mufunda J, Chimoskey JE, Matenga J, Musabayane C, Sparks HV Jr. Blood pressure response to acute changes in dietary sodium in young Zimbabwean men. J Hypertens 1992;10(3):279‐85. [DOI] [PubMed] [Google Scholar]
Muhlhauser 1996 {published data only}
- Muhlhauser I, Prange K, Sawicki PT, Bender R, Dworschak A, Schaden W, Berger M. Effects of dietary sodium on blood pressure in IDDM patients with nephropathy. Diabetologia 1996;39:212‐219. [DOI] [PubMed] [Google Scholar]
Mülhauser 1996 {published data only}
- Mülhauser I, Prange K, Sawicki PT, Bender R, Dworschak A, Schaden W, Berger M. Effects of dietary sodium on blood pressure in IDDM patients with nephropathy. Diabetologia 1996;39(2):212‐9. [DOI] [PubMed] [Google Scholar]
Myers 1983 {published data only}
- Myers J, Morgan T. The effect of sodium intake on the blood pressure related to age and sex. Clin Exp Hypertens 1983;A5:99‐118. [DOI] [PubMed] [Google Scholar]
Nakamura 2003 {published data only}
- Nakamura M, Aoki N, Yamada T, Kubo N. Feasibility and effect on blood pressure of 6‐week trial of low sodium soy sauce and miso (fermented soybean paste). Circ J 2003;67:530‐4. [DOI] [PubMed] [Google Scholar]
Nowson 1988 {published data only}
- Nowson CA, Morgan TO. Change in blood pressure in relation to change in nutrients effected by manipulation of dietary sodium and potassium. Clin Exp Pharmacol Physiol 1988;15:225‐42. [DOI] [PubMed] [Google Scholar]
Nowson 2003 {published data only}
- Nowson CA, Morgan TO, Gibbons C. Decreasing dietary sodium while following a self‐selected potassium‐rich diet reduces blood pressure. J Nutr 2003;133:4118‐23. [DOI] [PubMed] [Google Scholar]
Nowson 2004 {published data only}
- Nowson CA, Worsley A, Margerison C, Jorna MK, Frame AG, Torres SJ, Godfrey SJ. Blood pressure response to dietary modifications in free‐living individuals. J Nutr 2004;134:2322‐9. [DOI] [PubMed] [Google Scholar]
Nowson 2009 {published data only}
- Nowson CA, Wattanapenpaiboon N, Pachett A. Low‐sodium Dietary Approaches to Stop Hypertension‐type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res 2009;29:8‐18. [DOI] [PubMed] [Google Scholar]
Omland 2001 {published data only}
- Omland T, Johnson W, Gordon MB, Creager MA. Endothelial function during stimulation of renin‐angiotensin system by low‐sodium diet in humans. Am J Physiol Heart Circ Physiol 2001;280(5):H2248‐54. [DOI] [PubMed] [Google Scholar]
Omvik 1995 {published data only}
- Omvik P, Myking OL. Unchanged central hemodynamics after six months of moderate sodium restriction with or without potassium supplement in essential hypertension. Blood Pressure 1995;4(1):32‐41. [DOI] [PubMed] [Google Scholar]
Overlack 1993 {published data only}
- Overlack A, Ruppert M, Kolloch R, Gobel B, Kraft K, Diehl J, Schmitt W, Stumpe KO. Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Hypertension 1993;22:331‐8. [DOI] [PubMed] [Google Scholar]
Overlack 1995 {published data only}
- Overlack A, Ruppert M, Kolloch R, Kraft K, Stumpe KO. Age is a major determinant of the divergent blood pressure responses to varying salt intake in essential hypertension. Am J Hypertens 1995;8:829‐36. [DOI] [PubMed] [Google Scholar]
Palacios 2004 {published data only}
- Palacios C, Wigertz K, Martin BR, Jackman L, Pratt JH, Peacock M, McCabe G, Weaver CM. Sodium retention in black and white female adolescents in response to salt intake. J Clin Endocrinol Metab 2004;89:1858‐63. [DOI] [PubMed] [Google Scholar]
Palmer 1989 {published data only}
- Palmer RM, Osterweil D, Loon‐Lustig G, Stern N. The effect of dietary salt ingestion on blood pressure of old‐old subjects. A double‐blind, placebo‐controlled, crossover trial. J Am Geriatr Soc 1989;37:931‐6. [DOI] [PubMed] [Google Scholar]
Parfrey 1981 {published data only}
- Parfrey PS, Condon K, Wright P, Vandenburg MJ, Holly JM, Goodwin FJ, Evans SJ, Ledingham JM. Blood pressure and hormonal changes following alteration in dietary sodium and potassium in young men with and without a familial predisposition to hypertension. Lancet 1981;1(8212):113‐7. [DOI] [PubMed] [Google Scholar]
Parker 1990 {published data only}
- Parker M, Puddey IB, Beilin LJ, Vandongen R. Two‐way factorial study of alcohol and salt restriction in treated hypertensive men. Hypertension 1990;16:398‐406. [DOI] [PubMed] [Google Scholar]
Pechere‐Bertschi 2008 {published data only}
- Pechere‐Bertschi A, Maillard M, Bischof P, Fathi M, Burnier M. Hemodynamic effect of angiotensin II receptor blockade in postmenopausal women on a high‐sodium diet: A double‐blind, randomized, placebo‐controlled study. Current Therapeutic Research ‐ Clinical and Experimental 2008;69(6):467‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 1986 {published data only}
- Pedersen KE, Jest P, Klitgaard NA, Rokkedal Nielsen J, Johansen T. Effect of oral salt loading on blood pressure and lymphocyte sodium metabolism in borderline hypertension. Acta Med Scand 1986;714 (Suppl):81‐5. [DOI] [PubMed] [Google Scholar]
Perry 2003 {published data only}
- Perry CG, Palmer T, Cleland SJ, Morton IJ, Salt IP, Petrie JR, Gould GW, Connell JM. Decreased insulin sensitivity during dietary sodium restriction is not mediated by effects of angiotensin II on insulin action. Clin Sci (Lond). 2003;105(2):187‐94. [DOI] [PubMed] [Google Scholar]
Petrie 1998 {published data only}
- Petrie JR, Morris AD, Minamisawa K, Hilditch TE, Elliott HL, Small M, McConnell J. Dietary sodium restriction impairs insulin sensitivity in noninsulin‐dependent diabetes mellitus. J Clin Endocrinol Metab 1998;83:1552‐7. [DOI] [PubMed] [Google Scholar]
Pimenta 2009 {published data only}
- Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, Calhoun DA. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension 2009;54(3):475‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pogson 2009 {published data only}
- Pogson ZE, McKeever TM, Lewis SA, Pacey SJ, Antoniak MD, Britton JR, Fogarty AW. Does a low sodium diet modify heart rate variability? A randomised placebo‐controlled double‐blind trial. International Journal of Cardiology 2009;135(3):390‐3. [DOI] [PubMed] [Google Scholar]
Pomeranz 2002 {published data only}
- Pomeranz A, Dolfin T, Korzets Z, Eliakim A, Wolach B. Increased sodium concentrations in drinking water increase blood pressure in neonates. J Hypertens 2002;20:203‐7. [DOI] [PubMed] [Google Scholar]
Redon 1995 {published data only}
- Reódn J, Lozano JV, Figuera M, Rodriguez JC, Garrido J, Alés‐Marítnez JE, Alvarez‐Cantalapiedra I, Velasco‐Quintana J. Do changes in dietary salt influence blood pressure of hypertensive patients pharmacologically controlled with verapamil? The Salt‐Switching‐Study (SSS). J Hum Hypertens 1995;9(2):143‐7. [PubMed] [Google Scholar]
Redon‐Mas 1993 {published data only}
- Redon‐Mas J, Abellan‐Aleman J, Aranda‐Lara P, Figuera‐von Wichmann M, Luque‐Otero M, Rodicio‐Diaz JL, Ruilope‐Urioste LM, Velasco‐Quintana J. The VERSAL Study Group. Antihypertensive activity of verapamil: impact of dietary sodium. J Hypertens 1993;11:665‐71. [DOI] [PubMed] [Google Scholar]
Richards 1986 {published data only}
- Richards AM, Tonolo G, Cleland JG, Leckie BJ, McIntyre GD, Ingram M, Dargie HJ, Ball SG, Robertson JI. Plasma atrial natriuretic peptide: responses to modest and severe sodium restriction. J Hypertens (Suppl) 1986;4:S559‐63. [PubMed] [Google Scholar]
Ruilope 1992 {published data only}
- Ruilope LM, Casal MC, Guerrero L, Alcázar JM, Férnandez ML, Lahera V, Rodicio JL. Sodium intake does not influence the effect of verapamil in hypertensive patients with mild renal insufficiency. Drugs 1992;44(Suppl 1):94‐8. [DOI] [PubMed] [Google Scholar]
Ruilope 1993 {published data only}
- Ruilope LM, Lahera V. Influence of salt intake on the antihypertensive effect of carvedilol. J Hypertens Suppl 1993;11:S17‐9. [PubMed] [Google Scholar]
Ruppert 1991 {published data only}
- Ruppert M, Diehl J, Kolloch R, Overlack A, Kraft K, Gobel B, Hittel N, Stumpe KO. Short‐term dietary sodium restriction increases serum lipids and insulin in salt‐sensitive and salt‐resistant normotensive adults. Klin Wochenschr 1991;69 (Suppl 25):51‐7. [PubMed] [Google Scholar]
Ruppert 1994 {published data only}
- Ruppert M, Overlack A, Kolloch R, Kraft K, Lennarz M, Stumpe KO. Effects of severe and moderate salt restriction on serum lipids in nonobese normotensive adults. Am J Med Sci 1994;307 (Suppl 1):S87‐90. [PubMed] [Google Scholar]
Salvetti 1988 {published data only}
- Salvetti A, Bichisao E, Caiazza A, Bartolomei G, Cagianelli MA, Federighi G, Innocenti P, Loni C, Ferrari E, Saba G, et al. The combination of a low‐Na/high‐K salt with metoprolol in the treatment of mild‐moderate hypertension. A multicenter study. Am J Hypertens 1988;3 Pt 3:201S‐205S. [DOI] [PubMed] [Google Scholar]
Santello 1997 {published data only}
- Santello JL, Dichtchekenian V, Heimann JC. Effect of long‐term blood pressure control on salt sensitivity. J Med 1997;28(3‐4):147‐58. [PubMed] [Google Scholar]
Saptharishi 2009 {published data only}
- Saptharishi L, Soudarssanane M, Thiruselvakumar D, Navasakthi D, Mathanraj S, Karthigeyan M, Sahai A. Community‐based Randomized Controlled Trial of Non‐pharmacological Interventions in Prevention and Control of Hypertension among Young Adults. Indian Journal of Community Medicine 2009;34(4):329‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmid 1990 {published data only}
- Schmid M, Mann JF, Stein G, Herter M, Nussberger J, Klingbeil A, Ritz E. Natriuresis‐pressure relationship in polycystic kidney disease. J Hypertens 1990;8:277‐83. [PubMed] [Google Scholar]
Schorr 1997(a) {published data only}
- Schorr U, Beige J, Ringel J, Turan S, Kreutz R, Distler A, Sharma AM. Hpa II polymorphism of the atrial natriuretic peptide gene and the blood pressure response to salt intake in normotensive men. J Hypertens 1997;15:715‐8. [DOI] [PubMed] [Google Scholar]
Schorr 1997(b) {published data only}
- Schorr U, Turan S, Distler A, Sharma AM. Relationship between ambulatory and resting blood pressure responses to dietary salt restriction in normotensive men. Journal of Hypertension 1997;15(8):845‐9. [DOI] [PubMed] [Google Scholar]
Sciarrone 1990 {published data only}
- Sciarrone SE, Rouse IL, Rogers P, Beilin LJ. A factorial study of fat and fibre changes and sodium restriction on blood pressure of human hypertensive subjects. Clin Exp Pharmacol Physiol 1990;17(3):197‐201. [DOI] [PubMed] [Google Scholar]
Sciarrone 1992 {published data only}
- Sciarrone SE, Beilin LJ, Rouse IL, Rogers PB. A factorial study of salt restriction and a low‐fat/high‐fibre diet in hypertensive subjects. J Hypertens 1992;10:287‐98. [DOI] [PubMed] [Google Scholar]
Sharma 1990 {published data only}
- Sharma AM, Arntz HR, Kribben A, Schattenfroh S, Distler A. Dietary sodium restriction: adverse effect on plasma lipids. Klin Wochenschr 1990;68:664‐8. [DOI] [PubMed] [Google Scholar]
Sharma 1991 {published data only}
- Sharma AM, Ruland K, Spies KP, Distler A. Salt sensitivity in young normotensive subjects is associated with a hyperinsulinemic response to oral glucose. J Hypertens 1991;9:329‐35. [DOI] [PubMed] [Google Scholar]
Sharma 1993 {published data only}
- Sharma AM, Schorr U, Distler A. Insulin resistance in young salt‐sensitive normotensive subjects. Hypertension 1993;21:273‐9. [DOI] [PubMed] [Google Scholar]
Shore 1988 {published data only}
- Shore AC, Markandu ND, MacGregor GA. A randomized crossover study to compare the blood pressure response to sodium loading with and without chloride in patients with essential hypertension. J Hypertens 1988;6:613‐7. [DOI] [PubMed] [Google Scholar]
Sinaiko 1993 {published data only}
- Sinaiko AR, Gomez‐Marin O, Prineas RJ. Effect of low sodium diet or potassium supplementation on adolescent blood pressure. Hypertension 1993;21:989‐94. [DOI] [PubMed] [Google Scholar]
Singer 1991 {published data only}
- Singer DR, Markandu ND, Sugden AL, Miller MA, MacGregor GA. Sodium restriction in hypertensive patients treated with a converting enzyme inhibitor and a thiazide. Hypertension 1991;17:798‐803. [DOI] [PubMed] [Google Scholar]
Singer 1994 {published data only}
- Singer DR, Markandu ND, Buckley MG, Miller MA, Sagnella GA, Lachno DR, Cappuccio FP, Murday A, Yacoub MH, MacGregor GA. Blood pressure and endocrine responses to changes in dietary sodium intake in cardiac transplant recipients. Implications for the control of sodium balance. Circulation 1994;89(3):1153‐9. [DOI] [PubMed] [Google Scholar]
Singer 1995 {published data only}
- Singer DR, Markandu ND, Cappuccio FP, Miller MA, Sagnella GA, MacGregor GA. Reduction of salt intake during converting enzyme inhibitor treatment compared with addition of a thiazid. Hypertension 1995;25(5):1042‐4. [DOI] [PubMed] [Google Scholar]
Skrabal 1980 {published data only}
- Skrabal F, Aubock J, Hortnagl H, Braunsteiner H. Effect of moderate salt restriction and high potassium intake on pressor hormones, response to noradrenaline and baroreceptor function in man. Clinical Science 1980;59 Suppl 6:157s‐160s. [DOI] [PubMed] [Google Scholar]
Skrabal 1981 {published data only}
- Skrabal F, Aubock J, Hortnagl H. Low sodium/high potassium diet for prevention of hypertension: probable mechanisms of action. Lancet 1981;2:895‐900. [DOI] [PubMed] [Google Scholar]
Skrabal 1984(a) {published data only}
- Skrabal F, Herholz H, Neumayr M, Hamberger L, Ledochowski M, Sporer H, Hortnagl H, Schwarz S, Schonitzer D. Salt sensitivity in humans is linked to enhanced sympathetic responsiveness and to enhanced proximal tubular reabsorption. Hypertension 1984;6:152‐8. [PubMed] [Google Scholar]
Skrabal 1984(b) {published data only}
- Skrabal F, Gasser RW, Finkenstedt G, Rhomberg HP, Lochs, A. Low‐sodium diet versus low‐sodium/high‐potassium diet for treatment of hypertension. Klinische Wochenschrift 1984;62(3):124‐8. [DOI] [PubMed] [Google Scholar]
Skrabal 1985 {published data only}
- Skrabal F, Hamberger L, Cerny E. Salt sensitivity in normotensives with and salt resistance in normotensives without heredity of hypertension. Scand J Clin Lab Invest 1985;176 (Suppl):47‐57. [PubMed] [Google Scholar]
Staessen 1988 {published data only}
- Staessen J, Bulpitt CJ, Fagard R, Joossens JV, Lijnen P, Amery A. Salt intake and blood pressure in the general population: a controlled intervention trial in two towns. J Hypertens 1988;6(12):965‐73. [DOI] [PubMed] [Google Scholar]
Starmans‐Kool 2011 {published data only}
- Starmans‐Kool MJ, Stanton AV, Xu YY, Mc GTSA, Parker KH, Hughes AD. High dietary salt intake increases carotid blood pressure and wave reflection in normotensive healthy young men. J Appl Physiol 2011;110:468‐471. [DOI] [PubMed] [Google Scholar]
Steegers 1991 {published data only}
- Steegers EA, Lakwijk HP, Jongsma HW, Fast JH, Boo T, Eskes TK, Hein PR. (Patho)physiological implications of chronic dietary sodium restriction during pregnancy; a longitudinal prospective randomized study. Br J Obstet Gynaecol 1991;98:980‐7. [DOI] [PubMed] [Google Scholar]
Sullivan 1980 {published data only}
- Sullivan JM, Ratts TE, Taylor JC, Kraus DH, Barton BR, Patrick DR, Reed SW. Hemodynamic effects of dietary sodium in man: a preliminary report. Hypertension 1980;2:506‐14. [DOI] [PubMed] [Google Scholar]
Suppa 1988 {published data only}
- Suppa G, Pollavini G, Alberti D, Savonitto S. Effects of a low‐sodium high‐potassium salt in hypertensive patients treated with metoprolol: a multicentre study. J Hypertens 1988;6(10):787‐90. [PubMed] [Google Scholar]
Suzuki 2000 {published data only}
- Suzuki M, Kimura Y, Tsushima M, Harano Y. Association of insulin resistance with salt sensitivity and nocturnal fall of blood pressure. Hypertension 2000;35:864‐8. [DOI] [PubMed] [Google Scholar]
Takahashi 2006 {published data only}
- Takahashi Y, Sasaki S, Okubo S, Hayashi M, Tsugane S. Blood pressure change in a free‐living population‐based dietary modification study in Japan. Journal of Hypertension 2006;24(3):451‐8. [DOI] [PubMed] [Google Scholar]
Takashashi 2003 {published data only}
- Takashashi Y, Sasaki S, Takahashi M, Okubo S, Hayashi M, Tsugane S. A population‐based dietary intervention trial in a high‐risk area for stomach cancer and stroke: changes in intakes and related biomarkers. Prev Med 2003;37(5):432‐41. [DOI] [PubMed] [Google Scholar]
Teow 1985 {published data only}
- Teow BH, Nicolantonio R, Morgan TO. Sodium chloride preference and recognition threshold in normotensive subjects on high and low salt diet. Clin Exp Hypertens 1985‐86;A7:1681‐95. [DOI] [PubMed] [Google Scholar]
Thaler 1982 {published data only}
- Thaler BI, Paulin JM, Phelan EL, Simpson FO. A pilot study to test the feasibility of salt restriction in a community. N Z Med J 1982;95:839‐42. [PubMed] [Google Scholar]
TMHRG 1991 {published data only}
- TMHRG. The Treatment of Mild Hypertension Research Group. The treatment of mild hypertension study. A randomized, placebo‐controlled trial of a nutritional‐hygienic regimen along with various drug monotherapies. Archives of Internal Medicine 1991;151(7):1413‐1423. [DOI] [PubMed] [Google Scholar]
Todd 2010 {published data only}
- Todd AS, Macginley RJ, Schollum JB, Johnson RJ, Williams SM, Sutherland WH, Mann JI, Walker RJ. Dietary salt loading impairs arterial vascular reactivity. American Journal of Clinical Nutrition 2010;91(3):557‐64. [DOI] [PubMed] [Google Scholar]
Todd 2012 {published data only}
- Todd AS, Macginley RJ, Schollum JB, Williams SM, Sutherland WH, Mann JI, Walker RJ. Dietary sodium loading in normotensive healthy volunteers does not increase arterial vascular reactivity or blood pressure. Nephrology 2012;17:249‐256. [DOI] [PubMed] [Google Scholar]
Townsend 2007 {published data only}
- Townsend RR, Kapoor S, McFadden CB. Salt intake and insulin sensitivity in healthy human volunteers. Clinical Science 2007;113:141‐148. [DOI] [PubMed] [Google Scholar]
Tzemos 2008 {published data only}
- Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 2008;51(6):1525‐30. [DOI] [PubMed] [Google Scholar]
Uusitupa 1996 {published data only}
- Uusitupa M, Korhonen M, Litmanen H, Niskanen L, Vaisanen S, Rauramaa R. Effects of moderate salt restriction alone and in combination with cilazapril on office and ambulatory blood pressure. J Hum Hypertens 1996;10(5):319‐326. [PubMed] [Google Scholar]
Uzu 1999 {published data only}
- Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Determinants of circadian blood pressure rhythm in essential hypertension. Am J Hypertens 1999;12(1 Pt 1):35‐39. [DOI] [PubMed] [Google Scholar]
Uzu 2009 {published data only}
- Uzu T, Sakaguchi M, Yokomaku Y, Kume S, Kanasaki M, Isshiki K, Araki S, Sugiomoto T, Koya D, Haneda M, Kashiwagi A. Effects of high sodium intake and diuretics on the circadian rhythm of blood pressure in type 2 diabetic patients treated with an angiotensin II receptor blocker. Clinical and Experimental Nephrology 2009;13(4):300‐306. [DOI] [PubMed] [Google Scholar]
Vaidya 2009 {published data only}
- Vaidya A, Bentley‐Lewis R, Jeunemaitre X, Adler GK, Williams JS. Dietary sodium alters the prevalence of electrocardiogram determined left ventricular hypertrophy in hypertension. American Journal of Hypertension 2009;22(6):669‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
van BergeLandry 2004 {published data only}
- Berge‐Landry H, James GD. Serum electrolyte, serum protein, serum fat and renal responses to a dietary sodium challenge: allostasis and allostatic load. Ann Hum Biol 2004;31:477‐87. [DOI] [PubMed] [Google Scholar]
van Paassen 1996 {published data only}
- Paassen P, Zeeuw D, Navis G, Jong PE. Does the renin‐angiotensin system determine the renal and systemic hemodynamic response to sodium in patients with essential hypertension?. Hypertension 1996;27:202‐8. [DOI] [PubMed] [Google Scholar]
Vedovato 2004 {published data only}
- Vedovato M, Lepore G, Coracina A, Dodesini AR, Jori E, Tiengo A, Prato S, Trevisan R. Effect of sodium intake on blood pressure and albuminuria in Type 2 diabetic patients: the role of insulin resistance. Diabetologia 2004;47(2):300‐3. [DOI] [PubMed] [Google Scholar]
Visser 2008 {published data only}
- Visser FW, Boonstra AH, Titia Lely A, Boomsma F, Navis G. Renal response to angiotensin II is blunted in sodium‐sensitive normotensive men. American Journal of Hypertension 2008;21(3):323‐8. [DOI] [PubMed] [Google Scholar]
Vollmer 2001 {published data only}
- Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons‐Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, Karanja N, DASH‐Sodium Trial Collaborative Research Group. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH‐sodium trial. Ann Intern Med. 2001;135(12):1019‐1028. [DOI] [PubMed] [Google Scholar]
Weder 1991 {published data only}
- Weder AB, Egan BM. Potential deleterious impact of dietary salt restriction on cardiovascular risk factors. Klinische Wochenschrift 1991;69 Suppl 25:45‐50. [PubMed] [Google Scholar]
Wedler 1992 {published data only}
- Wedler B, Brier ME, Wiersbitzky M, Gruska S, Wolf E, Kallwellis R, Aronoff GR, Luft FC. Sodium kinetics in salt‐sensitive and salt‐resistant normotensive and hypertensive subjects. J Hypertens 1992;10:663‐9. [PubMed] [Google Scholar]
Weir 1995 {published data only}
- Weir MR, Dengel DR, Behrens MT, Goldberg AP. Salt‐induced increases in systolic blood pressure affect renal hemodynamics and proteinuria. Hypertension 1995;25:1339‐44. [DOI] [PubMed] [Google Scholar]
Weir 1997 {published data only}
- Weir MR, Hall PS, Behrens MT, Flack JM. Salt and blood pressure responses to calcium antagonism in hypertensive patients. Hypertension 1997;30:422‐7. [DOI] [PubMed] [Google Scholar]
Weir 1998 {published data only}
- Weir MR, Chrysant SG, McCarron DA, Canossa‐Terris M, Cohen JD, Gunter PA, Lewin AJ, Mennella RF, Kirkegaard LW, Hamilton JH, Weinberger MH, Weder AB. Influence of race and dietary salt on the antihypertensive efficacy of an angiotensin‐converting enzyme inhibitor or a calcium channel antagonist in salt‐sensitive hypertensives. Hypertension 1998;31(5):1088‐96. [DOI] [PubMed] [Google Scholar]
Weir 2001 {published data only}
- Weir MR, Smith DH, Neutel JM, Bedigian MP. Valsartan alone or with a diuretic or ACE inhibitor as treatment for African American hypertensives: relation to salt intake. Am J Hypertens 2001;14(7 Pt 1):665‐71. [DOI] [PubMed] [Google Scholar]
Weir 2010 {published data only}
- Weir MR, Yadao AM, Purkayastha D, Charney AN. Effects of high‐ and low‐sodium diets on ambulatory blood pressure in patients with hypertension receiving aliskiren. J Cardiovasc Pharmacol Ther 2010;15:356‐363. [DOI] [PubMed] [Google Scholar]
Whelton 1998 {published data only}
- Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, Cutler JA. TONE Collaborative Research Group. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). JAMA 1998;279:839‐46. [DOI] [PubMed] [Google Scholar]
Whitten 1980 {published data only}
- Whitten CF, Stewart RA. The effect of dietary sodium in infancy on blood pressure and related factors. Studies of infants fed salted and unsalted diets for five months at eight months and eight years of age. Acta Paediatr Scand Suppl 1980;279:1‐17. [PubMed] [Google Scholar]
Wing 1984 {published data only}
- Wing RR, Caggiula AW, Nowalk MP, Koeske R, Lee S, Langford H. Dietary approaches to the reduction of blood pressure: the independence of weight and sodium/potassium interventions. Prev Med 1984;13(3):233‐44. [DOI] [PubMed] [Google Scholar]
Wing 1998 {published data only}
- Wing LM, Arnolda LF, Harvey PJ, Upton J, Molloy D, Gabb GM, Bune AJ, Chalmers JP. Low‐dose diuretic and/or dietary sodium restriction when blood pressure is resistant to ACE inhibitor. Blood Pressure 1998;7(5‐6):299‐307. [DOI] [PubMed] [Google Scholar]
Yamakoshi 2006 {published data only}
- Yamakoshi J, Shimojo R, Nakagawa S, Izui N, Ogihara T. Hypotensive effects and safety of less‐sodium soy sauce containing ‐aminobutyric acid (GABA) on high‐normal blood pressure and mild hypertensive subjects. [Japanese]. Japanese Pharmacology and Therapeutics 2006;34(6):691‐709. [Google Scholar]
Yamamoto 1997 {published data only}
- Yamamoto, Hiroshi. Randomized controlled trial of salt‐restriction program for primary prevention of hypertension in the community. Journal of the Osaka City Medical Center 1997;46(3‐4):255‐67. [Google Scholar]
Yazici 2009 {published data only}
- Yazici M, Kaya A, Kaya Y, Albayrak S, Cinemre H, Ozhan H. Lifestyle modification decreases the mean platelet volume in prehypertensive patients. Platelets 2009;20(1):58‐63. [DOI] [PubMed] [Google Scholar]
Zhao 2009 {published data only}
- Zhao Q, Gu D, Chen J, Bazzano LA, Rao DC, Hixson JE, Jaquish CE, Cao J, Chen J, Li J, Rice T, He J. Correlation between blood pressure responses to dietary sodium and potassium intervention in a Chinese population. American Journal of Hypertension 2009;22(12):1281‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zoccali 1993 {published data only}
- Zoccali C, Mallamaci F, Leonardis D, Romeo M. Randomly allocated crossover study of various levels of sodium intake in patients with mild hypertension. J Hypertens 1993;11 (Suppl 5):S326‐7. [PubMed] [Google Scholar]
Zoccali 1994(a) {published data only}
- Zoccali C, Mallamaci F, Parlongo S. The influence of salt intake on plasma calcitonin gene‐related peptide in subjects with mild essential hypertension. J Hypertens 1994;12:1249‐53. [PubMed] [Google Scholar]
Zoccali 1994(b) {published data only}
- Zoccali C, Mallamaci F, Leonardis D. Assessment of the salt‐arterial pressure relationship in mild hypertensive subjects by 24‐hour ambulatory monitoring. Clinical Science 1994;87(6):635‐9. [DOI] [PubMed] [Google Scholar]
Zoccali 1996 {published data only}
- Zoccali C, Mallamaci F, Cuzzola F, Leonardis D. Reproducibility of the response to short‐term low salt intake in essential hypertension. J Hypertens 1996;14(12):1455‐9. [DOI] [PubMed] [Google Scholar]
Additional references
ALLHAT 2002
- ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981‐97. [DOI] [PubMed] [Google Scholar]
Asaria 2007
- Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet 2007;370:2044‐53. [DOI] [PubMed] [Google Scholar]
Berlin 2002
- Berlin JA, Santanna J, Schmid CH, Szczech LA, Feldman HI. Individual patient‐ versus group‐level data meta‐regressions for the investigation of treatment effect modifiers: ecological bias rears its ugly head. Stat Med 2002;21:371‐387. [DOI] [PubMed] [Google Scholar]
Bibbins‐Domingo 2010
- Bibbins‐Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010;362:590‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cappuccio 1991
- Cappuccio FP, MacGregor GA. Does potassium supplementation lower blood pressure? A meta‐analysis of published trials. J Hypertens 1991;9:465‐73. [DOI] [PubMed] [Google Scholar]
Cochrane 2011
- Cutting Down on Salt Doesn't Reduce Your Chance of Dying. http://eu.wiley.com/WileyCDA/PressRelease/pressReleaseId‐99517.html.
de Wardener 2004
- Wardener HE, He FJ, MacGregor GA. Plasma sodium and hypertension. Kidney Int 2004;66:2454‐66. [DOI] [PubMed] [Google Scholar]
Denton 1995
- Denton D, Weisinger R, Mundy NI, Wickings EJ, Dixson A, Moisson P, Pingard AM, Shade R, Carey D, Ardaillou R, Paillard F, Chapman J, Thillet J, Michel JB. The effect of increased salt intake on blood pressure of chimpanzees. Nature Medicine 1995;1:1009‐16. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 1996
- Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, Marmot M, for the Intersalt Cooperative Research Group. Intersalt revisited: further analyses of 24‐hour sodium excretion and blood pressure within and across populations. BMJ 1996;312:1249‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769‐73. [DOI] [PubMed] [Google Scholar]
Forte 1989
- Forte JG, Pereira Miguel JM, Pereira Miguel MJ, Padua F, Rose G. Salt and blood pressure: a community trial. J Human Hypertens 1989;3:179‐84. [PubMed] [Google Scholar]
Friedman 1990
- Friedman SM, McIndoe RA, Tanaka M. The relation of blood sodium concentration to blood pressure in the rat. J Hypertens 1990;8:61‐6. [DOI] [PubMed] [Google Scholar]
FSA 2008
- Food Standards Agency. Dietary sodium levels surveys, 22 July 2008. www.food.gov.uk/science/dietarysurveys/urinary.
Graudal 2012
- Graudal NA, Hubeck‐Graudal T, Jurgens G. ffects of low‐sodium diet vs. high‐sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). Am J Hypertens 2012;25:1‐15. [DOI] [PubMed] [Google Scholar]
Graudal 2011
- Graudal NA, Hubeck‐Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD004022.pub3] [DOI] [PubMed] [Google Scholar]
He 1998
- He FJ, Markandu ND, Sagnella GA, MacGregor GA. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension 1998;32:820‐4. [DOI] [PubMed] [Google Scholar]
He 2001
- He FJ, Markandu ND, MacGregor GA. Importance of the renin system for determining blood pressure fall with acute salt restriction in hypertensive and normotensive Whites. Hypertension 2001;38:321‐5. [DOI] [PubMed] [Google Scholar]
He 2003
- He FJ, MacGregor GA. How far should salt intake be reduced?. Hypertension 2003;42:1093‐9. [DOI] [PubMed] [Google Scholar]
He 2005
- He FJ, Markandu ND, Sagnella GA, Wardener HE, MacGregor GA. Plasma sodium: ignored and underestimated. Hypertension 2005;45:98‐102. [DOI] [PubMed] [Google Scholar]
He 2006
- He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: meta‐analysis of controlled trials. Hypertension 2006;48:861‐9. [DOI] [PubMed] [Google Scholar]
He 2010
- He FJ, MacGregor GA. Reducing population salt intake worldwide: from evidence to implementation. Prog Cardiovasc Dis 2010;52:363‐82. [DOI] [PubMed] [Google Scholar]
He 2011
- He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta‐analysis of outcome trials. Lancet 2011;378:380‐382. [DOI] [PubMed] [Google Scholar]
He 2011a
- He FJ, Appel LJ, Cappuccio FP, Wardener HE, MacGregor GA. Does reducing salt intake increase cardiovascular mortality?. Kidney Int 2011;80:696‐8. [DOI] [PubMed] [Google Scholar]
He 2012
- He FJ, MacGregor GA. Cardiovascular disease: Salt and cardiovascular risk. Nat Rev Nephrol 2012;8:134‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
IOM 2010
- Institute of Medicine Strategies to Reduce Sodium Intake in the United States, April 2010. www.iom.edu/Reports/2010/Strategies‐to‐Reduce‐Sodium‐Intake‐in‐the‐United‐States.aspx.
Karppanen 2006
- Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis 2006;49:59‐75. [DOI] [PubMed] [Google Scholar]
Lifton 1996
- Lifton RP. Molecular genetics of human blood pressure variations. Science 1996;272:676‐80. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Clinical Excellence (NICE). Guidance on the prevention of cardiovascular disease at the population level. June 2010. http://guidance.nice.org.uk/PH25.
O'Donnell 2011
- O'Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A, Probstfield J, Schmieder RE. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 2011;306:2229‐38. [DOI] [PubMed] [Google Scholar]
Paterna 2008
- Paterna S, Gaspare P, Fasullo S, Sarullo FM, Pasquale P. Normal‐sodium diet compared with low‐sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend?. Clin Sci (Lond) 2008;114:221‐30. [DOI] [PubMed] [Google Scholar]
Poulter 1990
- Poulter N, Khaw KT, Hopwood BEC, Mugambi M, Peart WS, Rose G, Sever PS. The Kenyan Luo migration study: observations on the initiation of a rise in blood pressure. BMJ 1990;300:967‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
PSC 2002
- Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903‐13. [DOI] [PubMed] [Google Scholar]
Riley 2010
- Riley RD, Lambert PC, Abo‐Zaid G. Meta‐analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340:c221. [DOI] [PubMed] [Google Scholar]
SACN 2003
- Scientific Advisory Committee on Nutrition, Salt and health. 2003. The Stationery Office. Available at http://www.sacn.gov.uk/pdf/saltfinal.pdf.
Smith‐Spangler 2010
- Smith‐Spangler CM, Juusola JL, Enns EA, Owens DK, Garber AM. Population strategies to decrease sodium intake and the burden of cardiovascular disease: a cost‐effectiveness analysis. Ann Intern Med 2010;152:481‐7, W170‐3. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care. Meta‐analysis in Context. BMJ Books, 2001:189‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stolarz‐Skrzypek 2011
- Stolarz‐Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovsky J, Kawecka‐Jaszcz K, Nikitin Y, Staessen JA. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 2011;305:1777‐85. [DOI] [PubMed] [Google Scholar]
Strazzullo 2009
- Strazzullo, P.D'Elia, L.Kandala, N. B.Cappuccio, F. P. Salt intake, stroke, and cardiovascular disease: meta‐analysis of prospective studies. BMJ 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2011
- Taylor, R.S.Ashton, K.E.Moxham, T.Hooper, L.Ebrahim, S. Reduced Dietary Salt for the Prevention of Cardiovascular Disease: A Meta‐Analysis of Randomized Controlled Trials (Cochrane Review). Am J Hypertens 2011;24:843‐53. [DOI] [PubMed] [Google Scholar]
Taylor 2011a
- Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whelton 2002
- Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J, National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 2002;288:1882‐1888. [DOI] [PubMed] [Google Scholar]
WHO 2003
- Joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases. 2003, Geneva. Available at http://whqlibdoc.who.int/trs/who_trs_916.pdf.
References to other published versions of this review
He 2002
- He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta‐analysis of randomised trials. Implications for public health. J Human Hypertens 2002;16:761‐70. [DOI] [PubMed] [Google Scholar]
He 2013
- He FJ, Li JF, MacGregor GA. Modest salt reduction lowers blood pressure in all ethnic groups at all levels of blood pressure without adverse consequences – A meta‐analysis of randomised trials. BMJ 2013;in print. [Google Scholar]


