Abstract
The evolution of antimicrobial-resistant strains jeopardizes the existing clinical drugs and demands new therapeutic interventions. Herein, we report the synthesis of cationic thiazolidine bearing a quaternary pyridinium group, in which thiazolidine was N-acylated with fatty acid to establish a hydrophilic–lipophilic balance that disrupts bacterial membranes. The bacterial growth inhibition assays and hemolytic activity against human red blood cells indicate that the N-acylated cationic thiazolidine (QPyNATh) inhibits Gram-positive bacteria at lower minimum inhibitory concentrations (MIC) and is selective for bacteria over mammalian cells. N-Acylation modulates MIC, and it is found that the N-palmitoylated compound, QPyN16Th, had the lowest MIC (1.95 μM) against Gram-positive, Enterococcus faecalis, Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA). In contrast, the N-myristoylated compound, QPyN14Th, showed the lowest MIC (31.25 μM) against Gram-negative, Escherichia coli, uropathogenic Escherichia coli, and Pseudomonas aeruginosa. At 1× MIC, QPyNATh permeabilizes the bacterial membrane, depolarizes the cytoplasmic membranes, and produces excess reactive oxygen species to kill the bacteria, as evidenced by live and dead staining. Interestingly, only QPyNATh containing a palmitoyl acyl chain demonstrated membrane-damaging activity at 2 μM concentrations, suggesting that the optimal hydrophilic–lipophilic balance enables QPyN16Th to selectively kill Gram-positive bacteria at lower doses. S. aureus develops resistance to ciprofloxacin quickly; however, no resistance to QPyN16Th is observed after several passages. As a proof of concept, the animal study revealed that QPyN16Th treatment reduced the bacterial burden in MRSA-infected zebrafish, allowing them to recover from infection and resume normal life. The results imply that lipidation and derivatizing thiazolidine with cationic charge offer an antimicrobial that is selective to treat Gram-positive bacterial infections, biocompatible, and less prone to develop resistance.
Lipidation modulates the Gram-selective antibacterial activity of QPyNATh.
Introduction
Infectious disease-causing pathogens are evolving and becoming more resistant to current treatments. It compels researchers to develop newer antimicrobials capable of circumventing the resistance mechanism. Host defense molecules are widely screened for developing new antimicrobials. Notably, antimicrobial peptides (AMPs) are demonstrated to be effective against several pathogens. However, the challenge with natural AMPs is their susceptibility to proteases, pH sensitivity, poor selectivity, and difficulty in large-scale isolation. Synthetic analogs of AMPs are rising, but the complicated synthesis and high cost prevent their development.1 Antimicrobial lipids (AMLs) are another class of molecules that hosts produce to combat microbial invasions,2 but they are less explored in new antimicrobial development. Fatty acids, part of skin lipid composition, prevent bacterial adherence by making the skin surface acidic.3 The vernix caseosa, a white material covering the fetus and infant skin, contains lipids that protect newborns from infection.4 In addition to those found in the skin, many other body lipids have been identified with antimicrobial activity and are known to support innate host defense.5,6 The lipids in saliva and oral mucosa prevent the colonization of Porphyromonas gingivalis, bacteria responsible for periodontitis.7
Lipids and lipoproteins are important for the host defense, yet microorganisms can exploit the host lipid network for replication and survival.8 For example, Helicobacter pylori uses host cholesterol to make cholesteryl glycosides that it inserts into its outer membrane to escape phagocytosis and T-cell activation.9 When Mycobacterium tuberculosis inhabits lipid-rich macrophages, fatty acids derived from the host triacylglycerol are imported and integrated into bacterial triacylglycerol.10 Thus, relying solely on natural AMLs may not be sufficient. Given pathogens co-opting on natural lipids, developing antimicrobial mimicking natural lipids may be an effective alternative to combat infectious microorganisms. Thus, lipidated thiazolidine was developed to combat bacteria and the influence of lipid acyl chain hydrophobicity in exerting effective antibacterial activity was studied.
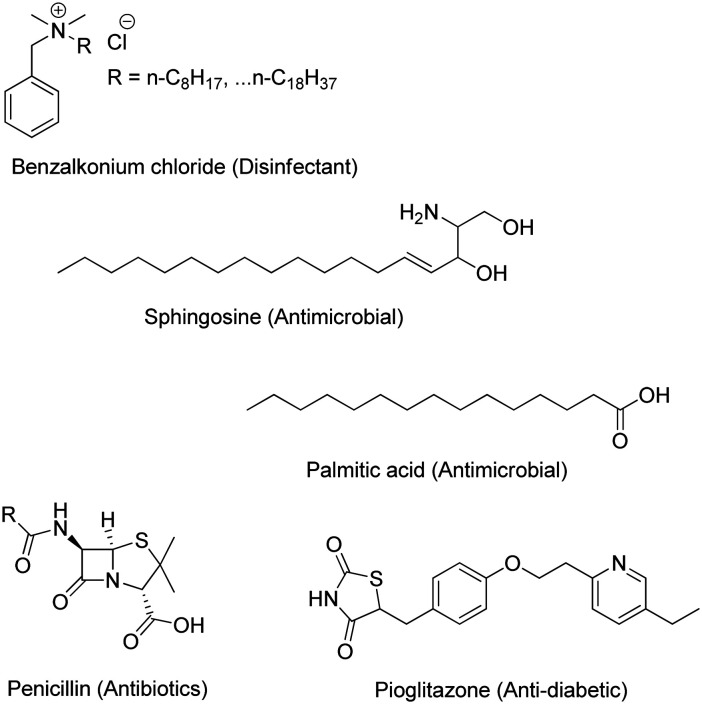
Thiazolidine is a five-member saturated heterocyclic compound consisting of a thioether and an amine group in the 1 and 3 positions. The possibility of substitution in positions 2, 4, and 5 enabled the synthesis of a wide range of thiazolidine-based bioactive compounds. Notably, pioglitazone, a generic drug used to treat type II diabetes, contains a thiazolidinedione group.11,12 The well-known antibiotic penicillin contains a fused ring of thiazolidine and β-lactam (Fig. 1).13 Herein, we report the synthesis of thiazolidine with quaternary pyridinium group substitution at position 2 and further acylation of the secondary amine at position 3 with fatty acids. Quaternary ammonium compounds are known disinfectants,14 and their cationic charge may enable them to target the negatively charged surface of bacterial membranes specifically.15 Similarly, fatty acids have natural antibacterial properties and provide the drug with optimal hydrophobicity for incorporation into membranes.16,17 This paper describes the antibacterial activity of quaternary pyridinium-tethered N-acylated thiazolidine (QPyNATh) against major bacterial groups. The findings emphasize the significance of lipidation of thiazolidine, particularly revealing the effect of fatty acid acyl chain length in exhibiting better activity. As a proof of concept, the therapeutic efficacy of the active compound was proved through treating bacteria-infected zebrafish, and the results are discussed.
Fig. 1. Representative structures of commercial drugs with long chain hydrocarbons and a thiazolidine core.
Results and discussion
Synthesis of quaternary N-acyl thiazolidine
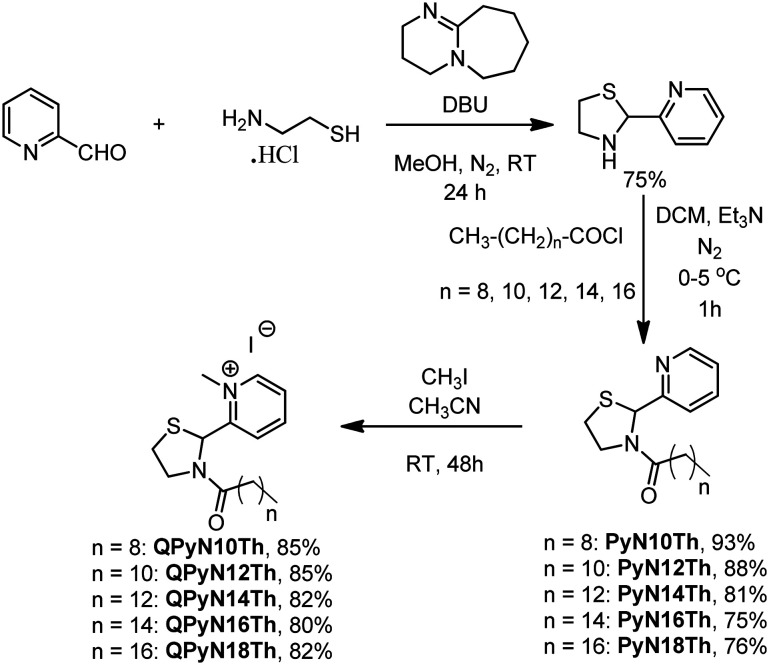
The preparation of quaternary N-acyl thiazolidine (QPyNATh) involves three steps (Scheme 1). First, cysteamine hydrochloride was allowed to react with pyridine aldehyde to obtain thiazolidine in good yield. Next, the thiazolidine was N-acylated using fatty acid chloride and subjected to methylation with methyl iodide to get the desired product in quantitative yield. 1H NMR, 13C NMR and HRMS confirmed the products (see ESI† Fig. S1–S11). The 1H NMR analysis of QPyN18Th showed that aromatic protons exhibit distinctive chemical shifts, with resonances at δ 9.35 (1H, doublet, J = 3.0 Hz), 8.56 (1H, triplet, J = 3.9 Hz), 8.42 (1H, doublet, J = 4.0 Hz), and 7.94 (1H, triplet, J = 3.3 Hz). Peaking at δ 6.39 (1H, singlet), a typical signature of the thiazolidine ring, a single methine proton has been identified. Furthermore, the diastereotopic methylene protons deshields in the 4.65–3.35 range, notably at 4.65 (1H, multiplet), 4.00 (1H, triplet of doublets, J = 4.8, 3.1 Hz), 3.58 (1H, multiplet), and 3.35 (1H, multiplet). At 4.55 (3H, singlet), the quaternary methyl group is observed. Signals are seen at 2.66 (1H, doublet of triplets, J = 7.7, 3.7 Hz) and 2.47 (1H, doublet of triplets, J = 8.0, 3.8 Hz) for the methylene group next to the amide functional group. All other methylene groups can be identified in the region of δ 1.64 to 1.56 (2H, multiplet) and δ 1.35 to 1.20 (28H, multiplet). The methyl group within the extended carbon chain is apparent within the range of δ 0.93 to 0.83 (3H, multiplet).
Scheme 1. Synthesis of quaternary N-acyl thiazoldines.
The amide carbon has been identified at δ 173.12 in the 13C NMR spectra. Notably, at δ 157.18, the quaternary carbon next to the quaternary nitrogen in the molecule perceives considerable deshielding. Similarly, the carbon atoms with C–H bonds at the alpha and para positions concerning the quaternary nitrogen also show signs of deshielding; they show up at δ 147.19 and δ 146.83, respectively, while the meta position shows up at δ 126.78 and δ 126.02. The distinctive methine carbon is identified at δ 60.26. At δ 51.21, the methylene carbon next to the thiazolidine nitrogen atom has been observed. The methyl group resulting from quaternization with methyl iodide is located at δ 46.74. The methylene carbon alpha to the cyclic amide gives a signal at δ 35.46. All other methylene carbons are found in the δ 31 to δ 22 range. The methyl carbon, on the other hand, is strongly shielded, appearing at δ 4.12. The mass of the compound QPyN18Th found (447.3428 (M − I)) from the analysis of HRMS exactly matched the theoretically calculated mass of 447.3403.
Antibacterial activity
The antibacterial activity of QPyNATh with varied acyl chain length was examined to understand the influence of lipidation. The antibacterial activity of QPyNATh has been evaluated against various Gram-negative and Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). The activity was expressed as minimum inhibitory concentration (MIC), the lowest concentration required for inhibiting bacterial growth (Table 1). The MIC determined by resazurin microtiter assay (REMA) shown in Table 1 indicates that the MIC values of Gram-positive bacteria are lower than those of Gram-negative bacteria. The MIC differs significantly with respect to the acyl chain length. The shorter acyl chain-length compounds that contained decanoyl and lauroyl groups showed higher MICs. Interestingly, QPyN14Th and QPyN16Th displayed lower MICs for Gram-negative and Gram-positive bacteria, respectively.
MIC of QPyNATh against bacteria determined by REMA.
| Molecule | MIC (μM) | |||||
|---|---|---|---|---|---|---|
| Gram-negative bacteria | Gram-positive bacteria | |||||
| E. coli | UPEC | P. aeruginosa | E. faecalis | S. aureus | MRSAa | |
| QPyN10Th | 2000 | 2000 | 2000 | 500 | 250 | 500 |
| QPyN12Th | 250 | 250 | 125 | 62.5 | 31.25 | 31.25 |
| QPyN14Th | 31.25 | 31.25 | 31.25 | 7.81 | 3.91 | 7.81 |
| QPyN16Th | 500 | 125 | 62.5 | 1.95 | 1.95 | 1.95 |
| QPyN18Th | 2000 | 2000 | 2000 | 7.81 | 1.95 | 15.63 |
Drug-resistant strain.
The difference in the MIC can be explained by considering thickness variation in the bacterial cell wall.18–20 The thick peptidoglycan outer layer of the Gram-positive bacteria cell wall necessitated a long palmitoyl acyl chain to overcome the cell wall hydrophilic–lipophilic balance (HLB) and allow QPyN16Th to permeate the membranes. In contrast, the hydrophobicity provided by the myristoyl acyl chain in QPyN14Th is sufficient to permeate the outer membranes of Gram-negative bacteria. At the same time, despite having a longer acyl chain, stearoyl containing QPyN18Th displayed a higher MIC, indicating that the higher hydrophobicity makes it challenging to permeate bacterial membranes. The results reveal that either lower or higher hydrophobicity influences the antibacterial activity, indicating that an optimal HLB is necessary to exert efficient antibacterial activity. Noteworthily, PyNATh (1-(2-(pyridine-2-yl)thiazolidin-3-yl)acyl-1-one) exhibited no antibacterial activity, indicating that cationic functionalization plays a crucial role in QPyNATh's antibacterial activity.
Selectivity assay
Following the determination of the MIC, we performed a hemolytic experiment to investigate the toxicity of QPyNATh to mammalian cells and determine the selectivity. The hemolytic concentration (HC) of QPyN10Th, QPyN12Th, QPyN14Th, QPyN16Th, and QPyN18Th was 8000, 2000, 500, 500, and 500 μM, respectively. HC50 values (concentration required to achieve 50% lysis of RBCs) of QPyN10Th, QPyN12Th, QPyN14Th, QPyN16Th, and QPyN18Th were 4032, 868, 283, 299, and 290 μM, respectively. Unlike MIC, a higher concentration was required for hemolysis, suggesting that QPyNATh is not toxic to RBCs. The analysis of the HC/MIC ratio revealed that QPyN14Th has a selectivity that is 16 times toxic to Gram-negative bacteria. While all QPyNATh compounds exhibit higher toxicity to Gram-positive bacteria than Gram-negative ones, QPyN16Th, in particular, exhibits 256-fold selective toxicity to Gram-positive bacteria (Table 2). The results indicate that QPyN16Th exhibits more selective toxicity to Gram-positive bacteria than the other QPyNATh compounds while remaining less toxic to human cells due to its delicate HLB. A previous study using peptidomimetic compounds that are membrane-active showed that molecular hydrophobicity plays a crucial role in exhibiting high toxicity to bacteria over mammalian cells.21
Selectivity of QPyNATh for bacteria compared with human red blood cells (RBC).
| Molecule | HC/MIC | |||||
|---|---|---|---|---|---|---|
| Gram-negative bacteria | Gram-positive bacteria | |||||
| E. coli | UPEC | P. aeruginosa | E. faecalis | S. aureus | MRSAa | |
| QPyN10Th | 4 | 4 | 4 | 16 | 32 | 16 |
| QPyN12Th | 8 | 8 | 16 | 32 | 64 | 64 |
| QPyN14Th | 16 | 16 | 16 | 64.02 | 127.878 | 64.02 |
| QPyN16Th | 1 | 4 | 8 | 256.41 | 256.41 | 256.41 |
| QPyN18Th | 0.25 | 2 | 0.25 | 64.020 | 256.41 | 31.98 |
Drug-resistant strain.
Time-kill kinetics
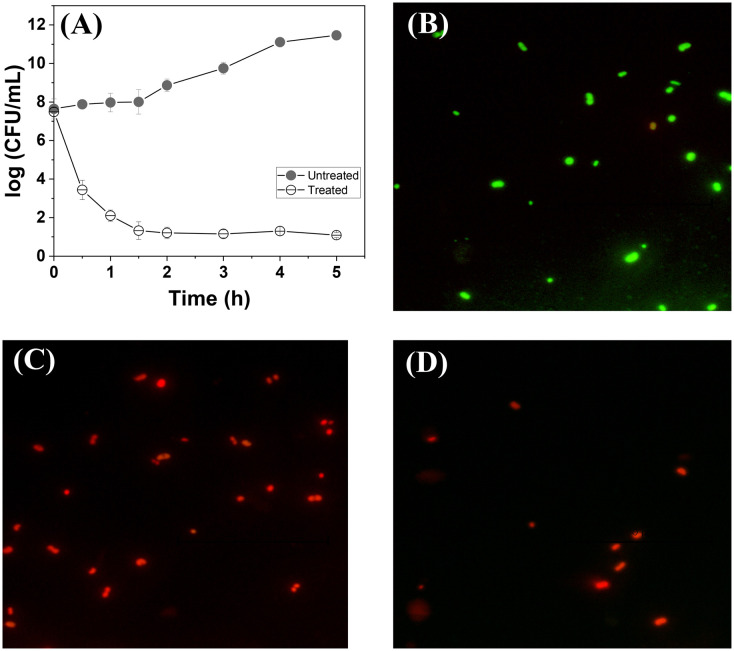
Following the observation of the selective antibacterial activity against Gram-positive bacteria, we studied the time-dependent antibacterial efficiency of QPyN16Th against MRSA. The viable cells that remained after treatment were followed over time and analyzed using the colony count method, as shown in CFU (Fig. 2A). As shown in Fig. 2A, QPyN16Th suppresses MRSA proliferation within 30 minutes and mostly killed the bacteria in 1 h, but the proliferation is unaffected in the untreated cultures. To confirm that cells were dead after 1 h of treatment, we treated MRSA with 1× MIC QPyN16Th and assessed live and dead cells using fluorescence-based assays (Fig. 2B–D). This experiment used a dual fluorescent dye: acridine orange (AO), which is membrane permeable, and propidium iodide (PI), which is membrane impermeable. Viable cells will only stain with acridine orange and fluoresce green. In contrast, dead cells with a damaged membrane will stain with both AO (green) and PI (red), with PI showing dominant red fluorescence due to resonance energy transfer.22 As seen in Fig. 2C, cells treated with QPyN16Th produced red fluorescent cells, as opposed to green fluorescent cells in the untreated control (Fig. 2B). The cells treated with membrane permeabilizing agent, Triton X-100,23 showed red fluorescent cells (Fig. 2D). The result suggests that QPyN16Th effectively affects bacterial membranes and kills them in a shorter time.
Fig. 2. (A) Time-kill curves of (n = 3, mean ± SD) of MRSA exposed to 1× MIC QPyN16Th. Live and dead staining of MRSA (B) untreated, (C) treated with 1× MIC QPyN16Th, and (D) treated with Triton X100. Viable cells with intact membranes appear green, and dead cells with compromised membranes appear red.
Antimicrobial mechanism
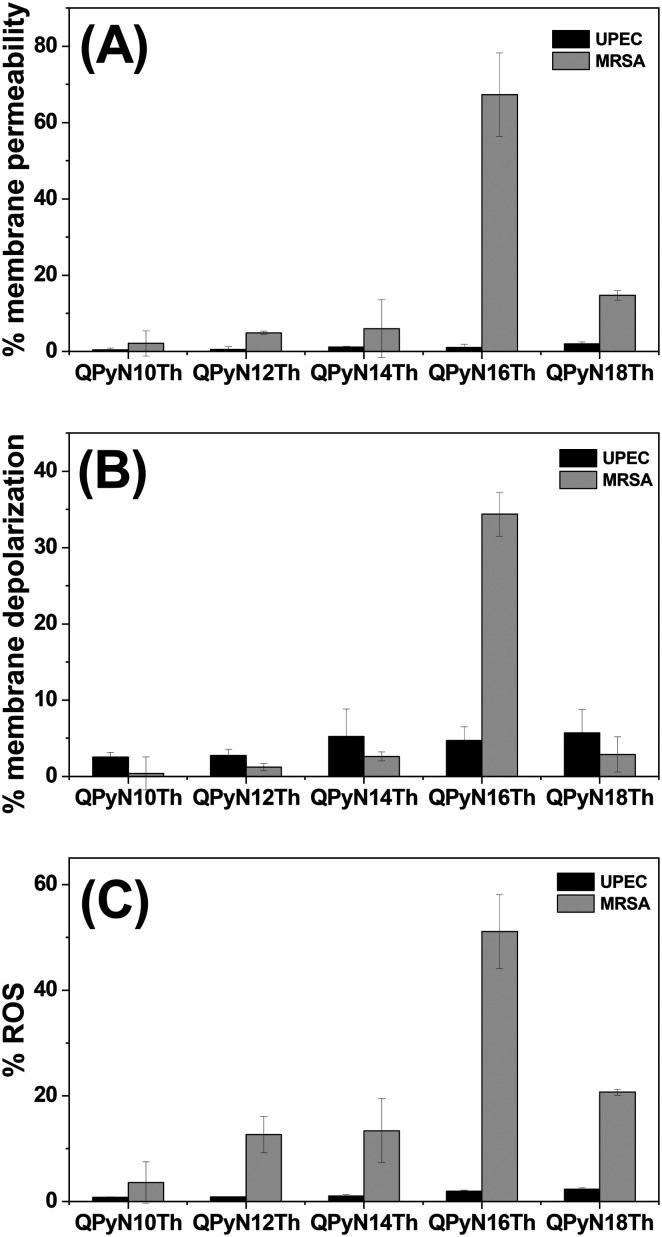
Membranes typically serve as the front line of protection. Therefore, the effect of QPyNATh on membrane integrity was studied. For this study, UPEC and MRSA were selected as representative Gram-negative and Gram-positive bacteria, respectively. Having observed the lowest MIC against Gram-positive bacteria, we investigated membrane integrity at 2 μM QPyNATh. Propidium iodide, a fluorescent probe, was chosen to monitor membrane permeability because it is impermeable to membranes and emits mild fluorescence. However, when PI permeates compromised membranes, it will emit red fluorescence.24 While testing the membrane permeability at 2 μM, we noted membrane permeability only in Gram-positive bacteria treated with QPyN16Th (Fig. 3A). This suggests that QPyNATh is selective, as well as that the acyl chain length has a significant impact on membrane permeability. Nevertheless, QPyNATh permeabilizes bacteria membranes at 1× MIC, and that membrane permeability was higher in longer acyl chain length, despite the difference in the 1× MIC of QPyNATh (see ESI† Fig. S12A and B).
Fig. 3. Antibacterial mechanism. (A) Membrane permeability, (B) membrane depolarization and (C) ROS generation in bacteria after treatment with 2 μM QPyNATh.
Next, the cytoplasmic membrane depolarization activity of QPyNATh was determined using DiSC3(5) (3,3′-dipropylthiadicarbocyanine iodide), a membrane potential sensitive dye.25 When DiSC3(5) intercalates into the cytoplasmic membrane of active cells, the fluorescence is quenched. Any disruption in membrane potential will lead to dye release and result in increased fluorescence. All five tested molecules showed membrane depolarization at 1× MIC, but the extent of depolarization differs significantly (see ESI† Fig. S12C and D). However, at 2 μM, only QPyN16Th caused significant membrane depolarization in Gram-positive bacteria, whereas no effect was observed in Gram-negative bacteria (Fig. 3B). Other QPyNATh compounds showed less effect on MRSA and UPEC membrane depolarization, which could be ascribed to the lower concentration of QPyNATh that was less than their MIC.
Most antibiotic-mediated bactericidal activity is accompanied by an increase in reactive oxygen species (ROS) production. 2′-7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) has been used as a probe to measure hydroxyl, peroxyl and other ROS activity within the cell.26 DCFH-DA crosses the membranes and is transformed into 2′-7′-dichlorodihydrofluorescein (DCFH) by intracellular esterase. DCFH is sensitive to ROS and oxidizes to fluorescent dichlorofluorescein (DCF). The measured fluorescence from DCF was used to determine ROS generation. The potential of QPyNATh to induce ROS generation in UPEC and MRSA was assessed at 2 μM (Fig. 3C). It is noted from Fig. 3C that only 2 μM QPyN16Th showed higher ROS production, specifically in Gram-positive bacteria. Other compounds with higher MICs than QPyN16Th performed poorly against UPEC and MRSA. At 1× MIC, QPyNATh induces excess ROS generation, and molecules with longer acyl chains produce more ROS (see ESI† Fig. S13A and B). The findings imply that lipidation plays an important role in QPyNATh antibacterial activity. At 2 μM QPyN16Th, the N-palmitoyl tail hydrophobicity allowed QPyNATh to insert into the bacterial membranes, compromising membrane integrity and producing excess ROS to cause cell death.
Drug-resistance study
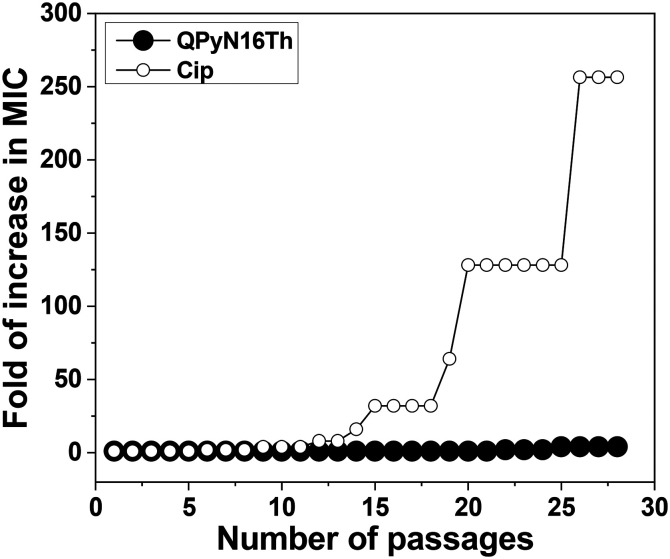
The demand for new drugs arises from the inherent resistance of bacteria to most of the existing antibiotics.27 Hence, QPyN16Th ability to develop resistance was evaluated against S. aureus. Ciprofloxacin (Cip) was chosen as a control antibiotic because quinolone-resistant S. aureus is the most common species in clinics. Cip exerts its bactericidal action by targeting DNA gyrase and topoisomerase IV, which in turn inhibits chromosomal DNA replication and segregation. The drug resistance analysis showed that Cip had a 256-fold increase in MIC against S. aureus, but QPyN16Th had a minimal (4-fold) increase in MIC after 28 passages (Fig. 4). These findings show that, unlike target-specific antibiotics, QPyNATh damaging bacterial cell membranes offers advantages since bacteria are less likely to develop resistance.
Fig. 4. Bacterial resistance studies of QPyN16Th and antibiotics. S. aureus developed rapid and high resistance to Cip but not to QPyN16Th even after 28 passages.
In vivo testing
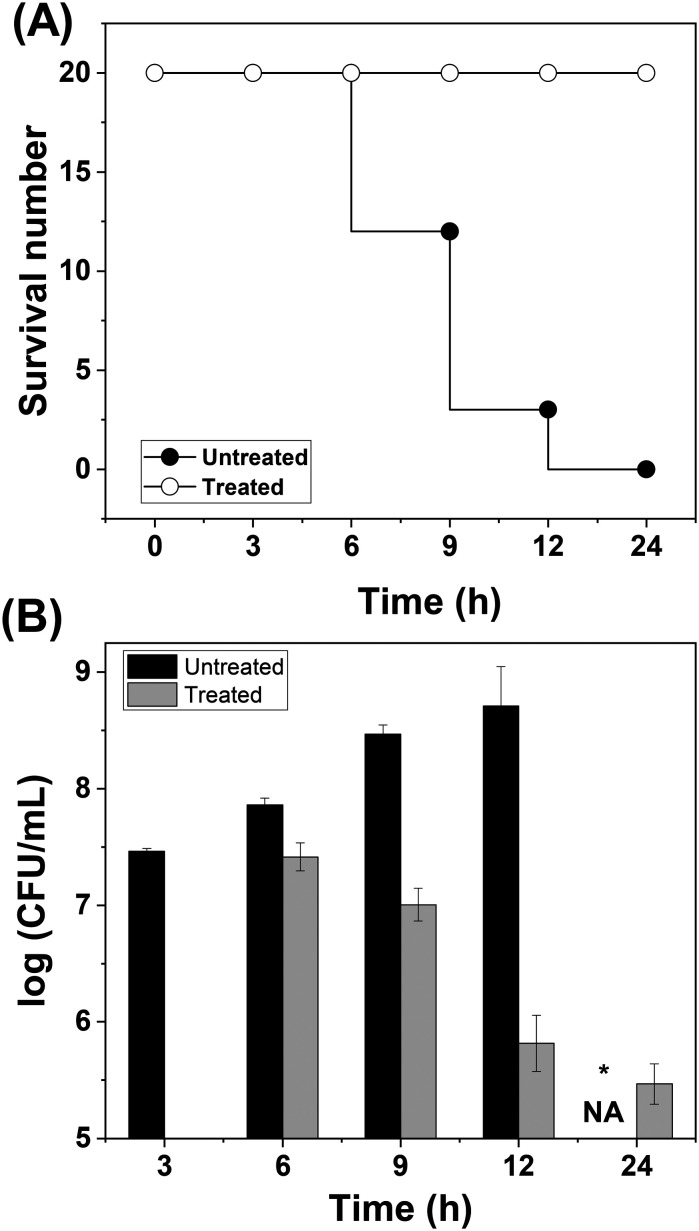
The successful exhibition of antibacterial activity in vitro enabled us to investigate the therapeutic potential of QPyN16Th in vivo. Zebrafish were chosen as a model because they have 70% genomic similarity to humans, and 84% of the genes responsible for human diseases are also present in zebrafish. Zebrafish are easier to handle than other animal models, allowing antimicrobials to be tested for efficacy against extracellular and intracellular bacterial infection.28 The fish were infected intramuscularly with MRSA, given three hours to survive without medical intervention, and then divided into two groups. QPyN16Th was used to treat one group while leaving the other untreated. The survival curve describing the fish mortality is shown in Fig. 5A. It is noted from Fig. 5A that the fish in the treated group recovered from the infection and resumed their regular behaviour, but the fish in the untreated group died within 9 to 14 h due to infection.
Fig. 5. (A) Survival curve. MRSA was intramuscularly injected and divided into two groups, each containing 20 fish. The time-dependent mortality rate was monitored and plotted. It has been noticed that fish treated with QPyN16Th recovered from infection. (B) Colony count assay. MRSA load in the muscle was determined by culturing on an LB-agar plate and reported as log (CFU mL−1). *NA – no fish live. Treatment started after the first 3 h of infection.
To investigate the bacterial load in infected and treated fish, we collected them at defined intervals, sacrificed them, and assessed the bacterial load in the muscle by plating them on LB-agar solidified media. The colonies that developed on the plates were manually counted and reported in CFU mL−1 (Fig. 5B). As seen in Fig. 5B, the number of bacterial colonies in the untreated group increases with time, resulting in a high bacterial burden that the animal was unable to withstand thus succumbing to infection. Strikingly, the fish that received QPyN16Th through intramuscular injection showed a decrease in bacterial colonies within the first three hours of treatment, and the bacterial load continued to decrease over the next few hours, resulting in fish survival from infection. The findings suggest that QPyN16Th has good therapeutic potential for treating MDR infection; nevertheless, more research on cell lines and higher animal models is needed before suggesting this chemical for next-phase testing.
Experimental
Materials
Pyridine-2-carboxaldehyde, cysteamine hydrochloride and thionyl chloride were purchased from Avra, India. DBU (1,8-diazabicyclo[5.4.0]undec-7-ene was procured from Spectro-Chem. Decanoic acid (10), lauric acid (12), myristic acid (14), palmitic acid (16), stearic acid (16), and 3,3′-dipropylthiacarbocyanine iodide (DiSC3(5)) were purchased from TCI, India. Propidium iodide (PI) and dichlorofluorescein diacetate (DCFH2-DA) were procured from SRL Chemicals. Methyl iodide was purchased from LOBA Chemie. Media used are obtained from Himedia, India.
Synthesis of quaternary N-acyl thiazolidines
Synthesis of 2-(pyridine-2-yl)thiazolidine
One equivalent of DBU and two equivalents of cysteamine hydrochloride dissolved in methanol were stirred for two min. To this reaction mixture, one equivalent of pyridine aldehyde was added under a nitrogen atmosphere. The reaction was allowed for 24 h at room temperature before being analyzed using thin layer chromatography (TLC). The reaction mass was extracted with ethyl acetate and water. The product obtained in the organic layers was dried with sodium sulphate and then purified by column chromatography (60–120 mesh size), yielding 75%.
2-(Pyridin-2-yl)thiazolidine
1H NMR: NMR (400 MHz, CDCl3) δ 8.59 (1H, d, J = 2.8 Hz), 7.65 (1H, td, J = 5.1, 1.2 Hz), 7.29 (1H, d, J = 5.2 Hz), 7.20 (1H, m), 5.60 (1H, s), 3.95–3.66 (1H, m), 3.16–3.13 (1H, m), 3.12–3.08 (1H, m), 3.08–3.04 (1H, m); 13C NMR: (100 MHz, CDCl3) δ 158.19, 149.86, 136.79, 123.09, 122.08, 72.78, 53.51, 37.03; HRMS: M. Wt calculated for [M + H]+ = 167.0637; found = 167.0648.
Synthesis of 1-(2-(pyridine-2-yl)thiazolidin-3-yl)acyl-1-one
First, fatty acid chloride with varying chain lengths was prepared by reacting three equivalents of thionyl chloride and one equivalent of fatty acid in DCM for one hour. The freshly synthesized fatty acid chlorides were added dropwise to the reaction mass comprising 2-(pyridine-2-yl)thiazolidine and triethyl amine under ice-cold conditions in the presence of a nitrogen environment. The mixture was stirred for 30 min and the product formation was judged by TLC. The crude mass was extracted using ethyl acetate and saturated sodium hydrogen carbonate aqueous solution. The organic layer was dried with sodium sulphate before being extracted in a rotavapor under low pressure and purified further by column chromatography (silica mesh size 60–120). The yield is in the range of 75–90%.
1-(2-(Pyridin-2-yl)thiazolidin-3-yl)decan-1-one (PyN10Th)
1H NMR: (400 MHz, CDCl3) δ 8.58 (1H, d, J = 4.3 Hz), 8.52 (1H, d, J = 4.2 Hz), 7.70 (1H, m), 7.62 (1H, m), 7.19 (4H, m), 6.38 (1H, s), 6.10 (1H, s), 4.37–4.29 (1H, m), 4.16–4.08 (1H, m), 4.03–3.94 (2H, m), 3.51–3.42 (1H, m), 3.12 (2H, m), 3.08–3.01 (1H, m), 2.45–2.37 (2H, m), 2.31 (1H, m), 2.01 (1H, m), 1.71–1.52 (4H, m), 1.31–1.14 (24H, m), 0.89–0.84 (6H, m). 13C NMR: (100 MHz, CDCl3) δ 171.98, 171.24, 161.05, 160.84, 149.78, 149.50, 137.23, 136.65, 122.82, 122.43, 120.32, 118.94, 65.57, 65.14, 50.48, 50.29, 35.26, 34.76, 31.85, 30.77, 29.42, 29.35, 29.26, 29.21, 29.18, 28.89, 24.86, 24.68, 22.64, 14.09. HRMS: M. Wt calculated for [M + H]+ = 321.1995; found = 321.2024.
1-(2-(Pyridin-2-yl)thiazolidin-3-yl)dodecan-1-one (PyN12Th)
1H NMR: (400 MHz, CDCl3) δ 8.58 (1H, d, J = 4.7 Hz), 8.52 (1H, d, J = 4.2 Hz), 7.70 (1H, td, J = 7.7, 1.7 Hz), 7.62 (1H, td, J = 7.7, 1.7 Hz), 7.25–7.11 (4H, m), 6.38 (1H, s), 6.10 (1H, s), 4.37–4.28 (1H, m), 4.15–4.07 (1H, m), 3.99 (2H, m), 3.52–3.44 (1H, m), 3.12 (2H, m), 3.06 (1H, m), 2.43–2.36 (2H, m), 2.30 (1H, m), 2.01 (1H, m), 1.70–1.53 (4H, m), 1.30–1.16 (32H, m), 0.88 (6H, t, J = 6.9 Hz). 13C NMR: (100 MHz, CDCl3) δ 171.96, 171.22, 161.07, 160.86, 149.81, 149.53, 137.20, 136.63, 122.80, 122.42, 120.32, 118.93, 65.59, 65.15, 50.47, 50.27, 35.26, 34.77, 31.89, 30.79, 29.60, 29.57, 29.49, 29.42, 29.35, 29.31, 29.19, 28.91, 24.86, 24.68, 22.67, 14.10. HRMS: M. Wt calculated for [M + H]+ = 349.2307; found = 349.2325.
1-(2-(Pyridin-2-yl)thiazolidin-3-yl)tetradecan-1-one (PyN14Th)
1H NMR: (400 MHz, CDCl3) δ 8.51 (1H, d, J = 4.4 Hz), 8.45 (1H, d, J = 4.3 Hz), 7.63 (1H, td, J = 7.7, 1.5 Hz), 7.55 (1H, td, J = 7.7, 1.5 Hz), 7.18–7.04 (4H, m), 6.31 (1H, s), 6.03 (1H, s), 4.26 (1H, dt, J = 12.2, 7.1 Hz), 4.04 (1H, m), 3.99–3.86 (2H, m), 3.41 (1H, dt, J = 11.1, 7.3 Hz), 3.13–3.02 (2H, m), 3.02–2.93 (1H, m), 2.37–2.29 (2H, m), 2.24 (1H, m), 1.94 (1H, m), 1.54 (4H, m), 1.28–1.09 (40H, m), 0.81 (6H, t, J = 6.8 Hz). 13C NMR: (100 MHz, CDCl3) δ 171.98, 171.23, 161.06, 160.85, 149.81, 149.53, 137.21, 136.64, 122.81, 122.43, 120.33, 118.93, 65.59, 65.15, 50.48, 50.28, 35.27, 34.77, 31.92, 30.79, 29.66, 29.64, 29.57, 29.50, 29.43, 29.42, 29.35, 29.29, 29.19, 28.91, 24.87, 24.69, 22.68, 14.11. HRMS: M. Wt calculated for [M + H]+ = 377.2620; found = 377.2645.
1-(2-(Pyridin-2-yl)thiazolidin-3-yl)hexadecan-1-one (PyN16Th)
1H NMR: (400 MHz, CDCl3) δ 8.58 (d, J = 4.2 Hz, 1H), 8.52 (d, J = 4.2 Hz, 1H), 7.70 (td, J = 7.7, 1.7 Hz, 1H), 7.62 (td, J = 7.7, 1.7 Hz, 1H), 7.25–7.11 (m, 4H), 6.38 (s, 1H), 6.10 (s, 1H), 4.33 (m, 1H), 4.11 (m, 1H), 3.99 (m, 2H), 3.48 (m, 1H), 3.13 (m, 2H), 3.05 (m, 1H), 2.45–2.37 (m, 2H), 2.31 (m, 1H), 2.01 (m, 1H), 1.66–1.56 (m, 4H), 1.33–1.15 (m, 48H), 0.88 (t, J = 6.9 Hz, 6H). 13C NMR: (100 MHz, CDCl3) δ 171.98, 171.24, 161.06, 160.85, 149.81, 149.53, 137.22, 136.64, 122.81, 122.43, 120.33, 118.93, 65.59, 65.15, 50.48, 50.28, 35.27, 34.77, 31.92, 30.79, 29.69, 29.65, 29.57, 29.50, 29.43, 29.36, 29.29, 29.19, 28.91, 24.87, 24.69, 22.69, 14.11. HRMS: M. Wt calculated for [M + H]+ = 405.2933; found = 405.2953.
1-(2-(Pyridin-2-yl)thiazolidin-3-yl)octadecan-1-one (PyN18Th)
1H NMR: (400 MHz, CDCl3) δ 8.58 (d, J = 4.4 Hz, 1H), 8.52 (d, J = 4.4 Hz, 1H), 7.73–7.66 (m, 1H), 7.61 (td, J = 7.7, 1.4 Hz, 1H), 7.17 (m, 4H), 6.38 (s, 1H), 6.09 (s, 1H), 4.38–4.29 (m, 1H), 4.11 (m, 1H), 3.99 (m, 2H), 3.48 (m, 1H), 3.18–3.09 (m, 2H), 3.08–3.00 (m, 1H), 2.38 (m, 2H), 2.35–2.26 (m, 1H), 2.06–1.97 (m, 1H), 1.66–1.53 (m, 4H), 1.34–1.11 (m, 56H), 0.88 (t, J = 6.8 Hz, 6H). 13C NMR: (100 MHz, CDCl3) δ 171.98, 171.20, 161.09, 160.88, 149.77, 149.55, 137.18, 136.60, 122.79, 122.41, 120.33, 118.93, 65.60, 65.22, 50.51, 50.26, 35.26, 34.75, 31.92, 30.80, 29.69, 29.65, 29.57, 29.50, 29.42, 29.35, 29.29, 29.20, 28.92, 24.87, 24.69, 22.68, 14.10. HRMS: M. Wt calculated for [M]+ = 432.3174; found = 432.3131.
Synthesis of 1-methyl-2-(3-acyl-thiazolidin-2-yl)pyridin-1-ium-iodide
To obtain quaternary N-acyl thiazolidines, 1-(2-(pyridine-2-yl)thiazolidin-3-yl)acyl-1-one dissolved in acetonitrile was allowed to react with excess methyl iodide for 48 h. After the reaction, the excess methyl iodide was removed by evaporating at reduced pressure, and the obtained product was dissolved in acetone and precipitated using hexane. The process is repeated several times to obtain the pure product. The yield is in the range of 80-93%.
2-(3-Decanoylthiazolidin-2-yl)-1-methylpyridin-1-ium iodide (QPyN10Th)
1H NMR: (400 MHz, CDCl3) δ 9.31 (1H, d, J = 6.0 Hz), 8.58 (1H, t, J = 7.6 Hz), 8.36 (1H, d, J = 7.4 Hz), 7.98 (1H, dd, J = 10.0, 3.7 Hz), 6.41 (1H, s), 4.63 (1H, m), 4.56 (3H, s), 4.03 (1H, td, J = 9.5, 6.1 Hz), 3.57 (1H, m), 3.37 (1H, m), 2.65 (1H, dt, J = 14.5, 7.8 Hz), 2.52–2.43 (1H, m), 1.59 (2H, dt, J = 15.0, 7.4 Hz), 1.27 (12H, m), 0.91–0.84 (3H, m). 13C NMR: (100 MHz, CDCl3) δ 173.11, 157.17, 147.19, 146.84, 126.79, 126.01, 60.26, 51.22, 46.76, 35.46, 31.86, 31.60, 29.44, 29.27, 29.22, 24.35, 22.66, 14.12. HRMS: M. Wt calculated for [M − I] = 335.2151; found = 335.1936.
2-(3-Dodecanoylthiazolidin-2-yl)-1-methylpyridin-1-ium iodide (QPyN12Th)
1H NMR: NMR (400 MHz, CDCl3) δ 9.36 (1H, d, J = 4.0 Hz), 8.58 (1H, t, J = 5.2 Hz), 8.42 (1H, d, J = 5.0 Hz), 7.97 (1H, t, J = 4.3 Hz), 6.40 (1H, s), 4.66 (1H, m) 4.55 (3H, s), 4.01 (1H, dd, J = 6.4, 2.3 Hz), 3.61–3.55 (1H, m), 3.39–3.33 (1H, m), 2.69–2.62 (1H, m), 2.50–2.43 (1H, m), 1.58 (2H, dd, J = 10.0, 5.0 Hz), 1.30–1.23 (16H, m), 0.87 (3H, m). 13C NMR: (100 MHz, CDCl3) δ 173.11, 157.20, 147.19, 146.83, 126.80, 126.02, 60.27, 51.22, 46.79, 35.47, 31.90, 31.63, 29.61, 29.50, 29.44, 29.33, 29.23, 24.36, 22.68, 14.13. HRMS: M. Wt calculated for [M − I] = 363.2465; found = 363.2504.
1-Methyl-2-(3-tetradecanoylthiazolidin-2-yl)pyridin-1-ium iodide (QPyN14Th)
1H NMR: NMR (400 MHz, CDCl3) δ 9.36 (1H, d, J = 3.6 Hz), 8.56 (1H, t, J = 5.1 Hz), 8.42 (1H, d, J = 5.3 Hz), 7.94 (1H, t, J = 4.2 Hz), 6.39 (1H, s), 4.66–4.64 (1H, m), 4.55 (3H, s), 4.01 (1H, dd, J = 10.4, 6.2 Hz), 3.64–3.54 (1H, m), 3.40–3.30 (1H, m), 2.73–2.60 (1H, m), 2.52–2.42 (1H, m), 1.58 (2H, dd, J = 9.6, 4.7 Hz), 1.26 (20H, m), 0.87 (3H, m). 13C NMR: (100 MHz, CDCl3) δ 173.08, 157.19, 147.18, 146.85, 126.78, 126.01, 60.26, 51.24, 46.76, 35.46, 31.90, 31.62, 29.66, 29.63, 29.50, 29.45, 29.34, 29.23, 24.35, 22.67, 14.11. HRMS: M. Wt calculated for [M − I] = 391.2778; found = 391.2826.
1-Methyl-2-(3-palmitoylthiazolidin-2-yl)pyridin-1-ium iodide (QPyN16Th)
1H NMR: NMR (400 MHz, CDCl3) δ 9.35 (1H, d, J = 4.0 Hz), 8.56 (1H, t, J = 5.1 Hz), 8.43 (1H, d, J = 5.0 Hz), 7.99–7.88 (1H, m), 6.39 (1H, s), 4.68–4.63 (1H, m), 4.55 (3H, s), 4.01 (1H, dd, J = 6.4, 2.3 Hz), 3.61–3.56 (1H, m), 3.38–3.32 (1H, m), 2.70–2.61 (1H, m), 2.51–2.42 (1H, m), 1.58 (2H, dd, J = 10.0, 4.9 Hz), 1.31–1.24 (24H, m), 0.88 (3H, t, J = 4.7 Hz). 13C NMR: (100 MHz, CDCl3) δ 173.10, 147.17, 126.72, 126.09, 60.23, 51.17, 46.63, 35.44, 31.92, 31.56, 29.69, 29.50, 29.44, 29.35, 29.23, 24.34, 22.68, 14.11; HRMS: M. Wt calculated for [M − I] = 419.3091; found = 419.2853.
1-Methyl-2-(3-stearoylthiazolidin-2-yl)pyridin-1-ium iodide (QPyN18Th)
1H NMR: NMR (400 MHz, CDCl3) δ 9.35 (1H, d, J = 3.0 Hz), 8.56 (1H, t, J = 3.9 Hz), 8.42 (1H, d, J = 4.0 Hz), 7.94 (1H, t, J = 3.3 Hz), 6.39 (1H, s), 4.65 (1H, m), 4.55 (3H, s), 4.00 (1H, td, J = 4.8, 3.1 Hz), 3.58 (1H, m), 3.35 (1H, m), 2.66 (1H, dt, J = 7.7, 3.7 Hz), 2.47 (1H, dt, J = 8.0, 3.8 Hz), 1.64–1.56 (2H, m), 1.35–1.20 (28H, m), 0.93–0.83 (3H, m). 13C NMR: (100 MHz, CDCl3) δ 173.12, 157.18, 147.19, 146.83, 126.78, 126.02, 60.26, 51.21, 46.74, 35.46, 31.92, 31.60, 29.71, 29.66, 29.52, 29.45, 29.36, 29.24, 24.36, 22.69, 14.12; HRMS: M. Wt calculated for [M − I] = 447.3403; found = 447.3428.
Determination of minimum inhibitory concentration
Antibacterial activity was tested in the following microorganisms: Enterococcus faecalis (ATCC29212), Staphylococcus aureus (MTCC3160), methicillin-resistant Staphylococcus aureus (ATCC 43300), Escherichia coli (MTCC723), uropathogenic Escherichia coli (MTCC 729), and Pseudomonas aeruginosa (MTCC1688). Typically, 100 μL of QPyNATh was added to a 96-well plate and serially diluted with Luria Bertani broth. About 50 μL of 0.1 OD595nm was seeded first, followed by 50 μL of LB broth for a total amount of 200 μL. The plate was incubated overnight at 37 °C in a shaker incubator. The solution's turbidity was determined using an ELISA plate reader. Then, 50 μL (0.01%) of resazurin was added to each well and incubated for 2 h at 37 °C. The color change from blue to pink indicated the presence of living cells.17
Hemolytic assay
All experiments were performed in accordance with the Indian Council of Medical Research (ICMR) guidelines and experiments were approved by the ethics committee at SASTRA University. Informed consents were obtained from human participants of this study. With the help of a doctor, 5 mL of blood from a healthy volunteer was collected and mixed with an equal proportion of freshly prepared anticoagulants.17 RBCs were isolated from the sample by centrifugation at 1300 rpm for 30 minutes at 4 °C. The cells were then washed in PBS buffer until the supernatant was clear. In hemolytic testing, 100 μL of QPyNATh was added to the first well and diluted serially. Each well received 100 μl of RBC (4%) in PBS buffer and was incubated for 2 h at 37 °C. The negative control consisted of untreated RBCs, whereas the positive control consisted of cells treated with 8 mM Triton X-100. After two hours of incubation, the supernatant was collected, and the absorbance of the released heme was checked in a plate reader at 540 nm.
Membrane permeability assay
The membrane permeability was evaluated in uropathogenic Escherichia Coli (MTCC 729) and methicillin-resistant Staphylococcus aureus (ATCC 43300). Briefly, 0.5 OD595nm bacterial cells suspended in PBS buffer were treated with QPyNATh at 37 °C for 2 h. Untreated cells were used as the negative control, whereas cells treated with 8 mM Triton X-100 were used as a positive control. After treatment, cells were centrifuged at 3000 rpm, resuspended in PBS buffer, and stained with 50 μM propidium iodide (PI). The fluorescence of PI was measured using a spectrofluorometer (excitation wavelength 535 nm, emission wavelength 617 nm). As a measure of membrane permeabilization, the uptake of PI was calculated using the equation:17
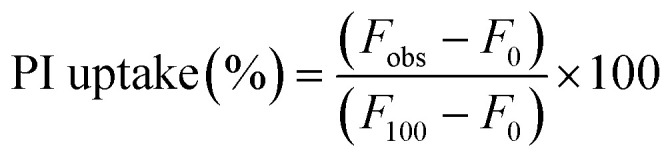 |
1 |
where F0 is the initial fluorescence of PI with bacteria in the absence of a drug; Fobs and F100 are the intensities of fluorescence observed at 2 μM QPyNATh concentration and 10 μM Triton X-100, respectively.
Membrane depolarization assay
Overnight grown bacterial cells (UPEC and MRSA) in LB media were collected by centrifugation at 3000 rpm for 15 minutes at 4 °C. The cells were rinsed twice with PBS before being suspended in HEPES buffer (7.2 pH) containing 250 mM sucrose and 5 mM MgSO4. 0.5 OD cells in HEPES buffer were treated with 3 μM membrane potential sensitive fluorescent dye 3,3′-dipropylthiacarbocyanine iodide (DiSC3(5)) and incubated at 37 °C for 1 hour to promote dye uptake by the cells.17 After incubation, the cells were rinsed with HEPES buffer to eliminate any unbound dye. The obtained cells were further disseminated in the HEPES buffer. QPyNATh was used to treat about 500 μL of DiSC3(5)-loaded cell culture. The DiSC3(5) fluorescence emission was observed at 670 nm (excitation). Untreated cells and cells treated with 10 μM Triton X-100 were used as the negative and positive control, respectively.
Reactive oxygen species assay
Typically, 0.5 OD595nm bacterial cells in PBS buffer were treated with QPyNATh at 37 °C for 2 h. After treatment, the cells were collected by centrifugation at 3000 rpm and washed thrice with PBS. The pellet was suspended in 1 mL PBS buffer containing 10 μM dichlorofluorescein diacetate (DCFH2-DA) and incubated for 30 min at 37 °C.17 After incubation, fluorescence emission was checked at 525 nm (excitation wavelength-485 nm). Untreated cells and cells treated with 10 μL of 30% H2O2 were used as the negative and positive control, respectively.
Time kill kinetics
An overnight MRSA culture in LB broth was adjusted to 0.1 OD595nm and treated with QPyN16Th at 1× MIC.21 Untreated cells served as the control. Cells were cultured at 37 °C, with 1 mL aliquots collected at a defined time point and inoculated aseptically into 20 mL LB-agar and incubated in a static incubator at 37 °C for 24 h. The number of colonies grown on the plate was manually counted and expressed as CFU mL−1. Three independent tests were performed, and the log CFU mL−1 was plotted against time.
Live and dead staining
The effect of QPyN16Th on bacterial membrane permeability was investigated using fluorescence microscopy with the probes, acridine orange (AO) and propidium iodide (PI).26 A 0.5 OD culture of bacteria was treated with QPyN16Th for one hour at 37 °C. Following the treatment, the cells were collected by centrifuging them at 3000 rpm for 10 minutes at 4 °C. The cells were resuspended in 10 mM PBS, pH of 7.4 and stained with an equal volume of 0.5 mM AO and PI. The excess AO and PI were removed by washing the cells with PBS and imaged in a fluorescence microscope using a green and red filter (Nikon Eclipse microscope).
Drug-resistance study
S. aureus (MTCC3160) was used to determine the drug resistance, where ciprofloxacin was used as the antibiotic control.21 Typically, 0.1 OD595nmS. aureus culture was exposed at 0.5× MIC of QPyN16Th and ciprofloxacin for 8 h at 37 °C. Then, cells were collected and their MIC was obtained by REMA as mentioned above. The MIC determined in the first passage was used to treat the cells for the next passage at 0.5× MIC. The experiment was repeated for 28 passages and MIC was determined in each passage and reported.
Drug testing in zebrafish
All experiments were performed in compliance with the CPCSEA guidelines for laboratory animal facilities (Central Act 26 of 1982) and the experiments were approved by the institutional ethics committee (CPCSEA-493/SASTRA/IAEC/RPP) of SASTRA University, India. Zebrafish were used to test the effectiveness of QPyN16Th in treating bacterial infections.22 Typically, 10 μL of 0.1 OD660nm MRSA culture was used to infect 20 adult fish intramuscularly. Following three hours, a group of fish received treatment with 2 μM QPyN16Th, whereas a control group received treatment with PBS buffer. Fish were collected and sacrificed, and their muscular tissues were removed at a defined time. Muscle tissues that had been dissected into around 30 mg pieces were homogenized, suitably diluted, and then plated on a sterile LB agar plate. The plates were incubated at 37 °C for 12 h. The colonies that grew on the plates were counted manually and reported in CFU mL−1.
Conclusions
In summary, we established a facile method for synthesizing the pyridyl derivative of thiazolidine. This strategy involves incorporating a fatty acid amide group by N-acylation and then methylating the N-acyl pyridyl thiazolidine to make them cationic. These processes enhance the compound's properties and potential uses, notably against antimicrobial-resistant microorganisms. QPyNATh exhibits higher selectivity for bacteria, particularly Gram-positive bacteria, than mammalian cells. N-Acylation influences antibacterial activity, with N-palmitoyl-containing cationic compounds having a lower MIC and killing MRSA in less time. A mechanistic study supports that QPyN16Th disrupts bacterial membranes and produces excess ROS, causing cell death. QPyN16Th is therapeutically effective in treating infected zebrafish by lowering the bacterial bioburden and curing the infection. The reported new series of thiazolidine was effective against resistant bacteria with specificity to Gram-positive ones with less chance of resistance development. Further research in higher animal models, as well as mechanistic study in infected cell lines, may aid in determining the specific mechanism of action of QPyN16Th, enabling clinical testing.
Data availability
The data supporting this article have been included as part of the ESI.†
Author contributions
AP: investigation, data curation, formal analysis, methodology, writing – original draft; VV: investigation, data curation, formal Analysis, writing – original draft; DKS: investigation, data curation, formal Analysis; SR: formal analysis, validation, writing – original draft; FA: resources, validation, visualization, writing – original draft; AV: conceptualization, methodology, funding acquisition, project administration, supervision, validation, visualization, writing – original draft, writing – review & editing.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
AV acknowledges the financial support given through the Indian Council of Medical Research, Government of India (67/7/2022-DDI/BMS). AP and DKS earnestly acknowledge the teaching assistantship from SASTRA Deemed University. FA acknowledges the fund by researchers supporting project number (RSP2024R364) at King Saud University, Riyadh, Saudi Arabia.
Electronic supplementary information (ESI) available: NMR; membrane integrity; ROS. See DOI: https://doi.org/10.1039/d4md00626g
References
- Gan B. H. Gaynord J. Rowe S. M. Deingruber T. Spring D. R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021;50(13):7820–7880. doi: 10.1039/D0CS00729C. https://dx.doi.org/10.1039/d0cs00729c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C. L. Antimicrobial Activity of Host-Derived Lipids. Antibiotics. 2020;9(2):75. doi: 10.3390/antibiotics9020075. https://dx.doi.org/10.3390/antibiotics9020075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Smeden J. Boiten W. A. Hankemeier T. Rissmann R. Bouwstra J. A. Vreeken R. J. Combined LC/MS-platform for analysis of all major stratum corneum lipids, and the profiling of skin substitutes. Biochim. Biophys. Acta. 2014;1841(1):70–79. doi: 10.1016/j.bbalip.2013.10.002. https://dx.doi.org/10.1016/j.bbalip.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Tollin M. Bergsson G. Kai-Larsen Y. Lengqvist J. Sjövall J. Griffiths W. Skúladóttir G. V. Haraldsson A. Jörnvall H. Gudmundsson G. H. Agerberth B. Vernix caseosa as a multi-component defence system based on polypeptides, lipids, and their interactions. Cell. Mol. Life Sci. 2005;62(19–20):2390–2399. doi: 10.1007/s00018-005-5260-7. https://dx.doi.org/10.1007/s00018-005-5260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. T. Jansen M. Yilma A. N. Nguyen A. Desharnais R. Porter E. Antimicrobial lipids: novel innate defense molecules are elevated in sinus secretions of patients with chronic rhinosinusitis. Am. J. Rhinol. Allergy. 2010;24(2):99–104. doi: 10.2500/ajra.2010.24.3444. https://dx.doi.org/10.2500/ajra.2010.24.3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C. Andersen C. J. Blesso C. N. The Role of Lipids in the Regulation of Immune Responses. Nutrients. 2023;15(18):3899. doi: 10.3390/nu15183899. https://dx.doi.org/10.3390/nu15183899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C. L. Walters K. S. Drake D. R. Dawson D. V. Blanchette D. R. Brogden K. A. Wertz P. W. Oral mucosal lipids are antibacterial against Porphyromonas gingivalis, induce ultrastructural damage, and alter bacterial lipid and protein compositions. Int. J. Oral Sci. 2013;5(3):130–140. doi: 10.1038/ijos.2013.28. https://dx.doi.org/10.1038/ijos.2013.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. E. Martinez J. J. Modulation of Host Lipid Pathways by Pathogenic Intracellular Bacteria. Pathogens. 2020;9(8):614. doi: 10.3390/pathogens9080614. https://dx.doi.org/10.3390/pathogens9080614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. Y. Yeh J. Y. Chen C. Y. Wu H. Y. Chiang M. H. Wu C. L. Lin H. J. Chiu C. H. Lai C. H. Helicobacter pylori cholesterol-α-glucosyltransferase manipulates cholesterol for bacterial adherence to gastric epithelial cells. Virulence. 2021;12(1):2341–2351. doi: 10.1080/21505594.2021.1969171. https://dx.doi.org/10.1080/21505594.2021.1969171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Shin S. J. Revolutionizing control strategies against Mycobacterium tuberculosis infection through selected targeting of lipid metabolism. Cell. Mol. Life Sci. 2023;80(10):291. doi: 10.1007/s00018-023-04914-5. https://dx.doi.org/10.1007/s00018-023-04914-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahiba N. Sethiya A. Soni J. Agarwal D. K. Agarwal S. Saturated Five-Membered Thiazolidines and Their Derivatives: From Synthesis to Biological Applications. Top. Curr. Chem. 2020;378(2):34. doi: 10.1007/s41061-020-0298-4. https://dx.doi.org/10.1007/s41061-020-0298-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long N. Le Gresley A. Wren S. P. Thiazolidinediones: An In-Depth Study of Their Synthesis and Application to Medicinal Chemistry in the Treatment of Diabetes Mellitus. ChemMedChem. 2021;16(11):1716–1735. doi: 10.1002/cmdc.202100177. https://dx.doi.org/10.1002/cmdc.202100177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa M. Verdino A. Soriente A. Marabotti A. The Odd Couple(s): An Overview of Beta-Lactam Antibiotics Bearing More Than One Pharmacophoric Group. Int. J. Mol. Sci. 2021;22(2):617. doi: 10.3390/ijms22020617. https://dx.doi.org/10.3390/ijms22020617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J. M. Quaternary ammonium disinfectants and antiseptics: tolerance, resistance and potential impact on antibiotic resistance. Antimicrob. Resist. Infect. Control. 2023;12(1):32. doi: 10.1186/s13756-023-01241-z. https://dx.doi.org/10.1186/s13756-023-01241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y. Niu L. N. Ma S. Li J. Tay F. R. Chen J. H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017;71:53–90. doi: 10.1016/j.progpolymsci.2017.03.001. https://dx.doi.org/10.1016/j.progpolymsci.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B. K. Jackman J. A. Valle-González E. R. Cho N. J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018;19(4):1114. doi: 10.3390/ijms19041114. https://dx.doi.org/10.3390/ijms19041114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniyan S. B. Sengan M. Subburethinam R. Veerappan A. Excellent Synergistic Activity of a Designed Membrane Acting Pyridinium Containing Antimicrobial Cationic N-Acylethanolamine with Isoniazid against Mycobacterium. New J. Chem. 2021;45:11937–11945. doi: 10.1039/D1NJ00776A. https://dx.doi.org/10.1039/D1NJ00776A [DOI] [Google Scholar]
- Mai-Prochnow A. Clauson M. Hong J. Murphy A. B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016;6:38610. doi: 10.1038/srep38610. https://dx.doi.org/10.1038/srep38610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörr T. Moynihan P. J. Mayer C. Editorial: Bacterial Cell Wall Structure and Dynamics. Front. Microbiol. 2019;10:2051. doi: 10.3389/fmicb.2019.02051. https://dx.doi.org/10.3389/fmicb.2019.02051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore M. C. Ritzl-Rinkenberger B. Cava F. An updated toolkit for exploring bacterial cell wall structure and dynamics. Fac. Rev. 2021;10:14. doi: 10.12703/r/10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konai M. M. Samaddar S. Bocchinfuso G. Santucci V. Stella L. Haldar J. Selectively targeting bacteria by tuning the molecular design of membrane-active peptidomimetic amphiphiles. Chem. Commun. 2018;54(39):4943–4946. doi: 10.1039/C8CC01926F. https://dx.doi.org/10.1039/c8cc01926f [DOI] [PubMed] [Google Scholar]
- Bala Subramaniyan S. Karnan Singaravelu D. Raman T. Ameen F. Veerappan A. Antimicrobial lipids loaded on lectin display reduced MIC, curtail pathogenesis and protect zebrafish from reinfection by immunomodulation. Microb. Pathog. 2024;193:106744. doi: 10.1016/j.micpath.2024.106744. https://dx.doi.org/10.1016/j.micpath.2024.106744 [DOI] [PubMed] [Google Scholar]
- Mattei B. Lira R. B. Perez K. R. Riske K. A. Membrane permeabilization induced by Triton X-100: The role of membrane phase state and edge tension. Chem. Phys. Lipids. 2017;202:28–37. doi: 10.1016/j.chemphyslip.2016.11.009. https://dx.doi.org/10.1016/j.chemphyslip.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Dharshini K. S. Ameen F. Anbazhagan V. Mechanistic Investigation on the Antibacterial Activity of Biogenic Silver Nanoparticles Prepared Using Root Extract of Sarsaparilla and Demonstrated their In Vivo Efficacy in Zebrafish Model. Curr. Microbiol. 2024;81(9):268. doi: 10.1007/s00284-024-03794-7. https://dx.doi.org/10.1007/s00284-024-03794-7 [DOI] [PubMed] [Google Scholar]
- Boix-Lemonche G. Lekka M. Skerlavaj B. A Rapid Fluorescence-Based Microplate Assay to Investigate the Interaction of Membrane Active Antimicrobial Peptides with Whole Gram-Positive Bacteria. Antibiotics. 2020;9(2):92. doi: 10.3390/antibiotics9020092. https://dx.doi.org/10.3390/antibiotics9020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniyan S. B. Vijayakumar S. Megarajan S. Kamlekar R. K. Anbazhagan V. Remarkable Effect of Jacalin in Diminishing the Protein Corona Interference in the Antibacterial Activity of Pectin-Capped Copper Sulfide Nanoparticles. ACS Omega. 2019;4(9):14049–14056. doi: 10.1021/acsomega.9b01886. https://dx.doi.org/10.1021/acsomega.9b01886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Liu D. Zhang Q. Guo P. Ding S. Shen J. Zhu K. Lin W. A Marine Antibiotic Kills Multidrug-Resistant Bacteria without Detectable High-Level Resistance. ACS Infect. Dis. 2021;7(4):884–893. doi: 10.1021/acsinfecdis.0c00913. https://dx.doi.org/10.1021/acsinfecdis.0c00913 [DOI] [PubMed] [Google Scholar]
- Neely M. N. The Zebrafish as a Model for Human Bacterial Infections. Methods Mol. Biol. 2017;1535:245–266. doi: 10.1007/978-1-4939-6673-8_16. https://dx.doi.org/10.1007/978-1-4939-6673-8_16 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been included as part of the ESI.†