Abstract
Purpose:
To determine if identifiable baseline patient characteristics predict who will benefit from pharmacomechanical catheter-directed thrombolysis (PCDT) of acute iliofemoral DVT.
Materials and Methods:
In the ATTRACT multicenter randomized trial, 381 acute iliofemoral DVT patients received PCDT and anticoagulation or anticoagulation alone. Post-hoc regression analyses evaluated correlations between baseline factors and venous clinical outcomes over 24 months. Interaction terms were examined to evaluate for differential effects by treatment arm.
Results:
Patients with clinically severe DVT (higher baseline Villalta score) experienced greater effects of PCDT in improving 24-month venous outcomes including moderate-or-severe post-thrombotic syndrome (PTS) (OR [95%CI] per unit increase in baseline Villalta score: PCDT 1.08 [1.01,1.15], Control 1.20 [1.12,1.29], p-interaction=0.03); PTS severity (between-arm differences in Villalta [p-interaction=0.004] and Venous Clinical Severity Scale [VCSS, p-interaction=0.002)] scores); and quality of life (between-arm difference in VEINES-QOL score, p-interaction=0.025). Patients with previous DVT had greater effects of PCDT on 24-month PTS severity than patients without previous DVT (mean [95%CI] between-arm difference in Villalta score: 4.2 [1.56,6.84] vs 0.9 [−0.44,2.26], p-interaction 0.03; VCSS score: 2.6 [0.94,4.21] vs 0.3 [−0.58,1.14], p-interaction=0.02). PCDT effects on some but not all outcomes were greater in patients presenting with left-sided DVT (Villalta PTS severity, p-interaction 0.04; venous ulcer, p-interaction 0.0499) or a non-compressible popliteal vein (PTS, p-interaction 0.02). PCDT effects did not vary by sex, race, ethnicity, BMI, symptom duration, hypertension, diabetes, or hypercholesterolemia.
Conclusion:
In patients with acute iliofemoral DVT, greater presenting clinical severity (higher baseline Villalta score) and a history of previous DVT predict enhanced benefits from PCDT.
Graphical Abstract
Introduction
Patients with acute iliofemoral deep vein thrombosis (DVT) frequently develop the post-thrombotic syndrome (PTS) (1). Catheter-directed thrombolysis (CDT, image-guided intra-thrombus fibrinolytic drug administration) and pharmacomechanical CDT (PCDT, includes mechanical thrombectomy devices) have been used to treat iliofemoral DVT for many years (2). In the iliofemoral DVT subgroup of the Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) Trial, PCDT led to thrombus reduction and restoration of venous patency in most treated patients, but did not reduce the occurrence of PTS over 24 months (3). PCDT did improve important secondary outcomes but the average size of these benefits and the associated improvements in health-related quality of life (QOL) were modest (4). As the trial also observed increased major bleeding in PCDT recipients, it is likely that PCDT is an optimal first-line treatment for some, but not all, iliofemoral DVT patients (5).
Published reports from ATTRACT have evaluated correlations of a limited number of pre-specified baseline factors with PCDT treatment effects, with a central focus upon the study’s primary outcome (cumulative occurrence of PTS over 24 months) (3–5). However, additional data elements were collected at study entry to characterize the study population including demographic factors, co-morbidities, and characteristics of the index DVT. Key secondary outcomes that appeared to be favorably influenced by PCDT included the severity of PTS, the occurrence of moderate-or-severe PTS, and venous disease-specific QOL (3–5). This study describes a post-hoc exploratory analysis aimed at identifying additional baseline predictors of PCDT treatment effect upon 24-month outcomes in patients with acute iliofemoral DVT.
Materials and Methods
Study Design, Patients, and Treatments
This study is a post-hoc analysis of the iliofemoral DVT subgroup of the ATTRACT trial, a Phase III, multicenter, open-label, assessor-blinded, randomized controlled trial. All patients provided written informed consent. The study was approved by the institutional review boards of all clinical centers (Appendix E1). The population, methods, and main outcomes of ATTRACT and its iliofemoral DVT subgroup have been previously described (3–5). Briefly, patients with acute symptomatic proximal DVT were randomly assigned to receive, or not receive, PCDT for initial DVT treatment at 56 U.S. clinical centers. Randomization was stratified by clinical center and by whether there was involvement of the iliac or common femoral vein (iliofemoral DVT), or not (femoral-popliteal DVT), at baseline. All study patients were to receive anticoagulant therapy and elastic compression stockings (BSN Medical); in addition, patients in the PCDT Arm underwent PCDT at a median of one day post-randomization. PCDT involved the intra-thrombus delivery of recombinant tissue plasminogen activator (rt-PA, alteplase, Activase ®, Genentech, South San Francisco, CA) by board-certified physicians using one of several methods, after which they used catheter aspiration, mechanical thrombectomy, balloon maceration, and/or stent placement to restore venous patency (5).
This analysis includes 381 ATTRACT patients with acute iliofemoral DVT who actually received their study-assigned treatment within 7 days (per-protocol study population) (Figure 1).
Figure 1 -. Patient Flow (CONSORT) Diagram.
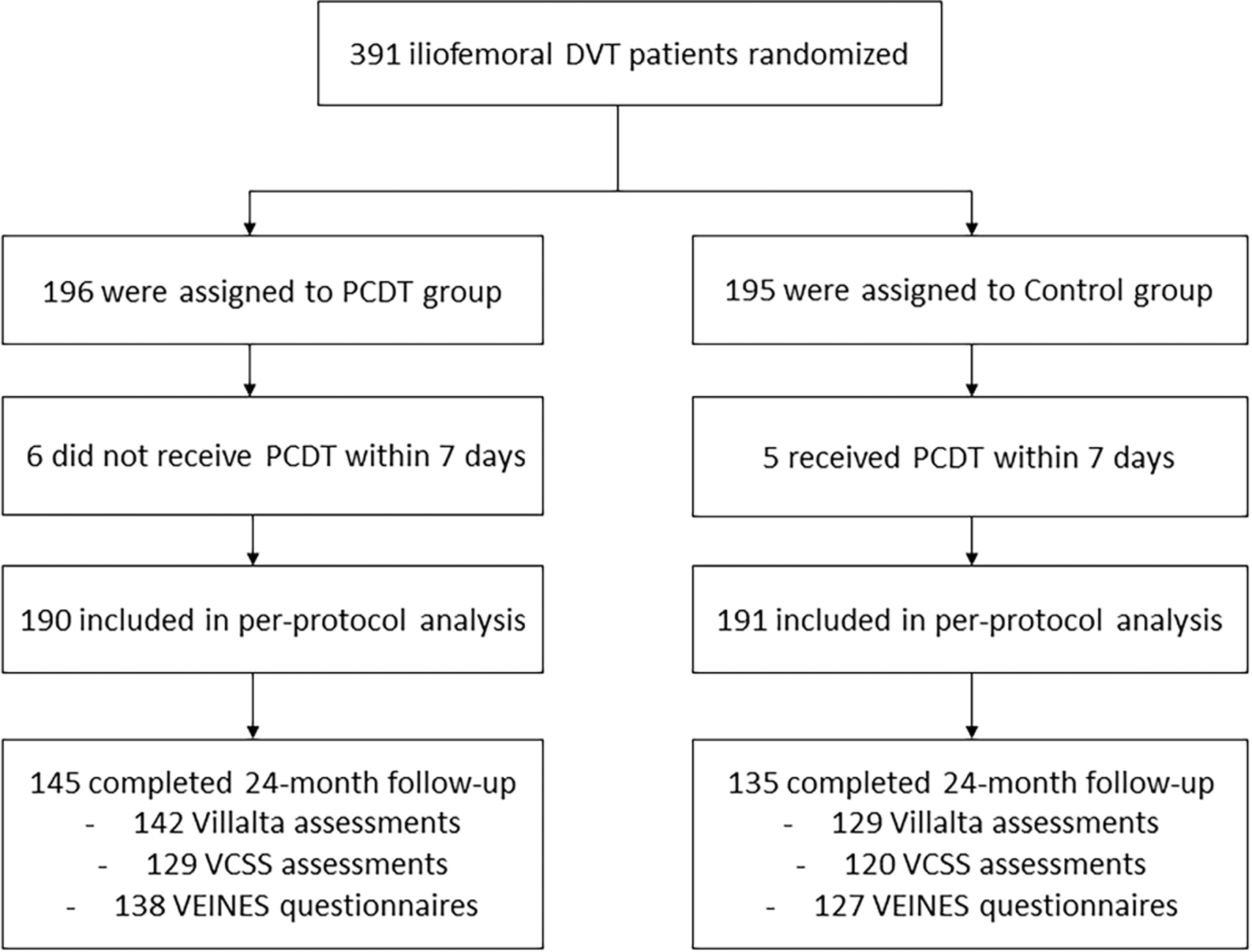
Patient flow and outcome data in the ATTRACT Trial (per-protocol analysis population).
Baseline Characteristics
Previous publications have summarized subgroup analyses for 11 categorically expressed baseline factors that were pre-specified in the study’s statistical analysis plan (3–5). This post hoc analysis considers a more detailed set of potential PCDT effect predictors collected at baseline including demographic characteristics (sex, continuous age, race, Hispanic/Latino ethnicity), medical history (hypertension, diabetes, hypercholesterolemia, previous DVT, continuous body-mass index [BMI]), and index DVT features (right or left leg, provoked or unprovoked, popliteal vein compressibility, continuous DVT symptom duration, continuous Villalta clinical severity scores). For some factors, categories that did not constitute at least 10% of the population were combined with an adjacent category for analysis (Appendix E2). The distributions of baseline factors were well-balanced between the two treatment groups (Table 1).
TABLE 1 –
Distribution of Baseline Variables
| Factor | Overall (N=381) | PCDT Arm (N=190) | Control Arm (N=191) | P Value |
|---|---|---|---|---|
| Age (years) | 51.0 [39.0,62.0] | 51.0 [38.0,62.0] | 52.0 [42.0,61.0] | 0.65 a |
| Sex | 0.72 b | |||
| Male | 203 (53.3%) | 103 (54.2%) | 100 (52.4%) | |
| Female | 178 (46.7%) | 87 (45.8%) | 91 (47.6%) | |
| Race | 0.28 b | |||
| White | 296 (77.7%) | 152 (80.0%) | 144 (75.4%) | |
| Other | 85 (22.3%) | 38 (20.0%) | 47 (24.6%) | |
| Ethnicity | 0.34 b | |||
| Not Hispanic or Latino | 352 (92.4%) | 178 (93.7%) | 174 (91.1%) | |
| Hispanic or Latino | 29 (7.6%) | 12 (6.3%) | 17 (8.9%) | |
| BMI (kg/m2) | 30.8 [27.0,36.7] | 30.9 [27.6,36.9] | 30.8 [25.5,36.4] | 0.26 a |
| Index Leg | 0.78 b | |||
| Left | 244 (64.0%) | 123 (64.7%) | 121 (63.4%) | |
| Right | 137 (36.0%) | 67 (35.3%) | 70 (36.6%) | |
| Previous DVT | 0.64 b | |||
| No | 295 (77.4%) | 149 (78.4%) | 146 (76.4%) | |
| Yes | 86 (22.6%) | 41 (21.6%) | 45 (23.6%) | |
| Provoked DVT | 0.42 b | |||
| No | 319 (83.7%) | 162 (85.3%) | 157 (82.2%) | |
| Yes | 62 (16.3%) | 28 (14.7%) | 34 (17.8%) | |
| Hypertension | 0.23 b | |||
| No | 226 (59.3%) | 107 (56.3%) | 119 (62.3%) | |
| Yes | 155 (40.7%) | 83 (43.7%) | 72 (37.7%) | |
| Diabetes | 0.41 b | |||
| No | 311 (81.6%) | 152 (80.0%) | 159 (83.2%) | |
| Yes | 70 (18.4%) | 38 (20.0%) | 32 (16.8%) | |
| High Cholesterol | 0.55 b | |||
| No | 270 (70.9%) | 132 (69.5%) | 138 (72.3%) | |
| Yes | 111 (29.1%) | 58 (30.5%) | 53 (27.7%) | |
| PV Compressible | 0.89 b | |||
| No | 273 (80.1%) | 135 (80.4%) | 138 (79.8%) | |
| Yes | 68 (19.9%) | 33 (19.6%) | 35 (20.2%) | |
| Total Villalta Score | 10.0 [6.0,14.0] | 10.0 [7.0,14.0] | 9.6 [6.0,14.0] | 0.23a |
| Symptom Duration (days) | 6.0 [3.0,9.0] | 6.0 [3.0,9.0] | 6.0 [3.0,9.0] | 0.42 a |
PCDT = pharmacomechanical catheter-directed thrombolysis
DVT = deep vein thrombosis
PV = popliteal vein
BMI = body-mass index
= Kruskal-Wallis test
= Pearson’s Chi-square test
Clinical Outcomes
Patient outcomes were assessed at 6, 12, 18, and 24 months after randomization by blinded clinician examiners. PTS was evaluated with the Villalta Scale, in which 5 patient-reported symptoms (pain, cramps, heaviness, pruritus, paresthesia) and 6 clinician-observed signs (edema, skin induration, hyperpigmentation, venous ectasia, redness, pain on calf compression) are scored 0–3 and summed together (6). PTS was also assessed using the modified Venous Clinical Severity Scale (VCSS) in which 9 items (8 signs, 1 symptom) are scored 0–3 and summed together (7).
Categorical clinical outcomes in this analysis were the cumulative occurrence over 24 months of: any PTS (Villalta ≥5 or an ulcer), moderate-or-severe PTS (Villalta ≥10 or an ulcer), severe PTS (Villalta ≥15 or an ulcer), and venous ulcer. Continuous clinical outcomes included PTS severity at 24 months as measured by the Villalta score (range, 0–33) and modified VCSS score (range, 0–27); for both scales, higher scores indicate more severe PTS (6,7). Patient-reported venous disease-specific QOL at 24 months was assessed using the Venous Insufficiency Epidemiologic and Economic Quality of Life Survey (baseline-adjusted VEINES-QOL and VEINES-Sym subscores, reflecting venous QOL and venous symptoms, respectively) (4,8).
Statistical Analysis
Descriptive statistics were used to summarize baseline patient characteristics. Continuous data were summarized using mean (standard deviation [SD]) and median (inter-quartile range [IQR]). Categorical variables were summarized as proportions. Kruskal-Wallis, Chi-Square, and Fisher’s Exact Tests (when appropriate) were performed to evaluate for associations of baseline variables with treatment response outcomes. Logistic regression was used to assess associations between independent variables and four binary outcomes (PTS, moderate-or-severe PTS, severe PTS, venous ulcer). Linear regression models were used to identify associations between baseline variables and four continuous outcomes at 24 months: Villalta, VCSS, VEINES-QOL, and VEINES-Sym scores. Each model examined the interaction term between the treatment assignment and baseline variables to assess whether there was a differential treatment effect for each level of the baseline variables. The odds ratios (OR) and their 95% confidence intervals (95%CI) were reported for logistic regression models. Model estimates, standard error, and mean differences were reported for linear regression models. To visualize the associations between treatment assignment and four continuous baseline variables of interest (Villalta score, DVT symptom duration, patient age, BMI), plots depicting the predicted probabilities of PTS and moderate-or-severe PTS as a function of the continuous baseline variables, with 95%CIs, were created for each treatment group based on the logistic regression model. Plots were also created to depict the mean scores at 24 months on the continuous outcome measures, with 95%CIs, as a function of the same four continuous baseline variables of interest.
A two-sided P value < 0.05 was considered statistically significant for these exploratory analyses. Analyses were conducted in SAS version 9.4 (SAS, Cary, NC).
Results
Demographical Factors
Patient sex, race (white versus non-white), and ethnicity (Hispanic/Latino versus not) were not associated with differential effects of PCDT upon binary (Figure 2, Appendix E3) or continuous (Figure 3, Appendix E4) outcomes. Younger patients receiving PCDT had nominally lower odds of developing moderate-or-severe PTS over 24 months, but this finding was not statistically significant (OR [95% CI] per 5-year increase in age: PCDT 1.20 [1.05, 1.40], Control 1.03 [0.91, 1.15], p-interaction=0.08). Predicted probability plots from this model (Figure 4, Appendices E5-E8) suggest that PCDT may be more likely to reduce moderate-or-severe PTS relative to Control treatment in younger patients up to approximately 650 years of age (Figure 4C).
Figure 2 – Baseline Predictors of PCDT Effect on 24-Month Binary Venous Outcomes.
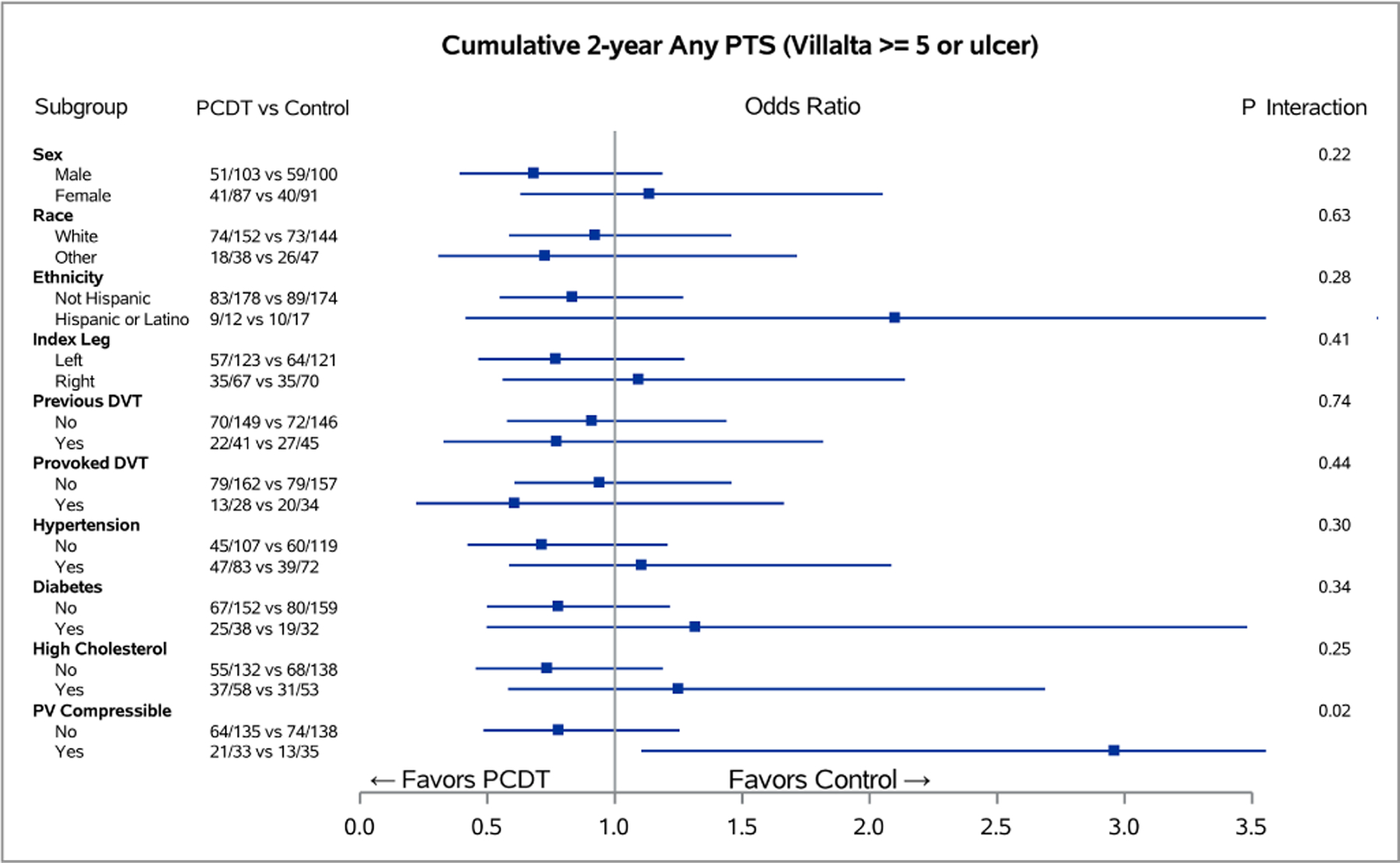
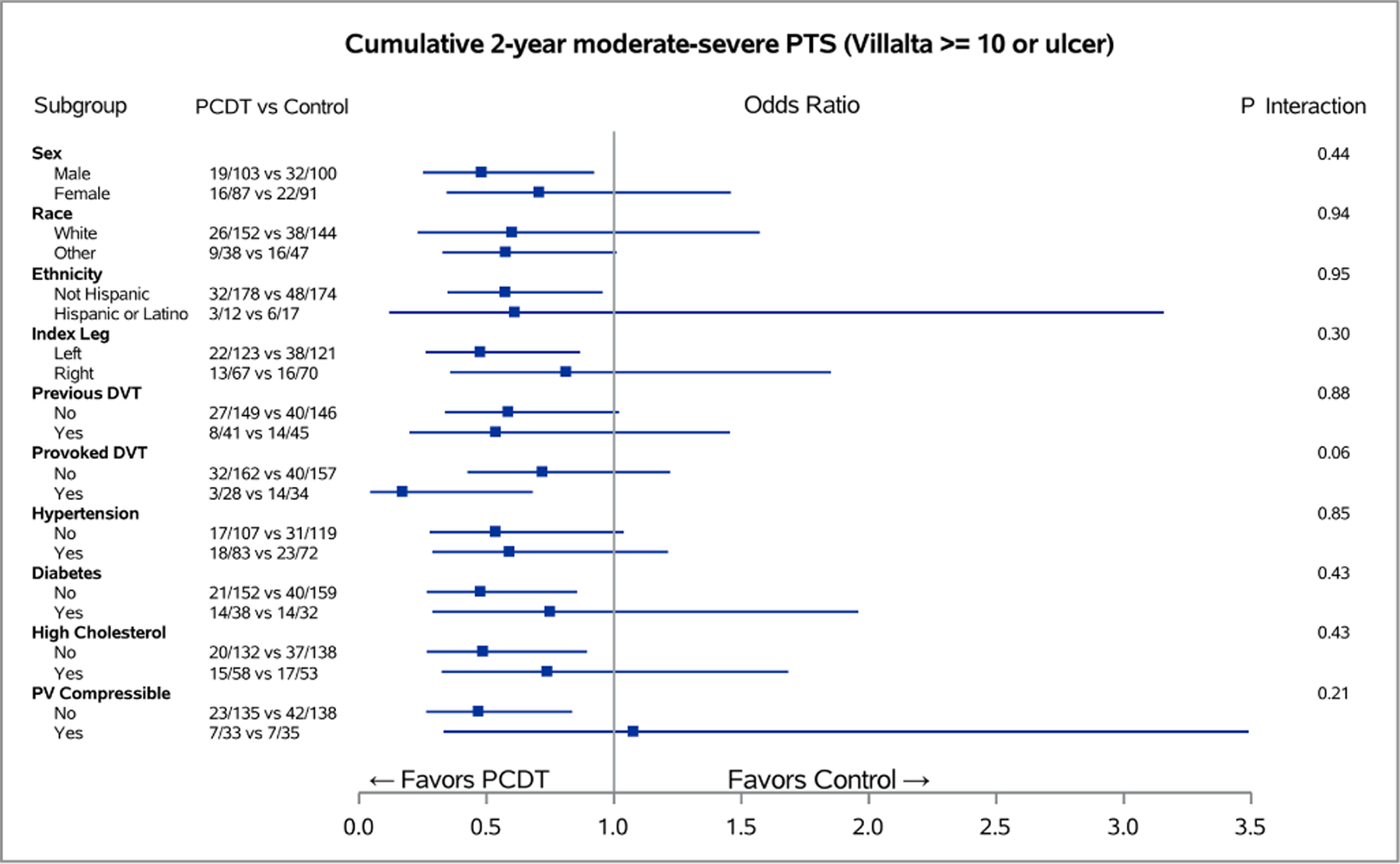
Forest plots of odds ratios for the 24-month cumulative occurrences of PTS (A) and moderate-or-severe PTS (B), in subgroups of ATTRACT patients with acute iliofemoral DVT for each level of baseline factors. The horizontal lines represent 95% confidence intervals (CIs).
Figure 3 – Baseline Predictors of PCDT Effect on 24-Month Continuous Venous Outcomes.
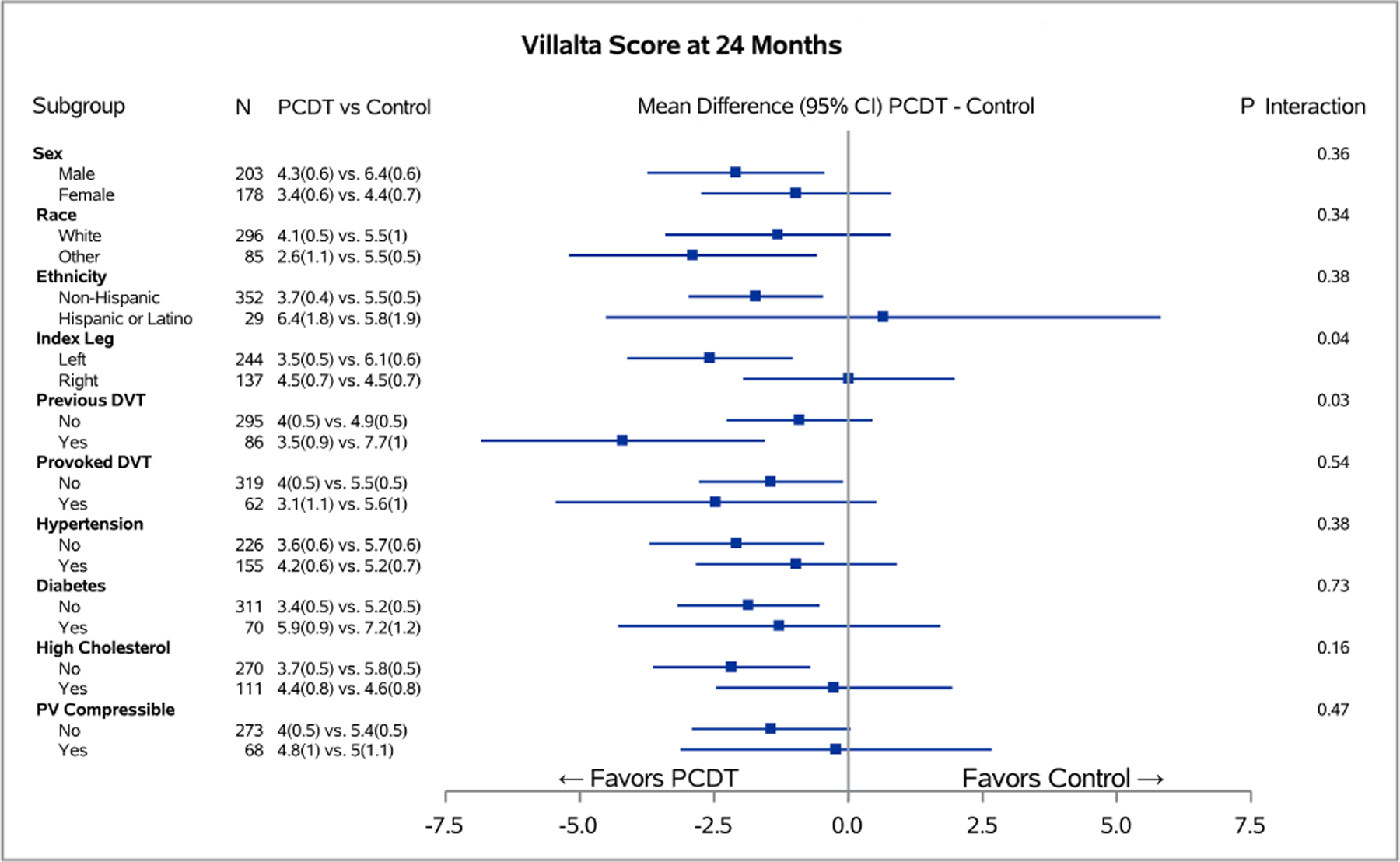
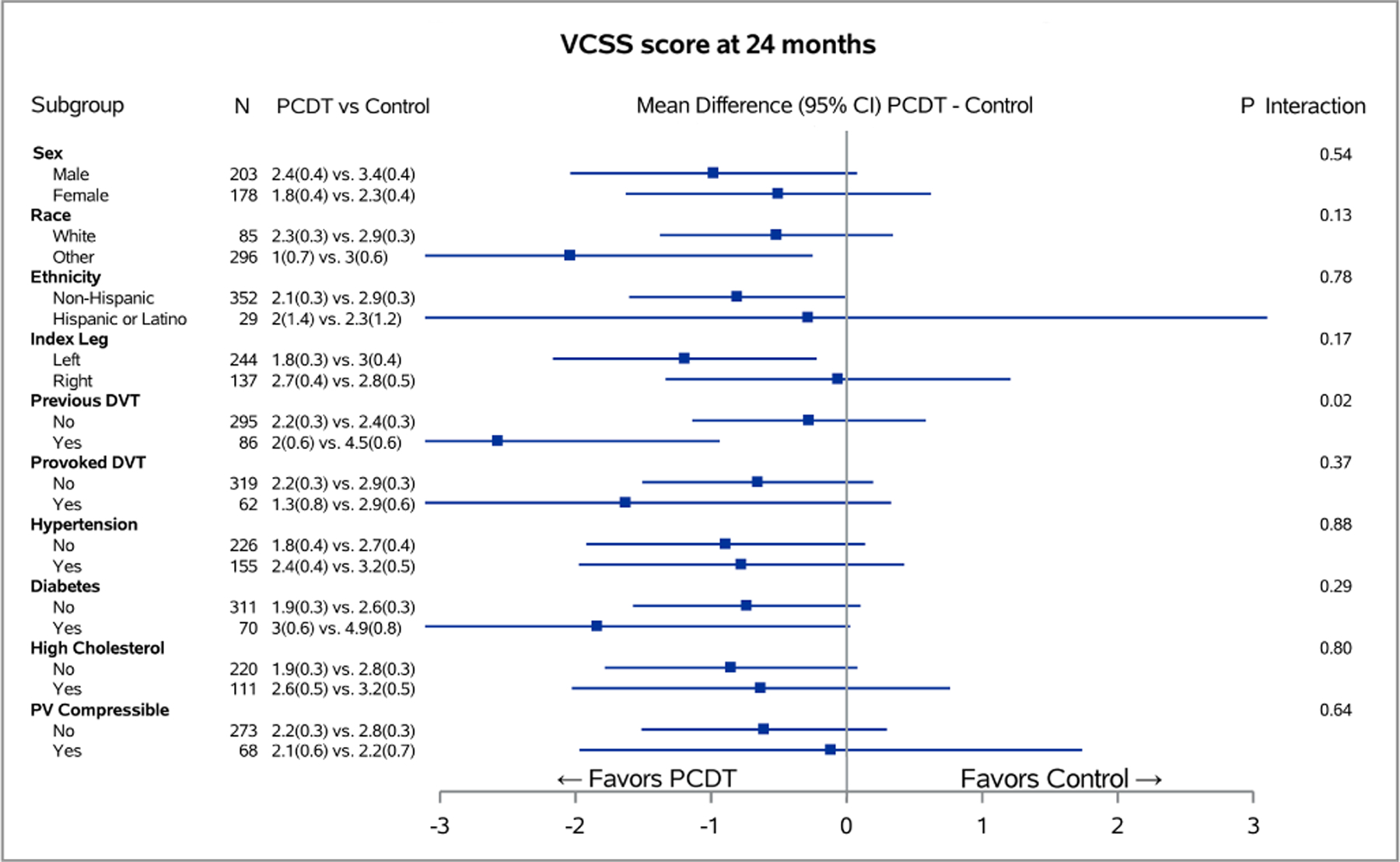
Associations of binary baseline factors with 24-month Villalta (A) and Venous Clinical Severity Scale (B) scores in subgroups of ATTRACT patients with acute iliofemoral DVT for each level of baseline factors. The horizontal lines represent 95% confidence intervals (CIs).
Figure 4 – Predicted Probabilities of Venous Outcomes by Continuous Baseline Factors.
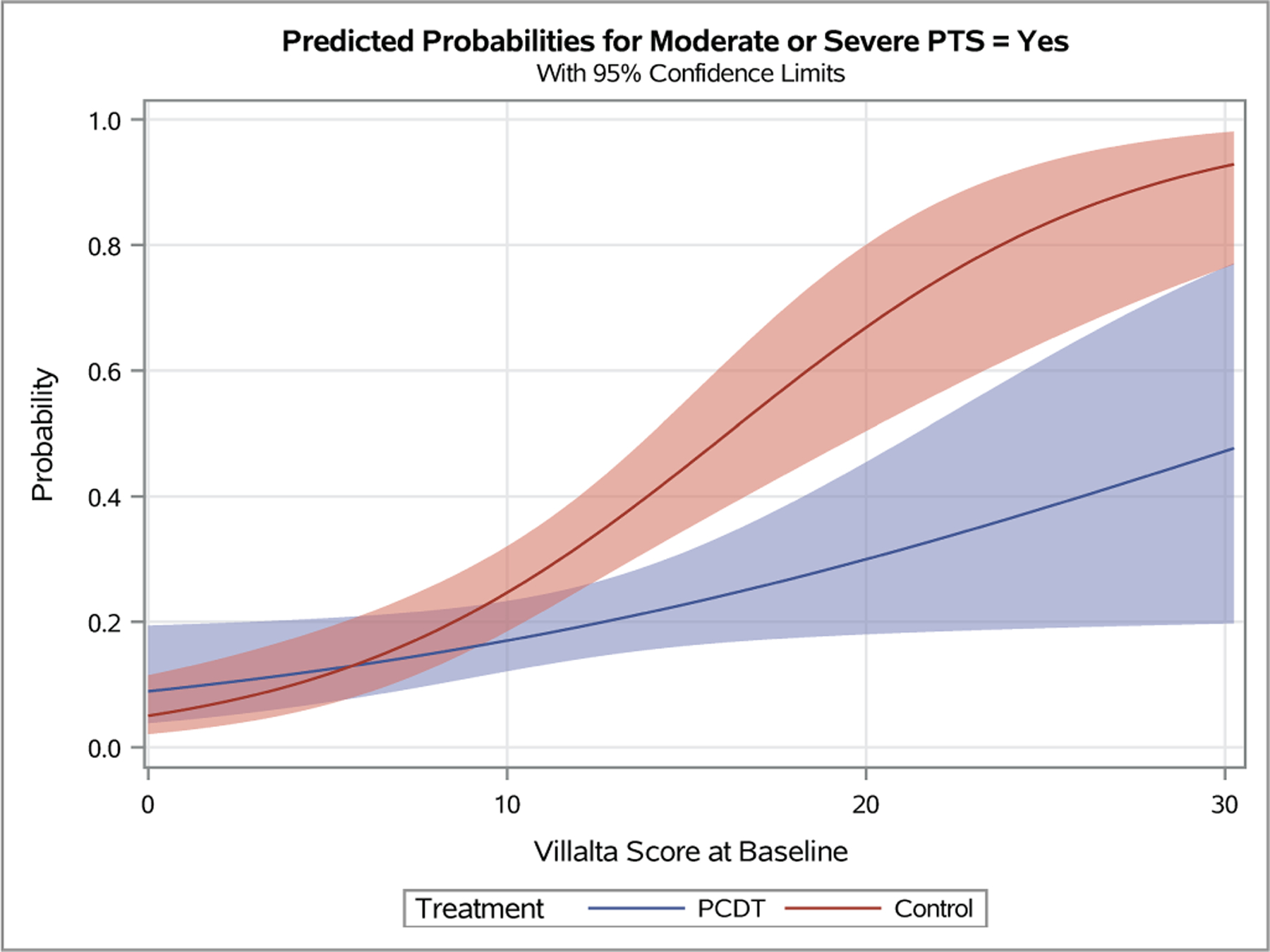
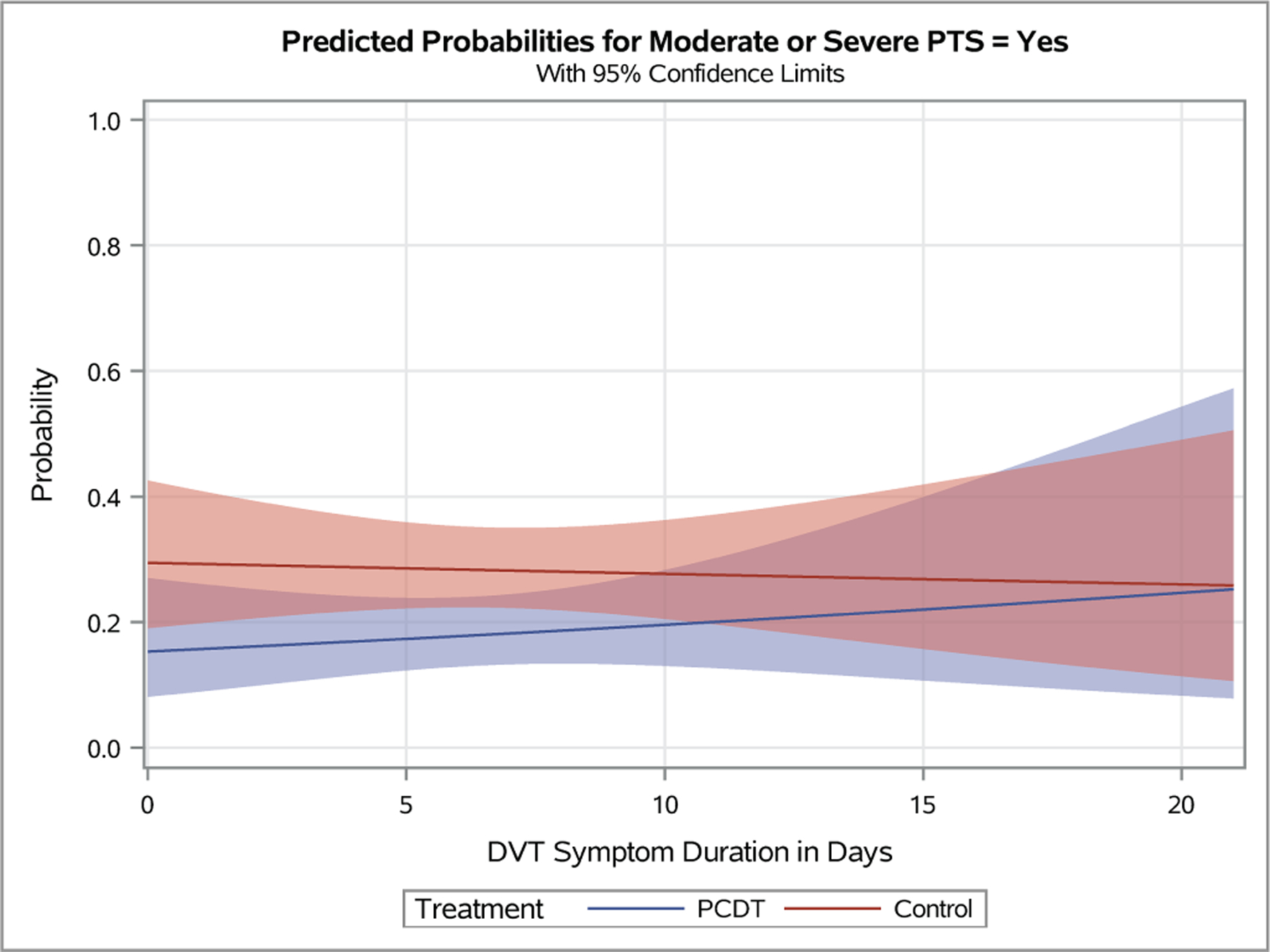
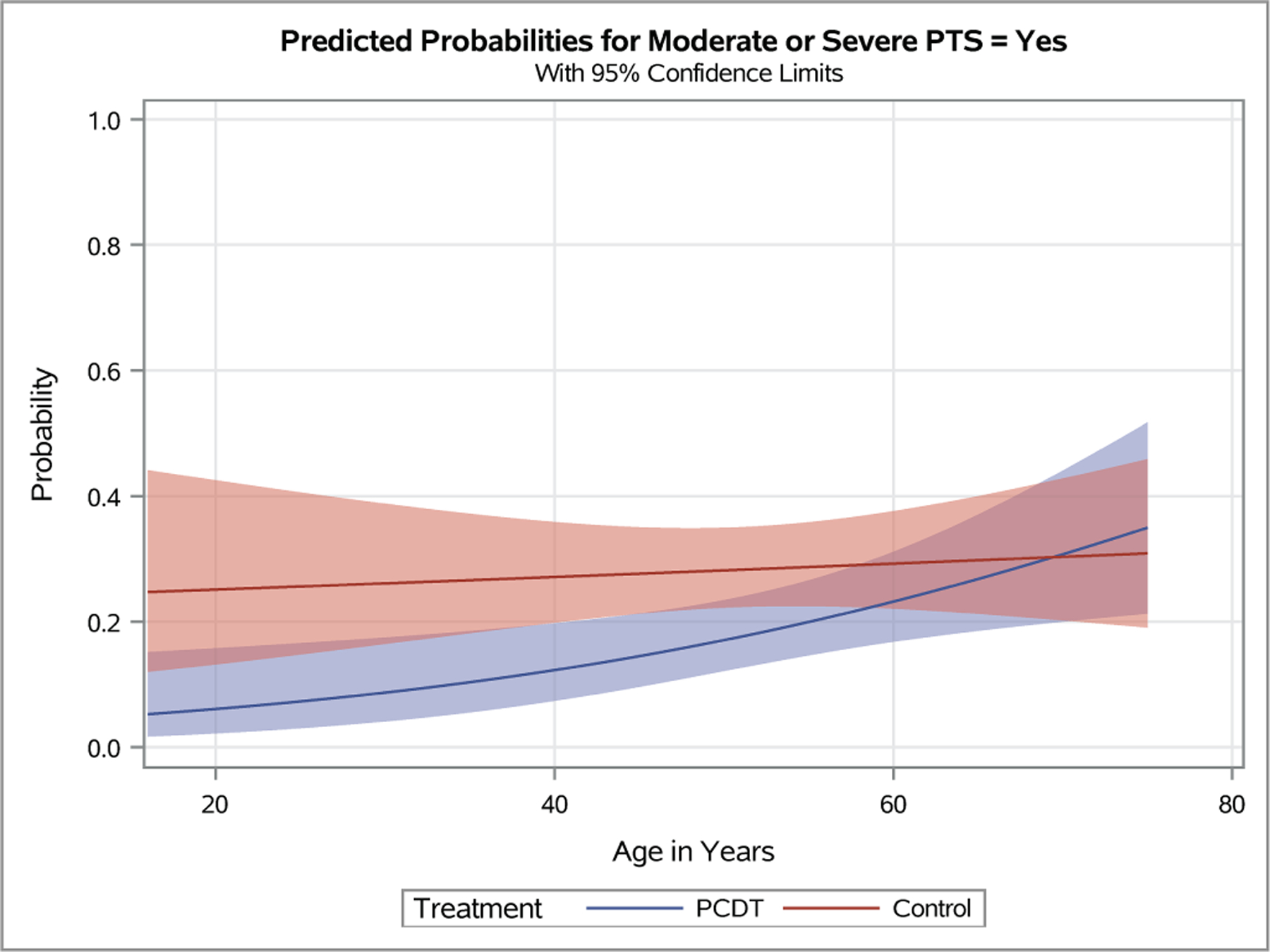
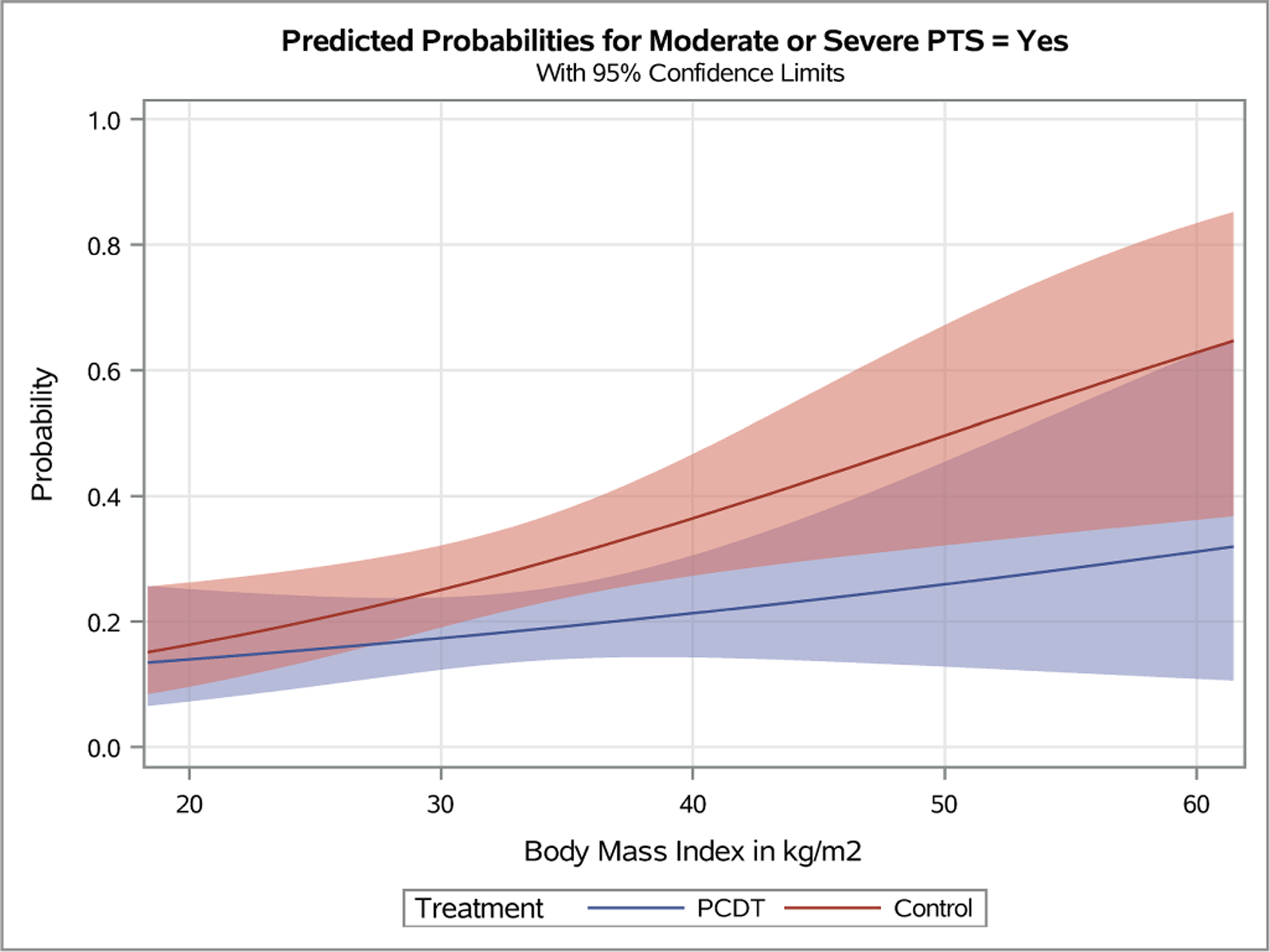
Plots depicting the predicted probabilities of moderate-or-severe PTS in patients with increasing baseline Villalta score (A), symptom duration (B), patient age (C), and body-mass index (D). The light red and blue colored bands represent the 95% confidence intervals (CIs) around the estimates for each respective treatment arm; the dark red/purple bands represent areas of overlap between the 95% CIs for the two treatment arms.
Medical History and Co-Morbidities
Patients with previous DVT experienced a greater effect of PCDT upon 24-month PTS severity than patients with no previous DVT (mean [95% CI] PCDT-Control difference in Villalta score 4.2 [1.56, 6.84] points versus 0.9 [−0.44, 2.26] points, p-interaction=0.03) (Figure 3A); and VCSS score (2.6 [0.94, 4.21] points versus 0.3 [−0.58, 1.14] points, p-interaction=0.02) (Figure 3B). PCDT’s effects upon binary outcomes (Figure 2) and QOL (Appendix E4) were nominally greater in patients with previous DVT compared to no previous DVT, but these findings were not statistically significant.
The impact of baseline continuous variables on PCDT effects on continuous outcomes are presented in Figure 5 and Appendices E9-E17. Continuous BMI was not a statistically significant predictor of PCDT treatment effect in the overall model (moderate-or-severe PTS: OR [95% CI] per 5-unit increase in BMI: PCDT 1.14 [0.90, 1.44], Control 1.31 [1.08, 1.59], p-interaction=0.36). Predicted probability plots from this model depict a gradual divergence of the PCDT and Control curves for moderate-or-severe PTS as the BMI increased (Figure 4D). The effect of PCDT appears prominent for patients with a very high BMI (moderate-or-severe PTS in patients with BMI > 40 kg/m2: OR 0.23 [0.07, 0.75]), but the 95% confidence intervals overlap with those for patients with BMI < 40 kg/m2 (OR 0.71 [0.41, 1.21]), perhaps reflecting the fact that these estimates are based on very small numbers of patients who had a BMI of that magnitude. BMI did not exert significant effects on between-arm differences in Villalta, VCSS, and VEINES-QOL scores (Appendices E15-E17) (). Pooled across treatment arms, patients with elevated BMI did have increased odds of developing moderate-or-severe PTS (p=0.006).
Figure 5 – Association of Continuous Baseline Factors with 24-Month Venous Outcomes.
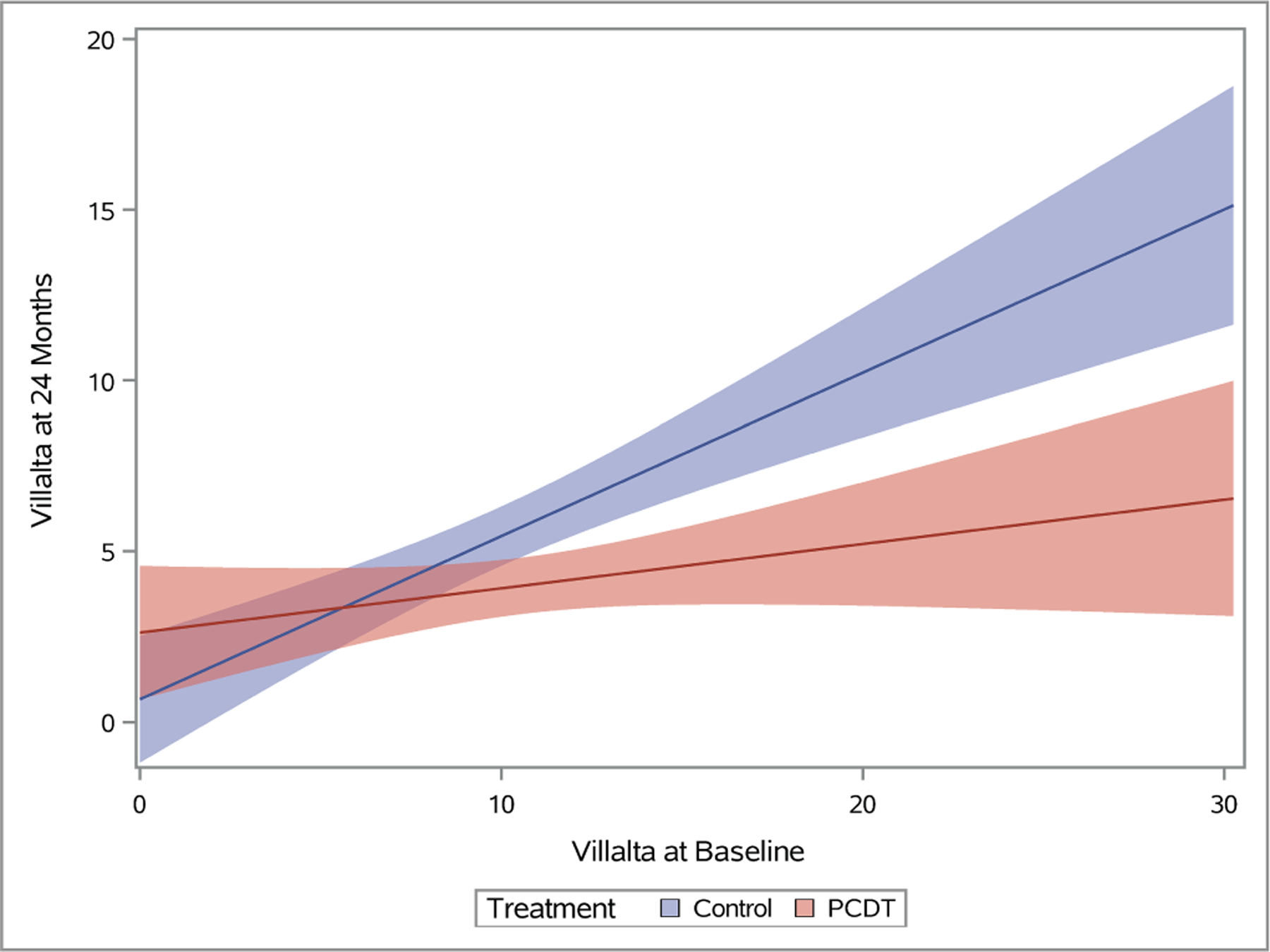
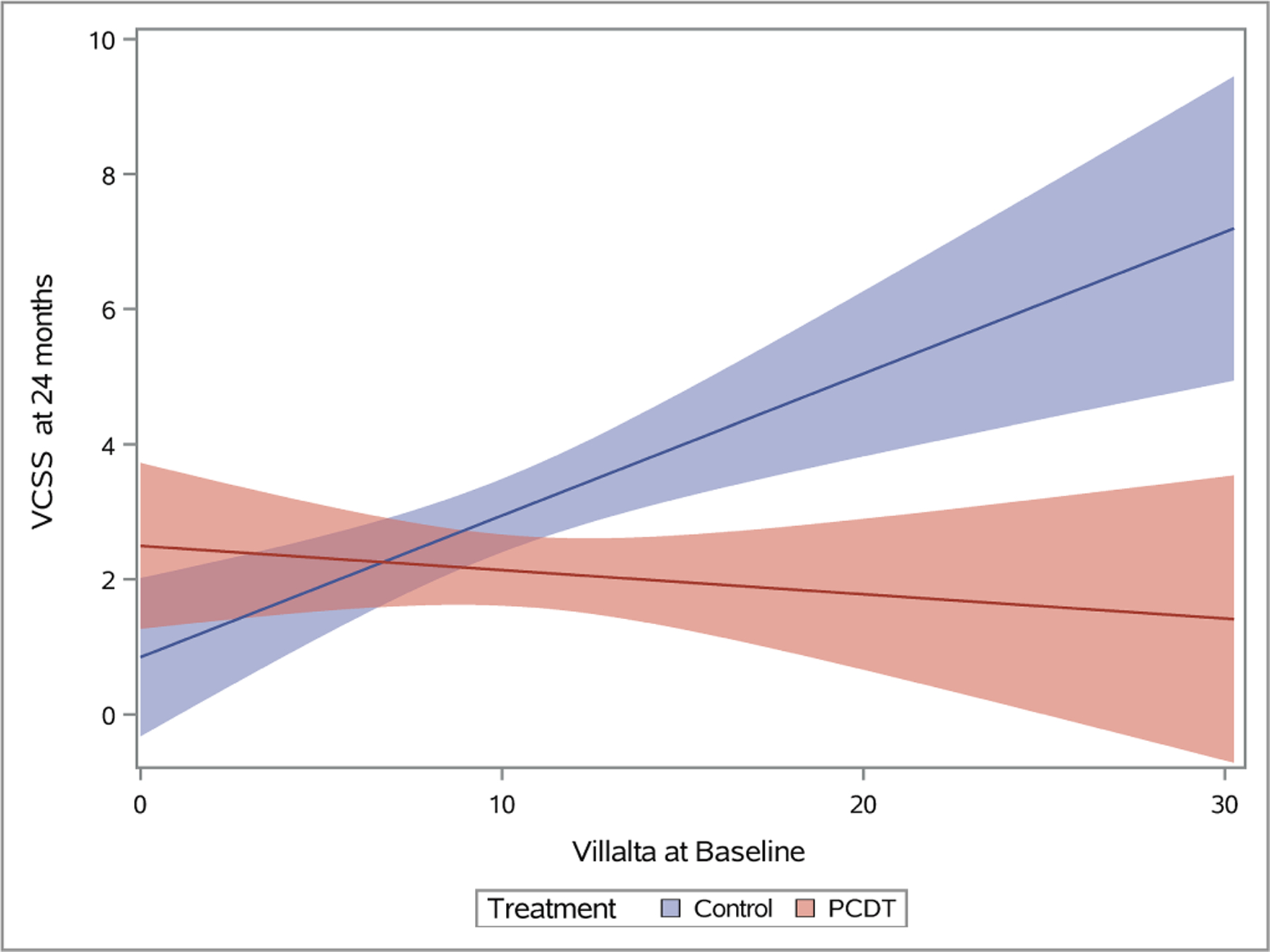
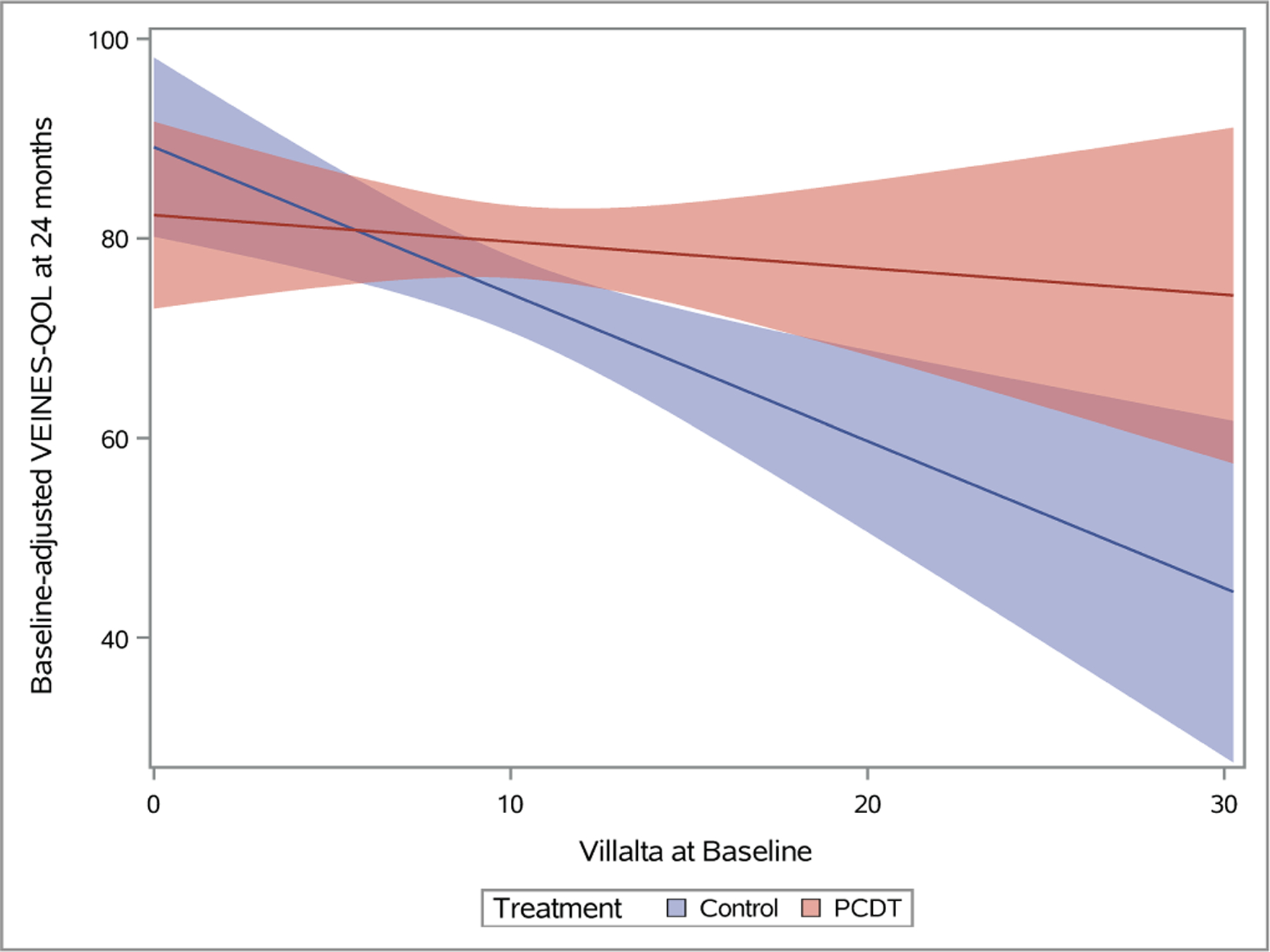
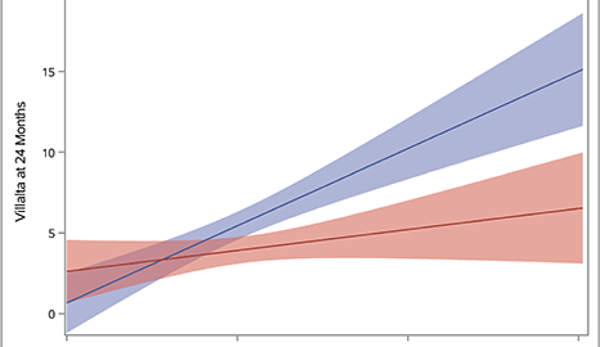
Plots depicting predicted mean scores for the Villalta (A), Venous Clinical Severity Scale (B), and baseline-adjusted VEINES-QOL (C) scores at 24 months as a function of increasing continuous baseline Villalta score. The light red and blue colored bands represent the 95% confidence intervals (CIs) around the estimates for each respective treatment arm; the dark red/purple bands represent areas of overlap between the 95% CIs for the two treatment arms.
A history of hypertension, diabetes, or hypercholesterolemia did not show a differential effect by treatment arm on clinical outcomes (Figures 2–3, Appendices E3-E4).
Characteristics of Presenting DVT Episode
A patient’s presenting DVT clinical severity was a key predictor of the effects of PCDT upon 24-month clinical outcomes in this analysis of iliofemoral DVT patients. Adjusted for treatment assignment, as the baseline Villalta score increased, the occurrence of PTS increased (p<0.0001 in logistic interaction model). Use of PCDT was associated with lower odds of moderate-or-severe PTS in patients with higher baseline Villalta scores (OR [95% CI] per unit increase in baseline Villalta score: PCDT 1.08 [1.01, 1.15], Control 1.20 [1.12, 1.29], p-interaction=0.03). This effect was also nominally apparent for PTS but was not statistical significant (OR [95% CI] per unit increase in baseline Villalta score: PCDT 1.06 [1.01, 1.13], Control 1.14 [1.07, 1.21], p-interaction=0.11). Predicted probability plots from the models suggest that relative to Control, PCDT is most likely to reduce moderate-or-severe PTS in patients with a baseline Villalta score that exceeds 10 (Figure 4A).
As the baseline Villalta score increased, the benefits of PCDT upon PTS severity and venous QOL also increased. For patients with a baseline Villalta score > 10, the mean 24-month Villalta (p-interaction=0.004) (Figure 5A) and VCSS (p-interaction=0.002) (Figure 5B) scores were lower in the PCDT Arm compared with the Control Arm, and the mean VEINES-QOL (p-interaction=0.025) (Figure 5C) and VEINES-Sym (p-interaction=0.02) scores were higher for PCDT compared with Control, with 95% confidence intervals that did not overlap.
DVT symptom duration did not predict the effects of PCDT on 24-month occurrences of PTS (OR [95% CI] per additional day of symptoms: PCDT 1.04 [0.96, 1.11], Control 1.00 [0.94, 1.06], p-interaction=0.46) or moderate-or-severe PTS (p-interaction=0.52), or on between-arm differences in 24-month Villalta or VCSS scores. Although gradual divergence of PCDT and Control curves for VEINES-QOL and VEINES-Sym is apparent as symptom duration increases, the 95% CIs around these curves overlapped at all symptom durations (Appendices E9-E11).
For all outcomes assessed over 24 months, PCDT was nominally more effective in patients with left leg DVT than right leg DVT, but the differences only reached statistical significance for PTS severity (mean [95% CI] PCDT-Control difference in Villalta score: left DVT 2.60 [1.04, 4.12] points, right DVT 0.01 [−1.96, 1.98] points; p-interaction=0.04) (Figure 3A) and venous ulcer (left leg: PCDT 2.4% versus Control 7.4%; right leg: PCDT 9.0% versus Control 4.3%; p-interaction=0.049) (Appendix E3). Similarly, patients with a non-compressible popliteal vein appeared to have nominally more favorable outcomes with PCDT than patients with a compressible popliteal vein, but significance was only seen for PTS: (compressible: PCDT 64% versus Control 37%; non-compressible: PCDT 47% versus Control 54%, p-interaction=0.02) (Figures 2–3). The presence of provoking risk factors at the time of the index DVT did not show a differential effect by treatment arm upon clinical outcomes (Figures 2–3).
Discussion
This analysis of the ATTRACT Trial found that in patients with acute iliofemoral DVT, (a) higher presenting clinical severity (baseline Villalta score) predicts greater benefits of PCDT (versus anticoagulation alone) upon PTS severity, moderate-or-severe PTS, and venous QOL over 24 months; (b) a history of previous DVT predicts greater PCDT effects in reducing 24-month PTS severity; (c) patients with left-sided DVT or a non-compressible popliteal vein at baseline may experience stronger PCDT effects on some 24-month outcomes, but these findings were not compelling in magnitude or consistency; and (d) PCDT treatment effects did not differ based on sex, race, Hispanic/Latino ethnicity, continuous BMI, continuous symptom duration (within trial parameters), hypertension, diabetes, hypercholesterolemia, or provoked DVT.
Although some societal guidelines now suggest use of CDT/PCDT for selected patients with acute iliofemoral DVT, evidence linking discernible baseline patient characteristics to the treatment effects of endovascular therapies has not been available to guide such decisions (9,10). In the randomized Catheter-Directed Venous Thrombolysis (CAVENT) Trial, baseline factors including symptom duration (≤21 days), thrombus extent, and side of DVT did not influence thrombolysis grade, residual thrombus score, patency, or PTS (11,12). Late patency was more frequent in women but PTS was similar for men and women. Left leg DVT predicted lower 24-month Villalta scores than right leg DVT, but PTS occurrence was similar in both legs. The randomized CAVA Trial, which studied ultrasound-assisted CDT for acute iliofemoral DVT, was unable to identify baseline modifiers of the treatment effects of the intervention (13).
A pre-specified subgroup analysis of ATTRACT found PCDT to lead to a higher PTS occurrence over 2 years in proximal DVT patients ≥ 65 years of age, compared with younger patients (p-interaction=0.04); age ≥ 65 years also predicted major bleeding (p<0.0001) (5,14). In the trial’s iliofemoral DVT subgroup, PCDT reduced the occurrence of moderate-or-severe PTS over 2 years in patients < 65 years old (PCDT 16% versus Control 30%) compared with patients ≥ 65 years old (PCDT 28% versus Control 19%) (p-interaction=0.04) (3). Hence, it has been noted that patient age is relevant to factor into PCDT treatment decisions. That conclusion is supported to some extent here by the predicted probability plots which suggest that moderate-or-severe PTS is less frequent in PCDT-treated patients up to around 65 years of age. However, viewed as a continuous variable, age was not a statistically significant predictor of PCDT effect in this study, perhaps partly due to the limited number of very young patients in the analysis.
In this analysis, a history of previous DVT predicted a stronger effect of PCDT upon PTS severity. In theory, this finding could be explained by (a) a greater ability of anticoagulation alone to permit restoration of a normal venous system in limbs with only acute thrombus (no previous event); and/or (b) a greater likelihood that patients with previous DVT had chronic venous abnormalities (compression, residual thrombus) that were addressed via adjunctive endovascular therapy during PCDT. Similarly, patients with a compressible popliteal vein may respond well to anticoagulation alone, and treatment of iliac vein compression in patients with left leg DVT could account for the finding of greater PCDT benefit in some analyses.
Randomized trials of CDT/PCDT have limited enrollment to patients with symptom duration ≤ 14–21 days. In a venogram analysis of ATTRACT PCDT recipients, patients randomized within 7 days of symptom onset had a higher rate of complete lysis (35% versus 23%, p-interaction=0.04) and nominally greater thrombus removal (88% versus 82%, p=0.06) than patients randomized 7–14 days after symptom onset (15). However, complete lysis did not lead to reduced PTS and the effect of PCDT on PTS prevention did not differ between these two groups. The current study, which analyzed symptom duration as a continuous variable, also does not suggest a major effect on clinical outcomes that would argue for expedited conduct of PCDT.
The current analysis identified greater presenting DVT severity (higher baseline Villalta score) as a key predictor of enhanced PCDT effect in improving 24-month venous outcomes. Patients with a baseline Villalta score >10 experienced very high rates of moderate-or-severe PTS. In these patients, PCDT reduces the probability of developing moderate-or-severe PTS by over one-third, with the projected effect increasing as the baseline clinical severity increases. These effects were also visualized in consistently larger differences between the PCDT and Control Arms in PTS severity scores and QOL in patients with severe clinical presentations.
The Villalta Scale was originally developed as a tool to identify and quantify PTS, and is endorsed for this purpose by the International Society of Hemostasis and Thrombosis (6). In ATTRACT, the Villalta scale was also used to grade the clinical severity of DVT in the acute phase. The correlations identified in this analysis suggest that using the Villalta Scale at the time of DVT diagnosis may help in stratifying a patient’s risk of developing clinically important PTS and in predicting the effects of more aggressive treatment.
The current analysis has several limitations. In ATTRACT, benefits of PCDT upon PTS severity and venous QOL were mainly apparent in the iliofemoral DVT subgroup. Stratification of randomization by baseline thrombus extent enhanced the study’s ability to evaluate PCDT treatment effects within each anatomic subgroup. For these reasons, the current analysis was limited to the iliofemoral subgroup; however, this reduced the sample size and statistical power. The study was not able to analyze genetic determinants or other biomarkers that might predict PCDT effects. Because this study was intended as an exploratory analysis that would transparently present the observed outcomes against the baseline factors, subgrouping of continuous scale data by arbitrarily selected threshold cut-points was not performed, and a P-value threshold of 0.05 was used for statistical significance. The predicted probability plots should be interpreted with care since for any variable, the number of patients at each point on the spectrum varied, precluding robust statistical comparisons. For these reasons, prospective confirmation of these findings in additional studies would be desirable.
In conclusion, in patients with acute iliofemoral DVT, PCDT is more effective in improving 24-month venous outcome in patients with more severe baseline clinical presentation or a history of previous DVT. Left-sided DVT and a non-compressible popliteal vein may also connote a greater likelihood to benefit from PCDT, but these apparent relationships are less conclusive since they were only seen for some outcomes evaluated. The findings of this analysis may help to inform the design of future studies evaluating new or existing thrombus removal strategies.
Supplementary Material
Research Highlights.
A post-hoc analysis of the iliofemoral DVT subgroup of the ATTRACT multicenter randomized trial was performed to identify baseline predictors of treatment effect of pharmacomechanical catheter-directed thrombolysis (PCDT) upon primary and secondary venous outcomes over 24 months.
Patients with higher baseline Villalta score experienced greater effects of PCDT in reducing post-thrombotic syndrome (PTS) severity, reducing the occurrence of moderate-or-severe PTS, and improving venous health-related quality of life over 24 months.
Patients with previous DVT also had greater effects of PCDT on 24-month PTS severity (mean PCDT-Control difference in Villalta score was 3.3 points, p-interaction=0.03; mean PCDT- Control difference in VCSS score was 2.3 points, p-interaction=0.02).
Acknowledgements:
The authors thank Dr. Andrei Kindzelski (NHLBI Project Officer), Dr. Clive Kearon (Chair, Data Coordinating Center, deceased), and the entire network of investigators and study staff at the ATTRACT Trial coordinating centers, core laboratories, and clinical centers (see Appendix).
Sources of Study Funding and In-Kind Support:
The ATTRACT Trial (ClinicalTrials.gov: NCT00790335) was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) (U01-HL088476, U01-HL088118); the Washington University Center for Translational Therapies in Thrombosis, supported by NHLBI (U54-HL112303); Boston Scientific; Covidien (now Medtronic); Genentech; the Society of Interventional Radiology Foundation; the Heart and Stroke Foundation of Canada (Investigator Award to Dr. Clive Kearon); and a Jack Hirsh Professorship in Thrombosis (to Dr. Kearon). Dr. Kahn is a Tier 1 Canada Research Chair holder and an investigator of the CanVECTOR Network, which holds grant funding from the Canadian Institutes of Health Research (CDT142654) and from the Fonds de recherche du Québec – Santé (File # 309911). BSN Medical donated compression stockings. This research was supported by the Washington University Institute of Clinical and Translational Sciences, funded by the National Center for Advancing Translational Sciences (UL1-TR00044810, UL1-TR002345, TL1-TR002344). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix: ATTRACT Study Leadership and Investigators
Steering Committee
Samuel Z. Goldhaber, MD (Chair) Harvard Medical School
David J. Cohen, MD, MSc St. Luke’s Mid America Heart Institute
Anthony J. Comerota, MD University of Michigan
Heather L. Gornik, MD, MHS, RVT Cleveland Clinic Heart & Vascular Institute
Michael R. Jaff, DO Harvard Medical School
Jim Julian, MMath McMaster University
Susan R. Kahn, MD, MSc McGill University, Jewish General Hospital
Clive Kearon, MB, PhD McMaster University Stephen Kee, MD (SIR Foundation) UCLA Medical Center
Andrei L. Kindzelski, MD, PhD National Heart, Lung, and Blood Institute
Lawrence Lewis, MD Washington University in St. Louis
Elizabeth Magnuson, ScD St. Luke’s Mid America Heart Institute
Mahmood K. Razavi, MD St. Joseph’s Vascular Institute
Timothy P. Murphy, MD Brown University
Suresh Vedantham, MD (Principal Investigator) Washington University in St. Louis
Clinical Coordinating Center
Mallinckrodt Institute of Radiology, Washington University in St. Louis, United States
Data Coordinating Center
Ontario Clinical Oncology Group, McMaster University, Hamilton, Canada
Health Economic Core Laboratory
Mid America Heart Institute, St. Luke’s Hospital, Kansas City, United States
Vascular Ultrasound Core Laboratory
VasCore, Massachusetts General Hospital, Boston, United States
ATTRACT Clinical Centers: Site Investigators
Adventist Midwest Health: Michael Sichlau – site PI, Athanasios Vlahos, Steven Smith, Quinn Thalheimer, Nisha Singh, Rekha Harting, John Gocke, Scott Guth, Neel Shah
Albert Einstein Medical Center: Paul Brady – site PI, Marvin Schatz, Mindy Horrow, Peyman Markazi, Leli Forouzan, Terence A.S. Matalon, David Hertzog
Allegheny General Hospital: Swapna Goday – site PI, Margaret Kennedy – previous site PI, Robert Kaplan, Thomas Campbell, Jamie Hartman, Elmer Nahum, Arvind Venkat
Ann Arbor VA Health Center: Venkataramu Krishnamurthy – site PI, John Rectenwald, Peter Henke, Jonathan Eliason, Jonathon Willatt, Guillermo Escobar
Baptist Cardiac and Vascular Institute: Shaun Samuels – site PI, Barry Katzen, James Benenati, Alex Powell, Constantino Pena, Howard Wallach, Ripal Gandhi
Central DuPage Hospital: Joseph Schneider – site PI, Stanley Kim, Farrah Hashemi, Joseph Boyle, Nilesh Patel, Michael Verta
Christiana Care Hospital: Daniel Leung – site PI, Marc Garcia – previous site PI, Phillip Blatt, Jamil Khatri, Dave Epstein, Randall Ryan, Tom Sweeny, Michael Stillabower, George Kimbiris, Tuhina Raman, Paul Sierzenski, Lelia Getto, Michael Dignazio, Paul Sierzenski, Mark Horvath
Cleveland Clinic Foundation: Heather Gornik – site PI, John Bartholomew, Mehdi Shishehbor, Frank Peacock, Douglas Joseph, Soo Hyum Kim, Natalia Fendrikova-Mahlay, Daniel Clair, Sean Lyden, Baljendra Kapoor, Gordon McLennon, Gregory Pierce, James Newman, James Spain, Amanjiit Gill, Aaron Hamilton, Anthony Rizzo, Woosup Park
Danbury Hospital: Alan Dietzek – site PI, Ira Galin, Dahlia Plummer, Richard Hsu, Patrick Broderick, Andrew Keller, Sameer Sayeed
Eastern Connecticut Hematology & Oncology Associates: Dennis Slater – site PI, Herb Lustberg, Jan Akus, Robert Sidman, Mandeep Dhami, Phillip Kohanski, Anca Bulgaru, Renuka Dulala, James Burch, Dinesh Kapur, Jie Yang
Florida Hospital: Mark Ranson – site PI, Alan Wladis, David Varnagy, Tarek Mekhail, Robert Winter, Manuel Perez-Izquierdo
Forsyth Medical Center: Stephen Motew – site PI, Robin Royd-Kranis, Raymond Workman, Scott Kribbs, Gerald Hogsette, Phillip Moore, Bradley Thomason, William Means, Richard Bonsall, John Stewart, Daniel Golwya
Gundersen Clinic, Ltd.: Ezana Azene – site PI, Wayne Bottner, William Bishop, Dave Clayton, Lincoln Gundersen, Jody Riherd, Irina Shakhnovich, Kurt Ziegelbein
Georgetown University: Thomas Chang – site PI, Karun Sharma – previous site PI, Sandra Allison, Fil Banovac, Emil Cohen, Brendan Furlong, Craig Kessler, Mike McCullough, Jim Spies
Henry Ford Health System: Judith Lin – site PI, Scott Kaatz, Todd Getzen, Joseph Miller, Scott Schwartz, Loay Kabbani, David McVinnie
Holy Name Medical Center: John Rundback – site PI, Joseph Manno, Richard Schwab, Randolph Cole, Kevin Herman, David Singh, Ravit Barkama, Amish Patel
Jobst Vascular Center: Anthony Comerota – site PI, John Pigott, Andrew Seiwert, Ralph Whalen, Todd Russell, Zakaria Assi, Sahira Kazanjian, Jonathan Yobbagy, Brian Kaminski, Allan Kaufman, Garett Begeman, Robert DiSalle, Subash Thakur
Maine Medical Center: Paul Kim – site PI, Marc Jacquet, Thomas Dykes, Joseph Gerding, Christopher Baker, Mark Debiasto, Derek Mittleider, George Higgins III, Steven Amberson, Roger Pezzuti, Thomas Gallagher PA-C
Massachusetts General Hospital: Robert Schainfeld – site PI, Stephan Wicky – previous site PI, Sanjeeva Kalva, Gregory Walker, Gloria Salazar, Benjamin Pomerantz, Virenda Patel, Christopher Kabrhel, Shams Iqbal, Suvranu Gangull, Rahmi Oklu, Scott Brannan
Mayo Clinic: Sanjay Misra – site PI, Haraldur Bjarnason – previous site PI, Aneel Ashrani, Michael Caccavale, Chad Fleming, Jeremy Friese, John Heit, Manju Kalra, Thanila Macedo, Robert McBane, Michael McKusick, Andrew Stockland, David Woodrum, Waldemar Wysokinski
Mease Countyside Hospital: Adarsh Verma – site PI, Andrew Davis – previous site PI, Jerry Chung, David Nicker, Brian Anderson, Robert Stein, Michael Weiss
Medical College of Wisconsin/Froedtert Hospital & Clinics: Parag Patel – site PI, William Rilling, Sean Tutton, Robert Hieb, Eric Hohenwalter, M. Riccardo Colella, James Gosset, Sarah White, Brian Lewis, Kellie Brown, Peter Rossi, Gary Seabrook
Medical University of South Carolina: Marcelo Guimaraes – site PI, J. Bayne Selby, William McGary, Christopher Hannegan, Jacob Robison, Thomas Brothers, Bruce Elliott, Nitin Garg, M. Bret Anderson, Renan Uflacker, Claudio Schonholz, Laurence Raney, Charles Greenberg
Oregon Health & Science University: John Kaufman – site PI, Frederick Keller, Kenneth Kolbeck, Gregory Landry, Erica Mitchell, Robert Barton, Thomas DeLoughery, Norman Kalbfleisch, Renee Minjarez, Paul Lakin, Timothy Liem, Gregory Moneta, Khashayar Farsad, Ross Fleischman, Loren French
Pepin Heart Hospital and Dr. Kiran C. Patel Research Institute: Vasco Marques – site PI, Yasir Al-Hassani, Asad Sawar, Frank Taylor
Phoenix Heart & Cardiovascular: Rajul Patel – site PI, Rahul Malhotra – previous site PI, Stanley Kim, Farah Hashemi, Joseph Boyle, Nilesh Patel, Marvin Padnick , Melissa Gurley, Fred Cucher, Ronald Sterrenberg, G. Reshmaal Deepthi, Gomes Cumaranatunge
Riverside Methodist Hospital: Sumit Bhatla – site PI, Darick Jacobs, Eric Dolen, Pablo Gamboa, L. Mark Dean, Thomas Davis, John Lippert, Sanjeev Khanna, Brian Schirf, Jeffrey Silber, Donald Wood, J. Kevin McGraw, Lucy LaPerna, Paul Willette
Rhode Island Hospital: Timothy Murphy – site PI, Joselyn Cerezo, Rajoo Dhangana, Sun Ho Ahn, Gregory Dubel, Richard Haas, Bryan Jay, Ethan Prince, Gregory Soares, James Klinger, Robert Lambiase, Gregory Jay, Robert Tubbs, Michael Beland, Chris Hampson, Ryan O’Hara, Chad Thompson, Michael Beland, Aaron Frodsham, Fenwick Gardiner, Abdel Jaffan, Lawrence Keating, Abdul Zafar
Providence Sacred Heart Medical Center & Children’s Hospital: Radica Alicic – site PI, Rodney Raabe – previous site PI, Jayson Brower, David McClellan, Thomas Pellow, Christopher Zylak, Joseph Davis, M. Kathleen Reilly, Kenneth Symington, Camerson Seibold, Ryan Nachreiner, Daniel Murray, Stephen Murray, Sandeep Saha, Gregory Luna
Southern Illinois University: Kim Hodgson – site PI, Robert McLafferty – previous site PI, Douglas Hood, Colleen Moore, David Griffen
St. Elizabeth Healthcare Edgewood (KY): Darren Hurst – site PI, David Lubbers, Daniel Kim, Brent Warren, Jeremy Engel, D. P. Suresh
St. Elizabeth Regional Medical Center (NE): Eric VanderWoude – site co-PI, Rahul Razdan – site co-PI, Mark Hutchins, Terry Rounsborg, Madhu Midathada, Daniel Moravec, Joni Tilford, Daniel Kim, Joni Beckman PA
St. Joseph Hospital: Mahmood Razavi – site PI, Kurt Openshaw, D. Preston Flanigan, Christopher Loh, Howard Dorne, Michael Chan
St. Luke’s Hospital and Health Network: Jamie Thomas – site PI, Justin Psaila, Michael Ringold, Jay Fisher, Any Lipcomb, Timothy Oskin
St. Luke’s Hospital: Brandt Wible – site PI, Brendan Coleman, David Elliott, Gary Gaddis, C. Doug Cochran
St. Vincent Medical Group: Kannan Natarajan – site PI, Stewart Bick, Jeffrey Cooke, Ann Hedderman, Anne Greist, Lorrie Miller, Brandon Martinez, Vincent Flanders, Mark Underhill
Stanford University Medical Center: Lawrence Hofmann – site PI, Daniel Sze, William Kuo, John Louie, Gloria Hwang, David Hovsepian, Nishita Kothary, Caroline Berube, Donald Schreiber, Brooke Jeffrey
Staten Island University Hospital: Jonathan Schor – site PI, Jonathan Deitch, Kuldeep Singh, Barry Hahn, Brahim Ardolic, Shilip Gupta
Temple University Hospital: Riyaz Bashir – site PI, Angara Koneti Rao, Manish Garg, Pravin Patil, Chad Zack, Gary Cohen, Frank Schmieder, Valdimir Lakhter
The Reading Hospital: David Sacks – site PI, Robert Guay, Mark Scott, Karekin Cunningham, Adam Sigal, Terrence Cescon, Nick Leasure, Thiruvenkatasamy Dhurairaj
TriHealth/Good Samaritan Hospital: Patrick Muck – site PI, Kurt Knochel, Joann Lohr, Jose Barreau, Matthew Recht, Jayapandia Bhaskaran, Ranga Brahmamdam, David Draper, Apurva Mehta, James Maher
University of Iowa: Melhem Sharafuddin – site PI, Steven Lentz, Andrew Nugent, William Sharp, Timothy Kresowik, Rachel Nicholson, Shiliang Sun, Fadi Youness, Luigi Pascarella
University of Illinois- Chicago: Charles Ray – site PI, Martha-Gracia Knuttinen – previous site PI, James Bui, Ron Gaba, Valerie Dobiesz, Ejaz Shamim, Sangeetha Nimmagadda, David Peace, Aarti Zain, Alison Palumto
University of Maryland: Ziv Haskal – site PI, Jon Mark Hirshon, Howard Richard, Avelino Verceles, Jade Wong-You-Chong, Bertrand Othee, Rahul Patel, Bogdan Iliescu
University of Michigan Hospitals and Health Centers: David Williams – site PI, Joseph Gemmete, Venkataramu Krishnamurthy, Wojciech Cwikiel, Kyung Cho, James Schields, Ranjith Vellody, Paula Novelli, Narasimham Dasika, Thomas Wakefield, John Rectenwald, Peter Henke, Jeffrey Desmond, James Froehlich, Minhajuddin Khaja
University of Minnesota: David Hunter – site PI, Jafar Golzarian, Erik Cressman, Yvonne Dotta, Nate Schmiechen
University of New Mexico: John Marek – site PI, David Garcia, Isaac Tawil, Mark Langsfeld
University of North Carolina: Stephan Moll – site PI, Matthew Mauro, Joseph Stavas, Charles Burke, Robert Dixon, Hyeon Yu, Blair Keagy, Kyuny Kim, Raj Kasthuri, Nigel Key
University of Pittsburgh: Rabih Chaer – site PI, Michael Makaroun, Robert Rhee, Jae-Sung Cho, Donald Baril, Luke Marone, Margaret Hseih, Kristian Feterik, Roy Smith, Geetha Jeyabalan, Jennifer Rogers
University of Utah Medical Center: Russel Vinik – site PI, Dan Kinikini, Larry Kraiss, Michelle Mueller, Robert Pendleton, Matthew Rondina, Mark Sarfati, Nathan Wanner, Stacy Johnson, Christy Hopkins, Daniel Ihnat
University of Virginia Health System: John Angle – site PI, Alan Matsumoto, Nancy Harthun, Ulku Turba, Wael Saad, Brian Uthlaut, Srikant Nannapaneni, David Ling, Saher Sabri, John Kern, B. Gail Macik, George Hoke, Auh Wahn Park, James Stone, Benjamin Sneed, Scott Syverud, Kelly Davidson, Aditya Sharma, Ziv Haskal, Luke Wilkins
Utah Valley Reginal Medical Center: Carl Black – site PI, Mark Asay, Daniel Hatch, Robert Smilanich, Craig Patten, S. Douglas Brown, Ryan Nielsen, William Alward, John Collins, Matthew Nokes
Wake Forest Baptist Health: Randolph Geary – site PI, Matthew Edwards, Christopher Godshall, Pavel Levy
Weill Cornell Medical College: Ronald Winokur – site PI, Akhilesh Sista – previous site PI, David Madoff, Kyungmouk Lee, Bradley Pua, Maria DeSancho, Raffaele Milizia, Jing Gao
Western Penn Allegheny Health System: Swapna Goday – site PI, Margaret Kennedy – previous site PI, Robert Kaplan, Thomas Campbell, Gordon McLean, Jamie Hartman, Elmer Nahum, Sanualah Khalid
Washington University in St. Louis: Suresh Vedantham – site PI, Larry Lewis, Nael Saad, Mark Thoelke, Robert Pallow, Seth Klein, Gregorio Sicard
Footnotes
Disclosures:
Siddhant Thukral, Samantha Lancia - nothing to disclose. Amber Salter: Unrelated support from American Heart Association. Susan R. Kahn: Grants from Canadian Institutes of Health Research, consulting for Alexion (unrelated). Suresh Vedantham: Grants from NHLBI, Boston Scientific, Covidien, Genentech, BSN Medical. In-kind support from Medi USA (unrelated).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008; 149:698–707. [DOI] [PubMed] [Google Scholar]
- 2.Vedantham S, Grassi CJ, Ferral H, et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol 2006, 17:417–434. [DOI] [PubMed] [Google Scholar]
- 3.Comerota AJ, Kearon C, Gu C, et al. ; ATTRACT Investigators. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis: analysis from a stratified multicenter randomized trial. Circulation 2019; 139:1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn SR, Julian JA, Kearon C, et al. ; ATTRACT Investigators. Quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep vein thrombosis. J Vasc Surg Venous Lymphat Disord 2020, 8:8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vedantham S, Goldhaber SZ, Julian J, et al. ; Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med 2017; 377:2240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn SR, Partsch H, Vedantham S, et al. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 2009; 7(5):879–883. [DOI] [PubMed] [Google Scholar]
- 7.Vasquez MA, Rabe E, McLafferty RB, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg 2010; 52(5):1387–1396. [DOI] [PubMed] [Google Scholar]
- 8.Lamping DL, Schroter S, Kurz X, et al. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg 2003; 37:410–419. [DOI] [PubMed] [Google Scholar]
- 9.Ortel T, Neumann I, Ageno W, et al. American Society of Hematology 2020 Guidelines for Management of Venous Thromboembolism: Treatment of Deep Vein Thrombosis and Pulmonary Embolism. Blood Adv 2020, 4(19):4693–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakkos SK, Gohel M, Baekgaard N, et al. European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. Eur J Vasc Endovasc Surg 2021; 61(1):9–82. [DOI] [PubMed] [Google Scholar]
- 11.Enden T, Haig Y, Klow NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012, 379:31–38. [DOI] [PubMed] [Google Scholar]
- 12.Haig Y, Enden T, Grøtta O, et al. ; CaVenT Study Group. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomized controlled trial. Lancet Haematol 2016; 3(2):e64–71. [DOI] [PubMed] [Google Scholar]
- 13.Notten P, ten Cate-Hoek AJ, Arnoldussen CWKP, et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. Lancet Haematol 2020; 7:e40–e49. [DOI] [PubMed] [Google Scholar]
- 14.Goldhaber SZ, Magnuson EA, Chinnakondepalli KM, et al. Catheter-directed thrombolysis for deep vein thrombosis: 2021 update. Vasc Med 2021, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razavi MK, Salter A, Goldhaber SZ, et al. ; ATTRACT Investigators. Correlation between post-procedure residual thrombus and clinical outcome in deep vein thrombosis patients receiving pharmacomechanical thrombolysis in a multi-center randomized trial. J Vasc Interv Radiol 2020; 31:1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


