Abstract
Acute myeloid leukemia (AML) carries poor survival and high recurrence rate. We conducted a retrospective analysis of AML patients (N=453) treated with chemotherapy only or chemotherapy + hematopoietic cell transplant (HCT) who maintained their first complete remission (CR) for ≥ 3 years. Prior comorbidities, new comorbidities, secondary malignancies, late relapse, and causes of death (COD) were documented. New comorbidities for chemotherapy only patients (n=304) included renal disease (10%), and osteopenia/osteoporosis (38%) for HCT patients (n=149). Incidence of hypertension was similar in the chemotherapy only cohort and chemotherapy + HCT cohort (14% vs 17%). Secondary malignancies occurred in 13%, commonly skin, prostate and breast cancers. Common COD included: secondary malignancy (4%), HCT complications (3%), and late relapses (5%). Overall, 12% had a late relapse. Median overall survival for chemotherapy only and HCT was 10.7 and 12.7 years, respectively. Long-term AML survivors need routine monitoring for comorbidities, secondary malignancies, and late relapses.
Keywords: acute myeloid leukemia, survivorship, remission, comorbidities
Introduction
Acute myeloid leukemia (AML) is the second most common adult leukemia in the US, with an estimated 19,940 new cases diagnosed in 2020 [1]. AML is a highly heterogeneous disease with a variety of cytogenetics and molecular alterations that are associated with various phenotypes and outcomes, and, increasingly, determine the use of specific therapies targeted to such abnormalities [2,3]. Treatment for AML remained unchanged for many decades resulting in modest improvements in survival, with advances due in part to improved supportive care and the use of hematopoietic cell transplant (HCT). Still, in 2020, an estimated 11,180 are expected to die from AML [1]. In the last few years, a flurry of new agents has received regulatory approval for various subsets of patients. Many of these agents have resulted in an improvement in overall survival compared to standard therapy raising the expectation of a more significant change in the survival expectations for more patients [4–8].
As survival rates of cancer improve, the need for research on long-term outcomes becomes increasingly important. Among those who are long-term cancer survivors, chronic diseases such as heart failure and diabetes, as well as psychologic consequences affect the patient’s quality of life and long-term outcomes [9–11]. It is essential that survivors are counseled on the importance of long-term follow-up both with specialists and primary care physicians to properly address their needs. Survivors of cancer are more likely to have poor health and lower quality of life which emphasizes the need to provide comprehensive care to long-term survivors [12,13].
Due to the historical high rate of recurrence and poor overall survival rate, characteristics of long-term survivorship in AML are relatively unknown. With the rapid evolution of therapies for AML, more attention will have to be paid to long-term survivorship. The incidence of new chronic disease as well as secondary malignancies requires investigation in long-term survivors of AML [14]. This study aims to investigate the characteristics and needs associated with long-term survivorship in AML with a focus on comorbidities and second malignancies in patients who have survived in first complete remission (CR) for 3 or more years.
Methods
We conducted a retrospective chart review of all patients with previously untreated AML who were treated at MD Anderson Cancer Center between 2000 and 2015. From that overall cohort, we identified all patients who achieved CR and maintained their first CR for at least 3 years after their initial therapy. The initial anti-leukemia treatment received, including whether a HCT was performed, was recorded.
Medical records were reviewed by one of the investigators (CKM) to identify comorbidities present prior to the start of treatment as well as comorbidities present at any time from the 3-year anniversary of achieving CR (including those that occurred from start of induction until the 3-year anniversary, provided they were still present at that time) until the last follow up. The comorbidities were grouped into 11 distinct categories: hypertension (HTN), dyslipidemia (DLD), chronic pulmonary disease, cardiac/cardiovascular disease, diabetes mellitus type 2 (DM), chronic Renal disease, depression, anxiety, gastroesophageal reflux disease, hypothyroidism, and osteopenia/osteoporosis. We also investigated any malignancies that developed after 3 years in CR. Late relapses (relapses occurring after 3 years) were recorded and, for those who had a late relapse, changes in karyotype and FLT3-ITD status were noted. Relapse was defined as the presence of >5% bone marrow blasts or extramedullary disease. Response after subsequent therapy for those with a late relapse was identified and patients with a second remission of at least 3 years were noted. The cause of death for all patients was investigated.
Statistical analysis
This was a descriptive analysis and data is presented as percentage of patients with given features and median and range for duration of the different events investigates. Overall survival was calculated using a Kaplan-Meier technique.
Results
A total of 2779 patients with AML were treated between 2000 and 2015 at MD Anderson Cancer Center and 1686 (61%) achieved a first complete remission (CR1). Among them, 453 (27%; 16% of all treated) maintained CR1 for at least 3 years and constitute the focus of this analysis. The initial therapy was chemotherapy alone for 304 (67%) and chemotherapy followed by HCT for 149 (33%). The median time from the initiation of induction chemotherapy to CR1 was 29 days [18–207] for the entire cohort, and it was similar for patients treated with chemotherapy alone and those who later received HCT. Induction therapy most frequently consisted of intermediate or high-dose cytarabine-based combinations (n=270, 89% of chemotherapy only cohort, and n=131, 88% of HCT cohort). Low-dose cytarabine-based combinations were used in 8% of both cohorts, and hypomethylating-agent based regimens in 1% and 3%, respectively. Consolidation therapy consisted of a median of 6 cycles [0–24] for the chemotherapy only cohort and 3 cycles [0–9] for the chemotherapy + HCT cohort. The median time to HCT from induction was 112 days [48–641] for the chemotherapy + HCT cohort. Baseline characteristics for the 453 AML survivors are presented in Table 1a and the age range by decade is presented in Table 1b. The median age was 52 years (range, 18 to 88 years) with a near equal number of male and female patients. A total of 91 (20%) patients had a FLT3 mutation, most frequently an internal tandem duplication. Six patients had both a FLT3-ITD and FLT3-D835 mutation. The median follow-up from the time of diagnosis was 86 months (range, 37 to 208 months), and from the 3-year anniversary of achieving CR1 45 months (1 to 172 months). The median survival for the all 453 patients was 10.7 years (range 0.1 to 14.3 years) from the 3-year anniversary of achieving CR1. Overall survival was similar for patients treated with chemotherapy alone (median 10.6 years, range 0.1 to 14.3 years) and for those treated with chemotherapy followed by HCT (median not reached, range 0.1 to 12.7 years) (Figure 1). We compared the observed survival in our patient population with the expected survival of the general population. The matching was performed based on age at diagnosis, gender, ethnicity, and diagnostic year to calculate expected survival of the general population. The median observed survival in the study population was significantly shorter than the expected survival in the general population (349 months; p<0.001).
Table 1a.
Baseline characteristics at the time of AML diagnosis.
| Variable | No. (%), Median [Range] | ||
|---|---|---|---|
| Chemotherapy (N=304) | HCT (N=149) | Total (N=453) | |
| Age | 52 [18–88] | 52 [20–75] | 52 [18–88] |
| Female | 145 (48) | 71 (48) | 216 (48) |
| ECOG PS | |||
| 0 | 79 (26) | 40 (27) | 119 (26) |
| 1 | 189 (62) | 94 (63) | 283 (62) |
| 2 | 31 (10) | 14 (9) | 45 (10) |
| NPM1 Mutation | |||
| Positive | 43 (14) | 24 (16) | 67 (15) |
| Negative | 94 (31) | 65 (44) | 159 (35) |
| Not Done | 167 (55) | 60 (40) | 227 (50) |
| FLT3-ITD Status | |||
| Positive | 24 (8) | 35 (23) | 59 (13) |
| Negative | 225 (74) | 97 (65) | 322 (71) |
| Not Done | 55 (18) | 17 (12) | 72 (16) |
| FLT3-D835 | |||
| Positive | 18 (6) | 14 (9) | 32 (7) |
| Negative | 231 (76) | 118 (79) | 349 (77) |
| Not Done | 55 (18) | 17 (12) | 72 (16) |
Abbreviations: HCT, hematopoietic stem cell transplant; ECOG PS, Eastern Cooperative Oncology Group; NPM1, Nucleophosmin 1; FLT3-ITD, fms related tyrosine kinase 3-internal tandem duplication; FLT3-D835, fms related tyrosine kinase 3-D835.
Table 1b:
Age Breakdown by Decade.
| Decade | n (%) |
|---|---|
| 18–19 | 4 (<1) |
| 20–29 | 45 (10) |
| 30–39 | 63 (14) |
| 40–49 | 78 (17) |
| 50–59 | 131 (29) |
| 60–69 | 97 (21) |
| 70–79 | 32 (7) |
| 80–89 | 3 (<1) |
Figure 1.
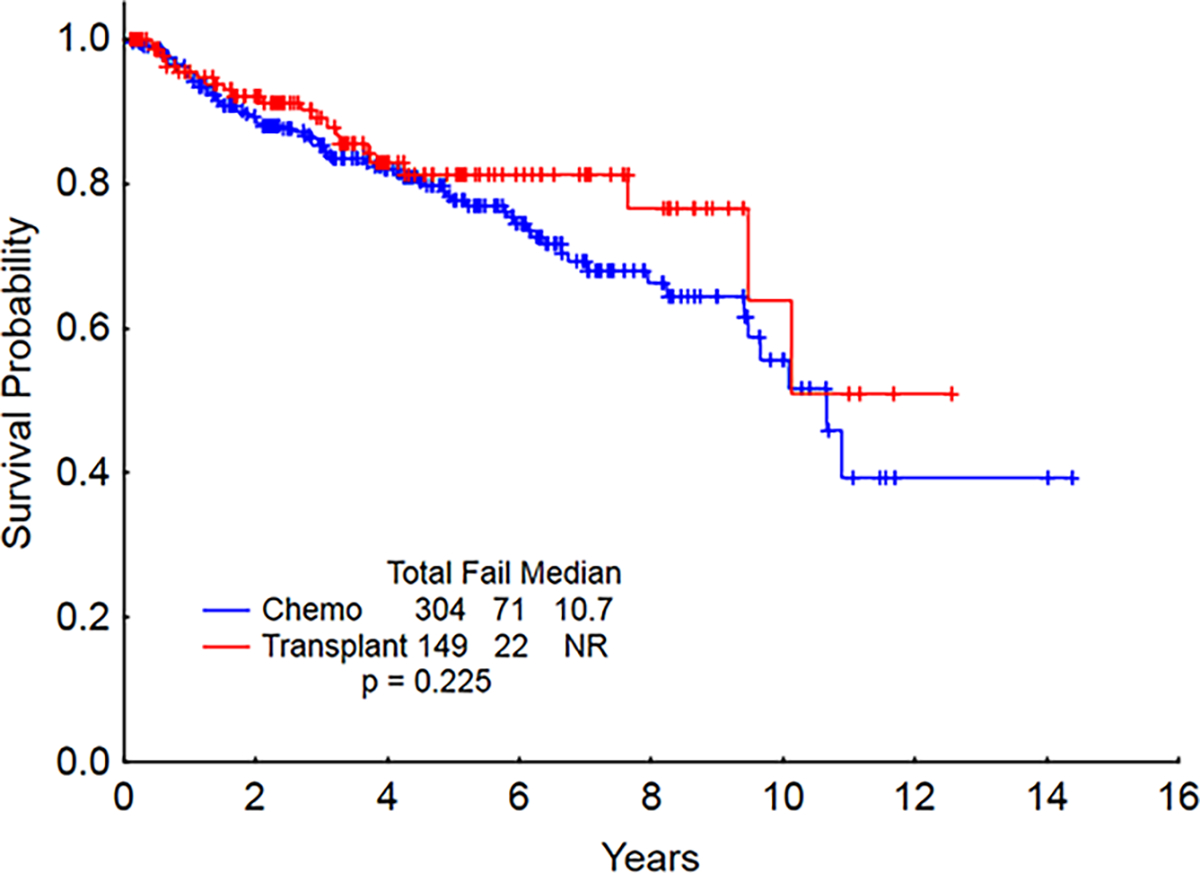
Overall survival starting at 3 years CR (CR: complete remission).
New Comorbidities
At baseline (i.e., at the time of diagnosis prior to the start of induction chemotherapy) patients had the following co-morbidities: hypertension (HTN) in 123 (27%) patients, dyslipidemia (DLD) in 74 (16%), chronic pulmonary disease in 46 (10%), cardiac disease in 43 (9%; most commonly coronary artery disease in 27 and atrial fibrillation in 15), diabetes mellitus (DM) in 41 (9%), depression in 21 (5%), chronic renal disease in 24 (5%), gastroesophageal reflux disease (GERD) in 21 (5%), hypothyroidism in 25 (5%), anxiety in 16 (4%), and osteopenia/osteoporosis in 7 (1%) (Table 2). New comorbidities that were present at or after the 3-year mark included: new onset HTN in 67 (15%) patients, osteopenia/osteoporosis in 67 (15%), chronic renal disease in 63 (14%), chronic pulmonary disease in 51 (11%), DLD in 42 (9%), GERD in 35 (8%), cardiac disease in 34 (7%; most commonly atrial fibrillation in 13 and congestive heart failure in 6), depression in 31 (7%), anxiety in 29 (6%), DM in 27 (6%), and hypothyroidism in 19 (4%). (Table 3). There were some imbalances in the frequency of the most common co-morbidities between the chemotherapy alone and HCT cohorts. For example, osteopenia/osteoporosis was seen in 38% of HCT patients but only in 3% of chemotherapy patients. Similarly, chronic renal disease was seen in 22% of HCT patients and 10% of chemotherapy patients, while chronic pulmonary disease was seen in 16% and 9%, respectively.
Table 2.
Comorbidities at the time of AML diagnosis.
| Variable | No. (%) | ||
|---|---|---|---|
| Chemotherapy (N=304) | HCT (N=149) | Total (N=453) | |
| Hypertension | 92 (30) | 31 (21) | 123 (27) |
| Dyslipidemia | 53 (17) | 21 (14) | 74 (16) |
| Pulmonary | 29 (9) | 17 (12) | 46 (10) |
| Cardiac Disease | 32 (10) | 11 (7) | 43 (9) |
| Diabetes Mellitus | 31 (10) | 10 (7) | 41 (9) |
| Depression | 11 (4) | 10 (7) | 21 (5) |
| Renal | 18 (6) | 6 (4) | 24 (5) |
| Gastrointestinal Reflux Disease | 12 (4) | 9 (6) | 21 (5) |
| Hypothyroidism | 19 (6) | 6 (4) | 25 (5) |
| Anxiety | 8 (3) | 8 (5) | 16 (4) |
| Osteopenia/Osteoporosis | 6 (2) | 1 (.7) | 7 (1) |
Abbreviations: AML, acute myeloid leukemia; HCT, hematopoietic stem cell transplant.
Table 3.
Comorbidities at the time of last follow up.
| Variable | No. (%) | ||
|---|---|---|---|
| Chemotherapy (N=304) | HCT (N=149) | Total (N=455) | |
| Hypertension | 42 (14) | 25 (17) | 67 (15) |
| Dyslipidemia | 21 (7) | 22 (15) | 42 (9) |
| Pulmonary | 27 (9) | 24 (16) | 51 (11) |
| Cardiac Disease | 24 (8) | 10 (7) | 34 (7) |
| Diabetes Mellitus | 15 (5) | 14 (9) | 27 (6) |
| Depression | 19 (6) | 12 (8) | 31 (7) |
| Renal | 30 (10) | 33 (22) | 63 (14) |
| Gastrointestinal Reflux Disease | 20 (6) | 15 (10) | 35 (8) |
| Hypothyroidism | 8 (2) | 11 (7) | 19 (4) |
| Anxiety | 15 (5) | 14 (10) | 29 (6) |
| Osteopenia Osteoporosis | 9 (3) | 57 (38) | 66 (15) |
Abbreviations: HCT, hematopoietic stem cell transplant.
Prior malignancies
Seventy-six patients (16%) had a history of another malignancy prior to the diagnosis of AML, with the most common being breast cancer in 23 (5%) patients, skin cancer in 19 (4%), lymphoma in 13 (3%), and prostate in 9 (2%) (Table 4). In chemotherapy cohort, a history of other hematologic malignancies was present in 9 patients (3%), with 8 having a history of lymphoma and 1 multiple myeloma. History of hematologic malignancies was present in 8 (5%) of HCT patients: 5 with history of lymphoma, 2 were small lymphocytic lymphoma/chronic lymphocytic leukemia, and 1 chronic myelomonocytic leukemia.
Table 4.
Prior malignancies before the diagnosis of AML.
| Variable | No. (%) | ||
|---|---|---|---|
| Chemotherapy (304) | HCT (149) | Total (453) | |
| Breast | 10 (3) | 13 (9) | 23 (5) |
| Skin | 9 (3) | 10 (7) | 19 (4) |
| Lymphoma | 8 (2) | 5 (3) | 13 (3) |
| Prostate | 6 (2) | 3 (2) | 9 (2) |
Abbreviations: AML, acute myeloid leukemia; HCT, hematopoietic stem cell transplant.
Second malignancies
Second malignancies occurred in 80 (17%) patients, with the most common being skin cancer in 34 (7%) patients, prostate cancer in 9 (2%), breast cancer in 6 (1%), and lymphoma in 5 (1%) (Table 5). Skin cancer occurred in 12% of HCT patients and 5% of chemo patients. Melanoma accounted for 21% of all skin cancers, with 5 instances diagnosed among patients who received HCT and 2 among those treated with chemotherapy only. Among patients who developed a second malignancy, 18 (4%) had a history of malignancy prior to the diagnosis of AML. Notably however, none of the subsequent malignancies (third malignancies in such instances) represented a relapse of a prior malignancy.
Table 5.
Second malignancies diagnosed by the time of last follow-up.
| Variable | No. (%) | ||
|---|---|---|---|
| Chemotherapy (304) | HCT (149) | Total (453) | |
| Skin | 16 (5) | 18 (12) | 34 (7) |
| Prostate | 5 (2) | 4 (3) | 9 (2) |
| Breast | 5 (2) | 1 (.7) | 6 (1) |
| Lymphoma | 5 (2) | 0 | 5 (1) |
| Colon | 3 (1) | 1 (.7) | 4 (.9) |
Abbreviations: HCT, hematopoietic stem cell transplant.
Late Relapses
Late relapses (i.e., after 3 years in CR1) occurred in 56 (12%) patients, including 46 (15%) treated with chemotherapy alone and 10 (7%) treated with HCT (Table 6). Such relapses occurred after a median CR duration of 7 years (3–17.3) and 6.5 years (4–15.7), respectively. A change in karyotype upon relapse compared to the original karyotype was seen in 54% of relapsed patients treated with chemotherapy only and in 60% of those that received HCT. The most common new cytogenetic abnormalities were 7q deletion (22%), trisomy 8 (11%), and 5q deletion (7%). Only 3 instances of new cytogenetic abnormalities (7q- in two, +8 in one) occurred among patients with an initial diploid karyotype. In addition, FLT3-ITD was a new abnormality (i.e., seen at the time of late relapse but not documented at the time of initial diagnosis) in 6% of relapsed patients (all treated with chemotherapy only); in contrast, FLT3-ITD was no longer detectable at the time of late relapse in 10% of HCT-treated patients. None of the chemotherapy-only patients with FLT3-ITD at baseline survived 3 years. FLT3-D835 appeared at the time of late relapse in 6% of patients treated with chemotherapy only and it was no longer detectable in 3%. No patient treated with HCT had a change in FLT3-D835 status. At relapse, TP53 mutation was seen in 7% of patients; however, TP53 status at the time of diagnosis was unknown for most of them.
Table 6.
Characteristics of late relapses.
| No. (%), Median [Range] | ||||||
|---|---|---|---|---|---|---|
| Treatment before relapse | No. of Relapses | Change in Karyotype | Change in FLT3 ITD Status | CR1 Duration (yrs) | Survival from relapse (mo) | HCT after relapse |
| Chemotherapy | 46 (15) | 25 (54) | 2 (4) | 7 [3 –17.3] | 20.45 [.10–95.59] | 14 (30) |
| HCT | 10 (7) | 6 (60) | 1 (10) | 6 [3.7–15.7] | 22.13 [2.15–51.05] | 4 (40) |
| Total | 56 (12) | 31 (55) | 3 (5) | 7 [3–17.3] | 22.20 [.10–95.59] | 18 (32) |
Abbreviations: HCT, hematopoietic stem cell transplant; CR1, first complete remission; FLT3-ITD, fms related tyrosine kinase 3-internal tandem duplication.
Among the 56 patients who had a late relapse, a second complete remission (CR2) was achieved in 29 (52%): 50% among those that had been treated with chemotherapy only and 60% among those that had received HCT. Eighteen of these 56 patients (1 HCT and 17 chemo) maintained their second complete remission for at least 3 years. Of patients who had received chemotherapy only and relapsed, 30% underwent an HCT while 4 patients initially treated with HCT received a second HCT. The median survival after late relapse was 20 months for the patients that had initially received chemotherapy only and 22 months for those that had received HCT.
Cause of Death
The most common cause of death (COD) for CR1 patients that had received HCT was transplant-related complications (GVHD in 3 patients, 2% and infection in 2 patients, 1%), second malignancy (2 patients, 1%), and myocardial infarction (MI) (1 patient, <1%). For patients treated with chemotherapy only, the most common COD was second malignancy (10 patients, 4%), MI (2 patients, <1%), and pneumonia (1 patient, <1%). Among patients who had a late relapse, the most common COD for those that had received an initial HCT was relapsed AML (4 patients, 40%), complications of the 2nd HCT (2 patients, 20%), and infection (1 patient, 10%); the most common COD for relapse chemotherapy patients was relapsed AML (21 patients, 46%), complications of HCT (8 patients, 17%), and second malignancy (4 patients, 9%).
Risk for Late Complications
We then did an analysis of correlation of new comorbidities, second malignancies, and late relapse with performance status, age (by decade), and the two most common baseline comorbidities (hypertension and dyslipidemia) (Table 7). The incidence of new comorbidities increased with age, with 78% of those aged 50–59 developing a new comorbidity. Second malignancies were most common among those aged 70–79. Late relapses were seen with increasing frequency with each decade. Patients with no baseline comorbidities had a lower incidence of late relapse than patients with HTN and/or DLD at baseline (7% vs 13% vs 13%). Second malignancies and the development of new comorbidities were also lower among those who had no baseline comorbidities. Baseline HTN and DLD also carried an increased risk of developing future comorbidities (69% among those with baseline HTN and 55% in those with DLD). Late relapse was more common in patients with a performance status of 0 or 1 vs 2 (18% vs 10% vs 6%).
Table 7:
New comorbidities, second malignancies, and late relapses by baseline characteristics.
| Baseline characteristics | N | n (%) | ||
|---|---|---|---|---|
| New Comorbidities | Second Malignancies | Late Relapse | ||
| PS 0 | 119 | 87 (73) | 25 (21) | 22 (18) |
| PS 1 | 283 | 195 (68) | 47 (16) | 31 (10) |
| PS 2 | 45 | 24 (53) | 6 (13) | 3 (6) |
| Hypertension | 123 | 85 (69) | 25 (20) | 17 (13) |
| Dyslipidemia | 74 | 41 (55) | 20 (27) | 10 (13) |
| No Comorbidities | 130 | 73 (56) | 19 (14) | 10 (7) |
| Age (years by decade) | ||||
| 18–19 | 4 | 1 (25) | 0 (0) | 0 (0) |
| 20–29 | 45 | 28 (62) | 0 (0) | 3 (6) |
| 30–39 | 63 | 43 (68) | 5 (7) | 4 (6) |
| 40–49 | 78 | 52 (66) | 7 (8) | 6 (7) |
| 50–59 | 131 | 103 (78) | 30 (23) | 18 (13) |
| 60–69 | 97 | 68 (70) | 26 (26) | 17 (17) |
| 70–79 | 32 | 19 (60) | 11 (34) | 7 (21) |
| 80–89 | 3 | 1 (33) | 1 (33) | 1 (33) |
Abbreviations: PS, ECOG performance status.
Discussion
As survival rates for AML and other cancers improve, analysis of long-term outcomes will become crucial in ensuring that patients are adequately managed, and all their medical needs addressed. It is estimated that by 2040 there will be approximately 26 million cancer survivors [15]. For leukemia specifically, the American Cancer Society and the National Cancer Institute estimate that in 2019 there were 256,790 male survivors and this figure would increase to 352,900 in 2030 (no data available for women as only top 10 diagnosis for cancer survivor are presented) [16]. These statistics however represent all leukemias, with no detail given to the various types of leukemia. Patient-centered care is an essential part of providing exemplary treatment to patients and recognizing the importance of all needs of such patients, in addition to treating and following the primary cancer, will improve satisfaction and long-term outcomes for patients [17]. Unfortunately, little attention has been given to survivorship in hematologic malignancies in general and in AML in particular. This might be in part due to the fact that the probability of cure has been stagnant for many years and all efforts were directed to improve the remission rates and the remission duration. With the availability of new therapies that are improving the long-term outcome of patients, it is important to address this important aspect of cancer management.
For this analysis we focused on patients who have been in remission for at least 3 years. This is based on our previous experience showing that, after 3 years, the risk of relapse decreases significantly [18]. It should be noted however that several organizations consider that cancer survivorship starts at the time of diagnosis and lasts for the duration of the patient’s life [15]. This is important as it makes emphasis on aspects of prevention and surveillance that are very relevant to the long-term well-being of cancer patients. However, with this being a retrospective analysis and in the context of very limited available data on long-term AML survivors, we focused on this aspect of survivorship in an attempt to understand the needs and complexities of what has been termed “permanent survivorship” for AML patients.
Our analysis shows that a significant number of patients develop co-morbidities. Hypertension, osteopenia/osteoporosis, renal dysfunction and pulmonary problems were the most frequently observed comorbidities, seen in >10% each. Identification of these health issues does not necessarily represent causality or association with the initial therapy. However, it is noticeable that osteopenia/osteoporosis and renal dysfunction are particularly common in patients that received a HCT (38% and 22%, respectively) compared to those treated with chemotherapy only (3% and 10%, respectively) suggesting some association with HCT. Osteopenia/osteoporosis is well recognized as a late complication of HCT. Khera et al reported it to occur in 23% of survivors of HCT with a variety of cancer diagnoses [19]. Diabetes mellitus and lung disease were also frequent occurring in 22.9% and 36.9% [19]. In our series, these complications occurred in 9% and 16% of patients respectively. This highlights the importance of continued follow-up of all health conditions. Teaming with primary care physicians and/or other specialists and developing care plans are important to obtain the best outcome for patients. To put these results in perspective, the average life expectancy in the United States is 78 years [20], and the matched comparison yielded a significantly inferior observed survival in our cohort. This shows that despite the initial good outcome from AML, factors such as late relapses, co-morbidities and perhaps other unrecognized factors shorten the life expectancy of leukemia survivors. Hypertension occurs in almost half of individuals in the United States, [21] while diabetes is seen at a rate of 10.5%, with increasing incidence over 65 years of age [22]. Approximately 8% of adults in the US experience depression at some point in their lifetime [23]. These incidences seem to be similar to what we observed in our patient population considering those with these diagnoses at baseline and those that had acquired them by the time of last follow-up.
Occurrence of second malignancies are a feature of survivorship in many cancers. SEER data from over 2 million patients diagnosed with cancer between 1992 and 2008 showed that 8.1% developed a second cancer [24]. This analysis however included the ten most common cancer diagnosis in both men and women and therefore no data was presented on occurrence of second cancers after a diagnosis of AML. Data has been reported on this topic for some hematologic malignancies. In CML, SEER data suggests an incidence of second cancers of 4% in the era of tyrosine kinase inhibitors [25]. In MPN, from a cohort of 9379 patients in Sweden, 1192 (12.7%) developed a second non-hematologic malignancy with represented a significantly higher risk than matched controls [26]. In this report we show that second cancers occurred in 17% of the population analyzed. More than half of these were skin cancers, with prostate, breast and lung representing the next largest cohorts. This is similar to the previous experience also from MDACC showing second cancers in 22 of 215 (10.2%) long-term survivors [18]. This stresses the need for continued vigilance and adherence to standard screening and early detection procedures for long-term survivors of AML.
Additionally, the incidence of late relapses (12%), while low, is still a cause for investigation and an indication of the necessity of long-term follow for patients in long-term complete remission of AML [27,28]. Because of the retrospective and historical nature of this analysis, the information on molecular changes from initial diagnosis to late relapse, and the assessment of whether late relapses may come from new emerging clones or resurgence of the initial clone is lacking. However, it is interesting that some changes in chromosome abnormalities and FLT3 mutations (the most commonly available genetic mutation available for analysis in this cohort of patients) were identified. In the context of the more current understanding of the emergence of new clones, particularly in the context of use of specific inhibitors, this poses a challenge for long term cures [29].
Our analysis has limitations that have to be recognized. The retrospective nature of the review makes it possible that some co-morbidities or malignancies may have not been collected. We also do not have data on other important aspects of long-term survivorship that may have a significant impact on patients’ well-being such as the emotional, functional and financial effects of AML and its therapy. We also recognize the treatment paradigms are changing with the introduction of novel agents that are not only increasing the survival probability but may also impact the long-term effects of therapy. We still think this analysis offers a valuable starting point to highlight the need for putting more emphasis on the survivorship aspects of patients with AML.
In summary, survivors of AML have important medical needs that need attention long after treatment for AML has ended. Other aspects of cancer survivorship, such as emotional, social and functional consequences, need further study and prospective evaluation. Importantly, as is increasingly recognized in cancer in general, survivorship needs to be addressed from the time of diagnosis and a plan of care designed with the patient to ensure that defeating cancer is associated with an optimal and holistic well-being for the patient.
Acknowledgements/Funding
This study was supported in part by the MD Anderson Cancer Center Support Grant CA016672 (PI: Dr. Ronald DePinho)
Footnotes
Disclosure of interest
The authors report no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020. Jan;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018. Aug 18;392(10147):593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012. Mar 22;366(12):1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med. 2017. Aug 3;377(5):454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019. Feb;33(2):379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J Clin Oncol. 2018. Sep 10;36(26):2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert J, Pautas C, Terre C, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019. Jan;104(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med. 2019. Oct 31;381(18):1728–1740. [DOI] [PubMed] [Google Scholar]
- 9.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004. Oct 15;101(8):1712–9. [DOI] [PubMed] [Google Scholar]
- 10.Harrington CB, Hansen JA, Moskowitz M, et al. It’s not over when it’s over: long-term symptoms in cancer survivors--a systematic review. Int J Psychiatry Med. 2010;40(2):163–81. [DOI] [PubMed] [Google Scholar]
- 11.Mehnert A, Koch U. Psychological comorbidity and health-related quality of life and its association with awareness, utilization, and need for psychosocial support in a cancer register-based sample of long-term breast cancer survivors. J Psychosom Res. 2008. Apr;64(4):383–91. [DOI] [PubMed] [Google Scholar]
- 12.Damlaj M, El Fakih R, Hashmi SK. Evolution of survivorship in lymphoma, myeloma and leukemia: Metamorphosis of the field into long term follow-up care. Blood Rev. 2019. Jan;33:63–73. [DOI] [PubMed] [Google Scholar]
- 13.Yabroff KR, Lawrence WF, Clauser S, et al. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004. Sep 1;96(17):1322–30. [DOI] [PubMed] [Google Scholar]
- 14.Reinhart CA, Sae-Hau M, Lee CA, et al. Blood cancer survivorship in NCI-Designated Cancer Centers: a study of services, gaps, and access barriers. J Cancer Surviv. 2020. Feb;14(1):43–47. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt M, Greenfield S, Stovall E, et al. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academy Press; 2006. [Google Scholar]
- 16.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019. Sep;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro CL. Cancer Survivorship. N Engl J Med. 2018. Dec 20;379(25):2438–2450. [DOI] [PubMed] [Google Scholar]
- 18.de Lima M, Strom SS, Keating M, et al. Implications of potential cure in acute myelogenous leukemia: development of subsequent cancer and return to work. Blood. 1997. Dec 15;90(12):4719–24. [PubMed] [Google Scholar]
- 19.Khera N, Storer B, Flowers ME, et al. Nonmalignant late effects and compromised functional status in survivors of hematopoietic cell transplantation. J Clin Oncol. 2011. Jan 1;30(1):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias E United States Life Tables, 2017. Natl Vital Stat Rep. 2019. Jun;68(7):1–66. [PubMed] [Google Scholar]
- 21.Ostchega Y, Fryar CD, Nwankwo T, et al. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018. NCHS Data Brief. 2020. Apr(364):1–8. [PubMed] [Google Scholar]
- 22.CDC. National Diabetes Statistics Report. 2020. [Google Scholar]
- 23.Brody DJ, Pratt LA, Hughes JP. Prevalence of Depression Among Adults Aged 20 and Over: United States, 2013–2016. NCHS Data Brief. 2018. Feb(303):1–8. [PubMed] [Google Scholar]
- 24.Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016. Oct;122(19):3075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki K, Kantarjian HM, O’Brien S, et al. Incidence of second malignancies in patients with chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Int J Hematol. 2019. May;109(5):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landtblom AR, Bower H, Andersson TM, et al. Second malignancies in patients with myeloproliferative neoplasms: a population-based cohort study of 9379 patients. Leukemia. 2018. Oct;32(10):2203–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma D, Kantarjian H, Faderl S, et al. Late relapses in acute myeloid leukemia: analysis of characteristics and outcome. Leuk Lymphoma. 2010. May;51(5):778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yilmaz M, Wang F, Loghavi S, et al. Late relapse in acute myeloid leukemia (AML): clonal evolution or therapy-related leukemia? Blood Cancer J. 2019. Jan 16;9(2):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Savage S, Schultz AR, et al. Clinical resistance to crenolanib in acute myeloid leukemia due to diverse molecular mechanisms. Nat Commun. 2019. Jan 16;10(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]


