Abstract
The genetic and antigenic variability of human respiratory syncytial virus (HRSV) strains isolated in Buenos Aires from 1995 to 2001 was evaluated by partial nucleotide sequencing of the G gene and enzyme-linked immunosorbent assay analysis with anti-G monoclonal antibodies. Phylogenetic analyses showed that 37 group A strains clustered into five genotypes, whereas 20 group B strains clustered into three genotypes. Group A showed more genetic variability than group B. A close correlation between genotypes and antigenic patterns was observed. Changes detected in the G protein of viruses from both groups included (i) amino acid substitutions and(ii) differences in protein length due to either changes in stop codon usage or sequence duplications. Three B strains from 1999 exhibited a duplication of 20 amino acids, while one B strain from 2001 had 2 amino acids duplicated. The comparison among Argentinean HRSV strains and viruses isolated in other geographical areas during different epidemics is discussed.
Human respiratory syncytial virus (HRSV) is the leading cause of viral lower respiratory tract infections (LRTI) among infants and young children in both developed and developing countries (37). Since the immune response produced by HRSV infection does not provide lasting protection, reinfections throughout life are common (15, 16). Antigenic variation may play a role in the ability of HRSV to escape the immune response and produce reinfections.
Studies with monoclonal antibodies (MAbs) against the G glycoprotein led to the recognition of two antigenic groups, A and B (1, 28). The variability of the G protein is located mainly in the ectodomain, which contains two hypervariable segments separated by a highly conserved region between amino acids 164 and 176 (4, 18, 40).
Genetic and antigenic studies also demonstrated the existence of distinct lineages within groups A and B. Viruses belonging to different genotypes within the same group may cocirculate during the same season, and most epidemics are produced by more than one viral genotype. Predominant genotypes are replaced by new ones during consecutive years (5, 26). Although most studies have been conducted on group A viruses, the available studies of group B viruses show that both antigenic groups seem to follow the same evolutionary pattern (10, 24, 31, 40).
In Argentina, HRSV was found to be the major viral pathogen for children under 5 years of age with acute LRTI: it was detected in 18 to 36% of cases (7, 45, 46). Most studies have focused on group characterization with monoclonal antibodies and clinical features associated with HRSV infection (6, 17, 35).
There is limited information regarding the molecular epidemiology of HRSV in South America (2, 11, 12, 24). In the present study, we describe the genetic diversity of group A and B isolates obtained during seven consecutive epidemic periods in Buenos Aires, Argentina (1995 to 2001). The correlation between the antigenic and genetic variability among group A strains is also shown.
MATERIALS AND METHODS
Viruses.
Nasopharyngeal aspirates were obtained from children under 5 years of age with LRTI admitted to three hospitals from Buenos Aires city between 1995 and 2001. Samples positive for HRSV by immunofluorescence were inoculated onto monolayers of HEp-2 cells growing in Earle's minimal essential medium supplemented with 2% fetal bovine serum. Four to seven blind passages were made until a cytopathic effect was evident 48 h postinfection. Cells were scraped and centrifuged at low speed for protein extraction.
Cell extracts and enzyme immunoanalysis.
Protein extracts were obtained from the pellets of HEp-2 cells infected with the different isolates. Pellets from 4 × 106 cells were resuspended in 100 μl of lysis buffer (1% n-octyl β-d-glucopyranoside, 10 mM Tris-HCl [pH 7.6], 140 mM NaCl, 5 mM EDTA), sonicated, and centrifuged at 12,000 rpm for 5 min.
Ninety-six-well vinyl plates (Costar, Cambridge, MA) were coated with protein extracts and incubated overnight at 4°C. Plates were blocked with 1% bovine serum albumin in phosphate-buffered saline. Dilutions of MAbs in 0.1% bovine serum albumin in phosphate-buffered saline were added to the plates. Bound antibodies were detected after incubation with biotin-labeled anti-mouse immunoglobulin and streptavidin-peroxidase (Amersham Life Science). Finally, plates were incubated with the substrate o-phenylenediamine dihydrochloride (Abbott Laboratories, North Chicago, IL), and the absorbance at 490 nm was measured.
MAbs used in the enzyme-linked immunosorbent assay (ELISA) were obtained using prototype strain A/Mon/3/88 (22), A/Long (13), or B/Mon/3/94 (I. Martinez et al., unpublished data) as the immunogen. This panel of antibodies included two that recognized epitopes conserved in all HRSVs isolated (021/1G and 021/21G), two antibodies specific for epitopes shared by all group A isolates (021/2G and 021/19G), two antibodies specific for group B isolates (B1G and B2G), and eight antibodies that recognized variable epitopes represented in some but not all group A isolates (021/5G, 021/7G, 021/8G, 021/9G, 25G, 59G, 63G, and 68G). These strain-specific MAbs are directed against epitopes that have been mapped in the C-terminal third of the G glycoprotein, except for 021/5G, which maps in the N-terminal hypervariable region (14, 22, 33, 34).
Seminested reverse transcription-PCR.
Total RNA was obtained from 1.1 × 105 HEp-2 cells infected with the different strains by using RNA extraction columns (QIAamp Viral RNA kit; QIAGEN), resuspended in 50 μl of RNase-free water, and stored at −70°C.
cDNA was obtained using 2 μg of RNA in a mix containing 300 ng of antisense primer LG3(−) (5′-GGCCCGGGAAGCTTTTTTTTTTTTTTT-3′), 0.5 mM each deoxynucleoside triphosphate (Promega), 200 U Moloney murine leukemia virus reverse transcriptase (Promega), and first-strand buffer (Promega), containing 250 mM Tris-HCl (pH 8.3), 375 mM KCl, and 15 mM MgCl2, in a final volume of 20 μl. The reaction mixture was incubated for 60 min at 42°C.
The first amplification of the G gene was performed by adding the synthesized cDNA to a tube containing 300 ng of both antisense primer LG3(−) and sense primer LG5(+) (5′-GGATCCCGGGGCAAATGCAAACATGTCC-3′, including the first 20 nucleotides of the G gene from A/Long strain), 2.5 mM MgCl2, 2.5 U Taq DNA polymerase (Promega), and PCR buffer (20 mM Tris-HCl [pH 8.4], 50 mM KCl) in a final volume of 100 μl. The amplification procedure included 5 min at 95°C and 35 cycles of denaturation at 94°C for 1.5 min, annealing at 71°C for 1.5 min, and extension at 72°C for 1.5 min, with a final extension at 72°C for 10 min.
Finally, the seminested PCR was carried out as follows: 1 μl of the first amplification reaction product was added to a tube containing 300 ng of primers LG3(−) and GA480(+) (5′-ACAAACCACCAAACAAACCC-3′) for group A or primers LG3(−) and GB496(+) (5′-GATGATTACCATTTTGAAGTGTTCA-3′) for group B, 50 μM each deoxynucleoside triphosphate, 2.5 U Taq DNA polymerase, 2.5 mM MgCl2, and PCR buffer in a final volume of 100 μl. The cycling protocol was the same as in the first amplification, except that annealing temperatures were 62°C for group A and 66°C for group B. Amplified products (450 bp for group A and 434 bp for group B) were purified from agarose gels using columns (QIAquick Gel Extraction kit; QIAGEN) and sequenced with the GA480(+) or GB496(+) primer using the Big-Dye Terminator sequencing kit (Applied Biosystems).
Phylogenetic analysis.
Sequences were aligned using Clustal X, version 1.81 (42). Phylogenetic analysis was carried out by the distance method, using the neighbor-joining algorithm, with MEGA, version 2.1, software (19). The topological accuracy of the trees was evaluated by the bootstrap method (1,000 replicates). Only values ≥70% are shown at the branch nodes.
Phylogenetic analyses were also carried out by the distance method and the maximum-parsimony criterion, using PAUP*, version 4.0b4a (41). Bootstrap values were obtained with 100 replicates. Trees were plotted with TreeView, version 1.6.6 (29). The sequence sets from both groups were also submitted to quartet maximum-likelihood reconstruction (TreePuzzle, version 4.0) (36) (data not shown).
Nucleotide sequence accession numbers.
Sequences analyzed in this study were submitted to GenBank with accession numbers AY667067 to AY667096 for group A sequences and accession numbers AY672685 to AY672701 and AY333362 to AY333364 for group B sequences.
RESULTS
Genetic analysis of HRSV strains.
One hundred twenty-nine HRSV strains isolated in Buenos Aires during seven consecutive epidemics (1995 to 2001) were antigenically characterized by their reactivity in ELISA with 14 MAbs against the G glycoprotein. Table 1 shows the yearly distribution of group A and B HRSV strains throughout the study period. Group A viruses predominated in all years except 1999, and group B viruses were not isolated in 1996 and 2000.
TABLE 1.
Yearly distribution of HRSV strains characterized by ELISA (groups A and B) in Argentina, from 1995 to 2002
| Yr |
n (%) of strains in antigenic group
|
|
|---|---|---|
| Group A | Group B | |
| 1995 | 5 (71) | 2 (29) |
| 1996 | 10 (100) | 0 (0) |
| 1997 | 4 (57) | 3 (43) |
| 1998 | 15 (75) | 5 (25) |
| 1999 | 18 (47) | 20 (53) |
| 2000 | 32 (100) | 0 (0) |
| 2001 | 12 (80) | 3 (20) |
| Total (n = 129) | 96 (74.4) | 33 (25.6) |
Among 96 group A strains, 17 different antigenic patterns were determined (M. C. Galiano et al., unpublished data). Since no strain-specific MAbs were available for group B viruses (n = 33), we could not further characterize those viruses antigenically.
For the genetic analysis, 37 strains representative of 14 out of 17 antigenic patterns were selected for group A. Since no antigenic differences were available for group B, 20 strains were randomly selected for sequencing. All sequences were denominated with the prefix BA (Buenos Aires), followed by the isolate number and year of isolation.
In order to place our genotype classification in the context of previous genotype designations (11, 25, 30, 31, 38, 44), 29 published sequences from group A and 21 from group B were added to the analysis. These selected sequences were representative of the genotypes described by the authors of the works cited above. Since most of the published sequences comprised the last 270 nucleotides of the G protein, the same fragment was considered in the BA strains.
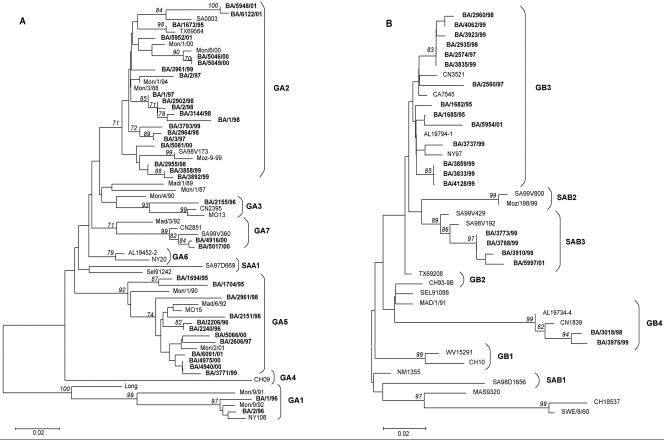
Figure 1A shows the phylogenetic tree obtained with the distance criterion for 66 group A strains. The analysis revealed two main branches: one included most of the BA strains, whereas the other comprised only strains of the GA1 genotype. As previously reported (11), strains BA/1/96 and BA/2/96 clustered with strains from GA1.
FIG. 1.
Phylogenetic trees of group A (A) and B (B) HRSV strains. Prototype strains A/Long (8) and B/CH18537 (18) were included in the analysis. Selected worldwide sequences representing described genotypes were retrieved from GenBank and included in the trees for their comparison (9, 11, 12, 24, 25, 27, 30-32, 39, 44). Trees were built using the neighbor-joining algorithm through the MEGA program. BA HRSV strains and genotypes are shown in boldface. Numbers in italics correspond to bootstrap values supporting the adjacent nodes; only bootstrap values greater than 70% are displayed.
Most BA strains clustered in the other main branch with viruses of genotypes GA2, GA3, GA5, and GA7. These genotypes showed bootstrap values of 85 to 100%, except for GA2, which displayed bootstrap values of 67% (not shown in the tree). None of the Argentinean viruses grouped with representative strains from genotype GA4, GA6, or SAA1.
The most represented genotypes among the BA sequences were GA2 (54%) and GA5 (32%). Strains with these two genotypes were isolated throughout the study period.
Phylogenetic analysis of group B strains showed that BA viruses clustered into three genotypes: GB3, GB4, and SAB3 (Fig. 1B). Fourteen out of 20 strains (70%) clustered with strains of the GB3 genotype. Although the topology of this cluster was similar among all the trees constructed, the bootstrap support was not significant. Strains BA/3018/98 and BA/3976/99 clustered in the GB4 genotype with a bootstrap value of 100%. The other four BA strains clustered in the SAB3 genotype, showing a bootstrap value of 84%. Again, strains representative of all years of the study clustered in GB3, except for 1996 and 2000, when group B strains were not isolated.
Genotype-antigenic-pattern correlation among BA strains from group A.
The antigenic variability of HRSV group A viruses was analyzed by reactivity with eight strain-specific MAbs: 021/5G, 021/7G, 021/8G, 021/9G, 25G, 59G, 63G, and 68G. Except for MAb 021/5G, all of these antibodies recognize epitopes that have been mapped in the C-terminal third of the G protein.
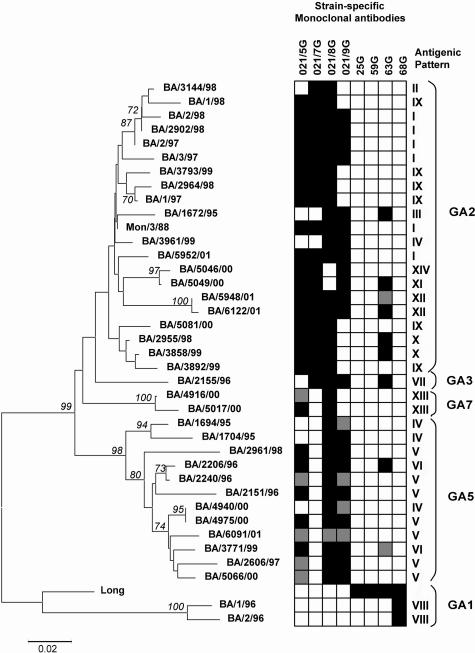
In order to correlate the genetic variability of the G protein from group A isolates with changes at the antigenic level, we compared the antigenic patterns obtained in the ELISA analysis with the genotypes observed in the 37 sequenced BA viruses from group A. A new tree, similar to the tree in Fig. 1, was built using the last 350 nucleotides from the C-terminal segment of the G protein (Fig. 2). This tree contained only BA sequences and the prototype strains A/Long and A/Mon/3/88, which were used to produce the MAbs. The tree was made by the distance method, using the neighbor-joining algorithm. Antigenic patterns shown by each virus are depicted near the tree for comparison.
FIG. 2.
Correlation between the genetic and antigenic analyses of group A strains isolated in Buenos Aires. Strains Mon/3/88 and Long, used to raise monoclonal antibodies, are also included. Trees were built with the neighbor-joining algorithm, using the last 350 nucleotides, which code for the C-terminal segment of the G protein. Antigenic patterns corresponding to each virus are displayed near the tree. Antigenic patterns were defined by the combination of reactivities with the eight strain-specific MAbs. Reactivity with strain-specific MAbs is represented in black (reactive), gray (weakly reactive), or white (nonreactive).
There was a clear correlation between genotypes and antigenic patterns, although some discrepancies were also observed. Thus, antigenic patterns were associated with certain genotypes but not with others, except for antigenic pattern IV, which was observed for one GA2 strain and three GA5 strains.
BA strains clustering in the GA1 genotype reacted only with MAb 68G, raised against A/Long strain, and lacked reactivity with any of the MAbs produced against A/Mon/3/88 (prefix 021). BA strains located in the other main branch reacted with all or some of the MAbs raised against A/Mon/3/88 strain. Only some of these strains reacted with MAb 63G, raised against A/Long.
Analysis of nucleotide sequences.
The average nucleotide identity was 93.3% (range, 82.2 to 100%) for group A strains and 96.4% (range, 88.9 to 100%) for group B strains, while the average amino acid identity was 86.9% (range, 62 to 100%) for group A and 93.4% (range, 78.9 to 100%) for group B.
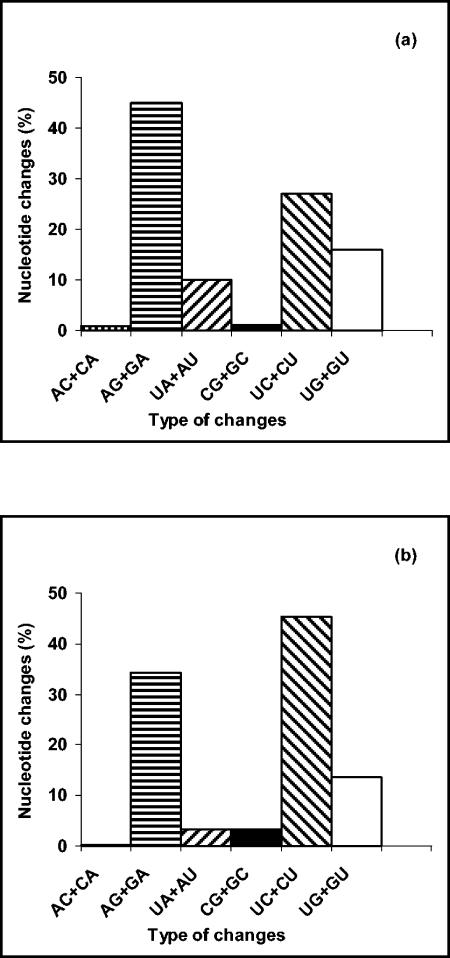
Pairwise comparisons of BA sequences showed that transitions were more frequent than transversions (72% versus 28% for group A viruses and 80% versus 20% for group B viruses). The most frequently observed changes among group A strains were AG+GA transitions, whereas among group B viruses the most frequent changes were CU+UC transitions (Fig. 3).
FIG. 3.
Frequencies of nucleotide changes in sequences of HRSV G protein (BA viruses) of group A (a) and group B (b). Sequences in the vRNA sense were compared pairwise for each virus, and the percentage of different changes shown in each figure was calculated. Since the ancestor sequence for each pair of viruses is unknown, changes involving the same nucleotides (i.e., AG or GA) were added together as in reference 22.
Analysis of amino acid sequences.
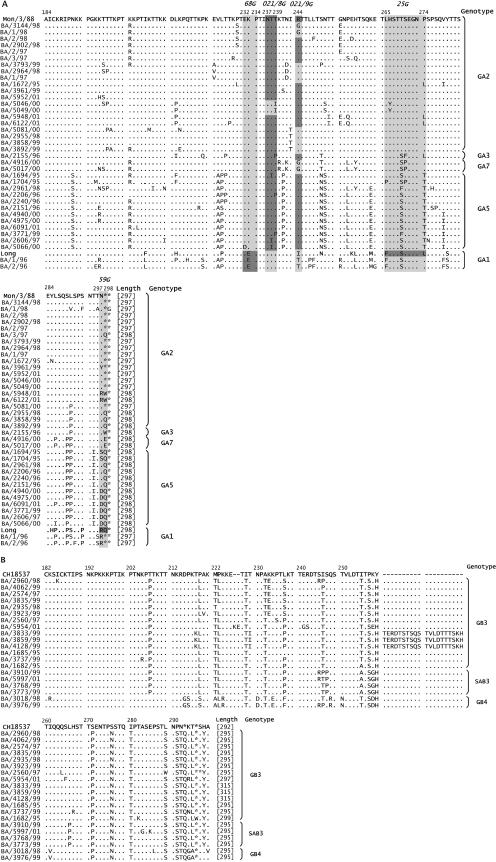
Figures 4A and B represent the deduced amino acid sequences of 37 group A and 20 group B viruses, respectively. Strains A/Mon/3/88, A/Long, and B/CH18537 are shown as reference sequences for each group.
FIG. 4.
Amino acid alignment of the second variable region of the G protein for group A (A) and group B (B) Argentinean strains. Strains A/Mon/3/88 and B/CH18537 are shown as consensus sequences. The genotype and protein length are shown at the end of each sequence. Locations of some group A strain-specific epitopes, as deduced from amino acid changes selected in escape mutants (22, 33, 34), are highlighted in panel A (light gray). Reactivities by ELISA (see Fig. 2) with strain-specific MAbs shown in italics above sequences are indicated by dark gray.
Differences in the length of the G protein were observed for strains of both groups, in most cases due to mutations in the first stop codon of the G gene. Among group B strains, BA/5954/01 showed a protein of 297 amino acids, due to a 6-nucleotide duplication, and strains BA/3833/99, BA/3859/99, and BA/4128/99 had a protein of 315 amino acids as a consequence of a 60-nucleotide duplication (43).
Amino acid substitutions were the most commonly observed changes. Some of them were specific for certain genotypes, e.g., N191S, T241P, and P274T for GA5. GA2 and GB3 showed a lack of genotype-specific amino acid substitutions and different lengths in the G protein of their strains, thus highlighting the heterogeneity of these genotypes.
The three group B strains isolated during 1999 that showed the 20-amino-acid duplication at position 260 also shared other amino acid changes that were not observed in the other isolates.
In an attempt to associate amino acid substitutions with the loss of strain-specific epitopes, we have represented in Fig. 4 the locations of certain epitopes based on amino acid changes selected in escape mutants (22, 33, 34), superimposed with changes related to loss of antibody reactivity found in the present study. Only certain amino acid changes could be clearly related to either gain or loss of specific epitopes.
For instance, GA1 strains that reacted with MAb 68G had E at position 233, whereas the rest of the BA strains, which had K at this position, did not react with antibody 68G.
Strains BA/5046/00 and BA/5049/00, which lack reactivity with MAb 021/8G, had the substitution 239T-I within the identified epitope limits. However, other amino acid changes, such as 238T-I and 239T-P, observed in some strains that reacted with 021/8G, did not seem to be relevant for the integrity of this epitope.
Epitope 021/9G was particularly illustrative. GA1 strains that did not react with this antibody showed the amino acid change, 244R-(I/T), previously described for other natural isolates (11, 21). BA/3144/98, BA1/98, and GA7 strains also showed a change at the same position (244R-G). The rest of the GA2 viruses lacking reactivity with 021/9G displayed changes at nearby locations, such as 243I-T and 242N-D, which may be related to loss of this epitope. This suggests that the limits of epitope 021/9G may be beyond the boundaries identified with escape mutants.
Lack of reactivity with MAb 25G in escape mutants and previous natural isolates (12, 33) has been related to the 265F-L or 274L-P change. All BA strains showed substitutions at one or both positions, and they lost reactivity with this MAb. Therefore, it seems that both amino acids are relevant for the integrity of the 25G epitope.
Only the Long strain reacted with MAb 59G. The change 298Q-stop, which was described for escape mutants (34), was also found in all strains sharing a G protein of 297 amino acids. Among the rest of the BA viruses, other amino acid substitutions located at positions 297 and 298 may be responsible for the lack of reactivity with MAb 59G. The change 295T-I was found exclusively in all GA5 strains and can be considered a genotype-specific substitution.
In summary, some amino acid changes found in BA viruses could be correlated with the loss or gain of certain epitopes. In some cases, these changes were within the epitope boundaries identified previously, but in other cases they were in the proximity of the epitope limits, indicating that these epitopes may expand larger segments of the G protein than previously suspected.
DISCUSSION
Knowledge of HRSV molecular epidemiology has been based mainly on studies done in developed countries (5, 26, 30, 31, 38). In this regard, few studies have been conducted in South America (2, 11, 12, 24). Our analysis showed extensive variability between the two groups of HRSV in Argentina and confirmed the higher variability, at the nucleotide and amino acid levels, within group A viruses than within group B viruses (24, 32, 40). In addition, amino acid sequences showed higher divergence than nucleotide sequences, reinforcing the notion of a selective pressure operating on HRSV, possibly mediated by the host immune response (10, 12, 26).
Phylogenetic analyses showed that BA strains from group A clustered into five previously described genotypes (30, 31), according to bootstrap values of 67 to 100%. These genotypes were placed in two different main branches of the tree (GA1 and GA2 to GA7). This last feature is in agreement with previous studies (11, 30, 38, 44).
Genotypes GA2 and GA5 were the predominant genotypes that cocirculated during most of the study period. A similar occurrence of group A genotypes was described in the neighbor city of Montevideo, Uruguay (11). Some authors (30, 44) previously suggested a transfer of viruses between communities, due to the similar occurrence of genotypes in areas close to each other. It was also emphasized that several strains would be introduced into or circulate endemically in communities but that a strain-specific component of the immune response might determine which genotype would predominate each year.
Argentinean group B strains clustered into three previously described genotypes (30, 31, 44). Genotype GB3 grouped 70% of B strains but with a low bootstrap value. GB3 was also present in every season when occurrence of B strains was detected.
As we described above, strains from group A were selected for sequencing on the basis of their different antigenic patterns shown in an ELISA with strain-specific anti-G MAbs. Comparison between antigenic patterns and genotypes observed for these BA strains allowed us to establish a clear and specific correlation between genotypes and antigenic patterns. Some genotypes were associated with more than one antigenic pattern. This would suggest that the antigenic variability was greater than the diversity observed with genotypes.
Transitions were detected more frequently than transversions, a phenomenon described previously (22, 24, 32). However, while AG+GA transitions were the predominant change among group A strains, UC+CU transitions were detected more frequently among B strains. These data are in accordance with those for strains from Mozambique (32). A model has been proposed in which reiterative A-to-G transitions might be generated by adenosine deaminases operating on short segments of double-stranded RNA secondary structures (23). It was suggested that this mechanism could contribute to the bias accumulation of either A-to-G changes, if adenosine deaminases operated on viral RNA (vRNA), or U-to-C changes, if they operated on cRNA, during the natural evolution of this virus.
The predicted amino acid sequences from both groups showed that the main changes were (i) amino acid substitutions (in some cases genotype specific) (30, 44) and (ii) different lengths of the G protein due to either changes in stop codon usage or nucleotide insertions leading to amino acid insertions. This last change was detected only in some group B isolates. Three strains isolated in 1999 showed a 60-nucleotide duplication. Sequence changes involving nucleotide insertions have been described rarely for HRSV natural isolates (12, 40). However, none of the nucleotide insertions previously described included a drastic change, such as the 60-nucleotide duplication found in the BA strains isolated in 1999. Immunoblot studies have shown that the 60-nucleotide duplication is actually translated into the G protein of these viruses (43). We have proposed a mechanism by which a partial vRNA secondary structure that might be formed during HRSV replication allows the viral polymerase to switch back to the original vRNA strand and copy the 60 nucleotides again.
BA/5954/01, a group B strain isolated in 2001, had a 6-nucleotide insertion in its G protein gene. Two other group B strains showing the same 2-amino-acid duplication at position 227 as BA/5954/01 were isolated in Montevideo, Uruguay, during the same epidemic (3). This finding reinforces the notion of transfer of viruses between close communities.
Regarding the correlation between amino acid substitutions and loss of integrity of strain-specific epitopes, we observed that in most cases the changes were located in relevant positions previously described for escape mutants but involved a different replacing amino acid. Nearby changes, which could be responsible for the loss of reactivity, were also observed. In contrast, some amino acid substitutions located at relevant positions had no effect on loss of epitopes.
In summary, the finding that certain amino acid changes in HRSV isolates may alter the integrity of some epitopes, together with the observed correlation between genetic and antigenic relatedness of viral isolates (Fig. 2), lends support to the idea that immune selection by antibodies may be one of the driving forces in HRSV evolution.
Acknowledgments
This work was supported by European Union grant ERBIC18CT980374 for the project “Molecular Epidemiology of Respiratory Syncytial Virus” (1999 to 2001).
We thank Carmen Ricarte, Beatriz Ebekian, and Cristina Juárez for technical assistance, Silvia Sanchez Puch for help with the ELISA analysis, Adriana Delfraro for advice on phylogenetic analysis, Valeria Melia for help with the English version of the manuscript, and pediatricians for collecting the samples.
REFERENCES
- 1.Anderson, L. J., J. C. Hierholzer, C. Tsou, R. M. Hendry, B. F. Fernie, Y. Stone, and K. McIntosh. 1985. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J. Infect. Dis. 151:626-633. [DOI] [PubMed] [Google Scholar]
- 2.Baumeister, E. G., D. S. Hunicken, and V. L. Savy. 2003. RSV molecular characterization and specific antibody response in young children with acute lower respiratory infection. J. Clin. Virol. 27:44-51. [DOI] [PubMed] [Google Scholar]
- 3.Blanc, A., A. Delfraro, S. Frabasile, and J. Arbiza. 2005. Genotypes of respiratory syncytial virus group B identified in Uruguay. Arch. Virol. 150:603-609. [DOI] [PubMed] [Google Scholar]
- 4.Cane, P. A., D. A. Matthews, and C. R. Pringle. 1991. Identification of variable domains of the attachment (G) protein of subgroup A respiratory syncytial viruses. J. Gen. Virol. 72:2091-2096. [DOI] [PubMed] [Google Scholar]
- 5.Cane, P. A., and C. R. Pringle. 1995. Evolution of subgroup A respiratory syncytial virus: evidence for progressive accumulation of amino acid changes in the attachment protein. J. Virol. 69:2918-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carballal, G., C. Videla, M. D. Sequeira, A. Mistchenko, P. V. Requeijo, and J. Arbiza. 2000. Respiratory syncytial virus: changes in prevalence of subgroups A and B among Argentinian children, 1990-1996. J. Med. Virol. 61:275-279. [DOI] [PubMed] [Google Scholar]
- 7.Carballal, G., C. M. Videla, M. A. Espinosa, V. Savy, O. Uez, M. D. Sequeira, V. Knez, P. V. Requeijo, C. R. Posse, and I. Miceli. 2001. Multicentered study of viral acute lower respiratory infections in children from four cities of Argentina, 1993-1994. J. Med. Virol. 64:167-174. [DOI] [PubMed] [Google Scholar]
- 8.Chanock, R. M., B. Roizman, and M. Myers. 1957. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent. I. Isolation, properties and characterization. Am. J. Hyg. 66:281-290. [DOI] [PubMed] [Google Scholar]
- 9.Choi, E. H., and H. J. Lee. 2000. Genetic diversity and molecular epidemiology of the G protein of subgroups A and B of respiratory syncytial viruses isolated over 9 consecutive epidemics in Korea. J. Infect. Dis. 181:1547-1556. [DOI] [PubMed] [Google Scholar]
- 10.Coggins, W. B., E. J. Lefkowitz, and W. M. Sullender. 1998. Genetic variability among group A and group B respiratory syncytial viruses in a children′s hospital. J. Clin. Microbiol. 36:3552-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frabasile, S., A. Delfraro, L. Facal, C. Videla, M. Galiano, M. J. de Sierra, D. Ruchansky, N. Vitureira, M. Berois, G. Carballal, J. Russi, and J. Arbiza. 2003. Antigenic and genetic variability of human respiratory syncytial viruses (group A) isolated in Uruguay and Argentina: 1993-2001. J. Med. Virol. 71:305-312. [DOI] [PubMed] [Google Scholar]
- 12.Garcia, O., M. Martin, J. Dopazo, J. Arbiza, S. Frabasile, J. Russi, M. Hortal, P. Perez-Brena, I. Martinez, B. Garcia-Barreno, and J. A. Melero. 1994. Evolutionary pattern of human respiratory syncytial virus (subgroup A): cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J. Virol. 68:5448-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Barreno, B., C. Palomo, C. Penas, T. Delgado, P. Perez-Brena, and J. A. Melero. 1989. Marked differences in the antigenic structure of human respiratory syncytial virus F and G glycoproteins. J. Virol. 63:925-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Barreno, B., A. Portela, T. Delgado, J. A. Lopez, and J. A. Melero. 1990. Frame shift mutations as a novel mechanism for the generation of neutralization resistant mutants of human respiratory syncytial virus. EMBO J. 9:4181-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glezen, W. P., L. H. Taber, A. L. Frank, and J. A. Kasel. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140:543-546. [DOI] [PubMed] [Google Scholar]
- 16.Henderson, F. W., A. M. Collier, W. A. Clyde, Jr., and F. W. Denny. 1979. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N. Engl. J. Med. 300:530-534. [DOI] [PubMed] [Google Scholar]
- 17.Imaz, M. S., M. D. Sequeira, C. Videla, I. Veronessi, R. Cociglio, E. Zerbini, and G. Carballal. 2000. Clinical and epidemiologic characteristics of respiratory syncytial virus subgroups A and B infections in Santa Fe, Argentina. J. Med. Virol. 61:76-80. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, P. R., M. K. Spriggs, R. A. Olmsted, and P. L. Collins. 1987. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc. Natl. Acad. Sci. USA 84:5625-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 20.Madhi, S. A., M. Venter, R. Alexandra, H. Lewis, Y. Kara, W. F. Karshagen, M. Greef, and C. Lassen. 2003. Respiratory syncytial virus associated illness in high-risk children and national characterisation of the circulating virus genotype in South Africa. J. Clin. Virol. 27:180-189. [DOI] [PubMed] [Google Scholar]
- 21.Martin, M. 1994. Ph.D. thesis. Análisis genético y antigénico de la glicoproteína G del virus respiratorio sincitial humano. Posible modelo evolutivo. Facultad de Ciencias, Universidad Autónoma de Madrid, Madrid, Spain.
- 22.Martinez, I., J. Dopazo, and J. A. Melero. 1997. Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J. Gen. Virol. 78:2419-2429. [DOI] [PubMed] [Google Scholar]
- 23.Martinez, I., and J. A. Melero. 2002. A model for the generation of multiple A to G transitions in the human respiratory syncytial virus genome: predicted RNA secondary structures as substrates for adenosine deaminases that act on RNA. J. Gen. Virol. 83:1445-1455. [DOI] [PubMed] [Google Scholar]
- 24.Martinez, I., O. Valdes, A. Delfraro, J. Arbiza, J. Russi, and J. A. Melero. 1999. Evolutionary pattern of the G glycoprotein of human respiratory syncytial viruses from antigenic group B: the use of alternative termination codons and lineage diversification. J. Gen. Virol. 80:125-130. [DOI] [PubMed] [Google Scholar]
- 25.Mazzulli, T., T. C. Peret, A. McGeer, D. Cann, K. S. MacDonald, R. Chua, D. D. Erdman, and L. J. Anderson. 1999. Molecular characterization of a nosocomial outbreak of human respiratory syncytial virus on an adult leukemia/lymphoma ward. J. Infect. Dis. 180:1686-1689. [DOI] [PubMed] [Google Scholar]
- 26.Melero, J. A., B. Garcia-Barreno, I. Martinez, C. R. Pringle, and P. A. Cane. 1997. Antigenic structure, evolution and immunobiology of human respiratory syncytial virus attachment (G) protein. J. Gen. Virol. 78:2411-2418. [DOI] [PubMed] [Google Scholar]
- 27.Mufson, M. A., R. B. Belshe, C. Orvell, and E. Norrby. 1987. Subgroup characteristics of respiratory syncytial virus strains recovered from children with two consecutive infections. J. Clin. Microbiol. 25:1535-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mufson, M. A., C. Orvell, B. Rafnar, and E. Norrby. 1985. Two distinct subtypes of human respiratory syncytial virus. J. Gen. Virol. 66:2111-2124. [DOI] [PubMed] [Google Scholar]
- 29.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 30.Peret, T. C., C. B. Hall, G. W. Hammond, P. A. Piedra, G. A. Storch, W. M. Sullender, C. Tsou, and L. J. Anderson. 2000. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J. Infect. Dis. 181:1891-1896. [DOI] [PubMed] [Google Scholar]
- 31.Peret, T. C., C. B. Hall, K. C. Schnabel, J. A. Golub, and L. J. Anderson. 1998. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J. Gen. Virol. 79:2221-2229. [DOI] [PubMed] [Google Scholar]
- 32.Roca, A., M. P. Loscertales, L. Quinto, P. Perez-Brena, N. Vaz, P. L. Alonso, and J. C. Saiz. 2001. Genetic variability among group A and B respiratory syncytial viruses in Mozambique: identification of a new cluster of group B isolates. J. Gen. Virol. 82:103-111. [DOI] [PubMed] [Google Scholar]
- 33.Rueda, P., T. Delgado, A. Portela, J. A. Melero, and B. Garcia-Barreno. 1991. Premature stop codons in the G glycoprotein of human respiratory syncytial viruses resistant to neutralization by monoclonal antibodies. J. Virol. 65:3374-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rueda, P., C. Palomo, B. Garcia-Barreno, and J. A. Melero. 1995. The three C-terminal residues of human respiratory syncytial virus G glycoprotein (Long strain) are essential for integrity of multiple epitopes distinguishable by antiidiotypic antibodies. Viral Immunol. 8:37-46. [DOI] [PubMed] [Google Scholar]
- 35.Salomón, H. E., J. C. Russi, M. Grandien, C. Orvell, M. M. Avila, M. Hortal, and M. Weissenbacher. 1988. Antigenic variants of the respiratory syncytial virus in Argentina and Uruguay. Rev. Argent. Microbiol. 20:147-150. (In Spanish.) [PubMed] [Google Scholar]
- 36.Schmidt, H. A., K. Strimmer, M. Vingron, and A. von Haeseler. 2002. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18:502-504. [DOI] [PubMed] [Google Scholar]
- 37.Selwyn, B. J., et al. 1990. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries. Rev. Infect. Dis. 12(Suppl. 8):S870-S888. [DOI] [PubMed] [Google Scholar]
- 38.Sullender, W. M. 2000. Respiratory syncytial virus genetic and antigenic diversity. Clin. Microbiol. Rev. 13:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullender, W. M., K. Anderson, and G. W. Wertz. 1990. The respiratory syncytial virus subgroup B attachment glycoprotein: analysis of sequence, expression from a recombinant vector, and evaluation as an immunogen against homologous and heterologous subgroup virus challenge. Virology 178:195-203. [DOI] [PubMed] [Google Scholar]
- 40.Sullender, W. M., M. A. Mufson, L. J. Anderson, and G. W. Wertz. 1991. Genetic diversity of the attachment protein of subgroup B respiratory syncytial viruses. J. Virol. 65:5425-5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swofford, D. L. 1998. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, Mass.
- 42.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trento, A., M. Galiano, C. Videla, G. Carballal, B. Garcia-Barreno, J. A. Melero, and C. Palomo. 2003. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J. Gen. Virol. 84:3115-3120. [DOI] [PubMed] [Google Scholar]
- 44.Venter, M., S. A. Madhi, C. T. Tiemessen, and B. D. Schoub. 2001. Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: identification of new subgroup A and B genotypes. J. Gen. Virol. 82:2117-2124. [DOI] [PubMed] [Google Scholar]
- 45.Videla, C., G. Carballal, A. Misirlian, and M. Aguilar. 1998. Acute lower respiratory infections due to respiratory syncytial virus and adenovirus among hospitalized children from Argentina. Clin. Diagn. Virol. 10:17-23. [DOI] [PubMed] [Google Scholar]
- 46.Weissenbacher, M., G. Carballal, M. Avila, H. Salomon, J. Harisiadi, M. Catalano, M. C. Cerqueiro, and P. Murtagh. 1990. Etiologic and clinical evaluation of acute lower respiratory tract infections in young Argentinian children: an overview. Rev. Infect. Dis. 12(Suppl. 8):S889-S898. [DOI] [PubMed] [Google Scholar]