Abstract
The staphylococcal methicillin resistance determinant, mecA, resides on a mobile genetic element, staphylococcus chromosomal cassette mec (SCCmec). The distribution of SCCmec in nature is limited to relatively few clonal complexes of related methicillin-resistant Staphylococcus aureus (MRSA). We have previously reported that some genetic backgrounds are restrictive of mecA and penicillin-binding protein 2a expression, which could account for the restricted clonal distribution of SCCmec in nature. In this study, we investigate the potential role of the host chromosome in the transformability and expression of mecA in 103 naturally occurring methicillin-susceptible S. aureus clinical isolates. The isolates, which had been genotyped previously by multilocus sequence typing, were classified into one of two mutually exclusive categories based on whether the isolates belonged to “major” MRSA lineages or to “other” lineages that are never or occasionally MRSA. We introduced mecA expressed on the low-copy-number plasmid pYK20 into each MSSA strain and assayed the phenotype of resistance to nafcillin by population analysis to assess the relationship between the stability of mecA expression and genetic background. Strains from the major MRSA lineages were more transformable with pYK20 and better able to maintain the plasmid and express resistance in comparison to strains from other lineages. These data support the hypothesis that the presence of mecA within relatively few clonal complexes is partly due to genetic factors that are permissive of mecA and its gene product.
Staphylococcus aureus is a frequent and important human pathogen both in the community and in hospitals (12). Until recently, community strains have been reliably susceptible to most antibiotics, but the prevalence of methicillin-resistant S. aureus (MRSA) is increasing. Methicillin resistance (that is, β-lactam-antibiotic-class resistance) is mediated by PBP2a, a bacterial cell wall synthetic penicillin-binding protein (PBP) with low-affinity binding to β-lactam antibiotics (6, 17). PBP2a is encoded by mecA, which is located on a mobile element, staphylococcal chromosomal cassette mec (SCCmec), which is horizontally transferable among staphylococcal species (8-10). Four types of SCCmec elements have been characterized. Types I, II, and III (34 to 66 kb) are principally found among hospital-associated MRSA (HA-MRSA) strains. SCCmec type IV (20 to 24 kb) was first identified in a community-associated MRSA (CA-MRSA) strain (13), and it is by far the predominant type found among community isolates. In contrast to HA-MRSA, CA-MRSA tends to be susceptible to most non-β-lactam antimicrobials.
Multilocus sequence typing (MLST) is a discriminatory genotyping technique used to characterize isolates of S. aureus based on sequence variation at seven housekeeping genes (3-5). The sequence variation is used to define sequence type (STs) that can be further organized into clonal complexes (CCs) of related STs. The majority of HA-MRSA isolates are members of five CCs or lineages: CC5, CC8, CC22, CC30, and CC45 (4). Additionally, CC1 represents a lineage strongly associated with emerging CA-MRSA infections (13). Of 468 MRSA isolates recorded in the MLST database (www.mlst.net) as of March 2004, 420 belong to CC1, CC5, CC8, CC22, CC30, or CC45. A number of “other” lineages can be found among the S. aureus species that are never or occasionally MRSA or infrequently isolated in general (4, 5, 14). Given the apparent mobility of SCCmec, its limited distribution among the possible S. aureus genotypes found in nature is striking.
Genetic background profoundly influences the methicillin resistance phenotype. Chromosomal genes located outside of SCCmec, for example, determine whether a strain is homogeneous (defined as 1% or more of cells expressing high-level resistance [16]) or heterogeneous (one cell in 106 expressing high-level resistance) in its pattern of resistance. Methicillin-susceptible variants of MRSA strains from which SCCmec was excised (11) were permissive of the introduction of unregulated mecA expressed on a low-copy-number plasmid, pYK20, whereas naïve strains (i.e., those in which mecA was not previously resident on the host chromosome) were restrictive and selected against mecA expression. This barrier to mecA could be overcome by providing β-lactamase regulatory genes blaR1-blaI (1) or homologous regulatory genes mecR1-mecI (7), which presumably act as compensatory elements that control mecA expression and permit the maintenance and expression of plasmid-expressed mecA. We hypothesized that this instability of mecA in some genetic backgrounds could play a role in the relatively restricted clonal distribution of MRSA in nature. In this study, we introduced the pYK20 plasmid expressing unregulated mecA into 103 methicillin-resistant S. aureus (MSSA) clinical isolates representing a variety of different genetic backgrounds to determine whether there was an association between specific genetic backgrounds, i.e., sequence types or clonal complexes and permissive or restrictive behavior with respect to the presence of mecA.
MATERIALS AND METHODS
Bacterial strains and culture condition.
One hundred three MSSA strains that we tested represented all of the tetracycline-sensitive MSSA strains from among 220 strains selected to span the global diversity of the species as described previously (14). Tetracycline-sensitive strains were required in order to avoid direct selection of mecA, which was introduced into recipient strains on a plasmid that encoded tetracycline resistance as the selectable marker. These strains were classified on the basis of ST and/or CC into one of two categories: (i) “major” MRSA lineages, defined as the most common and predominant MRSA lineages from both hospital and community sources and comprising CC1, CC5, CC8, CC22, CC30, and CC45; and (ii) “other” MRSA lineages, defined as those never or occasionally MRSA or infrequently isolated in general. Seventy-seven (75%) of the 103 strains produced β-lactamase. To eliminate the possibility that β-lactamase regulatory genes were compensating for the presence of mecA in an otherwise restrictive background, β-lactamase-negative variants were isolated from 33 of the 77 strains by curing the β-lactamase plasmid by subculture at 43°C.
The control strain, COLnex, is a tetracycline-susceptible, methicillin-susceptible variant of the β-lactamase-negative homogeneous methicillin-resistant strain COL, from which SCCmec has been eliminated (11).
All S. aureus strains and transformants were grown overnight at 37°C in trypticase soy broth or on trypticase soy agar (Difco Laboratories, Detroit, MI) with aeration, unless indicated otherwise. Tetracycline (Sigma Chemical Co., St. Louis, MO) was used at the concentration of 10 μg/ml. Nafcillin (Sigma) was used for population analysis to determine the methicillin resistance phenotype.
Detection of β-lactamase and blaZ.
β-Lactamase was detected by use of a nitrocefin disk (Becton, Dickinson and Company, Sparks, MD) after induction with 2-(2′-carboxyphenyl)benzoyl-6-aminopenicillanic acid (CBAP). The blaZ gene was detected by PCR amplification of DNA extracted from whole-cell lysates with primers 5′-AGTGCATGTAATTCAAACAGTTCA-3′ (205 nt to 182 nt in the blaZ gene [GenBank accession no. X04121]) and 5′-GTCTTACCGAAAGCAGC-3′ (50 nt to 71 nt in the blaZ gene). PCR was carried out with a Taq polymerase kit (QIAGEN) as follows: 3 min at 94°C, 30 cycles of 30 s at 94°C, 1 min at 57°C, and 90 s at 72°C, followed finally by 6 min at 70°C.
Plasmid and DNA manipulation.
The construction of plasmid pYK20 carrying mecA was done as described previously (11). Briefly, pYK20 was isolated from E. coli DH5α using standard procedures. It consists of constitutively expressed mecA cloned into a tetracycline-selectable S. aureus-E. coli shuttle vector, pAW8 (18). The 2.8-kb mecA insert was obtained by PCR amplification of COL mecA, its promoter, the first 223 nt of mecR1, plus a 249-nt stretch downstream of the stop codon, which includes a strong transcriptional terminator. MSSA strains were transformed by electroporation with pYK20 isolated from a COLnex transformant. MSSA transformants were selected by growth on tetracycline-containing agar. DNA manipulations were performed by standard methodologies (10, 19).
COLnex assay for defective mecA expression.
Mutations interfering with the expression of mecA in pYK20 transformants of MSSA strains were assayed using a reporter assay with COLnex, as previously described (11). Briefly, a representative colony of a transformant yielding the predicted 2.8-kb PCR mecA amplification product was regrown in broth containing 10 μg/ml of tetracycline. The pYK20 plasmid was extracted, purified, and introduced into the COLnex host strain by electroporation. After a 48-h incubation, COLnex (pYK20) transformants were selected on tetracycline-containing agar and replicated on trypticase soy agar containing tetracycline at a concentration of 10 μg/ml and nafcillin at concentrations of 0, 2.5, 10, or 100 μg/ml. After a 24-h incubation, CFU growing at each nafcillin concentration were counted and the proportion calculated relative to CFU growing on nafcillin-free agar. Plasmid-expressed mecA on pYK20 reproduces the homogeneous COL phenotype, which is 100% growth of CFU at 100 μg/ml of nafcillin. Mutations interfering with mecA expression of a functional gene product are detected by a heterogeneous phenotype or as susceptibility.
Population analysis.
Population analysis employed the agar plate method (15), in which approximately 108 CFU are quantitatively inoculated onto agar containing nafcillin concentrations of 0, 2.5, 10, or 100 μg/ml. Cultures were incubated for 48 to 72 h at 37°C, and colonies were counted at each concentration. Resistance phenotypes were scored based on the classification proposed by Tomasz et al. (16). Homogeneous resistance was defined as growth of ≥1 CFU in 102 growing on agar containing 100 μg/ml of nafcillin; class 3 heterogeneous resistance was defined as 1 CFU in 102 to 104 at 100 μg/ml of nafcillin; class 2 heterogeneous resistance was defined as 1 CFU in 105 to 106 at 100 μg/ml of nafcillin; and class 1 heterogeneous resistance was defined as ≤103 CFU of 108 growing at 2.5 or 10 μg/ml and no growth at 100 μg/ml of nafcillin.
Statistical analysis.
2 × 2 contingency tables were evaluated with Fisher's exact test.
RESULTS
Detection of β-lactamase plasmid.
The 103 MSSA strains and their classification into “major” MRSA lineages and “other” lineages by MLST are listed in Tables 1 and 2 (5). Seventy-seven percent (41/53) of the strains from major MRSA lineages produced β-lactamase; 72% (36/50) of the strains from other lineages produced β-lactamase.
TABLE 1.
Clonal distribution of MSSA strains related to major MRSA lineages
| Clonal complex | No. of strains
|
% blaZ positive | |
|---|---|---|---|
| Total | blaZ gene positivea | ||
| CC1 | 9 | 8 | 89 |
| CC5 | 8 | 6 (2) | 75 |
| CC8 | 5 | 3 (2) | 60 |
| CC22 | 10 | 8 | 80 |
| CC30 | 10 | 8 (4) | 80 |
| CC45 | 11 | 8 | 73 |
| Total | 53 | 41 (8) | 77 |
The values in parentheses are numbers of strains producing β-lactamase constitutively.
TABLE 2.
Clonal distribution of MSSA strains related to other MRSA lineages
| Clonal complex | No. of strains
|
% blaZ positive | |
|---|---|---|---|
| Total | blaZ gene positivea | ||
| CC9 | 4 | 4 | 100 |
| CC12 | 7 | 2 (1) | 29 |
| CC15 | 5 | 5 | 100 |
| CC25 | 5 | 5 (4) | 100 |
| CC51 | 4 | 4 | 100 |
| ST20 | 2 | 2 | 100 |
| ST59-296 | 3 | 1 | 33 |
| ST97 | 2 | 1 | 50 |
| ST101 | 2 | 0 | 0 |
| ST145-10 | 2 | 1 (1) | 50 |
| Otherb | 14 | 11 | 79 |
| Total | 50 | 36 (6) | 72 |
The values in parentheses are numbers of strains producing β-lactamase constitutively.
Other singletons are ST6, -7, -17, -19, -49, -50, -55, -182, -248, -264, -266, -301, -517, and -529.
Efficiency of plasmid transformation into each MSSA strain.
Of the 103 potential recipient strains for pYK20 transformation, 26 were naturally β-lactamase negative, 33 were successfully cured of β-lactamase, and 44 β-lactamase-producing strains could not be cured using our procedures. Eighty-two percent (84/103) of all strains were successfully transformed with pYK20 (Tables 3 and 4). Transformants were obtained with 86% (51/59) of the β-lactamase-negative strains and with 80% (35/44) of the β-lactamase-producing strains. β-Lactamase production therefore had no association with the transformability of the strains (P = 0.199). Ninety-six percent (51/53) of the strains from major MRSA lineages were transformable, whereas 66% (33/50) of the strains from other lineages were transformable. Thus, strains from the major MRSA lineages were more transformable with the mecA-expressing plasmid than were other lineages (P = 0.0001).
TABLE 3.
Clonal distribution of MSSA strains of other MRSA lineages according to efficiency of transformation with pYK20
| Clonal complex | Strainsa
|
|||
|---|---|---|---|---|
| Total no. | No. with the following efficiency of transformation:
|
|||
| ≤10−11 | 10−10 to 10−8 | 10−7 | ||
| CC1 | 9 (6) | 1 | 8 (6) | 0 |
| CC5 | 8 (2) | 1 (1) | 6 (1) | 1 |
| CC8 | 5 (2) | 0 | 4 (1) | 1 (1) |
| CC22 | 10 (6) | 0 | 6 (3) | 4 (3) |
| CC30 | 10 (5) | 0 | 9 (4) | 1 (1) |
| CC45 | 11 (4) | 0 | 8 (4) | 3 |
| Total (%) | 53 (100) | 2b (4) | 41 (77) | 10 (19) |
Values in parentheses are numbers of β-lactamase-producing strains.
No transformants were obtained for either strain.
TABLE 4.
Clonal distribution of MSSA strains of major MRSA lineages according to efficiency of transformation with pYK20
| Clonal complex | Strainsa
|
|||
|---|---|---|---|---|
| Total no. | No. with the following efficiency of transformation:
|
|||
| ≤10−11 | 10−10 to 10−8 | 10−7 | ||
| CC9 | 4 (4) | 0 | 4 (4) | 0 |
| CC12 | 7 | 3 | 4 | 0 |
| CC15 | 5 (1) | 4 (1) | 1 | 0 |
| CC25 | 5 (2) | 2 | 3 (2) | 0 |
| CC51 | 4 (1) | 2 | 2 (1) | 0 |
| ST20 | 2 (2) | 1 (1) | 1 (1) | 0 |
| ST59-296 | 3 | 3 | 0 | 0 |
| ST97 | 2 (1) | 1 | 1 (1) | 0 |
| ST101 | 2 | 0 | 1 | 1 |
| ST145-10 | 2 (1) | 2 (1) | 0 | 0 |
| Otherb | 14 (7) | 8 (6) | 5 (1) | 1 |
| Total (%) | 50 (100) | 26c (52) | 22 (44) | 2 (4) |
Values in parentheses are numbers of β-lactamase-producing strains.
Other singletons are ST6, -7, -17, -19, -49, -50, -55, -182, -248, -264, -266, -301, -517, and -529.
One or more transformants were obtained at this low efficiency for nine strains.
Stability of pYK20 in MSSA strains.
Another possible explanation for the limited distribution of mecA to a few major MRSA lineages is that the genomes of the major MRSA lineages are relatively permissive of the presence of intact mecA, whereas genomes that infrequently or never are found to harbor mecA are restrictive (11). If so, then the MSSA strains from major MRSA lineages would be expected to maintain functional mecA, whereas other lineages would be restrictive and select against expression of mecA. To test this hypothesis, pYK20 plasmids were purified from MSSA transformants and used to transform COLnex. Defective mecA expression was detected by the loss of the homogeneous resistance phenotype in transformants of COLnex, as briefly described in Materials and Methods.
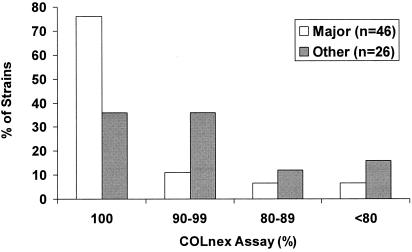
pYK20 purified from 76% (35/46) of the strains from major MRSA lineages yielded 100% of COLnex transformants with homogeneous resistance, whereas only 36% (9/25) of the strains from other lineages did the same (P = 0.0018) (Fig. 1). Thus, MSSA strains from the major MRSA lineages are more favorable to the presence of constitutively expressed mecA than MSSA strains from other lineages.
FIG. 1.
Percentages of MSSA (pYK20) transformants from major MRSA or other lineages that yield plasmids which when introduced into COLnex give 100%, 90 to 99%, 80 to 89%, or <80% of transformants expressing homogeneous resistance to nafcillin.
Phenotype of pYK20 transformants.
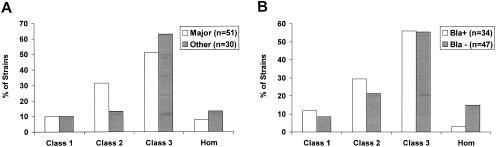
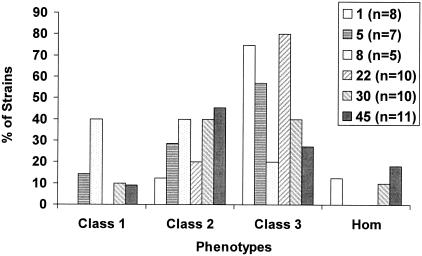
Population analysis was performed with the 84 pYK20 transformants, both β-lactamase positive and negative. Overall, the transformants, regardless of whether they were related to MRSA lineages or not, showed similar patterns of resistance (Fig. 2A). The majority of strains expressed class 2 or class 3 heterogeneous resistance. β-Lactamase-positive and β-lactamase-negative strains distributed among the various resistance phenotype classes in a similar fashion (Fig. 2B). Transformants of MSSA strains from the same CC tended to exhibit similar resistance phenotypes (Fig. 3). For example, CC8 transformants were relatively heterogeneous, with four of five strains showing either class 1 or class 2 resistance. CC1 transformants were relatively more resistant, with six of eight strains showing class 3 resistance.
FIG. 2.
(A) Distribution of MSSA (pYK20) transformants of major MRSA genotypes and other lineages according to resistance phenotype, i.e., homogeneous class (Hom) or heterogeneous class 1, class 2 or class 3. (B) Distribution of MSSA transformants separated according to the presence (Bla+) or absence (Bla−) of β-lactamase.
FIG. 3.
Distribution of MSSA (pYK20) transformants for strains of the six major MRSA CCs according to resistance phenotype, i.e., homogeneous class (Hom) or heterogeneous class 1, class 2 or class 3.
DISCUSSION
These results indicate that there is an association between the genetic background of a strain and its transformability by and maintenance of plasmid-expressed mecA. MSSA strains from major MRSA lineages were relatively easily transformed by electroporation of the pYK20 vector expressing mecA. In contrast, MSSA strains from other lineages were much less efficiently transformed. pYK20 transformants of MSSA strains from major MRSA lineages faithfully maintained mecA, whereas strains from other lineages were more likely to propagate plasmids defective in mecA expression. The distribution of SCCmec within S. aureus may be partly determined by strain properties that contribute to transformation efficiency and the stability of PBP2a expression. Accordingly, major MRSA lineages may be capable of acquiring the mecA gene more easily and maintaining PBP2a expression.
Results obtained with CC1 deserve special mention. mecA expression, as measured by the COLnex assay, was defective in three of seven CC1 transformants. CC1 has relatively recently been identified as an MRSA lineage but is strongly associated with community onset and not with hospital-related outbreaks of infection. CA-MRSA strains, including CC1 strains, commonly carry a type IV SCCmec (13). Interestingly, one of the CC1 MSSA strains examined, MSSA476, carries an SCC element that lacks mecA but expresses ccr genes that mediate excision and insertion. This host strain yielded a relatively high proportion of COLnex transformants that were defective in mecA expression. Perhaps the original mecA copy, assuming that it was once present, was deleted from the resident SCC element because of selection against mecA expression, as was observed with the plasmid-expressed mecA.
There was no significant difference in resistance phenotypes when comparing major MRSA lineages and other lineages. pYK20 transformants tended to show a characteristic resistance level to nafcillin within each CC. This result is consistent with the important effects that chromosomal loci outside of SCCmec have on resistance phenotypes (2), which should be similar within a defined genetic background.
The genetics or biochemical bases of the permissive and restrictive properties of potential mecA recipient genomes are unknown. Our results indicate that the major MRSA lineages may be favored recipients. On the other hand, MSSA strains other than those related to the major MRSA lineages also tolerated mecA to some extent. If the type IV SCCmec enjoys some advantage in its mobility, which seems likely given its smaller size and wider distribution among S. aureus genotypes compared to other SCCmec types (4), then receptive genomes among MSSA strains can and probably will be selected out. This does not bode well for prospects of limiting the spread of MRSA in either hospitals or the community.
Acknowledgments
This work was supported by Public Health Service grant AI46610 from the National Institute of Allergy and Infectious Diseases.
We thank Dong Hong for excellent technical assistance.
REFERENCES
- 1.Asheshov, E. H., and K. G. Dyke. 1968. Regulation of the synthesis of penicillinase in diploids of Staphylococcus aureus. Biochem. Biophys. Res. Commun. 30:213-218. [DOI] [PubMed] [Google Scholar]
- 2.Berger-Bachi, B. B., and M. L. Kohler. 1983. A novel site on the chromosome of Staphylococcus aureus influencing the level of methicillin resistance: genetic mapping. FEMS Microbiol. Lett. 20:305-309. [Google Scholar]
- 3.Crisostomo, M. I., H. Westh, A. Tomasz, M. Chung, D. C. Oliveira, and H. de Lencastre. 2001. The evolution of methicillin resistance in Staphylococcus aureus: similarity of genetic backgrounds in historically early methicillin-susceptible and -resistant isolates and contemporary epidemic clones. Proc. Natl. Acad. Sci. USA 98:9865-9870. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enright, M. C., D. A. Robinson, G. Randle, E. J. Feil, H. Grundmann, and B. G. Spratt. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 99:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feil, E. J., J. E. Cooper, H. Grundmann, D. A. Robinson, M. C. Enright, T. Berendt, S. J. Peacock, J. M. Smith, M. Murphy, B. G. Spratt, C. E. Moore, and N. P. Day. 2003. How clonal is Staphylococcus aureus? J. Bacteriol. 185:3307-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman, B. J., and A. Tomasz. 1984. Low-affinity penicillin-binding protein associated with β-lactam resistance in Staphylococcus aureus. J. Bacteriol. 158:513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiramatsu, K., K. Asada, E. Suzuki, K. Okonogi, and T. Yokota. 1992. Molecular cloning and nucleotide sequence determination of the regulator region of mecA gene in methicillin-resistant Staphylococcus aureus (MRSA). FEBS Lett. 298:133-136. [DOI] [PubMed] [Google Scholar]
- 8.Ito, T., Y. Katayama, K. Asada, N. Mori, K. Tsutsumimoto, C. Tiensasitorn, and K. Hiramatsu. 2001. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito, T., Y. Katayama, and K. Hiramatsu. 1999. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob. Agents Chemother. 43:1449-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katayama, Y., T. Ito, and K. Hiramatsu. 2000. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama, Y., H. Z. Zhang, D. Hong, and H. F. Chambers. 2003. Jumping the barrier to β-lactam resistance in Staphylococcus aureus. J. Bacteriol. 185:5465-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 13.Okuma, K., K. Iwakawa, J. D. Turnidge, W. B. Grubb, J. M. Bell, F. G. O'Brien, G. W. Coombs, J. W. Pearman, F. C. Tenover, M. Kapi, C. Tiensasitorn, T. Ito, and K. Hiramatsu. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 40:4289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson, D. A., and M. C. Enright. 2004. Evolution of Staphylococcus aureus by large chromosomal replacements. J. Bacteriol. 186:1060-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieradzki, K., T. Leski, J. Dick, L. Borio, and A. Tomasz. 2003. Evolution of a vancomycin-intermediate Staphylococcus aureus strain in vivo: multiple changes in the antibiotic resistance phenotypes of a single lineage of methicillin-resistant S. aureus under the impact of antibiotics administered for chemotherapy. J. Clin. Microbiol. 41:1687-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomasz, A., S. Nachman, and H. Leaf. 1991. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 35:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Utsui, Y., and T. Yokota. 1985. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 28:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada, A., and H. Watanabe. 1998. Penicillin-binding protein 1 of Staphylococcus aureus is essential for growth. J. Bacteriol. 180:2759-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, H. Z., C. J. Hackbarth, K. M. Chansky, and H. F. Chambers. 2001. A proteolytic transmembrane signaling pathway and resistance to beta-lactams in staphylococci. Science 291:1962-1965. [DOI] [PubMed] [Google Scholar]