Abstract
We investigated how lexical form similarity of referential candidates and ambiguity of following pronouns impact the encoding and retrieval of words from memory during sentence processing in younger and older adults. Critical sentences included two noun phrases (henceforth NPs) that were either phonologically and orthographically similar (Jason and Jacob/Jade) or dissimilar (Jason and Matt/Hannah), followed by a pronoun (e.g., he) that was either ambiguous or unambiguous (depending on the genders of the preceding NPs). We analyzed brain activity time-locked to the onsets of the second NP (NP2) and the pronoun to investigate the encoding and the retrieval of the NPs, respectively. During encoding NP2, older adults exhibited greater alpha power when NP1 had the same gender, whereas younger adults showed no such effect, suggesting an increased need for inhibition for older adults during encoding. Moreover, although both groups exhibited an increase in alpha power for similar NPs, only younger adults exhibited a theta power increase, suggesting similarity-induced inhibition for both groups, but an additional maintenance cost only for younger adults. During retrieval (i.e., on the pronoun), we found that both pronominal ambiguity and form similarity resulted in greater theta power for younger adults, suggesting full pronominal processing and therefore more difficult retrieval, but smaller theta/alpha power for older adults, suggesting good-enough processing and therefore easier retrieval. Together with complementary behavioral results, our findings suggest that older adults resort to good-enough referential processing when the retrieval of relevant representations is cognitively demanding.
Keywords: Aging, Language processing, Encoding, Retrieval, Time-frequency
1. Introduction
Language comprehension is a complicated cognitive process that involves encoding, maintenance and retrieval of information from the immediate past (e.g., Hofmeister, 2011; Jäger et al., 2017; Lewis et al., 2006; Lewis & Vasishth, 2005; McElree, 2000; McElree et al., 2003; Van Dyke & Lewis, 2003; Van Dyke & McElree, 2006). A prime example illustrating these general memory functions during sentence comprehension is long-distance dependency resolution including referential dependencies, which involve a referring expression such as a pronoun (e.g., she) triggering the retrieval of the memory representation associated with a previously-encoded antecedent (i.e., the character that the pronoun refers to). For example, when processing Jack noticed Sara on the street when he was drinking coffee on the balcony, comprehenders should encode the two characters (Jack and Sara), and when the pronoun he is being processed, the representation of the antecedent (Jack) needs to be retrieved in order for the referential dependency to be successfully resolved (Dell et al., 1983; Gernsbacher, 1989; Gerrig & McKoon, 1998; Lucas et al.,1990; MacDonald & MacWhinney, 1990; Sanford & Garrod, 1989; Sanford & Garrod, 2005). Memory processes typically decline as individuals age (Caplan & Waters, 2005; Park et al., 2002), particularly in relation to the ability to encode and retrieve perceptual, form-related information such as phonological or orthographic features (Burke et al., 1991; Burke & Shafto, 2011). The current study investigates whether and how the effects of lexical form similarity of referential candidates as well as the ambiguity of pronouns are modulated by cognitive aging.
1.1. The effects of semantic similarity, pronominal ambiguity, and cognitive ability on referential processing
Previous studies have demonstrated that semantically more similar referential candidates increase the processing difficulty of following pronouns; which has been attributed to interference during the retrieval of the memory representation of the antecedent (Fukumura et al., 2011; Patil et al, 2016; Parker & Phillips, 2017; also see Gordon et al., 2006; Jäger et al., 2017; Lewis et al., 2006; Martin et al., 2012; Van Dyke & McElree, 2006). Likewise, past research has shown that ambiguous pronouns are more difficult to process than unambiguous pronouns, presumably because ambiguous pronouns activate more than one referential candidate, creating retrieval interference for the antecedent (Karimi et al., 2014, 2018; Karimi & Ferreira, 2016a; Nieuwland & Van Berkum, 2006; van Berkum et al., 1999; Van Berkum et al., 2007). Moreover, past research has demonstrated that although individuals with greater working memory capacity exhibit processing difficulty for ambiguous relative to unambiguous pronouns, individuals with lower working memory spans exhibit no such difficulty, suggesting that high-span individuals entertain both referential interpretations permitted by ambiguous pronouns whereas low-span individuals quickly resolve ambiguous pronouns to the most activated representation, bypassing the ambiguity-induced resolution difficulty (Nieuwland & Van Berkum, 2006; Nicenboim et al., 2016).
1.2. The effects of lexical form similarity and cognitive aging on referential processing
Unlike semantic representations that contain meaning and conceptual associations, phonological and orthographic representations contain surface form information that is essential for speech perception. These distinct types of representations have been shown to be organized and accessed in different parts in the human brain (Hickok & Poeppel, 2007; Indefrey & Levelt, 2004), and may give rise to different similarity-based effects during language comprehension. Importantly, empirical evidence suggests that although older adults possess relatively intact semantic representations, access to phonological and orthographic representations may be more challenging for them (Burke et al., 1991; Burke & Shafto, 2011; Wilson et al., 2018). A theoretical account that provides an explanation for this observation is the Transmission Deficit Hypothesis (Burke et al., 1991, 2000). Based on this account, although aging negatively affects both semantic and surface form-related (phonological/orthographic) representations, phonological/orthographic representations are more vulnerable to cognitive decline due to having a smaller number of interconnections. Specifically, linguistic information is assumed to be stored in a large network of interconnected nodes, which can be divided into a semantic network representing word meanings, and a phonological/orthographic network representing perceptual features of words (e.g., Collins & Loftus, 1975; Luce & Pisoni, 1998). Importantly, while the semantic network is highly interconnected with numerous redundant connections, the phonological/orthographic network is more sparsely connected without much redundancy. Consequently, when connections undergo age-related decline, phonological/orthographic representations are more susceptible to connection losses.
Additionally, pronominal ambiguity necessarily increases retrieval demands because ambiguous pronouns activate multiple referential candidates. To alleviate processing demands, older adults have been shown to exhibit neural compensation or resort to good-enough/shallow processing for more complex sentences (Christianson et al., 2006; Gordon & Lowder, 2016; Payne et al. 2014; Payne & Stine-Marrow, 2017; Christianson, 2016; Ferreira et al. 2002; Ferreira & Patson, 2007; Karimi & Ferreira, 2016b), including referentially ambiguous pronouns (Karimi & Diaz, 2021). For example, Arslan et al. (2020) demonstrated that although younger and older adults exhibit the same brain response to referential violations (a P600 effect), only older adults exhibit an additional anterior negativity, suggesting that older adults may recruit additional brain regions to compensate for increased processing demands imposed by the violations. Moreover, Karimi and Diaz (2021) demonstrated that when referential candidates are phonologically/orthographically confusable, older adults may resort to good-enough processing by resolving the pronoun to the most accessible NP (Christianson, 2016; Ferreira et al. 2002; Ferreira & Patson, 2007; Karimi & Ferreira, 2016b), presumably to alleviate the burden on processing resources (Nieuwland & Van Berkum, 2006). Similarly, Lee and Lai (2024) demonstrated that older adults fail to exhibit the typical Nref effect as a function of pronominal ambiguity- a sustained, frontal negativity that is elicited for ambiguous relative to unambiguous pronouns (e.g., Van Berkum et al., 1999, Nieuwland & Van Berkum, 2006), suggesting that older adults probably lack the processing resources to engage in deep and elaborative referential processing. Thus, referential processing in the context of lexical form similarity and pronominal ambiguity may result in increased cognitive demands, which may be further compounded by cognitive aging.
In a previous study (Karimi & Diaz, 2021), we crossed Pronominal Ambiguity (Ambiguous vs. Unambiguous) and Lexical Form Similarity of the two preceding noun phrases (Similar vs. Dissimilar), across younger and older adults, using experimental sentences such as (1).
| (1) | |
| a) Similar-Ambiguous (Same Genders): | Jason laughed at Jacob when he was almost drunk and high. |
| b) Similar-Unambiguous (Different Genders): | Jade laughed at Jacob when he was almost drunk and high. |
| c) Dissimilar-Ambiguous (Same Genders): | Matt laughed at Jacob when he was almost drunk and high. |
| d) Dissimilar-Unambiguous (Different Genders): | Hannah laughed at Jacob when he was almost drunk and high. |
During encoding NP2 (Jacob), both younger and older adults showed smaller frontal N400 amplitudes (i.e., facilitation) for similar relative to dissimilar NPs, presumably due to phonological priming (e.g., Carreiras et al., 2005; Desroches et al., 2009; Humphreys et al., 1982; Lukatela & Turvey, 1994; c.f., Kush et al. 2015). However, during retrieval of the antecedents (i.e., while processing the pronoun), younger adults exhibited greater late posterior negativity (LPN) amplitudes on pronouns following similar compared to dissimilar NPs (in the 700ms-1100ms post-pronoun window), while older adults showed the opposite pattern. With regards to the effect of pronominal ambiguity, we observed no general effect of ambiguity during encoding or retrieval of the NPs, although a subset of younger adults with greater working memory spans did show the ambiguity effect, which is consistent with Nieuwland and Van Berkum (2006)’s results. To further investigate the apparent facilitatory effect of lexical form similarity for older adults, we carried out a post-hoc behavioral study, explicitly probing the antecedent of unambiguous pronouns to assess pronoun resolution accuracy1. The results revealed a significant effect of lexical form similarity on response accuracy for older but not younger adults. Specifically, while younger adults’ accuracy rates were unaffected by perceptual similarity, older adults had better access to NP1 (i.e., better accuracy) following similar NPs, and better access (accuracy) to NP2 following dissimilar NPs. Moreover, lexical form similarity resulted in slower reaction times for younger, but not older adults.
Together, these results suggest that older adults had lopsided access to the two NPs, and likely did not entertain both referential interpretations when processing the pronoun. Instead, older adults likely performed good-enough processing by quickly resolving the pronoun to the more available NP, because both NPs were not simultaneously available to them. If older adults had equal access to both NPs, the effect of lexical form similarity on accuracy rates should have emerged for both NPs equally, and they should have been slowed down by perceptual similarity (as younger adults were; see Lee & Lai, 2024 for similar results). Note that younger adults’ longer reaction times following perceptually more confusable NPs suggest that they performed full (as opposed to good-enough) referential processing (for more details about the post-hoc experiment, see Karimi & Diaz, 2021). One possibility for older adults’ lopsided access to the nouns could be that when the two nouns were similar, NP2 likely boosted the activation level of the preceding NP1 due to their shared phonological/orthographic features, leading to greater accessibility of NP1. However, when the nouns were dissimilar, encoding NP2 likely reduced NP1’s activation due to the diminished working memory capacity of older adults, leading to higher accessibility for NP2.
1.3. The present study
In our previous study (Karimi & Diaz, 2021), we focused on analyzing ERPs. However, time-frequency analyses enable the examination of aspects of the EEG signal that cannot be observed with ERPs, such as non-phase-locked activity in particular frequency bands (Cohen, 2014), and may therefore reveal different effects than we observed in the ERP study. Specifically, three aspects of our previous study merit further investigation. First, although we observed no age-related differences during encoding, it is possible that such differences actually existed but did not emerge as ERPs. Second, we failed to observe a pronominal ambiguity effect in the ERPs. Given that both lexical form similarity and pronominal ambiguity are expected to influence the retrieval of the antecedent’s memory representation, it is not clear why the ambiguity effect did not emerge in the ERPs. Third, it is not clear how lexical form similarity affects brain oscillations. In the current study, we re-analyzed the data from Karimi & Diaz (2021) adopting a time-frequency approach to focus on oscillatory activity. Note that because the gender congruence of the names determines the ambiguity of the pronoun, Gender Congruence (Same- vs. Different-genders) and Pronominal Ambiguity (Ambiguous vs. Unambiguous) are the same manipulation. However, to maximize clarity, we will refer to this manipulation as Gender Congruence when analyzing how the second-mentioned NP (NP2) was encoded after processing the first-mentioned NP (NP1), and as Pronominal Ambiguity when analyzing how the pronoun was processed.
Past research has shown that neural activity in a variety of frequency bands is implicated during both encoding and retrieval of linguistic information. Specifically, encoding words into working memory has been shown to lead to alpha and theta modulations. For example, alpha power has been reported to increase as the number of items held in memory increases during the delay period of working memory tasks (Jensen et al., 2002; Busch & Herrmann, 2003; Leiberg et al., 2006; Obleser et al., 2012). Such increases in alpha are argued to protect the storage of items in memory by functionally inhibiting task-irrelevant information and/or brain regions (Klimesch et al., 2007; Roux & Uhlhaas, 2014), as well as inhibiting interference from previously-encoded information (Melnik et al., 2017; Waldhauser et al. 2012). Also, theta power has been implicated in working memory operations including encoding new information (Klimesch et al., 1994, Klimesch et al., 1996, Klimesch et al., 1997a; 1997b), as well as the maintenance of information in working memory (Hsieh & Ranganath, 2014; Jensen, & Tesche, 2002; Onton et al., 2005). Similarly, theta oscillations have been implicated during memory retrieval. Specifically, theta power has been shown to increase on pronouns whose antecedent representations are harder to retrieve (Heine et al., 2006; Meyer et al., 2015), consistent with research demonstrating that retrieval of morpho-syntactic information is associated with increases in theta band oscillations (Bastiaansen et al., 2005; Bastiaansen & Hagoort, 2003; Meyer et al., 2015; also see Friese et al., 2013; Gruber et al., 2018). Another frequency band linked with referential processing is the gamma band oscillations, which have been argued to reflect integration of the retrieved representations with the incoming information (Coopmans & Cohn, 2022; Coopmans & Nieuwland, 2020; Nieuwland & Martin, 2017). In fact, consistent with two-stage models of referential processing (e.g., Almor & Nair, 2007; Garnham, 2001; Garrod & Sanford, 1994; Gernsbacher, 1989; McKoon & Ratcliff, 1980; Nieuwland & Martin, 2017; Sanford et al.,1983; Sturt, 2003), reactivation and integration of referential candidates are hypothesized to be associated with theta and gamma power, respectively (Coopmans & Nieuwland, 2020).
Importantly, although language processing generally remains intact with age (Shafto & Tyler, 2014; Diaz et al., 2021; 2022), working memory demands have been shown to complicate language processing for older adults (e.g., Arslan et al., 2020; Feier & Gerstman, 1980; Karimi & Diaz, 2021; Kemper, 1986; Kemper et al., 2009; Obler et al., 1991; Waters & Caplan, 2001). Moreover, cognitive aging has been shown to lead to diminished capacity to modulate neural oscillations in response to stimuli during task-related activities (Huizeling et al., 2021; Henry et al., 2017), or a functional shift in oscillatory neural response from greater cortical disinhibition to greater cortical inhibition (i.e., from a decrease to an increase in alpha-band power) to reduce information overload (e.g., Jensen & Mazaheri, 2010; Beese et al., 2019). This is because while an increase in alpha-band power is thought to have an inhibitory function (either of regions unrelated to the current task, or in which inhibition would support task performance), decreased alpha is associated with recruitment of task-relevant regions (Klimesch et al., 2007; Haegens et al., 2010; Jensen & Mazaheri, 2010). Consistent with these findings, encoding of information has been shown to be supported by cortical disinhibition (i.e., alpha suppression) in younger adults, but by cortical inhibition (i.e., alpha increase) in older adults (Beese et al., 2019). Moreover, cognitive aging has been associated with increased difficulty in long-distance dependency resolution in general (Stine-Morrow et al., 2000), and pronoun processing in particular (Reifegerste & Felser, 2017; Kahn & Till, 1991; Fotiadou et al., 2020).
These findings suggest that cognitive aging may impact the neural oscillatory mechanisms underlying pronoun processing, particularly for pronouns following same-gender and/or perceptually similar referential candidates, which may require increased processing efficiency. Thus, we expected to see modulations of theta, alpha and gamma oscillations when encoding and retrieving antecedents from memory during referential processing. Specifically, based on prior research, we expected an increase in alpha power when encoding is complicated by lexical form similarity and/or pronominal ambiguity, especially for older adults. We also expected an increase in theta power when retrieval was complicated by lexical form similarity and/or pronominal ambiguity, with relatively greater increase for older adults, unless they rely on good-enough processing, in which case we would expect the opposite results (namely, a theta power decrease for older adults). Finally, we expected an increase in gamma power for ambiguous compared to unambiguous pronouns, especially for older adults.
2. Method
2.1. Transparency and Openness
The de-identified data, materials and all the analytic code are available at https://osf.io/75qyz/ (see under “Time-Frequency”). The study design and hypotheses for this study were not pre-registered. As mentioned, we conducted a re-analysis of data previously reported, and a comprehensive description of the study protocol are available in the original publication: Karimi & Diaz (2021).
2.2. Participants
A total of 88 participants initially participated. However, data from 7 younger and 1 older participant was excluded due to noise, leaving 40 participants in each group (younger adults: mean age = 19.37, 31 females, 9 males; older adults: mean age = 67.22, 26 females, 14 males). A post-hoc power analysis revealed that 40 participants in each age group yields a statistical power of .96. All participants were right-handed, native speakers of American English, with no history of neurological or language-related disorders. They underwent MMSE screening (mean = 28.92, range = 27–30), passing without signs of cognitive impairment (Folstein et al., 1975). The study was approved by the Institutional Review Board at the Pennsylvania State University (PSU; protocol number: STUDY00009001). Participants provided written consent before participation. Data was collected during the 2018 academic year at PSU.
2.3. Materials
We created 100 experimental stimuli such as (1). Name pairs were selected using the government generated database, limiting name popularities and gender information to the 2000s (https://www.ssa.gov/OACT/babynames/decades/names2000s.html)2. Phonological and orthographic overlap varied across items, but overall similarity between critical conditions differed significantly, as intended (see “Manipulation Checks” in Results). Similarly, although the name gender interpretations might have slightly differed across participants due to variations in individual knowledge/experience and/or adaptations to diachronic change (Cain & Ryskin, 2023; Li & Siew, 2022), we obtained the Gender Congruence/Pronominal Ambiguity effect in both behavioral and EEG data (see below), suggesting that our gender manipulation was effective. The number of words between NP2 and the critical pronoun ranged from one to four. We used proper names instead of common nouns to isolate phonological/orthographic information, as older adults struggle more with proper names (Cohen & Faulkner, 1986; Lovelace & Twohig, 1990; Ramscar et al., 2014). Each experimental list included 60 fillers. We tagged 24 critical sentences and 20 fillers with comprehension questions to ensure engagement. The questions did not directly probe pronoun antecedents to avoid strategic processing (Stewart et al., 2007; Swets et al., 2008). The order of items within each list was randomized for each participant. The experiment was programmed with E-prime 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA).
2.4. Procedure
Participants first completed a verbal working memory test. They then sat approximately 90 cm from an LCD computer screen. Sentences were displayed one word at a time on the center of the screen, with each word shown for 200ms followed by a 300ms blank screen. A fixation cross preceded each sentence to center participants’ attention and minimize eye movements. Comprehension questions, if present, appeared after the sentence in full, prompting participants to respond with a true/false answer. They were given unlimited time to respond and initiate the next trial once ready. Participants were instructed to maintain high comprehension accuracy, minimize movement and blinking during trials. The experimental session included four blocks following a practice session of six trials. The session lasted approximately 40 and 55 minutes for younger and older adults, respectively.
2.5. EEG recording
EEG was recorded using a 32-channel ActiCHamp system with Ag/AgCl electrodes in an elastic cap (Brain Vision acticap). Electrodes below and to the side of each eye captured blinks and horizontal eye movements, while mastoid electrodes were used for offline re-referencing. Impedances were maintained below 20 kΩ, and data were sampled at 500Hz. EEG data was preprocessed in MATLAB (MathWorks Inc., Natick, MA) using EEGlab toolbox, including bandpass filtering (.01–102 Hz), epoching, and independent component analysis to remove blink-related components. Single-trial waveforms were inspected for artifacts such as drift, muscle activity, and eye movements, with approximately 2% of trials being excluded. Data were re-referenced to the average mastoids and analyzed for EEG power using time-frequency analyses based on modified scripts by Morales & Bowers (2022) and Cohen (2014) (Accessible at: https://osf.io/taed5/). Spectral power was computed by convolving the EEG data with complex Morlet wavelets (cycles ranging from 3 to 10) on a trial-by-trial basis.
Two epochs were extracted for each sentence: one time-locked to NP2 onset and another to the pronoun onset, representing encoding and retrieval of antecedents, respectively. Longer epochs (2000ms before to 3000ms after critical word onset) were used for optimal frequency resolution (Cohen, 2014; Keil et al., 2022). Baseline correction (−300 to −50 ms relative to critical word onset) was applied, noting that the screen was blank during the baseline period.
2.6. Statistical Analyses
We conducted Analysis of Variance using the ezANOVA function in R (Lawrence, 2011), with Age as the between-subjects factor. In addition to Lexical Form Similarity and Pronominal Ambiguity, we introduced Scalp Region with three levels, as a third within-subjects factor to capture the spatial location of any effects. The Frontal region included electrodes FP1/2, F3/4, F7/8, and Fz, the Central region included FC1/2, FC5/6, C3/4, CP1/2, CP5/6, and Cz, and the Posterior region included P3/4, P7/P8, O1/2, Pz, and Oz3. Thus, our final analyses utilized a mixed effects design with a 2×2×3 configuration depending on which within-subjects manipulation was emphasized: Age (Younger vs. Older) × [Lexical Form Similarity (Similar vs. Dissimilar), or, Gender Congruence/Pronominal Ambiguity (Same Gender/Ambiguous vs. Different Genders/Unambiguous)] × Scalp Region (Frontal vs. Central vs. Posterior).
3. Results
3.1. Manipulation checks
Before embarking on the main analyses, we performed two manipulation checks to assess the characteristics of the stimuli. First, a sentence completion experiment confirmed that our lexical form similarity manipulation did not introduce a bias to interpret the ambiguous pronouns in favor of one of the referential candidates for either age group. Past research has shown that contextual bias can render formally ambiguous pronouns virtually unambiguous (Nieuwland & Van Berkum, 2006). Second, we confirmed that the two NPs in the phonologically and orthographically similar condition were indeed more similar in terms of their pronunciations and spellings compared to the NPs in the dissimilar condition. Using the adist function in R, we calculated the Levenshtein distance between the pronunciations as well as the spellings of the two NPs. The name pronunciations were extracted from the Carnegie-Mellon Pronouncing Dictionary, version 0.7b (http://www.speech.cs.cmu.edu/cgi-bin/cmudict; see Karimi & Diaz, 2021, and supplemental materials for details on the manipulation checks).
3.2. Behavioral results: Comprehension Question Accuracy (during the EEG experiment)
We performed a generalized mixed-effects regression model to assess accuracy as a function of our manipulations. The model included random intercepts for subjects and by-item random slopes for the interaction between Age and Gender Congruence/Pronominal Ambiguity. The results revealed a significant main effect of Age, indicating that older adults were less accurate (mean = 91.4%) compared to younger adults (mean = 94.6%; β = −.58, SE = .27, z = −2.13, p = .03). Also, there was a main effect of Gender Congruence/Pronominal Ambiguity in the unexpected direction, with Same-Gender/Ambiguous condition having higher accuracy (mean = 94.3%) than the Different-Gender/Unambiguous condition (mean = 91.6%; β = .55, SE = .20, z = 2.65, p = .008). However, the effect of Gender Congruence/Pronominal Ambiguity was dependent on Age (β = −1.32, SE = .41, z = −3.19, p = .001). Specifically, younger adults exhibited greater accuracy than older adults in the Same-Gender/Ambiguous condition (β = −1.42, SE = .70, z = −2.02, p = .04), but not in the Different-Gender/Unambiguous condition (β = −.07, SE = .37, z = −.19, p = .84). No other effects were statistically reliable.
3.3. EEG results
As mentioned above, given the current literature on referential processing, we expected theta and alpha modulations during encoding the second NP, and theta and gamma modulations during processing the pronouns. We also observed some unpredicted delta, and simultaneous alpha/beta effects as a function of both pronominal ambiguity and lexical form similarity. The predicted (and analyzed) frequency bands are marked with solid lines, whereas the unpredicted effects are marked by dashed lines in the time-frequency plots, and are analyzed and reported in supplemental materials. We note that these unpredicted effects and the associated interpretations should be taken with caution.
For all EEG results, we always report the main effects of Age, Gender Congruence/Pronominal Ambiguity, Lexical Form Similarity, as well as their interactions. However, for brevity, we only report interactions with topographic regions when they were statistically significant. Moreover, when the higher order (3-way) interaction was significant, we did not follow up 2-way interactions. All the reported p values were corrected for sphericity where appropriate. For both age groups, the effects emerged on all electrodes during encoding, whereas during retrieval, the Pronominal Ambiguity effect was maximal at the frontal areas, and the Lexical Form Similarity effect at the parietal sites. Thus, the time-frequency plots during encoding are presented on all electrodes, while those during retrieval are presented for frontal and parietal sites for the Ambiguity and Similarity effects, respectively.
3.3.1. Gender Congruence effect during encoding
Figure 1 shows oscillation power time-locked to NP2 as a function of gender congruence of the referential candidates (first two rows), as well as the Gender Congruence effect (Same Gender – Different Genders; third row) across all electrodes for younger and older adults. Although younger adults did not exhibit any modulation of time-frequency power, older adults exhibited greater alpha power (8–13 Hz) around 350ms-550ms after NP2 onset when the two NPs had the same gender relative to when they had different genders.
Figure 1.
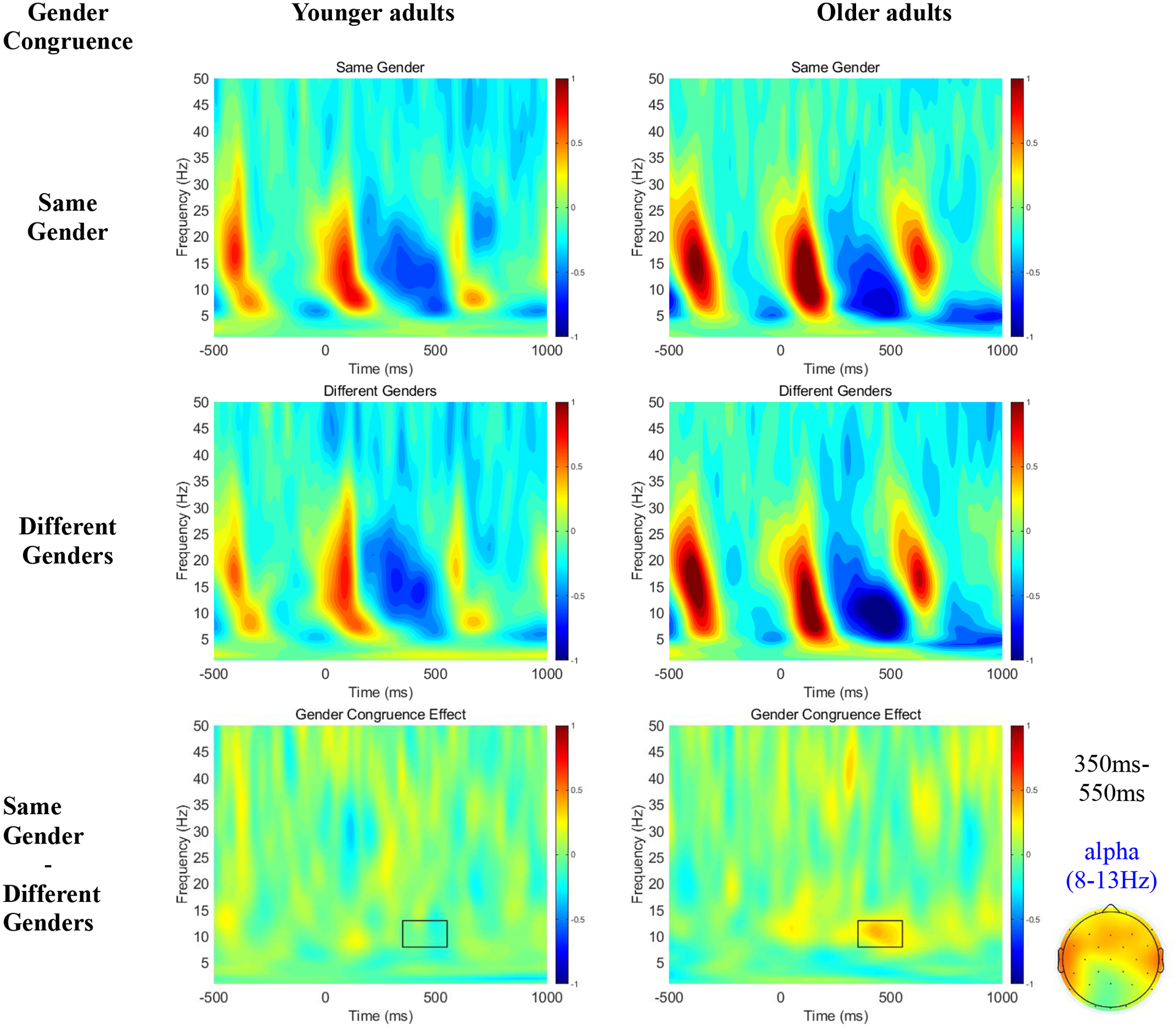
Time-Frequency plots collapsed over all electrodes as a function of Gender Congruence across younger and older adults during encoding (i.e., on NP2).
Table 1 reports mean alpha power for all conditions, as well as the results of our statistical tests on alpha power (8–13Hz) in the critical time window (350ms-550ms). The tests revealed a main effect of age on alpha power, with stronger overall alpha suppression (i.e., smaller alpha power) for older than younger adults4. There was no significant main effect of Gender Congruence. However, we observed a 2-way interaction between Age and Gender Congruence, with greater alpha power in the same- relative to different-genders condition only for older adults. Moreover, we also observed a significant 2-way interaction between Age and Scalp Region. Follow up simple effect analyses showed that the Age effect was significant only at frontal and central (but not parietal) electrodes.
Table 1.
Mean alpha power and ANOVA results for the effect of Gender Congruence during encoding.
| Time window & Frequency | Predictor | Mean power | Results |
|---|---|---|---|
| 350ms-550ms Alpha (8–13Hz) |
*Age | YAs = −.49 OAs = −.76 |
F(1,78) = 5.49, p = .02 |
| Gender Congruence | Same = −.59 Diff = −.67 |
F(1,78) = 1.20, p = .27 | |
| *Age × Gender Congruence | F(1,78) = 4.32, p = .04 | ||
| Gender Congruence effect for YAs | Same = −.53 Diff = −.45 |
F(1,39) = .59, p =.44 | |
| *Gender Congruence effect for OAs | Same = −.64 Diff = −.89 |
F(1,39) = 4.25, p =.04 | |
| *Age × Scalp Region | F(2,156) = 3.47, p = .049 | ||
| *Age effect at frontal regions | YAs = −.41 OAs = −.75 |
F(1,78) = 6.75, p = .01 | |
| *Age effect at central regions | YAs = −.38 OAs = −.74 |
F(1,78) = 8.87, p = .003 | |
| Age effect at parietal regions | YAs = −.69 OAs = −.81 |
F(1,78) = .79, p = .37 |
indicates statistically significant effects at p < .05.
3.3.2. Lexical Form Similarity effect during encoding
Figure 2 shows oscillation power time-locked to NP2 as a function of the lexical form similarity of the referential candidates (first two rows), as well as the Lexical Form Similarity effect (Similar – Dissimilar) across all electrodes for younger and older adults (third row). Although lexical form similarity resulted in increased theta power (4–8Hz) in younger adults in the 450ms-700ms window, it resulted in increased alpha power (8–13Hz) for both groups in the 250ms-400ms window.
Figure 2.
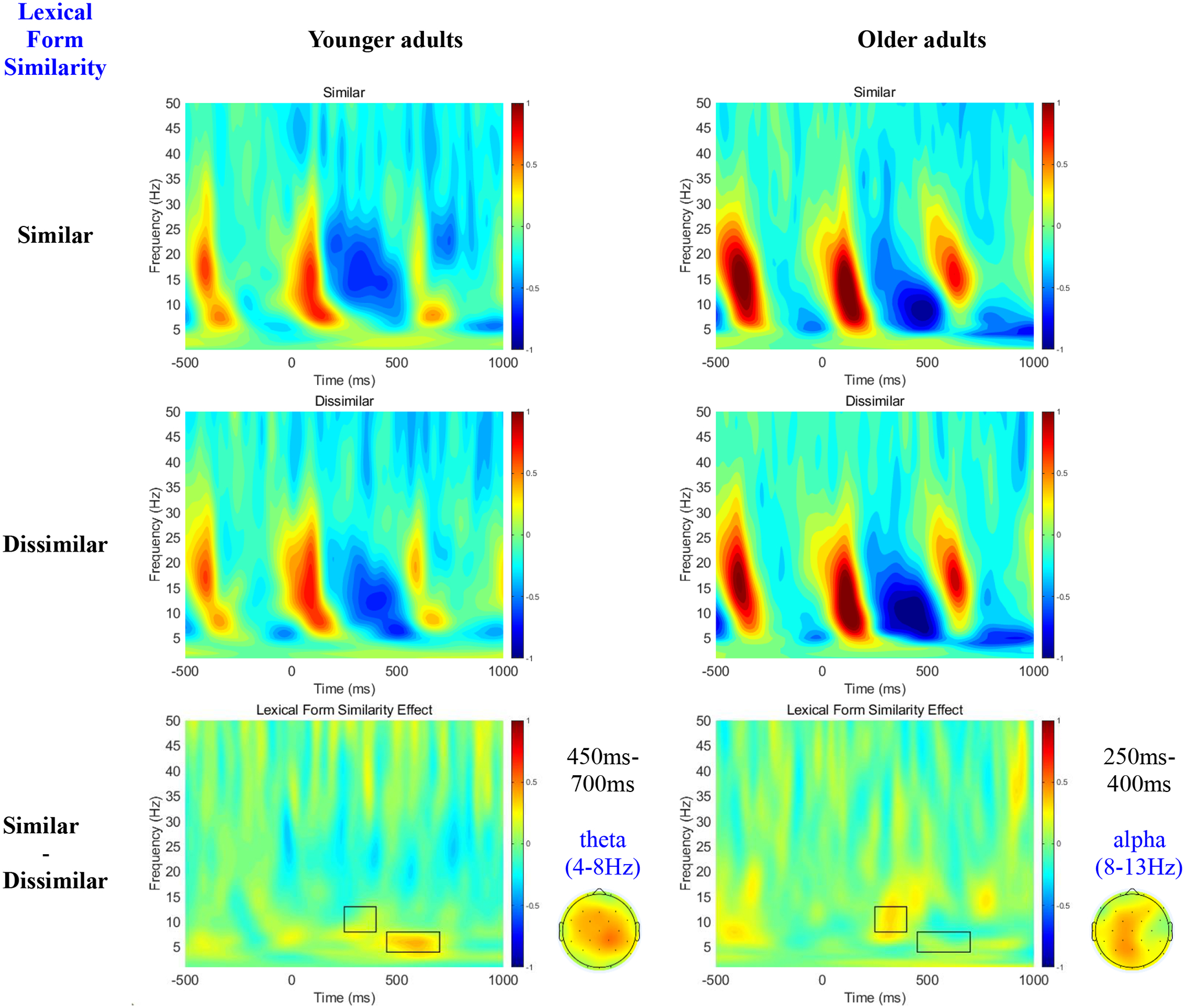
Time-Frequency plots collapsed over all electrodes as a function of Lexical Form Similarity across younger and older adults during encoding (i.e., on NP2).
Table 2 reports the mean theta and alpha power as well as the results of our statistical tests. As for theta power, we observed a main effect of Age, with younger adults exhibiting greater theta power than older adults, as well as a main effect of Lexical Form Similarity with greater theta when the two NPs were similar relative to when they were dissimilar. We also observed trends towards an Age×Lexical Form Similarity interaction, and an Age×Similarity×Scalp Region interaction. Follow up simple effects revealed that younger but not older adults exhibited greater theta power for similar than dissimilar NP2s, and that this greater theta power was significant only at the centro-parietal regions. As for alpha power, we only observed a main Lexical Form Similarity effect, with greater alpha power on NP2s in the phonologically/orthographically similar than in the dissimilar condition.
Table 2.
Mean alpha power and ANOVA results for the effect of Lexical Form Similarity during encoding.
| Time window & Frequency | Predictor | Mean power | Results |
|---|---|---|---|
| 450ms-700ms Theta (4–8Hz) |
*Age | YAs: −.11 OAs: −.33 |
F(1,78) = 4.53, p = .03 |
| *Lexical Form Similarity | Sim: −.15 Dissim: −.29 |
F(1,78) = 4.07, p = .04 | |
| Age × Lexical Form Similarity | F(1,78) = 3.29, p = .07 | ||
| Age × Lexical Form Similarity × Scalp Region | F(2,156) = 3.14, p =.06 | ||
| Lexical Form Similarity effect-YAs-Frontal | Sim: .05 Dissim: −.17 |
F(1,39) = 2.91, p =.09 | |
| * Lexical Form Similarity effect-YAs-Central | Sim: .07 Dissim: −.31 |
F(1,39) = 8.70, p =.005 | |
| * Lexical Form Similarity effect-YAs-Parietal | Sim: −.05 Dissim: −.26 |
F(1,39) = 4.19, p =.04 | |
| Lexical Form Similarity effect-OAs-Frontal | Sim: −.40 Dissim: −.32 |
F(1,39) = .41, p =.52 | |
| Lexical Form Similarity effect-OAs-Central | Sim: −.47 Dissim: −.43 |
F(1,39) = .08, p =.77 | |
| Lexical Form Similarity effect-OAs-Parietal | Sim: −.10 Dissim: −.26 |
F(1,39) = 2.41, p =.12 | |
| 250ms-400ms Alpha (8–13Hz) |
Age | YAs: −.47 OAs: −.60 |
F(1,78) = 1.84, p =.17 |
| * Lexical Form Similarity | Sim: −.45 Dissim: −.62 |
F(1,78) = 4.66, p =.03 | |
| Age × Lexical Form Similarity | F(1,78) = 1.06, p =.30 |
indicates statistically significant effects at p < .05.
3.3.3. Pronominal Ambiguity effect during retrieval
Figure 3 displays time-frequency plots at the frontal electrodes for each condition (first two rows), and for condition contrasts (third row) across younger and older adults. While younger adults exhibited greater theta power, older adults showed reduced theta power (4–8Hz) for ambiguous relative to unambiguous pronouns in the 800–1400 ms time window.
Figure 3.
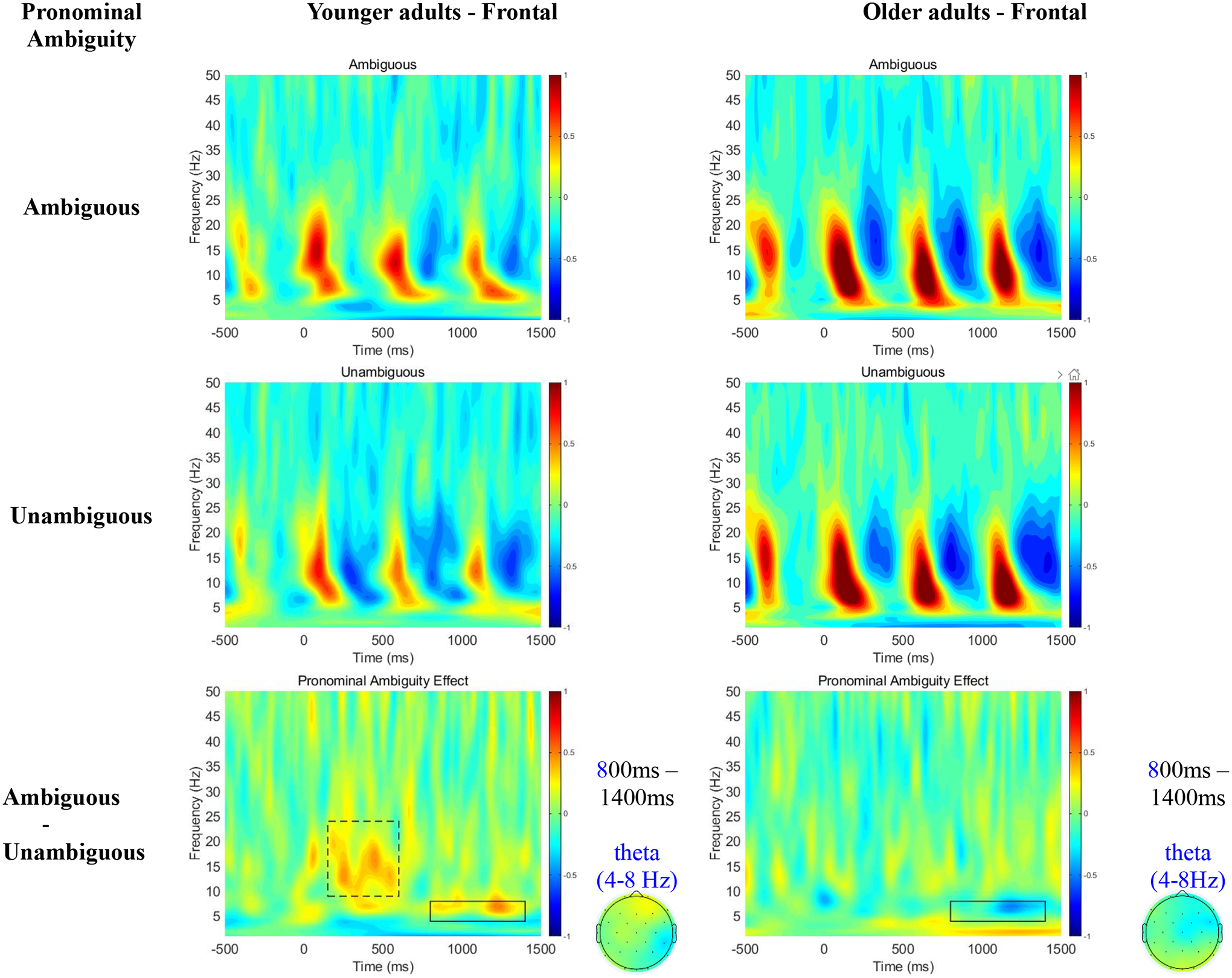
Time-Frequency plots collapsed at frontal electrodes as a function of Pronominal Ambiguity across younger and older adults during retrieval (i.e., on the pronoun).
Table 3 reports mean theta power as well as the results of our statistical tests for the effect of pronominal ambiguity. We observed no main effects of Age or Pronominal Ambiguity. But the 3-way interaction between Age, Pronominal Ambiguity and Scalp Region was significant. Simple analyses revealed that the ambiguity effect flipped at the frontal regions across age groups, with younger adults exhibiting greater, but older adults showing smaller theta power for ambiguous relative to unambiguous pronouns (although no simple effects reached statistical significance).
Table 3.
Mean theta power and ANOVA results for the effect of Pronominal Ambiguity during retrieval.
| Time window & Frequency | Predictor | Mean Power | Results |
|---|---|---|---|
| 800ms-1400ms Theta (4–8Hz) |
Age | YAs: .02 OAs: .10 |
F(1,78) = .72, p = .39 |
| Pronominal Ambiguity | Amb: .05 Unamb: .08 |
F(1,78) = .10, p = .74 | |
| Age × Pronominal Ambiguity | F(1,78) = .27, p = .59 | ||
| * Age × Pronominal Ambiguity × Scalp Region | F(2,156) = 4.11, p = .03 | ||
| Pronominal Ambiguity effect-YA-Frontal | Amb: .14 Unamb: .00 |
F(1,39) = .86, p = .35 | |
| Pronominal Ambiguity effect-YA-Central | Amb: −.00 Unamb: .02 |
F(1,39) = .02, p = .87 | |
| Pronominal Ambiguity effect-YA-Parietal | Amb: −.04 Unamb: .02 |
F(1,39) = .39, p = .53 | |
| Pronominal Ambiguity effect-OA-Frontal | Amb: .14 Unamb: .28 |
F(1,39) = 1.33, p = .25 | |
| Pronominal Ambiguity effect-OA-Central | Amb: .01 Unamb: .15 |
F(1,39) = 1.17, p = .28 | |
| Pronominal Ambiguity effect-OA-Parietal | Amb: .05 Unamb: −.01 |
F(1,39) = .28, p = .59 |
indicates statistically significant effects at p < .05.
Because past research has shown that the referential ambiguity effect emerges for individuals with higher working memory span (e.g., Nieuwland & van Berkum, 2006), we investigated this potential interaction, and observed greater delta power (1–4Hz) for ambiguous relative to unambiguous pronouns only for high-span individuals. This is consistent with recent findings showing that delta activity may reflect reinstatement of antecedent representations during referential processing (Ding et al., 2023; Slaats et al., 2023). The full results of this analysis are reported in the supplemental materials5.
3.3.4. Lexical Form Similarity effect during retrieval
Figure 4 shows the time-frequency plots at the parietal electrodes as a function of lexical form similarity for each single condition (first two rows), and for the condition contrasts (third row). Similar to the ambiguity effect, while younger adults showed theta power (4–8 Hz) increase for the Similar relative to the Dissimilar condition in the 550ms-1500ms time window, older adults showed a discontinuous theta/alpha power decrease (4–10Hz) in the 0ms-1100ms time window.
Figure 4.
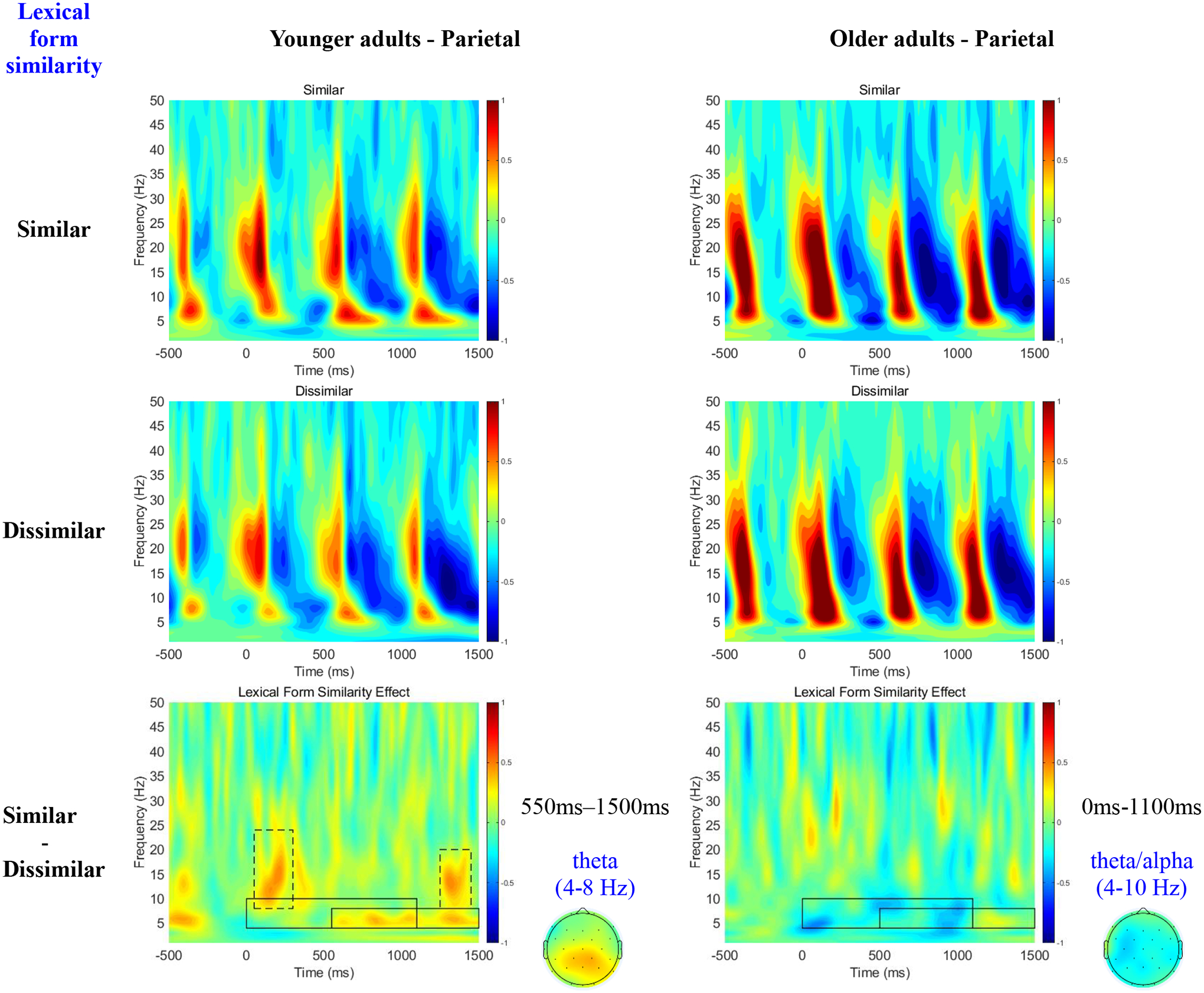
Time-Frequency plots collapsed at frontal electrodes as a function of Lexical Form Similarity across younger and older adults during retrieval (i.e., on the pronoun).
Table 4 reports the mean theta/alpha power in each condition, as well as the results of our statistical tests. We analyzed two time-windows as indicated by the solid boxes in Figure 4. With regards to theta power (4–8Hz) in the 550ms-1500ms window, while we observed no main effects of Age or Lexical Form Similarity, the 3-way interaction between Age, Lexical Form Similarity and Scalp Region was significant. Simple analyses revealed that the Lexical Form Similarity effect was significant only for younger adults and only at the central and parietal (but not frontal) regions. With regards to theta/alpha power (4–10Hz) in the 0ms-1100ms window, we observed a main effect of Age, with older adults exhibiting greater theta/alpha power than younger adults. The main effect of Lexical Form Similarity was not significant, but the 3-way interaction between Age, Lexical Form Similarity and Scalp Region was. Simple analyses revealed that older adults exhibited smaller theta/alpha power in the Similar than in the Dissimilar condition only at the central and parietal (but not frontal) regions.
Table 4.
Mean theta power and ANOVA results for the effect of Lexical Form Similarity during retrieval.
| Time window & Frequency | Predictor | Mean power | Results |
|---|---|---|---|
| 550–1500ms theta (4–8Hz) |
Age | YAs: .06 OAs: .16 |
F(1,78) = 1.32, p =.25 |
| Lexical Form Similarity | Sim: .12 Dissim: .10 |
F(1,78) = .07, p =.78 | |
| Age × Lexical Form Similarity | F(1,78) = 3.62, p =.06 | ||
| *Age × Lexical Form Similarity × Scalp Region | F(2,156) = 3.87, p =.04 | ||
| Lexical Form Similarity Effect-YAs-Frontal | Sim: .10 Dissim: .11 |
F(1,39) = .00, p =.95 | |
| * Lexical Form Similarity Effect-YAs-Central | Sim: .13 Dissim: −.07 |
F(1,39) = 4.15, p =.04 | |
| * Lexical Form Similarity Effect-YAs-Parietal | Sim: .15 Dissim: −.07 |
F(1,39) = 6.19, p =.01 | |
| Lexical Form Similarity Effect-OAs-Frontal | Sim: .27 Dissim: .32 |
F(1,39) = .17, p =.67 | |
| Lexical Form Similarity Effect-OAs-Central | Sim: .03 Dissim: .20 |
F(1,39) = 2.51, p =.12 | |
| Lexical Form Similarity Effect-OAs-Parietal | Sim: .02 Dissim: .12 |
F(1,39) = 1.02, p =.32 | |
| 0–1100ms theta/alpha (4–10Hz) |
*Age | YAs: −.02 OAs: .17 |
F(1,78) = 6.51, p =.01 |
| Lexical Form Similarity | Sim: .04 Dissim: .11 |
F(1,78) = 1.50, p =.22 | |
| Age × Lexical Form Similarity | F(1,78) = 3.80, p =.05 | ||
| *Age × Lexical Form Similarity × Scalp Region | F(2,156) = 5.89, p =.008 | ||
| Lexical Form Similarity Effect-YAs-Frontal | Sim: −.01 Dissim: .09 |
F(1,39) = .88, p =.35 | |
| Lexical Form Similarity Effect-YAs-Central | Sim: .00 Dissim: −.09 |
F(1,39) = .93, p =.33 | |
| Lexical Form Similarity Effect-YAs-Parietal | Sim: .02 Dissim: −.12 |
F(1,39) = 2.97, p =.09 | |
| Lexical Form Similarity Effect-OAs-Frontal | Sim: .24 Dissim: .37 |
F(1,39) = 1.66, p =.20 | |
| * Lexical Form Similarity Effect-OAs-Central | Sim: −.04 Dissim: .22 |
F(1,39) = 7.20, p =.01 | |
| * Lexical Form Similarity Effect-OAs-Parietal | Sim: .01 Dissim: .21 |
F(1,39) = 4.85, p =.03 |
indicates statistically significant effects at p < .05.
4. Discussion
We investigated the effects of pronominal ambiguity as well as lexical form similarity on the encoding and retrieval of referential candidates during sentence processing across younger and older adults. Critical sentences included two noun phrases (NP1 and NP2) that were either phonologically/orthographically similar (e.g., Jason/Jade and Jacob) or dissimilar (e.g., Matt/Hannah and Jacob), followed by a pronoun (e.g., he) that was either ambiguous or unambiguous depending on the genders of the two NPs. By time-locking the brain activity to the onsets of NP2 (Jacob) and the pronoun (he), we were able to investigate the potential effect of lexical form similarity on the encoding and retrieval of the memory representations of the referential candidates, respectively. In the following, we will first discuss the results of encoding and retrieval processes separately. We then discuss the overall results and their broader implications.
4.1. Encoding same- vs. different-gender nouns
When encoding a second NP, older adults showed greater alpha power increase when the second NP had the same gender as the first NP. Since alpha power increases have been shown to reflect inhibition of irrelevant memory representations and/or neural populations (e.g., Klimesch et al., 2007; Roux & Uhlhaas, 2014), this pattern of results suggest that older adults relied more heavily on cortical inhibition to encode same- relative to different-gender nouns. These results are consistent with prior findings demonstrating that encoding efficiency tends to be achieved through cortical inhibition (alpha power increase) in older adults, but through cortical disinhibition (alpha suppression) in younger adults (Beese et al., 2019). However, as discussed below, the cortical inhibition on the part of older adults did not aid subsequent retrieval when processing pronouns (see sections 4.3 and 4.4).
4.2. Encoding phonologically/orthographically similar vs. dissimilar nouns
When encoding a second NP, younger adults showed increased theta power when the second NP was perceptually (i.e., phonologically and orthographically) similar to NP1 than when two NPs were dissimilar, suggesting greater maintenance cost for younger than for older adults (Gevins et al., 1997; Jensen & Tesche, 2002), and consistent with prior research showing that greater theta power during encoding predicts retrieval success at a subsequent point (Klimesch et al., 1996; 1997a–b; Sederberg et al., 2003). Despite different theta powers, both age groups showed greater alpha power for NP2s following perceptually similar than dissimilar NPs, suggesting that both groups exerted greater cortical inhibition with increasing surface form similarity. This is not surprising given that encoding two similar nouns is more susceptible to proactive interference, heightening the need for inhibition. Thus, the overall pattern of results during encoding perceptually similar nouns suggests that younger adults encode and maintain the memory representations associated with both nouns. However, although older adults seem to encode both NPs (initially), they may not maintain both noun representations (at least not efficiently). Importantly, we observed the consequence of such lopsided maintenance during retrieval as a function of lexical form similarity (see below).
4.3. Retrieving memory representations associated with ambiguous pronouns
When processing ambiguous pronouns (i.e., when retrieving the memory representation associated with the potential antecedents), younger adults exhibited greater theta power when the pronouns were ambiguous than when they were unambiguous, but older adults showed the opposite pattern. The latency of this effect matches many prior studies (e.g., Karimi et al., 2018; Nieuwland, 2014; Nieuwland et al., 2019; Martin et al., 2012). Because theta power has been empirically linked to antecedent retrieval during referential processing (e.g., Heine et al., 2006; Meyer et al., 2015), this pattern of results should, on the surface, be taken to reflect easier (not harder) retrieval for older relative to younger adults. However, given that older adults have been shown to experience more (not less) difficulty during memory retrieval (e.g., Bowles & Poon, 1985; Burke & Light, 1981), these results open up the possibility that older adults likely performed shallow/good-enough processing by avoiding elaborative referential processing (Lee & Lai, 2024). However, younger adults likely performed a full retrieval operation by activating the memory representations of both referential candidates, leading to greater retrieval difficulty. This interpretation is consistent with the accuracy results during the EEG experiment, where we observed significantly lower accuracy rates for older than younger adults, especially when the pronouns were ambiguous (see section 3.2). These results are also consistent with past research showing that individuals with smaller working memory capacity (which correspond to older adults in our study; see supplementary materials) exhibit less difficulty during pronoun resolution, presumably because they circumvent entertaining both referential interpretations by “immediately [taking] on the first referential commitment that comes to mind” (Nieuwland & Van Berkum, 2006; page 156). To further corroborate the role of working memory capacity in referential processing, we also demonstrated that the effect of pronominal ambiguity emerges only for individuals with greater working memory spans as greater delta power for ambiguous relative to unambiguous pronouns. These results are consistent with recent research showing that delta oscillations may reflect reinstatement of antecedent representations during referential processing (Ding et al., 2023; Slaats et al., 2023).
4.4. Retrieving memory representations of phonologically/orthographically similar vs. dissimilar words
Similar to the pronominal ambiguity effect, when processing the pronouns, younger adults exhibited greater theta power than older adults when the preceding NPs were perceptually (i.e., phonologically and orthographically) more similar. In addition, we also observed modulation of low-frequency bands (4–10Hz, theta and alpha) at the 0ms-1100ms window such that older adults exhibited smaller theta/alpha power on pronouns following similar than dissimilar NPs. On the surface, these results may seem to suggest that lexical form similarity causes more retrieval difficulty for younger than older adults. However, this may also simply show that older adults perform good-enough pronoun processing, whereas younger adults fully process both NPs, which complicates retrieval difficulty. This interpretation is in line with our post-hoc behavioral study where we showed that, unlike younger adults, older adults likely had access to the representation of one (but not both) of the referential candidates when processing the pronoun, thereby facilitating referential resolution via a good-enough processing mechanism. Interestingly, a good-enough interpretation of these results is consistent with the pattern of results during encoding phonologically/orthographically similar NPs. As mentioned above, younger (but not older) adults exhibited theta increase for phonologically/orthographically similar NPs. Since past research has established that increasing maintenance demands leads to an increase in theta power (e.g., Hsieh & Ranganath, 2014; Jensen, & Tesche, 2002; Onton et al., 2005), the encoding results seem to suggest that maintenance cost was greater for younger than older adults. This most likely implies that younger adults maintained both noun representations in working memory whereas older adults likely maintained only one, leading to the observed facilitation for older adults during retrieval.
4.5. Overall discussion and theoretical implications
Put together, the pattern of results observed in this study suggests that older adults are less efficient when encoding referential candidates, which leads to good-enough referential resolution when the memory representation of the antecedent needs to be retrieved. Evidence for inefficient encoding for older adults came from their reliance on neural inhibition during encoding, even in the absence of lexical form similarity (when the two referential candidates only shared gender information). Moreover, older adults did not exhibit enhanced theta activity when encoding similar-sounding/-looking referential candidates. Since theta power has been linked to maintenance cost (e.g., Hsieh et al., 2011; Hsieh & Ranganath, 2014; Scheeringa et al., 2009), this suggests that older adults may have maintained the memory representation of one of the referential candidates, not both.
Evidence for good-enough retrieval came from the observation that older adults exhibited a facilitation (rather than the expected hinderance) on ambiguous relative to unambiguous pronouns, as well as on pronouns following two perceptually similar relative to dissimilar nouns. Because ambiguous pronouns have been shown to be more difficult to process than unambiguous pronouns, and also because similar-sounding/-looking NPs are expected to be more (not less) confusable, the apparent ease of retrieval for older adults suggests good-enough referential resolution whereby both referential options are not fully considered when the pronoun is being resolved. In contrast to older adults, younger adults seem to have performed full and elaborative (as opposed to good-enough) sentence processing by encoding and maintaining both referential representations, and entertaining both referential interpretations when processing the critical pronouns, leading to more retrieval difficulty (Lee & Lai, 2024). Based on our post-hoc experiment (see above), older adults seem to perform good-enough referential processing by quickly attaching the pronoun to one of the referential candidates to alleviate the cognitive burden of having to entertain two referential interpretations. Another way in which older adults’ referential processing could be good-enough is that the memory representations for the two NPs were merged into a single representation due to their shared (gender or perceptual) features. Then, the retrieval operation would return this single, merged representation, leading to an apparent facilitation6.
Our results have important implications for theories of language processing in general and theories of referential processing in particular. Specifically, previous research has demonstrated involvement of general memory processes for successful retrieval of referential candidates during pronoun processing (e.g., Coopmans & Cohn, 2022; Coopmans & Nieuwland, 2020; Karimi et al., 2018; Martin, 2018; Nieuwland et al., 2007; Nieuwland & Martin, 2017; Nieuwland & Martin, 2017). Our results clearly show engagement of memory retrieval during pronoun processing as manifested by greater theta power for younger adults, consistent with previous research showing that theta power reflects morpho-semantic retrieval. Our results are also generally consistent with cue-based retrieval models of language processing (e.g., Lewis et al., 2006; Lewis & Vasishth, 2005). Under these models, a key factor determining the retrieval difficulty of previously-encoded information is interference, which may arise when a retrieval cue matches multiple memory items (as in the case of ambiguous pronouns). Consistent with cue-based models, our results showed a pronominal ambiguity effect for younger adults, such that relative to unambiguous pronouns, ambiguous pronouns led to greater theta power at the frontal scalp regions, suggesting greater retrieval difficulty for ambiguous than unambiguous pronouns. In fact, prior research on referential processing has established a pronominal ambiguity effect known as the Nref, which is elicited for ambiguous relative to unambiguous pronouns (Van Berkum et al., 1999, Nieuwland & Van Berkum, 2006), suggesting greater retrieval difficulty for ambiguous pronouns.
However, despite the efficiency of the cue-based models in explaining processing difficulty during referential processing, these theories, in their current form, do not offer a straightforward explanation for the observed effect of lexical form similarity. An important assumption of cue-based retrieval theories is that stored representations in memory are accessed through semantic and/or morpho-syntactic features (such as gender, number, animacy etc.), but not through phonological or orthographic cues (e.g., Jäger et al., 2017; Lewis et al., 2006; Lewis & Vasishth, 2005). These theories assume that attentional resources are very limited, and once incoming linguistic information is encoded, it is rapidly transmitted to the long-term memory store, access to which is made possible through semantic and/or morpho-syntactic codes. Thus, perceptual information (such as phonological and/or orthographic features) may affect how incoming words are encoded, with shared perceptual features leading to encoding interference, but not how they are retrieved (Kush et al., 2015). Nonetheless, our results clearly show an effect of lexical form similarity during retrieval, with more similar items leading to more retrieval difficulty. Thus, our results call for a modification of cue-based theories to include phonological and orthographic information as potential cues to retrieval.
Our results also have implications for models of working memory. Under some frameworks, working memory is phonologically mediated, meaning that the phonological codes of words are actively maintained during language processing (Acheson & MacDonald, 2011; Caramazza et al., 1981; Gibson, 1998; Shankweiler & Crain, 1986). These theories are in line with findings from the memory literature showing that word lists with phonological overlap are more difficult to recall compared with word lists without phonological overlap (e.g., Baddeley, 1966, 2018; Craik, 1968). Thus, under these accounts, lexical form similarity should produce interference during both encoding and retrieval of word representations during sentence processing. Because we observed clear effects of lexical form similarity during both encoding and retrieval of referential candidates, our results lend support to theories that assume a phonologically-mediated working memory store.
Our results also have important implications for theories of cognitive decline. First, our results show that older adults seem to rely more heavily on cognitive inhibition when encoding linguistic information, as manifested by greater alpha power during encoding the second NP regardless of lexical form similarity. Alpha power has been associated with allocation of attention away from external stimuli and onto internal memory representations (Jensen et al., 2002; Jensen & Mazaheri, 2010; Roux & Uhlhaas, 2014), as well as with functional inhibition of task-irrelevant brain areas or distracting neural activity (Foxe & Snyder, 2011; Jensen & Mazaheri, 2010; Mazaheri et al., 2014; Klimesch et al., 2007). Thus, the greater alpha power for NP2 on the part of older adults seems to indicate greater interference from NP1, which heightens the need for inhibition for older adults. These results are also in line with recent research showing that cognitive aging is associated with a shift from cortical disinhibition to inhibition, with younger adults’ maximizing encoding efficiency with disinhibition and older adults with cortical inhibition (Beese et al., 2019). Second, relative to younger adults, older adults exhibited a diminished ability to maintain multiple representations in memory during encoding, as manifested by smaller theta power during encoding NP2 when NP1 was phonologically/orthographically similar. Previous research has shown that theta power increases during working memory maintenance (e.g., Hsieh et al., 2011; Hsieh & Ranganath, 2014; Scheeringa et al., 2009). Thus, the theta power results raise the possibility that older adults were probably not able to maintain both NP representations in working memory when encoding phonologically/orthographically similar words. Third, older adults exhibited good-enough processing on the pronoun, by resolving the pronoun to the more available NP, without entertaining both referential interpretations. Thus, our results are consistent with previous research showing working memory decline in older adults (Caplan & Waters, 2005; Park et al., 2002), particularly in relation to the ability to encode and retrieve phonological/orthographic information (Burke et al., 1991; Burke & Shafto, 2011). Moreover, our results are consistent with previous studies showing age-related decline in processing phonological/orthographic information (Brown & McNeill, 1966; Burke et al., 1991; Cross & Burke, 2004; Diaz et al., 2014; 2019; 2021; Kemper et al., 1992; Rizio et al., 2017; Taylor & Burke, 2002; Zhang et al., 2019), as well as with the Transmission Deficit Hypothesis of cognitive decline maintaining that phonological processes are more vulnerable to loss than semantic processes.
One aspect of our predictions and results merits further discussion. Specifically, despite previous reports (e.g., Coopmans & Nieuwland, 2020; Coopmans & Cohn, 2022), we did not observe modulations of gamma activity during retrieval, which is assumed to reflect integration of referring expressions (such as pronouns) with preceding context. This discrepancy may have been caused by two design differences. First, unlike our study, Coopmans and Nieuwland (2020) manipulated “integration” using antecedent repetitions and discourse coherence violations. Interestingly, past research has demonstrated increased gamma activity for primed/repeated stimuli (Schneider et al., 2008). Moreover, it could be the case that gamma oscillations are elicited only if a referential expression explicitly violates a discourse representation. Note that our figures do not show the gamma range to maximize the clarity of our results at the ranges where we observed reliable differences. However, figures displaying the whole range of results are provided as supplementary materials. Importantly, our results also imply that a two-stage process of referential resolution (e.g., Almor & Nair, 2007; Garnham, 2001; Garrod & Sanford, 1994; Gernsbacher, 1989; McKoon & Ratcliff, 1980; Nieuwland & Martin, 2017; Sanford et al.,1983; Sturt, 2003) may apply only when there is an explicit need to integrate an incoherent referential interpretation. When the current discourse involves no incoherent referential interpretation, a single-stage resolution process including only the retrieval (reactivation) of the felicitous representation may suffice for referential processing.
5. Conclusion
In this study, we investigated age-related differences in encoding and retrieval of words (referential candidates) by measuring the power of brain oscillations during sentence processing. The results showed that although older adults may initially encode multiple representations in memory, they may not maintain those representations throughout the entire sentence. The direct consequence of this lack of maintenance was shallow processing of pronouns following the referential candidates. Our results have important implications for cue-based retrieval models of language processing by highlighting the roles that perceptual information and cognitive aging play during language processing.
Supplementary Material
Public Significance Statement:
In this project, we show that cognitive aging is associated with poor maintenance of information that is phonologically and orthographically confusable (for example, two similar-sounding/-looking names), which undermines subsequent retrieval of that information during language comprehension. Specifically, some of the confusable information (for example, one of the names) seems to drop out of the focus of attention during memory maintenance for older adults.
Acknowledgments
This research was supported by the National Institutes of Health’s National Institute on Aging awarded to HK (R15 AG073945-01A1), and to MTD (R01 AG034138). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. A draft of this manuscript and all materials, analyses, and R Scripts for this project can be found at: https://osf.io/75qyz/ (see under “Time-Frequency”).
Hossein Karimi, Department of Psychology, Mississippi State University; Megan Boudewyn, Department of Psychology, University of California, Santa Cruz; David van den Heever, Department of Agricultural and Biomedical Engineering, Mississippi State University, and Michele Diaz, Department of Psychology, Pennsylvania State University. We thank the staff and scientists at the Social, Life, & Engineering Sciences Imaging Center and the Center for Language Science, where the experiment was conducted. The ideas and data appearing in the manuscript have not been disseminated before (e.g., at a conference or meeting, posted on a listserv, shared on a website).
Footnotes
Forty younger (age range: 18–29, mean age = 19.66, 27 females, 13 males), and 38 older adults (age range: 60–78, mean age = 65.32, 24 females, 14 males) took part in this study. We used the same critical stimuli and fillers as in the EEG experiment, tagging the critical sentences with two-choice comprehension questions such as Who was almost drunk and high? [Hannah - Jacob]. For more details see Karimi & Diaz (2021).
Our logic here was that this decade’s names would be familiar to both groups. Most younger adults grew up in this decade, and the older adults experienced this decade and likely had grandchildren who grew in this decade.
As suggested by an anonymous reviewer, we also investigated hemisphere-specific effects (Rugg & Dickens, 1982; Klimesch, 1999; Klimesch et al., 1997a). We opted not to include these analyses in the main text so that the results more closely match the ERP analysis reported in Karimi and Diaz (2021). However, all analyses including Hemisphere as a topographical factor are available at: https://osf.io/75qyz/ (see under “Time-Frequency”).
Note that an overall greater alpha power for younger adults is in relation to the baseline period, and does not necessarily imply that younger adults had greater raw alpha power during encoding NP2.
Note that these results demonstrate that the effect of Pronominal Ambiguity is determined by a complex interplay between age, working memory, and brain activity at distinct frequency bands and time windows.
We thank an anonymous reviewer for offering this interesting alternative possibility.
References
- Arslan S, Palasis K, & Meunier F (2020). Electrophysiological differences in older and younger adults’ anaphoric but not cataphoric pronoun processing in the absence of age-related behavioural slowdown. Scientific Reports, 10(1), 19234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson DJ, & MacDonald MC (2011). The rhymes that the reader perused confused the meaning: Phonological effects during on-line sentence comprehension. Journal of Memory and Language, 65(2), 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almor A, & Nair VA (2007). The form of referential expressions in discourse. Language and Linguistics Compass, 1(1e2), 84e99. [Google Scholar]
- Baddeley AD (1966). The influence of acoustic and semantic similarity on long-term memory for word sequences. Quarterly Journal of Experimental Psychology, 18(4), 302–309. [DOI] [PubMed] [Google Scholar]
- Baddeley AD (2018). Short-term memory for word sequences as a function of acoustic, semantic and formal similarity. Quarterly Journal of Experimental Psychology. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, & Hagoort P (2003). Event-induced theta responses as a window on the dynamics of memory. Cortex, 39(4–5), 967–992. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, & Hagoort P (2015). Frequency-based segregation of syntactic and semantic unification during online sentence level language comprehension. Journal of cognitive neuroscience, 27(11), 2095–2107. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, Van Der Linden M, Ter Keurs M, Dijkstra T, & Hagoort P (2005). Theta responses are involved in lexical—Semantic retrieval during language processing. Journal of cognitive neuroscience, 17(3), 530–541. [DOI] [PubMed] [Google Scholar]
- Beese C, Vassileiou B, Friederici AD, & Meyer L (2019). Age differences in encoding-related alpha power reflect sentence comprehension difficulties. Frontiers in aging neuroscience, 11, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles NL, & Poon LW (1985). Aging and retrieval of words in semantic memory. Journal of Gerontology, 40(1), 71–77. [DOI] [PubMed] [Google Scholar]
- Brown R, & McNeill D (1966). The “tip of the tongue” phenomenon. Journal of Verbal Learning and Verbal Behavior, 5(4), 325–337. [Google Scholar]
- Burke DM, & Light LL (1981). Memory and aging: the role of retrieval processes. Psychological bulletin, 90(3), 513. [PubMed] [Google Scholar]
- Burke DM, MacKay DG, & James LE (2000). Theoretical approaches to language and aging. In Models of cognitive aging (pp. 204–237). Oxford University Press. [Google Scholar]
- Burke DM, MacKay DG, Worthley JS, & Wade E (1991). On the tip of the tongue: What causes word finding failures in young and older adults?. Journal of memory and language, 30(5), 542–579. [Google Scholar]
- Burke DM, & Shafto MA (2011). Language and aging. In The handbook of aging and cognition (pp. 381–451). Psychology Press. [Google Scholar]
- Busch NA, & Herrmann CS (2003). Object-load and feature-load modulate EEG in a short-term memory task. Neuroreport, 14(13), 1721–1724. [DOI] [PubMed] [Google Scholar]
- Cain E, & Ryskin R (2023). Diachronic Language Change and Its Influence on Lexico-semantic Representations Across the Lifespan. In Proceedings of the Annual Meeting of the Cognitive Science Society (Vol. 45, No. 45). [Google Scholar]
- Caplan D, & Waters G (2005). The relationship between age, processing speed, working memory capacity, and language comprehension. Memory, 13(3–4), 403–413. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Berndt RS, Basili AG, & Koller JJ (1981). Syntactic processing deficits in aphasia. Cortex, 17(3), 333–347. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Ferrand L, Grainger J, & Perea M (2005). Sequential effects of phonological priming in visual word recognition. Psychological Science, 16(8), 585–589. [DOI] [PubMed] [Google Scholar]
- Christianson K (2016). When language comprehension goes wrong for the right reasons: Good-enough, underspecified, or shallow language processing. Quarterly journal of experimental psychology, 69(5), 817–828. [DOI] [PubMed] [Google Scholar]
- Christianson K, Williams CC, Zacks RT, & Ferreira F (2006). Younger and older adults’“ good-enough” interpretations of garden-path sentences. Discourse processes, 42(2), 205–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX (2014). Analyzing neural time series data: theory and practice. MIT press. [Google Scholar]
- Cohen G, & Faulkner D (1986). Memory for proper names: Age differences in retrieval. British Journal of Developmental Psychology, 4(2), 187–197. [Google Scholar]
- Collins AM, & Loftus EF (1975). A spreading-activation theory of semantic processing. Psychological Review, 82(6), 407–428. [Google Scholar]
- Coopmans CW, & Cohn N (2022). An electrophysiological investigation of co-referential processes in visual narrative comprehension. Neuropsychologia, 172, 108253. [DOI] [PubMed] [Google Scholar]
- Coopmans CW, & Nieuwland MS (2020). Dissociating activation and integration of discourse referents: Evidence from ERPs and oscillations. cortex, 126, 83–106. [DOI] [PubMed] [Google Scholar]
- Craik FIM (1968). Two components in free recall. Journal of Verbal Learning and Verbal Behavior, 7(6), 996–1004. [Google Scholar]
- Crespo-Garcia M, Cantero JL, & Atienza M (2012). Effects of semantic relatedness on age-related associative memory deficits: the role of theta oscillations. Neuroimage, 61(4), 1235–1248. [DOI] [PubMed] [Google Scholar]
- Cross ES, & Burke DM (2004). Do alternative names block young and older adults’ retrieval of proper names? Brain and Language, 89(1), 174–181. [DOI] [PubMed] [Google Scholar]
- Cummins TD, & Finnigan S (2007). Theta power is reduced in healthy cognitive aging. International journal of psychophysiology, 66(1), 10–17. [DOI] [PubMed] [Google Scholar]
- Desroches AS, Newman RL, & Joanisse MF (2009). Investigating the time course of spoken word recognition: Electrophysiological evidence for the influences of phonological similarity. Journal of Cognitive Neuroscience, 21(10), 1893–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, & Madden DJ (2014). Age-related differences in the neural bases of phonological and semantic processes. Journal of Cognitive Neuroscience, 26(12), 2798–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, Truong T-K, & Madden DJ (2019). Age-related differences in the neural bases of phonological and semantic processes in the context of task-irrelevant information. Cognitive, Affective, & Behavioral Neuroscience, 19(4), 829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Karimi H, Troutman SB, Gertel VH, Cosgrove AL, & Zhang H (2021). Neural sensitivity to phonological characteristics is stable across the lifespan. NeuroImage, 225, 117511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Zhang H, Cosgrove AL, Gertel VH, Troutman SB, & Karimi H (2022). Neural sensitivity to semantic neighbors is stable across the adult lifespan. Neuropsychologia, 171, 108237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushanova J, & Christov M (2014). The effect of aging on EEG brain oscillations related to sensory and sensorimotor functions. Advances in medical sciences, 59(1), 61–67. [DOI] [PubMed] [Google Scholar]
- ElShafei HA, Fornoni L, Masson R, Bertrand O, & Bidet-Caulet A (2020). Age-related modulations of alpha and gamma brain activities underlying anticipation and distraction. PLoS One, 15(3), e0229334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feier CD, & Gerstman LJ (1980). Sentence comprehension abilities throughout the adult life span. Journal of Gerontology, 35(5), 722–728. [DOI] [PubMed] [Google Scholar]
- Ferreira F, Bailey KG, & Ferraro V (2002). Good-enough representations in language comprehension. Current directions in psychological science, 11(1), 11–15. [Google Scholar]
- Ferreira F, & Patson ND (2007). The ‘good enough’approach to language comprehension. Language and linguistics compass, 1(1–2), 71–83. [Google Scholar]
- Fotiadou G, Pérez-Muñoz AI, & Tsimpli IM (2020). Anaphora resolution and word-order across adulthood: Ageing effects on online listening comprehension. Glossa: a journal of general linguistics, 5(1), 71. [Google Scholar]
- Foxe JJ, & Snyder AC (2011). The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Frontiers in psychology, 2, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese U, Köster M, Hassler U, Martens U, Trujillo-Barreto N, & Gruber T (2013). Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded EEG. Neuroimage, 66, 642–647. [DOI] [PubMed] [Google Scholar]
- Fukumura K, Van Gompel RP, Harley T, & Pickering MJ (2011). How does similarity-based interference affect the choice of referring expression?. Journal of Memory and Language, 65(3), 331–344. [Google Scholar]
- Gajewski PD, & Falkenstein M (2014). Age-related effects on ERP and oscillatory EEG-dynamics in a 2-back task. Journal of Psychophysiology. [Google Scholar]
- Garnham A (2001). Mental models and the interpretation of anaphora. Hove, UK: Psychology Press. [Google Scholar]
- Garrod SC, & Sanford AJ (1994). Resolving sentences in a discourse context: How discourse representation affects language understanding. In Gernsbacher MA (Ed.), Handbook of psycholinguistics (pp. 675e698). San Diego, CA: Elsevier Academic Press. [Google Scholar]
- Gazzaley A,Clapp W, Kelley J, McEvoy K, Knight RT, & D’Esposito M (2008). Agerelated top-down suppression deficit in the early stages of cortical visual memory processing. Proceedings of the National Academy of Sciences, 105(35), 13122–13126. doi: 10.1073/pnas.0806074105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernsbacher MA (1989). Mechanisms that improve referential access. Cognition, 32(2), 99e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, & Yu D (1997). High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cerebral cortex (New York, NY: 1991), 7(4), 374–385. [DOI] [PubMed] [Google Scholar]
- Gibson E (1998). Linguistic complexity: Locality of syntactic dependencies. Cognition, 68(1), 1–76. [DOI] [PubMed] [Google Scholar]
- Gordon PC, Hendrick R, Johnson M, & Lee Y (2006). Similarity-based interference during language comprehension: Evidence from eye tracking during reading. Journal of experimental psychology: Learning, Memory, and Cognition, 32(6), 1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PC, Lowder MW, & Hoedemaker RS (2016). Reading in normally aging adults. Cognition, language and aging, 165–191. [Google Scholar]
- Gruber MJ, Hsieh LT, Staresina BP, Elger CE, Fell J, Axmacher N, & Ranganath C (2018). Theta phase synchronization between the human hippocampus and prefrontal cortex increases during encoding of unexpected information: a case study. Journal of Cognitive Neuroscience, 30(11), 1646–1656. [DOI] [PubMed] [Google Scholar]
- Haegens S, Osipova D, Oostenveld R, & Jensen O (2010). Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Human brain mapping, 31(1), 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarmann HJ, & Cameron KA (2005). Active maintenance of sentence meaning in working memory: Evidence from EEG coherences. International journal of psychophysiology, 57(2), 115–128. [DOI] [PubMed] [Google Scholar]
- Heine A, Tamm S, Hofmann M, Bösel RM, & Jacobs AM (2006). Event-related theta activity reflects memory processes in pronoun resolution. Neuroreport, 17(18), 1835–1839. [DOI] [PubMed] [Google Scholar]
- Henry MJ, Herrmann B, Kunke D, & Obleser J (2017). Aging affects the balance of neural entrainment and top-down neural modulation in the listening brain. Nature communications, 8(1), 15801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nature reviews neuroscience, 8(5), 393–402. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, HajiHosseini A, & Baker TE (2012). ERPs and EEG oscillations, best friends forever: Comment on Cohen et al. Trends in Cognitive Sciences, 16, 192. [DOI] [PubMed] [Google Scholar]
- Hsieh LT, & Ranganath C (2014). Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. Neuroimage, 85, 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LT, Ekstrom AD, & Ranganath C (2011). Neural oscillations associated with item and temporal order maintenance in working memory. Journal of Neuroscience, 31(30), 10803–10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizeling E, Wang H, Holland C, & Kessler K (2021). Changes in theta and alpha oscillatory signatures of attentional control in older and middle age. European Journal of Neuroscience, 54(1), 4314–4337. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Evett LJ, & Taylor DE (1982). Automatic phonological priming in visual word recognition. Memory & Cognition, 10(6), 576–590. [DOI] [PubMed] [Google Scholar]
- Indefrey P, & Levelt WJ (2004). The spatial and temporal signatures of word production components. Cognition, 92(1–2), 101–144. [DOI] [PubMed] [Google Scholar]
- Jäger LA, Engelmann F, & Vasishth S (2017). Similarity-based interference in sentence comprehension: Literature review and Bayesian meta-analysis. Journal of Memory and Language, 94, 316–339. [Google Scholar]
- Jensen O, Gelfand J, Kounios J, & Lisman JE (2002). Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cerebral cortex, 12(8), 877–882. [DOI] [PubMed] [Google Scholar]
- Jensen O, & Mazaheri A (2010). Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Frontiers in human neuroscience, 4, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, & Tesche CD (2002). Frontal theta activity in humans increases with memory load in a working memory task. European journal of Neuroscience, 15(8), 1395–1399. [DOI] [PubMed] [Google Scholar]
- Jost K, Bryck RL, Vogel EK, & Mayr U (2011). Are old adults just like low working memory young adults? Filtering efficiency and age differences in visual working memory. Cerebral cortex, 21(5), 1147–1154. doi: 10.1093/cercor/bhq185 [DOI] [PubMed] [Google Scholar]
- Kahn HJ, & Till RE (1991). Pronoun reference and aging. Developmental neuropsychology, 7(4), 459–475. [Google Scholar]
- Karimi H, & Diaz M (2021). Age-related differences in the retrieval of phonologically similar words during sentence processing: Evidence from ERPs. Brain and language, 220, 104982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi H, & Ferreira F (2016a). Informativity renders a referent more accessible: Evidence from eyetracking. Psychonomic bulletin & review, 23, 507–525. [DOI] [PubMed] [Google Scholar]
- Karimi H, & Ferreira F (2016b). Good-enough linguistic representations and online cognitive equilibrium in language processing. Quarterly journal of experimental psychology, 69(5), 1013–1040. [DOI] [PubMed] [Google Scholar]
- Karimi H, Fukumura K, Ferreira F, & Pickering MJ (2014). The effect of noun phrase length on the form of referring expressions. Memory & cognition, 42, 993–1009. [DOI] [PubMed] [Google Scholar]
- Karimi H, Swaab TY, & Ferreira F (2018). Electrophysiological evidence for an independent effect of memory retrieval on referential processing. Journal of Memory and Language, 102, 68–82. [Google Scholar]
- Keil A, Bernat EM, Cohen MX, Ding M, Fabiani M, Gratton G, … & Weisz N (2022). Recommendations and publication guidelines for studies using frequency domain and time-frequency domain analyses of neural time series. Psychophysiology, 59(5), e14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper S (1986). Imitation of complex syntactic constructions by elderly adults. Applied psycholinguistics, 7(3), 277–287. [Google Scholar]
- Kemper S, Kynette D, & Norman S (1992). Age differences in spoken language. In West RL, & Sinnott JD (Eds.), Everyday memory and aging: Current research and methodology (pp. 138–152). Springer. [Google Scholar]
- Kemper S, Schmalzried R, Herman R, Leedahl S, & Mohankumar D (2009). The effects of aging and dual task demands on language production. Aging, Neuropsychology, and Cognition, 16(3), 241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain research reviews, 29(2–3), 169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, & Pachinger T (1996). Theta band power in the human scalp EEG and the encoding of new information. NeuroReport, 7, 1235–1240. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger Th., & Ripper B (1997a). Brain oscillations and human memory performance: EEG correlates in the upper alpha and theta bands. Neuroscience Letters, 238, 9–12. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, & Ripper B (1997b). Theta synchronization in a memory task. Psychophysiology, 34, 169–176. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, & Hanslmayr S (2007). EEG alpha oscillations: the inhibition–timing hypothesis. Brain research reviews, 53(1), 63–88. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, & Schwaiger J (1994). Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalography and clinical Neurophysiology, 91(6), 428–441. [DOI] [PubMed] [Google Scholar]
- Kush D, Johns CL, & Van Dyke JA (2015). Identifying the role of phonology in sentence-level reading. Journal of Memory and Language, 79–80, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MA (2011) ez: Easy analysis and visualization of factorial experiments. R package version 3.0-0. http://CRAN.R-project.org/package=ez [Google Scholar]
- Lee CL, & Lai CH (2024). Age-related differences in understanding pronominal reference in sentence comprehension: An electrophysiological investigation. Psychology and Aging, 39(3), 275–287. 10.1037/pag0000775 [DOI] [PubMed] [Google Scholar]
- Leiberg S, Lutzenberger W, & Kaiser J (2006). Effects of memory load on cortical oscillatory activity during auditory pattern working memory. Brain research, 1120(1), 131–140. [DOI] [PubMed] [Google Scholar]
- Lewis RL, & Vasishth S (2005). An activation-based model of sentence processing as skilled memory retrieval. Cognitive Science, 29(3), 375–419. [DOI] [PubMed] [Google Scholar]
- Lewis RL, Vasishth S, & Van Dyke JA (2006). Computational principles of working memory in sentence comprehension. Trends in cognitive sciences, 10(10), 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, & Siew CSQ (2022). Diachronic semantic change in language is constrained by how peopleuse and learn language. Memory & Cognition, 50(6), 1284–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace EA, & Twohig PT (1990). Healthy older adults’ perceptions of their memory functioning and use of mnemonics. Bulletin of the Psychonomic Society, 28 (2), 115–118. [Google Scholar]
- Luce PA, & Pisoni DB (1998). Recognizing spoken words: The neighborhood activation model. Ear and Hearing, 19(1), 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukatela G, & Turvey MT (1994). Visual lexical access is initially phonological: 2. Evidence from phonological priming by homophones and pseudohomophones. Journal of Experimental Psychology: General, 123(4), 331–353. [DOI] [PubMed] [Google Scholar]
- Martin AE (2018). Cue integration during sentence comprehension: Electrophysiological evidence from ellipsis. PloS one, 13(11), e0206616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AE, Nieuwland MS, & Carreiras M (2012). Event-related brain potentials index cue-based retrieval interference during sentence comprehension. NeuroImage, 59(2), 1859–1869. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, van Schouwenburg MR, Dimitrijevic A, Denys D, Cools R, & Jensen O (2014). Region-specific modulations in oscillatory alpha activity serve to facilitate processing in the visual and auditory modalities. Neuroimage, 87, 356–362. [DOI] [PubMed] [Google Scholar]
- McKoon G, & Ratcliff R (1980). The comprehension processes and memory structures involved in anaphoric reference. Journal of Verbal Learning and Verbal Behavior, 19(6), 668e682. [Google Scholar]
- Melnik N, Mapelli I, & Özkurt TE (2017). Modulation of alpha oscillations is required for the suppression of semantic interference. Neurobiology of Learning and Memory, 144, 11–18. [DOI] [PubMed] [Google Scholar]
- Meyer L, Grigutsch M, Schmuck N, Gaston P, & Friederici AD (2015). Frontal–posterior theta oscillations reflect memory retrieval during sentence comprehension. Cortex, 71, 205–218. [DOI] [PubMed] [Google Scholar]
- Murty DV, Manikandan K, Kumar WS, Ramesh RG, Purokayastha S, Javali M, … & Ray S (2020). Gamma oscillations weaken with age in healthy elderly in human EEG. NeuroImage, 215, 116826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicenboim B, Logačev P, Gattei C, & Vasishth S (2016). When high-capacity readers slow down and low-capacity readers speed up: Working memory and locality effects. Frontiers in psychology, 7, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwland MS, & Martin AE (2017). Neural oscillations and a nascent corticohippocampal theory of reference. Journal of cognitive neuroscience, 29(5), 896–910. [DOI] [PubMed] [Google Scholar]
- Nieuwland MS, Petersson KM, & Van Berkum JJA (2007). On sense and reference: Examining the functional neuroanatomy of referential processing. NeuroImage, 37(3), 993–1004. [DOI] [PubMed] [Google Scholar]
- Nieuwland MS, & Van Berkum JJ (2006). Individual differences and contextual bias in pronoun resolution: Evidence from ERPs. Brain research, 1118(1), 155–167. [DOI] [PubMed] [Google Scholar]
- Obler LK, Fein D, Nicholas M, & Albert ML (1991). Auditory comprehension and aging: Decline in syntactic processing. Applied psycholinguistics, 12(4), 433–452. [Google Scholar]
- Obleser J, Wöstmann M, Hellbernd N, Wilsch A, & Maess B (2012). Adverse listening conditions and memory load drive a common alpha oscillatory network. Journal of Neuroscience, 32(36), 12376–12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onton J, Delorme A, & Makeig S (2005). Frontal midline EEG dynamics during working memory. Neuroimage, 27(2), 341–356. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, & Smith PK (2002). Models of visuospatial and verbal memory across the adult life span. Psychology & Aging, 17(2), 299–320. [PubMed] [Google Scholar]
- Parker D, & Phillips C (2017). Reflexive attraction in comprehension is selective. Journal of Memory and Language, 94, 272–290. [Google Scholar]
- Patil U, Vasishth S, & Lewis RL (2016). Retrieval interference in syntactic processing: The case of reflexive binding in English. Frontiers in Psychology, 7, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Grison S, Gao X, Christianson K, Morrow DG, & Stine-Morrow EA (2014). Aging and individual differences in binding during sentence understanding: Evidence from temporary and global syntactic attachment ambiguities. Cognition, 130(2), 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, & Stine-Morrow EA (2017). The effects of home-based cognitive training on verbal working memory and language comprehension in older adulthood. Frontiers in Aging Neuroscience, 9, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramscar M, Hendrix P, Shaoul C, Milin P, & Baayen H (2014). The myth of cognitive decline: Non-linear dynamics of lifelong learning. Topics in Cognitive Science, 6(1), 5–42. [DOI] [PubMed] [Google Scholar]
- Reifegerste J, & Felser C (2017). Effects of aging on interference during pronoun resolution. Journal of Speech, Language, and Hearing Research, 60(12), 3573–3589. [DOI] [PubMed] [Google Scholar]
- Rizio AA, Moyer KJ, & Diaz MT (2017). Neural evidence for phonologically based language production deficits in older adults: An fMRI investigation of age-related differences in picture-word interference. Brain and Behavior, 7(4), e00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, & Uhlhaas PJ (2014). Working memory and neural oscillations: alpha–gamma versus theta–gamma codes for distinct WM information?. Trends in cognitive sciences, 18(1), 16–25. [DOI] [PubMed] [Google Scholar]
- Rugg MD, & Dickens AMJ (1982). Dissociation of alpha and theta activity as a function of verbal and visuospatial tasks. Electroencephalography and Clinical Neurophysiology, 53(2), 201–207. [DOI] [PubMed] [Google Scholar]
- Sanford AJ, Garrod SC, Lucas A, & Henderson R (1983). Pronouns without explicit antecedents? Journal of Semantics, 2(3e4), 303e318. [Google Scholar]
- Scheeringa R, Petersson KM, Oostenveld R, Norris DG, Hagoort P, & Bastiaansen MC (2009). Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage, 44(3), 1224–1238. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, & Madsen JR (2003). Theta and gamma oscillations during encoding predict subsequent recall. Journal of Neuroscience, 23(34), 10809–10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaert K, Mazaheri A, & Hagoort P (2018). Binding language: structuring sentences through precisely timed oscillatory mechanisms. European Journal of Neuroscience, 48(7), 2651–2662. [DOI] [PubMed] [Google Scholar]
- Shafto MA, & Tyler LK (2014). Language in the aging brain: The network dynamics of cognitive decline and preservation. Science, 346(6209), 583–587. [DOI] [PubMed] [Google Scholar]
- Shankweiler D, & Crain S (1986). Language mechanisms and reading disorder: A modular approach. Cognition, 24(1), 139–168. [DOI] [PubMed] [Google Scholar]
- Stewart AJ, Holler J, & Kidd E (2007). Shallow processing of ambiguous pronouns: Evidence for delay. Quarterly Journal of Experimental Psychology, 60(12), 1680–1696. [DOI] [PubMed] [Google Scholar]
- Stine-Morrow EAL, Ryan S, & Leonard JS (2000). Age differences in on-line syntactic processing. Experimental Aging Research, 26, 315–322. [DOI] [PubMed] [Google Scholar]
- Sturt P (2003). The time-course of the application of binding constraints in reference resolution. Journal of Memory and Language, 48(3), 542e562. [Google Scholar]
- Swets B, Desmet T, Clifton C, & Ferreira F (2008). Underspecification of syntactic ambiguities: Evidence from self-paced reading. Memory & Cognition, 36, 201–216. [DOI] [PubMed] [Google Scholar]
- Taylor JK, & Burke DM (2002). Asymmetric aging effects on semantic and phonological processes: Naming in the picture-word interference task. Psychology and Aging, 17(4), 662–676. [DOI] [PubMed] [Google Scholar]
- Van Berkum JJ, Brown CM, & Hagoort P (1999). Early referential context effects in sentence processing: Evidence from event-related brain potentials. Journal of memory and language, 41(2), 147–182. [Google Scholar]
- Van Berkum JJA, Koornneef AW, Otten M, & Nieuwland MS (2007). Establishing reference in language comprehension: An electrophysiological perspective. Brain Research, 1146, 158–171. [DOI] [PubMed] [Google Scholar]
- Van Dyke JA, & McElree B (2006). Retrieval interference in sentence comprehension. Journal of memory and language, 55(2), 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, & Knight RT (2015). Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biological psychiatry, 77(12), 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Kramer MA, Case J, Lepage KQ, Tempesta ZR, Knight RT, & Gazzaley A (2015). Age-related changes in 1/f neural electrophysiological noise. Journal of neuroscience, 35(38), 13257–13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhauser GT, Johansson M, & Hanslmayr S (2012). Alpha/beta oscillations indicate inhibition of interfering visual memories. Journal of Neuroscience, 32(6), 1953–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters GS, & Caplan D (2001). Age, working memory, and on-line syntactic processing in sentence comprehension. Psychology and aging, 16(1), 128. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Potter KW, & Cowell RA (2018). Recognition memory shielded from semantic but not perceptual interference in normal aging. Neuropsychologia, 119, 448–463. [DOI] [PubMed] [Google Scholar]
- Yan Q, & Zhang Q (2022). Theta Band (4–8 Hz) Oscillations Reflect Online Processing of Rhythm in Speech Production. Brain Sciences, 12(12), 1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Eppes A, & Diaz MT (2019). Task difficulty modulates age-related differences in the behavioral and neural bases of language production. Neuropsychologia, 124, 254–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


