Abstract
Background:
Periprosthetic infection after breast reconstruction is not uncommon and can result in loss of the implant pocket and negative patient outcomes. Management of these infections typically involves removal of the prosthesis, treatment with antibiotics, and delayed reconstruction upon infection resolution. The impact of adjunctive use of negative pressure wound therapy with instillation and dwell (NPWTi-d) on breast pocket salvage rates, time to implant reinsertion, and related outcomes was examined.
Methods:
A systematic literature search using PubMed, Cochrane, OVID, Scopus, and Embase was conducted to identify peer-reviewed articles written in English and published between January 2004 and April 2023 that examined NPWTi-d use in the breast pocket with a history of periprosthetic infection after breast reconstruction.
Results:
Of the 1703 publications, 6 studies met inclusion criteria, representing 115 patients and 122 breasts. The overall breast pocket salvage rate with NPWTi-d across studies was approximately 92%. In the 6 studies that included prosthesis type and radiation history, overall salvage rates were 97.8% (45 of 46) for pockets containing implants and 93.8% (15 of 16) for pockets containing tissue expanders. Salvage rates were 85.7% (12 of 14) and 91.7% (53 of 58) for irradiated and nonirradiated breasts, respectively. Mean time to implant reinsertion ranged from 2.3 to 10.3 days.
Conclusions:
In this review, antibiotic therapy along with adjunctive use of NPWTi-d for periprosthetic infections after breast reconstructions was associated with high rates of breast pocket salvage and reduced time to implant reinsertion. Larger prospective and randomized trials are needed to better understand and optimize the effectiveness of NPWTi-d in this population.
Takeaways
Question: How effective is negative pressure wound therapy with instillation and dwell (NPWTi-d) in the management of periprosthetic infection after implant-based breast reconstruction?
Findings: This systematic review demonstrated that NPWTi-d for periprosthetic infections after breast reconstructions was associated with a 92% breast pocket salvage rate. Salvage rates were 85.7% and 91.7% for irradiated and nonirradiated breasts, respectively.
Meaning: Antibiotic therapy along with adjunctive use of NPWTi-d for periprosthetic infections after breast reconstructions was associated with high rates of breast pocket salvage and reduced time to implant reinsertion.
INTRODUCTION
Implant-based breast reconstruction remains the most common form of reconstruction after mastectomy, as approximately 75% of eligible patients undergo this procedure.1 Standard protocols exist to prevent periprosthetic infection after reconstruction; however, infection rates as high as 35.4% have been reported.2,3 The current standard of care (SOC) for the management of these infections includes removal of the prosthesis, treatment with antibiotics, and delayed reconstruction upon resolution of the infection4 based on the belief that a severely infected breast pocket cannot be adequately sterilized without removal of the implant for an extended period of time.
In addition to increased duration of treatment, delayed reconstruction can lead to loss or contracture of the implant pocket and aesthetic morbidity, requiring the patient to undergo additional alloplastic or autologous breast reconstruction.5,6 Additional procedures and time without a reconstructed breast are often detrimental to patient health and quality of life.5 Delayed reconstruction may pose additional risks to patients who have received radiation therapy because they are more prone to serious infections and wound-healing disorders.5,6
The use of negative pressure wound therapy with instillation and dwell time (NPWTi-d) may provide a means to thoroughly cleanse the existing breast pocket, thus facilitating early reinsertion of a new implant. NPWTi-d combines traditional NPWT with wound cleansing by delivering a topical wound solution at a controlled rate through a tube to a foam dressing. Once the fluid dwells in the wound for a selected amount of time, the topical wound solution is removed during the subsequent negative pressure cycle.7 NWPTi-d allows for the promotion of granulation tissue development; removal of debris, devitalized tissue, and infectious materials; and the maintenance of a moist wound environment.7 This system eliminates the need for frequent dressing changes, making it beneficial for wound care that requires daily cleansing.8
NPWTi-d has been shown to reduce bacterial burden, decrease the number of surgical debridements, increase the likelihood of wound closure, and shorten time to closure across various wound types, including chronic, traumatic, and infected wounds.9 A few small studies have examined the use of NPWTi-d for breast pocket salvage after infection and have reported high rates of successful salvage5,6,10–13; however, no large clinical trials have been conducted. Given the lack of robust data regarding the use of NPWTi-d for breast pocket salvage, we conducted a systematic review to consolidate existing evidence and better understand the impact of NPWTi-d (3M Veraflo Therapy; Solventum Corp, Maplewood, Minn.) in the management of periprosthetic infection after implant-based breast reconstruction on breast pocket salvage rates, time to implant reinsertion, and related outcomes.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed in this review.14 The protocol was registered in Prospero under registration ID: CRD42023446552.
Literature Search
A literature search for full-text articles written in English and published between January 1, 2004, and April 1, 2023, was performed using PubMed, Cochrane, OVID, Scopus, and Embase databases in August of 2023. The search strategy used the terms: (“Lavage” OR “instill” OR “instillation” OR “irrigated” OR “irrigation” OR “topical solution” OR “topical wound solution” OR “topic solution” OR “VERAFLO” OR “VERAFLOW” OR “Veraflo dressing” OR “Veraflo cleanse dressing” OR “Veraflo cleanse choice dressing” OR “Ulta”) AND (“Negative Pressure Wound Therapy” OR “NPWT” OR “vacuum assisted closure” OR “vacuum sealing” OR “NPWTi” OR “NPWTi-d”) AND (“breast” or “implant”).
Eligibility Criteria
Only peer-reviewed studies that mentioned the use of NPWTi-d (3M Veraflo Therapy; Solventum Corp) in the treatment of infected breast pockets and used primary data (clinical trials, randomized control trials, case series with at least 5 patients) were included. Studies that featured a pediatric population or investigated the use of instillation into the thoracic or abdominal cavity were excluded.
Literature Screening, Data Extraction, and Analysis
Two independent blind reviewers conducted title and abstract screening to remove duplicate studies, followed by a full review of studies that seemed to meet the inclusion criteria to confirm eligibility. A third reviewer resolved any inconsistencies between reviewers. The following data were extracted and entered into a data collection form: year of publication, study design, sample size, age, prosthesis type, percentage of breast pockets salvaged, hospital length of stay (LOS), time to infection, time to reinsertion of prosthesis, inflammatory markers, antibiotic regimen, instillation solution type, NPWTi-d device settings, and complications. Descriptive statistics were performed using Python 3.12.2.
RESULTS
Results of the Literature Search
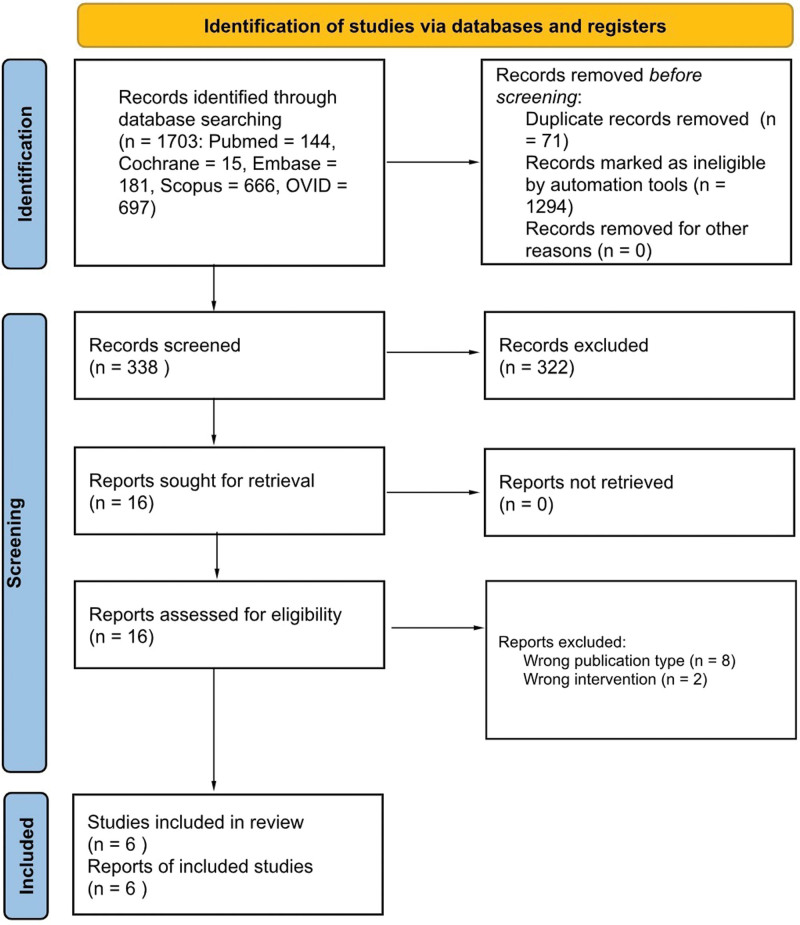
A total of 1703 publications were identified through database searching (Fig. 1). After removing duplicates (n = 71) and ineligible records (n = 1294) identified by automation tools, titles and abstracts of 338 records were screened for relevant content. Sixteen studies were identified that warranted full-text review with 6 meeting the criteria for inclusion.
Fig. 1.
A diagram that shows the search strategy.
Study Characteristics
The studies were conducted in Australia,6,11,13 Germany,5 the United Kingdom,12 and the United States10 and published between 2016 and 2021. (See table, Supplemental Digital Content 1, which displays the characteristics of the 6 included studies. http://links.lww.com/PRSGO/D578.) Three studies were retrospective cohort studies,5,10,12 with 2 reporting outcomes for a control group of patients who did not receive NPWTi-d.10,12 Two studies were case series.11,13 The other study was a prospective cohort study of patients who received NPWTi-d.6 NPWTi-d was applied to 86 of 115 patients and 92 of 122 breasts across the studies. Mean follow-up after reimplantation ranged from 6 to 39.4 months.
Patient and Breast Characteristics
The mean age of patients who received NPWTi-d ranged from 44 to 49 years. Across the included studies, 68.5% (63 of 92) of the infected prostheses for patients who received NPWTi-d were implants, and 31.5% (29 of 92) were tissue expanders. Approximately 19% (14 of 72) of breasts were irradiated in the 5 studies that reported radiation status. Only Meybodi et al6 explicitly described the reconstruction technique used, reporting 5 patients (16.7%) with prepectoral reconstruction and 25 patients (83.3%) with retropectoral reconstruction.
Time to Infection, Bacterial Burden, and Inflammatory Markers
The interval between reconstruction surgery and subsequent infection ranged from 37.5 to 243.4 days with a median of 54.8 days. Various bacterial species were detected from intraoperative cultures, with methicillin-sensitive Staphylococcus aureus, methicillin-resistant S. aureus, Serratia marcescens, and Pseudomonas aeruginosa being the most common. Three studies reported C-reactive protein (CRP) levels and 2 reported leukocytes/white blood cell count at presentation of infection.
Antibiotic Therapy
Intravenous (IV) antibiotics were given in all 6 studies. Antognoli et al10 initiated empirical broad-spectrum IV antibiotics upon admission for infection with narrowing of therapy based on wound cultures. Patients were discharged on oral or home infusion of IV antibiotics for 14 days or less.10 Greuner et al5 initiated antibiotics perioperatively during prosthesis explantation with continued use until 5 days after wound closure or reimplantation. Antibiotic therapy was adjusted based on wound cultures.5 In the case study, Meybodi et al13 reported that IV antibiotics were guided by microbiological sensitivities and were given from explantation of the infected prosthesis until reinsertion. In the subsequent cohort study, IV antibiotics were individualized by the infectious disease unit and initialized upon admission for infection. Patients were transitioned to 3 weeks or more of oral antibiotics at discharge.6
Surgical Technique
In all included studies, the prosthesis was removed, and the breast pocket was washed out before the placement of NPWTi-d. Antognoli et al abraded the capsule with a Bovie scratch pad to remove biofilm and irrigated the breast pocket with hydrogen peroxide, Betadine, Clorpactin, and/or bacitracin.10 Any nonintegrated biologic mesh and/or necrotic tissue was removed, followed by immediate placement of NPWTi-d.10 After washout, Cheong et al11 packed the cavity with rolls of sterile foam, achieved an airtight seal, and connected it via tubing to NPWTi-d for cavity irrigation and maintenance. Meybodi et al6 performed debridement/curettage of the pocket until healthy tissue was reached and washed it with betadine and normal saline before NPWTi-d placement.
NPWTi-d Settings
Across studies, NPWTi-d was placed immediately after explantation of the infected prosthesis. Two studies instilled normal saline.11,12 Meybodi et al primarily used saline but allowed the solution type to be changed to acetic acid or a betaine/polyhexanide solution based on the bacteria type and/or severity of the infection.6,13 Antognoli et al and Gruener et al used a betaine/polyhexanide solution and polyhexanide solution, respectively, for installation.5,10 NPWTi-d device settings included instillation of 80–400 mL of topical wound solutions (dependent on breast envelope size) (Supplemental Digital Content 1, http://links.lww.com/PRSGO/D578). Dwell times ranged from 10 to 20 minutes, followed by continuous negative pressure for 1–4 hours at −75 to −125 mm Hg. Dressing change frequency ranged from 1 to 5 days.
Criteria for Reinsertion of Prosthesis
Criteria for prosthesis reinsertion varied. Antognoli et al10 determined reinsertion was safe when a patient showed signs of clinical improvement and had completed multiple cycles of NPWTi-d. Thus, reinsertion timing was based mostly on surgeon preference and operating room (OR) schedule.10 Cheong et al11 reinserted breast implants when the wound cavity was clean with healthy granulation tissue. Gruener et al5 considered reimplantation after resolution of clinical signs of infection including redness, swelling, pain, decreased serum CRP and leukocytes, and absence of pus in the exudate. Haque et al12 also required wounds to be clean and healthy with improved inflammatory markers and negative tissue cultures before reinsertion. Similarly, Meybodi et al required healthy granulation tissue and at least one negative culture before reinsertion.6,13
Primary Outcome: Breast Pocket Salvage Rate
The overall breast pocket salvage rate across the 6 studies was approximately 92% (Table 1). All breast pockets were salvaged in 3 of the studies.5,11,12 Five studies documented salvage rate by the type of prosthesis at the time of infection, and a combined 97.8% (45 of 46) of pockets containing implants and 93.8% (15 of 16) of pockets containing tissue expanders were salvaged. Across the 5 studies that documented breast pocket salvage rates based on radiation history, the salvage rate was 85.7% (12 of 14) for irradiated breasts versus 91.7% (53 of 58) for nonirradiated breasts. The causes of breast pocket salvage failure included abandonment of protocol to avoid delays in adjuvant chemotherapy, unhealed skin defects, chronic seroma of the breast pocket, and erythema with an exposed implant and murky drainage (Table 2).
Table 1.
Breast Pocket Salvage Rates for Patients Receiving NPWTi-d
| Antognoli et al10 | Cheong et al11 | Gruener et al5 | Haque et al12 | Meybodi et al13 | Meybodi et al6 | Total | |
|---|---|---|---|---|---|---|---|
| Overall breast pockets salvaged, n/d (%) | 16/17 (94) | 6/6 (100) | 13/13 (100) | 20/20 (100) | 5/6 (83) | 25/30 (83) | 85/92 (92.4) |
| Implants | 6/7 (86) | 4/4 (100) | 13/13 (100) | 20/20 (100) | 2/2 (100) | NR | 45/46 (97.8) |
| Tissue expanders | 10/10 (100) | 2/2 (100) | 0/0 (0) | 0/0 (0) | 3/4 (75) | NR | 15/16 (93.8) |
| Irradiated | 4/4 (100) | 3/3 (100) | 4/4 (100) | NR | 0/1 (0) | 1/2 (50) | 12/14 (85.7) |
| Nonirradiated | 12/13 (92) | 3/3 (100) | 9/9 (100) | NR | 5/5 (100) | 24/28 (86) | 53/58 (91.7) |
Table 2.
Causes of Breast Pocket Salvage Failure
| Causes of Breast Pocket Salvage Failure, n (%) | Cases (N = 7) |
|---|---|
| Protocol abandoned due to concerns about delays in adjuvant chemotherapy | 2 (28.6) |
| Unhealed skin defect (flap ischemia or diathermy burn) remote to incision site | 2 (28.6) |
| Chronic seroma of prosthesis pocket | 1 (14.3) |
| Erythema and exposed implant with murky periprosthetic drainage | 1 (14.3) |
| Unknown—patient lost to follow-up | 1 (14.3) |
Secondary Outcomes
Bacterial Burden and Inflammatory Markers after NPWTi-d
Only Gruener et al reported on bacterial burden, obtained from swabs of the implant pocket, and inflammatory markers after NPWTi-d therapy. (See table, Supplemental Digital Content 2, which displays the other reported outcome measures. http://links.lww.com/PRSGO/D579.) A statistically significant reduction in mean bacterial burden (−1.16 points, P = 0.0002), CRP levels (−39.8 mL/L, P = 0.0002), and leukocyte count (−2.33 µL, P = 0.0002) was observed.
Time to Negative Culture and Reinsertion of Prosthesis
Mean time to negative culture was 5.2 days in the 1 study that reported this measure.6 Mean time from prosthesis removal to reinsertion varied among studies. Antognoli et al inserted a new prothesis within 1 to 4.9 days of explantation with a mean time of 2.3 days.10 Cheong et al11 reinserted implants within 7 days, and Gruener et al5 were able to achieve wound closure within 8.5 days on average. Haque et al12 found that mean time to reinsertion was 10.3 days for patients who received NPWTi-d versus 247.5 days for those receiving SOC (P < 0.001).
Return Trips to the OR
In 1 study, patients returned to the OR once for definitive NPWTi-d removal with placement of the new prosthesis.10 In the other studies, patients returned to the OR multiple times for breast pocket examination, debridement, washout, and dressing changes. The average number of return trips ranged from 2.3 to 3.4 in the studies conducted by Haque et al and Meybodi et al.6,12,13
Hospital LOS
Mean hospital LOS was shortest (4.4 d) in the study conducted by Antognoli et al.10 Mean LOS was between 11.5 and 12 days in 3 of the studies.5,6,13 Haque et al12 reported that patients who received NPWTi-d had a significantly shorter LOS than the control group (7.1 versus 11.9, P < 0.004). Patients in the Cheong et al study spent 7 days or more in the hospital, though the average LOS was not reported.11
Follow-up Office Visits and Hospitalizations
Patients who received NPWTi-d had fewer office visits and hospitalizations than the control group in the studies conducted by Antognoli et al (11 versus 24 office visits, P = 0.002; 2 versus 4 hospitalizations, P = 0.002) and Haque et al (12 versus 14.2 office visits, P = 0.491; 1 versus 2.1 hospitalizations, P < 0.001).10,12
Patient Satisfaction
The lone study that reported on patient satisfaction found that mean BREAST-Q scores were higher for breast satisfaction (55.0 versus 39.7, P = 0.032) and implant satisfaction (6.2 versus 4.9, P = 0.061) for patients who received NPWTi-d compared with SOC.12
Complications
Complications were reported in 4 studies. In 1 study, capsular contractures affected 2 (12.5%) patients who received NPWTi-d.10 Haque et al12 noted that all patients who received NPWTi-d had reinsertion of a new implant without requiring additional skin or soft tissue cover, unlike three patients in the control group. Meybodi et al6,13 reported that there were no issues upon follow-up for the case studies and that there was no record of capsular contraction or recurrent infection for patients included in the larger cohort study.
Costs
In 1 of the 2 studies that examined cost of care, Antognoli et al estimated NPWTi-d could result in a cost savings of $6475 per patient through reduced office visits and hospitalizations.10 Haque et al found that mean costs for patients who received NPWTi-d were significantly higher (£14,343 versus £8920, P < 0.001) than for patients who received SOC due to higher numbers of procedures and longer LOS.12
DISCUSSION
This systematic review examined use of NPWTi-d in the adjunctive management of periprosthetic breast infections after implant-based breast reconstruction and the impact on breast pocket salvage rates, time to implant reinsertion, and related outcomes. The results of this review suggest that NPWTi-d along with infection treatment protocols is an effective option for preserving the breast pocket after an infected prosthesis. More than 92% of breast pockets managed with NPWTi-d and systemic antibiotics were salvaged across the 6 studies with individual study salvage rates ranging from 83% to 100%. Most patients received a new implant within 2–10 days of removal of the infected prosthesis.
The observed overall breast pocket salvage rate with NPWTi-d is relatively high given reported salvage rates as low as 33%–64% in some studies where treatment for periprosthetic infections varied but included antibiotics, irrigation, immediate implant exchange, capsulectomy, pulse lavage, curettage, debridement, primary closure, and/or flap coverage.15–19 However, some studies utilizing similar interventions have reported much higher salvage rates.4,20–24 The variation in salvage rates could be attributed to variations in the severity of infections of included patients. Spear and Seruya18 observed a salvage rate of 100% for breasts with mild infections compared with 30.8% for breasts with severe infections.
Although none of the included studies classified infections and salvage rates based on severity, Meybodi et al6 reported that all study patients met the definition of severe periprosthetic infection that previously contraindicated salvage attempt due to high risk of failure. Despite this risk, the salvage rate with NPWTi-d was 83%.6 Similarly, Antognoli et al did not exclude patients based on severity of infection and successfully salvaged the breast pockets of several patients with exposed implants, purulent drainage, and severe sepsis using NPWTi-d along with systemic antibiotics.10 Salvage rates also may be impacted by the type of prosthesis in situ at the time of infection25 and the use of radiation.20 However, the high salvage rates for both implants (97.8%) and tissue expanders (93.8%) as well as for irradiated (85.7%) and nonirradiated breasts (91.7%) for patients who received NPWTi-d indicate that this therapy may be effective for patients with various risk factors for salvage failure.
The success of adjunctive NPWTi-d for the management of periprosthetic infections may be attributed to the instillation component’s wound cleansing and ability to remove infectious materials and reduce bioburden, as has been demonstrated in vitro26 and by one of the included studies that found statistically significant reductions in bacterial levels, CRP, and leukocyte count after NPWTi-d.5 In addition, NPWTi-d may reduce edema, and for periprosthetic breast infections, it is important to achieve a wound bed that is both free of inflammation and has had a maximum decontamination of the bacterial burden before reinsertion to reduce risk of further infection. The negative pressure aspect of NPWTi-d has been associated with improved granulation tissue formation in several studies, which may also contribute to successful breast pocket salvage. Reduction of bioburden and bacterial levels and improved granulation tissue formation allow for an ideal environment for healing and earlier reinsertion of the prosthesis. Earlier reinsertion prevents shrinkage of the breast pocket and preserves the skin envelope, avoiding the need for re-expansion and return trips to the OR that increase risk of subsequent infection. These factors may contribute to higher salvage rates observed with NPWTi-d across the included studies.
Additional benefits of NPWTi-d may include improved patient satisfaction and reductions in healthcare utilization and costs. However, the reconstruction type and salvage protocol can influence these outcomes. Patients in the study conducted by Antognoli et al underwent reconstructions that were direct to implant without interval tissue expanders, and the salvage protocol involved the single application of NPWTi-d for 1–4 days with implant replacement upon signs of clinical improvement. Patients who received NPWTi-d had significantly shorter LOS and fewer hospitalizations and office visits than patients who received SOC resulting in a significant reduction of $6475 per patient in modeled treatment costs.10
In comparison, the salvage protocols for other studies required patients to return to the OR every few days for wound review, debridement, washout, and dressing changes with implant reinsertion occurring only when wounds were deemed healthy and cultures were negative. In the study conducted by Hague et al, patients who received NPWTi-d had more surgeries than patients receiving SOC because they returned to the OR every 2–3 days per protocol. Despite only having 1 hospitalization for both explantation and reinsertion compared with multiple hospitalizations for patients who received SOC, patients with NPWTi-d had a significantly longer average LOS by 4.8 days resulting in significantly higher costs of approximately £5423.12 However, Haque et al noted that if a patient requires soft-tissue coverage the costs of pedicled flap, free-flap reconstruction, or preimplant lipofilling alone would make use of NPWTi-d less costly than SOC or cost-neutral.12 Also, patients with NPWTi-d received new implants within days of implant removal instead of waiting months and had higher levels of postreconstruction breast satisfaction compared with patients who received SOC.
Limitations
This review is subject to several limitations. The included studies were mostly small retrospective studies and case series. The majority lacked control groups and featured heterogeneous populations with differences in reconstruction procedures and salvage protocols including NPWTi-d settings and antibiotic use. Furthermore, there was variability in the outcomes reported and duration of follow-up. The review only included studies that used NPWTi-d from a single manufacturer, as the installation and dwell feature is unique to this device. Modest complications were reported with NPWTi-d, limited to 2 cases of capsular contracture in a singular study.10 Although current data are limited, NPWTi-d is generally well tolerated and a safe option for the adjunctive management of breast pocket periprosthetic infections. The scarcity of data on patient-reported and aesthetic outcomes as well as race and ethnicity are other limitations. Additionally, there is likely publication bias, as studies with favorable or significant results tend to be more frequently published, which may influence overall findings. Larger controlled studies are warranted to further elucidate and enhance the therapeutic efficacy of NPWTi-d in this population.
CONCLUSIONS
Based on the findings of this literature review, NPWTi-d shows promise as an effective adjunctive intervention in conjunction with antibiotic therapy for breast pocket salvage after periprosthetic infection, even in cases previously deemed unsuitable for salvage. Given the severity of these infections that can lead to patient morbidity, multiple reoperations, and unsatisfactory outcomes,5 it is crucial to optimize therapeutic approaches. Traditional approaches often involve significant delays in the reinsertion of new implants, contributing to patient distress, delays in adjuvant treatment, and risk of loss of the breast pocket.10,12 Alternatively, the integration of NPWTi-d with antibiotic therapy seems to support efficient infection management, facilitate breast pocket wound bed preparation, and enable implant replacement within a single admission; hence, potentially preserving the breast pocket and improving aesthetic results.
DISCLOSURE
Dr. Galiano is a consultant and receives funding from 3M/KCI. Drs. Collinsworth and Griffin are employees of Solventum. The other authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.American Society of Plastic Surgeons. Plastic surgery statistics report 2020. Available at https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf. [Google Scholar]
- 2.Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–2274. [DOI] [PubMed] [Google Scholar]
- 3.Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg. 2011;127:514–524. [DOI] [PubMed] [Google Scholar]
- 4.Spear SL, Howard MA, Boehmler JH, et al. The infected or exposed breast implant: management and treatment strategies. Plast Reconstr Surg. 2004;113:1634–1644. [DOI] [PubMed] [Google Scholar]
- 5.Gruener JS, Horch RE, Geierlehner A, et al. Is instillational topical negative pressure wound therapy in peri-prosthetic infections of the breast effective? A pilot study. J Pers Med. 2022;12:2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meybodi F, Sedaghat N, Elder E, et al. Salvaging the unsalvageable: negative pressure wound therapy for severe infection of prosthetic breast reconstruction. Plast Reconstr Surg Glob Open. 2021;9:e3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leow K, Tey J, Tan A, et al. Negative pressure wound therapy with instillation and dwell time modifications for lower limb wounds with the waterfall technique: a case series. Wounds. 2020;32:E120–E125. [PubMed] [Google Scholar]
- 8.Delapena S, Fernández LG, Foster KN, et al. Negative pressure wound therapy with instillation and dwell time for the management of complex wounds: a case series. Wounds. 2020;32:E96–E100. [PubMed] [Google Scholar]
- 9.Gabriel A, Camardo M, O’Rorke E, et al. Effects of negative-pressure wound therapy with instillation versus standard of care in multiple wound types: systematic literature review and meta-analysis. Plast Reconstr Surg. 2021;147:68S–76S. [DOI] [PubMed] [Google Scholar]
- 10.Antognoli LE, Singh DP, Choudhry S, et al. Rinse but don’t repeat: single application V.A.C. VERAFLO salvages infected breast prostheses. Plast Reconstr Surg Glob Open. 2021;9:e3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheong JY, Goltsman D, Warrier S. A new method of salvaging breast reconstruction after breast implant using negative pressure wound therapy and instillation. Aesthetic Plast Surg. 2016;40:745–748. [DOI] [PubMed] [Google Scholar]
- 12.Haque S, Kanapathy M, Bollen E, et al. Patient-reported outcome and cost implication of acute salvage of infected implant-based breast reconstruction with negative pressure wound therapy with instillation (NPWTi) compared to standard care. J Plast Reconstr Aesthet Surg. 2021;74:3300–3306. [DOI] [PubMed] [Google Scholar]
- 13.Meybodi F, Sedaghat N, French J, et al. Implant salvage in breast reconstruction with severe peri-prosthetic infection. ANZ J Surg. 2017;87:E293–E299. [DOI] [PubMed] [Google Scholar]
- 14.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozturk C, Ozturk CN, Platek M, et al. Management of expander- and implant-associated infections in breast reconstruction. Aesthetic Plast Surg. 2020;44:2075–2082. [DOI] [PubMed] [Google Scholar]
- 16.Reish RG, Damjanovic B, Austen WG, Jr, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. 2013;131:1223–1230. [DOI] [PubMed] [Google Scholar]
- 17.Song JH, Kim YS, Jung BK, et al. Salvage of infected breast implants. Arch Plast Surg. 2017;44:516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spear SL, Seruya M. Management of the infected or exposed breast prosthesis: a single surgeon’s 15-year experience with 69 patients. Plast Reconstr Surg. 2010;125:1074–1084. [DOI] [PubMed] [Google Scholar]
- 19.Yii NW, Khoo CT. Salvage of infected expander prostheses in breast reconstruction. Plast Reconstr Surg. 2003;111:1087–1092. [DOI] [PubMed] [Google Scholar]
- 20.Prince MD, Suber JS, Aya-Ay ML, et al. Prosthesis salvage in breast reconstruction patients with periprosthetic infection and exposure. Plast Reconstr Surg. 2012;129:42–48. [DOI] [PubMed] [Google Scholar]
- 21.Sarfati I, Millochau J, Meredith I, et al. Salvaging the infected breast implant: results of a retrospective series of 80 consecutive cases. J Plast Reconstr Aesthet Surg. 2020;73:2232–2238. [DOI] [PubMed] [Google Scholar]
- 22.Sforza M, Andjelkov K, Husein R, et al. Will 1-stage implant salvage after periprosthetic breast infection ever be routine? A 6-year successful experience. Aesthet Surg J. 2014;34:1172–1178. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y, Hu X, Du Z, et al. Minimally invasive and innovative management of prosthesis infections in endoscopic-assisted breast reconstruction. Aesthetic Plast Surg. 2023;48:266–272. [DOI] [PubMed] [Google Scholar]
- 24.Yeo H, Lee D, Kim JS, et al. Strategy for salvaging infected breast implants: lessons from the recovery of seven consecutive patients. Arch Plast Surg. 2021;48:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franchelli S, Pesce M, Baldelli I, et al. Analysis of clinical management of infected breast implants and of factors associated to successful breast pocket salvage in infections occurring after breast reconstruction. Int J Infect Dis. 2018;71:67–72. [DOI] [PubMed] [Google Scholar]
- 26.Tahir S, Malone M, Hu H, et al. The effect of negative pressure wound therapy with and without instillation on mature biofilms in vitro. Materials (Basel). 2018;11:811. [DOI] [PMC free article] [PubMed] [Google Scholar]