Abstract
Recent studies have speculated on the possible role of the mother in transmitting Helicobacter pylori infection to their children. In an attempt to either prove or disprove this supposition, we investigated the rates of infection of children born to H. pylori-positive mothers from birth to 5 years of age using serology and the stool antigen test. When infection of the children did occur, the strains from the children were compared to those of their mothers using DNA analysis. Sixty-nine of the 350 pregnant mothers (19.7%) had a positive serology for H. pylori. Fifty-one children underwent serological examinations and stool antigen tests at 4 to 6 days after birth, followed by 1, 3, and 6 months. They were continuously given the stool antigen test at 4- to 6-month intervals until the age of 5 years. Gastric juice samples were collected from the infected children and their mothers for culture and DNA analyses using a random amplified polymorphic DNA fingerprinting method. None of the 51 children acquired H. pylori infection during the first year of life. Of the 44 children enrolled in a 5-year follow-up study, five (11%) acquired H. pylori infection. They acquired the infection at the age of 1 year 2 months, 1 year 3 months, 1 year 6 months, 1 year 8 months, and 4 years 4 months. Random amplified polymorphic DNA fingerprinting confirmed that the strains of the five children exhibited DNA fingerprinting patterns identical to those of their mothers. These findings suggest that mother-to-child transmission is the most probable cause of intrafamilial spread of H. pylori.
Helicobacter pylori has been implicated as an important etiological factor in a variety of gastroduodenal disorders, including chronic gastritis, peptic ulcer disease, and as a risk factor for developing gastric malignancies (14, 22). Previous studies suggest that early childhood is a period of high risk for acquiring the H. pylori infection (8). However, the mode of acquisition has remained unclear. Intrafamilial clustering has been described in various studies (7, 9. 20). In intrafamilial H. pylori infections, the infected parents, particularly the infected mothers, have been considered the likely source of transmission (18). Those previous epidemiological studies, however, were not based on molecular biological analysis of H. pylori strains, but based mainly on serologic tests or the urea breath test; therefore, the evidence for this possible path of infection has been indirect. In only a few studies the same or similar strains of H. pylori have been identified among families by using the DNA typing technique (4, 10, 16, 23).
The first aim of this study was to investigate the prevalence of H. pylori infection in children born to H. pylori-positive mothers from birth to 5 years of age using serology and the stool antigen test. The second aim was to compare the strains between the infected children and their mothers for possible DNA matches. Because children under five years have the closest contact with their mothers, we assumed that they were at an increased risk of acquiring H. pylori through this pathway. To clarify the assumption of mother-to-child infection, the entire genome of H. pylori isolates cultured from the gastric juice of infected children and their mothers was studied by PCR-based random amplified polymorphic DNA (RAPD) fingerprinting method.
MATERIALS AND METHODS
Subjects.
During a period of 25 months, from August 1997 to August 1999, we studied 350 pregnant women who came to our outpatient clinic for routine prenatal follow-ups. Together with standard prenatal screening analysis, a 1-ml venous blood aliquot was taken for a serologic test to detect H. pylori antibodies. All infants born to seropositive mothers were invited to undergo serological examinations and the H. pylori stool antigen test (HpSA) at 4 to 6 days after birth, 1, 3, and 6 months, as well as an HpSA test at 4- to 6-month intervals until the age of 5 years. Fifty-one mothers of children born during the period from March 1998 to March 2000 allowed their children to be part of the study. Forty-four out of 51 children were included in a 5-year follow-up study ending in March 2004. The children, whose HpSA test turned out to be positive underwent the serum H. pylori immunoglobulin G test and a gastric juice culture to confirm the acquisition of H. pylori infection. Then the H. pylori isolates from the gastric juice of the index children and their corresponding mothers were characterized by the RAPD fingerprinting method after obtaining written informed consent from the mothers.
Serology.
Serum titers of IgG and IgA antibodies for H. pylori were measured using commercial enzyme immunoassay (EIA) kits (HM-CAP and PP-CAP, respectively; Enteric Products, New York, NY). For both assays, EIA values of more than 2.2, 1.8 to 2.2, and less than 1.8 were defined as positive, indeterminate, and negative, respectively.
Stool antigen test.
Stool samples were stored at −20°C until analyzed. An enzyme immunoassay kit (Premier Platinum HpSA, Meridian Diagnostics Inc, Cincinnati, Ohio) with a polyclonal anti-H. pylori rabbit antibody adsorbed to microwells was used to detect H. pylori antigen in the stool according to the manufacturer's instructions. Values of more than 0.120, 0.100 to 0.119, and less than 0.100 were defined as positive, indeterminate, and negative, respectively.
Culture of gastric juice.
Gastric juice samples were aspirated through a sterile nasogastric tube and H. pylori was isolated from the gastric juice by culturing at 37°C in a microaerophilic atmosphere (10% O2 and 15% CO2) on H. pylori-selective agar plates (Nissui Plate Helicobacter Agar, Nissui Phamaceuticals, Tokyo, Japan) containing trimethoprim, polymyxin B, vancomycin and amphotericin B to inhibit the growth of microbes other than H. pylori for up to 7 days. Identification of H. pylori was done by Gram staining, and the production of oxidase, catalase, and urease. Single colonies were subcultured on Nissui Plate helicobacter agar under the same microaerophilic atmosphere mentioned above at 37°C for 48 h. In the end, H. pylori isolates were obtained from 5 children and their corresponding mothers. H. pylori colonies were suspended in a brain heart infusion broth (Nissui Phamaceuticals) and then stored at −80°C.
RAPD fingerprinting method.
The extraction of H. pylori genomic DNA and the PCR-based RAPD DNA pattern were performed in accordance with the processes described previously (1, 11). Genomic DNA was isolated via SepaGene (Sanko Junyaku, Tokyo, Japan). PCR was carried out in a final volume of 50 μl containing 20 ng template DNA, 20 pmol primer, 1.5 mM MgCl2, 1.25 U of Taq DNA polymerase (Promega, WI, USA), 0.1 mM deoxynucleoside triphosphates (Promega) in 19 mM Tris-HCl (pH 8.3) containing 50 mM KCl and Triton X-100 (1%, vol/vol). For the analysis of the RAPD fingerprinting, random primers from DNA Oligomer (12) set A-1 (NIPPON GENE, Toyama, Japan) were screened for amplification of H. pylori DNA. Four primers, A04 (5′-ATCAGCGCACCA-3′), A05 (5′-AGCAGCGCCTCA-3′), A07 (5′-TGCCTCGCACCA-3′), and A08 (5′-GCCCCGTTAGCA-3′) were suitable for this study. A Gene Amp PCR System 9600-R cycler was used for amplification. The cycling program was 35 cycles of 94°C for 2 min, 38°C for 2 min, 72°C for 2 min, followed by a final incubation at 72°C for 10 min. Ten-microliter aliquots of product were electrophoresed in 2% agarose gels containing 0.5 μg/ml of ethidium bromide in the gel while using a 1× Tris-acetate-EDTA running buffer. The pHY marker was used as a size marker.
RESULTS
Serology.
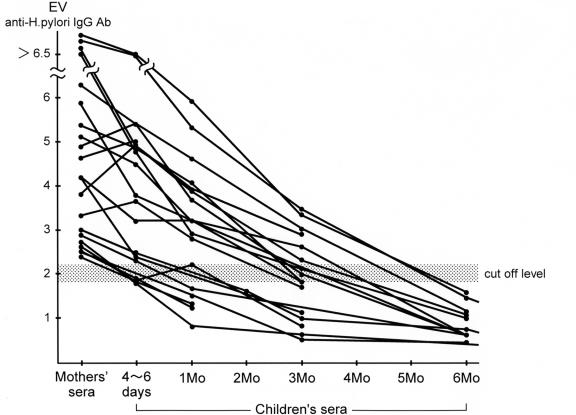
Sixty-nine of the 350 mothers (19.7%) had a positive serology on both the IgG and the IgA for H. pylori. Passive transplacental antibodies of H. pylori IgG were detected at 4 to 6 days after birth though these antibodies disappeared between 3 and 6 months of age in all 51 children born to H. pylori-positive mothers (Fig. 1). All 51 children tested negative for H. pylori IgA antibodies during follow-ups until six months. The five children who tested positive on the HpSA on follow-ups up to 5 years of age were examined by serology. Three children were positive for the IgG antibody, but two (case 1 and case 2) were negative for IgG antibodies (Table 1).
FIG. 1.
Kinetics of anti-H. pylori IgG antibody in children born to H. pylori-positive mothers from 4 to 6 days to six months after birth. Anti-H. pylori IgG antibody titers in the mothers are expressed as an initial value as a control.
TABLE 1.
Age at acquisition and H. pylori status of five cases who acquired H. pylori infection during the follow-up period
| Case no. (sex) | Age at infection (yr, mo) | HpSA (OD) | H. pylori IgG (E.V.a) | Culture (gastric juice) |
|---|---|---|---|---|
| 1 (F) | 1y2mo | 1.330 | 1.3 | + |
| 2 (M) | 1y5mo | 2.5 < | 1.5 | + |
| 3a (F) | 1y6mo | 0.136 | 3.5 | + |
| 3b (M) | 1y8mo | 0.124 | 4.3 | + |
| 4 (M) | 4y4mo | 0.863 | 3.9 | + |
E.V., EIA values.
Stool antigen test.
During the first year of life 51 children tested negative for stool antigen as judged by HpSA. Because no stool samples were available for 7 children after 1 year of age, only 44 out of the original 51 were included in the final analysis of the follow-up study (3 years 6 months to 5 years, median 4 years 4 months). However, 3 were positive for stool antigen at 4 to 6 days after birth, only to test negative later at 1 month. Since they continued to test negative for H. pylori IgA antibodies until 6 months of age, these results were considered false positive. Of the 44 children, five (11%) tested positive for HpSA during the follow-up period. The age at acquisition of H. pylori infection was 1 year 2mo, 1 year 3 months, 1 year 6 months, 1 year 8mo and 4 years 4 months, respectively (Table 1). Case 3a, born in December 1998, and case 3b, born in February 2000, were siblings. During the follow-up period, another two children tested positive for HpSA at 3 years 5 months and 3 years 7 months. Since no isolates were harvested from their gastric juice samples and both tested negative for stool antigen thereafter, these results were considered false positive.
Culture of gastric juice and RAPD fingerprinting analysis.
Cultures of the gastric juice of five children were H. pylori positive (Table 1). Another two children who tested positive transiently for HpSA had their gastric juice samples cultured, with negative results. We performed DNA analysis of cultured H. pylori isolates from the gastric juice samples of these 5 children and their mothers using PCR-based RAPD fingerprinting method. For the fingerprinting of H. pylori DNA, we used at least two primers. All the H. pylori strains of these children exhibited identical DNA fingerprinting patterns to those taken from their corresponding mothers (Fig. 2).
FIG. 2.
RAPD fingerprint patterns of genomic DNAs from H. pylori isolates from the five cases and their mothers of family 1 to family 4 obtained by RAPD analysis with primers A04, A05, A07, and A08. PCR of H. pylori obtained from gastric juice was performed as described in the text. Family numbers are shown under the lanes. PHY marker, a size marker; C, child; M, mother; 3a and 3b are siblings.
DISCUSSION
Blecker et al. (5) demonstrated that H. pylori IgG antibodies cross the placental barrier, and out of 67 infants born to seropositive mothers only one infant tested positive on the 13C-urea breath test at the age of 12 to 15 months. Ashorn et al. (3) found that only one infant out of 21 who had transplacental H. pylori IgG antibodies remained seropositive throughout the follow-up period, and no new seroconversions occurred in the remaining 20 infants. On the contrary, they found ten originally cord-blood negative children seroconverted up to the age of 2 years (5.1%). We also examined 51 children born to seropositive mothers by using HpSA. None were infected during the first year of life.
From these follow-up studies in developed countries, infants born to H. pylori-positive mothers did not appear to have an increased risk of acquiring H. pylori infection during the first year of life. Considering the fact that most of the infants received a combination of breast-and formula- feeding, and the majority of those received only breast milk during the first few months of their lives, breast-feeding may play a large role in preventing the acquisition of H. pylori infection during the first year of life. Although the literature on H. pylori and breast-feeding practices is limited, a few studies have hinted at the protective effect of breast milk against acquisition of H. pylori infection. Thomas et al. (21) demonstrated that a high level of H. pylori IgA antibodies in the mother's breast milk protects against an early onset of infection. The mechanism by which human milk might reduce the rate of acquisition of H. pylori infection was examined in an in vitro study that evaluated the inhibiting effect of human milk on the adherence of H. pylori to gastric cells (6).
In our findings, out of the 44 children, five (11%) acquired H. pylori infection in the follow-up study. In the five children whose HpSA test was positive, only three were positive for IgG antibodies. The discrepancy between the HpSA test results and IgG serology might be due to the fact that different antigens were used. HM-CAP uses a mixture of high-molecular-weight cell-associated proteins the strains of which were isolated from an American patient. Recently Okuda et al. (15) demonstrated that urine-HpELISA, which uses a sonicated extract of a strain isolated from a Japanese patient, was a reliable method even in children.
The age at acquisition of infection was 1 year 2 months, 1 year 3 months, 1 year 6 months, 1 year 8 months, and 4 years 4 months, respectively. The peak period of probable infection appeared to be between the ages of one and five years (3, 17), and in this period the mother was usually the family member with the closest contact with the child. These findings were very consistent with those of our own. Possible vectors of transmission may be the common use of spoons or chopsticks, the sharing of the teats of feeding bottles, and even by the chewing or testing of the child's food. Evidence that H. pylori might be transmitted by saliva comes from a study by Megraud et al. (13), who found a higher risk of H. pylori associated with the custom of the premastication of food when mothers feed their children in western Africa. Recently, Sinha et al. (19) demonstrated H. pylori positive PCR in maternal saliva and in the water in which children's soothers (pacifiers) were stored suggesting a plausible route of mother-to-child oral transmission.
It has been suggested that gastric juice may play an important role in the natural route of transmission. Intermittent reflux may facilitate the passage of viable organisms into the mouth, from where they may be transmitted to other individuals. We successfully isolated H. pylori from the gastric juice taken from all the five children and their mothers. This information is important since gastric juice can be aspirated through a nasogastric tube without gastroduodenoscopy. Allaker et al. (2) reported that H. pylori was detected in the gastric juice of only 3 out of 22 infected children who were cultured. The low level of detection was considered largely due to the difficulty in collecting gastric juice samples, as well as problems in the culture technique. In our experience several factors are important for a successful culture of H. pylori from gastric juice. Gastric juice samples aspirated from a nasogastric tube should be sent to a laboratory room as soon as possible and then cultured on the H. pylori-selective plates directly by streak culture using a sterile cotton swab and a microloop without centrifugation.
Many studies have suggested the possibility of intrafamilial infection (7, 9, 18, 20). The study of children in rural Colombia suggested that a major mode of transmission of H. pylori infection might be from older to younger siblings (9), especially in populations in which larger families are common. Tindberg et al. (20) concluded that intrafamilial infection is far more prevalent than child-to-child transmission outside the family, such as in a nursery school. To the contrary, Malaty et al. (12) highlighted the importance of factors outside of the home in the transmission, and concluded that children living in the most crowded day-care centers were at greater risk for H. pylori acquisition. Rothenbacher et al. (18) determined the infection status of the children by urea breath test and that of their parents through the study of salivary antibodies. The odds ratio for H. pylori infection of the child was 3.9 when the mother was saliva positive and was 2.0 when the father was saliva positive. This suggests that the mother may play a key role in the spread of the infection. Although our study also provides strong evidence for a transmission pathway from mothers to children, the following limitations must be considered: we were not able to control for fathers' and siblings' infection status because we measured only the H. pylori infection of the accompanying mothers.
By using DNA typing technique, the same or similar strains of H. pylori have been identified among family members (4, 10, 16, 23). Roma-Giannikou et al. (16) supported the intrafamilial transmission by conducting genetic analysis among 32 members of 11 families. They used formalin-fixed, paraffin-embedded material of antral mucosa. RAPD fingerprinting confirmed that closely related H. pylori strains were involved in the intrafamilial dispersion. To our knowledge, this is the first time that the RAPD fingerprinting method has been applied in H. pylori isolated from the gastric juice samples aspirated from children and mothers during the follow-up study from birth to 5 years of age.
Colonization usually begins in childhood and, once established, may persist throughout life. Knowledge of the epidemiology and mode of transmission of H. pylori is important to prevent its spread, as well as to identify high-risk populations, especially in areas that have high rates of gastric cancer, as is the case in Japan.
In conclusion, the rate of H. pylori acquisition of children born to H. pylori-positive mothers was 11% during the follow-up period from birth to 5 years of age. The identical DNA fingerprinting patterns between the five children and their mothers suggest the spread from mother to child as the pathway for H. pylori transmission in these cases.
REFERENCES
- 1.Akopyanz, N., N. O. Bukanov, T. U. Westblom, S. Kresovich, and D. E. Berg. 1992. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 20:5137-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaker, R. P., K. A. Young, J. M. Hardie, P. Domizio, and N. J. Meadows. 2002. Prevalence of Helicobacter pylori at oral and gastrointestinal sites in children: evidence for possible oral-to-oral transmission. J. Med. Microbiol. 51:312-317. [DOI] [PubMed] [Google Scholar]
- 3.Ashorn, M., M. Maki, M. Hallstorm, M. Uhari, H. K. Akerblom, J. Viikari, and A. Miettinen. 1995. Helicobacter pylori infection in Finnish children and adolescents. A serologic cross-sectional and follow-up study. Scand. J. Gastroenterol. 30:876-879. [DOI] [PubMed] [Google Scholar]
- 4.Bamford, K. B., J. Bickley, J. S. A. Collins, B. T. Johnston, S. Potts, V. Boston, R. J. Owen, and J. M. Sloan. 1993. Helicobacter pylori: comparison of DNA fingerprints provides evidence for intrafamilial infection. Gut. 34:1348-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blecker, U., S. Lanciers, E. Keppens, and Y. Vandenplas. 1994. Evolution of Helicobacter pylori positivity in infants born from positive mothers. J. Pediatr. Gastroenterol. Nutr. 19:87-90. [DOI] [PubMed] [Google Scholar]
- 6.Clyne, M., J. Thomas, L. Weaver, and B. Drumm. 1997. In vitro evaluation of the role of antibodies against Helicobacter pylori in inhibiting adherence of the organism to gastric cells. Gut. 40:731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drumm, B., G. I. Perez-Perez, M. J. Blaser, and P. M. Sherman. 1990. Intrafamilial clustering of Helicobacter pylori infection. N. Engl. J. Med. 322:359-363. [DOI] [PubMed] [Google Scholar]
- 8.Goodman, K., and P. Correa. 1995. The transmission of Helicobacter pylori, a critical review of the evidence. Int. J. Epidemiol. 24:875-887. [DOI] [PubMed] [Google Scholar]
- 9.Goodman, K., and P. Correa. 2000. Transmission of Helicobacter pylori among siblings. Lancet 355:358-362. [DOI] [PubMed] [Google Scholar]
- 10.Han, S.-R., H.-C. E. Zschausch, H.-G. W., Meyer, T. Shneider, M. Loos, S. Bhakdi, and M. J. Maeurer. 2000. Helicobacter pylori: Clonal population structure and restricted transmission within families revealed by molecular typing. J. Clin. Microbiol. 38:3635-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura, K., S. Hayashi, H. Isogai, E. Isogai, T. Sugiyama, M. Asaka, T. Anbo, A. Imamura, T. Nishikawa, T. Kubota, and N. Fujii. 1998. Restriction fragment polymorphism in Helicobacter pylori associated with gastric cancer and duodenal ulcer. Biosci. Microflora. 17:61-64. [Google Scholar]
- 12.Malaty, H., N. Logan, and D. Graham. 2001. Helicobacter pylori infection in preschool and school-aged minority children: Effect of socioeconomic indicators and breastfeeding practices. Clin. Diagn. Lab. Immunol. 32:1387-1392. [DOI] [PubMed] [Google Scholar]
- 13.Megraud. F. 1995. Transmission of Helicobacter pylori: faecal-oral versus oral-oral route. Aliment. Phamacol. Ther. 9(Suppl. 2):85-91. [PubMed] [Google Scholar]
- 14.NIH Consensus Development Panel on Helicobacter pylori in peptic ulcer disease. 1994. Helicobacter pylori in peptic ulcer disease. JAMA 272:65-69. [PubMed] [Google Scholar]
- 15.Okuda, M., T. Nakazawa, M. Booka, E. Miyashiro, and N. Yoshikawa. 2004. Evaluation of a urine antibody test for Helicobacter pylori in Japanese children. J. Pediatr. 144:196-199. [DOI] [PubMed] [Google Scholar]
- 16.Roma-Giannikou, E., A. Karameris, B. Balatsos, J. Panayiotou, Z. Manida, C. Van-Vliet, Rokkas, N. Skandalis, and C. Kattamis. 2003. Intrafamilial spread of Helicobacter pylori: a genetic analysis. Helicobacter. 8:15-20. [DOI] [PubMed] [Google Scholar]
- 17.Rothenbacher, D., J. Inceoglu, G. Bode, and H. Brenner. 2000. Acquisition of Helicobacter pylori infection in a high risk population occurs within the two years of life. J. Pediatr. 136:744-748. [PubMed] [Google Scholar]
- 18.Rothenbacher, D., M. M. Winkler, T. Gonser, G. Adler, and H. Brenner. 2002. Role of infected parents in transmission of Helicobacter pylori to their children. Pediatr. Infect. Dis. J. 21:674-679. [DOI] [PubMed] [Google Scholar]
- 19.Sinha, S. K., B. Martin, B. D. Gold, Q. Song, M. Sargent, and C. N. Bernstein. 2004. The incidence of Helicobacter pylori acquisition in children of a Canadian first nations community and the potential for parent-to-child transmission. Helicobacter. 9:59-68. [DOI] [PubMed] [Google Scholar]
- 20.Tindberg, Y., C. Bengtsson, F. Granath, M. Blennow, O. Nyren, and M. Granstrom. 2001. Helicobacter pylori infection in Swedish school children lack of evidence of child-to-child transmission outside the family. Gastroenterology. 121:310-316. [DOI] [PubMed] [Google Scholar]
- 21.Thomas, J. E., S. Austin, A. Dale, P. McClean, M. Harding, W. A. Coward, and L. T. Weaver. 1993. Protection by human milk IgA against Helicobacter pylori infection in infancy. Lancet 342:121. [DOI] [PubMed] [Google Scholar]
- 22.Uemura, N., S. Okamoto, S. Yamamoto, N. Matsumura, S. Yamaguchi, M. Yamakido. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784-789. [DOI] [PubMed] [Google Scholar]
- 23.Wang, J. T., J. C. Sheu, J. T. Lin, T. H. Wang, and M. S. Wu. 1993. Direct DNA amplification and restriction pattern analysis of Helicobacter pylori antibodies in children and their parents. J. Infect. Dis. 168:1544-1548. [DOI] [PubMed] [Google Scholar]