Abstract
Background:
Gastric cancer is the third most common malignant tumor with the second highest mortality rate in the world, and radical gastrectomy is the main treatment method, but the operation needs a long period of time to carry out and has strong surgical trauma stimulation, which is likely to cause sympathetic nerve excitement and stress reaction in the body. Therefore, the selection of appropriate anesthetic medication regimen and anesthesia method has an important impact on the intraoperative management and postoperative recovery of patients. This study aims to compare the clinical effects of dexmedetomidine alone in combination with propofol, etomidate and propofol-etomidate mixture in the treatment of radical gastrectomy for gastric cancer.
Methods:
A total of 90 patients undergoing elective radical gastrectomy were randomly divided into the propofol group (group P), the etomidate group (group E), and the etomidate–propofol mixture group (group PE). Anesthesia induction was performed under the monitoring of bispectral index anesthesia depth. The same pumping drugs were used in 3 groups: 0.1 to 0.3 μg/kg·min remifentanil, 0.5 μg/kg·h dexmedetomidine, and 5 to 10 μg/kg·min rocuronium. The primary outcome indicator was the hemodynamic conditions. The secondary outcome indicators included awakening time and time to accurately answer questions after operation, the prevalence of postoperative respiratory depression and adverse events, the incidence of postoperative cognitive dysfunction, and preoperative and postoperative Montreal Cognitive Assessment and Mini-Mental State Examination scores.
Results:
Among the 3 groups of patients, the use rate of vasoactive drugs in group P was higher (P < .05); the systolic blood pressure, diastolic blood pressure, and heart rate of group P at T1 to T4 were significantly lower than those of groups E and PE (P < .05); the systolic blood pressure, diastolic blood pressure, and heart rate of group E in T2, T4, and T6 were significantly higher than those of groups P and PE (P < .05). The wake-up time after operation and the time to accurately answer the questions were longer in group E than in groups P and PE (P < .05). The incidence of postoperative respiratory depression in group P was higher than that in groups E and PE (P < .05). The Montreal Cognitive Assessment score of group P was lower than that of groups E and PE 7 days after operation (P < .05).
Conclusion:
Dexmedetomidine combined with propofol–etomidate mixture is a better anesthesia drug combination.
Keywords: dexmedetomidine, etomidate, gastric cancer, postoperative cognition, propofol
1. Introduction
As the third most common malignant tumor in the world,[1] the incidence of gastric cancer in China has been much higher than that in other parts of the world. Gastric cancer is one of the top priorities of cancer prevention and treatment in China.[2–4] There are a variety of radical gastrectomy procedures, but there is no doubt that irrespective of the operation method, anesthesia plays a major role in the success of these operations. Selecting the appropriate anesthesia medication scheme and anesthesia method has an important impact on the intraoperative management and postoperative recovery of patients.
At present, commonly used sedative and hypnotic drugs for clinical anesthesia include etomidate and propofol, which have significant differences in characteristics. Propofol is characterized by rapid effect, short-term effect, and complete awakening. However, the conventional use of propofol has an obvious inhibitory effect on the respiratory and circulatory system, and the degree of inhibition is related to the dose of propofol.[5,6] Etomidate is a nonbarbiturate imidazole derivative. It has the advantages of rapid onset, short action time, and complete awakening, and has been widely used in clinical practice. However, relevant reports have pointed out that using etomidate alone can induce muscle tremors, postoperative nausea, and vomiting in patients. Long-term use of etomidate can also affect adrenal cortex function.[7] In recent years, some scholars have mixed propofol and etomidate in a certain ratio and achieved good clinical anesthesia effects. Propofol–etomidate mixture (Etofol) refers to mixing propofol and etomidate in a certain ratio (mixing in a 1:1 ratio in this study). It has been confirmed that the mixed use of propofol and etomidate does not produce new substances and can be safely used in clinical anesthesia. Compared with propofol or etomidate alone, it can significantly reduce adverse reactions such as injection pain and muscle tremors and has a good clinical application prospect.[8,9]
Radical gastrectomy for gastric cancer needs a long period of time to carry out and has strong surgical trauma stimulation, which is likely to cause sympathetic nerve excitement and stress reaction in the body. Dexmedetomidine, as an α2 adrenergic receptor agonist widely used in clinical perioperative anesthesia, has good sedative, hypnotic, analgesic, and anti-sympathetic effects; it inhibits inflammatory reaction significantly and provides lung protection. At present, dexmedetomidine is widely used in clinical anesthesia.[10,11] However, the combination of dexmedetomidine and which anesthetic drug produces the best anesthetic effect with the lowest side effects remains unclear.
Therefore, the aim of this study was to explore the clinical effects of dexmedetomidine combined separately with propofol, etomidate, and propofol–etomidate mixture in radical gastrectomy for gastric cancer.
2. Data and methods
2.1. General information
In this study, the consent from the Ethics Committee of the First Affiliated Hospital of Dali University was obtained, and the informed consent form for anesthesia was signed with the patients and their family members before operation. A total of 90 patients undergoing elective radical gastrectomy for gastric cancer who were admitted to the First Affiliated Hospital of Dali University from January 2019 to January 2021 were selected as the research participants; they were of ages 44 to 73 years, including 39 males and 51 females whose weights were in the range of 44 to 75 kg and American Society of Anesthesiologist grade II to III. Patients were routinely forbidden to consume any liquids and were required to fast before the operation. They had no previous history of cerebral thrombosis and cerebral infarction, no history of head trauma, no history of diabetes, no history of hypertension, no history of arrhythmia, no history of asthma, no history of cognitive diseases such as senile dementia, no history of endocrine system diseases, no recent upper respiratory tract infection, and no language or limb dysfunction. The duration of the operation was longer than 4 hours, the amount of bleeding was >20% of the patient’s own body blood volume, and patients with propofol, etomidate, and fat emulsion allergy were excluded in the statistical scope. To reduce the experimental error, the selected cases were all performed by the same group of surgeons and anesthesiologists. Bispectral index (BIS) was used to monitor the depth of anesthesia during induction and intraoperative maintenance, to control the depth of anesthesia to the same level as possible.
2.2. Grouping methods
A total of 90 patients undergoing elective radical gastrectomy were randomly divided into the propofol group (group P), etomidate group (group E), and etomidate–propofol mixture group (group PE), with 30 cases placed in each group using the numerical table method. For anesthesia induction and intraoperative maintenance, propofol was selected in group P, etomidate in group E, and propofol–etomidate mixture (1:1 mixture) in group PE. The rest of the treatment and medication were the same, and none of the selected patients took medication before the operation.
2.3. Experimental methods
Immediately after entering the room, the patients were administered oxygen by face mask, were monitored using a noninvasive cuff blood pressure, as well as their blood oxygen saturation, heart rate (HR), and electrocardiogram parameters were monitored. The venous channel of the upper limb was opened and the BIS value was monitored with the BIS alarm parameter set to 60 to 70. The integrity of the tracheal catheter cuff was checked and oxybuprocaine mucilage was evenly applied to the front two-thirds of the catheter for standby. After the anesthesia machine was checked and parameters were set based on the patients’ condition, the patients; vital signs were observed for 5 minutes, and the current oxygen saturation, HR, systolic blood pressure (SBP), and diastolic blood pressure (DBP) values of the patient were recorded after the vital signs stabilized.
2.4. Anesthesia methods
Under the deep anesthesia induction while undergoing BIS anesthesia monitoring, the inducer in group P was: propofol 1.5 to 2.0 mg/kg, the inducer in the group E was: etomidate 0.2 to 0.6 mg/kg, and the inducer in the group PE was: propofol–etomidate mixture injection (0.25 mL/s). Three groups of the same inducers were: sufentanil 0.4 µg/kg, rocuronium 0.6 to 0.8 mg/kg. The patients’ BIS values were monitored when injecting propofol, etomidate, and propofol–etomidate mixture; the injection was stopped when the BIS value reached 55, and after 3 minutes, intubation was done with a visual laryngoscope. According to the patient’s end-expiratory carbon dioxide concentration (PETCO2), the respiratory parameters were further adjusted, and PETCO2 was controlled within 35 to 40 mm Hg. At this time, right internal jugular vein puncture and catheterization were performed, and central venous pressure was monitored, while arterial puncture and catheterization were performed simultaneously. Continuous invasive blood pressure observation was performed to monitor arterial SBP and DBP. Intravenous anesthesia was maintained during the operation. After 5 minutes of intubation, group P was pumped with propofol 0.3 mL/kg·h, group E with etomidate 0.3 mL/kg·h, and group PE with propofol–etomidate mixture 0.3 mL/kg·h. The same pumping drugs were used in 3 groups: 0.1 to 0.3 μg/kg·min remifentanil, 0.5 μg/kg·h dexmedetomidine, and 5 to 10 µg/kg·min rocuronium. During the operation, PETCO2 was controlled within the range of 35 to 45 mm Hg. The pumping doses of the 3 drugs were adjusted according to the patients’ HR, blood pressure, and BIS value, and the BIS value was maintained in the range of 45 to 50. Atropine 0.5 mg/time was intravenously administered when the HR was below 55 beats/min during the operation, and ephedrine 5 mg/time was intravenously administered when the blood pressure during the operation was <20% of the basic blood pressure at time of entrance to the operating room until the blood pressure returned to normal. Dexmedetomidine pumping and additional muscle relaxants were stopped 30 minutes before the end of the operation. A total of 100 mL (8 mg) ondansetron was administered intravenously to prevent postoperative nausea and vomiting. Propofol, etomidate, and propofol–etomidate mixture pumping were stopped 10 minutes before the end of the operation and 0.1 µg/kg sufentanil was added. Remifentanil pump injection was stopped at the end of skin suture. At the end of the operation, neostigmine, naloxone, flumazenil, and other antagonists were not used. When the indication for extubation was met, the endotracheal tube was removed after adequate sputum aspiration, and the patients were sent to the postanesthesia care unit for continued resuscitation.
2.5. Evaluation indicators
In this study, the primary outcome indicator was the hemodynamic conditions. The secondary outcome indicators included awakening time and time to accurately answer questions after operation, the prevalence of postoperative respiratory depression and adverse events, the incidence of postoperative cognitive dysfunction (POCD), and preoperative and postoperative Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) scores.
2.6. Statistical methods
SPSS 24.0 statistical software was used to analyze and process the data, and the measurement data are expressed by x ± s. The height, weight, age, awakening time, bleeding volume, operation duration, systolic blood pressure, diastolic blood pressure, HR, MMSE score, and MoCA score of patients in the 3 groups were compared among the 3 groups using single factor analysis of variance. The use of vasoactive drugs, occurrence of POCD, American Society of Anesthesiologist grade, sex, and other counting data of patients in the 3 groups were compared using the test or Fisher exact test. P < .05 was considered as the difference with statistical significance.
3. Results
3.1. Comparison of general conditions of patients
There was no significant difference in general data of patients in the 3 groups (P > .05). The utilization rate of vasoactive drugs in group P was significantly higher than those in groups E and PE (P < .05) (Table 1).
Table 1.
Comparison of general data among the 3 groups (n = 30, ±s).
| General conditions | Group P | Group E | Group PE | P-value |
|---|---|---|---|---|
| ASA grade (I/II) | 13/17 | 12/18 | 15/15 | .730 |
| Age (years) | 57.5 ± 5.2 | 57.3 ± 7.3 | 57.7 ± 6.1 | .970 |
| Gender (male/female) | 12/18 | 14/16 | 13/17 | .873 |
| Height (cm) | 161.7 ± 7.3 | 161.8 ± 8.5 | 163.3 ± 6.1 | .732 |
| Body weight (kg) | 60.1 ± 8.5 | 60.3 ± 7.6 | 61.0 ± 7.6 | .900 |
| Operation time (min) | 206.3 ± 14.0 | 204.5 ± 15.8 | 205.5 ± 14.2 | .890 |
| Amount of bleeding (mL) | 196.7 ± 39.2 | 195.0 ± 41.1 | 188.3 ± 38.7 | .714 |
| Years of education (years) | 6.7 ± 1.3 | 6.5 ± 1.1 | 6.6 ± 1.2 | .817 |
| Vasoactive drug use (yes/no) | 12/18 | 4/26 | 6/24 | .044 |
| Type of surgery (total gastrectomy/distal subtotal gastrectomy/proximal subtotal gastrectomy) | 8/12/10 | 9/13/8 | 7/14/9 | .960 |
Group P, the propofol group; group E, the etomidate group; group PE, the etomidate–propofol mixture group.
ASA = American Society of Anesthesiologist.
3.2. Comparison of hemodynamics of patients in 3 groups at different time points
3.2.1. Comparison of SBP of patients in 3 groups at different time points
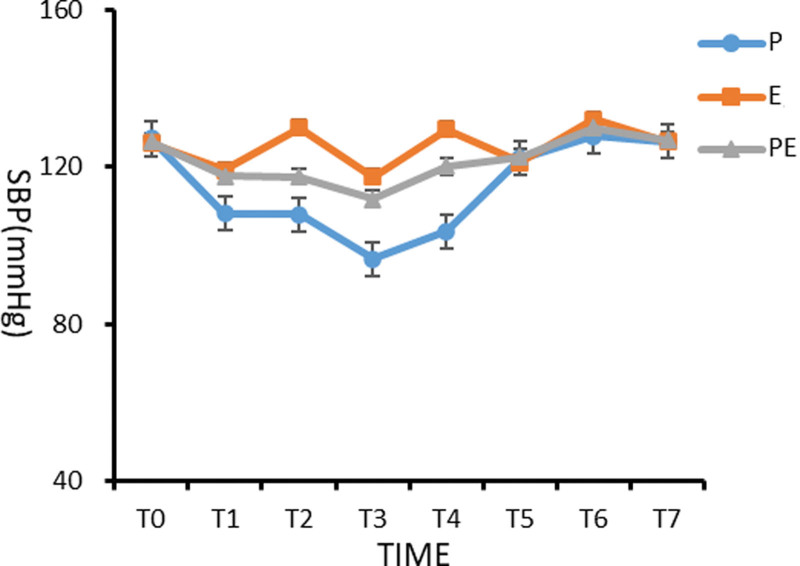
There was no significant difference in SBP of patients between the 3 groups before induction (T0) (P > .05). Compared with the basic value (T0), the systolic blood pressure of patients in the 3 groups decreased significantly from 3 minutes after induction (T1) to the beginning of operation (T4), with statistical significance (P < .05). At the moment of intubation (T2) and extubation (T6), the systolic blood pressure of patients in group E was significantly higher than that of patients in groups P and PE, with statistical significance (P < .05). Compared with those in groups E and PE, the systolic blood pressure in group P decreased significantly from 3 minutes (T1) after induction to the beginning of operation (T4), and the difference was statistically significant (P < .05), as shown in Figure 1.
Figure 1.
Comparison of systolic blood pressure (SBP) of patients in the 3 groups at different time points.
3.2.2. Comparison of DBP of patients in the 3 groups at different time points
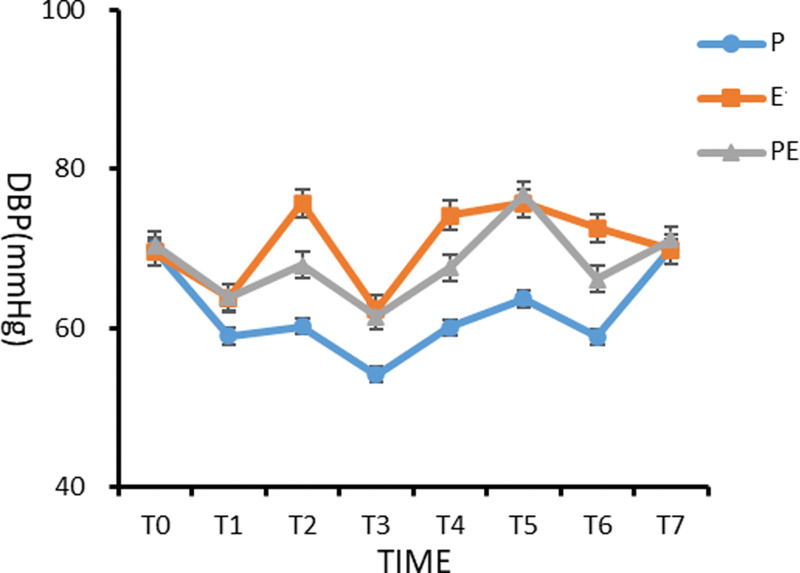
There was no significant difference in DBP between the 3 groups before induction (T0) (P > .05). Compared with the basic value (T0) in group P, the diastolic blood pressure from 3 minutes before induction (T1) to the moment of extubation (T6) was significantly lower, and the difference was statistically significant (P < .05). At the time of intubation (T2) and extubation (T6), the DBP of patients in group E was significantly higher than those in groups P and PE (P < .05). The diastolic blood pressure of patients in 3 groups from 3 minutes (T1) after induction to the beginning of operation (T4) was significantly lower in group P than in groups E and PE, and the difference was statistically significant (P < .05), as shown in Figure 2.
Figure 2.
Comparison of diastolic blood pressure (DBP) of patients in the 3 groups at different time points.
3.2.3. Comparison of HR of patients in the 3 groups at different time points
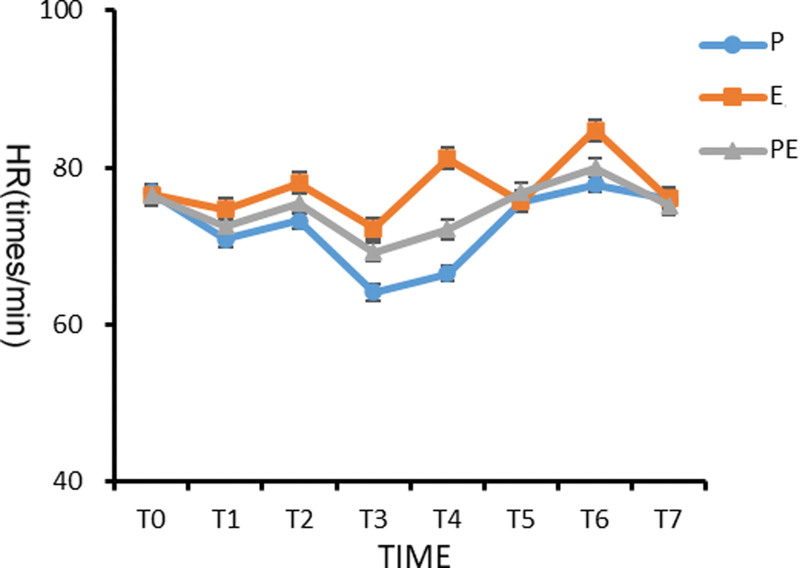
There was no significant difference in HR between the 3 groups before induction (T0), and there was no statistical significance (P > .05). The HRs from T1 to T4 (3 minutes before induction to the start of operation) in the groups P and PE were significantly lower than the basic value (T0), and the differences were statistically significant (P < .05). Group E had higher HR immediately after intubation (T2) and extubation (T6) than the groups P and PE (P < .05). Compared with patients in groups E and PE, the HR of patients in group P decreased significantly from 3 minutes (T1) after induction to the beginning of operation (T4) (P < .05), as shown in Figure 3.
Figure 3.
Comparison of heart rate (HR) of patients in the 3 groups at different time points.
3.3. Comparison of awakening time and time to accurately answer questions after operation
After stopping the drug pumping operation, the patients were patted on the shoulder and their name was softly called, and the awakening time after operation was recorded when the patients responded to the tapping and calling. Two basic preset questions were asked—1. What is your name? 2. Do you feel any discomfort? If the patients could answer the questions accurately, the time of answering the question was recorded accurately. It was found that the recovery time and correct answer time of experimental group E were much longer than those of groups P and PE, and the difference was statistically significant (P < .05), as shown in Table 2.
Table 2.
Comparison of awakening time and accurate time to answer questions after surgery (±s, minutes).
| Group | Number of cases | Wake-up time after surgery | Accurate time to answer questions |
|---|---|---|---|
| Group P | 30 | 6.73 ± 1.39† | 16.57 ± 1.48† |
| Group E | 30 | 8.03 ± 2.31* | 17.77 ± 2.46* |
| Group PE | 30 | 7.03 ± 1.25† | 16.80 ± 1.38† |
| P-value | .011 | .031 |
Group P, the propofol group; group E, the etomidate group; group PE, the etomidate–propofol mixture group.
Indicates that compared with group P, P < .05.
Indicates that compared with group E, P < .05.
3.4. Prevalence of postoperative respiratory depression and adverse events and comparison of the incidence of postoperative POCD among the 3 groups
Three patients in group P had postoperative respiratory depression, but none in groups E and PE, and the difference was statistically significant (P < .05). Postoperative adverse events of nauseas were common, though postoperative vomiting was rare. The incidence of postoperative adverse events in groups P and PE was far lower than that in group E, but the difference was not statistically significant (P > .05). The incidence of POCD in group P was higher than that in groups E and PE, but there was no statistical difference (P > .05), as shown in Table 3.
Table 3.
Comparison of the incidence of postoperative respiratory depression and adverse events and the incidence of postoperative POCD.
| Group | n | Postoperative respiratory depression | Postoperative adverse events | Incidence of POCD | |
|---|---|---|---|---|---|
| Nausea | Vomiting | ||||
| Group P | 30 | 3/27 | 3/27 | 0/30 | 7/23 |
| Group E | 30 | 0/30 | 8/22 | 1/29 | 4/26 |
| Group PE | 30 | 0/30 | 4/26 | 0/30 | 3/27 |
| P value | 6.207 | 3.360 | 2.022 | 2.139 | |
| χ 2 | 0.045 | 0.186 | 0.364 | 0.333 | |
Group P, the propofol group; group E, the etomidate group; group PE, the etomidate–propofol mixture group.
POCD = postoperative cognitive dysfunction.
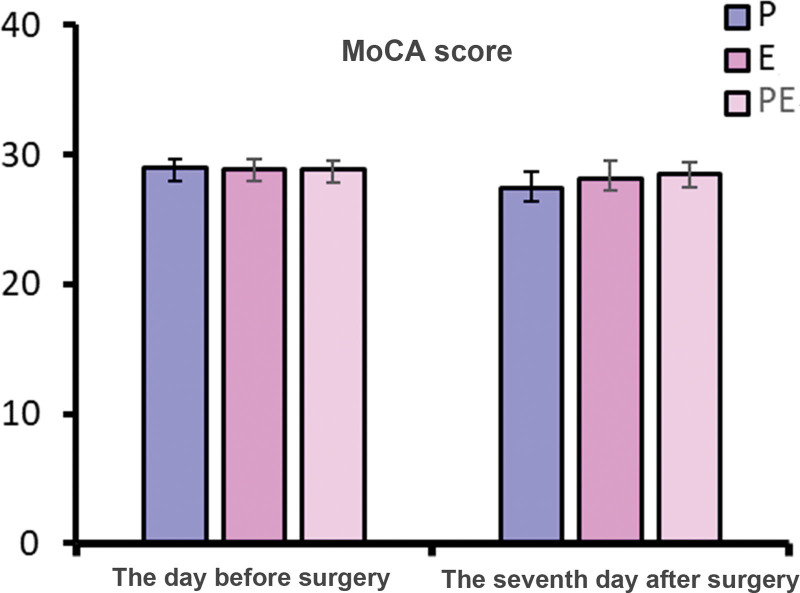
3.5. Comparison of preoperative and postoperative MoCA and MMSE scores
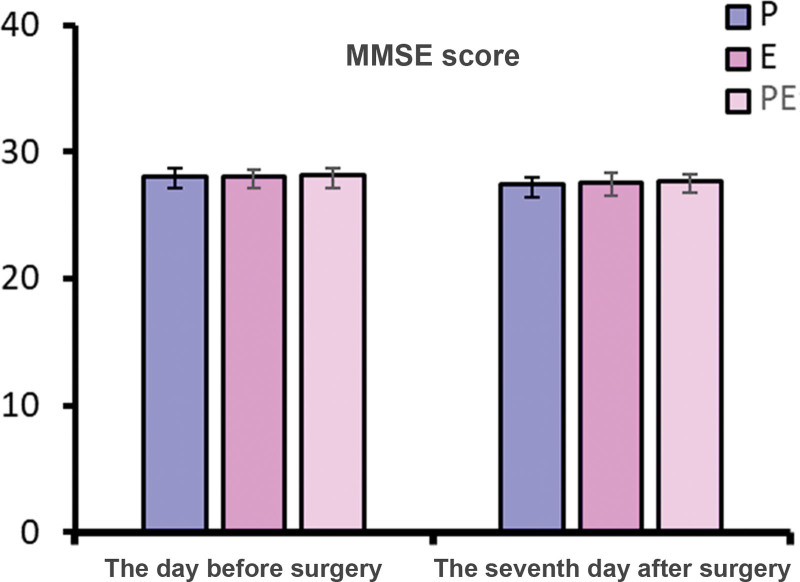
There was no significant difference in MMSE scores of 1 day before operation and of 7 days after operation and the MoCA scores of 1 day before operation among the 3 groups (P > .05). On the 7th day after operation, MoCA scores of patients in group P were lower than those in groups E and PE (P < .05), as shown in Figures 4 and 5.
Figure 4.
Comparison of preoperative and postoperative MMSE scores. MMSE = Mini-Mental State Examination.
Figure 5.
Comparison of preoperative and postoperative MoCA scores. MoCA = Montreal Cognitive Assessment.
4. Discussion
4.1. Comparison of effects of dexmedetomidine combined with propofol, etomidate, and propofol–etomidate mixture in general anesthesia for radical gastrectomy
Dexmedetomidine, as an α2 nephron receptor agonist, has relatively strong sedative, analgesic, and antianxiety functions due to its rapid drug metabolism, and it has no inhibitory effect on respiration.[12–14] The pharmacological effect of dexmedetomidine is to inhibit sympathetic nerve excitation by exciting the α2 adrenergic receptor in the locus coeruleus. Dexmedetomidine acts on nerve cells to open the Ca2+ and terminal K+ channels, and further hyperpolarization of cell membrane produces postsynaptic inhibition, which acts on α2 receptors of spinal presynaptic and postsynaptic membranes, thus controlling the release of norepinephrine, and further inhibiting blood pressure and HR.[15,16] Studies have shown that the analgesic results of dexmedetomidine (µg) and tramadol (mg) have a constant dose-quantitative relationship, with a ratio of 1: 1. The analgesic effect has a capping effect and is not directly proportional to the drug dose.[17,18]
In this study, SBP, DBP, and HR of patients in the 3 groups at different time points were compared, and it was found that the SBP, DBP, and HR from T1 to T4 in group P were significantly lower than those in groups E and PE (P < .05). The SBP, DBP, and HR in group E were significantly higher than those in groups P and PE at T2, T4, and T6 (P < .05). The data showed that propofol, etomidate, and propofol–etomidate mixture could achieve the purpose of rapid anesthesia. However, in group E, the systolic blood pressure, diastolic blood pressure, and HR increased more significantly at the time of immediate intubation (T2), the beginning of operation (T4), and immediate extubation (T6), indicating that etomidate had a weaker inhibition effect on the hemodynamic fluctuation caused by intubation, extubation stimulation, and skin incision than in case of propofol or the propofol–etomidate mixture. The SBP, DBP, and HR of patients in group P were significantly lower than those in groups E and PE from T1 to T4 (3 minutes after induction-the start of operation) and T6 (immediately after extubation). The use of vasoactive drugs in patients in group P was also significantly higher than those in groups E and PE, suggesting that propofol inhibited the circulatory system significantly. Therefore, whether it is anesthesia induction or anesthesia maintenance during the operation, the propofol–etomidate mixture has more stable hemodynamic performance. In line with our finding, Liu et al[19] demonstrated that the combined use of etomidate and propofol appeared to maintain cardiopulmonary stability with minimal side effects in older hypertensive patients scheduled for gastroscopy.
Comparison of postoperative wake-up time of 3 groups of patients showed that the wake-up time in group E was significantly longer than that in groups P and PE, and the difference was statistically significant (P < .05). The wake-up time in group PE was slightly longer than that in group P, but there was no significant difference between the 2 groups (P > .05). The accurate answer time of patients in group E was significantly longer than those in groups P and PE, and the difference was statistically significant (P < .05). The accurate answer time in group PE was slightly longer than that in group P, but there was no significant difference between the 2 groups (P > .05). Therefore, patients who used propofol or the propofol–etomidate mixture recovered more quickly after operation.
The comparison of adverse reactions among the 3 groups showed that patients using propofol or the propofol–etomidate mixture had less adverse events after operation.
The comparison of postoperative cognitive function showed that the differences in MMSE scores 1 day before operation and 7 days after operation as well as MoCA score 1 day before operation among the 3 groups were not statistically significant (P > .05). The MoCA scores of the patients in group P 7 days after operation was lower than those in the groups E and PE, and the difference was statistically significant (P < .05). The incidence of postoperative POCD in group P was higher than that in groups E and PE, but the difference was not statistically significant (P > .05). According to the scores of MoCA scale, patients using etomidate or the propofol–etomidate mixture had better cognitive function recovery and lower incidence of POCD after the operation. However, the study of Du et al[20] showed that there was no significant difference in the incidence of postoperative anesthesia-related adverse reactions between the joint group (anesthesia induction with etomidate and propofol) and the etomidate group.
Dexmedetomidine does not cause respiratory depression, however, the respiratory depression effect of propofol is significant. In short daytime procedures such as painless gastroscopy, cystoscopy, hysteroscopy, and other procedures that require preservation of spontaneous breathing, the combination of propofol and dexmedetomidine not only provides excellent sedation, but also reduces the occurrence of respiratory depression. It has been found that the combination of etomidate and dexmedetomidine can reduce the adverse effects such as nausea, vomiting, muscle tremor, etc, and at the same time ensure safe and effective anesthesia, smooth respiratory and circulatory functions, and achieve the purpose of reducing apoptosis of brain cells to achieve brain protection. The immediate effect is to make the patient’s awakening more rapid and complete, and to achieve the purpose of cognitive function protection in the long term. In outpatient surgical anesthesia that requires preservation of spontaneous respiration, both dexmedetomidine and etomidate have no inhibitory effect on spontaneous respiration, and the side effects of etomidate are reduced by the combination of drugs, which has been widely used in the clinic. For the special group of geriatric patients, due to the degradation of their basic physical health, their tolerance to anesthetic drugs and surgical stimuli has significantly decreased compared with the younger group. Elderly patients are prone to greater hemodynamic fluctuations during the perioperative period, and the risk of performing surgery and anesthesia is significantly higher than that of the average patient. Previous studies have pointed out that compared with etomidate alone for surgical anesthesia in elderly patients, etomidate combined with dexmedetomidine for surgical anesthesia in elderly patients performs better in terms of anesthetic sedation, prevention of postoperative respiratory depression, and perioperative hemodynamic stability. At the same time, the combination of the 2 drugs can also reduce intraoperative stress response and postoperative agitation, which greatly reduces the surgical and anesthetic risks in elderly patients, making it an ideal drug choice for high-risk and elderly surgical patients. Consistent with these findings, the results of the present study showed that patients in the combined group had smooth intraoperative hemodynamic performance, the advantage of good recovery of cognitive function was very obvious, and postoperative respiratory depression basically did not occur.
There were some limitations in the present study. It was a single-center and small sample size study, and the results need to be further verified by multicenter and larger sample size studies in the future.
In summary, under the guidance of BIS values, we more accurately studied the clinical anesthesia effects of dexmedetomidine combined with propofol, etomidate, and propofol–etomidate mixture respectively in the induction of general anesthesia and intraoperative maintenance for patients undergoing radical gastrectomy. We made a detailed comparison from 4 aspects, that is, hemodynamic stability, awakening time, occurrence of postoperative adverse events, and postoperative cognitive function. The results are as follows—dexmedetomidine combined with propofol anesthesia has fewer postoperative adverse events and enables patients to wake up quicker, however, the stability of the circulatory system during the operation is poor, there is obvious postoperative respiratory depression, and postoperative cognitive function recovery is poor. Under the anesthesia mode of dexmedetomidine combined with etomidate, the circulatory system of the patients is more stable during operation, the occurrence of postoperative respiratory depression is reduced, and postoperative cognitive function recovers well. However, intubation, extubation, and skin incision easily cause an increase in blood pressure, and the postoperative awakening time is relatively long, with a high incidence of adverse events. The combined use of propofol and etomidate can also reduce the dose of propofol and etomidate, which is not only reflected in the clinical anesthesia experience, but has also been proved to have a synergistic effect at the molecular level in foreign studies.[21] Under the anesthesia mode of dexmedetomidine combined with propofol–etomidate mixture, the cardiovascular system of the patients during the operation is stable, the patients wake up quickly after the operation, the incidence of adverse events is low, and the postoperative cognitive function recovery is good. In conclusion, the use of propofol–etomidate mixture for anesthesia induction and anesthesia maintenance during the operation for patients undergoing radical gastrectomy has more advantages.
Author contributions
Conceptualization: Ji Liu, Jinqiu Yang, Guangfen Yin.
Investigation: Xiyang Yang, Tao Li, Ruoyu Li, Ai Wang.
Methodology: Tao Li.
Resources: Ruoyu Li, Ai Wang.
Software: Ai Wang.
Supervision: Guangfen Yin.
Writing – original draft: Ji Liu, Jinqiu Yang, Guangfen Yin.
Writing – review & editing: Ji Liu, Jinqiu Yang, Guangfen Yin.
Abbreviations:
- BIS
- bispectral index
- DBP
- diastolic blood pressure
- ECG
- electrocardiogram
- HR
- heart rate
- MMSE
- Mini-Mental State Examination
- MoCA
- Montreal Cognitive Assessment
- POCD
- postoperative cognitive dysfunction
- SBP
- systolic blood pressure
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Liu J, Yang J, Yang X, Yin G, Li T, Li R, Wang A. Application of dexmedetomidine combined with propofol–etomidate mixture in radical gastrectomy under general anesthesia. Medicine 2024;103:44(e39669).
JL and JY contributed equally to this work.
Contributor Information
Ji Liu, Email: liujiljmw@21cn.com.
Jinqiu Yang, Email: yangqianyangyqy8@21cn.com.
Xiyang Yang, Email: yangqianyangyqy8@21cn.com.
Tao Li, Email: liruoyulrya0@21cn.com.
Ruoyu Li, Email: liruoyulrya0@21cn.com.
Ai Wang, Email: wangaiw9h@21cn.com.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Akira Y, Tai O, Tetsuji Y. Changing trends in cancer incidence of upper aerodigestive tract and stomach in Japanese alcohol-dependent men (1993–2018). Cancer Med. 2020;9:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bai HX. Advances in the incidence and mortality of gastric cancer in China. Electronic J Clin Med Literature. 2019;6:192. [Google Scholar]
- [4].Han C, Wu Q, Ni Z, et al. Correlation analysis between incidence of gastric cancer and statistical yearbook indicators in China from 1989 to 2014. J Canc Control Treat. 2019;32:434–40. [Google Scholar]
- [5].Sahinovic MM, Struys MMRF, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018;57:1539–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang J, Duan J, Xie C, Yu Y, Lu Y. Comparison between intravenous nalbuphine and lidocaine in reducing propofol-induced injection pain during gastroscopy: a randomized controlled trial. Pain Ther. 2020;9:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xiao-Chun S, Xing A, Yan C, et al. Etomidate-remifentanil is more suitable for monitored anesthesia care during gastroscopy in older patients than propofol-remifentanil. Med Sci Monitor. 2015;21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liao Y, et al., “The Component Analysis of Propofol and Etomidate Mixture and Its Clinical Efficacy,” 2015 Seventh International Conference on Measuring Technology and Mechatronics Automation, Nanchang, 2015:1152–1155. [Google Scholar]
- [9].Zhou X, Li B-X, Chen L-M, et al. Etomidate plus propofol versus propofol alone for sedation during gastroscopy: a randomized prospective clinical trial. Surg Endosc. 2016;30:5108–16. [DOI] [PubMed] [Google Scholar]
- [10].Liu YE, Tong CC, Zhang YB, Jin H-X, Gao Y, Hou M-X. Effect of dexmedetomidine on rats with renal ischemia-reperfusion injury and the expression of tight junction protein in kidney. Int J Clin Exp Med. 2015;8:18751–7. [PMC free article] [PubMed] [Google Scholar]
- [11].Liu Z, Wang Y, Wang Y, et al. Dexmedetomidine attenuates inflammatory reaction in the lung tissues of septic mice by activating cholinergic anti-inflammatory pathway. Int Immunopharmacol. 2016;35:210–6. [DOI] [PubMed] [Google Scholar]
- [12].McMorrow SP, Abramo TJ. Dexmedetomidine sedation: uses in pediatric procedural sedation outside the operating room. Pediatr Emerg Care. 2012;28:292–6. [DOI] [PubMed] [Google Scholar]
- [13].Mostafa RH, Ibrahim IM, Ayoub AH. Effect of perioperative dexmedetomidine infusion on blood glucose levels in non-diabetic morbid obese patients undergoing laparoscopic bariatric surgery. Egyptian J Anaesth. 2018;34:75–81. [Google Scholar]
- [14].Kim SH, Kim DH, Shin S, Kim SJ, Kim TL, Choi YS. Effects of dexmedetomidine on inflammatory mediators after tourniquet-induced ischemia-reperfusion injury: a randomized, double-blinded, controlled study. Minerva Anestesiol. 2018;85:279–87. [DOI] [PubMed] [Google Scholar]
- [15].Lewis SR, Nicholson A, Smith AF, et al. Alpha-2 adrenergic agonists for the prevention of shivering following general anaesthesia. Cochrane Database Syst Rev. 2015;8:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Funai Y, Pickering AE, Uta D, et al. Systemic dexmedetomidine augments inhibitory synaptic transmission in the superficial dorsal horn through activation of descending noradrenergic control: an in vivo patch-clamp analysis of analgesic mechanisms. Pain. 2014;155:617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guneli E, Yavasoglu NUK, Apaydin S, et al. Analysis of the antinociceptive effect of systemic administration of tramadol and dexmedetomidine combination on rat models of acute and neuropathic pain. Pharmacol Biochem Behav. 2007;88:9–17. [DOI] [PubMed] [Google Scholar]
- [18].Li WB, Cai YL, Li AX, Zhu H. Application of dexmedetomidine combined with etomidate in interventional anesthesia for stroke. Modern Diagnosis Treat. 2016;27:1040–1. [Google Scholar]
- [19].Liu Y, Huang Y, Wang R, Zhai Y, Huang K, Ren Z. Sedation with a 1:1 mixture of etomidate and propofol for gastroscopy in hypertensive elderly patients. J Clin Hypertens (Greenwich). 2023;25:778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Du J, Bie X, Zhu D. Application of etomidate and propofol mixture in hematoma removal in patients with intracranial epidural hematoma. Am J Transl Res. 2021;13:8403–8. [PMC free article] [PubMed] [Google Scholar]
- [21].Bansal V, Sisodia RS, Tiwari D, et al. A comparative study of effect of propofol, etomidate lipuro and propofol–etomidate lipuro admixture on haemodyanamic response and on BIS values at induction of general anaesthesia—A RCT. Int J Health Clin Res. 2020;3:61–7. [Google Scholar]