Abstract
This research aims to explore the diagnostic value of computed tomography (CT) indicators in patients with stable chronic obstructive pulmonary disease (COPD) in a plateau of China, and to find out the correlation between CT indexes and lung function and symptoms. This study screened out 53 stable COPD patients and 53 healthy people through inclusion and exclusion criteria in Hongyuan county, Aba Prefecture, Sichuan Province, between July 2020 and December 2020, and then collected their baseline data, conducted lung function tests and chest CT scans, and collected COPD Assessment Test (CAT), modified Medical Research Council Dyspnea Scale (mMRC) scores. The CT indexes of the 2 groups were compared, binary logistic regression was used to analyze the influence of COPD, the receiver operating characteristic (ROC) curve was used to evaluate the diagnostic value of the CT indexes for COPD. The Spearman test was used to understand the correlation analysis between the CT indexes and lung function, symptom score, and the number of acute exacerbations. Multiple linear regression was used to analyze the influencing factors of lung function. The percentage of low-attenuation areas less than −950 Hounsfield units (%LAA−950; t = −4.387,P = 0), percentage of wall area (WA%; t = −4.501, P = 0), and thickness–diameter ratio (TDR; t = −4.779, P = 0) in the COPD group were higher than those in the normal group. ROC shows that: %LAA−950 (P = .047) and TDR (P = .034) were independent influence in COPD in the plateau. %LAA−950 combined with TDR (AUC = 0.757, P < .001) had the value of diagnosis of COPD in the plateau. All 3 indexes are negatively correlated with lung function, and positively correlated with the symptoms and the number of acute exacerbations. Multiple linear regression analysis showed that the main factors for decrease of ratio of measurement to prediction of forced expiratory volume to the first second (FEV1%) included %LAA−950 (OR = −0.449, P < .001) and WA% (OR = −0.516, P < .001). CT indexes have a certain diagnostic value in patients with stable COPD at high altitude.
Keywords: COPD, CT, diagnostic value, high altitude
Advances in knowledge.
In Plateau of China, CT indexes (%LAA−950, WA%, TDR) were negatively correlated with lung function, acute exacerbation and positively correlated with the symptoms. Multiple linear regression analysis showed that the main factors affecting FEV1% included %LAA−950 and WA%. This study suggests CT indexes of CPOD that may allow the diagnosis of this disease in Tibetan Plateau of China, where there is a lack of pulmonary function tests.
1. Introduction
Chronic obstructive pulmonary disease (COPD) was reported as the third leading cause of death globally by the World Health Organization in 2020. Studies indicate that the prevalence of COPD in the Chinese population aged 40 and above is 9.9%, significantly higher than those under 40.[1] In high-altitude areas, the prevalence of COPD is around 10%, with Asia experiencing higher rates compared to Europe and the Americas.[2] A study conducted in Xinjiang and Tibet, China, found that the prevalence of COPD in individuals aged 15 and above is 8.5%, which was significant correlation observed in those over 40 years old.[3] Our previous research in the Hongyuan county, located at an altitude above 3000 m, revealed the COPD prevalence of 12.16% in individuals aged 40 and above.[4] The higher prevalence of COPD in high-altitude regions of China may be associated with prolonged exposure to indoor pollutants, Han ethnicity, female, age.[3,4]
The primary pathological changes in COPD involve emphysema and airway narrowing. Research indicates that 25% to 30% of patients with lung tissue injury may not show a spontaneous decrease in forced expiratory volume in 1 second (FEV1). It is only when lung tissue damaged exceed 30% that patients exhibit alterations in lung function.[5] Some patients without evidence of airflow limitation may display structural lung disease on chest imaging, including emphysema, gas trapping, and thickening of airway walls.[6] Thus, while lung function testing remains the gold standard for COPD diagnosis, it may not fully reflect the complexity of the disease.
Computed tomography (CT) has the advantages of noninvasiveness, objectivity, high accuracy, and ease of cooperation. It can detect lung damage earlier, and CT scanning can describe and quantify the pathological changes in lungs of COPD in vivo by quantifying emphysema and bronchial wall thickness. The parameter for evaluating emphysema and airway wall include percentage of low-attenuation areas less than −950 Hounsfield units (%LAA−950), thickness–diameter ratio (TDR) and wall area percentage (WA%).[7] Chest CT images can be used for COPD identification and severity classification.[8] However, there is still a lack of relevant studies on plateau population.
In high-altitude Tibetan regions of China, insufficient awareness of COPD among healthcare professionals and the lack of lung function testing equipment result in the inability to diagnose the disease.[9] This study aims to explore the assessment and diagnostic value of CT scans in stable COPD patients in high-altitude areas.
2. Research objects and methods
2.1. Research object
2.1.1. Source of objects
We selected 53 stable COPD patients aged 40 years and above as the case group and 53 age-matched healthy individuals as the control group based on inclusion and exclusion criteria from our previous cross-sectional study.[4] We collected the following information from 2 groups of individuals: gender, age, ethnicity, chest CT parameters (%LAA−950, TDR, WA%), and lung function test parameters (FEV1%; forced vital capacity, FVC; ratio of measured maximal mid expiratory flow to predicted value, MMEF%; ratio of measured maximum expiratory flow at 25% of FVC to predicted value, MEF25%; ratio of measured maximum expiratory flow at 50% of FVC to predicted value, MEF50%). We also collected the Chronic Obstructive Pulmonary Disease Assessment Test (CAT) score, modified Medical Research Council Dyspnea Scale (mMRC), frequency of acute exacerbations within 1 year (the number of instances within a year when respiratory symptoms worsen, requiring additional treatment) of case group.[10] This study was approved by the Ethics Committee of the third hospital of Mianyang, all participants (or their legal representatives) provided informed written consent.
2.1.2. Inclusion criteria for patients with COPD
Conformity to the diagnostic criteria of the Global Initiative for Chronic Obstructive Lung Disease in 2019[11] (FEV1/FVC < 0.7 after bronchodilator inhalation), age between 40 and 80 years, Stable condition (no acute exacerbations within the past 12 weeks). Inclusion criteria for subjects in normal group: FEV1/FVC ≥ 0.70 after bronchodilator inhalation, age between 40 and 80 years, No previous diagnosis of chronic airway diseases such as COPD, asthma, or bronchiectasis.
2.1.3. Exclusion criteria
Inability to cooperate with CT examination, incomplete lung field visualization, or significant artifacts in CT images that impede analysis; history of chest deformities or lung resection surgery; diagnosis of bronchial asthma, bronchiectasis, lung collapse, lung consolidation, extensive pleural effusion, lung cancer, or other space-occupying lung diseases; inability to complete questionnaire surveys, inability to cooperate with lung function tests; and history of acute exacerbation within the past 12 weeks or current treatment with antibiotics or systemic steroids.
2.2. Research methods
2.2.1. Pulmonary function test method
Using the spiro-PD portable lung function instrument from the United States, respiratory doctors with 5 years of attending physician experience completed lung function tests on all subjects in an independent, quiet room. Qualified pulmonary ventilation function tests are repeated more than 3 times, with an interval of more than 1 minute between each time. During the measurement process, the subject is required to have no cough, early or sudden stop, air leakage and mouthpart obstruction, and the maximum difference between FEV1 and FVC of each time is within 0.2 L. For those with airflow limitation, a bronchodilation test was performed, and one of the best detection values was taken. In order to avoid the impact of inhaled bronchodilators on the quantitative measurement of airways, pulmonary function tests (including bronchodilation tests) of all selected patients were performed within 3 days after CT scanning.
2.2.2. Chest CT
In order to ensure the consistency of scans and parameters obtained, 2 radiologists with 10 years of experience participating in the scans and CT parameter analysis were uniformly trained and subjected to quality control by investigator. Before the scan, the radiologist trained the subjects to perform the maximum deep-inhalation breath-hold and calm-expiration breath-hold, and instructed them to breathe and hold the breath according to the operator’s voice prompts during the scan. The US GE optima520 16-slice spiral CT machine was used. The patient was placed in the supine position, with both arms raised to hold the head, and no contrast medium was injected. The whole lung scan was performed from the lung apex to the lung base when breath-holding at the end of the deepest inspiration and breath-holding at the end of calm expiration respectively. The slice thickness was 0.6 to 1.0 mm. The raw data obtained after scanning were stored in DICOM format and imported into the SYNAPSE 3D software package (FUJIFILM, Japan) for measurements. Subsequently, the researchers and 2 associate senior radiologists cross-verified the data. We collected %LAA−950, TDR = wall thickness (T)/average outer diameter (D), and the percentage of wall area (wall area percentage, WA%) = tube wall area/outer cross-sectional area × 100%=(outer cross-sectional area - inner cross-sectional area)/outer cross-sectional area × 100%. They calculated the WA% and TDR of the midpoint cross-section of the 3rd and 4th grade bronchi of 5 segments, including the upper tongue segment of the left lung, the posterior basal segment of the left lower lung, the right upper apical segment, the right lateral segment of the middle lung, and the right lower lung posterior basal segment. And the average values of WA% and TDR of the above 5 bronchial segments were calculated as the final results.[12–14]
2.2.3. The mMRC
CAT scale scores and frequency of acute exacerbations were used to grade the degree of dyspnea in patients with COPD. The questionnaire was completed under the guidance of the researcher, and the scores were recorded truthfully and returned by the assessor on the spot.
2.3. Statistical analysis
Statistical analysis was performed on the data using SPSS 17.0 software. The Kolmogorov-Smirnov test was performed to determine whether the samples conformed to a normal distribution, and the measurement data conforming to the normal distribution were expressed as mean ± standard deviation, categorical data was represented using frequencies. The measurement data conforming to the normal distribution were analyzed by independent sample t test, and the categorical data were analyzed by chi-square test. Binary logistic regression was used to analyze the influence of COPD, the ROC curve was used to evaluate the auxiliary diagnostic value of CT indicators for COPD. The Spearman test was used to analyze the correlation of CT indicators with lung function and symptoms. Multiple linear regression was used to analyze the influencing factors of lung function. P < .05 means the difference is statistically significant.
3. Result
3.1. Clinical baseline information
A total of 53 stable COPD subjects and 53 normal subjects were included according to the baseline inclusion criteria. Demographic and clinical information were collected, and chest CT examinations, questionn-aires and pulmonary function tests were completed. All Continuous data followed a normal distribution. There were no significant differences in gender, age, and ethnicity between the 2 groups. The %LAA−950, WA%, and TDR in the COPD group were higher than those in the normal control group, and the difference was statistically significant (Table 1).
Table 1.
Clinical baseline information.
| Groups | Normal group (n = 53) | COPD group (n = 53) | X2/t value | P value |
|---|---|---|---|---|
| Tibetan/Han | 43/10 | 37/16 | 1.835 | .176 |
| Male/Female | 36/17 | 40/13 | 0.744 | .388 |
| Age | 58.15 ± 11.485 | 60.58 ± 13.592 | -0.996 | .322 |
| %LAA−950 | 1.41 ± 1.992 | 5.69 ± 6.83 | -4.387 | .000 |
| WA% | 41.88 ± 7.89 | 51.16 ± 12.77 | -4.501 | .000 |
| TDR | 0.25 ± 0.067 | 0.33 ± 0.098 | -4.779 | .000 |
| FEV1% | 105.54 ± 12.83 | 75.81 ± 20.42 | 8.97 | <.001 |
| MEF25% | 104.83 ± 38.23 | 39.44 = 19.27 | 11.117 | <.001 |
| MEF50% | 105.35 ± 24.14 | 48.50 ± 26.91 | 11.447 | <.001 |
| MMEF% | 89.74 ± 20.7 | 76.28 ± 20.23 | 3.385 | .001 |
| mMRC | – | 1.40 ± 0.90 | – | – |
| CAT | – | 13.34 ± 6.20 | – | – |
| Acute exacerbations in 1 year | – | 0.57 ± 1.185 | – | – |
| CDPD classification (I/II/III/IV) | – | (22/18/5/8) | – | – |
%LAA−950 = percentage of low attenuation areas less than −950 Hounsfield units, CAT = COPD Assessment Test, COPD = chronic obstructive pulmonary disease, FEV1% = ratio of measurement to prediction of forced expiratory volume to the first second, MEF25% = ratio of measured maximum expiratory flow at 25% of FVC to predicted value, MEF50% = ratio of measured maximum expiratory flow at 50% of FVC to predicted value, MMEF% = ratio of measured maximal mid expiratory flow to predicted value, mMRC = modified Medical Research Council Dyspnea Scale, TDR = thickness–diameter ratio, WA% = percentage of wall area.
3.2. The diagnostic value of CT indexes in high altitude chronic
3.2.1. Obstructive pulmonary at high altitude
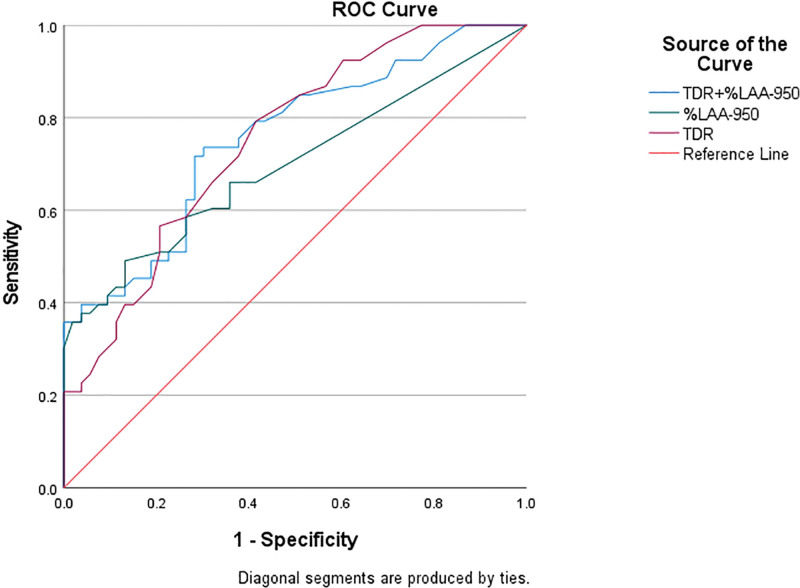
We included age, gender, ethnicity, %LAA−950, WA%, and TDR in the binary logistic regression equation, employing the forward stepwise method to select risk factors, which showed that %LAA−950 and TDR were the independent risk factors (Table 2). The ROC curve was used to explore the diagnostic value of the CT indexes (%LAA−950, TDR) in COPD in plateau area. The indicators of %LAA−950 and TDR all had diagnostic value in high altitude COPD (all P < .05). Among them, the combined diagnosis of %LAA−950 and TDR has better specificity and sensitivity (Fig. 1 and Table 3).
Table 2.
Binary logistic regression analysis of COPD.
| Variables | β | Standard error | Wald X2 | OR | 95%CI | P value |
|---|---|---|---|---|---|---|
| TDR | 0.079 | 0.037 | 4.478 | 1.082 | 1.006–1.165 | .034 |
| %LAA−950 | 0.172 | 0.087 | 3.937 | 1.187 | 1.002–1.407 | .047 |
COPD = chronic obstructive pulmonary disease, %LAA−950 = percentage of low attenuation areas less than −950 Hounsfield units, TDR = thickness–diameter ratio.
Figure 1.
The receiver-operating characteristic (ROC) curve analysis of TDR and %LAA−950 and their association with confirmed chronic obstructive pulmonary disease. %LAA−950 = percentage of low attenuation areas less than −950 Hounsfield units, ROC = receiver-operating characteristic, TDR = thickness–diameter ratio.
Table 3.
ROC curve analysis results of CT indicators in auxiliary diagnosis of COPD.
| Variables | AUC | P value | Diagnostic cut point | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|
| TDR | 0.752 | <.001 | 0.255 | 0.792 | 0.585 | 0.377 |
| %LAA−950 | 0.692 | .001 | 3.210 | 0.491 | 0.858 | 0.359 |
| TDR + %LAA−950 | 0.757 | <.001 | 0.429 | 0.717 | 0.7174 | 0.434 |
AUC = area under the curve, COPD = chronic obstructive pulmonary disease, CT = computed tomography, %LAA−950 = percentage of low attenuation areas less than −950 Hounsfield units, ROC = receiver operating characteristic, TDR = thickness–diameter ratio.
3.3. Correlation analysis of CT indicators with pulmonary function and symptoms in stable COPD patients at high altitude
The study showed that the 3 indicators of %LAA−950, WA% and TDR are negatively correlated with FEV1%, MEF25%, MMEF%, and MEF50%, and positively correlated with the number of acute exacerbations, CAT score, MMRC score, and COPD classification (Table 4).
Table 4.
The R value (P value) of the correlation analysis between CT indicators and symptoms/lung function of COPD.
| Variables | FEV1% | Acute exacerbations in 1 year | CAT score | mMRC score | CDPD classification | MEF25% | MEF50% | MMEF% |
|---|---|---|---|---|---|---|---|---|
| WA% | −0.863 (0.000) | 0.611 (0.000) | 0.635 (0.000) | 0.735 (0.000) | 0.561 (0.000) | −0.418 (0.02) | −0.505 (0.00) | −0.295 (0.032) |
| TDR | −0.890 (0.000) | 0.811 (0.000) | 0.725 (0.000) | 0.749 (0.000) | 0.464 (0.000) | −0.439 (0.01) | −0.427 (0.01) | −0.330 (0.016) |
| %LAA−950 | −0.846 (0.000) | 0.810 (0.000) | 0.709 (0.000) | 0.760 (0.000) | 0.331 (0.016) | −0.426 (0.01) | −0.313 (0.023) | −0.392 (0.04) |
CAT = COPD Assessment Test, COPD = Chronic obstructive pulmonary disease, FEV1% = ratio of measurement to prediction of forced expiratory volume to the first second, %LAA−950 = percentage of low attenuation areas less than −950 Hounsfield units, MEF25% = ratio of measured maximum expiratory flow at 25% of FVC to predicted value, MEF50% = ratio of measured maximum expiratory flow at 50% of FVC to predicted value, MMEF% = ratio of measured maximal mid expiratory flow to predicted value, mMRC = Modified Medical Research Council Dyspnea Scale, TDR = thickness–diameter ratio, WA% = percentage of wall area.
3.4. Exploring the impact of CT indicators on lung function of COPD
FEV1% was the dependent variable, and ethnicity, sex, age, WA%, TDR, %LAA−950 were included in the analysis. Collinearity analysis showed that variables had multicollinearity, TDR (variance inflation factor, VIF = 5.194) was deleted, and the remaining variables were included in multiple linear regression analysis. Residual P–P graph and scatter plot suggested that the residual basically follows the normal distribution; Durbin Watson = 1.572, indicating that the data are independent of each other; collinearity statistics showed that the VIF values were <5, indicating that there is no correlation between the data; ANOVA analysis, F = 55.119, P < .001, indicates that the model fitting equation is statistically significant; adjusted R2 = 0.854, indicating that the model fits well. The results showed that the main factors affecting FEV1% included %LAA−950, WA%. The regression equation was obtained: FEV1% = 127.679 − 1.373 × %LAA−950 − 0.845 × WA%. It suggested that %LAA−950, WA% had a negative correlation with FEV1% (Tables 5 and 6).
Table 5.
Model summary and ANOVA analysis.
| Model | R | R 2 | Adjust R2 | Standard estimated error | Durbin–Watson | F | P |
|---|---|---|---|---|---|---|---|
| 1 | 0.924a | 0.854 | 0.839 | 8.389 | 1.572 | 55.119 | <.001 |
Table 6.
Multivariate linear regression analysis of FEV1%.
| Variables | β | Standard error | t | OR | 95%CI | P value | Tolerance | VIF |
|---|---|---|---|---|---|---|---|---|
| Gender | 1.989 | 2.775 | 0.717 | 0.041 | −3.594, 7.572 | .477 | 0.805 | 1.241 |
| WA% | −0.845 | 0.138 | −6.133 | −0.516 | −1.122, −0.568 | <.001 | 0.815 | 1.227 |
| Age | −0.035 | 0.095 | −0.366 | −0.023 | −0.227, 0.157 | .716 | 0.913 | 1.074 |
| Ethnicity | −2.742 | 2.781 | −0.986 | −0.061 | −8.336, −2.853 | .329 | 0.404 | 2.472 |
| %LAA−950 | −1.373 | 0.268 | −5.124 | −0.449 | −1.912, −0.834 | <.001 | 0.437 | 2.286 |
FEV1% = ratio of measurement to prediction of forced expiratory volume to the first second, %LAA−950 = percentage of low attenuation areas less than −950 Hounsfield units, VIF = variance inflation factor, WA% = percentage of wall area.
4. Discussion
Due to lack of pulmonary function instruments and related technical personnel in plateau areas in China, patients with COPD in plateau areas cannot be diagnosed. Emphysema is quantitatively evaluated by measuring the %LAA−950 at the end of deep inspiration to the total lung volume. Previous studies found that %LAA−950 was negatively correlated with exercise endurance, 6-minute walking distance, and positively correlated with dyspnea score in COPD patients, which may be caused by the negative correlation between %LAA−950 and peak oxygen intake.[15–17] %LAA−950 was negatively associated with FEV1%, but not with disease progression,[16,18] however Oh et al[19] found that high emphysema index was independently correlated with frequent exacerbations, and McAllister et al[20] also found that CT-based assessment of emphysema could predict the acute onset of chronic lower respiratory diseases. The differences in the results may be caused by the subjects who was included and how they were measured. Previous studies of lung cancer patients and war-exposed populations had found that the more severe the emphysema, the lower the MMEF.[21,22]Our results of study in plateau area were similar to those of previous studies and indicated that %LAA−950 was positively correlated with symptom score, acute exacerbation, and disease severity in stable COPD patients, and negatively correlated with lung function (FEV1%, MEF25%, MMEF%, MEF50%), and was a risk factor for FEV1%.
Airway wall thickening and lumen narrowing are caused by enlarged mucus glands, smooth muscle hypertrophy and airway wall fibrosis in COPD patients. This process mainly occurs in small airways <2 mm in diameter.[23] However, there are still some difficulties in direct measurement of small airways with a diameter of <2 mm by CT. It has been suggested that inflammatory and remodeling processes also affect the larger airways, and therefore, airway remodeling of segmental and subsegmental airways can be used as a surrogate measure of small airway remodeling.[24,25] Some studies have found a negative correlation between airway wall thickness and MMEF in COPD patients,[26] and airway collapse is related to MEF25,[27] indicating that airway wall thickness and lumen diameter in affect the respiratory function of COPD patients. Previous studies have found that WA% is negatively correlated with exercise endurance, oxygen pulse and peak workload in COPD patients, and positively correlated with CAT score,[28] and WA% is negatively correlated with FEV1%.[29] Han et al[30] found that for every 1mm increase in bronchial wall thickness, the annual deterioration frequency of COPD patients increased by 1.84 times. This study found that WA% and TDR were negatively correlated with lung function and positively correlated with CAT score, MMRC score and COPD grade in COPD in plateau area, similar to previous findings.
The rate of FEV1 decline faster in COPD patients than that in normal people, and FEV1/FVC < 0.7 is the standard for the diagnosis of COPD. Previous study found that the severity of emphysema was positively correlated with the decline rate of FEV1 in patients with COPD patients.[31] Regardless of whether the patient had airflow restriction, the thicker the airway wall and the greater the degree of emphysema, the faster the FEV1 decline.[32,33] Emphysema and TDR were negatively correlated with FEV1/FVC in COPD patients.[34,35] This study showed that %LAA−950 combined with TDR was a good indicator for the diagnosis of COPD in plateau area, with sensitivity and specificity of 71.1%. To sum up, CT indicators have a certain diagnostic value in patients with COPD at high altitude. Therefore, CT indicators can assist in the diagnosis of COPD in high altitude regions where patients are lack of pulmonary function tests and have low compliance with examination.
Limitations: The COPD patients in this study included Han and Tibetan. Due to the small sample size, this study did not conduct subgroup analysis according to ethnicity; The COPD patients in this study were not uniformly screened according to the therapeutic drugs before completing the CT. Some studies have shown that glucocorticoids can reduce the thickness and area of the tube wall, which has a certain impact on the subsequent measurement data; This study is not subdivided according to altitude, and some indicators may be affected by changes in altitude; This study is mainly aimed at patients in the stable stage, and however, many patients have discovered the disease for the first time, so the symptoms are not obvious, and there is a lack of data on patients with worse lung function and more obvious symptoms; Because a portable spirometer was used, the data of RV/TCL could not be obtained.
5. Conclusion
In plateau area, %LAA−950, WA% and TDR were negatively correlated with lung function, and positively correlated with the symptoms, acute exacerbation. Multiple linear regression analysis showed that the main factors for FEV1% included %LAA−950 and WA%. %LAA−950 combined with TDR was a good indicator for the diagnosis of COPD, with sensitivity and specificity of 71.1%. CT indicators have a certain diagnostic value in patients with COPD at high altitude. For areas lacking pulmonary function tests and patients who cannot cooperate with examination, CT indicators can assist in the diagnosis of COPD.
Acknowledgments
Health Commission of Sichuan Province 20PJ267.
Author contributions
Conceptualization: Xingxiong Zou, Bowen Tian, Qingqing Lin, Junjie Xia.
Data curation: Bowen Tian, Qingqing Lin, Junjie Xia, Yu Qiu.
Formal analysis: Xingxiong Zou, Bowen Tian, Qingqing Lin, Junjie Xia, Yu Qiu.
Funding acquisition: Xingxiong Zou, Bowen Tian, Qingqing Lin, Junjie Xia.
Investigation: Xingxiong Zou, Bowen Tian, Junjie Xia, Yu Qiu.
Methodology: Xingxiong Zou, Bowen Tian, Junjie Xia, Ling Huang, Wenjun Li.
Project administration: Xingxiong Zou, Bowen Tian, Junjie Xia, Yu Qiu, Ling Huang.
Resources: Xingxiong Zou, Bowen Tian, Junjie Xia, Ming Yang.
Software: Xingxiong Zou, Bowen Tian, Junjie Xia, Ling Huang, Feng Gao.
Supervision: Xingxiong Zou, Bowen Tian, Junjie Xia.
Validation: Xingxiong Zou, Bowen Tian, Junjie Xia.
Visualization: Xingxiong Zou, Junjie Xia, Yu Qiu.
Writing – original draft: Xingxiong Zou, Junjie Xia.
Writing – review & editing: Xingxiong Zou, Junjie Xia.
Abbreviations:
- CAT
- COPD Assessment Test
- COPD
- chronic obstructive pulmonary disease
- CT
- computed tomography
- FEV1%
- ratio of measurement to prediction of forced expiratory volume to the first second
- FEV1
- forced expiratory volume to the first second
- FVC
- forced vital capacity
- %LAA−950 =
- percentage of low-attenuation areas less than –950 Hounsfield units
- MEF25%
- ratio of measured maximum expiratory flow at 25% of FVC to predicted value
- MEF50%
- ratio of measured maximum expiratory flow at 50% of FVC to predicted value
- MMEF%
- ratio of measured maximal mid expiratory flow to predicted value
- mMRC
- modified Medical Research Council Dyspnea Scale
- ROC
- receiver operating characteristic
- TDR
- thickness–diameter ratio
- WA%
- percentage of wall area
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Zou X, Tian B, Lin Q, Xia J, Qiu Y, Huang L, Li W, Yang M, Gao F. Diagnostic value of CT in patients with stable chronic obstructive pulmonary disease at high altitude: Observational study. Medicine 2024;103:44(e40291).
XZ and BT contributed to this article equally.
Contributor Information
Xingxiong Zou, Email: 308592390@qq.com.
Bowen Tian, Email: 363092767@qq.com.
Qingqing Lin, Email: 406505082@qq.com.
Yu Qiu, Email: 847968918@qq.com.
Ling Huang, Email: 2118626061@qq.com.
Wenjun Li, Email: 1292240286@qq.com.
Ming Yang, Email: 250850268@qq.com.
Feng Gao, Email: 405226505@qq.com.
References
- [1].Bao H, Fang L, Wang L. A meta-analysis of the prevalence of COPD in people aged 40 years and older in China from 1990 to 2014. Chin J Epidemiol. 2016;37:119–24. Chinese. [Google Scholar]
- [2].Xiong H, Huang Q, He C, et al. Prevalence of chronic obstructive pulmonary disease at high altitude: a systematic review and meta analysis. PeerJ. 2020;8:e8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guo Y, Xing Z, Shan G, et al. .Prevalence and risk factors for COPD at high altitude: a large cross-sectional survey of subjects living between 2,100-4,700 m above sea level. Front Med (Lausanne). 2020;7:581763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xia JJ, Zou XX, Qiu Y, et al. Investigation and analysis of risk factors and psychological status of chronic obstructive pulmonary disease in permanent residents aged 40 or older in Hongyuan County, Aba Prefecture, Sichuan Province. Int J Chron Obstruct Pulmon Dis. 2023;18:827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pratt PC, Kilburn KH. A modern concept of the emphysemas based on correlations of structure and function. Hum Pathol. 1970;1:443–63. [DOI] [PubMed] [Google Scholar]
- [6].Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2021 report). [EB/OL]. https://goldcopd.org/. Accessed November 17, 2020. [Google Scholar]
- [7].Wang M, Aaron CP, Madrigano J, et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA. 2019;322:546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li Z, Liu L, Zhang Z, et al. A novel CT-based radiomics features analysis for identification and severity staging of COPD. Acad Radiol. 2022;29:663–73. [DOI] [PubMed] [Google Scholar]
- [9].Expert consensus compilation of COPD screening in China, grass- roots working Committee of Respiratory Physician Branch of Chinese Medical Doctor Association. Chinese county chronic obstructive pulm- onary disease screening expert consensus(2020). Chin Med J (Engl). 2021;101:989–994. (Chinese). [Google Scholar]
- [10].Zhou Z, Zhou A, Zhao Y, Chen P. Evaluating the clinical COPD questionnaire: a systematic review. Respirology. 2017;22:251–62. [DOI] [PubMed] [Google Scholar]
- [11].Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2020 report[EB/OL].(2019-11-05) [2019-11-30]. https://goldcopd.org/gold-reports/. Accessed XXX [Google Scholar]
- [12].Wang Z, Gu S, Leader JK, et al. Optimal threshold in CT quantification of emphysema. Eur Radiol. 2013;23:975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gu S, Fuhrman C, Meng X, et al. Computerized identification of airway wall in CT examinations using a 3D active surface evolution approach. Med Image Anal. 2013;17:283–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Subramanian DR, Gupta S, Burggraf D, et al. Emphysema-and airway-dominant COPD phenotypes defined by standardised quantitative computed tomography. Eur Respir J. 2016;48:92–103. [DOI] [PubMed] [Google Scholar]
- [15].Rodrigues Sousa S, Nunes Caldeira J, Rodrigues C. COPD phenotypes by computed tomography and ventilatory response to exercise. Pulmonology. 2024;30:222–9. [DOI] [PubMed] [Google Scholar]
- [16].Kim WJ, Silverman EK, Hoffman E, et al. NETT Research Group. CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Suzuki T, Tada Y, Kawata N, et al. Influence of pulmonary emphysema on COPD assessment test-oriented categorization in GOLD document. Int J Chron Obstruct Pulmon Dis. 2015;10:1199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Boer E de, Nijholt IM, Jansen S, et al. Optimization of pulmonary emphysema quantification on CT scans of COPD patients using hybrid iterative and post processing techniques: correlation with pulmonary function tests. Insights Imaging. 2019;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Oh Y-M, Sheen S-S, Park JH, et al. Emphysematous phenotype is an independent predictor for frequent exacerbation of COPD. Int J Tuberc Lung Dis. 2014;18:1407–14. [DOI] [PubMed] [Google Scholar]
- [20].McAllister DA, Ahmed FS, Austin JHM, et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PLoS One. 2014;9:e93221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hatayama O, Kobayashi T, Fujimoto K, Kubo K. Utility of single-slice high-resolution CT in upper lung field combined with low-dose spiral CT for lung-cancer screening in the detection of emphysema. Intern Med. 2007;46:1519–25. [DOI] [PubMed] [Google Scholar]
- [22].Baradaran Mahdavi MM, Rafati M, Ghanei M, Arabfard M. Computer-assisted evaluation of small airway disease in CT scans of Iran-Iraq war victims of chemical warfare by a locally developed software: comparison between different quantitative methods. BMC Med Imaging. 2023;23:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dournes G, Laurent F. Airway remodelling in asthma and COPD: findings, similarities, and differences using quantitative CT. Pulm Med. 2012;2012:670414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tiddens HA, Paré PD, Hogg JC, Hop WC, Lambert R, de Jongste JC. Cartilaginous airway dimensions and airflow obstruction in human lungs. Am J Respir Crit Care Med. 1995;152:260–6. [DOI] [PubMed] [Google Scholar]
- [25].Nakano Y, Wong JC, Jong P A de, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–6. [DOI] [PubMed] [Google Scholar]
- [26].Ronish BE, Couper DJ, Barjaktarevic IZ, et al. Forced expiratory flow at 25%-75% links COPD physiology to emphysema and disease severity in the SPIROMICS cohort. Chronic Obstr Pulm Dis. 2022;9:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kloth C, Thaiss WM, Ditt H, et al. .Segmental bronchi collapsibility: computed tomography-based quantification in patients with chronic obstructive pulmonary disease and correlation with emphysema phenotype, corresponding lung volume changes and clinical parameters. J Thorac Dis. 2016;8:3521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Woodruff PG, Barr RG, Bleecker E, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sverzellati N, Lynch DA, Pistolesi M, Kauczor H-U, Grenier PA, Wilson C, Crapo JD. Physiologic and quantitative computed tomography differences between centrilobular and panlobular emphysema in COPD. Chronic Obstr Pulm Dis. 2014;1:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Han MK, Kazerooni EA, Lynch DA, et al. COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPD Gene study: associated radiologic phenotypes. Radiology. 2011;261:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Celli BR, Locantore N, Tal-Singer R, et al. ECLIPSE Study Investigators. Emphysema and extrapulmonary tissue loss in COPD: a multi- organ loss of tissue phenotype. Eur Respir J. 2018;51:1702146. [DOI] [PubMed] [Google Scholar]
- [32].Mohamed Hoesein FAA, Jong P A de, Lammers J-WJ, et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J. 2015;45:644–51. [DOI] [PubMed] [Google Scholar]
- [33].Ohara T, Hirai T, Sato S, et al. Longitudinal study of airway dimensions in chronic obstructive pulmonary disease using computed tomography. Respirology. 2008;13:372–8. [DOI] [PubMed] [Google Scholar]
- [34].Orlandi I, Moroni C, Camiciottoli G, et al. Chronic obstructive pulmonary disease: thin-section CT measurement of airway wall thickness and lung attenuation. Radiology. 2005;234:604–10. [DOI] [PubMed] [Google Scholar]
- [35].Mohamed Hoesein FA, de Jong PA, Lammers JW, et al. Contribution of CT quantified emphysema, air trapping and airway wall thickness on pulmonary function in male smokers with and without COPD. COPD. 2014;11:503–9. [DOI] [PubMed] [Google Scholar]