Abstract
Two methods were compared for decreasing bacterial contamination in the BacT/Alert mycobacterial culture detection system. Two concentrations, 0.5 ml (standard amount) and 1.0 ml, of mycobacterial antibiotic supplement were evaluated. Contamination rates were 14% and 6% for the standard and the doubled concentrations, respectively. This difference was statistically significant (P < 0.005).
Digestion and decontamination of clinical specimens from nonsterile body sites is required for optimal recovery of mycobacterial species, which can be quickly overgrown by contaminating bacteria. For this reason, commercial broth culture systems utilize lyophilized antibiotics, which are reconstituted and used for supplementation of standard broth media to decrease bacterial contamination.
Our laboratory experienced two 1-month episodes of increased bacterial contamination in less than a year following implementation of the BacT/Alert mycobacterial culture detection system (MB/BacT system; bioMerieux, Durham, N.C.). The interval between these events was approximately 4 months. These rates were 23.4% (27/115) and 17.9% (41/229). Similar contamination was not seen in the corresponding solid media cultures (4.5% and 13.5%, respectively). Gram-positive cocci predominated among the contaminants, leading us to question the concentration and stability of the gram-positive active agents in the mycobacterial antibiotic supplement (MAS). The manufacturer states that the concentration of vancomycin in the reconstituted MAS vials should be 5.0 μg/ml. The goals of this study were (i) to evaluate the concentration of gram-positive antimicrobials, specifically vancomycin, in the MAS by using both analytical and bioassay methods and (ii) to compare the manufacturer's recommended protocol (0.5 ml MAS) with an alternative method (1.0 ml MAS) to decrease bacterial contamination in both seeded and clinical specimens (n = 100) in the MB/BacT system.
Digestion and decontamination of study specimens was performed using standard methods (7) and a commercially available reagent containing N-acetyl-l-cysteine (0.375 gm), trisodium citrate (0.1 M), and sodium hydroxide (4%) (Scientific Device Laboratories, Des Plaines, Illinois). The following were added to individual BacT/Alert mycobacteria processing (MP) bottles: the standard amount (0.5 ml) of MAS as recommended by the manufacturer and twice the standard amount of MAS (1.0 ml) according to a validated protocol from the Arkansas Department of Health (S. F. Tidwell and T. Clark, Abstr. 98th Gen. Meet. Am. Soc. Microbiol. 1998, abstr. A-34, p. 43, 1998.). This protocol differs from the standard method only in the amount of MAS used. Mycobacteria recovered from positive cultures were identified using standard methods (5, 7, 10). Seeded MP bottles were inoculated with approximately 105 CFU/ml for each of the following mycobacterial species: Mycobacterium xenopi, M. asiaticum, M. mucogenicum, M. chelonae, M. abscessus, and M. tuberculosis. These mycobacterial species were selected for “seeding” experiments since they are infrequently encountered in our laboratory. Other mycobacterial species, such as M. avium, are commonly recovered in clinical specimens. Both seeded and clinical samples were inoculated in duplicate with one vial for each concentration of MAS tested.
We examined the possibility that increases in contamination were the result of insufficient decontamination and digestion of clinical samples to remove normal flora prior to inoculation of MP culture bottles. In such a case, the increase in contamination would be unrelated to the MAS in use. Decontamination efficiency of clinical specimens prior to inoculation of MP bottles was examined by comparing the survival of contaminating bacteria, pre- and postdigestion. Specimens were plated using a calibrated 10-μl loop on agar plates containing 5% sheep's blood before and after digestion. Plates were incubated for 48 h at 37°C, and the bacterial CFU/ml in the specimen was determined.
The vancomycin concentration in several vials of MAS was determined using two methods: a standard analytical protocol and a bioassay (3). Vials tested included those in use during the time of increased bacterial contamination and those in use when the contamination rates decreased to below threshold levels (threshold, ≤11%) (2). The analytical assay (EMIT vancomycin assay; Syva Company, Dade Behring) was performed by the Clinical Chemistry Laboratory of the Johns Hopkins Hospital by using the manufacturer's specific instructions. The bioassay was done according to established standard protocols (3). Briefly, an ATCC strain (29213) of Staphylococcus aureus and a patient isolate, both susceptible to 1.0 μg/ml vancomycin, were grown on blood agar media for 24 h and inoculated on 5 ml of Mueller-Hinton broth (MHB). The Mueller-Hinton broth tubes were incubated in a water bath at 37°C until the turbidity reached a 0.5 McFarland standard concentration. Subsequently, a 1:10 dilution was made of each isolate. Four milliliters of each 1:10 dilution was added to a conical tube of liquefied media and inverted to mix thoroughly. The media were then poured into 150-mm plates and allowed to harden. A 5-mm sterile, metal tube was used to punch holes in the media for testing. Each hole was then inoculated with 5 μl of vancomycin (concentrations: 0.5 to 20 μg/ml in twofold dilutions) and MAS in triplicate. Plates were allowed to prediffuse for 5 h at room temperature and were incubated overnight at 37°C. Mean zone sizes were calculated for vancomycin and MAS. All assays were performed in duplicate.
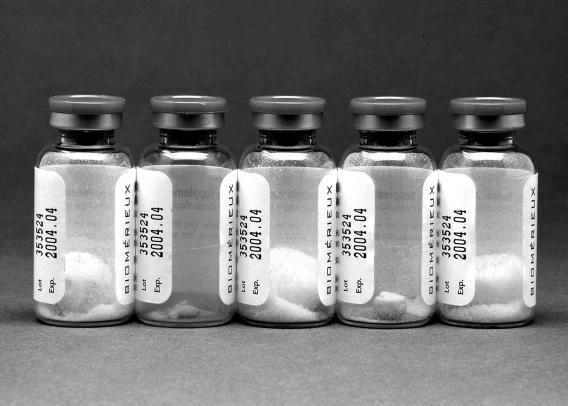
The concentration of vancomycin in the vial of MAS associated with the first contamination event was 1.4 μg/ml by the EMIT analytical assay and <2.5 μg/ml by the bioassay. This is below the manufacturer's stated concentration of 5.0 μg/ml. Several vials of this lot exhibited evidence of “extreme meltback,” in which excess water trapped in the rubber stopper of the vial during lyophilization saturates the pellet over time (Fig. 1). A new vial/lot of MAS, not associated with increased contamination, had >9.0 μg/ml. At this concentration, vancomycin may inhibit the growth of mycobacteria. However, use of this vial resolved the contamination issue. Preculture digestion and decontamination methods used in this study were determined to be adequate. Bacterial flora in predigested sputum specimens (19/19) was too numerous to count. Postdigestion, 79% (15/19) were negative for bacterial growth. The remaining 21% (4/19) produced only 1 colony (∼104 CFU/ml in the original specimen). During the clinical study, the contamination rate averaged 14% with the standard protocol (14/100), whereas the Arkansas protocol decreased contamination to 6% (6/100). This difference was statistically significant (P < 0.005). The mycobacterial positivity rate was 7% (7/100; 6 M. avium complex, 1 M. kansasii).
FIG. 1.
Vials from a single lot of MAS. Note evidence of “extreme meltback” in the vial second from the left and to a lesser extent in the vial fourth from the left.
Seeded cultures and clinical specimens were used to determine differences in the recovery of various mycobacterial species and the time to detection between the standard and Arkansas protocols used in this study. In the seeded study, the Arkansas protocol resulted in an average increase in time to detection of 2.3 days versus the standard protocol (Table 1). However, due to the higher contamination rate observed with the standard protocol in the clinical study, which required redigestion and reincubation, the time to detection of positive cultures with the Arkansas protocol was decreased by 0.8 days relative to the standard protocol (Table 2).
TABLE 1.
Comparison of time to detection between the standard and Arkansas protocols with various species of mycobacteria in a seeded study
| Mycobacterial species | Time to detection (days)a
|
|
|---|---|---|
| MAS | ARK | |
| M. xenopi | 10.4 | 11.6 |
| M. asiaticum | 6.7 | 6.8 |
| M. mucogenicum | 6.5 | 15.0 |
| M. chelonae | 7.9 | 8.1 |
| M. abscessus | 1.7 | 1.7 |
| M. tuberculosis | 9.2 | 13.0 |
MAS, standard protocol; ARK, Arkansas protocol.
TABLE 2.
Comparative time to detection of growth in the MB/BacT system between the standard and Arkansas protocols with acid-fast bacillus-positive clinical samples
| M. avium clinical specimen | Time to detection (days)a
|
|
|---|---|---|
| MAS | ARK | |
| 1* | 19.9 | 9.0 |
| 2* | 15.6 | 9.5 |
| 3 | 16.7 | 20.2 |
| 4 | 11.3 | 14.0 |
| 5 | 6.7 | 6.3 |
| 6 | 6.2 | 6.3 |
| 7 | 9.7 | 14.7 |
MAS, standard protocol; ARK, Arkansas protocol. * denotes specimens which required redigestion and subsequent reincubation due to bacterial contamination.
Prior studies have demonstrated higher bacterial contamination rates with predominantly gram-positive organisms in the MB/BacT system than with other methods, despite the addition of vancomycin to the MAS (1, 2, 6, 8, 11, 12). This study evaluated the MAS concentration of vancomycin and compared the manufacturer's recommended protocol with another method in an effort to decrease bacterial contamination in the MB/BacT system. The inconsistency in vancomycin concentration between vials in this investigation may be related to the “extreme meltback” observed in several of the vials/lots of MAS associated with the increased contamination rates. Saturation of the lyophilized pellets could decrease the potency of antibiotics, such as vancomycin, whose stability is affected in aqueous solutions (4, 9). By increasing the amount of MAS, contamination rates were significantly reduced compared with the standard method and negated the requirement for redigestion of affected cultures. Taken together, differences between the two methods in time to detection were negligible for all species of mycobacteria studied.
In summary, two periods of increased contamination of the MB/BacT system were directly related to lower concentrations of vancomycin in the antibiotic supplement. We suspect that this was due to the observed phenomenon of extreme meltback. These findings have been reported to the Food and Drug Administration. These problems would not have been detected without continuous monitoring of contamination rates.
REFERENCES
- 1.Alcaide, F., M. A. Benitez, J. M. Escriba, and R. Martin. 2000. Evaluation of the BACTEC MGIT 960 and the MB/BacT systems for recovery of mycobacteria from clinical specimens and for species identification by DNA Accuprobe. J. Clin. Microbiol. 38:398-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin, W. H., K. B. Waites, A. Beverly, L. Gibbs, M. Waller, S. Nix, S. A. Moser, and M. Willert. 1998. Comparison of the MB/BacT system with a revised antibiotic supplement kit to the BACTEC 460 system for the detection of mycobacteria in clinical specimens. J. Clin. Microbiol. 36:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filburn, B. H., V. H. Shull, Y. M. Tempera, and J. D. Dick. 1983. Evaluation of an automated fluorescence polarization immunoassay for vancomycin. Antimicrob. Agents Chemother. 24:216-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuhrman, L. C., Jr., and R. T. Stroman. 1998. Stability of vancomycin in an extemporaneously compounded ophthalmic solution. Am. J. Health Syst. Pharm. 55:1386-1388. [DOI] [PubMed] [Google Scholar]
- 5.Heifets, L. 1997. Mycobacteriology laboratory. Clin. Chest Med. 18:35-53. [DOI] [PubMed] [Google Scholar]
- 6.Manterola, J. M., F. Gamboa, E. Padilla, J. Lonca, L. Matas, A. Hernandez, M. Gimenez, P. J. Cardona, B. Vinado, and V. Ausina. 1998. Comparison of a nonradiometric system with BACTEC 12B and culture on egg-based media for recovery of mycobacteria from clinical specimens. Eur. J. Clin. Microbiol. Infect. Dis. 17:773-777. [DOI] [PubMed] [Google Scholar]
- 7.Metchock, B., F. S. Nolte, and R. J. Wallace, Jr. 1999. Mycobacterium, p. 399-437. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 8.Piersimoni, C., C. Scarparo, A. Callegaro, C. P. Tosi, D. Nista, S. Bornigia, M. Scagnelli, A. Rigon, G. Ruggiero, and A. Goglio. 2001. Comparison of MB/BacT ALERT 3D system with radiometric BACTEC system and Löwenstein-Jensen medium for recovery and identification of mycobacteria from clinical specimens: a multicenter study. J. Clin. Microbiol. 39:651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pikal, M. J., and S. Shah. 1992. Moisture transfer from stopper to product and resulting stability implications. Dev. Biol. Stand. 74:165-179. [PubMed] [Google Scholar]
- 10.Reisner, B. S., A. M. Gatson, and G. L. Wood. 1994. Use of Gen-Probe AccuProbes to identify Mycobacterium avium complex, M. tuberculosis complex, Mycobacterium kansasii, and Mycobacterium gordonae directly from BACTEC TB broth cultures. J. Clin. Microbiol. 32:2995-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roggenkamp, A., M. W. Hornef, A. Masch, B. Aigner, I. B. Autenrieth, and J. Heesemann. 1999. Comparison of MB/BacT and Bactec 460 TB systems for recovery of mycobacteria in a routine diagnostic laboratory. J. Clin. Microbiol. 37:3711-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohner, P., B. Ninet, C. Metral, S. Emler, and R. Auckenthaler. 1997. Evaluation of the MB/BacT system in comparison to the BACTEC 460 system and solid media for isolation of mycobacteria from clinical specimens. J. Clin. Microbiol. 35:3127-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]