Abstract
Objectives
Duchenne muscular dystrophy (DMD) is a heritable disorder that causes a rapid and progressive loss of ambulatory skills. There is no curative therapy for this pathology, that is currently managed with a combination of physiotherapy and pharmacological interventions limiting the progression of the disease (e.g. corticosteroids, cardiac medications). However, a new opportunity is represented by gene therapy, a promising treatment that, however, requires significant expertise during the whole delivery of care and a solid organisational infrastructure. An organisational strategy that could effectively support its delivery to DMD patients in Italy is the hub-and-spoke model. However, an accurate portrait of the present network of DMD centres of expertise in Italy and of their readiness in the delivery of gene therapy is paramount, to facilitate access to this experimental medicine in the future.
Methods
In this context, the present study aimed to map the DMD centres of expertise in Italy and later evaluate their preparedness in terms of gene therapy delivery. For this purpose, a series of items was proposed to 30 centres in Italy, of which 20 responded.
Results
After assessing the readiness of the involved centres in terms of patient preparation, therapy infusion, close surveillance, and long-term follow-up, we proposed a suitable organizational model, namely a flexible hub-and-spoke model, for the delivery of gene therapy in the Italian DMD network and solutions to tackle the challenges emerged from the survey.
Conclusion
Overall, the present study detected an adequate readiness of the Italian DMD centres of expertise, despite observing a significant room for improvement in digital infrastructures, culture, and training.
Key words: Duchenne muscular dystrophy, gene therapy, healthcare management, hub-and-spoke, lean management, delivery of care
Background
Duchenne muscular dystrophy (DMD) is a neuromuscular disorder that causes a progressive loss of ambulatory skills in patients 1. Associated with a mutation on the X chromosome and with a recessive character, DMD affects males symptomatically, while females usually show a mild or asymptomatic phenotype 2. This rare disease affects 1 in 5,000 live-born males and, according to an investigation of the Italian Federation of Primary Care Paediatricians (FIMP) in 2019, affects about 2,000 patients in Italy, even if no official estimates are yet available 3-6.
There is no curative therapy for DMD, currently managed with a combination of physiotherapy and pharmacological interventions limiting the progression of the disease (e.g. corticosteroids, cardiac medications) 7. A new treatment opportunity is represented by gene therapy, which has the potential to restore dystrophin production by providing an engineered form of the protein (mini-dystrophin) 8.
This novel therapy requires significant expertise on the part of health professionals who organize and follow the patient journey and should thus be associated with the implementation of a new organizational model that combines a solid system governance with an effective communication between all the involved stakeholders.
To facilitate the gene therapy delivery and to access to treatment in the future, it is crucial to map the present network of DMD centres of expertise in Italy and assess their readiness for the delivery and monitoring the gene therapy. An organisational strategy that could effectively support its delivery to DMD patients in Italy is the hub-and-spoke model 9,10. This model implies the centralisation of complex healthcare services in a limited number of major healthcare centres (hub), supported by secondary centres (spoke) in the delivery of basic services and long-term monitoring. Leveraging on a hierarchical network, the hub-and-spoke model tackles the logistic hurdles that characterise the delivery of advanced therapies, while optimising the resource use in the involved healthcare network 9.
The hub-and-spoke model
Theoretical framework and previous experiences
The hub-and-spoke design is based on arranging the service delivery in a network: hub centres offers a complete set of healthcare services, usually including complex and skill intensive services and advanced technologies, while spoke centres mostly focus on long-term monitoring and routine care 9,11.
From an operation management perspective, the hub-and-spoke model supports the development of a value stream and the minimisation of waste 12. For instance, it improves the allocation of resources by logically distributing skilled professionals and assets in the network, minimising the duplication of functions. Working on a structured task division, this organisational design contributes to minimise other relevant wastes, such as waiting lists and transportation, while increasing the consistency of operations and strengthening command and control in the network 9. Indeed, the hub-and-spoke model has been reported to improve treatment access at a local level, even in remote areas, and to reduce patient mobility across regions 13,14. Patients can indeed receive routine health services locally, avoiding unnecessary transportation to hubs, if not for highly specialised procedures (e.g, therapy infusion). While contributing to a reduction of transportation costs, this design holds a great potential in reducing waiting lists, evenly distributing the workload across the network and relieving pressure from the hubs 13,15.
Both aspects are relevant to the Italian context, where inter-regional mobility constitutes a complex public health issue, and long waiting lists challenge healthcare organisations, negatively impacting patient satisfaction 16,17.
The benefits of adopting a hub-and-spoke model are not limited to cost saving and managerial efficacy, as this set-up enables a patient-centred delivery of care.
However, this configuration has also potential downsides, especially when lacking proper infrastructures and leadership. Poor communication between centres can lead to over-processing patient information and congesting the hubs. Also, the absence of proper governance may result in professionals perceiving spokes as secondary elements, increasing staff dissatisfaction and worsening quality of care 9.
Of note, the hub-and-spoke model has been already applied in the setting of DMD and gene therapy as described by Heslop et al, who documented its feasibility in the institutional and clinical framework of the United Kingdom 18. With similar results, this model has been successfully applied in other rare disease settings, such as haemophilia 19.
The hub-and-spoke in Italy: regulatory background
The reorganisation of the Italian hospital network based on the hub-and-spoke model was set off by the Ministerial Decree n. 70 in 2015, with a policy of power decentralisation that had its origins in the Italian hospital care reform of 1968 20. The change initiated by this reform was reinforced by the 2001 Italian constitutional reform, which delegated the legislation power to the single Italian regions, increasing the agency of local institutions and raising awareness on the importance of public health expenditure and cost savings.
The hub-and-spoke implementation was thus highly encouraged and proposed as an effective strategy in various healthcare settings 20.
In the following paragraphs, we describe the readiness of Italian DMD centres of expertise in terms of regulatory, clinical, and digital infrastructures and address the pros and cons of adopting the hub-and-spoke model for delivering the gene therapy to DMD patients.
Results
Centres preparedness
This study aimed to map the DMD centres of expertise in Italy and later evaluate the preparedness of these centres in terms of gene therapy delivering and long-term patient follow-up. For this purpose, a survey was administered to 30 centres in Italy, of which 20 completed the questionnaire. All participants provided their informed consent for data processing before accessing the survey.
The survey was validated by two field experts and featured regulatory, clinical, and organisational requirements. Also, it assessed the ability of centres not meeting the criteria necessary for the therapy infusion, but that could cooperate in terms of long-term follow-up.
The survey results allowed to map a standard DMD patient journey, where different functions were assigned either to the hub, to the spoke, or both. An overview of this task division is reported in Figure 1.
Figure 1.
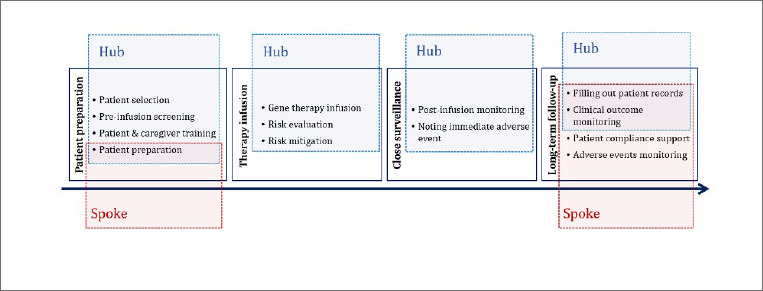
Responsibilities of hubs and spokes in the DMD patient journey of gene therapy in Italy.
Of the 20 DMD centres that completed the survey, 80.0% reported a prior involvement in the provision of other gene therapies. The presence of adequate organisational and regulatory infrastructures likely suggests their participation in other gene therapy clinical trials, that usually require to meet and continuously monitor specific standards. Additionally, the responding DMD centres displayed a marked propensity to participate in a network for the delivery of the gene therapy, declaring the possibility of increasing the number of followed patients, up to 30.0% and beyond.
When inquired about the possibility to dedicate a multidisciplinary team to a gene therapy protocol, responders did not highlight major difficulties and appeared capable to cover all the main professional profiles needed, except for what concerns physiotherapists and psychologists, who appear to be lacking particularly for the long-term follow-up.
Patient preparation
The responders indicated among the hub responsibilities the following functions: patient selection, screening, and preparation. However, the contribution of spoke centres was considered appropriate already at this stage, especially in the dissemination of information to patients and caregivers. Additionally, due to their grip on the territory, spokes were considered fundamental for the pre-assessment of eligible patients.
Pre-infusion patient evaluation includes assessing prior exposures to the used adeno-associated virus (AAV) with appropriate immunological testing, as well as pondering pros and cons of gene therapy together with patients and caregivers. The survey results highlighted the necessity to further develop such testing, which currently can be performed in less than half of the responding centres (45.0%).
Next, appropriate patient selection poses the question of when it is optimal to administer the therapy. While treating an older patient implies facing a more advanced pathology, treating a very young patient could later result in diluting the mini-dystrophin in the organism as the patient grows up.18 Considering the current impossibility to repeat gene therapy doses, due to its “one-off” nature, it is crucial to carefully choose the optimal administration time. In this regard, the responders consider more than 40.0% of the 0- to 4-year-old patients and 50.0% of the 5- to 9-year-old patients as potential gene therapy candidates. DMD centres in Italy would then treat patients with different ages similarly, albeit tending to treat more often children of a relatively older age range. A similar trend is observed for infusing patients coming from another region, considered eligible between 30.0% (0-4 years old) and 35.0% (5-9 years old). The observed decrease of 10.0% and 15.0% respectively is probably due to the logistics required for the treatment of these patients 21.
Before starting the treatment, the risks for possible adverse events and the expectations related to therapy must be evaluated together with patients and caregivers. In fact, while resulting in significant changes to the patients’ lifestyle, the durability of dystrophin expression following a gene therapy treatment remains uncertain 22,23.
Therapy infusion
Hubs play a major role in all the functions concerning the gene therapy infusion, from contacting the pharmacy for the product preparation to the management of infusion-related adverse reactions.
Among the 20 responders, at least 10 constitute potential hubs and fulfilled all the criteria related to the standardised reception, management, delivery, and disposal of experimental viral medicines (Tab. I). They also declared the presence of adequate hospital and pharmacy infrastructures. In particular, the survey highlighted that healthcare professionals in 80.0% of the included centres already received training on the safe handling of viral vectors used in gene therapy.
Table I.
Checklist for assessing the readiness of DMD centres of expertise in Italy in the delivery and/or monitoring of gene therapy: patient preparation, therapy infusion, and post-infusion close surveillance.
| Selected items* | Responsible | N centres that fulfil the item | % on total |
|---|---|---|---|
| Regulatory aspects, quality, and clinical risk management | |||
| Previous experience with gene therapy | Hub | 16 | 80.0% |
| Standard protocol for the reception of experimental viral medicines | Hub | 16 | 80.0% |
| Standard gene therapy administration protocol in line with national GCP guidelines | Hub | 10 | 50.0% |
| Digital systems | |||
| Centralised patient registry, patient check-in, check-out, and transfer | Hub/Spoke | 20 | 100.0% |
| Shared database with other gene therapy delivery and/or monitoring sites | Hub/Spoke | 9 | 45.0% |
| Possibility to provide medical services via telemedicine | Hub/Spoke | 14 | 70.0% |
| Clinical activities | |||
| Previous training on the safe handling of gene therapy viral vectors | Hub | 16 | 80.0% |
| Standard protocol for gene therapy risk assessment | Hub | 14 | 70.0% |
| Formalised process for the continuity of care between disease stages | Hub/Spoke | 15 | 75.0% |
| Pharmacy and hospital infrastructure | |||
| Biological safety cabinet at least Class II A2 and dry ice-appropriate ventilation system | Hub | 18 | 90.0% |
| Protocol for receiving the gene therapy (constant temperature between -60°C/-90°C) | Hub | 19 | 95.0% |
| Possibility to perform immunological tests for prior exposure to AAV9 | Hub/Spoke | 9 | 45.0% |
| *this table reports a selected number of criteria that appeared particularly relevant to the listed functions. The complete survey can be found in the supplementary material | |||
| GCP = good clinical practice; AAV9 =adenoassociated virus 9 | |||
Close surveillance
After the gene therapy infusion, hub centres must be prepared to carry out a close surveillance to monitor potential post-infusions adverse events. In this phase, it may be required to organise the accommodation of patients travelling from another region. Among the 20 surveyed centres, 11 have a formal agreement with a facility that could host patients and caregivers during the infusion and post-infusion monitoring and may thus be preferred by patients coming from far away. Additionally, all responding centres have a centralised patient registry and digital systems for managing patient admissions and transfers.
DMD centres in Italy appear ready to monitor post-infusion adverse events, with over 85.0% of responders fulfilling all criteria related to the treatment of common as well as more rare and serious adverse events. While most of the responding centres allocated close surveillance functions exclusively to the hubs, spokes can support the network at a local level, registering adverse events and supporting patients when needed.
Long-term follow-up
The follow-up of gene therapy patients includes the monitoring of clinical outcomes and adverse events, the patients’ psychological support and the data collection. At this stage, a close cooperation between hub and spoke centres is deemed fundamental. Spoke centres constitute the front-line for patients needing therapy-related assistance, as well as for everything concerning routine examinations and long-term adverse events. In this regard, 85.0% of the survey participants have an intensive care unit to address such emergencies.
Most responders already implemented standard protocols to continuously assess quality improvement (e.g, based on ISO 9000 standards) and present a clinical risk management unit.
Currently, the surveyed DMD centres appear significantly prepared to function as spoke centres, with 95.0% of them having sufficient staff and facilities to monitor patients long-term (for at least 5 years).
Also, the 75.0% of the responders can perform all the major DMD clinical outcome assessments, from speed and skill improvement tests to measure the changes in the levels of mini-dystrophin in the muscle or creatine kinase in the blood. An exception is represented by the possibility of performing the liquid chromatography–mass spectrometry (LC-MS), available only in the 30.0% of the involved centres. This result appears in line with the fact that LC-MS for DMD is usually performed abroad, in the frame of international multicentre clinical trials (expert opinion).
Regarding the management of adverse events, the survey results are not fully in line with the perceptions of the filed experts involved in the study, who attributed the confidence displayed by the centres to their previous experience with gene therapy for other pathologies, such as spinal muscular atrophy (SMA).
Optimal information transfer is another key requirement for a successful patient monitoring, particularly during follow-up, and it depends upon established information systems and digital infrastructures. In this regard, the centres reported an adequate availability of digital technologies and processes, with 75.0% of them having a protocol for the computerised transmission of clinical documentation, and 70.0% of them able to provide telemedicine services to patients. Finally, the effective follow-up of gene therapy patients requires a multi-disciplinary team providing clinical and psychological support, for which the 20 responders display sufficient human resources and expertise (Tab. II).
Table II.
Checklist for assessing the readiness of DMD centres of expertise in Italy in the delivery and/or monitoring of gene therapy: long-term follow-up.
| Selected items* | Responsible | N centres that fulfil the item | % on total |
|---|---|---|---|
| Clinical preparedness | |||
| Standard protocol for the long-term follow-up of gene therapy patients | Spoke | 15 | 75.0% |
| Possibility to monitor gene therapy patients for at least 5 years | Spoke | 19 | 95.0% |
| Presence of a multi-disciplinary team qualified in the follow-up of gene therapy | Spoke | 19 | 95.0% |
| Computerised system for the annotation of mortality or adverse events during follow-up | Hub/Spoke | 16 | 80.0% |
| Monitoring of clinical outcomes | |||
| Measuring skill acquisition through NSAA, PODCI, or speed test | Hub/Spoke | 18 | 90.0% |
| Performing the nuclear magnetic resonance | Hub/Spoke | 20 | 100.0% |
| Measuring creatine kinase changes by blood test | Hub/Spoke | 20 | 100.0% |
| Measure changes in mini-dystrophin expression in the muscle by LC-MS | Hub/Spoke | 6 | 30.0% |
| Monitoring of adverse events | |||
| Treat serious adverse events associated with gene therapy | Spoke | 18 | 90.0% |
| Treat hypersensitivity events to the gene therapy used | Spoke | 19 | 95.0% |
| Monitor possible incidental events of carcinogenesis related to the AAV insertion | Spoke | 17 | 85.0% |
| Presence of an intensive care unit to manage emergencies and acute adverse events | Spoke | 17 | 85.0% |
| *this table reports a selected number of criteria that appeared particularly relevant to the listed functions. The complete survey can be found in the supplementary material | |||
| AAV =adenoassociated virus; LC-MS = liquid chromatography mass spectrometry; NSAA = North Start Ambulatory Assessment; PODCI = Paediatric Outcomes Data Collection Instrument | |||
Major hurdles and set-up challenges
Digital infrastructures
The delivery of gene therapy requires prime communication between hub and spoke centres. In a recent study, Heslop et al. underlined the need for an early liaison with all the stakeholders involved due to the significant impact of new gene therapies implementation on hospital infrastructures and processes 18.
As previously mentioned, hub and spoke centres rely on established digital infrastructures for the follow-up of DMD patients and for an optimal information transfer. Additionally, digital infrastructures support continuity of care between different disease stages and child development. This can include also standardised procedures and a shared repository in the network 10,18. However, more than half of the responders (55.0%) indicated the lack of data sharing with other centres, which could hinder the long-term patient monitoring. The field experts backed up this result, pointing out the absence, to date, of an Italian national database that would facilitate data sharing in the network, emphasising the need for a development of such infrastructures. Also, the benefits of implementing electronic devices in the management of gene therapy delivery have been previously described in the literature and seem overall an essential requirement 24.
The lack of adequate digital systems could ultimately represent a major bottleneck and hamper the information flow between centres, impairing patient access and hindering the data collection at the basis of clinical outcome monitoring.
Additionally, adopting the hub-and-spoke model would significantly impact the digital sector by challenging present infrastructures and requiring the development of top-notch digital technologies.
Culture and training
Another critical aspect of the hub-and-spoke model actualisation is the need for a cultural change surrounding the roles and functions of the spokes. Especially in the light of an innovative treatment such as gene therapy, professionals active in the spoke centres might potentially develop dissatisfaction for not being involved in the primary therapy delivery. This might result in them seeking job opportunities in the hubs, contributing to increase the healthcare service inequity at the periphery of the network. The survey revealed that 50.0% of responders do not contemplate letting the patient being followed long-term by a spoke centre, confirming the relevancy of this issue in Italy.
A possible strategy to tackle this hurdle is to develop effective awareness campaigns explaining the determinant role of spokes in the most time- and resource-intensive steps of the gene therapy journey (i.e. long-term follow-up), thus constituting an indispensable element of this integrated care model 18.
Proposed solutions
The hub-and-spoke design has been already adopted in Italy for other care settings and it has been proven highly promising in the context of gene therapy in other countries 18,19.
Particularly noticeable is the high number of DMD centres that could constitute hubs in the network, with at least 10 among the surveyed sites fulfilling all criteria strictly related to the hub function (Tabs. I, II). However, the survey highlighted the need for enhancing the capacity to perform certain complementary health services (AAV testing, LC-MS), as well as the need for strengthening digital infrastructures and data sharing in the network. Of note, the field experts ascribed the suboptimal information transfer to the current absence of a national database, calling for a nation-wide intervention.
Considering the territorial and administrative peculiarities of different Italian regions, a promising development of the standard hub-and-spoke model could be represented by a modifiable design tailored to the country specificities 24. This implies a flexible task division between hubs and spokes depending on their expertise and available infrastructures, allowing to adjust the load of responsibilities on demand.
This modifiable design would also allow to adapt the task-division presented in Figure 1, to address the needs and features of the network. For example, while functions such as patient preparation and pre-selection were assigned to hubs by most of the responders, they well align with the expertise of spoke centres, that could leverage their knowledge and reach on the territory.
Major critical aspects of adopting the hub-and-spoke model have been already described in the literature. While an early liaison with all the stakeholders is important to support an effective coordination, hub and spoke centres should receive appropriate training aimed to guarantee an adequate support to patients and caregivers, and to manage their expectations related to the treatment 18.
Aside from strengthening and re-evaluating national digital systems and infrastructures, which are currently not sufficient to support a seamless information flow between DMD centres in Italy, a challenging hurdle is represented by the culture surrounding the role of the spokes. Over 50% of the survey responders do in fact not contemplate the participation of a centre other than the hub in the patient follow-up. It is therefore important to propound the benefits of adopting a structured task-division at a local level, that would facilitate access to care, avoiding lengthy and costly transportations to the hubs 15.
Notably, the findings of this study suggest a potential positive effect of adopting the hub-and-spoke model on the Italian healthcare inter-regional mobility, that still constitutes a major challenge for public health management and financing 16. In this light, optimising the patient flow would consequently reduce overall costs for the Italian healthcare system 13.
Conclusions
Overall, this study aimed to assess the presence of clinical and organisational infrastructures necessary for the delivery and monitoring of gene therapy in Italy and detected an adequate readiness of the Italian DMD centres of expertise, thus proposing a flexible hub-and-spoke model as a potential organisational design for the gene therapy delivery in the Italian DMD network. The survey, however, highlighted the need for improving digital infrastructure and organisational culture. Only a good synergy between hospitals and local healthcare organisations will allow to take full advantage of the significant readiness of DMD centres in Italy and to provide, by delivering the gene therapy, a great opportunity for patients and their families.
Conflict of interests statement
MP declare to have received in the last 5 years payments or honoraria for lectures, presentations, advisory board, speakers’ bureaus, manuscript writing or educational events from the following commercial sources: Sarepta, PTC, Biogen, Roche, Novartis. EB has received in the last 5 years payments or honoraria for lectures and presentations from the following commercial sources: Roche, Biogen, Novartis, Pfizer, PTC. RD and FG are employees of Pfizer; they contributed to writing and reviewing the manuscript. BP and FS declare to have received in the last 5 years payments or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from the following commercial sources: Allergan, Amgen, Astellas, Eli Lilly, Janssen Cilag, Nestle´ HS, Novartis, Novo Nordisk, Pfizer, Servier, Takeda, Teva; in addition, both received consulting fees from UCB. All other authors declare that they have no competing interests.
Funding
MD, BP, and FS were paid consultants to Pfizer in connection with the development of the manuscript. MP is an employee of the Catholic University of Rome and received funding from Pfizer in connection with the development of this manuscript. EB is an employee of the Bambino Gesù Children’s Hospital IRCCS of Rome and received funding from Pfizer in connection with the development of this manuscript. ER, RDV and FG are Pfizer employees.
Authors contribution
MP recruited the centres of expertise and carried out the data collection together with MD and BP. MD and BP analysed the data regarding the centres’ preparedness. MP, MD, BP, and EB carried out the data quality check and interpretation. MP and MD developed the manuscript. EB, BP, FS, RDV, FG, ER and DD contributed to the development and writing of the manuscript. All authors read and approved the final manuscript.
Ethical consideration
Not applicable.
History
Received: April 2, 2024
Accepted: June 10, 2024
Figures and tables
References
- 1.Mercuri E, Muntoni F. Muscular dystrophies. Lancet. 2013;381;845-860. https://doi.org/10.1016/s0140-6736(12)61897-2. 10.1016/s0140-6736(12)61897-2 [DOI] [PubMed] [Google Scholar]
- 2.Ryder S, Leadley RM, Armstrong N, et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12:79. https://doi.org/10.1186/s13023-017-0631-3. 10.1186/s13023-017-0631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doria M, Annicchiarico G, Rachele C, et al. Raccomandazioni per il riconoscimento precoce delle malattie neuromuscolari (focus sulla Distrofia Muscolare Duchenne). (Federazione Italiana Medici Pediatri (FIMP), 2019). [Google Scholar]
- 4.Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17:347-361. https://doi.org/10.1016/s1474-4422(18)30025-5. 10.1016/s1474-4422(18)30025-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17:251-267. https://doi.org/10.1016/s1474-4422(18)30024-3. 10.1016/s1474-4422(18)30024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018;17:445-455. https://doi.org/10.1016/s1474-4422(18)30026-7. 10.1016/s1474-4422(18)30026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Alfonso G. Distrofia Muscolare di Duchenne https://www.parentproject.it/duchenne-e-becker-la-patologia/> 2021.
- 8.Elangkovan N, Dickson G. Gene Therapy for Duchenne Muscular Dystrophy. J Neuromuscul Dis. 2021;8:S303-s316. https://doi.org/10.3233/jnd-210678. 10.3233/jnd-210678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elrod JK, Fortenberry JL, Jr. The hub-and-spoke organization design: an avenue for serving patients well. BMC Health Serv Res. 2017;17:457. https://doi.org/10.1186/s12913-017-2341-x. 10.1186/s12913-017-2341-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huitema AA, Harkness K, Heckman GA, et al. The Spoke-Hub-and-Node Model of Integrated Heart Failure Care. Can J Cardiol. 2018;34:863-870. https://doi.org/10.1016/j.cjca.2018.04.029. 10.1016/j.cjca.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 11.Srivastava S, Datta V, Garde R, et al. Development of a hub and spoke model for quality improvement in rural and urban healthcare settings in India: a pilot study. BMJ Open Qual 9. 2020. https://doi.org/10.1136/bmjoq-2019-000908. 10.1136/bmjoq-2019-000908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallam CRA, Contreras C. Lean healthcare: scale, scope and sustainability. Int J Health Care Qual Assur. 2018;31:684-696. https://doi.org/10.1108/ijhcqa-02-2017-0023. 10.1108/ijhcqa-02-2017-0023 [DOI] [PubMed] [Google Scholar]
- 13.Calabrò RS, Manuli A, De Cola MC, et al. Innovation technology in neurorehabilitation: introducing a hub and spoke model to avoid patient “migration” in Sicily. J Health Organ Manag. Ahead-of-print 2020. https://doi.org/10.1108/jhom-07-2019-0200. 10.1108/jhom-07-2019-0200 [DOI] [PubMed] [Google Scholar]
- 14.Miele GM, Caton L, Freese TE, et al. Implementation of the hub and spoke model for opioid use disorders in California: Rationale, design and anticipated impact. J Subst Abuse Treat. 2020;108:20-25. https://doi.org/10.1016/j.jsat.2019.07.013. 10.1016/j.jsat.2019.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devarakonda S. Hub and spoke model: making rural healthcare in India affordable, available and accessible. Rural Remote Health. 2016;16:3476. [PubMed] [Google Scholar]
- 16.Brenna E, Spandonaro F. Regional incentives and patient cross-border mobility: evidence from the Italian experience. Int J Health Policy Manag. 2015;4:363-372. https://doi.org/10.15171/ijhpm.2015.65. 10.15171/ijhpm.2015.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Groß SE, Pfaff H, et al. Waiting time, communication quality, and patient satisfaction: An analysis of moderating influences on the relationship between perceived waiting time and the satisfaction of breast cancer patients during their inpatient stay. Patient Educ Couns. 2020;103:819-825. https://doi.org/10.1016/j.pec.2019.11.018. 10.1016/j.pec.2019.11.018 [DOI] [PubMed] [Google Scholar]
- 18.Heslop E, Turner C, Irvin A, et al. Gene therapy in Duchenne muscular dystrophy: Identifying and preparing for the challenges ahead. Neuromuscul Disord. 2021;31:69-78. https://doi.org/10.1016/j.nmd.2020.10.001. 10.1016/j.nmd.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miesbach W, Chowdary P, Coppens M, et al. Delivery of AAV-based gene therapy through haemophilia centres-A need for re-evaluation of infrastructure and comprehensive care: A Joint publication of EAHAD and EHC. Haemophilia. 2021;27:967-973. https://doi.org/10.1111/hae.14420. 10.1111/hae.14420 [DOI] [PubMed] [Google Scholar]
- 20.Gazzetta Ufficiale della Repubblica Italiana (G.U.) 4 giugno 2015, n. 127. Ministero della Salute (2015). https://www.gazzettaufficiale.it/eli/gu/2015/06/04/127/sg/pdf
- 21.Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993. https://doi.org/10.1007/s10900-013-9681-1. 10.1007/s10900-013-9681-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noone D, Coffin D, Pierce GF. Reimbursing the value of gene therapy care in an era of uncertainty. Haemophilia 2021;27:12-18. https://doi.org/10.1111/hae.14218. 10.1111/hae.14218 [DOI] [PubMed] [Google Scholar]
- 23.Stephenson AA, Flanigan KM. Gene editing and modulation for Duchenne muscular dystrophy. Prog Mol Biol Transl Sci. 2021;182:225-255. https://doi.org/10.1016/bs.pmbts.2021.01.029. 10.1016/bs.pmbts.2021.01.029 [DOI] [PubMed] [Google Scholar]
- 24.Miesbach W, Eichler H, Holstein K, et al. Electronic diaries in the management of haemophilia gene therapy: Perspective of an expert group from the German, Austrian and Swiss Society on Thrombosis and Haemostasis (GTH). Haemophilia. 2022;28:264-269. https://doi.org/10.1111/hae.14516. 10.1111/hae.14516 [DOI] [PubMed] [Google Scholar]


