Abstract
Aim: No pharmacotherapeutic treatment has been established for metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH). This trial compared the effects of pemafibrate and omega-3-acid ethyl ester on hepatic function in patients with hypertriglyceridemia complicated by MASLD.
Methods: Patients with hypertriglyceridemia complicated by MASLD were enrolled, randomly assigned to the pemafibrate or omega-3-acid ethyl ester group, and followed for 24 weeks. The primary endpoint was the change in alanine aminotransferase (ALT) from baseline to week 24. The secondary endpoints included other hepatic enzymes, lipid profiles, and hepatic fibrosis biomarkers.
Results: A total of 80 patients were enrolled and randomized. The adjusted mean change in ALT from baseline to week 24 was significantly lower in the pemafibrate group (-19.7±5.9 U/L) than in the omega-3-acid ethyl ester group (6.8±5.5 U/L) (intergroup difference, -26.5 U/L; 95% confidence interval, -42.3 to -10.7 U/L;p=0.001). Pemafibrate significantly improved the levels of other hepatic enzymes (aspartate aminotransferase and gamma-glutamyl transpeptidase), lipid profiles (triglycerides, total cholesterol, high-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol), and hepatic fibrosis biomarkers (Mac-2 binding protein glycan isomer and Fibrosis-4 index). No cases of discontinuation due to adverse drug reactions were identified in either group, and there were no safety concerns.
Conclusions: Pemafibrate is recommended over omega-3-acid ethyl ester for lipid management and MASLD treatment in patients with hypertriglyceridemia complicated by MASLD. The study results may contribute to the development of future treatment strategies for patients with MASLD/MASH.
Keywords: Pemafibrate, Omega-3-acid ethyl ester, Metabolic dysfunction-associated steatotic liver disease, Hepatic function, Hepatic fibrosis
Clinical trial registration: Japan Registry for Clinical Trials (jRCT) (registration no.: jRCTs041200011; https://jrct.niph.go.jp/latest-detail/jRCTs041200011).
Introduction
Steatotic liver disease is a pathological condition characterized by triglyceride (TG) deposition in hepatocytes, leading to liver damage. Hepatic steatosis, defined as the pathological condition in which 5% or more of hepatocytes in liver tissues have lipid droplets, is a common complication of diabetes mellitus and obesity. Steatotic liver disease in conjunction with at least one of five cardiometabolic risk factors (obesity, glucose metabolism disorder, hypertension, hypertriglyceridemia, or hypo-high-density-lipoprotein (HDL) cholesterolemia) is referred to as metabolic dysfunction-associated steatotic liver disease (MASLD) 1) . Steatohepatitis with the cardiometabolic risk factors, in which inflammation and fibrosis occur in the liver, is referred to as metabolic dysfunction-associated steatohepatitis (MASH) 1) . Previous reports have indicated that MASLD is a risk factor for type 2 diabetes mellitus and cardiovascular diseases 2 , 3) , is associated with obesity and metabolic syndrome, and causes abnormal glucose tolerance, insulin resistance, and dyslipidemia 4 , 5) . MASH often results in severe outcomes, including cirrhosis, hepatocellular carcinoma, and death 6 - 8) . The prevalence of MASLD and MASH is increasing globally, with an increase of up to 20–30% and 2–6%, respectively 9 - 12) , in the obese population 6) .
Lifestyle modification through diet and exercise therapy for weight reduction is recommended to manage MASLD and MASH 6 - 8) ; nevertheless, maintaining lifestyle modification for a long period of time is often difficult 13) . Vitamin E and pioglitazone have been recommended as pharmacotherapeutics for MASLD/MASH, and eicosapentaenoic acid ethyl ester/docosahexaenoic acid 14) and sodium-glucose cotransporter 2 inhibitors 15 , 16) have been reported to ameliorate MASLD/MASH. However, these agents have not been approved for MASLD/MASH, and no pharmacotherapeutic treatment for MASLD/MASH has been established.
Pemafibrate, a selective peroxisome proliferator-activated receptor α modulator 17) , has been shown to reduce the TG levels 18) and has been approved for dyslipidemia in Japan. A previous study using an animal model reported that pemafibrate ameliorated MASH-related pathological conditions, such as hepatic steatosis, hepatocyte ballooning, and hepatic fibrosis 19) . Furthermore, clinical studies revealed that pemafibrate improved the hepatic enzyme levels in patients with hypertriglyceridemia 18 , 20) , particularly in patients exhibiting higher aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase (γ-GTP) levels than the upper limit of the normal range 21) . Based on these previous studies, we expected that pemafibrate would exert beneficial effects on MASLD/MASH.
Aim
The present study aimed to compare the effects of pemafibrate and eicosapentaenoic acid ethyl ester/docosahexaenoic acid, which can reportedly improve MASLD/MASH 14) , on hepatic function in patients with hypertriglyceridemia complicated by MASLD.
Methods
The “P rospective, multicenter, O pen-label, R andomized, omega-3-acid ethyl ester-controlled TR ial to A ssess the I mpact of T he pemafibrate on liver function in hypertriglyceridemia patients with non-alcoholic fatty liver disease” (PORTRAIT) study was a multicenter randomized controlled trial (RCT) conducted at three medical institutions in Japan ( Supplementary Table 1 ) . Patient enrollment was conducted from May 2020 to August 2021, and each enrolled patient was followed for 24 weeks. The trial protocol was approved by the Certified Clinical Research Review Board of Aichi Medical University Hospital (approval number: 2019-CR004), which is certified by Japan’s Ministry of Health, Labour and Welfare. After obtaining approval from the certified clinical review board, this study was registered on May 2, 2020, at the Japan Registry for Clinical Trials (jRCT; registration no.: jRCTs041200011), which is a clinical trial registration established by Japan’s Ministry of Health, Labour and Welfare, in accordance with the requirements of the Clinical Trials Act. This study adhered to the ethical principles laid down in the Declaration of Helsinki, Clinical Trials Act, and other relevant legal regulations in Japan. Third-party entities (Soiken, Inc., Osaka, Japan) performed data management, monitoring, audits, and statistical analyses. All authors vouched for the completeness and accuracy of the data, as well as their analyses, and for the fidelity of the trial to the protocol.
Supplementary Table 1. Participating medical institutions.
| Participating medical institution | Responsible investigator | Number of enrolled patients |
|---|---|---|
| Aichi Medical University Hospital | Sumida Yoshio | 39 |
| Ogaki Municipal Hospital | Yasuda Satoshi | 21 |
| Naka Kinen Clinic | Osonoi Takeshi | 20 |
Patients with hypertriglyceridemia complicated by MASLD were enrolled in this study after obtaining written informed consent. A complete list of the inclusion and exclusion criteria is provided in Supplementary Table 2 . The enrolled patients were randomly assigned into either the pemafibrate group or omega-3-acid ethyl ester group, with an allocation ratio of approximately 1:1. Depending on the ALT and TG levels at the consenting and medical institutions (i.e., allocation factors), random allocation was performed by a computer program located at the registration center using a minimization method. Pemafibrate (0.2 mg/day) was administered for 24 weeks in the pemafibrate group, whereas omega-3-acid ethyl ester (2 g/day) was administered for 24 weeks in the omega-3-acid ethyl ester group. The patients were followed up at baseline prior to the initiation of treatment with the study agents and at 4, 12, and 24 weeks after treatment initiation. The observation schedules and items are detailed in Supplementary Table 3 .
Supplementary Table 2. Eligibility criteria.
| Inclusion criteria | Patients who met all of the following criteria were included in this study: |
|
1. Patients with fasting TG levels ≥ 150 mg/dL and <500 mg/dL within 12 weeks prior to the provision of their consent | |
| 2. Patients diagnosed with steatosis by abdominal ultrasound within 6 months prior to the provision of their consent | |
|
3. Male patients with ALT levels >40 IU/L or female patients with ALT levels >30 IU/L within 12 weeks prior to the provision of their consent | |
| 4. Male and female patients aged ≥ 20 years | |
| 5. Patients who provided written informed consent | |
| Exclusion criteria | Patients who met any of the following criteria were excluded from this study: |
| 1. Patients with ALT levels ≥ 250 IU/L | |
| 2. Patients who were deemed to be excessive drinkers (ethanol intake of ≥ 210 g/week for male patients or ≥ 140 g/week for female patients) | |
| 3. Patients who used fibrates within 12 weeks prior to the provision of their consent | |
| 4. Patients who used omega-3 fatty acid agents or relevant supplements within 6 months prior to the provision of their consent | |
| 5. Patients who used drugs prohibited in this study (e.g., pioglitazone, vitamin E, SGLT2 inhibitors, GLP-1 receptor antagonists, study agent, control agent, or any drug/supplement with the same indications) within 12 weeks prior to the provision of their consent | |
| 6. Patients with BMI of <22 kg/m2 | |
| 7. Patients showing unstable glycemic control (HbA1c of 8% or higher within 12 weeks prior to the provision of their consent) | |
| 8. Patients with cirrhosis | |
| 9. Patients with plasma creatinine levels ≥ 1.5 mg/dL | |
| 10. Patients with cholelithiasis or biliary obstruction | |
| 11. Patients who had chronic liver diseases with any of the following steatosis except for NAFLD: | |
| (i) Viral hepatitis (hepatitis B, hepatitis C) | |
| (ii) Autoimmune hepatitis | |
| (iii) Primary biliary cholangitis | |
| (iv) Primary sclerosing cholangitis | |
| (v) Drug-induced liver injury | |
| (vi) Endocrine/metabolic hepatitis (hyperthyroidism, Wilson disease, hemochromatosis, or alpha-1 antitrypsin deficiency) | |
| 12. Patients who had a malignant tumor or who were expected to experience recurrence of a malignant tumor after amelioration | |
| 13. Patients who underwent blood collection or blood donation of ≥ 200 mL within 12 weeks prior to the provision of their consent | |
| 14. Patients with a history of severe drug hypersensitivity (e.g., anaphylactic shock) | |
| 15. Patients who were breastfeeding, pregnant, possibly pregnant, or planning to be pregnant | |
| 16. Patients who were participating in other interventional trials | |
| 17. Patients who had a history of hypersensitivity to or had contraindications to the study agent or control agent in this study | |
| 18. Patients who required a legal representative for the provision of consent | |
| 19. Patients who were considered to be unsuitable for study participation by the investigators |
TG, triglyceride; ALT, alanine aminotransferase; SGLT2, sodium-glucose cotransporter 2; GLP-1, glucagon-
like peptide 1; BMI, body mass index; NAFLD, non-alcoholic fatty liver disease.
Supplementary Table 3. Observation schedule and items.
| Consenting | Week 0 (baseline) | Week 4±1 week | Week 12±2 weeks | Week 24±2 weeks | |
|---|---|---|---|---|---|
| Eligibility information | 〇 | ||||
| Eligibility criteria, allocation factors | 〇 | ||||
| Characteristics of patients | 〇 | ||||
| Physical examinations | 〇 | 〇 | |||
| Medication data | 〇 | 〇 | 〇 | 〇 | |
| General blood tests | 〇 | 〇 | 〇 | 〇 | |
| Special blood tests | 〇 | 〇 | 〇 | 〇 | |
| Chest tomography | △ | △ | |||
| Magnetic resonance imaging | △ | △ | |||
| Virtual touch quantification | △ | △ | |||
| Adverse event | ← 〇 → | ||||
〇, mandatory; △, optional.
For this study, the primary endpoint was the change in ALT levels from baseline to week 24. The secondary endpoints included the lipid profiles (TG, total cholesterol [TC], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and non-HDL-C), hepatic fibrosis biomarkers (AST, γ-GTP, type IV collagen 7S, Mac-2 binding protein glycosylation isomer [M2BPGi], and Fibrosis-4 [FIB-4] index), inflammation biomarker (high-sensitivity C-reactive protein), and glucose metabolism biomarkers (hemoglobin A1c [HbA1c], fasting plasma glucose, and insulin). A full list of the endpoints is provided in Supplementary Table 4 .
Supplementary Table 4. Primary and secondary endpoints.
| Primary endpoint | Change in the ALT levels from baseline to week 24 |
|---|---|
| Secondary endpoints | (i) Percent change in the ALT levels from baseline to week 24 |
| (ii) Change and percent change in the ALT levels from baseline to week 4 or week 12 | |
|
(iii) Change and percet change in the following items from baseline to each observation point (items marked with * were evaluated using both change and percent change) | |
| Observation items | |
| - Body weight, BMI, waist circumference | |
| - Blood pressure (diastolic blood pressure/systolic blood pressure, office blood pressure in the sitting position) | |
| - Lipid biomarkers (TG, TC, LDL-C, HDL-C, non-HDL-C) | |
| - Hepatic biomarkers (AST*, γ-GTP, type IV collagen 7S, M2BPGi, FIB- 4 index*) | |
| - Inflammation biomarker (hsCRP) | |
| - Glucose metabolism biomarkers (HbA1c, fasting plasma glucose, insulin) | |
| - CT (L/S ratio) (optional) | |
| - Liver stiffness (virtual touch quantification) (optional) | |
| - MRI (liver fat content, liver stiffness) (optional) | |
| - Other general biochemical tests (platelet count, total protein, albumin, A/G ratio, urea nitrogen, uric acid, creatinine, eGFR, total bilirubin, lactic acid, pyruvic acid, direct bilirubin, LDH, ALP, ChE) | |
| (iv) Frequency of adverse events or diseases |
ALT, alanine aminotransferase; BMI, body mass index; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL- C, high-density lipoprotein cholesterol; AST, aspartate aminotransferase; γ-GTP, gamma-glutamyl transpeptidase; M2BPGi, Mac-2 binding protein glycosylation isomer; FIB-4 index, Fibrosis-4 index; hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; ALP, alkaline phosphatase, ChE, cholinesterase.
The sample size was determined to provide adequate power for the intergroup comparison of the change in ALT levels from baseline to week 24. With reference to the data of previous studies 21 , 22) , the change in ALT levels from baseline with standard deviations was assumed to be -18.5±23.0 IU/L in the pemafibrate group and -3.0±23.0 IU/L in the omega-3-acid ethyl ester group. For a two-sided test with a statistical power of 80%, the minimum sample size required to achieve a significance level of 0.05 was determined to be 36 patients per group. Assuming a dropout rate of approximately 10%, the planned sample size was set at 80 patients (i.e., 40 patients per group).
The primary and secondary endpoints were analyzed using data from the “full analysis set” population, which included all enrolled study patients who were subsequently randomized to one of the study treatments but excluded those who seriously violated the protocol. Sensitivity analysis was performed using the “per-protocol” population, excluding patients who violated the eligibility criteria, received prohibited agents and treatments, or exhibited poor medication adherence. All p-values were two-sided, and statistical significance was set at a p-value of <0.05. The adjusted mean change in each continuous variable was estimated using analysis of covariance (ANCOVA), with the treatment groups as the fixed effect and with the baseline ALT and TG levels and medical institutions as the covariates. All statistical analyses were performed by a third-party entity (Soiken Inc.).
Results
Patients’ Background
A total of 199 patients were evaluated for eligibility. Of these patients, 80 (pemafibrate group, n=39; omega-3-acid ethyl ester group, n=41) were enrolled and randomized in this study ( Fig.1 ) . The baseline patient characteristics were well balanced between the groups ( Table 1 ) .
Fig.1.
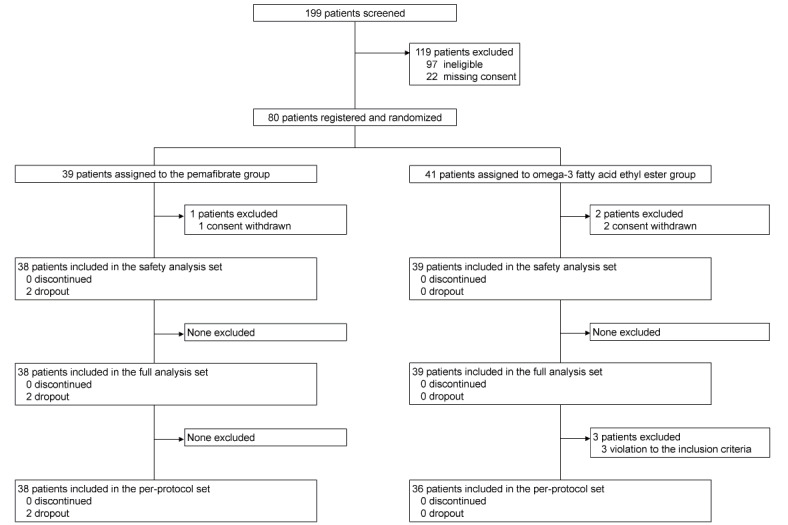
Study flow chart showing patient enrollment, allocation, and analysis
Table 1. Backgrounds of patients.
|
Pemafibrate group (n= 38) |
Omega-3-acid ethyl ester group (n= 39) |
p value | |||
|---|---|---|---|---|---|
| n | n | ||||
| Age (years) | 38 | 51.8±12.8 | 39 | 53.5±14.0 | 0.57 |
| Female sex [n (%)] | 38 | 14 (36.8) | 39 | 14 (35.9) | 0.93 |
| Height (cm) | 38 | 164.8±9.3 | 38 | 164.6±8.6 | 0.92 |
| Weight (kg) | 38 | 77.8±14.2 | 38 | 76.4±13.2 | 0.68 |
| Waist circumference (cm) | 20 | 99.2±11.7 | 21 | 95.2±9.1 | 0.23 |
| Systolic blood pressure (mmHg) | 30 | 134.8±16.9 | 28 | 136.1±11.3 | 0.73 |
| Diastolic blood pressure (mmHg) | 30 | 83.5±12.7 | 28 | 85.2±10.7 | 0.57 |
| Comorbidity | |||||
| Diabetes mellitus | 38 | 15 (39.5) | 39 | 18 (46.2) | 0.55 |
| Hypertension | 38 | 16 (42.1) | 39 | 25 (64.1) | 0.05 |
| Hepatic disease | 38 | 0 (0.0) | 39 | 0 (0.0) | - |
| Medication | |||||
| Antidiabetic agent | 38 | 9 (23.7) | 39 | 12 (30.8) | 0.49 |
| Biguanide | 38 | 8 (21.1) | 39 | 9 (23.1) | 0.83 |
| Glinide | 38 | 1 (2.6) | 39 | 0 (0.0) | 0.49* |
| α- glucosidase inhibitor | 38 | 4 (10.5) | 39 | 5 (12.8) | 1.00* |
| DPP-4 inhibitor | 38 | 1 (2.6) | 39 | 2 (5.1) | 1.00* |
| Antihypertensive agent | 38 | 12 (31.6) | 39 | 17 (43.6) | 0.28 |
| Angiotensin receptor blocker | 38 | 10 (26.3) | 38 | 13 (34.2) | 0.45 |
| Diuretic agent | 38 | 2 (5.3) | 38 | 4 (10.5) | 0.67* |
| Calcium antagonist | 38 | 7 (18.4) | 38 | 8 (21.1) | 0.77 |
| Hypolipidemic agent | 38 | 13 (34.2) | 39 | 12 (30.8) | 0.75 |
| Statin | 38 | 10 (26.3) | 39 | 11 (28.2) | 0.85 |
| Ezetimibe | 38 | 3 (7.9) | 39 | 2 (5.1) | 0.67* |
Data are presented as mean±standard deviation for continuous variables and as n (%) for categorical variables.
Chi-squared and two-sample t-tests were performed for categorical and continuous variables, respectively.
*Intergroup comparison was conducted using Fisher’s exact test, as it did not meet the requirement of the chi-squared test.
Hepatic Enzymes
The adjusted mean change in ALT levels from baseline to week 24 (i.e., the primary endpoint) was significantly lower in the pemafibrate group (-19.7±5.9 U/L) than in the omega-3-acid ethyl ester group (6.8±5.5 U/L) (intergroup difference, -26.5 U/L; 95% confidence interval [CI], -42.3 to -10.7 U/L; p=0.001) ( Fig.2 ) . Additionally, the adjusted mean change in ALT levels was significantly lower in the pemafibrate group (-18.9±3.3 U/L and -20.9±4.4 U/L, respectively) than in the omega-3-acid ethyl ester group (0.8±3.2 U/L and 6.4±4.1 U/L, respectively) at week 4 (intergroup difference, -19.7 U/L; 95% CI, -28.7 to -10.8 U/L; p<0.001) and week 12 (intergroup difference, -27.3 U/L; 95% CI, -39.1 to -15.6 U/L; p<0.001).
Fig.2. Changes in hepatic enzymes.
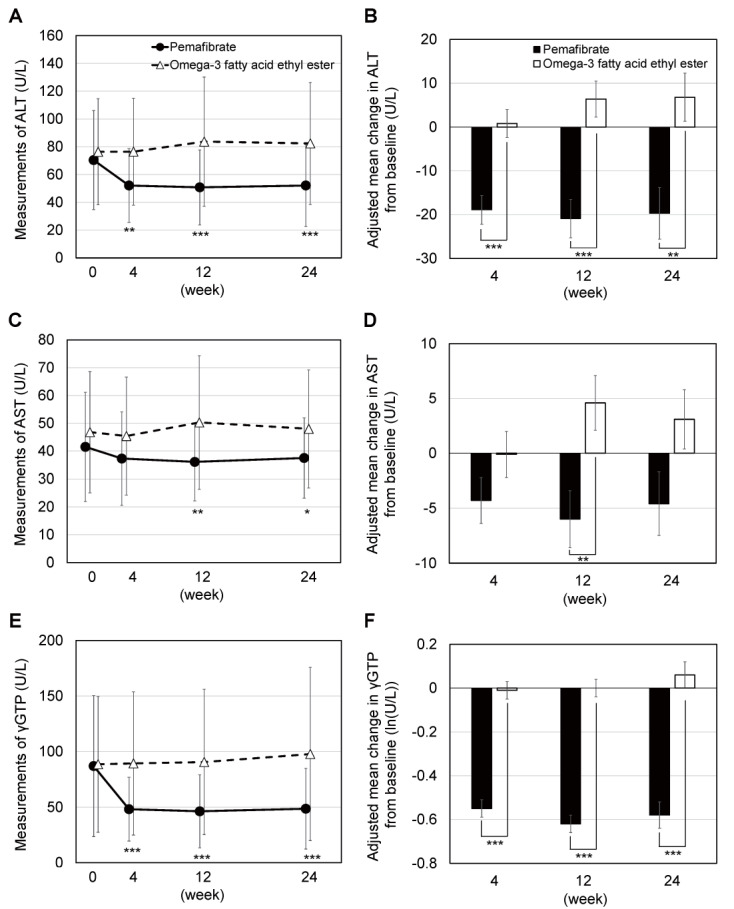
A two-sample t-test was performed for the intergroup comparison of measurements. Because the γ-GTP measurements were not distributed normally, a two-sample t-test was performed using log-transformed values. The adjusted mean change in each continuous variable was estimated using analysis of covariance (ANCOVA), with the treatment groups as the fixed effect and with the baseline ALT and TG levels and medical institutions as the covariates. *, **, and *** represent p<0.05, p<0.01, and p<0.001, respectively. ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GTP, gamma-glutamyl transpeptidase; TG, triglyceride.
As for other hepatic enzymes, the adjusted mean change in AST levels was significantly lower at week 12 in the pemafibrate group (-6.0±2.6 U/L) than in the omega-3-acid ethyl ester group (4.6±2.5 U/L) (intergroup difference, -10.6 U/L; 95% CI, -17.7 to -3.6 U/L; p=0.004). The adjusted mean change in AST levels was also numerically lower in the pemafibrate group than in the omega-3-acid ethyl ester group at week 4 (p=0.15) and week 24 (p=0.05); however, the difference was not statistically significant. The adjusted mean change in γ-GTP levels was significantly lower in the pemafibrate group than in the omega-3-acid ethyl ester group at weeks 4, 12, and 24 (p<0.001 for all).
Lipid Profiles
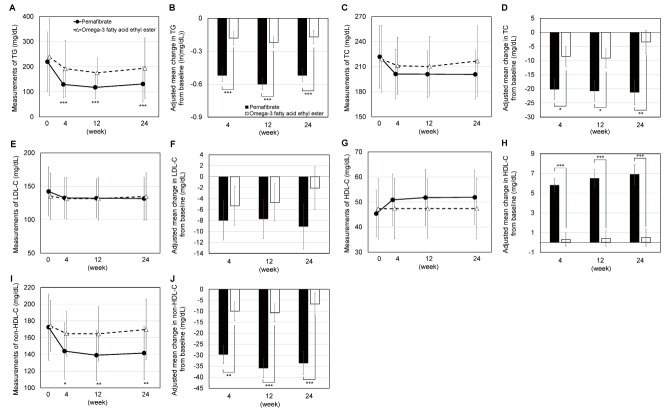
The adjusted mean changes in TG (p<0.001 for all), TC (p=0.021, 0.021, and 0.004, respectively), and non-HDL-C (p=0.001, <0.001, and <0.001, respectively) levels were significantly lower in the pemafibrate group than in the omega-3-acid ethyl ester group at weeks 4, 12, and 24. Conversely, the adjusted mean change in HDL-C levels (p<0.001 for all) was significantly higher at weeks 4, 12, and 24 ( Fig.3 ) . The adjusted mean change in LDL-C levels was lower in the pemafibrate group than in the omega-3-acid ethyl ester group at weeks 4, 12, and 24, albeit without statistically significant difference (p=0.59, 0.53, and 0.21, respectively).
Fig.3. Changes in lipid profiles.
A two-sample t-test was performed for the intergroup comparison of measurements. Because the TG measurements were not distributed normally, a two-sample t-test was performed using log-transformed values. The adjusted mean change in each continuous variable was estimated using analysis of covariance (ANCOVA), with the treatment groups as the fixed effect and with the baseline ALT and TG levels and medical institutions as the covariates. *, **, and *** represent p<0.05, p<0.01, and p<0.001, respectively. TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Hepatic Fibrosis Biomarkers
The adjusted mean change in M2BPGi was significantly lower in the pemafibrate group than in the omega-3-acid ethyl ester group at weeks 4, 12, and 24 (p<0.001 for all) ( Table 2 ) . M2BPGi significantly decreased in the pemafibrate group from baseline to week 4 (change from baseline -0.18±0.33, p=0.002), 12 (change from baseline -0.15±0.32, p=0.009), and 24 (change from baseline: -0.15±0.43, p=0.038), but it did not significantly decreased or rather tended to increase in the omega-3-acid ethyl ester group (week 4: change from baseline 0.00±0.13, p=0.96, week 12: change from baseline 0.03±0.19, p=0.27, week 24: change from baseline 0.04±0.14, p=0.05). Similarly, the adjusted mean change in the FIB-4 index was significantly lower in the pemafibrate group than in the omega-3-acid ethyl ester group at week 12 (p=0.014). The FIB-4 index significantly decreased in the pemafibrate group from baseline to week 12 (change from baseline -0.10±0.26, p=0.024), but not in the omega-3-acid ethyl ester group (change from baseline 0.05±0.31, p=0.33). No other intragroup significant change was observed at week 4 and 24 both in the pemafibrate group and the omega-3-acid ethyl ester group. No significant intergroup difference in the adjusted mean change in type IV collagen 7S was observed, however, it significantly decreased in the pemafibrate group from baseline to week 24 (change from baseline -0.46±0.75, p<0.001), but not in the omega-3-acid ethyl ester group (change from baseline -0.19±0.96, p=0.22). No other intragroup significant change was observed at week 4 and 12 both in the pemafibrate group and the omega-3-acid ethyl ester group.
Table 2. Changes in hepatic fibrosis biomarkers.
| Week | Pemafibrate group | Omega-3-acid ethyl ester group | Intergroup difference in adjusted mean change (95% CI) | p value | |||
|---|---|---|---|---|---|---|---|
| Measurement (n) | Adjusted mean change from baseline (SE) | Measurement (n) | Adjusted mean change from baseline (SE) | ||||
| Type IV collagen 7S (ng/mL) | 0 | 4.03±0.67 (37) | 4.23±0.96 (38) | ||||
| 4 | 4.05±0.92 (38) | 0.03 (0.12) | 4.40±1.12 (39) | 0.17 (0.11) | -0.14 (-0.46, 0.18) | 0.39 | |
| 12 | 3.81±0.85 (35) | -0.27 (0.15) | 4.38±1.27 (39) | 0.05 (0.14) | -0.33 (-0.73, 0.07) | 0.10 | |
| 24 | 3.56±0.75 (36) | -0.49 (0.14) | 4.06±1.19 (39) | -0.17 (0.13) | -0.31 (-0.69, 0.06) | 0.10 | |
| M2BPGi | 0 | 0.80±0.84 (38) | 0.74±0.39 (39) | ||||
| 4 | 0.62±0.57 (38) | -0.16 (0.03) | 0.74±0.39 (39) | 0.00 (0.03) | -0.16 (-0.24, -0.08) | <0.001 | |
| 12 | 0.63±0.59 (35) | -0.14 (0.03) | 0.77±0.45 (39) | 0.03 (0.03) | -0.17 (-0.26, -0.08) | <0.001 | |
| 24 | 0.65±0.50 (36) | -0.13 (0.03) | 0.78±0.43 (39) | 0.04 (0.03) | -0.18 (-0.27, -0.08) | <0.001 | |
| FIB-4 index | 0 | 1.25±0.81 (38) | 1.32±0.75 (39) | ||||
| 4 | 1.20±0.75 (38) | -0.06 (0.03) | 1.27±0.70 (39) | -0.05 (0.03) | -0.01 (-0.09, 0.07) | 0.78 | |
| 12 | 1.13±0.71 (36) | -0.10 (0.05) | 1.37±0.79 (39) | 0.06 (0.05) | -0.16 (-0.29, -0.03) | 0.014 | |
| 24 | 1.20±0.73 (36) | -0.04 (0.05) | 1.35±0.74 (39) | 0.05 (0.05) | -0.09 (-0.22, 0.05) | 0.21 | |
Data are presented as mean±standard deviation (n) for measurements or as mean (standard error [SE]) for adjusted mean changes from baseline. The adjusted mean change in each continuous variable was estimated using analysis of covariance (ANCOVA), with the treatment groups as the fixed effect and with the baseline alanine aminotransferase and triglyceride levels and medical institutions as the covariates.
95% CI, 95% confidence interval; M2BPGi, Mac-2 binding protein glycosylation isomer; FIB-4 index, Fibrosis-4 index.
Glucose Metabolism Biomarkers
The adjusted mean changes in glucose metabolism biomarkers (i.e., HbA1c, fasting plasma glucose, and insulin) did not significantly differ between the groups, except for fasting plasma glucose at week 4 (p=0.045) ( Table 3 ) .
Table 3. Changes in glucose metabolism biomarkers.
| Week | Pemafibrate group | Omega-3-acid ethyl ester group | Intergroup difference in adjusted mean change (95% CI) | p value | |||
|---|---|---|---|---|---|---|---|
| Measurement (n) | Adjusted mean change from baseline (SE) | Measurement (n) | Adjusted mean change from baseline (SE) | ||||
| HbA1c (%) | 0 | 6.2±0.6 (38) | 6.3±0.7 (39) | ||||
| 4 | 6.2±0.6 (38) | 0.0 (0.0) | 6.4±0.7 (39) | 0.0 (0.0) | 0.0 (-0.1, 0.0) | 0.42 | |
| 12 | 6.3±0.7 (35) | 0.1 (0.0) | 6.4±0.9 (39) | 0.1 (0.0) | 0.0 (-0.2, 0.1) | 0.60 | |
| 24 | 6.4±0.6 (35) | 0.1 (0.1) | 6.6±1.4 (39) | 0.2 (0.1) | -0.1 (-0.4, 0.3) | 0.60 | |
| Plasma glucose (mg/dL) | 0 | 119.5±31.8 (38) | 116.2±17.5 (39) | ||||
| 4 | 113.2±20.2 (37) | -5.3 (2.8) | 120.0±21.5 (39) | 2.5 (2.7) | -7.8 (-15.4, -0.2) | 0.045 | |
| 12 | 113.1±16.2 (36) | -5.9 (3.2) | 116.5±26.3 (38) | -1.6 (3.0) | -4.3 (-12.9, 4.3) | 0.32 | |
| 24 | 118.7±20.4 (36) | -1.3 (4.5) | 126.0±38.3 (39) | 7.9 (4.2) | -9.2 (-21.3, 3.0) | 0.14 | |
| Insulin (μIU/mL) | 0 | 30.3±25.2 (33) | 27.2±17.3 (34) | ||||
| 4 | 19.3±10.8 (26) | 25.1±15.7 (29) | |||||
| 12 | 17.7±6.8 (32) | 19.9±13.4 (36) | |||||
| 24 | 22.1±16.6 (32) | 23.0±14.5 (36) | |||||
|
Insulin (log-transformed) [ln(μIU/mL)] |
0 | 3.17±0.68 (33) | 3.12±0.62 (34) | ||||
| 4 | 2.84±0.46 (26) | -0.34 (0.14) | 3.02±0.68 (29) | -0.14 (0.12) | -0.19 (-0.49, 0.11) | 0.21 | |
| 12 | 2.79±0.44 (32) | -0.30 (0.09) | 2.81±0.60 (36) | -0.32 (0.08) | 0.03 (-0.21, 0.27) | 0.82 | |
| 24 | 2.93±0.54 (32) | -0.16 (0.09) | 2.96±0.60 (36) | -0.13 (0.08) | -0.03 (-0.27, 0.21) | 0.82 | |
Data are presented as mean±standard deviation (n) for measurements or as mean (standard error [SE]) for adjusted mean changes from baseline. The adjusted mean change in each continuous variable was estimated using analysis of covariance (ANCOVA), with the treatment groups as the fixed effect and with the baseline alanine aminotransferase and triglyceride levels and medical institutions as the covariates.
HbA1c, hemoglobin A1c; 95% CI, 95% confidence interval.
Body Composition
The adjusted mean changes in body weight, body mass index (BMI), and waist circumference did not significantly differ between the groups ( Table 4 ) .
Table 4. Changes in body composition.
| Week | Pemafibrate group | Omega-3-acid ethyl ester group | Intergroup difference in adjusted mean change (95% CI) | p value | |||
|---|---|---|---|---|---|---|---|
| Measurement (n) | Adjusted mean change from baseline (SE) | Measurement (n) | Adjusted mean change from baseline (SE) | ||||
| Body weight (kg) | 0 | 77.8±14.2 (38) | 76.4±13.2 (38) | ||||
| 24 | 78.9±15.4 (35) | 0.6 (0.4) | 76.4±13.2 (38) | 0.0 (0.4) | 0.6 (-0.6, 1.7) | 0.30 | |
| BMI (kg/m2) | 0 | 28.5±4.1 (38) | 28.1±3.7 (38) | ||||
| 24 | 29.0±4.5 (35) | 0.2 (0.2) | 28.1±3.8 (38) | 0.0 (0.1) | 0.2 (-0.2, 0.6) | 0.39 | |
| Waist circumference (cm) | 0 | 99.2±11.7 (20) | 95.2±9.1 (21) | ||||
| 24 | 100.0±12.1 (16) | 0.8 (1.3) | 96.1±7.1 (17) | 1.0 (1.6) | -0.3 (-2.9, 2.4) | 0.84 | |
Data are presented as mean±standard deviation (n) for measurements or as mean (standard error [SE]) for adjusted mean changes from baseline. The adjusted mean change in each continuous variable was estimated using analysis of covariance (ANCOVA), with the treatment groups as the fixed effect and with the baseline alanine aminotransferase and triglyceride levels and medical institutions as the covariates.
BMI, body mass index; 95% CI, 95% confidence interval
Inflammation Biomarker
As a biomarker for inflammation, high sensitivity C-reactive protein (hsCRP) was assessed. Since the values did not distribute normally, ANCOVA was performed using log-transformed values. Although no significant difference in the adjusted mean changes was observed ( Table 5 ) , it significantly decreased in the pemafibrate group from baseline to week 12 (change in the log-transformed value from baseline -0.26±0.75, p=0.046), but not in the omega-3-acid ethyl ester group (change in the log-transformed value from baseline 0.11±0.84, p=0.44). No other intragroup significant change was observed at week 4 and 24 both in the pemafibrate group and the omega-3-acid ethyl ester group.
Table 5. Changes in inflammatory biomarker.
| Week | Pemafibrate group | Omega-3-acid ethyl ester group |
Intergroup difference in adjusted mean change (95% CI) (log- transformed) |
p value | |||
|---|---|---|---|---|---|---|---|
| Measurement (n) | Adjusted mean change from baseline (SE) (log- transformed) | Measurement (n) | Adjusted mean change from baseline (SE) (log-transformed) | ||||
| hsCRP (ng/mL) | 0 | 1731.4±1515.3 (38) | 1967.6±1949.5 (39) | ||||
| 4 | 1926.3±3667.7 (38) | -0.15 (0.15) | 2823.9±4514.5 (39) | 0.18 (0.15) | -0.33 (-0.75, 0.10) | 0.13 | |
| 12 | 1355.4±1276.1 (35) | -0.23 (0.13) | 3128.3±5392.1 (39) | 0.12 (0.12) | -0.35 (-0.71, 0.01) | 0.06 | |
| 24 | 3705.3±8547.4 (36) | 0.10 (0.14) | 1827.0±2041.8 (39) | -0.13 (0.13) | 0.23 (-0.14, 0.61) | 0.22 | |
Data are presented as mean±standard deviation (n) for measurements or as mean (standard error [SE]) for adjusted mean changes from baseline. The adjusted mean change for log-transformed value was estimated using analysis of covariance (ANCOVA), with the treatment groups as the fixed effect and with the baseline alanine aminotransferase and triglyceride levels and medical institutions as the covariates.
hsCRP, high-sensitivity C-reactive protein; 95% CI, 95% confidence interval
Safety
The adverse events that occurred during the study period are summarized in Table 6 . No deaths were reported in either group, and the frequency of non-serious/serious adverse events did not differ between the groups. The most frequent adverse event was an increase in blood pyruvic acid levels, which occurred in 5 patients (13.2%) in the pemafibrate group and 8 patients (20.5%) in the omega-3-acid ethyl ester group.
Table 6. Adverse events.
| Pemafibrate group | Omega-3-acid ethyl ester group | |||
|---|---|---|---|---|
| Number of patients in the safety analysis set | 38 | 39 | ||
| Death | 0 (0.0) | 0 (0.0) | ||
| Any adverse event | 9 (23.7) | 12 (30.8) | ||
| Any serious adverse event | 1 (2.6) | 0 (0.0) | ||
| Name of adverse event | Total | Serious | Total | Serious |
| Increase in blood pyruvic acid levels | 5 (13.2) | 0 (0.0) | 8 (20.5) | 0 (0.0) |
| Hyperlactacidemia | 2 (5.3) | 0 (0.0) | 2 (5.1) | 0 (0.0) |
| Diverticulitis | 1 (2.6) | 1 (2.6) | 0 (0.0) | 0 (0.0) |
| Hypoproteinemia | 1 (2.6) | 0 (0.0) | 1 (2.6) | 0 (0.0) |
| Decrease in blood ALP levels | 1 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Malaise | 1 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pruritus | 1 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Retinal tear | 1 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Folliculitis | 0 (0.0) | 0 (0.0) | 2 (5.1) | 0 (0.0) |
| Dizziness | 0 (0.0) | 0 (0.0) | 1 (2.6) | 0 (0.0) |
| Epistaxis | 0 (0.0) | 0 (0.0) | 1 (2.6) | 0 (0.0) |
| Gastroesophageal reflux disease | 0 (0.0) | 0 (0.0) | 1 (2.6) | 0 (0.0) |
| Hyperglycemia | 0 (0.0) | 0 (0.0) | 1 (2.6) | 0 (0.0) |
| Increase in blood ALP levels | 0 (0.0) | 0 (0.0) | 1 (2.6) | 0 (0.0) |
| Migraine | 0 (0.0) | 0 (0.0) | 1 (2.6) | 0 (0.0) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (2.6) | 0 (0.0) |
Data are represented as n (%). ALP, alkaline phosphatase.
Discussion
The present study revealed that, compared with omega-3-acid ethyl ester, pemafibrate significantly improved the hepatic enzymes (ALT, AST, and γ-GTP), lipid profiles (TG, TC, HDL-C, and non-HDL-C), and hepatic fibrosis biomarkers (M2BPGi and FIB-4 index) despite the short intervention period of 24 weeks. These results suggest that pemafibrate not only shows superior efficacy against dyslipidemia but also has multi-aspect beneficial effects in patients with hypertriglyceridemia complicated by MASLD. These results are consistent with the findings of a recent RCT investigating pemafibrate versus placebo (PROMINENT trial), which reported an improvement in TG, very-low-density lipoprotein, remnant cholesterol, and apolipoprotein C-III levels with pemafibrate 23) . Another RCT on pemafibrate versus placebo showed that pemafibrate significantly reduced liver stiffness, suggesting the amelioration of hepatic fibrosis 24) . A recent retrospective study also reported that pemafibrate reduced the shear wave velocity (liver stiffness) 25) . Importantly, this study showed a significant improvement in lipid profiles and hepatic fibrosis biomarkers with pemafibrate, as compared with omega-3-acid ethyl ester, which has been reported to improve hepatic steatosis 14) . Recently, an RCT of pemafibrate versus omega-3-acid ethyl ester (PROUD48 study) reported that pemafibrate significantly reduced the lipid profiles and the levels of fasting apoB-48 (an indicator of postprandial hypertriglyceridemia) and other hepatic enzymes in patients with dyslipidemia receiving statin treatment 26) . Given that only approximately one-fourth of the enrolled patients in this study (10 cases (26.3%) in the pemafibrate group and 11 cases (28.2%) in the omega-3-acid ethyl ester group) used statins at baseline, the results of this study and the PROUD48 study may complementarily support the evidence regarding the efficacy of pemafibrate, as compared with that of omega-3-acid ethyl ester. While, although M2BPGi was significantly improved in the pemafibrate group, type IV collagen 7S did not show significant improvement in the pemafibrate group compared with the omega-3-acid ethyl ester group. One reason of this discrepancy might be that M2BPGi influences not only the hepatic fibrosis but also inflammation 27 , 28) . The fact that hsCRP significantly decreased in the pemafibrate group might support the speculation.
The latest treatment guidelines for MASLD/MASH in Japan recommend pioglitazone for patients with MASLD/MASH complicated by type 2 diabetes mellitus 29) . However, pioglitazone has not been approved for MASLD/MASH and no pharmacotherapeutic treatment has been established for MASLD/MASH. In contrast, recent practice guidance for MASLD issued by the American Association for the Study of Liver Diseases (AASLD) recommends fibrates or a combination therapy with fibrate and omega-3 fatty acids or icosapent ethyl for patients with MASLD and severe hypertriglyceridemia 30) . The results of this study suggest that pemafibrate has beneficial effects in patients with hypertriglyceridemia complicated by MASLD; hence, the use of pemafibrate over omega-3-acid ethyl ester for lipid management and MASLD treatment may be recommended for patients with hypertriglyceridemia complicated by MASLD. Our study results may contribute to the development of treatment strategies for MASLD/MASH.
With respect to safety, pemafibrate did not affect glucose metabolism, as the changes in HbA1c, fasting blood glucose, and insulin levels did not significantly differ between the pemafibrate and omega-3-acid ethyl ester groups. No specific adverse events were observed after pemafibrate administration. These results confirm the safety of pemafibrate in patients with hypertriglyceridemia complicated by MASLD.
This study has several limitations. First, this was an open-label trial that lacked blinding of both patients and physicians, which might have resulted in some bias. Nonetheless, this study employed objectively measurable endpoints such as ALT, AST, and γ-GTP; thus, we believe that the open-label study design minimally biased the study results. Second, this study used surrogate endpoints, such as hepatic enzymes (ALT, AST, and γ-GTP), lipid profiles (TG, TC, HDL-C, and non-HDL-C), and hepatic fibrosis biomarkers (M2BPGi and FIB-4 index), and did not investigate hard endpoints, such as the onset of cardiovascular diseases or death. Additionally, histological examination or hepatic elastography was not conducted in this study. However, considering that the AASLD practice guidance has indicated the correlation between ALT and FIB-4 index improvements and histological fibrosis reduction 30 , 31) , the significant improvement in these surrogate endpoints with pemafibrate, as compared with omega-3-acid ethyl ester, might reflect the beneficial effects of pemafibrate in patients with hypertriglyceridemia complicated by MASLD. Third, this study was conducted only at medical institutions in Japan. Therefore, the generalizability of our study results to other countries or patients of other ethnicities remains unknown, and further international studies are required. Fourth, this study enrolled a relatively small number of patients (n=80) and employed a relatively short intervention period (24 weeks); however, it did not investigate the long-term efficacy of pemafibrate in ameliorating hepatic fibrosis and suppressing cancer and cardiovascular diseases. The PROMINENT study, which enrolled patients with type 2 diabetes mellitus who had a history of cardiovascular disease or were at a high risk for cardiovascular diseases, reported that pemafibrate did not suppress the onset of cardiovascular diseases 23) . In comparison, more than half of the patients in this study did not have diabetes mellitus, and only one patient had a history of cardiovascular disease. Future studies should investigate the long-term efficacy of pemafibrate in ameliorating hepatic fibrosis or preventing cardiovascular diseases in patients with hypertriglyceridemia without diabetes mellitus or a history of cardiovascular disease. In addition, while the PROMINENT study enrolled patients 80% of which were treated with high-intensity statins, majority of enrolled patients in this PORTRAIT study did not use statins. Since the numbers of patients treated with statins were small in this study (10 cases (26.3%) in the pemafibrate group and 11 cases (28.2%) in the omega-3-acid ethyl ester group), further large-scale clinical trials are required to assess the effects of statins in combination with pemafibrate or omega-3-acid ethyl ester.
Conclusions
The results of this study indicated that, compared with omega-3-acid ethyl ester, pemafibrate significantly improved the levels of hepatic enzymes (ALT, AST, and γ-GTP), lipid profiles (TG, TC, HDL-C, and non-HDL-C), and hepatic fibrosis biomarkers (M2BPGi and FIB-4 index). These results suggest that pemafibrate is beneficial in patients with hypertriglyceridemia complicated by MASLD, as compared with omega-3-acid ethyl ester. The study results may contribute to the development of future treatment strategies for patients with MASLD/MASH.
Acknowledgements of Grant Support
This study was financially supported by Kowa Company, Ltd., Tokyo, Japan. The funding sponsor had no role in the study design; in the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the article for publication.
Acknowledgements
The authors thank all clinical staff for their assistance in the execution of this study, as well as Soiken Inc. for the technical assistance during the launch and execution of this study. Arata Yoneda from Soiken Inc. provided medical support for this manuscript. The research fund provided by Kowa Company, Ltd. covered the fees for technical assistance and medical writing. The manuscript has been edited by a professional native English editor at Editage.
Conflict of Interest
Yoshio Sumida received a speaker’s bureau fee from Kowa Company, Ltd. Hidenori Toyoda received a speaker’s bureau fee from AbbVie GK, Abbott Japan LLC, Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Terumo Corporation, FUJIFILM Wako Pure Chemical Corporation, Eisai Co., Ltd., and Kowa Company, Ltd. Kiyoaki Ito received research funds from AbbVie GK, Gilead Sciences K.K., and Bristol Myers Squibb Company. Takeshi Osonoi received research funds from Kowa Company, Ltd. Masashi Yoneda received research funds from Kowa Company, Ltd., AbbVie GK, Bayer Yakuhin Ltd., Otsuka Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., and Sumitomo Pharma Co. Ltd.; a consulting fee from Nitto Denko Corporation; and speaker’s bureau fees from Otsuka Pharmaceutical Co., Ltd. and Sumitomo Pharma Co. Ltd. Additionally, Masashi Yoneda holds leadership positions in the Japanese Society of Internal Medicine, Japanese Society of Gastroenterology, Japanese Society of Hepatology, and Japan Society of Neurovegetative Research. Satoshi Yasuda, Satoshi Kimoto, Kazumasa Sakamoto, and Yukiomi Nakade have no conflicts of interest to declare.
References
- 1).Rinella ME, Lazarus JV, Ratziu V et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol, 2023; 79: 1542-1556 [DOI] [PubMed] [Google Scholar]
- 2).Targher G, Kendrick J, Smits G, Chonchol M. Relationship between serum gamma-glutamyltransferase and chronic kidney disease in the United States adult population. Findings from the national health and nutrition examination survey 2001–2006. Nutr Metab Cardiovasc Dis, 2010; 20: 583-590 [DOI] [PubMed] [Google Scholar]
- 3).Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care, 2007; 30: 2940-2944 [DOI] [PubMed] [Google Scholar]
- 4).Akbar DH, Kawther AH. Nonalcoholic fatty liver disease in Saudi type 2 diabetic subjects attending a medical outpatient clinic: Prevalence and general characteristics. Diabetes Care, 2003; 26: 3351-3352 [DOI] [PubMed] [Google Scholar]
- 5).Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol, 2004; 19: 854-858 [DOI] [PubMed] [Google Scholar]
- 6).The Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2014. Nankodo, 2014 [Google Scholar]
- 7).Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology, 2012; 55: 2005-2023 [DOI] [PubMed] [Google Scholar]
- 8).National Institute for Health and Care Excellence. on-Alcoholic Fatty Liver Disease: Assessment and Management. NICE guideline, https: //www.nice.org.uk/guidance/ng49 [NG49]; 2016 [PubMed] [Google Scholar]
- 9).Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology, 2016; 64: 73-84 [DOI] [PubMed] [Google Scholar]
- 10).Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: A multicenter large retrospective study. J Gastroenterol, 2012; 47: 586-595 [DOI] [PubMed] [Google Scholar]
- 11).Williams R, Ashton K, Aspinall R, Bellis MA, Bosanquet J, Cramp ME et al. Implementation of the Lancet Standing Commission on Liver Disease in The UK. Lancet, 2015; 386: 2098-2111 [DOI] [PubMed] [Google Scholar]
- 12).Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther, 2011; 34: 274-285 [DOI] [PubMed] [Google Scholar]
- 13).Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology, 2010; 52: 79-104 [DOI] [PubMed] [Google Scholar]
- 14).Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: Results from the Welcome* study. Hepatology, 2014; 60: 1211-1221 [DOI] [PubMed] [Google Scholar]
- 15).Shiba K, Tsuchiya K, Komiya C, Miyachi Y, Mori K, Shimazu N et al. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep, 2018; 8: 2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Fujimori N, Tanaka N, Kimura T, Sano K, Horiuchi A, Kato N et al. Long-term luseogliflozin therapy improves histological activity of non-alcoholic steatohepatitis accompanied by type 2 diabetes mellitus. Clin J Gastroenterol, 2020; 13: 83-89 [DOI] [PubMed] [Google Scholar]
- 17).Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol, 2015; 62: 720-733 [DOI] [PubMed] [Google Scholar]
- 18).Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S et al. Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: A multicenter, placebo-controlled, double-blind, randomized trial. J Atheroscler Thromb, 2018; 25: 521-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Honda Y, Kessoku T, Ogawa Y, Tomeno W, Imajo K, Fujita K et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci Rep, 2017; 7: 42477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Ishibashi S, Yamashita S, Arai H, Araki E, Yokote K, Suganami H et al. Effects of K-877, a novel selective PPARalpha modulator (SPPARMα), in dyslipidaemic patients: A randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis, 2016; 249: 36-43 [DOI] [PubMed] [Google Scholar]
- 21).Yokote K, Yamashita S, Arai H, Araki E, Suganami H, Ishibashi S. A pooled analysis of pemafibrate Phase II/III clinical trials indicated significant improvement in glycemic and liver function-related parameters. Atheroscler Suppl, 2018; 32: 154-155 [Google Scholar]
- 22).Dasarathy S, Dasarathy J, Khiyami A, Yerian L, Hawkins C, Sargent R et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol, 2015; 49: 137-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Das Pradhan A, Glynn RJ, Fruchart JC, MacFadyen JG, Zaharris ES, Everett BM et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med, 2022; 387: 1923-1934 [DOI] [PubMed] [Google Scholar]
- 24).Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther, 2021; 54: 1263-1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Sugimoto R, Iwasa M, Eguchi A, Tamai Y, Shigefuku R, Fujiwara N et al. Effect of pemafibrate on liver enzymes and shear wave velocity in non-alcoholic fatty liver disease patients. Front Med (Lausanne), 2023; 10: 1073025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Takeda Y, Sakuma I, Hiramitsu S, Okada M, Ueda S, Sakurai M. The effects of pemafibrate and omega-3 fatty acid ethyl on apoB-48 in dyslipidemic patients treated with statin: A prospective, multicenter, open-label, randomized, parallel group trial in Japan (PROUD48 study). Front Cardiovasc Med, 2023; 10: 1094100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Takakusagi S, Sato K, Marubashi K et al. Impact of M2BPGi on the Hepatocarcinogenesis after the Combination Therapy with Daclatasvir and Asunaprevir for Hepatitis C. Biomedicines, 2021; 9: 660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Migita K, Horai Y, Kozuru H et al. Serum cytokine profiles and Mac-2 binding protein glycosylation isomer (M2BPGi) level in patients with autoimmune hepatitis. Medicine (Baltimore), 2018; 97: e13450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).The Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. 2nd ed. Nankodo, available at https: //www.jsge.or.jp/guideline/guideline/ [Google Scholar]
- 30).Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology, 2023; 77: 1797-1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Rinella ME, Dufour JF, Anstee QM, Goodman Z, Younossi Z, Harrison SA et al. Non-invasive evaluation of response to obeticholic acid in patients with NASH: Results from the REGENERATE study. J Hepatol, 2022; 76: 536-548 [DOI] [PubMed] [Google Scholar]