Abstract
Background
Polycystic Ovary Syndrome (PCOS) represents a complex endocrine disorder characterized by a significant interplay with metabolic dysfunction and obesity. This research endeavors to elucidate the causal dynamics among plasma metabolites, proteins, and PCOS, alongside Body Mass Index (BMI), to pinpoint prospective therapeutic interventions.
Methods
This investigation employed Mendelian randomization (MR) analyses combined with data derived from the National Health and Nutrition Examination Survey (NHANES) to explore the relationships between 1,400 plasma metabolites and PCOS, factoring in BMI adjustments. Additionally, the study examined the influence of plasma proteins and performed a retrospective cross-sectional analysis focusing on retinol consumption and testosterone levels.
Results
MR analyses showed metabolite Glycosyl-N-(2-hydroxynervonoyl)-sphingosine (GNS) and protein Keratin 19 (KRT19) were identified as significant markers in the context of PCOS and BMI adjustments. A Phenome-Wide Association Study (PheWAS) underscored the linkage between KRT19 and BMI, while gene-drug interaction findings demonstrated a connection between KRT19 and retinol. Analysis for NHANES data disclosed a negative correlation between retinol intake and testosterone levels, particularly within normal weight and obese cohorts, suggesting the feasibility of dietary interventions for PCOS management.
Conclusion
The study sheds light on the intricate interactions between plasma metabolites, proteins, and PCOS, considering BMI variations, and highlights KRT19 protein as a promising therapeutic target. The outcomes support the integration of retinol consumption into dietary strategies to regulate testosterone levels and potentially alleviate PCOS symptoms, underscoring the necessity for personalized nutritional and therapeutic approaches in the effective management of PCOS.
Keywords: PCOS, Mendelian randomization, drug target, NHANES, retinol intake
Introduction
Polycystic Ovary Syndrome (PCOS) is a complex endocrine pathology in women who are at reproductive age and is related to metabolic disorder (1). The diagnostic criteria for PCOS contains three distinct clinical characteristics—menstrual irregularities, hyperandrogenism (androgen excess), and the detection of polycystic ovaries via ultrasound—espoused by the National Institute of Health, Rotterdam, and Androgen Excess Society (2, 3). Currently, although great progresses have been made in both the acknowledgement of PCOS and the therapeutics (4–7), some limitations including unsatisfactory cure rate, undiscovered mechanism and disorders relapse still remain (8). Hence, it will be helpful to investigate the disease characteristics and therapeutic interventions for understanding and management for patients with PCOS.
Although PCOS is related to metabolic disorder, the interplay between PCOS and metabolic dysfunction is still intricate, manifesting through a myriad of facets. A variety of researches demonstrate that metabolic anomalies, including impaired glucose tolerance, reduced insulin sensitivity, and perturbed lipid metabolism, are intricately associated with PCOS (9–13). Insulin resistance, a major metabolic aberration in PCOS, is significantly correlated with increased metabolic risks such as dysglycemia, dyslipidemia, and hepatic steatosis (13). Approximately 50–70% of women with PCOS are disturbed by insulin resistance (14). Hence, it is necessary to investigate the correlation between metabolite and PCOS. Numerous studies have emphasized the differential metabolite between PCOS and normal people (15–17), but the casual correlations between them are less to be clarified.
Notably, among obese individuals, the PCOS tends to be aggravated. Obesity is defined as an overabundance of body fat and the body fat content is measured by the body mass index (BMI), calculated by the percentage of weight (in kilograms) to height squared (square meters). According to the definition from the World Health Organization (WHO), a BMI of 25–29 kg/m2 is named overweight; higher BMI (30 kg/m2) is named obesity (18). Some studies found that the prevalence of metabolic syndrome increases in overweight or obese patients with PCOS, highlighting the exacerbating role of excess weight on the metabolic phenotype of the syndrome (19–21). The amplification of metabolic defects in PCOS is further exacerbated by obesity (12, 22). Women with PCOS tend to exert higher obesity rates and a more deleterious metabolic profile, particularly in Hispanic and African American descent (23). An evidence underscores that PCOS may modulate the impact of genetic variants associated with BMI, intimating a genetic interrelation between obesity and PCOS (24). Genome-wide cross-trait analyses have also unveiled loci shared between PCOS and obesity-related traits, underscoring a genetic and causal linkage between the two conditions (25). Furthermore, PCOS has been independently linked to elevated BMI (26). The influence of BMI on the pathophysiology of PCOS contains insulin resistance, hormonal disequilibrium, and reproductive outcomes. Insulin resistance is an intrinsic defect of PCOS and is magnified by a high BMI regardless of PCOS status (14). Additionally, the interrelation between BMI and metabolism as well as endocrine dysregulation in women with PCOS has been documented (27). In essence, the literature substantiates a complex and bidirectional relationship between BMI and PCOS, involving in genetic, metabolic, reproductive, and endocrine domains (24). These evidences demonstrated that BMI as a covariate might unveil novel insights into the etiology of PCOS.
Retinol is a vital vitamin A form, is crucial for cell proliferation, apoptosis, differentiation, and metabolism (28). Numerous studies have demonstrated that Vitamin A can influence the development of obesity and the development of obesity-related diseases, including insulin resistance (29). Retinol-binding protein 4 (RBP4), an important protein in Vitamin A metabolism, have also been emphasized to be involved in the PCOS metabolic disorder (30) and might be a diagnostic markers in PCOS (31). In our analysis, Retinol was also found to be a pivotal drug target in PCOS. Hence, underscoring the correlation of Retinol with PCOS is necessary for personalized nutritional and therapeutic approaches in the effective management of PCOS.
Randomized controlled trials (RCTs) are a gold standard means to test hypotheses at a population level (32, 33). However, RCTs require intensive human resources, cost, and time, and some interventional strategies are not suitable for RCT-dependent assessments. Recently, Mendelian randomization (MR) has gained attention as a complementary method of exploring the casual relationship between the exposure and outcome in diseases, which utilizing the genetic variants with strong associations with exposure as the instrumental variables (IVs). The three main hypotheses of MR are: (1) relevance. Single-nucleotide polymorphisms (SNPs) from genome-wide association studies (GWAS) are used as IVs, and the selected IVs are strongly correlated with exposure; (2) independence. IVs are not associated with other confounding factors; (3) exclusivity. IVs affect outcomes only by exposure (34). This approach might avoid the confounding bias of conventional epidemiological investigations (35). In this study, we conducted a detailed MR analysis alongside a retrospective cross-sectional study to explore the potential causal relationships among plasma metabolites, plasma proteins, and PCOS. Our study highlights KRT19 protein as a viable therapeutic target and supports the integration of retinol consumption into dietary strategies to regulate testosterone levels and potentially alleviate PCOS symptoms. These findings would enrich our understanding to PCOS and enhance therapeutic strategies for patients with PCOS.
Materials and methods
Study design
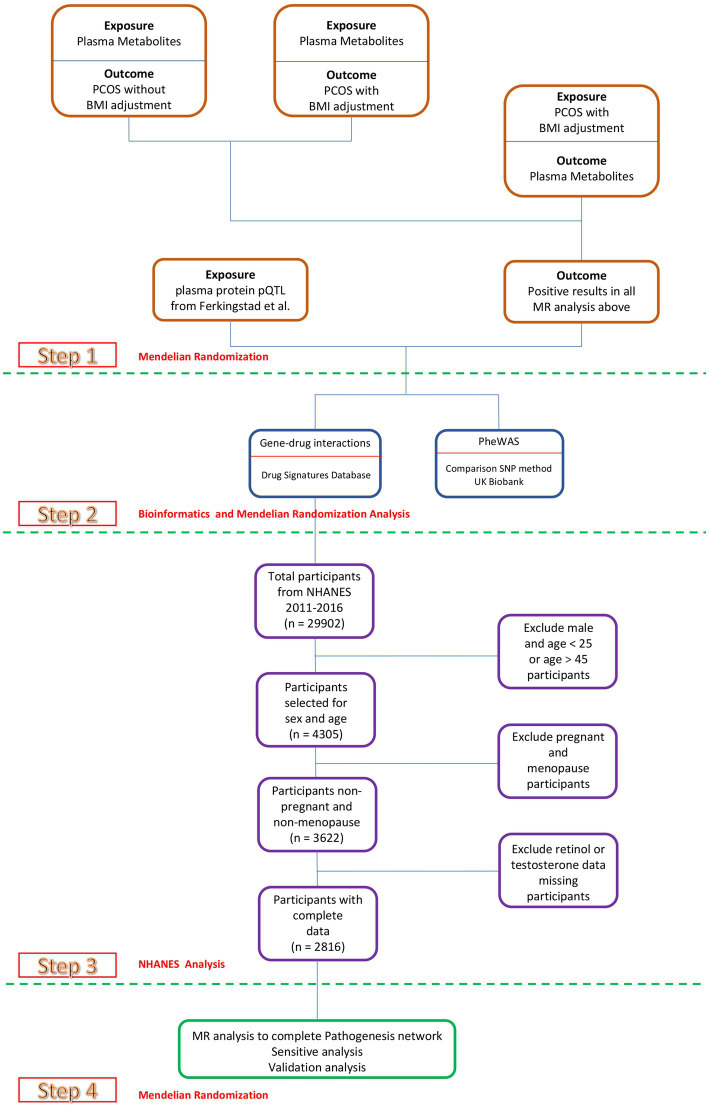
MR is a method to investigate the casual relationship between exposure and outcome. This study was conducted in accordance with the STROBE guidelines (36), ensuring a robust epidemiological investigation. As previously mentioned, the validity of MR hinges on three critical assumptions (37). Hence, in our methodology, we meticulously followed these assumptions. Our analysis employed a two-sample MR approach to examine the casual effects of 1,400 plasma metabolites on PCOS, with or without adjusting for BMI. Subsequently, we reversed the order of association, conducting another two-sample MR to explore the casual effects of PCOS (with adjustment) on the 1,400 plasma metabolites. Additionally, MR was utilized to elucidate the causal relationships between plasma proteins and the positive metabolites identified in the initial analyses. Significant positive plasma proteins were then selected for the construction of a Phenome-Wide Association Study (PheWAS). Drug signature analysis was also conducted focusing on the identified positive plasma proteins.
Our comprehensive analysis revealed retinol as the primary outcome of interest. Consequently, we retrieved data from National Health and Nutrition Examination Survey (NHANES), an ongoing study that provides invaluable health-related information on the U.S. adult and child populations, to conduct a retrospective cross-sectional study. This study aimed to investigate the relationships between retinol intake and blood testosterone levels, providing new insights into potential nutritional interventions for PCOS (Figure 1).
Figure 1.
Study design. Overview of the MR study design.
GWAS summary data sources
Data for PCOS were sourced from the investigation conducted by Jaakko et al. (38). The novel genetic variants associated with PCOS within these specific population groups were obtained using data from the FinnGen project and the Estonian Biobank (EstBB). Within the FinnGen cohort, PCOS cases were identified among women documented with the International Classification of Diseases (ICD)-10 code E28.2, ICD-9 code 256.4, or ICD-8 code 256.90. The control group comprised all female participants devoid of a PCOS diagnosis, without imposing any further exclusions. This criterion contained a cohort of 797 cases against 140,558 controls. In the EstBB framework, PCOS identification hinged on the ICD-10 code E28.2, and contained 2,812 cases and 89,230 controls. For the GWAS data adjusted for BMI, a refined association analysis was conducted. It included 482 PCOS cases (60.5% of the original cohort) and 91,631 FinnGen controls (65.2%). Similarly, for the validation dataset, 2,137 PCOS cases (75%) and 68,690 EstBB controls (76.9%) were analyzed with BMI adjustment.
The data concerning 1,400 plasma metabolites were derived from the studies conducted by Chen et al. (39). Additionally, plasma protein pQTL GWAS data were sourced from the research by Ferkingstad et al. (40), while genome-wide association studies (GWAS) data on female bioavailable testosterone levels were obtained from Ruth et al. (41). The analysis also incorporated publicly available GWAS summary datasets for Body Mass Index (BMI) from the MRC-IEU, encapsulating a total sample size of 461,460 individuals. These GWAS summary datasets are meticulously cataloged in Table 1, providing a structured overview of the data underpinning our analysis.
Table 1.
Description of the contributing GWAS studies.
| Traits | PMID | Case size | Sample size | Ancestry |
|---|---|---|---|---|
| PCOS not adjusted for BMI | 34791234 | 3,609 | 229,788 | European |
| PCOS adjusted for BMI | 34791234 | 2,619 | 160,321 | European |
| Plasma Metabolites | 36635386 | NA | 8,299 | European |
| Plasma Protein pQTL | 34857953 | NA | 35,559 | European |
| Female Bioavailable Testosterone | 32042192 | NA | 188,507 | European |
| Body Mass Index | UK Biobank | NA | 461,460 | European |
SNP selection
We selected exposure-associated independent SNPs with an r2 threshold of 0.001 and a distance criterion of ≥10,000 kb, based on their genome-wide (GW) significance levels (p < 1.0 × 10−5 or p < 5.0 × 10−8) (42). The F-statistic (F-stat) served as an indicator of instrument strength (IS) and is related to the variance explained by the phenotype. An F-stat value of at least 10 suggests a reduced likelihood of instrument bias (IB) in MR analyses (43). Details regarding the number of SNPs for each causal inference phenotype, along with their respective scale units and F-stats, are documented in Supplementary Table S1.
Mendelian randomization analysis
First, SNPs were harmonized to the exposure and outcome in an allele-specific manner to ensure proper alignment of effects. Wherever applicable, if any instrumental SNPs for the exposure were absent in the corresponding outcome dataset, a proxy was incorporated using GVs in the linkage disequilibrium (r2 > 0.8).
We employed an inverse variance-weighted (IVW) meta-analysis approach for MR analysis (44), in addition to the weighted median (45) and MR-Egger regression (MR-ER) approach. Multiple testing was addressed using the false discovery rate (FDR) approach.
Sensitive analysis for MR
The MR-ER method was further used to assess any potential impacts of the directional pleiotropy (DP) (46) and to perform the MR-pleiotropy residual sum and outlier (MR-PRESSO) method. Data heterogeneity was determined by Cochran’s Q test (47). Leave-one-out (LOO) analysis indicated the influences of a single SNP on overall estimates. To uncover potential reverse causation (RC), genetic instruments were chosen following a strategy akin to that used in MR analysis (48).
“TwoSampleMR v0.5.8,” “MRPRESSO v1.0,” and “MendelianRandomizaiton v0.9.0” packages in R v4.3.1 (source codes: https://github.com/studentyaoshi/MR) were applied for statistical analyses.
Phenome-wide association studies
To assess the potential implications of key plasma proteins, a Phenome-Wide Association Study (PheWAS) was conducted. A control SNP-set was compiled to benchmark against the plasma protein-associated SNPs for PheWAS analyses, ensuring matches in minor allele frequency (±5%), gene density around SNPs (±50%), proximity to the nearest gene (±50%), and the number of linked SNPs indicating haplotype block size (R^2 ≥ 0.50) (±50%) (49). Data from the UK Biobank was utilized to identify trait associations with the notable plasma proteins. SNP-trait associations that reached nominal significance (p < 0.01) were advanced for trait-enrichment analysis (50). The PheWAS explored the relationships between SNPs of significant plasma proteins and over 2000 phenotypic traits, contrasting these findings against the control SNP-set outcomes. Fisher’s exact tests were employed to discern the prevalence of trait associations with significant plasma protein SNPs relative to control SNPs, aiming to identify traits disproportionately associated with plasma proteins risk variants. The FDR correction method was applied to Fisher’s exact p-values for trait enrichment to mitigate the risk of false positives due to multiple testing.
Gene-drug interactions
Drug Signatures Database (DSigDB) was used to identify drug molecules that interact with plasma proteins through the Enrichr platform.1
NHANES analysis
The NHANES study collects comprehensive data through home interviews and health screenings conducted at mobile examination centers. The interviews cover areas such as demographics, socioeconomics, diet, health. And the examination includes detailed medical, dental, physiological assessments, and laboratory tests by professional staff. Dietary data, focusing on intake over the past 24 h, are derived from a two-day recall survey to analyze food and beverage consumption and their nutritional values. Specifically, the study examines total two-day retinol intake and blood testosterone levels from 2011 to 2016, following the ethical standards set by the 1964 Declaration of Helsinki. The study protocol received approval from the National Center for Health Statistics Research Ethics Review Committee, ensuring all participants provided informed consent.
In alignment with the guidelines stipulated by the Centers for Disease Control and Prevention (CDC), our study employed weighted sampling methodologies, complemented by stratification and clustering techniques, to ensure a representative reflection of the United States population. The demographic attributes of study participants were showed as mean ± standard deviation (SD) for continuous variables and percentages for categorical variables. To explore the associations between retinol intake and testosterone levels, we applied multivariable linear regression models. Model 1 was devoid of any covariates. Model 2 incorporated adjustments for age, race, educational attainment, and marital status. Model 3 expanded upon Model 2 by further adjusting for hypertension, diabetes, abdominal diameter, smoking status, and alcohol consumption. Moreover, we conducted a subgroup analysis, stratified by BMI categories: normal weight (BMI < 25), overweight (25 ≤ BMI < 30), and obesity (BMI ≥ 30), employing multivariable regression techniques. To address potential non-linear relationships between retinol intake and testosterone levels, we utilized smoothing techniques and generalized additive models. Statistical analyses were performed utilizing R v4.3.1 2 and EmpowerStats v4.0 3, with a predetermined significance threshold set at p < 0.05 for denoting statistical significance.
Data availability
All relevant data for this study are included in the article or uploaded as Supplementary material. Full GWAS summary statistics for the exposure and outcome data used in the manuscript can be found at https://www.ebi.ac.uk/gwas, https://gwas.mrcieu.ac.uk/.
Results
Step 1. Metabolic related druggable targets for BMI adjusting PCOS
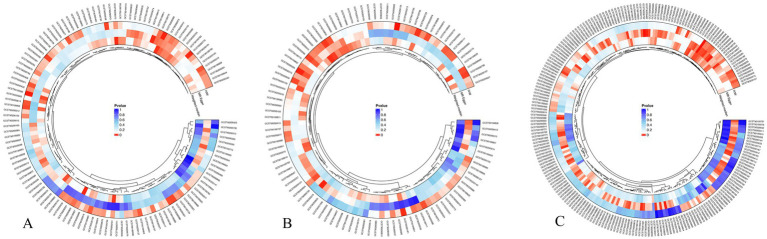
In this investigation, we aimed to explore the causal correlations between plasma metabolites and PCOS, with a particular emphasis on adjustments for BMI. In our preliminary analysis, MR analysis was conducted to assess the casual effects of approximately 1,400 plasma metabolites on PCOS under two distinct conditions: with and without BMI adjustment. After adjusting for the FDR, no outcomes of IVW reached statistical significance were discovered. Hence, a threshold of p-value less than 0.05 was adopted to denote statistical significance. PCOS without BMI adjustment revealed 77 significantly associated plasma metabolites, comprising of 36 beneficial and 41 deleterious metabolites (Figure 2A). Within the context of PCOS adjusted for BMI, we identified 62 plasma metabolites with significant correlations—divided evenly into 31 metabolites with beneficial and 31 with deleterious effects (Figure 2B). Remarkably, 20 metabolites were causally correlated with PCOS regardless of BMI adjustment, including 12 beneficial and 8 deleterious metabolites.
Figure 2.
MR analysis between plasma metabolites and PCOS. (A) The casual association between plasma metabolites and PCOS without BMI adjustment. (B) The causal association between plasma metabolites and PCOS with BMI adjustment. (C) The casual association between PCOS with BMI adjustment and plasma metabolites.
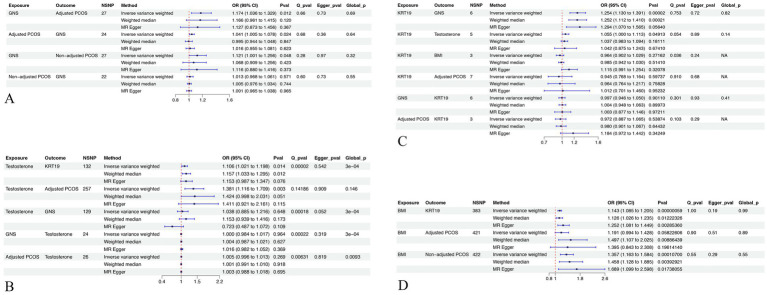
Subsequently, we explored the effects of PCOS with BMI adjustment on the same set of 1,400 plasma metabolites. T A total of 64 metabolites were demonstrated to be significantly affected by PCOS with BMI adjustment, featuring 23 with beneficial and 41 with deleterious effects (Figure 2C). When intersected with the 20 metabolites identified in the initial phase, only the levels of Glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18:1/24:1(2OH)) (GNS) were consistently observed in both sets of positive results, exerting an effect on PCOS (95% Confidence Interval [CI]: 1.0363 to 1.3294, p = 0.0117) and being influenced by PCOS (95% CI, 1.0053 to 1.0779, p = 0.0241) (Figure 3A; Supplementary Tables S1, S2).
Figure 3.
MR analysis and sensitive analysis of related exposures and outcomes. (A) MR and sensitive analysis related to GNS. (B) MR and sensitive analysis related to Testosterone. (C) MR and sensitive analysis related to KRT19. (D) MR and sensitive analysis related to BMI. GNS, Glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18:1/24:1(2OH)). KRT19, Keratin 19. NSNP, number of single-nucleotide polymorphism. Q_pval, p value of Cochran’s Q test. Egger_pval, p value of MR-ER method. Global_p, p value of MR-PRESSO.
Expanding our scope to the effects of plasma proteins in conjunction with GNS, and after FDR adjustment, Keratin 19 (KRT19) was found to exhibit a significant positive association (95% CI: 1.1301 to 1.3912, pFDR = 0.0251), whereas BIRC2 (95% CI: 0.836 to 0.9248, pFDR = 0.0022) and ING4 (95% CI, 0.8748 to 0.9506, pFDR = 0.0251) displayed significant negative relationships (Figure 3C; Supplementary Table S2). Based on these findings, KRT19 emerges as a viable druggable target for the management of PCOS, particularly when adjustments for BMI were considered.
Step 2. PheWAS and gene-drug interactions for KRT19
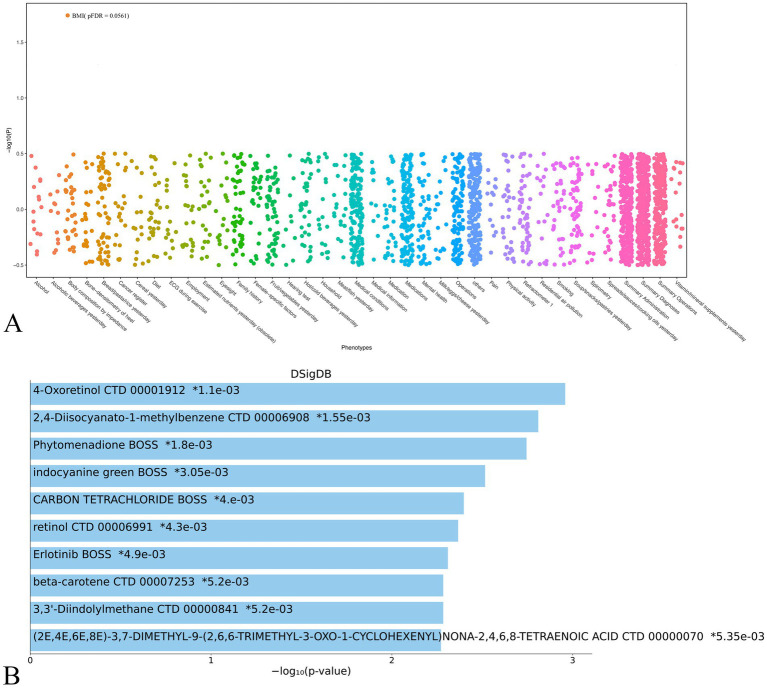
The results from the PheWAS can be interpreted to delineate the correlations between the genetic determination of protein expression and the incidence of specific diseases or traits. Initially, we identified that six SNPs were associated with KRT19 through prior MR analyses. These were subsequently subjected to clumping, with each being matched to four control SNPs (Supplementary Table S3). Following this, PheWAS analyses were conducted utilizing the UK Biobank database to examine each KRT19-associated variant and its control counterparts for associations with 2,502 traits. After adjustment for FDR, only BMI was demonstrated to be a statistically significant with KRT19 SNPs over control SNPs, achieving a significance level of pFDR<0.2 (pFDR = 0.0561) (Figure 3D; Supplementary Table S4). Further MR analysis revealed that BMI exhibited a positive effect on KRT19 expression (95% Confidence Interval (CI): 1.0847 to 1.2048, p = 5.8506e-7) (Figure 3D; Supplementary Table S4).
Subsequently, based on the DSigDB database, Enrichr analysis was conducted to explore the interplay between KRT19 and potential drug candidates (Figure 4). The results highlighted the significance of Vitamin A metabolites—specifically 4-Oxoretinol, retinol, and beta-carotene—among the top 10 drug candidates for KRT19, meriting further attention (Figure 4B).
Figure 4.
PheWAS and gene-drug interactions for KRT19. (A) Manhattan plot of the PheWAS analysis results for KRT19 in UK Biobank database. (B) Bar chart of top enriched terms of KRT19 from the DSigDB gene set library. The top 10 enriched terms for the input gene set are displayed based on the −log10(p-value), with the actual p-value shown next to each term. The term at the top has the most significant overlap with the input query gene set.
Step 3. NHANES analysis between retinol and blood testosterone
Data from 29,902 individuals registered in NHANES database spanning from 2011 to 2016 were initially considered. Following a meticulous final screening process, a cohort of 2,816 individuals who satisfied the specified inclusion and exclusion criteria were selected for enrollment (Figure 1). These individuals were subsequently categorized into three groups based on their BMI, with the characteristics of each group detailed in Table 2.
Table 2.
Weighted characteristics of study samples based on BMI.
| Normal weight | Overweight | Obesity | p value | |
|---|---|---|---|---|
| BMI <25 (n = 1,017) | BMI > =25, <30 (n = 715) | BMI > =30 (n = 1,084) | ||
| Age | 31.464 ± 7.797 | 32.406 ± 7.250 | 32.921 ± 7.312 | <0.001 |
| BMI | 21.825 ± 1.986 | 27.325 ± 1.464 | 37.223 ± 6.326 | <0.001 |
| Abdominal Diameter | 17.040 ± 1.578 | 20.284 ± 1.722 | 25.844 ± 3.479 | <0.001 |
| Retinol Intake (mcg) | 364.854 ± 268.146 | 357.853 ± 246.628 | 368.491 ± 289.725 | 0.72 |
| Testosterone Level (ng/dL) | 26.270 ± 16.163 | 28.275 ± 27.345 | 27.668 ± 15.782 | 0.08 |
| Race | <0.001 | |||
| Mexican American | 92 (6.999%) | 156 (15.877%) | 215 (15.151%) | |
| Other Hispanic | 108 (7.282%) | 82 (9.19%) | 109 (7.879%) | |
| Non-Hispanic White | 385 (63.733%) | 227 (54.926%) | 336 (51.623%) | |
| Non-Hispanic Black | 125 (7.043%) | 150 (12.273%) | 341 (20.365%) | |
| Other Race | 307 (14.943%) | 100 (7.735%) | 83 (4.982%) | |
| Education | <0.001 | |||
| Below High Scholl | 105 (8.417%) | 138 (13.905%) | 202 (14.725%) | |
| High School | 141 (13.604%) | 119 (15.151%) | 236 (20.717%) | |
| Above High School | 771 (77.979%) | 458 (70.944%) | 646 (64.558%) | |
| Marital Status | 0.05 | |||
| Yes | 588 (60.55%) | 433 (64.262%) | 604 (58.354%) | |
| No | 429 (39.45%) | 282 (35.738%) | 480 (41.646%) | |
| Alcohol Drink | <0.001 | |||
| Yes | 635 (78.783%) | 414 (71.297%) | 662 (71.939%) | |
| No | 272 (21.217%) | 219 (28.703%) | 313 (28.061%) | |
| Smoke | 0.02 | |||
| Yes | 260 (30.73%) | 180 (28.783%) | 359 (34.932%) | |
| No | 757 (69.27%) | 535 (71.217%) | 725 (65.068%) | |
| Hypertension | <0.001 | |||
| Yes | 65 (5.979%) | 78 (11.152%) | 289 (23.389%) | |
| No | 952 (94.021%) | 637 (88.848%) | 795 (76.611%) | |
| Diabetes | <0.001 | |||
| Yes | 4 (0.225%) | 27 (2.581%) | 88 (7.4%) | |
| No | 1,013 (99.775%) | 688 (97.419%) | 996 (92.6%) |
Mean ± SD for continuous variables, p-values were obtained using the weighted linear regression model and Kruskal-Wallis rank sum test. % for categorical variables and p value was calculated by weighted chi-square test.
In the comprehensive analysis conducted under Model 3—which accounted for confounding factors such as age, race, educational attainment, marital status, hypertension, diabetes, abdominal diameter, smoking status, and alcohol consumption—a significant negative correlation between retinol intake and testosterone levels was identified (Table 3).
Table 3.
The association between retinol intake (mcg) and testosterone (ng/dL).
| Model | Total | BMI = <25 | BMI > =25, <30 | BMI > =30 |
|---|---|---|---|---|
| β (95% CI) p value | β (95% CI) p value | β (95% CI) p value | β (95% CI) p value | |
| Model 1 | −0.004 (−0.007, −0.002) 0.00178 | −0.004 (−0.008, −0.000) 0.04277 | −0.002 (−0.010, 0.006) 0.62966 | −0.006 (−0.009, −0.002) 0.00053 |
| Model 2 | −0.004 (−0.007, −0.002) 0.00194 | −0.004 (−0.008, −0.000) 0.03658 | −0.001 (−0.010, 0.007) 0.73320 | −0.006 (−0.009, −0.003) 0.00024 |
| Model 3 | −0.004 (−0.007, −0.001) 0.00473 | −0.005 (−0.009, −0.000) 0.02933 | −0.000 (−0.010, 0.010) 0.99756 | −0.006 (−0.009, −0.003) 0.00059 |
No covariate was adjusted in Model 1. Model 2 indicates that analysis was adjusted for age, race, educational attainment, and marital status. Model 3 indicates Model 2 adjustment plus the adjustment for hypertension, diabetes, abdominal diameter, smoking status, and alcohol consumption.
Subgroup analyses revealed that among the normal weight (BMI ≤ 25) and obesity groups (BMI ≥ 30), significant negative correlations between retinol intake and testosterone levels were observed (Table 3). Conversely, in the overweight group (BMI ≥ 25, < 30), no significant correlation was detected.
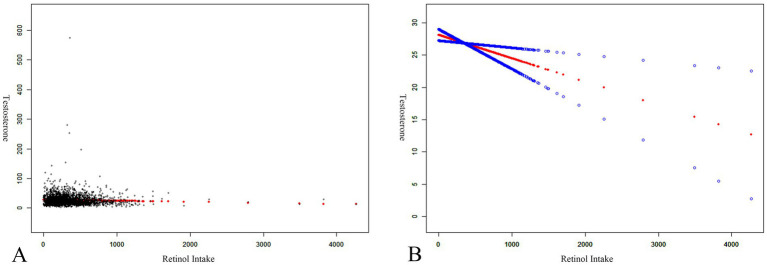
Further examination was conducted to elucidate non-linear relationships between retinol intake and testosterone levels through smooth curve fittings and generalized additive models, illustrated in Figure 5. The results indicated that retinol intake was negatively correlated with testosterone levels.
Figure 5.
The association between retinol intake (mcg) and testosterone (ng/dL). (A) Each black point represents a sample. (B) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence bands derived from the fit.
Step 4. Pathogenesis network of BMI adjusting PCOS
Upon synthesizing the results obtained, we developed a conceptual framework illustrating the influence of BMI and retinol on the PCOS after adjusting for BMI. However, certain interactions between mediators remained unanalyzed. To clarify these interactions, MR analysis was employed, the results of which were depicted in Figure 3B. The analysis revealed that testosterone displayed a significant positive association with both BMI-adjusted PCOS (95% Confidence Interval (CI): 1.1158 to 1.7089, p = 0.003) and Keratin 19 (KRT19) (95% CI: 1.0273 to 1.1716, p = 0.0057). Concurrently, KRT19 exhibited a significant positive association with testosterone (95% CI: 1.000 to 1.113, p = 0.0491) (Figure 3C). However, no association was found between BMI-adjusted PCOS and testosterone (Figure 3B, p = 0.269).
To validate the association between BMI and PCOS, we investigate the correlations between BMI and PCOS data with or without BMI adjusting. Positive associations were observed exclusively in the cases where BMI was adjusted. Further investigations were conducted to explore the direct associations between testosterone and GNS, KRT19 and BMI-adjusted PCOS, non- adjusted PCOS and GNS, yet no positive findings emerged. Additional reverse causality analyses and sensitivity analyses pertinent to all MR study were also performed, with results detailed in Supplementary Table S5. The results indicated that no heterogeneity and no horizontal pleiotropy were observed between the genetic IVs, indicating the robust of the MR analysis.
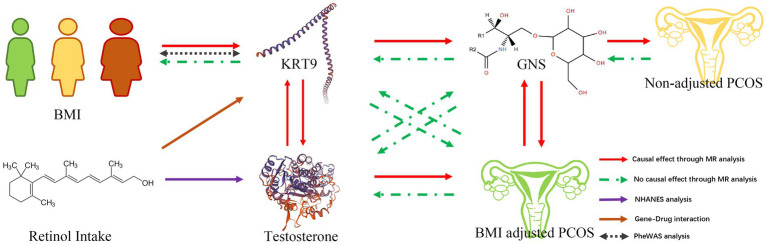
Based on the comprehensive analysis of the data collected, we were able to delineate a clear pathogenetic network for BMI-adjusted PCOS, as illustrated in Figure 6. This enhanced understanding underscores the complex interplay between BMI, retinol, genetic factors, and plasma metabolites in the development of PCOS, particularly when adjusted for BMI.
Figure 6.
Pathogenesis network of BMI adjusting PCOS. Pathogenesis network overview of BMI adjusting PCOS.
Discussion
PCOS is recognized as a prevalent endocrine disorder affecting women of reproductive age. The prevalence of obesity in women diagnosed with PCOS is notable, intensifying the syndrome’s clinical symptoms and adding to its complexity (51). Therefore, the exclusion of BMI as a confounding factor plays a crucial role in uncovering the underlying causes of PCOS. In this study, we employed MR analysis alongside analyses of data from NHANES to explore plasma metabolites and druggable targets that were associated with PCOS after adjusting for BMI. Through MR and gene-drug interactions analysis, KRT19 emerged as a potential therapeutic target, with its effects mediated by GNS and influenced by both BMI and retinol intake. Subsequent multivariable linear regression analysis on individuals from NHANES demonstrated that retinol intake could decrease testosterone levels in individuals of normal weight and those classified as obese, but it did not have a significant effect on the overweight group. These findings suggested that retinol intake and BMI may influence the development of PCOS through mechanisms mediated by KRT19 and GNS.
Glycosyl-N-(2-hydroxynervonoyl)-sphingosine (GNS) plays a pivotal role in cell growth, proliferation, apoptosis, and metabolic regulation (52–54). The critical involvement of GNS in cellular survival and metabolic stability, particularly within adipocytes, and its interconnection with cholesterol regulation (55), highlight its significance. Decreases in plasma sphingomyelins, which may be related to GNS, could reflect changes in lipoprotein cholesterol or deficiencies in sphingomyelin synthesis (56). GNS has also been linked to diabetic polyneuropathy in type 2 diabetes, emphasizing its importance in metabolic and neurological disorders (56). Further research by Guan et al. (57) indicates an inverse relationship between GNS levels and physical activity. In a Puerto Rican study (58) assessing metabolites and cognitive function in a non-diabetic population, lower GNS levels are associated with better cognitive performance, suggesting its potential impact on cognitive health. These insights collectively underscore the critical role of GNS in health and disease, warranting more in-depth exploration. In our investigation, findings indicated a bidirectional relationship between GNS and PCOS. Specifically, GNS could contribute to the exacerbation of PCOS, while simultaneously, the presence of PCOS might also lead to an increase in GNS levels. This reciprocal interaction underscores the complexity of the metabolic interplays underlying PCOS, highlighting GNS’s significant role in this dynamic.
KRT19, a constituent of the keratin family, plays a significant role in numerous processes associated with cancer (59–62). In our investigation, KRT19 functioned as a central node within the pathogenesis network for PCOS modulated by BMI. It has been observed that KRT19 can enhance the progression of PCOS via GNS and is positively influenced by BMI. Furthermore, KRT19 shares a bidirectional relationship with testosterone levels and is subject to modulation through retinol consumption. The molecular mechanisms of KRT19 include several pathways, such as the MET-ERK1/2-AP1 and SP1 axis (63), the potentiation of Notch1 signaling by Linc-KILH (64), DNA methylation-associated allelic inactivation (65), and immune infiltration (66). However, these findings do not definitively elucidate the results we obtained. A more detailed investigation into the role of KRT19 in PCOS is warranted for a comprehensive understanding.
Nutritional supplements and complementary therapies have been applicated in the treatment of PCOS (7, 67, 68). Retinol, a vital vitamin A form, is crucial for cell proliferation, apoptosis, differentiation, and metabolism (28). It is obtained from dietary beta-carotene and retinyl esters. In the gut, retinol converts to retinyl esters, then to chylomicrons for liver transport. There, it is stored or becomes retinol and its derivatives (69). Mammalian cells can absorb retinol via STRA6, metabolizing it into retinal and retinoic acid forms like 4-oxo-retinoic acid. Our research identified 4-oxoretinol, retinol, and beta-carotene as top KRT19 drug candidates, with beta-carotene converting to retinol, then to 4-oxoretinol. Thus, we focused on retinol for our study, emphasizing its therapeutic potential against KRT19. Retinol has also been demonstrated to be related to insulin resistance (29). In PCOS, RBP4, an important protein in Vitamin A metabolism, has also been emphasized to be involved in the PCOS metabolic disorder (30, 31). Our findings indicated that for women aged 20–45, consuming retinol can decrease testosterone levels in individuals with a BMI below 25 and above 30, with no significant impact observed within the 25 ≤ BMI < 30 range. In women with a normal BMI, a deficiency in retinol intake may increase testosterone levels and GNS through KRT19, potentially leading to the development of PCOS, which can further result in weight gain. However, for those who are overweight, retinol intake does not effectively modify testosterone levels, may lead to a situation where GNS and non-adjusted PCOS, present a one-way relationship. This pattern suggests that retinol intake could play a role in the prevention of PCOS among women with a normal BMI, yet its capacity to serve as an effective treatment in overweight individuals is limited.
Limitations
This research is subject to several constraints. Initially, our analysis focused on the pathogenic effects of proteins reported in various studies, where discrepancies in measurement techniques across these studies could have introduced biases into our findings. Additionally, while the MR and NHANES study populations and datasets encompass diverse racial and ethnic groups, the inherent inconsistencies among these groups might have led to biases in the results. Moreover, the casual correlation between GNS and PCOS, as well as the targets KRT19 in PCOS are not fully confirmed in the present analysis. More experiment validation and clinical validation are necessary to performed to improve this aspect of knowledge.
Conclusion
Employing a variety of methodologies, including Mendelian randomization, gene-drug interaction analyses, and retrospective cross-sectional studies, our investigation reveals that circulating KRT19 could potentially induce BMI-adjusted PCOS via mechanisms involving GNS and testosterone. Both BMI and retinol consumption appear to modulate KRT19, influencing its impact on PCOS. Notably, retinol intake might contribute to PCOS prevention in women with a normal BMI by affecting KRT19 activity. Furthermore, KRT19 emerges as a promising metabolic-related, druggable target for PCOS management and treatment.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Footnotes
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by this study used only publicly available, deidentified summary statistics, and specific ethical review and informed consent had been obtained in all the original studies. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PC: Conceptualization, Data curation, Software, Visualization, Writing – original draft, Writing – review & editing. SN: Visualization, Writing – review & editing. Q-FL: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. LO-Y: Conceptualization, Supervision, Validation, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1434648/full#supplementary-material
References
- 1.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. (2010) 8:41. doi: 10.1186/1741-7015-8-41, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. (2010) 25:544–51. doi: 10.1093/humrep/dep399 [DOI] [PubMed] [Google Scholar]
- 3.Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. (2016) 106:6–15. doi: 10.1016/j.fertnstert.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 4.Gu Y, Zhou G, Zhou F, Li Y, Wu Q, He H, et al. Gut and vaginal microbiomes in PCOS: implications for Women's health. Front Endocrinol (Lausanne). (2022) 13:808508. doi: 10.3389/fendo.2022.808508, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou G, Gu Y, Zhou F, Zhang H, Zhang M, Zhang G, et al. Adipocytes-derived extracellular vesicle-miR-26b promotes apoptosis of cumulus cells and induces polycystic ovary syndrome. Front Endocrinol (Lausanne). (2021) 12:789939. doi: 10.3389/fendo.2021.789939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou G, Gu Y, Zhou F, Zhang M, Zhang G, Wu L, et al. The emerging roles and therapeutic potential of extracellular vesicles in infertility. Front Endocrinol (Lausanne). (2021) 12:758206. doi: 10.3389/fendo.2021.758206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calcaterra V, Cena H, Sottotetti F, Hruby C, Madini N, Zelaschi N, et al. Low-calorie ketogenic diet: potential application in the treatment of polycystic ovary syndrome in adolescents. Nutrients. (2023) 15:3582. doi: 10.3390/nu15163582, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. (2021) 106:e1071–83. doi: 10.1210/clinem/dgaa839 [DOI] [PubMed] [Google Scholar]
- 9.Liao B, Qiao J, Pang Y. Central regulation of PCOS: abnormal neuronal-reproductive-metabolic circuits in PCOS pathophysiology. Front Endocrinol (Lausanne). (2021) 12:667422. doi: 10.3389/fendo.2021.667422, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niu Z, Lin N, Gu R, Sun Y, Feng Y. Associations between insulin resistance, free fatty acids, and oocyte quality in polycystic ovary syndrome during in vitro fertilization. J Clin Endocrinol Metab. (2014) 99:E2269–76. doi: 10.1210/jc.2013-3942, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng X, Huang Q, Long S, Zhong Q, Mo Z. Mitochondrial dysfunction in polycystic ovary syndrome. DNA Cell Biol. (2020) 39:1401–9. doi: 10.1089/dna.2019.5172, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Torchen LC. Cardiometabolic risk in PCOS: more than a reproductive disorder. Curr Diab Rep. (2017) 17:137. doi: 10.1007/s11892-017-0956-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber TM, Dimitriadis GK, Andreou A, Franks S. Polycystic ovary syndrome: insight into pathogenesis and a common association with insulin resistance. Clin Med (Lond). (2016) 16:262–6. doi: 10.7861/clinmedicine.16-3-262, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H, Oh JY, Sung YA, Chung H. Is insulin resistance an intrinsic defect in asian polycystic ovary syndrome? Yonsei Med J. (2013) 54:609–14. doi: 10.3349/ymj.2013.54.3.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang AY, Lalia AZ, Jenkins GD, Dutta T, Carter RE, Singh RJ, et al. Combining a nontargeted and targeted metabolomics approach to identify metabolic pathways significantly altered in polycystic ovary syndrome. Metabolism. (2017) 71:52–63. doi: 10.1016/j.metabol.2017.03.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F, Chen Z, Chen M, Chen G, Huang Q, Yang X, et al. Reduced stress-associated FKBP5 DNA methylation together with gut microbiota dysbiosis is linked with the progression of obese PCOS patients. NPJ Biofilms Microbiomes. (2021) 7:60. doi: 10.1038/s41522-021-00231-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Liu Y, Lv J, Zhang D, Hu K, Li J, et al. Differential Lipidomic characteristics of children born to women with polycystic ovary syndrome. Front Endocrinol (Lausanne). (2021) 12:698734. doi: 10.3389/fendo.2021.698734, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Kim HS, Lee HJ, Oh JY, Sung YA. Metabolic effects of polycystic ovary syndrome in adolescents. Ann Pediatr Endocrinol Metab. (2015) 20:136–42. doi: 10.6065/apem.2015.20.3.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mu L, Zhao Y, Lai Y, Li R, Qiao J. Insulin resistance and beta-cell dysfunction and the relationship with cardio-metabolic disorders among women with polycystic ovary syndrome. Clin Endocrinol. (2018) 89:779–88. doi: 10.1111/cen.13832, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Gu Y, Zhou G, Zhou F, Wu Q, Ma C, Zhang Y, et al. Life modifications and PCOS: old story but new Tales. Front Endocrinol (Lausanne). (2022) 13:808898. doi: 10.3389/fendo.2022.808898, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zore T, Joshi N, Lizneva D, Azziz R. Polycystic ovarian syndrome: long-term health consequences. Semin Reprod Med. (2017) 35:271–81. doi: 10.1055/s-0037-1603096 [DOI] [PubMed] [Google Scholar]
- 23.Helvaci N, Yildiz BO. The impact of ageing and menopause in women with polycystic ovary syndrome. Clin Endocrinol. (2022) 97:371–82. doi: 10.1111/cen.14558, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Brower MA, Hai Y, Jones MR, Guo X, Chen YDI, Rotter JI, et al. Bidirectional Mendelian randomization to explore the causal relationships between body mass index and polycystic ovary syndrome. Hum Reprod. (2019) 34:127–36. doi: 10.1093/humrep/dey343, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Zhu Z, Kraft P, Deng Q, Stener-Victorin E, Jiang X. Genomic correlation, shared loci, and causal relationship between obesity and polycystic ovary syndrome: a large-scale genome-wide cross-trait analysis. BMC Med. (2022) 20:66. doi: 10.1186/s12916-022-02238-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baqer LS, Ahmeid MS, Al-Obaidi AH. Evaluation the effect of metformin on hormones SERUM levels in women with polycystic ovary syndrome. Tikrit J Pure Sci. (2023) 22:1–5. doi: 10.25130/tjps.v22i9.865 [DOI] [Google Scholar]
- 27.El-Hafez H, Elrakhawy MM, El-Aziz SA, El-Eshmawy MM. Thyroid function and volume are associated with anthropometric measurements and insulin resistance in Egyptian women with polycystic ovary syndrome. J Diabetes Metab. (2013) 4:2. doi: 10.4172/2155-6156.1000288 [DOI] [Google Scholar]
- 28.Bar-El Dadon S, Reifen R. Vitamin a and the epigenome. Crit Rev Food Sci Nutr. (2017) 57:2404–11. doi: 10.1080/10408398.2015.1060940 [DOI] [PubMed] [Google Scholar]
- 29.Blaner WS. Vitamin a signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. (2019) 197:153–78. doi: 10.1016/j.pharmthera.2019.01.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lingaiah S, Morin-Papunen L, Piltonen T, Sundström-Poromaa I, Stener-Victorin E, Tapanainen JS. Serum retinol-binding protein 4 levels in polycystic ovary syndrome. Endocr Connect. (2019) 8:709–17. doi: 10.1530/EC-19-0116, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sopher AB, Gerken AT, Blaner WS, Root JM, McMahon DJ, Oberfield SE. Metabolic manifestations of polycystic ovary syndrome in nonobese adolescents: retinol-binding protein 4 and ectopic fat deposition. Fertil Steril. (2012) 97:1009–15. doi: 10.1016/j.fertnstert.2012.01.111, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann TJ, Choquet H, Yin J, Banda Y, Kvale MN, Glymour M, et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics. (2018) 210:499–515. doi: 10.1534/genetics.118.301479, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanley K. Design of randomized controlled trials. Circulation. (2007) 115:1164–9. doi: 10.1161/CIRCULATIONAHA.105.594945 [DOI] [PubMed] [Google Scholar]
- 34.Lawlor DA. Commentary: two-sample Mendelian randomization: opportunities and challenges. Int J Epidemiol. (2016) 45:908–15. doi: 10.1093/ije/dyw127, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekula P, del Greco M F, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Burgess S, Butterworth A, Malarstig A, Thompson SG. Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ. (2012) 345:e7325. doi: 10.1136/bmj.e7325 [DOI] [PubMed] [Google Scholar]
- 38.Tyrmi JS, Arffman RK, Pujol-Gualdo N, Kurra V, Morin-Papunen L, Sliz E, et al. Leveraging northern European population history: novel low-frequency variants for polycystic ovary syndrome. Hum Reprod. (2022) 37:352–65. doi: 10.1093/humrep/deab250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Lu T, Pettersson-Kymmer U, Stewart ID, Butler-Laporte G, Nakanishi T, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet. (2023) 55:44–53. doi: 10.1038/s41588-022-01270-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferkingstad E, Sulem P, Atlason BA, Sveinbjornsson G, Magnusson MI, Styrmisdottir EL, et al. Large-scale integration of the plasma proteome with genetics and disease. Nat Genet. (2021) 53:1712–21. doi: 10.1038/s41588-021-00978-w, PMID: [DOI] [PubMed] [Google Scholar]
- 41.The Endometrial Cancer Association Consortium. Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. (2020) 26:252–8. doi: 10.1038/s41591-020-0751-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsson SC, Burgess S, Michaelsson K. Association of Genetic Variants Related to Serum calcium levels with coronary artery disease and myocardial infarction. JAMA. (2017) 318:371–80. doi: 10.1001/jama.2017.8981, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. (2012) 21:223–42. doi: 10.1177/0962280210394459, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulinskaya E, Dollinger MB. An accurate test for homogeneity of odds ratios based on Cochran's Q-statistic. BMC Med Res Methodol. (2015) 15:49. doi: 10.1186/s12874-015-0034-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pers TH, Timshel P, Hirschhorn JN. SNPsnap: a web-based tool for identification and annotation of matched SNPs. Bioinformatics. (2015) 31:418–20. doi: 10.1093/bioinformatics/btu655, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semmes EC, Vijayakrishnan J, Zhang C, Hurst JH, Houlston RS, Walsh KM. Leveraging genome and phenome-wide association studies to investigate genetic risk of acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. (2020) 29:1606–14. doi: 10.1158/1055-9965.EPI-20-0113, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez Paris V, Solon-Biet SM, Senior AM, Edwards MC, Desai R, Tedla N, et al. Defining the impact of dietary macronutrient balance on PCOS traits. Nat Commun. (2020) 11:5262. doi: 10.1038/s41467-020-19003-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexaki A, Clarke BA, Gavrilova O, Ma Y, Zhu H, Ma X, et al. De novo sphingolipid biosynthesis is required for adipocyte survival and metabolic homeostasis. J Biol Chem. (2017) 292:3929–39. doi: 10.1074/jbc.M116.756460, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang ZQ, Lee SY, Kim HJ, Kim JR, Kim SJ, Hong IK, et al. Endotoxin activates de novo sphingolipid biosynthesis via nuclear factor kappa B-mediated upregulation of Sptlc2. Prostaglandins Other Lipid Mediat. (2011) 94:44–52. doi: 10.1016/j.prostaglandins.2010.12.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivanova MM, Agoulnik IU, ME LL. Editorial: sphingolipid metabolism and cancer. Front Oncol. (2022) 12:1049494. doi: 10.3389/fonc.2022.1049494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swain E, Stukey J, McDonough V, Germann M, Liu Y, Sturley SL, et al. Yeast cells lacking the ARV1 gene harbor defects in sphingolipid metabolism. Complementation by human ARV1. J Biol Chem. (2002) 277:36152–60. doi: 10.1074/jbc.M206624200, PMID: [DOI] [PubMed] [Google Scholar]
- 56.Rumora AE, Guo K, Alakwaa FM, Andersen ST, Reynolds EL, Jørgensen ME, et al. Plasma lipid metabolites associate with diabetic polyneuropathy in a cohort with type 2 diabetes. Ann Clin Transl Neurol. (2021) 8:1292–307. doi: 10.1002/acn3.51367, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guan M, Dong TS, Subramanyam V, Guo Y, Bhatt RR, Vaughan A, et al. Improved psychosocial measures associated with physical activity may be explained by alterations in brain-gut microbiome signatures. Sci Rep. (2023) 13:10332. doi: 10.1038/s41598-023-37009-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palacios N, Lee JS, Scott T, Kelly RS, Bhupathiraju SN, Bigornia SJ, et al. Circulating plasma metabolites and cognitive function in a Puerto Rican cohort. J Alzheimers Dis. (2020) 76:1267–80. doi: 10.3233/JAD-200040, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang J, Zhuo H, Zhang X, Jiang R, Ji J, Deng L, et al. A novel biomarker Linc00974 interacting with KRT19 promotes proliferation and metastasis in hepatocellular carcinoma. Cell Death Dis. (2014) 5:e1549. doi: 10.1038/cddis.2014.518, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Xu X, Peng C, Qin Y, Gao T, Jing J, et al. BRAF(V600E)-induced KRT19 expression in thyroid cancer promotes lymph node metastasis via EMT. Oncol Lett. (2019) 18:927–35. doi: 10.3892/ol.2019.10360, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z, Moresco P, Yan R, Li J, Gao Y, Biasci D, et al. Carcinomas assemble a filamentous CXCL12-keratin-19 coating that suppresses T cell-mediated immune attack. Proc Natl Acad Sci USA. (2022) 119:e2119463119. doi: 10.1073/pnas.2119463119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saha SK, Kim K, Yang GM, Choi HY, Cho SG. Cytokeratin 19 (KRT19) has a role in the reprogramming of Cancer stem cell-like cells to less aggressive and more drug-sensitive cells. Int J Mol Sci. (2018) 19:1423. doi: 10.3390/ijms19051423, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhee H, Kim HY, Choi JH, Woo HG, Yoo JE, Nahm JH, et al. Keratin 19 expression in hepatocellular carcinoma is regulated by fibroblast-derived HGF via a MET-ERK1/2-AP1 and SP1 Axis. Cancer Res. (2018) 78:1619–31. doi: 10.1158/0008-5472.CAN-17-0988 [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Xu X, Zhang Z, Xue C, Kong Z, Wu S, et al. Linc-KILH potentiates Notch1 signaling through inhibiting KRT19 phosphorylation and promotes the malignancy of hepatocellular carcinoma. Int J Biol Sci. (2021) 17:768–80. doi: 10.7150/ijbs.52279, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krüger J, Fischer A, Breunig M, Allgöwer C, Schulte L, Merkle J, et al. DNA methylation-associated allelic inactivation regulates keratin 19 gene expression during pancreatic development and carcinogenesis. J Pathol. (2023) 261:139–55. doi: 10.1002/path.6156, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Mi L, Liang N, Sun H. A comprehensive analysis of KRT19 combined with immune infiltration to predict breast Cancer prognosis. Genes (Basel). (2022) 13:1838. doi: 10.3390/genes13101838, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alesi S, Ee C, Moran LJ, Rao V, Mousa A. Nutritional supplements and complementary therapies in polycystic ovary syndrome. Adv Nutr. (2022) 13:1243–66. doi: 10.1093/advances/nmab141, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Che X, Chen Z, Liu M, Mo Z. Dietary interventions: a promising treatment for polycystic ovary syndrome. Ann Nutr Metab. (2021) 77:313–23. doi: 10.1159/000519302, PMID: [DOI] [PubMed] [Google Scholar]
- 69.Yadav AS, Isoherranen N, Rubinow KB. Vitamin a homeostasis and cardiometabolic disease in humans: lost in translation? J Mol Endocrinol. (2022) 69:R95–R108. doi: 10.1530/JME-22-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data for this study are included in the article or uploaded as Supplementary material. Full GWAS summary statistics for the exposure and outcome data used in the manuscript can be found at https://www.ebi.ac.uk/gwas, https://gwas.mrcieu.ac.uk/.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.