Abstract
Oral Campylobacter species are rarely reported to cause extraoral infections. Here we present three cases of extraoral abscess caused by an oral Campylobacter sp. and a Streptococcus sp. The Campylobacter species were all isolated anaerobically and identified by sequencing analysis of the 16S rRNA gene. The cases included a breast abscess caused by Campylobacter rectus and a non-group A beta-hemolytic streptococcus in a patient with lymphoma, a liver abscess caused by Campylobacter curvus and an alpha-hemolytic streptococcus in a patient with complicated ovarian cancer, and a postobstructive bronchial abscess caused by C. curvus and group C beta-hemolytic Streptococcus constellatus in a patient with lung cancer. The abscesses were drained or resected, and the patients were treated with antibiotics with full resolution of the lesions. The C. curvus cases are likely the first reported infections by this organism, and the C. rectus case represents the second such reported extraoral infection.
The genus Campylobacter currently contains 16 species. Most species are zoonotic, and Campylobacter jejuni and Campylobacter coli are the major human pathogens, mainly causing diarrhea (7). Several Campylobacter species are commonly found in the oral cavity, and a few, such as C. rectus, C. gracilis, and C. showae, have been implicated in human periodontal disease (6, 8). Extraoral infections by these organisms, however, are rarely reported (7). Campylobacter curvus, originally isolated from human sources without much medical information (11), is also found orally but not related to periodontal health or disease (6). Thus, the medical significance for C. curvus and its reservoirs in addition to the gingiva are largely unknown. Here we report three cases of extraoral abscess caused by a streptococcus and an oral Campylobacter sp., C. rectus or C. curvus. The cases occurred from April 2003 to September 2004 at The University of Texas M. D. Anderson Cancer Center in Houston, Texas, a 500-bed tertiary care cancer hospital. Approximately 2,100 wound and abscess specimens were cultured annually.
Case 1.
A 32-year-old woman who had just completed anticancer chemotherapy a week previously for her large-cell lymphoma presented to the emergency department for fever of 1-day duration and gradually worsening tenderness of the left breast for a few days. Physical examination revealed hypotension (97/64 mm Hg), fever (38.5°C), and an indurated, tender, reddened area around the left nipple. Laboratory examination revealed anemia (hemoglobin, 85 g/liter) and profound neutropenia (white blood cells [WBC], 0.2 × 109/liter) that had not been present 11 days earlier. Thus, the diagnosis of breast cellulitis with neutropenia was made. The cellulitis likely developed from the site of a previous nipple piercing that was removed 1 month earlier, prior to the start of chemotherapy. The patient was admitted to the hospital, and empirical antibiotic treatment with vancomycin, clindamycin, and aztreonam was initiated.
With antibiotic treatment, the patient's fever and breast cellulitis improved. Her neutropenia resolved in a week with treatment with granulocyte-stimulating factor. A percutaneous drainage of the breast infection was performed 10 days after onset, and the content showed many WBC with cultures growing a non-group A beta-hemolytic streptococcus and a gram-negative anaerobe that could not be identified by routine anaerobic test methods. With control of the infection, she was discharged from the hospital. The infection eventually resolved 4 weeks after onset, and no relapse occurred during the 2-month follow-up.
Case 2.
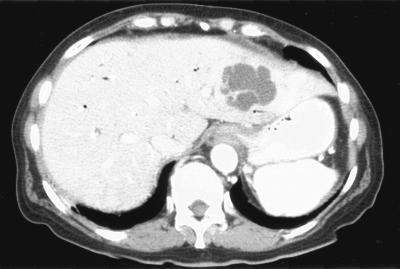
A 68-year-old woman with a complicated cancer history presented for diagnosis and management of newly found liver lesions. The patient had had stage IV ovarian cancer for 5 years and undergone tumor reduction surgery and many cycles of radiation therapy and various chemotherapy regimens. A year and half earlier, bile duct obstruction and dilatation were found secondary to tumor metastasis to the duodenum, and a biliary stent was placed, which had been relatively problem-free since. Six months earlier, she had had significant gastrointestinal bleeding that required blood transfusions and arterial embolization of the gastroduodenal artery for control. Four weeks earlier, she was hospitalized for neutropenic fever (39.6°C with nadir neutrophil count of 0.31 × 109/liter) that developed 6 days after another cycle of Taxotere treatment. The neutropenia resolved 4 days later, but she continued to have low-grade fever that was initially thought to be secondary to urinary tract infection, for which she had been taking ciprofloxacin. A significant leukocytosis from her normalized baseline (WBC, 14.3 × 109/liter from 4.7 × 109/liter) was noted 1 day before imaging studies of the abdomen and pelvis; the computed tomography revealed three liver lesions: a 5.5-cm one in the left lobe (Fig. 1) and two in the right lobe (6.5 cm and 1.6 cm). These findings raised the question of tumor metastasis versus infection. Pertinent negative findings included normal liver function tests and the lack of significant abnormality of the bile duct and its stent on imaging studies. The patient also had diabetes mellitus requiring insulin.
FIG. 1.
Computed tomography showing the 5.5-cm left lobe liver abscess caused by alpha-hemolytic Streptococcus sp. and Campylobacter curvus in case 2. Other imaging slices showed the right lobe abscesses caused by Enterococcus sp. and Escherichia coli.
An ultrasound-guided fine-needle aspiration of the left-side liver lesion was performed. The aspirate showed marked acute inflammation without evidence of tumor, and cultures grew an alpha-hemolytic streptococcus and an anaerobe that was barely reactive biochemically. The liver abscesses were drained percutaneously, and the same alpha-hemolytic streptococcus was again isolated from the same lesion. The drainage content from the right-side liver lesion grew Enterococcus sp. and Escherichia coli. The patient was treated with piperacillin and tazobactam; the leukocytosis resolved 13 days after the diagnostic procedure and antibiotic therapy, or 4 weeks after its initial rise. At completion of 6 weeks of antibiotics, follow-up imaging studies showed full resolution of the lesions.
Case 3.
A 59-year-old man underwent surgery for resection of a right upper lobe lung cancer (stage T2N0M0). During the operation, an area of pneumonia distal to the tumor was noted with expression of some purulent discharge from a segmental bronchus. The discharge was sent for microbiology workup. Two months earlier, the patient had had an episode of pneumonia that eventually led to the diagnosis of lung cancer. That pneumonia resolved with antibiotic therapy, and he completed the course of amoxicillin-clavulanate 10 days prior to surgery. After the course of antibiotic, however, the patient had complained of worsening shortness of breath and occasional headache despite lack of fever and chills.
A Gram stain of the discharge showed many WBC, and cultures grew a streptococcus and a gram-negative anaerobe. The streptococcus was beta-hemolytic and carried Lancefield group C antigen. The anaerobe was nonreactive on routine tests and thus not identified. The infected lung tissue was resected along with the tumor, and the patient recovered from the operation. He has been monitored for 11/2 years without recurrence.
Microbiologic studies.
To identify the anaerobic organisms, sequencing analysis of the 16S rRNA gene was performed (3). The method amplified and sequenced a large portion of the gene from each organism, and the sequences were queried to the GenBank (National Center for Biotechnology Information, National Institutes of Health) for best matches (1). The organism from case 1 matched completely (532 of 532 nucleotides) with C. rectus and C. showae (GenBank accessions AF550659 and AF550655) (2) in the middle region of the 16S rRNA gene. To differentiate between the two species, the head region of the gene was amplified and sequenced; the best GenBank match was with C. rectus (96.7%, 265/274) (L04317) (13). Together for both regions, the matches were 98.9%, consistent with the identification of C. rectus or a variant of it. The organisms from cases 2 and 3 both matched best, 100% (530/530) and 99.8% (573/574), respectively, with C. curvus (AF550652) (2). The matches with other Campylobacter species were all less than 99.4%. Thus, these organisms were confidently identified as C. curvus. In addition, the curved morphology of the C. curvus strains was also appreciated. The streptococcus from case 3 was identified as Streptococcus constellatus by the sequencing method. The streptococci from the other cases were not identified to species level.
None of the Campylobacter strains grew under microaerophilic conditions (GasPak). They also failed to grow on the selective Campy agar (BBL, Becton Dickinson Microbiology Systems, Cockeysville, MD). None reacted on API 20A (BioMerieux, Marcy-I'Etoile, France) or RapID ANA II (Remel, Norcross, GA).
The clinical and microbiologic data of the cases are summarized in Table 1.
TABLE 1.
Cases of Campylobacter rectus and Campylobacter curvus infections
| Feature | Case 1 | Case 2 | Case 3 | |
|---|---|---|---|---|
| Age in yrs, sexa | 32, F | 68, F | 59, M | |
| Underlying disease and risk | Lymphoma, neutropenia, nipple piercing | Ovarian cancer, surgery, GIb bleeding, biliary stent, diabetes, and neutropenic fever | Lung cancer, obstruction | |
| Site of infection | Breast abscess | Liver abscess | Pneumonia with abscess | |
| Organisms | Non-group A beta-hemolytic Strepto- coccus sp., Campylobacter rectus | Alpha-hemolytic Streptococcus sp., Campylobacter curvus | Group C beta-hemolytic Streptococcus constellatus, Campylobacter curvus | |
| Management | Drainage, antibiotics | Drainage, antibiotics | Resected with tumor | |
| Outcome | Cured | Cured | Cured |
F, female; M, male.
GI, gastrointestinal.
Conclusions.
The species C. rectus and C. curvus were established in the early 1980s as Wolinella recta and Wolinella curva, respectively (10, 11). They were transferred to the genus Campylobacter in 1991 based on phylogenetic studies (12). C. rectus has been implicated in periodontitis (6, 8), and a case of human chest wall infection with coisolation of Actinomyces viscosus, a gram-positive anaerobe, has been reported (9). This patient did not have known predispositions. The dental and medical significance of C. curvus is unknown. To our knowledge, the present C. curvus cases represent the first report of disease caused or contributed to by the organism.
Oral bacteria are a common cause of abscesses that may form from direct local extension to distant sites through lymphohematogenous spread. Streptococci and anaerobes, such as Fusobacterium nucleatum and a species of Peptostreptococcus, are the frequent culprits (4). Our cases further add C. rectus and C. curvus to the list of such anaerobes. The pathogenesis of our cases was related to the underlying cancer and its complications. In case 1, neutropenia and nipple piercing were the probable risk factors for the abscess formation, and oral contamination or hematogenous seeding after chemotherapy-related mucositis and neutropenia was the likely source. In case 2, multiple risk factors existed, including the history of surgery, tumor metastasis in the abdomen with resultant compromise of local anatomy and circulation, neutropenic fever, gastrointestinal bleeding, and diabetes. At diagnosis, the liver abscess was already advanced (5.5 cm); hence, it was likely that the organisms were seeded during the time of neutropenia that occurred 4 weeks earlier and was followed by persistent low-grade fever. The source of organisms was either the oral cavity or the gastrointestinal tract, although the latter as a reservoir has not been documented. In case 3, the infection from beta-hemolytic streptococcus and C. curvus likely resulted from aspiration of oral flora and cancer obstruction. In all patients, the infection further complicated cancer management significantly.
Our cases occurred during a 1.5-year period, suggesting that these oral Campylobacter infections may be not so rare among cancer patients. The lack of previous recognition was likely due to the difficulty of identification. Had the sequencing method for the 16S rRNA gene not been used for the current organisms, they would have been unidentified as well. These strains and the earlier clinical strain (9) were also isolated anaerobically, contrary to a previous study showing that C. rectus and C. curvus are microaerophilic, not anaerobic (5). We attribute this difference to strain variations. The coisolation of a Streptococcus sp., many of which are part of oral and gastrointestinal flora and are well known to cause various infections, and an oral Campylobacter sp. is unlikely to be incidental, and the interaction may warrant further investigation.
ADDENDUM IN PROOF
Abbott et al. newly showed strong evidence that Campylobacter curvus causes gastroenteritis and diarrhea, which supports the notion of the gastrointestinal tract as a reservoir for C. curvus, in addition to the oral cavity (S. L. Abbott, M. Waddington, D. Lindquist, J. Ware, W. Cheung, J. Ely, and J. M. Janda, J. Clin. Microbiol. 43:585-588, 2005).
Acknowledgments
This work was supported in part by a University Cancer Foundation grant (to X.Y.H.) from The University of Texas M. D. Anderson Cancer Center and by the National Institutes of Health grant CA16672 for the Sequencing Core Facility.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Gorkiewicz, G., G. Feierl, C. Schober, F. Dieber, J. Kofer, R. Zechner, and E. L. Zechner. 2003. Species-specific identification of campylobacters by partial 16S rRNA gene sequencing. J. Clin. Microbiol. 41:2537-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han, X. Y., A. S. Pham, J. J. Tarrand, P. K. Sood, and R. Luthra. 2002. Rapid and accurate identification of mycobacteria by sequencing hypervariable regions of the 16S ribosomal RNA gene. Am. J. Clin. Pathol. 118:796-801. [DOI] [PubMed] [Google Scholar]
- 4.Han, X. Y., S. J. Weinberg, S. Prabhu, S. J. Hassenbusch, G. N. Fuller, J. J. Tarrand, and D. P. Kontoyiannis. 2003. Fusobacterial brain abscess: a review of five cases and an analysis of possible pathogenesis. J. Neurosurg. 99:693-700. [DOI] [PubMed] [Google Scholar]
- 5.Han, Y. H., R. M. Smibert, and N. R. Krieg. 1991. Wolinella recta, Wolinella curva, Bacteroides ureolyticus, and Bacteroides gracilis are microaerophiles, not anaerobes. Int. J. Syst. Bacteriol. 41:218-222. [DOI] [PubMed] [Google Scholar]
- 6.Macuch, P. J., and A. C. Tanner. 2000. Campylobacter species in health, gingivitis, and periodontitis. J. Dent. Res. 79:785-792. [DOI] [PubMed] [Google Scholar]
- 7.Nachamkin, I. 2003. Campylobacter and Arcobacter, p. 585-608. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 8.Siqueira, J. F., Jr., and I. N. Rocas. 2003. Campylobacter gracilis and Campylobacter rectus in primary endodontic infections. Int. Endod. J. 36:174-180. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel, C. A., and G. Telford. 1984. Isolation of Wolinella recta and Actinomyces viscosus from an actinomycotic chest wall mass. J. Clin. Microbiol. 20:1187-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanner, A. C. R., S. Badger, C.-H. Lai, M. A. Listgarten, R. A. Visconti, and S. S. Socransky. 1981. Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from humans with periodontal disease. Int. J. Syst. Bacteriol. 31:432-445. [Google Scholar]
- 11.Tanner, A. C. R., M. A. Listgarten, and J. L. Ebersole. 1984. Wolinella curva sp. nov.: “Vibrio succinogenes” of human origin. Int. J. Syst. Bacteriol. 34:275-282. [Google Scholar]
- 12.Vandamme, P., E. Falsen, R. Rossau, B. Hoste, P. Segers, R. Tytgat, and J. De Ley. 1991. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int. J. Syst. Bacteriol. 41:88-103. [DOI] [PubMed] [Google Scholar]
- 13.Wesley, I. V., L. Schroeder-Tucker, A. L. Baetz, F. E. Dewhirst, and B. J. Paster. 1995. Arcobacter-specific and Arcobacter butzleri-specific 16S rRNA-based DNA probes. J. Clin. Microbiol. 33:1691-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]