Abstract
Adult ADHD is associated with increased risk for suicide attempts, as indicated by investigations of population- and community-based cohorts. However, there is little data regarding suicide attempts in a clinical setting. To address this, we used a comprehensively phenotyped clinical adult ADHD (aADHD) cohort to assess to which extent comorbidity, psychosocial adversity, personality, and ADHD symptoms contribute to suicidal behavior in ADHD. Furthermore, we investigated a triallelic variation in the serotonin transporter-linked polymorphic region (5-HTTLPR), which has previously been associated with suicidal behavior. Depression, substance use, eating, and posttraumatic stress disorders were independently associated with past suicide attempts, whereas anxiety, somatoform, and obsessive–compulsive spectrum disorders showed no association. Pulmonary diseases also showed an association with suicidal behavior. Psychosocial factors including occupational status, marital status/living situation, externalizing behavior and psychiatric family history were strongly associated with past suicide attempts. ADHD symptoms of inattention and hyperactivity/impulsivity were not associated with past suicide attempts after adjustment for psychiatric comorbidity and psychosocial adversity. However, the personality trait of neuroticism fully mediated the association between depression and suicidal behavior. 5-HTTLPR was not associated with suicidal behavior, but an interaction with ADHD symptoms and subtype was found. Our data suggest that psychiatric comorbidity and psychosocial adversity are key factors for suicidal behavior in aADHD, with neuroticism representing a critical mediator of the association between depression and suicidality. Further research, preferentially with longitudinal study designs is needed to better understand causal factors for suicidal behavior to enable effective preventive action.
Supplementary Information
The online version contains supplementary material available at 10.1007/s44192-024-00103-3.
Keywords: ADHD, Suicide attempt, Psychosocial adversity, Comorbidity, Serotonin, 5-HTTLPR
Introduction
Adult patients living with attention-deficit/hyperactivity disorder (ADHD) face multiple challenges in daily life due to their disorder. They have to cope with problems that arise from ADHD core symptoms resulting in distraction, forgetfulness, restlessness, impulsiveness, and emotional irritability [1]. It is thought that these symptoms are caused by complex alterations in fronto-striatal and fronto-limbic brain circuits, which impact executive and affective processing leading to cognitive disturbances and emotional-motivational difficulties [2]. Consequently, adults with ADHD tend to have lower work and financial success [3], and impaired social and romantic relationships [4, 5]. Furthermore, aADHD patients often suffer from comorbid psychiatric and somatic diseases, with comorbidity being usually the rule rather than the exception [6, 7]. Consequently, ADHD patients are significantly more likely to die prematurely, largely due to a higher rate of lethal (traffic) accidents [8, 9]. Previous studies also suggested an association of ADHD with suicide attempts [10–16], potentially due to the associated high mental disease burden and affective distress. Thus, the consequences of living with ADHD can not only be detrimental for social functioning and professional life, but may actually threaten life.
Our research focused on what has been called “The Dark Side of ADHD” in a recent article, namely suicidal behavior [12]. Suicide and suicide attempts severely affect relatives and friends of a patient, and pose an emergency for psychiatric health professionals. Suicide attempts generally follow psychologically stressful situations and mental states from which attempters ‘do not see a way out’, with more than 90% of suicides ascribable to psychiatric disorders [17]. Female sex, psychosocial adversity, psychiatric disorders, and also somatic morbidities (e.g. cardiovascular and pulmonary diseases) have been identified as general risk factors for suicidality [18–22]. Choices of action are influenced by personal beliefs and attitudes towards the self and the environment, moral values, and spontaneous emotions and urges, all depending on the individual biological, environmental, and socio-cultural background, part of which might not be empirically accessible. Questions regarding cognition, motivation, and impulses driving suicidal behavior can only be approximated by statistical methods, due to their complexity. Nonetheless, reliable, identifiable factors which influence the decision to attempt suicide are critical, as they can inform and improve individual risk assessment and clinical decision-making to enable effective preventive action. Next to individual mental, psychosocial, and health-related factors, genetic variation has been suggested as a hereditary risk factor for suicide attempts [23].
Among the neurobiological factors associated with suicidal behavior, early studies focused on the role of the serotonergic system [24]. The first studies on this topic suggested biochemical alterations with lower metabolites of serotonin in hindbrain regions [25] and in the cerebrospinal fluid [26] of suicide victims. SPECT/PET studies implicated altered serotonin transporter (5-HTT, SERT, SLC6A4) and receptor profiles in suicidal patients, with lower 5-HTT and lower 5-HT1A availability in suicide attempters [27–29]. Lower 5-HTT binding potential was also described in a study of postmortem brain tissue from suicide victims, who had previously been diagnosed with major depression [30]. In a prospective study investigating SLC6A4 mRNA expression levels in peripheral blood mononuclear cells, lower baseline SLC6A4 expression predicted suicidal ideation, and early increases in SLC6A4 expression levels preceded suicide attempts [31]. Data for the neurochemical involvement of serotonin and the 5-HTT gene in suicidal behavior has been supported by genetic studies, with initial twin studies suggesting an overall heritability for suicidal behavior of up to 55% [32]. 5-HTT is a classical reuptake transporter crucial for synaptic serotonin availability and the action of serotonin reuptake inhibiting antidepressants [33]. 5-HTT expression depends on the activity of its promotor region which is influenced by a 44 base pair (bp) insertion/deletion polymorphism, termed 5-HTTLPR, with a short (S) allele reducing 5-HT expression and a long (L) allele increasing 5-HT expression [34, 35]. Additionally, SNP rs25531 (chr17:30,237,328, GRCh38.p14) within the L allele of the length polymorphic region also affects 5-HTT expression [36]. The G allele of SNP rs25531 associated with the L-allele (LG) leads to lower serotonin transporter expression comparable to the expression level in S allele carriers, whereas LA carriers exhibit higher expression levels [37]. Numerous studies have investigated the possible effect of 5-HTTLPR on suicidality with inconsistent results. The first meta-analysis found no general association between 5-HTTLPR and suicidal behavior, but revealed an association of suicide attempts with the S allele in subjects with the same psychiatric diagnosis, and an association of the S allele with violent suicide attempts [38]. A second meta-analysis found a general association between 5-HTTLPR genetic variation and suicidal behavior [39]. The third and most recent meta-analysis found only an association of low expressing S and LG alleles with violent suicide attempts but not with general suicidal behavior [40]. Due to these inconsistent findings, it has been discussed that the 5-HTTLPR-mediated effect on 5-HTT expression might only play a role under certain environmental conditions, e.g. in interaction with (early life) stress [41].
Although not extensively covered by previous research, some variables (such as female sex and comorbid depressive or anxiety disorder) have already been described as associated with suicidal behavior in aADHD [12, 13], consistent with previously reported general risk factors for suicide attempts [19, 42, 43]. However, previous studies on suicidal behavior in aADHD investigated large-scale surveys or public health registries [12, 13]. Data from deeply phenotyped clinical cohorts is currently missing. We therefore utilised our large clinical aADHD cohort to investigate which comorbid conditions and psychosocial factors are associated with suicidal behavior in ADHD, and to which extent these factors contribute to a history of suicide attempt, with the aim to deliver a more relevant and complete picture of suicidal behavior in ADHD. Along with comorbidity and psychosocial adversity, we also explored the possible role of ADHD subtype (i.e. predominantly inattentive, predominantly hyperactive/impulsive or combined symptom expression), ADHD core symptoms, and personality factors for suicidal behavior. Lastly, we investigated whether genetic variation in 5-HTTLPR (L/S allele; rs25531 SNP) was associated with suicidality, to attempt to clarify previously contradictory reports.
Participants and methods
Adult ADHD cohort
Our cross-sectional cohort of aADHD patients was recruited within the framework of a DFG-funded clinical research group on ADHD (CRU125) between 2003 and 2011. Detailed descriptions of the study cohort, its recruitment, and comorbidity patterns with axis-I and axis-II psychiatric diagnoses were previously published [6, 44]. Study participants were adult patients (age range 18–65 years) with an ADHD diagnosis based on DSM-IV criteria recruited from in- and outpatients of the Department of Psychiatry, Psychosomatics and Psychotherapy at the Center of Mental Health in Würzburg, Germany. Exclusion criteria were IQ lower than 80, comorbid bipolar disorder, and schizophrenic psychoses. To study suicidal behavior we included all study participants from whom clinical interview data on past suicide attempts were available (n = 927). Sex and age distribution were balanced with 461 female patients (mean age: 35.7 years, SD = 9.4) and 466 male patients (mean age: 33.4, SD = 10.9).
Clinical characterization
Past suicide attempts were documented by asking “did you ever try to kill yourself?” followed by detailed questions on suicidal intent. Suicide attempts were then categorized according to the Feuerlein scale differentiating between serious suicide attempt (SSA) with clear intent to die, non-habitual deliberate self-harm (DSH), parasuicidal pause (SP) and parasuicidal gesture (SG) [45]. A semi-structured clinical interview was conducted to assess current DSM-IV symptoms of inattention and hyperactivity/impulsivity. ADHD subtype, i.e. inattentive (IA) subtype (n = 240), hyperactive/impulsive (HI) subtype (n = 71), and combined (C) subtype (n = 616) was diagnosed according to the number of symptoms with at least 6/9 symptoms required for the respective syndromic domain to be rated as positive. Lifetime comorbidity with axis-I psychiatric disorders was assessed by the SCID-I interview based on DSM-IV diagnostic criteria. However, for the construction of broader disease categories we applied newer DSM-V criteria (e.g. by excluding obsessive–compulsive disorder and PTSD from anxiety disorders). SCID-I interview data were available for 868 patients (436 female, 432 male). Depressive disorders included major depression, dysthymia and mood disorders due to a general medical condition. Anxiety disorders included panic disorder, agoraphobia, social phobia, generalized anxiety disorder, and anxiety disorder due to a general medical condition. Substance use disorders (SUD) included both lifetime abuse and dependency from all relevant substance groups. The spectrum of obsessive–compulsive disorders included OCD, hypochondria and body dysmorphic disorder. Somatoform disorders included somatization disorder, somatoform disorder and pain disorder. Anorexia nervosa, bulimia nervosa, and binge eating were included in the category of eating disorders. Posttraumatic stress disorder (PTSD) was also assessed. Additionally, we interviewed patients about their general medical record. Comorbid somatic diseases were assigned to the broad categories of autoimmune (including allergies), musculoskeletal, pulmonary, cardiovascular, endocrine, neurologic, skin, gastrointestinal, urogenital, blood, and neoplastic diseases. Psychosocial adversity factors were constructed to represent five different psychosocial life domains, which were rated positive if impaired. These were occupational status (unskilled and/or unemployed or retired early), positive psychiatric family history in 1st or 2nd degree relatives, externalizing/oppositional behavior (involved in physical fights several times and/or reported by police or law suit with condemnation), marital and living situation (divorced, more than two times married, living separately and/or living alone) and educational attainment (class repetition and/or school dropout). Additionally, we used NEO-PI-R self-rating questionnaires to assess the Big Five personality traits of neuroticism, extraversion, openness, agreeableness, and conscientiousness [46]. NEO-PI-R data were available for 761 patients (82.1%, 394 female, 367 male).
Genotyping of the triallelic 5-HTTLPR
DNA was extracted from EDTA blood using standard methods based on the protocol from [47]. We genotyped the 5-HTTLPR by polymerase chain reaction (PCR) with previously established primers (f-5’-TGC CGC TCT GAA TGC CAG CAC, r-5’-GGG ATT CTG GTG CCA CCT AGA CG), leading to a PCR product of 419 bp for the S allele and 462 bp for the L allele. The PCR product was then digested with MspI for 3 h at 37 °C, leading to distinguishable band sizes for LA vs. LG alleles, depending on rs25531 genotype. Detailed information regarding the genotyping procedure is given in the supplementary information.
Statistical analysis
All statistical analyses were performed with SPSS version 29 (IBM). Reported p values are nominal. For the investigation of sex-dependence of suicide attempts, we performed two-sided chi-square analyses for suicide attempt vs. no suicide attempt and female vs. male sex, both for all suicide attempts and for the different subtypes of suicidal behavior according to the Feuerlein scale [45]. To clarify the role of psychiatric comorbidity for suicidal behavior we regressed past suicide attempts on different psychiatric disease categories by means of multivariable binary logistic regression analyses with age and sex as additional covariates. We then built a comorbidity score as sum of all significant psychiatric comorbidities and tested the performance of models with different quantitative disease load (e.g. 0 comorbities vs. 1 or more comorbidities, 0 vs. 1 vs. 2 or more comorbidities, etc.) in subsequent logistic regression models. The comorbidity score comprising 4 levels with 0, 1, 2, 3 or more psychiatric comorbidities performed best and was therefore used as a covariate in further regression models of dimensional ADHD symptom load and personality traits.
An analogous procedure was undertaken for psychosocial adversity by first assessing the significance of the different psychosocial variables as independent variables in a multivariable regression model with past suicide attempt as the dependent variable adjusting for age and sex, and then building an overall psychosocial adversity score as the sum of psychosocial variables significantly associated with past suicide attempts. Next, we investigated if investigator-rated DSM-IV ADHD symptom counts of inattention and hyperactivity/impulsivity and personality traits (assessed by the NEO-PI-R questionnaire) were associated with past suicide attempts independent of age, sex, psychiatric comorbidity, and psychosocial adversity by employing multivariable logistic regression models. Finally, mediation models were utilized with significant comorbidities (X) as independent state variables, significant personality trait variables as mediating variables (M), and past suicide attempt as an independent variable (Y), to disentangle the contribution of state vs. trait variables for suicidal behavior in ADHD patients. To this end, we used PROCESS for SPSS version 4.2 beta by Andrew F. Hayes [48] to calculate the direct and indirect effect of X on Y with a number of bootstrap samples of 5,000 and for a 95% confidence interval.
For the genetic association study we grouped low-expressing allele combinations S and LG as S’ vs. the high-expressing LA allele. We then calculated S-dominant, and S-L-codominant inheritance models with genotype as an independent variable, and age, sex, comorbidity scores, and psychosocial adversity scores as covariates, with past suicide attempt as a binary dependent variable by binary logistic regression.
Results
Association of past suicide attempts with sex and ADHD subtype
The overall frequency of lifetime suicide attempts was 20.9%. Suicide attempts were much more frequent in female (26.9%) compared to male (15%) study participants (OR = 2.08 [1.50, 2.89], p = 8.81 × 10–6). Analyses of suicidal behavior subcategories according to the Feuerlein scale revealed a similar trend, with an increased frequency of severe suicide attempts with clear intent to die (OR = 1.61 [1.00, 2.60], p = 0.049) and also non-habitual deliberate self-harm (OR = 2.56 [1.45, 4.51], p = 7.93 × 10–4) in female participants. Parasuicidal behavior (parasuicidal gesture and parasuicidal pause) was not associated with sex (see Table 1). An association between suicidal behavior and ADHD subtype occurred only for parasuicidal gestures, which were more common in patients with combined (6.3%) than in patients with predominantly inattentive (1.7%) and predominantly hyperactive/impulsive (1.4%) subtypes (chi-square analysis, p = 0.006) (see Supplementary Table 1).
Table 1.
Sex-dependence of past suicide attempts
| Attempt | No attempt | Total | OR [95% CI] | Chi-square p-value |
|
|---|---|---|---|---|---|
| All SA | |||||
| Female | 124 (26.9%) | 337 (73.1%) | 461 | 2.08 [1.50, 2.89] | <0.001 |
| Male | 70 (15.0%) | 396 (85.0%) | 466 | ||
| Total | 194 (20.9%) | 733 (79.1%) | 927 | ||
| SSA | |||||
| Female | 46 (10.0%) | 415 (90.0%) | 461 | 1.61 [1.00, 2.60] | 0.049 |
| Male | 30 (6.4%) | 436 (93.6%) | 466 | ||
| Total | 76 (8.2%) | 851 (91.8%) | 927 | ||
| DSH | |||||
| Female | 43 (9.3%) | 418 (90.7%) | 461 | 2.56 [1.45, 4.51] | < 0.001 |
| Male | 18 (3.9%) | 448 (96.1%) | 466 | ||
| Total | 61 (6.6%) | 866 (93.4%) | 927 | ||
| SG | |||||
| Female | 27 (5.9%) | 434 (94.1%) | 461 | 1.64 [0.88, 3.06] | 0.114 |
| Male | 17 (3.6%) | 449 (96.4%) | 466 | ||
| Total | 44 (4.7%) | 883 (95.3%) | 927 | ||
| SP | |||||
| Female | 8 (1.7%) | 453 (98.3%) | 461 | 1.63 [0.53, 5.02] | 0.391 |
| Male | 5 (1.1%) | 461 (98.9%) | 466 | ||
| Total | 13 (1.4%) | 914 (98.6%) | 927 | ||
SA Suicide attempt, SSA Severe suicide attempt (with clear intent to die), DSH Non-habitual deliberate self-harm, SG Parasuicidal gesture, SP Parasuicidal pause, Chi-Square analyses, OR Odds ratio, CI Confidence interval. Level of significance was set at p < 0.05 and significant values are displayed in bold
Association of past suicide attempts with comorbidity
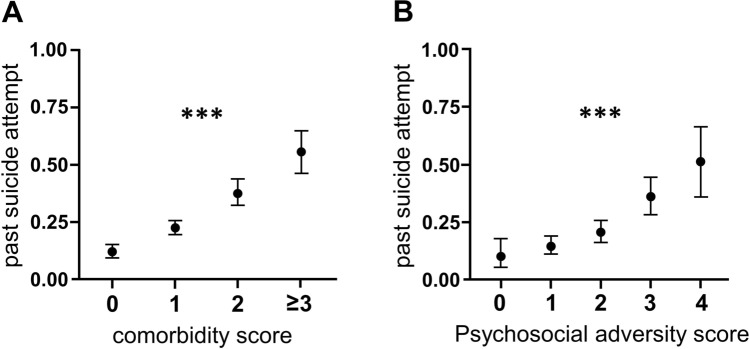
Past suicide attempts were associated with depressive disorders (OR = 2.18 [1.48, 3.20], p = 7.57 × 10–5), substance use disorders (OR = 2.00 [1.39, 2.89], p = 1.96 × 10–4), eating disorders (2.44 [1.43, 4.17], p = 0.001), and posttraumatic stress disorder (OR = 2.58 [1.14, 5.82], p = 0.023). Somatoform disorders and OCD spectrum disorders were not associated with past suicide attempts. Anxiety disorders showed an association with suicidal behavior only in the univariable analysis (OR = 1.81, p = 0.001), but not in the multivariable logistic regression model with adjustment for other psychiatric comorbidities (p = 0.127) (see Table 2). Psychiatric comorbidity had an additive effect on suicidal behavior, as depicted by comorbidity scores (0 vs. 1 vs. 2 vs. 3 and more comorbid psychiatric disorders significantly associated with past suicide attempts; OR = 2.32 [1.87, 2.88], p = 2.00 × 10–14; see Fig. 1). Multivariable logistic regression revealed an association of past suicide attempts with pulmonary diseases (OR = 2.14 [1.03, 4.43], p = 0.041), but with none of the other somatic diseases (see Supplementary Table 2, adjusted for age, sex, comorbidity score, and psychosocial score).
Table 2.
Association of past suicide attempts with psychiatric comorbidity
| n (%) | β (SE) | OR [95% CI] | p-value | |
|---|---|---|---|---|
| Depressive disorder | 488 (56.2) | 0.78 (0.20) | 2.18 [1.48, 3.20] | < 0.001a |
| SUD | 318 (34.3) | 0.69 (0.19) | 2.00 [1.39, 2.89] | <0.001a |
| Eating disorder | 77 (8.3) | 0.89 (0.27) | 2.44 [1.43, 4.17] | 0.001a |
| PTSD | 29 (3.1) | 0.95 (0.42) | 2.58 [1.14, 5.82] | 0.023a |
| Anxiety disorder | 208 (22.4) | 0.30 (0.20) | 1.35 [0.92, 2.00] | 0.127a |
| Somatoform disorder | 26 (2.8) | 0.40 (0.48) | 1.49 [0.58, 3.83] | 0.414 |
| OCD spectrum | 24 (2.6) | −0.74 (0.64) | 0.48 [0.14, 1.69] | 0.251 |
| Comorbidity score* | – | 0.84 (0.11) | 2.32 [1.87, 2.88] | < .001 |
Multivariable logistic regression model
OR Odds ratio, CI Confidence interval, avariables were also significant on the p < .05 level in a univariable regression model, * sum of lifetime psychiatric comorbidities significantly associated with past suicide attempts (depressive disorder, SUD, eating disorder, PTSD), SUD Substance use disorder, PTSD posttraumatic stress disorder, OCD Obsessive–compulsive disorder. Covariates: Age, sex. P values < .05 in bold
Fig. 1.
Graphs depict probability of past suicide attempt dependent on psychiatric comorbidity and psychosocial adversity scores. *** p < 0.001
Association of past suicide attempts with psychosocial adversity
Occupational status (OR = 2.18 [1.49, 3.18], p = 5.9 × 10–5), psychiatric family history (OR = 2.08 [1.35, 3.21], p = 8.9 × 10–4), externalizing behavior (OR = 1.81 [1.23, 2.66], p = 0.002) and marital/living situation (OR = 1.64 [1.14, 2.34], p = 0.007) all were associated with past suicide attempts (see Table 3). Among the investigated factors of psychosocial adversity only educational attainment was not associated with past suicide attempts (OR = 0.96 [0.68, 1.38], p = 0.840). A general psychosocial adversity score that was calculated as the sum of all factors which were significantly associated with suicidal behavior, i.e. work situation, psychiatric family history, externalizing behavior, and marital/living, was strongly associated with past suicidal behavior (OR = 1.90 [1.60, 2.27], p = 8.27 × 10–13, see Table 1 and Fig. 1).
Table 3.
Association of past suicide attempts with psychosocial adversity
| n (%) | β (SE) | OR [95% CI] | p-value | |
|---|---|---|---|---|
| Occupational status1 | 205 (22.1) | 0.78 (0.19) | 2.18 [1.49, 3.18] | < 0.001a |
| Psychiatric family history2 | 630 (68.0) | 0.73 (0.22) | 2.08 [1.35, 3.21] | < 0.001a |
| Externalizing behavior3 | 328 (35.4) | 0.59 (0.20) | 1.81 [1.23, 2.66] | 0.002a |
| Marital/living situation4 | 333 (35.9) | 0.49 (0.18) | 1.64 [1.14, 2.34] | 0.007a |
| Educational attainment5 | 385 (41.5) | −0.04 (0.18) | 0.96 [0.68, 1.38] | 0.840 |
| Psychosocial adversity score | – | 0.64 (0.09) | 1.90 [1.60, 2.27] | < 0.001 |
Multivariable logistic regression model
OR Odds ratio, CI Confidence interval, avariables were also significant on the p < .05 level in a univariable regression model. 1 unskilled and/or unemployed or early retired, 2 positive for psychiatric disorder in 1st and/or 2nd degree relatives, 3 involved in physical fights several times and/or reported by police or law suite with condemnation, 4 divorced, ≥ two times married, living separately and/or living alone, 5 class(es) repeated and/or dropped out of school. Covariates: Age, sex. P values < .05 in bold
Association of past suicide attempts with dimensional ADHD and personality traits
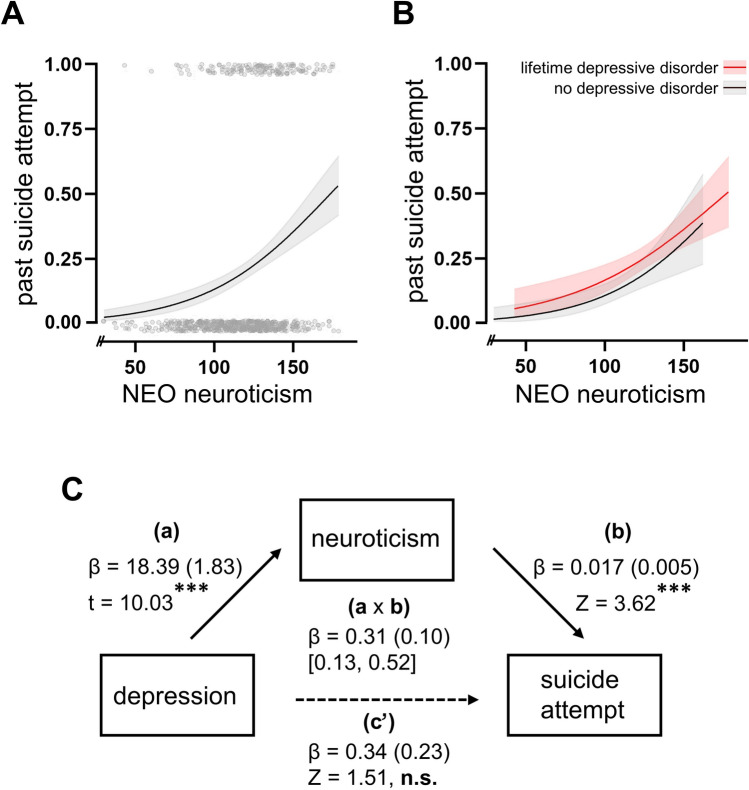
ADHD core symptoms of inattention and hyperactivity/impulsivity were not associated with past suicide attempts in a multivariable logistic regression model when adjusted for age, sex, comorbidity score, and psychosocial adversity score (see Table 4). The only personality trait associated with past suicide attempts was neuroticism (OR = 1.012 [1.002, 1.023], p = 0.019). The NEO extraversion scale (OR = 0.988 [0.979, 0.997], p = 0.008) showed an association with suicidal behavior only when investigated as univariable independent variable (see Table 4). Mediation analyses revealed that neuroticism fully mediated the association between depressive disorders and past suicide attempts (see Fig. 2), as well as the association between SUD and suicide attempts. For the latter, both regression between SUD and NEO neuroticism (β =−12.91, p < 0.001) and between NEO neuroticism and past suicide attempts (β = 0.014, p 0.003) were significant. The bootstrapped indirect effect was −0.183 with a 95% CI ranging from −0.358 to −0.057. The association between eating disorders and suicide attempts, and PTSD and suicide attempts, was not mediated by neuroticism.
Table 4.
Association of dimensional ADHD scores and personality traits with past suicide attempts
| β (SE) | OR [95% CI] | p-value | |
|---|---|---|---|
| ADHD symptoms | |||
| Inattention | 0.081 (0.063) | 1.085 [0.959, 1.227] | 0.196 |
| Hyperactivity/impulsivity | 0.068 (0.050) | 1.070 [0.971, 1.180] | 0.173 |
| Big 5 personality traits | |||
| Neuroticism | 0.012 (0.005) | 1.012 [1.002, 1.023] | 0.019a |
| Extraversion | −0.009 (0.006) | 0.991 [0.980, 1.002] | 0.115a |
| Openness | 0.002 (0.006) | 1.002 [0.990, 1.013] | 0.771 |
| Agreeableness | 0.003 (0.006) | 1.003 [0.991, 1.016] | 0.822 |
| Conscientiousness | 0.001 (0.005) | 1.001 [0.991, 1,012] | 0.907 |
Separate multivariable logistic regression models for ADHD symptoms and Big Five personality traits, OR Odds ratio, CI Confidence interval, avariables were also significant on the p < .05 level in a univariable regression model. Covariates: Age, sex, psychosocial adversity score, comorbidity score. P values < .05 in bold
Fig. 2.
Neuroticism as a mediator between depression and suicidal behavior
Probability of past suicide attempt dependent on NEO self-ratings of neuroticism without (A) and with differentiation between lifetime depressive disorder (B). Neuroticism fully mediates the association between lifetime depressive disorder and past suicide attempt (C), *** p < 0.001, solid lines are regression curves and shaded areas show 95% confidence intervals. Covariates: Age, sex, psychosocial adversity score, comorbidity score
Association of past suicide attempts with 5-HTT genotypes
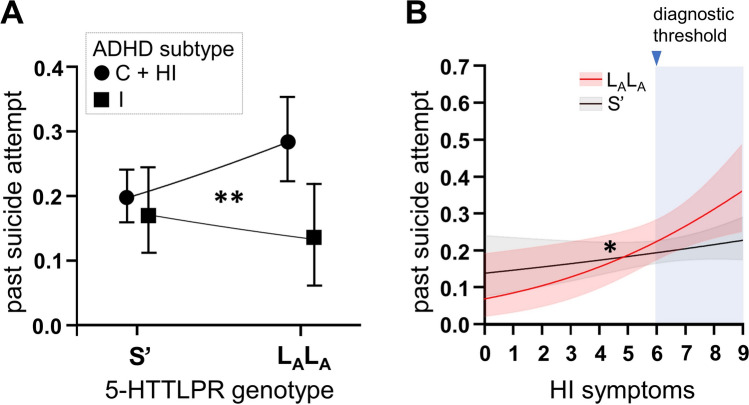
Distribution of the triallelic 5-HTTLPR did not differ from Hardy–Weinberg equilibrium in the full cohort (Chi-square test, p = 0.59), the group of suicide attempters (Chi-square test, p = 0.77), or in the group of non-attempters (Chi-square test, p = 0.71). Genotyping call rate was > 0.99. Allelic distribution of the 5-HTTLPR, and the triallelic polymorphism is given in Supplementary Table 3. The triallelic 5-HTTLPR was not associated with past suicide attempts in the full cohort after adjustment for age, sex, comorbidity, and psychosocial adversity (S’ dominant model: OR = 1.34 [0.89, 2.01], p = 0.158, codominant model: p = 0.369). However, there was an interaction effect between 5-HTTLPR genotype and ADHD subtype for the S’ dominant model (p = 0.019). Homozygous LA allele carriers in the predominantly inattentive had less suicide attempts than LALA carriers with combined or predominantly hyperactive/impulsive subtype (OR = 0.28 [0.11, 0.72], p = 0.008). No such genotype-dependent effect on past suicide attempts occurred in S’ allele carriers (OR = 1.20 [0.71, 2.01], p = 0.50). However, there was a contrary effect of more suicide attempts in the LALA (vs. S’ group) in patients with combined/hyperactive-impulsive subtype (OR = 1.86 [1.18, 2.94], p = 0.008), whereas in the predominantly inattentive patient group LALA carriers tended to have less suicide attempts than S’ allele carriers (OR = 0.41 [0.15, 1.13], p = 0.085). In line with this finding, there was an interaction effect of 5-HTTLPR genotype x DSM-IV hyperactive/impulsive ADHD symptoms (OR = 1.33 (1.06, 1.68), p = 0.015), see Fig. 3.
Fig. 3.
ADHD subtype and symptom-dependent effect of 5-HTTLPR genotype on suicidal behavior. 5-HTTLPR genotype x ADHD subtype interaction under an S-dominant model (A) and interaction effect for 5-HTTLPR genotype x investigator-rated DSM-IV HI symptoms (B). * < 0.05, ** < 0.01, logistic regression with past suicide attempts as binary outcome, and 5-HTTLPR genotype, ADHD subtype or DSM-IV symptom counts (and respective interaction terms) as independent variables, covariates: age, sex, psychiatric comorbidity, psychosocial adversity scores
Discussion
We performed a cross-sectional study of suicidal behavior in a large clinical cohort of aADHD patients to investigate the contribution of general and ADHD-specific factors to suicidal behavior in ADHD. This investigation is highly relevant for clinical practice, as our cohort was directly recruited from help-seeking in- and outpatients with a broad spectrum of psychiatric comorbidity [6]. Our findings of a higher rate of past suicide attempts in females, and the strong association of suicidality with psychiatric comorbidity and psychosocial adversity, are in accordance with previous observations in the general (psychiatric) population [19, 49, 50]. Frequency of lifetime suicide attempts in our aADHD cohort was slightly higher than the numbers reported by previous studies [11, 12], likely due to different sampling strategies. We also found an association of pulmonary diseases with suicidal behavior, highlighting the need to consider both psychiatric and somatic comorbidity in ADHD [7]. The association of neuroticism with past suicide attempts withstood adjustment for psychiatric comorbidity and psychosocial adversity, and fully mediated the association of depression and SUD with suicidality. This suggests an urgent need for psychotherapeutic interventions for low self-esteem and neurotic conflict processing, possibly independently of acute depressive episodes. Although our data do not support a general association of the triallelic 5-HTTLPR variants with suicidal behavior in ADHD, the interaction of 5-HTTLPR with clinical ADHD subtypes and investigator-rated ADHD symptoms suggests a possible differential effect of the serotonergic system in different disease courses, which should be addressed in future investigations. Therefore, future genetic studies in larger cohorts should also consider subtype-specific effects and investigate the interaction between genetic variation and different ADHD dimensions.
Previous studies have reported suicide attempt prevalence rates of ~ 15% in ADHD patients [11, 12]. Our results showed an even higher rate, with a past suicide attempt in about every fifth patient, and females twice as likely to attempt suicide as males. The higher rates of suicidal behavior in our study may be due to the use of a clinical cohort with an overall higher disease burden than in previously investigated community and population-based (health registry) samples [11, 12]. Therefore, our data may specifically reflect the clinical situation. The high prevalence of suicide attempts in ADHD patients reflects the high impact of the disorder on life quality, and suggests a need for special programs for suicide prevention or respective modules of psychoeducation to be considered in clinical practice.
We found that psychiatric comorbidity, and different factors of psychosocial adversity such as unemployment, early retirement, being divorced, living alone, delinquency, aggressive behavior, and psychiatric family history, were significantly associated with past suicide attempts. Educational attainment, (i.e. class(es) repeated or dropped out of school) was the only psychosocial factor not associated with past suicide attempts. This finding is in contrast with previous studies [51], and might be due to the fact that we investigated an adult cohort, in which environmental factors after graduation, (such as a protective family environment, stable interpersonal relationships, and work success) might negate past problems with school performance. The association of depressive disorders, SUD, eating disorders, and PTSD with suicidal behavior is consistent with previous reports of risk factors for suicide attempts [42, 52–54]. However, we found that anxiety disorders were not independently associated with past suicide attempts in our multivariable model, despite previous evidence for anxiety disorders as an independent risk factor for suicidal behavior [43]. This lack of association in our cohort may be due to the high comorbidity of anxiety disorders with other psychiatric disorders, as anxiety disorders only occurred as a single comorbidity in 12% of patients. Somatoform disorders and OCD spectrum disorders were not associated with past suicide attempts, suggesting these disorders do not play a role in suicidal behavior in ADHD, contradictory to earlier studies that found an increased risk for suicide attempts also with these disorders [55, 56]. However, it should be taken into account that the total number of somatoform disorder and OCD diagnoses in the investigated ADHD cohort was relatively low and therefore likely underpowered.
Our data also revealed a significant association between pulmonary diseases and past suicide attempts in ADHD patients for the first time. Interestingly, ADHD has previously been associated with asthmatic disease [7]. Pulmonary diseases, especially asthmatic and chronic obstructive pulmonary disease, have also been independently associated with suicidal behavior in other studies [57, 58]. Hence, our reported association between pulmonary diseases and suicidality in ADHD implies severely decreased quality of life in ADHD patients with comorbid pulmonary diseases [59].
After adjusting for psychiatric comorbidity and psychosocial adversity, ADHD symptoms were not associated with past suicide attempts, suggesting that suicidal behavior in ADHD may not be uniquely associated with ADHD-specific behavioral correlates of impulsive decision making, alterations in locomotor control, or inattention. We therefore suggest that suicide attempts in ADHD may occur as a consequence of the complex burden of living with ADHD, with its negative effects on psychosocial functioning and the increased risk for psychiatric comorbidity. Given that affected patients often describe suicide attempts as an impulsive act and previous studies suggest associations between suicidal behavior and impulsivity [60] the lack of association between impulsivity and suicidal behavior in our ADHD cohort is remarkable. However, it has to be considered that ADHD patients are already on an upper extreme of trait impulsivity and likely more homogeneous with regard to impulsive behaviors than patients without ADHD. Hence, the potential influence of impulsivity on suicidal behavior might be harder to detect in ADHD cohorts. On the other side, evidence on the association of impulsivity with suicidal behavior is mixed. A meta-analysis found an only small association [61] in line with the notion that self-reported impulsivity of suicide attempts seems to correlate poorly with trait impulsivity [62, 63]. Moreover, only some but not all facets of impulsivity seem to be associated with suicidal behavior [63].
Importantly, our data revealed a significant association of past suicide attempts with neuroticism, that withstood adjustment for psychiatric comorbidity and psychosocial adversity. The association of neuroticism with suicidality has been repeatedly described before [64, 65]. However, full mediation of the association between depression/SUD and suicidal behavior by neuroticism is of note, as it suggests that in this patient group not primarily depression and stress lead to suicide attempts, but also a more general anxious thinking style and negative self-view followed by maladaptive coping strategies. This implies that psychotherapeutic interventions for neurotic conflicts and low self-esteem should be considered as preventive for suicidal behavior in adult patients with ADHD.
This study investigates genetic variation of the 5-HTTLPR in association with suicidal behavior in aADHD patients for the first time. Our findings did not find a general association of the triallelic 5-HTTLPR polymorphism with suicide attempts in this diagnostic group. Data did reveal an interaction effect between 5-HTTLPR genotype and ADHD subtype, but this result should be interpreted with caution until it can be independently replicated. Previous research on the 5-HTTLPR and suicidal behavior has emphasized the importance of gene by environment interactions, especially the role of early life-stress and childhood trauma, with differential effects of 5-HTTLPR genetic variation reported to be dependent on childhood adversity [66, 67]. We do not have data on childhood trauma or other early life adversity in our cohort, and therefore were not able to investigate a gene by environment interaction. However, our data suggested a possible interaction between 5-HTTLPR genotype and ADHD subtype, and between hyperactive/impulsive symptoms and an increased risk for suicidal behavior in LALA carriers, which can be built upon in future studies.
A major limitation of our study is its cross-sectional design, which does not allow the investigation of possible causal inferences for comorbidity, psychosocial adversity, personality traits, 5-HTTLPR allelic variation on suicidal behavior. Due to its retrospective nature our study cannot shed light on factors potentially associated with suicidal behavior at the time of its occurrence, such as acute psychiatric conditions or medication. Longitudinal studies within the adulthood trajectory of ADHD, and bridging the gap between childhood and adulthood ADHD, are therefore needed to gain more insight. This would allow assessment of the reciprocal relationship between adverse and favorable living conditions, health-related outcomes of mental and somatic health, psychosocial well-being, and suicidal behavior, with the overarching aim of developing more targeted suicide prevention strategies.
In conclusion, psychiatric comorbidity and psychosocial adversity are major factors associated with suicidal behavior in aADHD, and seem to have a greater effect on risk than ADHD-specific symptoms. Especially high neuroticism should be further evaluated as possible risk factor for suicidal behavior in ADHD, which is also amenable for cognitive-behavioral interventions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all funding agencies that supported our research. We are grateful to all patients for their participation in the study and their constant enthusiasm despite the challenges of living with ADHD. We also would like to thank Gabriela Ortega and Nicole Steigerwald for their excellent technical support with regard to primer design and genotyping.
Author contributions
GCZ designed the study, carried out statistical analyses, and wrote the first draft of the manuscript. SG, AB, MH, RVM, and TMK contributed to data collection and analysis. MR, CPJ, AR, SKS, and KPL supervised the project and/or had leading roles in the CRU 125 project, underlying this study. All authors contributed to discussion of the study design, manuscript writing, and approved the final version of the manuscript.
Funding
This study was supported by the DFG (Project No. 413657723; Clinician Scientist-Program UNION CVD to GCZ, and CRU 125), and the European Commission Horizon 2020 Program Project Eat2beNICE (No 728018), and Serotonin and Beyond (No. 953327) to KPL.
Data availability
All research data underlying this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
This study was conducted according to the latest guidelines of the Declaration of Helsinki. All procedures have been reviewed and approved by the Ethics Committee of the University of Würzburg under ethical approval number 141/03. All participants volunteered to take part in this study and gave written informed consent.
Competing interest
CPJ received speaker's fees from derCampus, Daiichi Sankyo, Jansse-Cilag, Eli Lilly and Co, Shire, Novartis, and Medice. SKS received author's and speaker's honoraria from Takeda and Medice Arzneimittel Pütter GmbH & Co KG. AR has received honoraria from and/or serves on advisory boards for Medice, Shire/Takeda, SAGE/Biogen, Janssen, Boehringer Ingelheim, and cyclerion. All declared COI are not directly related to this work. None of the other authors reports any financial conflict of interest related to this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bjerrum MB, Pedersen PU, Larsen P. Living with symptoms of attention deficit hyperactivity disorder in adulthood: a systematic review of qualitative evidence. JBI Database System Rev Implement Rep. 2017;15(4):1080–153. 10.11124/JBISRIR-2017-003357. [DOI] [PubMed] [Google Scholar]
- 2.Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48(2):194–215. 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Jangmo A, Kuja-Halkola R, Perez-Vigil A, Almqvist C, Bulik CM, D’Onofrio B, et al. Attention-deficit/hyperactivity disorder and occupational outcomes: the role of educational attainment, comorbid developmental disorders, and intellectual disability. PLoS ONE. 2021;16(3): e0247724. 10.1371/journal.pone.0247724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eakin L, Minde K, Hechtman L, Ochs E, Krane E, Bouffard R, et al. The marital and family functioning of adults with ADHD and their spouses. J Atten Disord. 2004;8(1):1–10. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J, Faraone SV, Spencer TJ, Mick E, Monuteaux MC, Aleardi M. Functional impairments in adults with self-reports of diagnosed ADHD: A controlled study of 1001 adults in the community. J Clin Psychiatry. 2006;67(4):524–40. [DOI] [PubMed] [Google Scholar]
- 6.Gross-Lesch S, Dempfle A, Reichert S, Jans T, Geissler J, Kittel-Schneider S, et al. Sex- and subtype-related differences in the comorbidity of adult ADHDs. J Atten Disord. 2016;20(10):855–66. 10.1177/1087054713510353. [DOI] [PubMed] [Google Scholar]
- 7.Instanes JT, Klungsoyr K, Halmoy A, Fasmer OB, Haavik J. Adult ADHD and comorbid somatic disease: a systematic literature review. J Atten Disord. 2018;22(3):203–28. 10.1177/1087054716669589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalsgaard S, Ostergaard SD, Leckman JF, Mortensen PB, Pedersen MG. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190–6. 10.1016/S0140-6736(14)61684-6. [DOI] [PubMed] [Google Scholar]
- 9.Brunkhorst-Kanaan N, Libutzki B, Reif A, Larsson H, McNeill RV, Kittel-Schneider S. ADHD and accidents over the life span - A systematic review. Neurosci Biobehav Rev. 2021;125:582–91. 10.1016/j.neubiorev.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Babinski DE, Neely KA, Ba DM, Liu G. Depression and suicidal behavior in young adult men and women with ADHD: evidence from claims data. J Clin Psychiatry. 2020. 10.4088/JCP.19m13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eddy LD, Eadeh HM, Breaux R, Langberg JM. Prevalence and predictors of suicidal ideation, plan, and attempts, in first-year college students with ADHD. J Am Coll Health. 2020;68(3):313–9. 10.1080/07448481.2018.1549555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller-Thomson E, Riviere RN, Carrique L, Agbeyaka S. The dark side of ADHD: factors associated with suicide attempts among those with adhd in a national representative canadian sample. Arch Suicide Res. 2020. 10.1080/13811118.2020.1856258. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Chan BSM, Huang C, Cui X, Liu J, Lu J, et al. Suicidal behaviors and attention deficit hyperactivity disorder (ADHD): a cross-sectional study among Chinese medical college students. BMC Psychiatry. 2021;21(1):258. 10.1186/s12888-021-03247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen VC, Chan HL, Wu SI, Lee M, Lu ML, Liang HY, et al. Attention-deficit/hyperactivity disorder and mortality risk in Taiwan. JAMA Netw Open. 2019;2(8): e198714. 10.1001/jamanetworkopen.2019.8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald C, Dalsgaard S, Nordentoft M, Erlangsen A. Suicidal behaviour among persons with attention-deficit hyperactivity disorder. Br J Psychiatry. 2019. 10.1192/bjp.2019.128. [DOI] [PubMed] [Google Scholar]
- 16.Septier M, Stordeur C, Zhang J, Delorme R, Cortese S. Association between suicidal spectrum behaviors and Attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;103:109–18. 10.1016/j.neubiorev.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Bertolote JM, Fleischmann A. Suicide and psychiatric diagnosis: a worldwide perspective. World Psychiat. 2002;1(3):181–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Compton MT, Thompson NJ, Kaslow NJ. Social environment factors associated with suicide attempt among low-income African Americans: the protective role of family relationships and social support. Soc Psychiatry Psychiatr Epidemiol. 2005;40(3):175–85. 10.1007/s00127-005-0865-6. [DOI] [PubMed] [Google Scholar]
- 19.Canetto SS, Sakinofsky I. The gender paradox in suicide. Suicide Life Threat Behav. 1998;28(1):1–23. [PubMed] [Google Scholar]
- 20.Tidemalm D, Langstrom N, Lichtenstein P, Runeson B. Risk of suicide after suicide attempt according to coexisting psychiatric disorder: Swedish cohort study with long term follow-up. BMJ. 2008;337: a2205. 10.1136/bmj.a2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen BD, Stenager E, Mogensen CB, Erlangsen A. The association between heart diseases and suicide: a nationwide cohort study. J Intern Med. 2020;287(5):558–68. 10.1111/joim.13025. [DOI] [PubMed] [Google Scholar]
- 22.Chung JH, Han CH, Park SC, Kim CJ. Suicidal ideation and suicide attempts in chronic obstructive pulmonary disease: the Korea national health and nutrition examination survey (KNHANES IV, V) from 2007–2012. NPJ Prim Care Respir Med. 2014;24:14094. 10.1038/npjpcrm.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullins N, Kang J, Campos AI, Coleman JRI, Edwards AC, Galfalvy H, et al. Dissecting the shared genetic architecture of suicide attempt, psychiatric disorders, and known risk factors. Biol Psychiatr. 2022;91(3):313–27. 10.1016/j.biopsych.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nordstrom P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6:12–21. 10.1097/00004850-199206006-00003. [DOI] [PubMed] [Google Scholar]
- 25.Bourne HR, Bunney WE Jr, Colburn RW, Davis JM, Davis JN, Shaw DM, et al. Noradrenaline, 5-hydroxytryptamine, and 5-hydroxyindoleacetic acid in hindbrains of suicidal patients. Lancet. 1968;2(7572):805–8. 10.1016/s0140-6736(68)92459-8. [DOI] [PubMed] [Google Scholar]
- 26.Asberg M, Traskman L, Thoren P. 5-HIAA in the cerebrospinal fluid A biochemical suicide predictor? Arch Gen Psychiatry. 1976;33:10. 10.1001/archpsyc.1976.01770100055005. [DOI] [PubMed] [Google Scholar]
- 27.Pantazatos SP, Melhem NM, Brent DA, Zanderigo F, Bartlett EA, Lesanpezeshki M, et al. Ventral prefrontal serotonin 1A receptor binding: a neural marker of vulnerability for mood disorder and suicidal behavior? Mol Psychiatry. 2022;27(10):4136–43. 10.1038/s41380-022-01671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh YW, Ho PS, Chen CY, Kuo SC, Liang CS, Ma KH, et al. Incongruent reduction of serotonin transporter associated with suicide attempts in patients with major depressive disorder a positron emission tomography study with 4-[18F]-ADAM. Int J Neuropsychopharmacol. 2014. 10.1093/ijnp/pyu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartlett EA, Zanderigo F, Stanley B, Choo TH, Galfalvy HC, Pantazatos SP, et al. In vivo serotonin transporter and 1A receptor binding potential and ecological momentary assessment (EMA) of stress in major depression and suicidal behavior. Eur Neuropsychopharmacol. 2023;70:1–13. 10.1016/j.euroneuro.2023.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Dwork AJ, Mann JJ, et al. Serotonin receptors and suicide, major depression, alcohol use disorder and reported early life adversity. Transl Psychiatr. 2018;8(1):279. 10.1038/s41398-018-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consoloni JL, Ibrahim EC, Lefebvre MN, Zendjidjian X, Olie E, Mazzola-Pomietto P, et al. Serotonin transporter gene expression predicts the worsening of suicidal ideation and suicide attempts along a long-term follow-up of a major depressive episode. Eur Neuropsychopharmacol. 2018;28(3):401–14. 10.1016/j.euroneuro.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Voracek M, Loibl LM. Genetics of suicide: a systematic review of twin studies. Wien Klin Wochenschr. 2007;119(15–16):463–75. 10.1007/s00508-007-0823-2. [DOI] [PubMed] [Google Scholar]
- 33.Schloss P, Williams DC. The serotonin transporter: a primary target for antidepressant drugs. J Psychopharmacol. 1998;12(2):115–21. 10.1177/026988119801200201. [DOI] [PubMed] [Google Scholar]
- 34.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–4. 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 35.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–31. 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5(1):32–8. 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- 37.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–26. 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin PY, Tsai G. Association between serotonin transporter gene promoter polymorphism and suicide: results of a meta-analysis. Biol Psychiatry. 2004;55(10):1023–30. 10.1016/j.biopsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Li D, He L. Meta-analysis supports association between serotonin transporter (5-HTT) and suicidal behavior. Mol Psychiatry. 2007;12(1):47–54. 10.1038/sj.mp.4001890. [DOI] [PubMed] [Google Scholar]
- 40.Fanelli G, Serretti A. The influence of the serotonin transporter gene 5-HTTLPR polymorphism on suicidal behaviors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:375–87. 10.1016/j.pnpbp.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Delli Colli C, Borgi M, Poggini S, Chiarotti F, Cirulli F, Penninx B, et al. Time moderates the interplay between 5-HTTLPR and stress on depression risk: gene x environment interaction as a dynamic process. Transl Psychiatry. 2022;12(1):274. 10.1038/s41398-022-02035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844–50. 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 43.Nepon J, Belik SL, Bolton J, Sareen J. The relationship between anxiety disorders and suicide attempts: findings from the national epidemiologic survey on alcohol and related conditions. Depress Anxiety. 2010;27(9):791–8. 10.1002/da.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacob CP, Gross-Lesch S, Reichert S, Geissler J, Jans T, Kittel-Schneider S, et al. Sex- and subtype-related differences of personality disorders (Axis II) and personality traits in persistent ADHD. J Atten Disord. 2016;20(12):1056–65. 10.1177/1087054714521293. [DOI] [PubMed] [Google Scholar]
- 45.Feuerlein W. Attempted suicide or parasuicidal action? Tendency of suicidal behavior. Nervenarzt. 1971;42(3):127–30. [PubMed] [Google Scholar]
- 46.Costa, P. T., & McCrae, R. R. (1992). Revised NEO personality inventory (NEO-PI-R) and NEO Five factor inventory: professional manual. Odessa, FL: Psychological assessment resources.
- 47.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: observations, recommendations, and implementation. Behav Res Ther. 2017;98:39–57. 10.1016/j.brat.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Singhal A, Ross J, Seminog O, Hawton K, Goldacre MJ. Risk of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194–204. 10.1177/0141076814522033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruffaerts R, Demyttenaere K, Borges G, Haro JM, Chiu WT, Hwang I, et al. Childhood adversities as risk factors for onset and persistence of suicidal behaviour. Br J Psychiatry. 2010;197(1):20–7. 10.1192/bjp.bp.109.074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosoff DB, Kaminsky ZA, McIntosh AM, Davey Smith G, Lohoff FW. Educational attainment reduces the risk of suicide attempt among individuals with and without psychiatric disorders independent of cognition: a bidirectional and multivariable Mendelian randomization study with more than 815,000 participants. Transl Psychiatry. 2020;10(1):388. 10.1038/s41398-020-01047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilcox HC, Conner KR, Caine ED. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug Alcohol Depend. 2004;76:S11-19. 10.1016/j.drugalcdep.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Pisetsky EM, Thornton LM, Lichtenstein P, Pedersen NL, Bulik CM. Suicide attempts in women with eating disorders. J Abnorm Psychol. 2013;122(4):1042–56. 10.1037/a0034902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krysinska K, Lester D. Post-traumatic stress disorder and suicide risk: a systematic review. Arch Suicide Res. 2010;14(1):1–23. 10.1080/13811110903478997. [DOI] [PubMed] [Google Scholar]
- 55.Albert U, De Ronchi D, Maina G, Pompili M. Suicide risk in obsessive-compulsive disorder and exploration of risk factors: a systematic review. Curr Neuropharmacol. 2019;17(8):681–96. 10.2174/1570159X16666180620155941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torres ME, Lowe B, Schmitz S, Pienta JN, Van Der Feltz-Cornelis C, Fiedorowicz JG. Suicide and suicidality in somatic symptom and related disorders: a systematic review. J Psychosom Res. 2021;140: 110290. 10.1016/j.jpsychores.2020.110290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sampaio MS, Vieira WA, Bernardino IM, Herval AM, Flores-Mir C, Paranhos LR. Chronic obstructive pulmonary disease as a risk factor for suicide: a systematic review and meta-analysis. Respir Med. 2019;151:11–8. 10.1016/j.rmed.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Cheng J, Li Y, He R, Choudhry AA, Jiang J, et al. Suicidality among patients with asthma: a systematic review and meta-analysis. J Affect Disord. 2019;256:594–603. 10.1016/j.jad.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 59.Ali R, Ahmed N, Salman M, Daudpota S, Masroor M, Nasir M. Assessment of quality of life in bronchial asthma patients. Cureus. 2020;12(10): e10845. 10.7759/cureus.10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McHugh CM, Chun Lee RS, Hermens DF, Corderoy A, Large M, Hickie IB. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51–60. 10.1016/j.jpsychires.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 61.Anestis MD, Soberay KA, Gutierrez PM, Hernandez TD, Joiner TE. Reconsidering the link between impulsivity and suicidal behavior. Pers Soc Psychol Rev. 2014;18(4):366–86. 10.1177/1088868314535988. [DOI] [PubMed] [Google Scholar]
- 62.May AM, Klonsky ED. “Impulsive” suicide attempts: What do we really mean? Personal Disord. 2016;7(3):293–302. 10.1037/per0000160. [DOI] [PubMed] [Google Scholar]
- 63.Cole AB, Littlefield AK, Gauthier JM, Bagge CL. Impulsivity facets and perceived likelihood of future suicide attempt among patients who recently attempted suicide. J Affect Disord. 2019;257:195–9. 10.1016/j.jad.2019.07.038. [DOI] [PubMed] [Google Scholar]
- 64.Peters EM, John A, Bowen R, Baetz M, Balbuena L. Neuroticism and suicide in a general population cohort: results from the UK Biobank Project. BJPsych Open. 2018;4(2):62–8. 10.1192/bjo.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hafferty JD, Navrady LB, Adams MJ, Howard DM, Campbell AI, Whalley HC, et al. The role of neuroticism in self-harm and suicidal ideation: results from two UK population-based cohorts. Soc Psychiatry Psychiatr Epidemiol. 2019;54(12):1505–18. 10.1007/s00127-019-01725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32(9):2046–52. 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- 67.Vai B, Serretti A, Poletti S, Mascia M, Lorenzi C, Colombo C, et al. Cortico-limbic functional connectivity mediates the effect of early life stress on suicidality in bipolar depressed 5-HTTLPR*s carriers. J Affect Disord. 2020;263:420–7. 10.1016/j.jad.2019.11.142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All research data underlying this study are available from the corresponding author upon reasonable request.
Not applicable.