Abstract
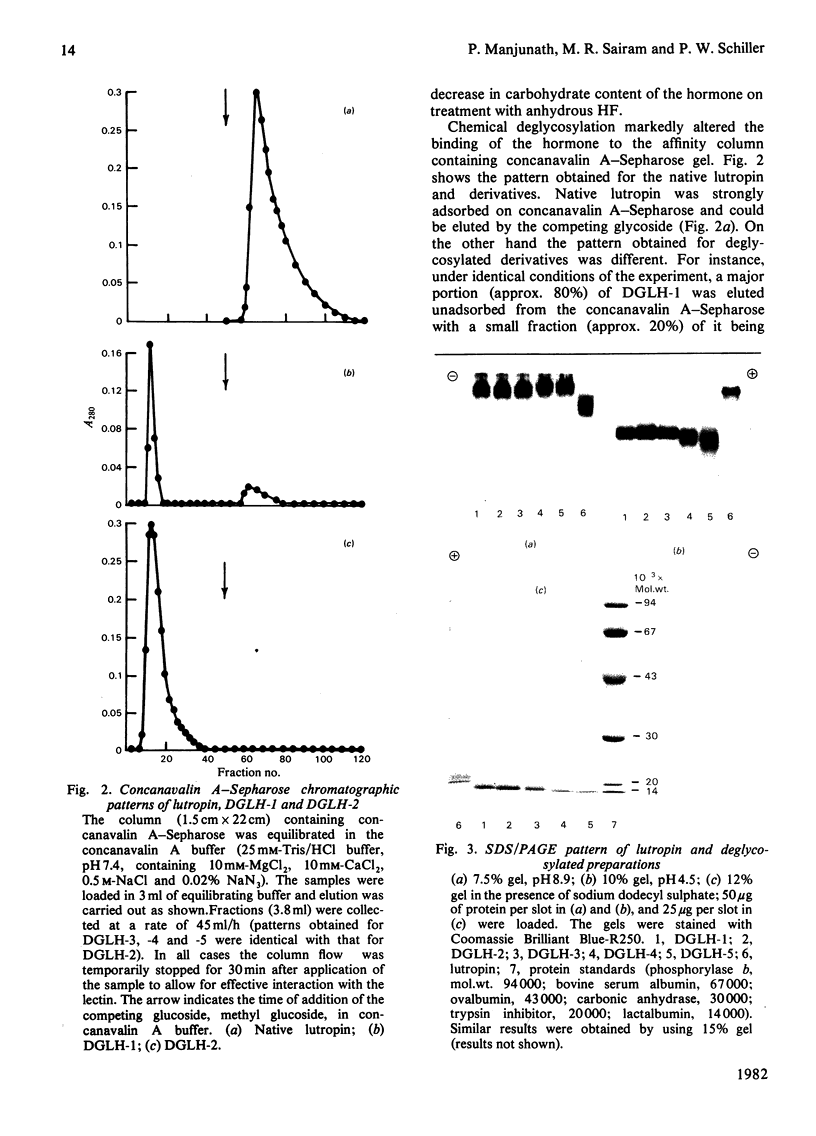
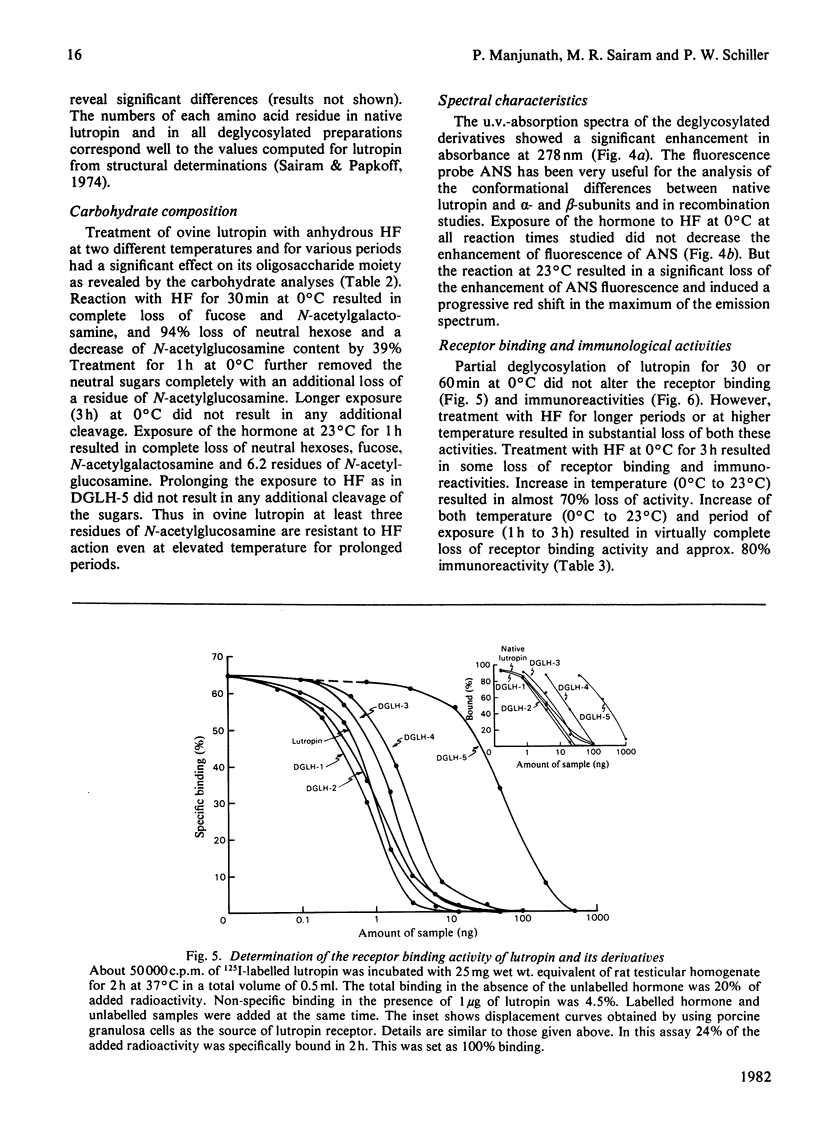
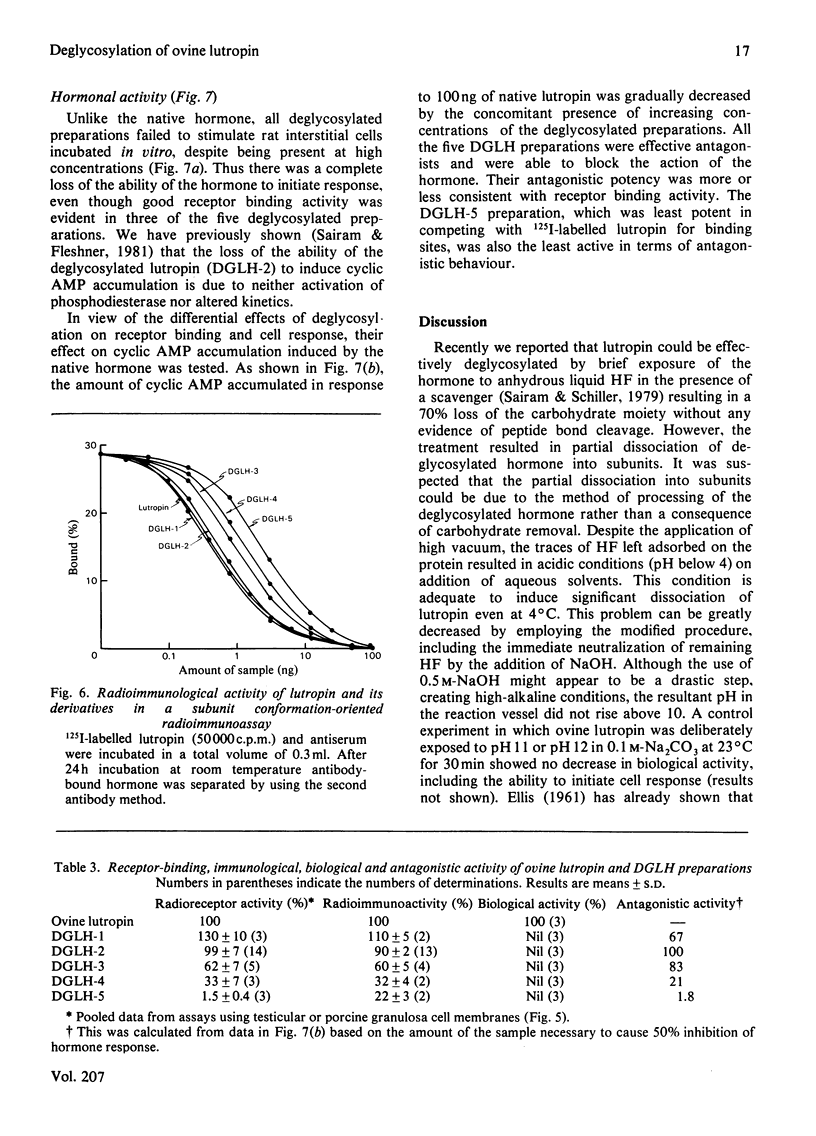
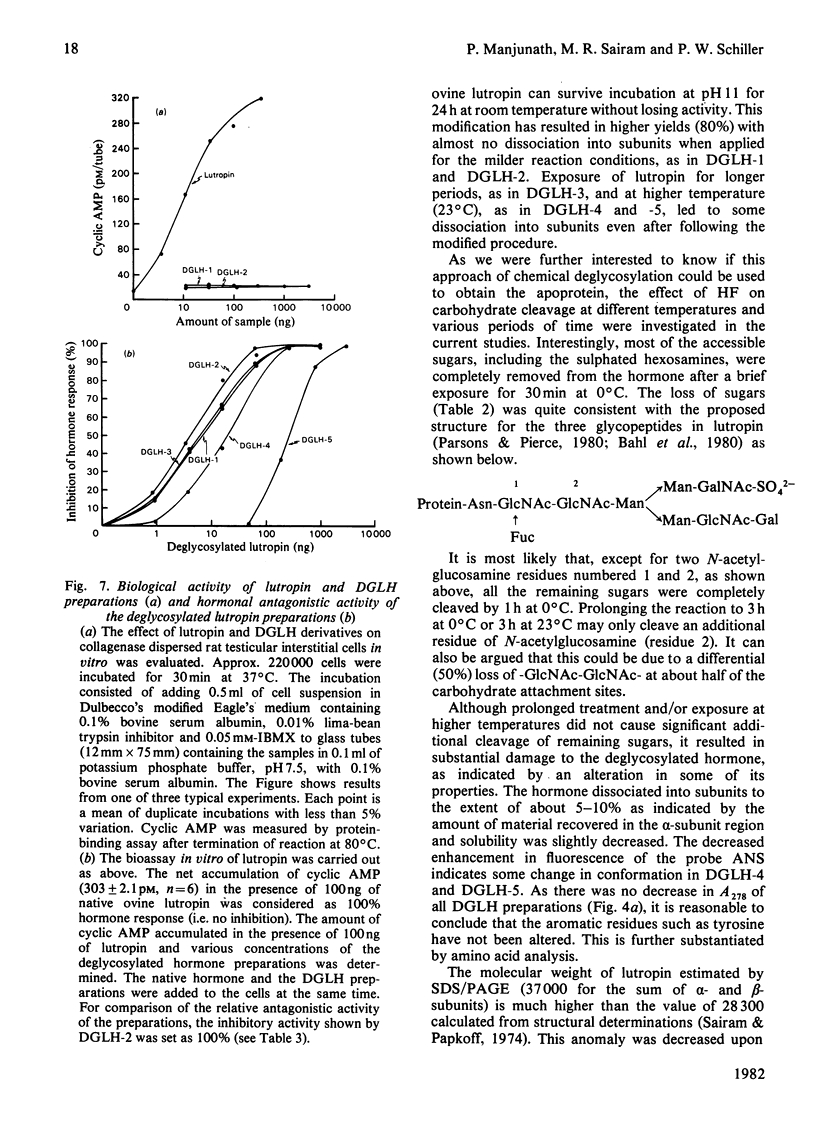
The oligomeric glycoprotein hormone, ovine lutropin was treated with anhydrous HF at 0°C for 30, 60 and 180 min and at 23°C for 60 and 180 min. The products, designated deglycosylated lutropin 1 (DGLH-1) to deglycosylated lutropin 5 (DGLH-5) respectively, were characterized by gel filtration, concanavalin A–Sepharose binding, disc electrophoresis, amino acid analysis, carbohydrate composition and spectral properties. The preparations were also evaluated for receptor binding activity and immunological activity and bioassayed in vitro in collagenase-dispersed rat interstitial cells. In DGLH-1, fucose and galactosamine were removed completely, and there was a 94% decrease in hexoses and 39% decrease in N-acetylglucosamine. Reaction with HF at 0°C for 1 or 3h led to removal of all hexoses and additional loss of hexosamines. Reactions at 23°C for either 1 or 3h were not of additional value in deglycosylation and none of the reaction conditions yielded the apohormone. All the five deglycosylated hormone preparations were not retained on immobilized-concanavalin A columns and on Sephadex G-100 they were eluted with an increased Ve/V0 ratio consistent with the loss of carbohydrate residues. Loss of all but the last of the N-acetylglucosamine residues decreased the abnormality of lutropin on sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, but did not eliminate it. Receptor binding activities of DGLH-1 and DGLH-2 were not different from that of the native hormone, but that of DGLH-3 was slightly decreased and the products obtained at 23°C (DGLH-4 and DGLH-5) had lower activity. Immunoreactivities followed a similar pattern. None of the derivatives had activity in the bioassay in vitro. All of the five derivatives inhibited the action of the native hormone in the bioassay in vitro. Their hormonal antagonistic activity was consistent with the receptor binding activity, with DGLH-5 being the least potent in this respect. The DGLH-4 and DGLH-5 preparations had undergone conformational changes as revealed by 8-anilinonaphthalene-1-sulphonate fluorescence, but this did not result in loss of quaternary structure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl O. P., Reddy M. S., Bedi G. S. A novel carbohydrate structure in bovine and ovine luteinizing hormones. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1192–1199. doi: 10.1016/0006-291x(80)90078-9. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham K. C., Bolotin C. Intrinsic and extrinsic fluorescence probes of subunit interactions in ovine lutropin. Arch Biochem Biophys. 1978 Nov;191(1):134–145. doi: 10.1016/0003-9861(78)90075-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manjunath P., Sairam M. R. Biochemical, biological, and immunological properties of chemically deglycosylated human choriogonadotropin. J Biol Chem. 1982 Jun 25;257(12):7109–7115. [PubMed] [Google Scholar]
- McKelvy J. F., Lee Y. C. Microheterogeneity of the carbohydrate group of aspergillus oryzae alpha-amylase. Arch Biochem Biophys. 1969 Jun;132(1):99–110. doi: 10.1016/0003-9861(69)90341-5. [DOI] [PubMed] [Google Scholar]
- Parsons T. F., Pierce J. G. Oligosaccharide moieties of glycoprotein hormones: bovine lutropin resists enzymatic deglycosylation because of terminal O-sulfated N-acetylhexosamines. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7089–7093. doi: 10.1073/pnas.77.12.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Sairam M. R. The effects of interstitial cell-stimulating hormone, its subunits, and recombinants on isolated rat Leydig cells. Arch Biochem Biophys. 1975 Mar;167(1):294–300. doi: 10.1016/0003-9861(75)90465-8. [DOI] [PubMed] [Google Scholar]
- Sairam M. R. Deglycosylation of ovine pituitary lutropin subunits: effects on subunit interaction and hormone activity. Arch Biochem Biophys. 1980 Oct 1;204(1):199–206. doi: 10.1016/0003-9861(80)90024-7. [DOI] [PubMed] [Google Scholar]
- Sairam M. R., Fleshner P. Inhibition of hormone-induced cyclic AMP production and steroidogenesis in interstitial cells by deglycosylated lutropin. Mol Cell Endocrinol. 1981 Apr;22(1):41–54. doi: 10.1016/0303-7207(81)90101-5. [DOI] [PubMed] [Google Scholar]
- Sairam M. R. Immunological relationships among ovine glycoprotein hormones. J Endocrinol. 1979 Nov;83(2):149–155. doi: 10.1677/joe.0.0830149. [DOI] [PubMed] [Google Scholar]
- Sairam M. R., Manjunath P. Comparison of the thermal stability characteristics of native and deglycosylated ovine pituitary lutropin. Int J Pept Protein Res. 1982 Mar;19(3):315–320. doi: 10.1111/j.1399-3011.1982.tb03044.x. [DOI] [PubMed] [Google Scholar]
- Sairam M. R., Schiller P. W. Receptor binding, biological, and immunological properties of chemically deglycosylated pituitary lutropin. Arch Biochem Biophys. 1979 Oct 1;197(1):294–301. doi: 10.1016/0003-9861(79)90248-0. [DOI] [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]



