Abstract
We designed a prospective multicenter cohort study to clarify a long-term, > 10-year prevalence and aggravation of cervical spine instabilities in rheumatoid arthritis (RA). In 2001–2002, 634 outpatients were enrolled, and 233 (36.8%) were followed for > 10 years. Cervical spine instability was defined as atlantoaxial subluxation (AAS, > 3-mm atlantodental interval), vertical subluxation (VS, < 13-mm Ranawat value), and subaxial subluxation (SAS, ≥ 2-mm irreducible vertebral translation). The aggravation was determined as ≥ 2 mm decrease of the Ranawat value in VS, ≥ 2-mm increase of slippage in SAS, and these new developments. The prevalence of VS and SAS increased during both the initial and last > 5 years (all, p ≤ 0.049). While VS aggravation was associated with pre-existing AAS (p = 0.007) and VS (p = 0.002), SAS aggravation correlated with pre-existing VS (p = 0.002). Multivariable analysis found hand mutilating changes (odds ratio [OR] = 4.048, p = 0.008), RA duration ≥ 5 years (OR = 3.711, p = 0.013), C-reactive protein (CRP) level ≥ 3.8 mg/dL (OR = 2.187, p = 0.044), and previous joint surgery (OR = 2.147, p = 0.021) as predictors for VS aggravation. Pre-existing VS (OR = 2.252, p = 0.024) and CRP ≥ 1.0 mg/dL (OR = 2.139, p = 0.013) were disclosed as predictors for SAS aggravation. Low disease activity and clinical remission before the development of VS and advanced peripheral joint destruction are essential to prevent progressive cervical spine instability in RA.
Keywords: Rheumatoid arthritis (RA), Cervical spine instability, Atlantoaxial subluxation (AAS), Vertical subluxation (VS), Subaxial subluxation (SAS), Natural history and course
Subject terms: Musculoskeletal system, Rheumatology, Rheumatic diseases, Musculoskeletal abnormalities, Rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder with unknown etiology, affecting 0.5–1% of the adult population1,2. This disease arises chronic inflammation in the synovial joints in extremities and spine, which results in characteristic deformities and carries a substantial burden not only for the individual but also for the society3. The cervical spine is a particularly common involvement in RA, which can lead to three distinctive instabilities: atlantoaxial subluxation (AAS)4–10, vertical subluxation (VS) of the axis11,12, and subaxial subluxation (SAS)13. Each instability can progress over time alone or in combination of other instabilities. Cautiously, these lesions can cause intractable neck pain and irreversible neurological damage with non-ambulation, respiratory dysfunction, and even sudden death in the worst scenario due to the potential development of spinal cord, cranial nerve, brainstem, and vertebral artery compression14. It is thus fundamental to understand the natural history of cervical spine involvement including the new onset and aggravation of instabilities. However, many prior investigations have been limited to the cross-sectional or retrospective design during < 10 years15. Because of drastic changes in RA treatment, e.g. biological agents, more recent prevalence, progression pattern, and predictive risk factors of cervical spine instabilities based on a longer-period observation are necessary.
We started a prospective multicenter cohort study of cervical spine, hand, and finger involvement in established RA fulfilling the “classical” or “definite” criteria in 2001–200216,17. At > 5-year radiographic examination in 2006–2008, 43.6% of patients without any cervical spine instabilities newly developed AAS, VS, and/or SAS with the increased prevalence of VS and SAS, which was further accelerated in those with pre-existing instability18–20. Thereafter, we further followed our RA cohort for a longer period of additional > 5 years. At > 10-year radiographic examination in 2012–2013, more plausible predictive risk factors for the development of severe cervical spine instabilities which could cause compression myelopathy were identified as mutilating changes in the hands, concomitant corticosteroid treatment, and previous peripheral joint surgery21. Meanwhile, treatment aiming at low disease activity and clinical remission was a key factor associated with the absence of instability22. However, the detailed natural course of instabilities for > 10 years were not the subject of previous studies. Therefore, the objective of the present study was to clarify the > 10-year prevalence and progression pattern—focusing on the aggravation—of cervical spine instabilities in patients with RA.
Methods
Study design and ethics statement
The current prospective observational study was performed at 21 research facilities in Hyogo, Japan. The sample size calculation was not applicable because of the explorative design. This study was designed from the same data collection cohort as our previous report21.
The study protocol was approved by the institutional review board at each facility. Written informed consent was obtained from each patient at the study enrollment. The present study was conducted in accordance with the principles of the Declaration of Helsinki and the laws and regulations of Japan.
Patients
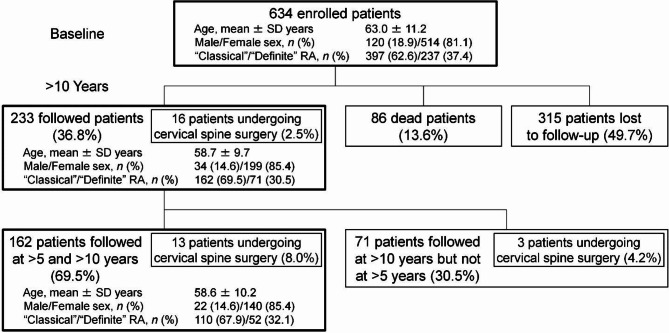
In 2001–2002, 634 Japanese outpatients who fulfilled the American Rheumatism Association 1958 criteria for ‘‘classical’’ or ‘‘definite’’ RA16 as well as the American College of Rheumatology 1987 revised criteria for RA17 were enrolled. Cervical spine instability was radiographically identified with the definition of three types. In 2012–2013, 233 (36.8%) were prospectively followed as outpatients every 3 months and radiographically assessed again after > 10 years. During the study period, 16 (6.9%) of 233 patients underwent cervical spine surgery, because of compression myelopathy in all cases. The observed myelopathy was associated with AAS alone (n = 5), VS alone (n = 1), SAS alone (n = 1), combined AAS and VS (n = 1), combined AAS and SAS (n = 1), combined VS and SAS (n = 3), or combined AAS, VS, and SAS (n = 4). The mean follow-up period was 11.0 ± 1.9 years. Letter and telephone survey revealed that 86 (13.6%) of 634 patients had died; thus, the final follow-up rate was 50.3% (Fig. 1).
Fig. 1.
Number of outpatients, fulfilling the American Rheumatism Association 1958 criteria for “classical” or “definite” RA, enrolled at baseline and prospectively followed as outpatients, patients undergoing cervical spine surgery, dead patients, or patients lost to follow-up at > 5 and > 10 years. RA rheumatoid arthritis, SD standard deviation.
Then, 162 (69.5%) of 233 patients had radiographic checkups both at > 5 (2006–2008) and > 10 (2012–2013) years, who were additionally analyzed to clarify time-course changes in the involvement of cervical spine instabilities. Of 162 patients, 13 (8.0%) received cervical spine surgery, due to myelopathy in all cases. The detected myelopathy was associated with AAS alone (n = 5), VS alone (n = 1), combined VS and SAS (n = 3), or combined AAS, VS, and SAS (n = 4). The mean follow-up period was 11.0 ± 0.3 years (Fig. 1).
Radiographs
Lateral cervical spine radiographs were obtained in full-flexion, neutral, and full-extension positions under a standardized protocol (exposure time 80 ms, distance 150 cm, current 250 mA, and voltage 72 kV) with the placement of a scale marker18–21. Cervical spine instability was defined as three types: AAS with the anterior atlantodental interval (ADI) of > 3 mm4,5, VS with the Ranawat value of < 13 mm11, and SAS with irreducible vertebral translation of ≥ 2 mm without osteophyte formation13. To simplify the classification of instabilities, VS combined with AAS was categorized as VS. The aggravation was determined as follows: ≥2-mm decrease of the Ranawat value in pre-existing VS, ≥ 2 mm increase of vertebral slippage and/or at multiple segments in pre-existing SAS, and new development of VS and SAS. In addition, baseline C2–C7 angle was measured to evaluate the sagittal alignment of the cervical spine. Radiographs were measured twice at a 1-week interval by each of four rheumatologists blinded to this study. The prevalence and aggravation of cervical spine instabilities were set based on the average measurement values. The presence of pre-existing instabilities was included as explanatory variables for the aggravation of VS and SAS, based on a prior report23.
Bilateral hand radiographs providing views of the wrist, carpal, and fingers were taken to classify the severity of peripheral joint destruction into five categories: Steinbrocker classification stages I–IV24 and mutilating changes25,26. As previously established26, ≥ 3 of 10 ‘‘mutilans fingers’’ in the bilateral hands were considered mutilating changes25. Radiographs were reviewed twice at a 1-week interval by another three rheumatologists blinded to this study. Steinbrocker stages and mutilating changes were determined by majority decision. Baseline RA severity was extracted as explanatory variables for VS and SAS aggravation, since it clinically depends on the number of joints with erosion within the first 5–10 years27.
Demographic, clinical, and disease characteristics
Demographic, clinical, and disease characteristics of patients were recorded at baseline. Age < 55 years10,13 and ≥ 65 years28, male sex29, RA duration ≥ 5 years27, and the presence of surgically treated peripheral joints for RA28,30,31 were marked as potential predictive risk factors of cervical spine instability. Serum C-reactive protein (CRP) level when ≥ 3.8 mg/dL, correlated with myelopathic deterioration32, or when > 1.0 mg/dL, indicating unsuccessful RA remission33, and the positivity for rheumatoid factor (RF), associated with subluxation7,10, were also listed. Thus, these were considered as explanatory variables for VS and SAS aggravation. Moreover, serum CRP level was measured again at > 10-year follow-up to evaluate time-course changes in disease activity during the study period.
Treatments
To treat RA, patients received intensive use of disease-modifying antirheumatic drugs (DMARDs): methotrexate (MTX) (≤ 8 [1999– in Japan] and ≤ 16 [2011–] mg/week), salazosulfapyridine (≤ 1000 mg/day), D-penicillamine (≤ 100 mg/day), intramuscular gold (≤ 25 mg/2 weeks), and tacrolimus (1.5–3.0 mg/day [2005–]). Oral corticosteroids (≤ 10-mg/day prednisolone) were allowed to relieve RA symptoms. In more aggressive cases, biological agents such as infliximab (3 mg/kg/8 weeks [2003–]), etanercept (10–25 mg/twice a week [2005–]), adalimumab (40 mg/2 weeks [2008–]), tocilizumab (8 mg/kg/4 weeks [2008–]), abatacept (125 mg/week [2010–]), golimumab (50–100 mg/4 weeks [2011–]), and certolizumab (200 mg/2 weeks [2013–]) or a Janus kinase (JAK) inhibitor tofacitinib (5–10 mg/day [2013–]) were applied. Patients were categorized based on drug administration at baseline and throughout ≥ 5 years during > 10 years. Patients who had therapy with tacrolimus, biological agents, or tofacitinib for ≥ 3 years were similarly identified, because these drugs had not been approved for RA at baseline. Consequently, as no patients underwent ≥ 5-year treatment with tacrolimus or tofacitinib, the use of corticosteroids, MTX, other DMARDs, and biological agents was recorded as potential predictors for VS and SAS aggravation.
Statistical analysis
Descriptive statistics for continuous variables are presented as the mean ± standard deviation, categorical variables as the frequency (percentages). The intraclass correlation coefficient (ICC) and Cohen’s kappa coefficient (κ) were calculated to assess the intraobserver and interobserver reliability for parametric and nonparametric radiographic parameters, respectively. In the entire cohort (n = 233), the prevalence of cervical spine instabilities at the baseline and endpoint of > 10-year follow-up was compared using the chi-squared test. In the subcohort (n = 162), the prevalence of instabilities at baseline, > 5-year follow-up, and > 10-year follow-up was also compared using the chi-squared test.
The chi-squared test for categorical variables and the Student’s t-test for continuous variables were used to assess differences between patients with and without the aggravation of VS and SAS at > 10-year follow-up. Univariable and multivariable logistic regression models were developed to identify independent predictive risk factors for the aggravation of VS and SAS. Variables with p < 0.050 in univariable analysis were eligible for the inclusion as potential predictors into multivariable logistic regression analysis, although CRP level at > 10-year follow-up was excluded to establish predictive models. With regard to variables having two thresholds, e.g. age and CRP, to avoid overfitting, one with a lower odds ratio (OR) in univariable analysis was not included in multivariable analysis.
All statistical tests with statistical significance set at p values of < 0.050 and < 0.010 were performed using SPSS 28.0 (IBM Corp., Armonk, NY).
Results
Intraobserver and interobserver reliability
In quantitative measurements of cervical spine radiographs, the intraobserver reliability by ICC for parametric ADI, Ranawat value, subaxial vertebral translation, and C2–C7 angle was 0.91–0.95, 0.81–0.85, 0.80–0.84, 0.82–0.85 respectively. The interobserver reliability by ICC was 0.96, 0.91, 0.91, and 0.90, respectively. In qualitative assessments of hand radiographs, the intraobserver and interobserver reliability by κ for nonparametric Steinbrocker stages and mutilating changes was 0.75–0.81 and 0.87, respectively. All ICC and κ values indicated an acceptable reproducibility.
Baseline demographic, clinical, and disease characteristics of dead or dropped-out patients at > 10-year follow-up
In this study cohort (n = 634), 86 (13.6%) were passed away while 315 (49.7%) were lost to follow-up during > 10 years. At baseline, the mean age of dead or dropped-out patients (n = 401) was 65.2 ± 11.3 years, which was older than 58.7 ± 9.7 years in the followed patients (n = 233) (p < 0.001). Then, 86 (21.4%) dead or dropped-out patients were male, whereas men in the followed patients were 34 (14.6%) (p = 0.034). While serum CRP level was 2.2 ± 2.8 mg/dL in the dead or dropped-out patients and 2.1 ± 2.6 mg/dL in the followed patients (p = 0.720), the distribution of Steinbrocker stages I–II, stages III–IV, and mutilating changes were 97 (24.2%), 246 (61.3%), and 58 (14.5%), respectively, in the dead or dropped-out patients versus 44 (18.9%), 167 (71.7%), and 22 (9.4%), respectively, in the followed patients (p = 0.027). Furthermore, the prevalence of pre-existing AAS, VS, and SAS in the dead or dropped-out patients was 138 (34.4%), 103 (25.7%), and 67 (16.7%), respectively, which were also higher than those of followed patients (AAS, 30.5%; VS, 19.3%; SAS, 7.7%) (p = 0.421). Factors associated with the study participation partially exists in demographic, clinical, and disease characteristics of patients.
The prevalence of cervical spine instabilities during > 10 years
In 233 patients undergoing radiographic measurements at baseline and > 10-year follow-up, the number of those without any cervical spine instability decreased from 114 (48.9%) at baseline to 47 (20.2%) at endpoint (p < 0.001). The prevalence of AAS showed no statistically significant increase from 71 (30.5%) to 75 (32.2%) (p = 0.689). However, patients with VS significantly increased in number from 45 (19.3%) to 93 (39.9%) (p < 0.001). Patients with SAS alone also significantly increased from 3 (1.3%) to 18 (7.7%) (p < 0.001). Furthermore, SAS combined with or without other instabilities significantly increased from 18 (7.7%) to 92 (39.5%) (p < 0.001). Consequently, patients with at least one instability significantly increased from 119 (51.1%) to 186 (79.8%) (p < 0.001) (Fig. 2).
Fig. 2.
The prevalence of cervical spine instability comprising AAS, VS, and SAS at baseline and > 10-year follow-up in 233 patients. To simplify, VS combined with AAS was categorized as VS. The chi-squared test was used for the comparison. AAS atlantoaxial subluxation, VS vertical subluxation, SAS subaxial subluxation.
In 162 patients undergoing consecutive radiographic measurements at baseline, > 5-year follow-up, and > 10-year follow-up, the number of those without any cervical spine instability significantly decreased from 85 (52.5%) to 46 (28.4%) during the initial > 5 years (p < 0.001), which further decreased to 32 (20.4%) during the last > 5 years but did not reach statistical significance (p = 0.069). The prevalence of AAS was 44 (27.2%) at baseline, 55 (34.0%) at > 5-year follow-up, and 48 (29.6%) at > 10-year follow-up, presenting no significant difference between both the initial and last > 5-year periods (p = 0.185 and p = 0.622, respectively). Meanwhile, VS significantly increased from 30 (18.5%) at baseline, 50 (30.9%) at > 5-year follow-up (p = 0.010), and consistently to 67 (41.4%) at > 10-year follow-up (p = 0.049). Also, SAS with or without other instabilities significantly increased from 8 (4.9%), 42 (25.9%) (p < 0.001), to 60 (37.0%) (p = 0.031) during > 10 years (Fig. 3).
Fig. 3.
The prevalence of cervical spine instability comprising AAS, VS, and SAS at baseline, > 5-year follow-up, and > 10-year follow-up in 162 consecutively followed patients. To simplify, VS combined with AAS was categorized as VS. The chi-squared test was used for the comparison. AAS atlantoaxial subluxation, VS vertical subluxation, SAS subaxial subluxation.
Predictive risk factors for the aggravation of VS
In 233 patients, the aggravation of VS was observed in 70 (30.0%) including 48 cases with newly developed VS and 22 cases with ≥ 2-mm decrease of the Ranawat value in pre-existing VS. When 45 patients without pre-existing VS were excluded (n = 188), 26 (36.6%) of 71 with pre-existing AAS experienced the aggravation of VS, while 22 (18.8%) of 117 without pre-existing AAS demonstrated the aggravation of VS (p = 0.007). Then, 22 (48.9%) of 45 with pre-existing VS further exhibited the aggravation of VS, whereas 48 (25.5%) of 188 without pre-existing VS displayed the aggravation of VS (p = 0.002). Similarly, 7 (38.9%) of 18 with pre-existing SAS had the trend toward the aggravation of VS compared to 63 (29.3%) of 215 without pre-existing SAS, although not reaching statistical significance (p = 0.394) (Table 1).
Table 1.
The aggravation of VS and SAS based on pre-existing cervical spine instability in 233 patients at > 10-year follow-up.
| Aggravated instability | Pre-existing instability | Aggravation | p | |||
|---|---|---|---|---|---|---|
| n | n | % | ||||
| VS | AAS | (+) | 71 | 26 | 36.6 | 0.007‡ |
| (−) | 117 | 22 | 18.8 | |||
| VS | (+) | 45 | 22 | 48.9 | 0.002‡ | |
| (−) | 188 | 48 | 25.5 | |||
| SAS | (+) | 18 | 7 | 38.9 | 0.394 | |
| (−) | 215 | 63 | 29.3 | |||
| SAS | AAS | (+) | 71 | 26 | 36.6 | 0.182 |
| (−) | 117 | 32 | 27.4 | |||
| VS | (+) | 45 | 25 | 55.6 | 0.002‡ | |
| (−) | 188 | 58 | 30.9 | |||
| SAS | (+) | 18 | 9 | 50.0 | 0.184 | |
| (−) | 215 | 74 | 34.4 | |||
Calculated by the chi-squared test.
AAS atlantoaxial subluxation, SAS subaxial subluxation, VS vertical subluxation.
‡p < 0.010.
In 162 consecutively followed patients, when 30 without pre-existing VS were excluded (n = 132), pre-existing AAS more frequently presented the aggravation of VS in 12 (27.3%) at > 5 years and 17 (38.6%) at > 10 years of 44 patients than 12 (12.8%) at > 5 years and 24 (25.5%) at > 10 years of 88 patients without pre-existing AAS (p = 0.030 and p = 0.097, respectively). Moreover, 14 (46.7%) at > 5 years and 16 (53.3%) at > 10 years of 30 patients with pre-existing VS showed the aggravation of VS, which were significantly higher than 20 (15.2%) at > 5 years and 37 (28.0%) at > 10 years of 132 patients without pre-existing VS (p < 0.001 and p = 0.008, respectively). However, pre-existing SAS was not significantly associated with the aggravation of VS both at > 5 and > 10 years (p = 0.674 and p > 0.999, respectively), possibly due to the small sample size (Supplementary Table S1).
In the comparison between patients with and without the aggravation of VS, those with VS aggravation tended to have a longer duration of RA (p = 0.010)—particularly ≥ 5 years (p < 0.001), higher level of CRP at baseline (p = 0.052)—especially ≥ 3.8 mg/dL (p = 0.009), higher frequency of previous peripheral joint surgery (p < 0.001), corticosteroid administration (p = 0.008), and mutilating changes in the hands (p < 0.001) at baseline. Male patients tended to be uncommon (p = 0.035). In addition, patients with CRP levels at > 10-year follow-up ≥ 3.8 mg/dL (p = 0.024) and > 1.0 mg/dL (p = 0.001) tended to develop the aggravation of VS (Table 2).
Table 2.
Baseline and > 10-year demographic, clinical, and disease characteristics of 233 patients with and without the aggravation of VS at > 10-year follow-up.
| Patients with VS aggravation | Patients without VS aggravation | p | |
|---|---|---|---|
| (n = 70) | (n = 163) | ||
| Age, mean ± SD years | 58.6 ± 9.4 | 58.7 ± 9.8 | 0.918 |
| <55 years, n (%) | 21 (30.0) | 56 (34.4) | 0.806 |
| 55–64 years, n (%) | 27 (38.6) | 58 (35.6) | |
| ≥65 years, n (%) | 22 (31.4) | 49 (30.1) | |
| Male sex, n (%) | 5 (7.1) | 29 (17.8) | 0.035† |
| RA duration, mean ± SD years | 16.3 ± 10.1 | 12.1 ± 10.8 | 0.010‡ |
| ≥5 years, n (%) | 65 (92.9) | 119 (73.0) | < 0.001‡ |
| CRP, mean ± SD mg/dL at baseline | 2.7 ± 2.9 | 2.0 ± 2.7 | 0.052 |
| ≥3.8 mg/dL, n (%) | 20 (28.6) | 23 (14.1) | 0.009‡ |
| >1.0 mg/dL, n (%) | 43 (61.4) | 93 (57.1) | 0.535 |
| CRP, mean ± SD mg/dL at > 10-year follow-up | 2.0 ± 1.7 | 1.6 ± 3.2 | 0.314 |
| ≥3.8 mg/dL, n (%) | 12 (17.1) | 12 (7.4) | 0.024† |
| >1.0 mg/dL, n (%) | 47 (67.1) | 72 (44.2) | 0.001‡ |
| RF positive, n (%) | 59 (84.3) | 132 (81.0) | 0.548 |
| Previous joint surgery, n (%) | 47 (67.1) | 66 (40.5) | < 0.001‡ |
| Treatments | |||
| Corticosteroids, n (%) | 54 (77.1) | 96 (58.9) | 0.008‡ |
| MTX, n (%) | 35 (50.0) | 63 (38.7) | 0.108 |
| Other DMARDs, n (%) | 33 (47.1) | 90 (55.2) | 0.258 |
| Biological agents, n (%) | 8 (11.4) | 11 (6.7) | 0.231 |
| Steinbrocker stages and mutilating changes | |||
| Stages I–II, n (%) | 6 (8.6) | 38 (23.3) | < 0.001‡ |
| Stages III–IV, n (%) | 49 (70.0) | 118 (72.4) | |
| Mutilating changes, n (%) | 15 (21.4) | 7 (4.3) | |
| C2–C7 angle | 11.2 ± 14.9 | 13.4 ± 13.0 | 0.261 |
Calculated by the chi-squared test and student’s t-test.
CRP C-reactive protein, DMARD disease-modifying antirheumatic drug, MTX methotrexate, RA rheumatoid arthritis, RF rheumatoid factor, SD standard deviation.
†p < 0.050, ‡p < 0.010.
In an additional analysis of 45 patients with pre-existing VS, 5 (83.3%) of 6 patients with CRP level at >10-year follow-up ≥ 3.8 mg/dL developed the aggravation of VS, while 17 (43.6%) of 39 patients with CRP < 3.8 mg/dL developed the aggravation of VS (p = 0.070).
In logistic regression analysis, univariable models showed that the following variables had p < 0.050: mutilating changes (p < 0.001), previous joint surgery (p < 0.001), RA duration ≥ 5 years (p = 0.002), pre-existing VS (p = 0.003), corticosteroid administration (p = 0.009), and CRP level at baseline ≥ 3.8 mg/dL (p = 0.010). Male sex (p = 0.041) had a negative correlation with VS aggravation. To distinguish predictive risk factors for the aggravation of VS, CRP level at > 10-year follow-up was not included. Thereafter, we designed a multivariable logistic regression model including these variables, identifying mutilating changes (OR = 4.048, 95% confidence interval [CI] = 1.432–11.438, p = 0.008), RA duration ≥ 5 years (OR = 3.711, 95% CI = 1.279–10.769, p = 0.013), CRP level ≥ 3.8 mg/dL (OR = 2.187, 95% CI = 1.021–4.685, p = 0.044), and previous joint surgery (OR = 2.147, 95% CI = 1.122–4.108, p = 0.021) as significant independent predictive risk factors for the aggravation of VS (Table 3).
Table 3.
Univariable and multivariable logistic regression models of 233 patients to identify predictive risk factors for the aggravation of VS at > 10-year follow-up.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| < 55 years | 0.886 | 0.490–1.603 | 0.689 | |||
| ≥ 65 years | 1.066 | 0.582–1.954 | 0.835 | |||
| Male sex | 0.355 | 0.132–0.961 | 0.041† | 0.477 | 0.166–1.373 | 0.170 |
| RA duration ≥ 5 years | 4.807 | 1.816–12.720 | 0.002‡ | 3.711 | 1.279–10.769 | 0.013† |
| CRP ≥ 3.8 mg/dL | 2.435 | 1.233–4.809 | 0.010† | 2.187 | 1.021–4.685 | 0.044† |
| CRP > 1.0 mg/dL | 1.199 | 0.676–2.125 | 0.535 | |||
| RF positive | 1.260 | 0.593–2.675 | 0.548 | |||
| Previous joint surgery | 3.003 | 1.667–5.411 | < 0.001‡ | 2.147 | 1.122–4.108 | 0.021† |
| Corticosteroids | 2.355 | 1.243–4.464 | 0.009‡ | 1.679 | 0.832–3.391 | 0.148 |
| MTX | 1.587 | 0.902–2.792 | 0.109 | |||
| Other DMARDs | 0.723 | 0.413–1.269 | 0.259 | |||
| Biological agents | 1.783 | 0.684–4.645 | 0.236 | |||
| Steinbrocker stages III–IV | 0.890 | 0.481–1.647 | 0.710 | |||
| Mutilating changes | 6.078 | 2.354–15.690 | < 0.001‡ | 4.048 | 1.432–11.438 | 0.008‡ |
| Pre-existing AAS | 1.549 | 0.855–2.807 | 0.149 | |||
| Pre-existing VS | 2.790 | 1.427–5.453 | 0.003‡ | 1.449 | 0.675–3.109 | 0.341 |
| Pre-existing SAS | 1.535 | 0.569–4.141 | 0.397 | |||
| C2–C7 angle | 0.988 | 0.967–1.009 | 0.262 | |||
AAS atlantoaxial subluxation, CI confidence interval, CRP C-reactive protein, DMARD disease-modifying antirheumatic drug, MTX methotrexate, OR odds ratio, RA rheumatoid arthritis, RF rheumatoid factor, SAS subaxial subluxation, VS vertical subluxation.
†p < 0.050, ‡p < 0.010.
Predictive risk factors for the aggravation of SAS
In 233 patients, the aggravation of SAS was shown in 83 (35.6%) including 74 without pre-existing SAS and 9 with pre-existing SAS, all of whom newly developed SAS at other segments and one additionally developed ≥ 2-mm increase of vertebral slippage in the segment of pre-existing SAS. Of 45 patients with pre-existing VS, 25 (55.6%) displayed the aggravation of SAS, while 58 (30.9%) of 188 without pre-existing VS demonstrated SAS aggravation (p = 0.002). Meanwhile, pre-existing AAS and SAS had the similar trend but did not reach any significant association with the aggravation of SAS (p = 0.182 and p = 0.184, respectively) (Table 1).
In 162 consecutively followed patients, 13 (43.3%) at > 5 years and 15 (50.0%) at > 10 years of 30 with pre-existing VS exhibited the aggravation of SAS, significantly higher than 23 (17.4%) at > 5 years and 41 (31.1%) at > 10 years of 132 patients without pre-existing VS (p = 0.002 and p = 0.049, respectively). On the other hand, pre-existing AAS and SAS was not significantly associated with the aggravation of SAS both at > 5 and > 10 years (p = 0.543–0.719 and p = 0.378–0.449, respectively) (Supplementary Table S1).
In the comparison between patients with and without the aggravation of SAS, those with SAS aggravation tended to have an older age (p = 0.073)—notably ≥ 65 years (p = 0.017), longer RA duration (p = 0.044), higher CRP level at baseline (p = 0.133)—especially ≥ 3.8 mg/dL (p = 0.045) and > 1.0 mg/dL (p = 0.003), higher frequency of previous joint surgery (p = 0.008), corticosteroid administration (p = 0.006), Steinbrocker stages III–IV or mutilating changes (p = 0.015), and larger C2–C7 angle (p = 0.073) at baseline. Similarly to VS aggravation, male patients tended to be uncommon (p = 0.048). Meanwhile, CRP level at > 10-year follow-up had no significant association with the aggravation of SAS (p = 0.509–>0.999) (Table 4).
Table 4.
Baseline and > 10-year demographic, clinical, and disease characteristics of 233 patients with and without the aggravation of SAS at > 10-year follow-up.
| Patients with SAS aggravation | Patients without SAS aggravation | p | |
|---|---|---|---|
| (n = 83) | (n = 150) | ||
| Age, mean ± SD years | 60.2 ± 8.9 | 57.8 ± 10.0 | 0.073 |
| <55 years, n (%) | 18 (21.7) | 59 (39.3) | 0.017† |
| 55–64 years, n (%) | 33 (39.8) | 52 (34.7) | |
| ≥65 years, n (%) | 32 (38.6) | 39 (26.0) | |
| Male sex, n (%) | 7 (8.4) | 27 (18.0) | 0.048† |
| RA duration, mean ± SD years | 15.5 ± 11.1 | 12.3 ± 10.4 | 0.044† |
| ≥5 years, n (%) | 69 (83.1) | 115 (76.7) | 0.246 |
| CRP, mean ± SD mg/dL at baseline | 2.6 ± 2.5 | 2.0 ± 2.9 | 0.133 |
| ≥3.8 mg/dL, n (%) | 21 (25.3) | 22 (14.7) | 0.045† |
| >1.0 mg/dL, n (%) | 59 (71.1) | 77 (51.3) | 0.003‡ |
| CRP, mean ± SD mg/dL at > 10-year follow-up | 1.6 ± 1.6 | 1.8 ± 3.4 | 0.613 |
| ≥3.8 mg/dL, n (%) | 10 (12.0) | 14 (9.3) | 0.509 |
| >1.0 mg/dL, n (%) | 42 (50.6) | 77 (51.3) | > 0.999 |
| RF positive, n (%) | 69 (83.1) | 122 (81.3) | 0.732 |
| Previous joint surgery, n (%) | 50 (60.2) | 63 (42.0) | 0.008‡ |
| Treatments | |||
| Corticosteroids, n (%) | 63 (75.9) | 87 (58.0) | 0.006‡ |
| MTX, n (%) | 33 (39.8) | 65 (43.3) | 0.597 |
| Other DMARDs, n (%) | 44 (53.0) | 79 (52.7) | 0.960 |
| Biological agents, n (%) | 9 (10.8) | 10 (6.7) | 0.265 |
| Steinbrocker stages and mutilating changes | |||
| Stages I–II, n (%) | 8 (9.6) | 36 (24.0) | 0.015† |
| Stages III–IV, n (%) | 64 (77.1) | 103 (68.7) | |
| Mutilating changes, n (%) | 11 (13.3) | 11 (7.3) | |
| C2–C7 angle | 14.9 ± 14.3 | 11.6 ± 13.1 | 0.073 |
Calculated by the chi-squared test and Student’s t-test.
CRP C-reactive protein, DMARD disease-modifying antirheumatic drug, MTX methotrexate, RA rheumatoid arthritis, RF rheumatoid factor, SD standard deviation.
†p < 0.050, ‡p < 0.010.
In logistic regression analysis, univariable models presented the following variables with p < 0.050: pre-existing VS (p = 0.002), CRP levels at baseline ≥ 3.8 mg/dL (p = 0.047) and > 1.0 mg/dL (p = 0.004), corticosteroid administration (p = 0.007), previous joint surgery (p = 0.008), and patients aged ≥ 65 years (p = 0.047). Patients aged < 55 years (p = 0.012) and male sex (p = 0.053) tended to have a negative correlation with SAS aggravation. To find predictors for the aggravation of SAS, CRP level at > 10-year follow-up was not analyzed. We therefore designed a multivariable logistic regression model including these variables, except for categorical variables with a lower OR in age (< 55 years) and CRP (≥ 3.8 mg/dL). This model identified pre-existing VS (OR = 2.252, 95% CI = 1.111–4.562, p = 0.024) and CRP > 1.0 mg/dL (OR = 2.139, 95% CI = 1.176–3.892, p = 0.013) as significant independent predictors for the aggravation of SAS (Table 5).
Table 5.
Univariable and multivariable logistic regression models of 233 patients to identify predictive risk factors for the aggravation of SAS at > 10-year follow-up.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| < 55 years | 0.463 | 0.254–1.843 | 0.012† | |||
| ≥ 65 years | 1.786 | 1.007–3.168 | 0.047† | 1.786 | 0.971–3.287 | 0.062 |
| Male sex | 0.420 | 0.174–1.011 | 0.053 | |||
| RA duration ≥ 5 years | 1.500 | 0.754–2.984 | 0.248 | |||
| CRP ≥ 3.8 mg/dL | 1.971 | 1.008–3.853 | 0.047† | |||
| CRP > 1.0 mg/dL | 2.331 | 1.315–4.131 | 0.004‡ | 2.139 | 1.176–3.892 | 0.013† |
| RF positive | 1.131 | 0.558–2.292 | 0.732 | |||
| Previous joint surgery | 2.092 | 1.212–3.613 | 0.008‡ | 1.645 | 0.912–2.966 | 0.098 |
| Corticosteroids | 2.281 | 1.254–4.150 | 0.007‡ | 1.820 | 0.965–3.433 | 0.064 |
| MTX | 0.863 | 0.500–1.489 | 0.597 | |||
| Other DMARDs | 1.014 | 0.593–1.735 | 0.960 | |||
| Biological agents | 1.703 | 0.663–4.374 | 0.269 | |||
| Steinbrocker stages III–IV | 1.537 | 0.829–2.850 | 0.172 | |||
| Mutilating changes | 1.931 | 0.798–4.668 | 0.144 | |||
| Pre-existing AAS | 1.558 | 0.908–2.672 | 0.107 | |||
| Pre-existing VS | 2.802 | 1.442–5.445 | 0.002‡ | 2.252 | 1.111–4.562 | 0.024† |
| Pre-existing SAS | 1.905 | 0.725–5.005 | 0.191 | |||
| C2–C7 angle | 0.982 | 0.969–1.002 | 0.054 | |||
AAS atlantoaxial subluxation, CI confidence interval, CRP C-reactive protein, DMARD disease-modifying antirheumatic drug, MTX methotrexate, OR odds ratio, RA rheumatoid arthritis, RF rheumatoid factor, SAS subaxial subluxation, VS vertical subluxation.
†p < 0.050, ‡p < 0.010.
Discussion
This > 10-year prospective multicenter cohort study demonstrated time-course changes in the prevalence and aggravation of cervical spine instabilities in 2001–2013. Among the entire cohort followed at > 10 years (n = 233) and consecutive subcohort followed both at > 5 and > 10 years (n = 162), pre-existing AAS was associated with VS aggravation while pre-existing VS had a strong association with VS and SAS aggravation. Mutilating changes, RA duration ≥ 5 years, CRP level ≥ 3.8 mg/dL, and previous joint surgery were identified as independent predictive risk factors for the aggravation of VS. Pre-existing VS and CRP > 1.0 mg/dL were independent predictors for the aggravation of SAS.
The prevalence of cervical spine instabilities in RA varies, depending on multiple factors including disease duration, radiological criteria, follow-up period, and paradigm shifts in the treatment. Hence, many studies focused on changes in the prevalence and progression pattern of instabilities; however, prior investigations have been largely limited due to the cross-sectional or retrospective design with a shorter-period observation. Additionally, some studies concentrated only to AAS without evaluating VS and SAS7,9, which are more strongly associated with neurological dysfunction and even sudden death13,34. We studied longitudinal changes in the prevalence and progression pattern of cervical spine instabilities in patients with clinically relevant, symptomatic “classical” or “definite” RA during a relatively longer period of > 10 years. Among our cohort, the > 10-year prevalence of VS and SAS consistently increased, whereas AAS did not significantly increase. This trend could be explained by the two phenomena of AAS; the ADI increase as AAS aggravation and the ADI decrease as subclinical VS aggravation with the upward migration of the odontoid process by the destruction of the atlantoaxial facet joints31,34,35. We therefore focused on the aggravation of VS and SAS.
First, we clarified the association of VS and SAS aggravation with pre-existing cervical spine instability. Our prospective cohort studies at > 5-year follow-up (n = 267 and n = 228) clarified the association of VS and SAS aggravation with pre-existing VS and SAS18,20. A more recent 3.9-year retrospective follow-up study in the early biological agent era (n = 91) identified pre-existing cervical spine involvement as an independent predictive risk factor for the progression of instabilities36. Another 4.4-year retrospective follow-up study (n = 101) also demonstrated pre-existing AAS as a predictor for VS progression23. A 6.1-year different retrospective follow-up study in the era fully treated by biological agents (n = 87) found the association of pre-existing SAS with aggravated instabilities37. In the present study, pre-existing AAS without pre-existing VS was associated with the increased prevalence of VS. Notably, 48.9% in the entire cohort and 53.3% in the subcohort of patients with pre-existing VS (Ranawat value < 13 mm) developed ≥ 2-mm decrease of the Ranawat value during > 10 years. In VS, Ranawat value ≤ 10 mm is at risk of compression myelopathy14,38,39. Moreover, pre-existing VS was strongly associated with SAS aggravation. When surgically treating VS, spine surgeons should carefully consider the risk of SAS aggravation, which potentially leads to subaxial myelopathy.
Based on this study observation, larger C2–C7 angle, indicating the increase in cervical lordosis, could affect the aggravation of SAS relative to that of VS. Further studies are needed to clarify the involvement of the sagittal alignment in the pathomechanism of cervical spine instabilities.
Next, we examined the association of patients’ demographic, clinical, disease, and treatment characteristics with the aggravation of VS and SAS. Baseline longer RA duration, higher CRP level, previous joint surgery, corticosteroid administration, and mutilating changes were candidate predictive risk factors for the aggravation of VS. In addition to these variables, baseline age ≥ 65 years and Steinbrocker stages III–IV were also associated with the aggravation of SAS. Longer RA duration and higher CRP level at baseline imply the importance of low disease activity and clinical remission. This is in agreement with the current treat-to-target and early intervention strategy40. In fact, insufficient RA control with CRP level exceeding 1.0 mg/L at > 10-year follow-up was also associated with the aggravation of VS. Previous joint surgery and Steinblocker stages III–IV or mutilating changes reflect peripheral joint erosion and destruction, potentially collaborating with cervical spine involvement. Cervical spine instability was radiographically detected in 40.8–61.1% of patients with RA who underwent total hip or knee arthroplasty30,41. Advanced Steinblocker stages and mutilating changes are at higher risk of instability19,21,26,28,31,32,35,42–44. While concomitant corticosteroids are a known predictor of instability6,7,13,45, 10-mg/day prednisolone within 2 years increases the benefits of DMARDs and has joint-sparing properties in early RA46. A 4-year prospective study (n = 186) detected a lower dosage of oral corticosteroids as a risk factor of large joint destruction in the lower extremities47. Therefore, high disease activity requiring concomitant corticosteroids rather than corticosteroids themselves might impact on the development of cervical spine instabilities in RA.
Based on our multivariable logistic regression analysis, hand mutilating changes, RA duration ≥ 5 years, CRP level ≥ 3.8 mg/dL, and previous joint surgery were identified as independent predictive risk factors for the aggravation of VS. In addition, pre-existing VS and CRP > 1.0 mg/dL were also independent predictors for the aggravation of SAS. Interestingly, CRP is a common indicator of VS and SAS aggravation; however, the threshold was different. In a meta-analysis (n = 2750), CRP level was a predictive risk factor of cervical spine involvement29. Our findings of consistent association between no VS and SAS progression and CRP level < 1.0 mg/dL support CRP < 1.0 mg/dL as one of the reliable indicators for low disease activity and clinical remission48. Pre-existing VS was identified as a predictive risk factor for the aggravation of SAS but not VS. This might be due to the distribution of VS aggravation. Of 70 patients with the aggravation of VS during > 10-year follow-up, 22 (31.4%) with pre-existing VS developed ≥ 2-mm decrease of the Ranawat value and 48 (68.6%) without pre-existing VS developed the new onset of VS. The number of pre-existing VS might affect the statistical power in multivariable analysis.
Taken together, acquiring low disease activity before erosive joint destruction is essential to avert the > 10-year aggravation of cervical spine instabilities in RA, e.g. the introduction of biological agents. In this study, although the use of biological agents was not associated with no VS and SAS aggravation, it does not indicate no effectiveness of biological agents. As biological agents had been approved since 2003, 2 years after the 2001–2002 start of our surveillance in Japan, our results should be carefully interpreted. The incidence of cervical spine instabilities was still high at 31.8 to 41.8% in patients during the beginning era of biologic agents42,43. However, biological agents could prevent the new onset of cervical spine involvement in RA but fail to control the progression of pre-existing lesions36,49. In fact, a more recent cross-sectional study (n = 1333) reported the decreased prevalence of AAS by 50%, VS by 75%, and SAS by 5% in 2015, compared to in 199950.
There are several limitations in this study. First, the follow-up rate was relatively low. In this study, 86 (13.6%) of 634 patients had died at 10-year follow-up. The comparison of baseline age, sex, RA severity, and cervical spine instability between followed patients versus dead or dropped-out patients indicate that many older, advanced RA outpatients could drop out due to polyarthralgia, worsening general condition, and/or cervical spine problems. Dropouts thus appear to be not randomly chosen in this study. Second, our data lacks clinical remission criteria such as the disease activity score-28 and magnetic resonance imaging findings, because of the study start in 2001–2002 and the last follow-up in 2012–2013. Moreover, our survey does not reflect the effect of more recent treatment options such as JAK inhibitors. Further investigation including these data would be desirable to validate the current study results.
In conclusion, this > 10-year prospective multicenter cohort study of 233 outpatients with RA elucidated the increased prevalence and aggravation of cervical spine instabilities, particularly VS and SAS, which was the most prominent in patients with pre-existing VS. Pre-existing VS itself, mutilating changes in the hands, RA duration ≥ 5 years, residual CRP level > 1.0 mg/dL—particularly ≥ 3.8 mg/dL, and previous peripheral joint surgery were predictive risk factors for further aggravation of instabilities. Low disease activity and clinical remission before the development of advanced peripheral joint erosion and destruction are essential to prevent progressive cervical spine instability in patients with RA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the patients for their permission to use the data. The authors also thank all the members of Hyogo Organization for Spinal Disorders (Kobe, Japan) for their help to acquire the data.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Yurube had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: Kanda, Yurube, Hirata, Sumi. Acquisition of data: Kanda, Yurube, Hirata, Sumi. Analysis and interpretation of data: Kanda, Yurube, Hirata, Sumi.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yutaro Kanda and Takashi Yurube.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78429-9.
References
- 1.Alamanos, Y. & Drosos, A. A. Epidemiology of adult rheumatoid arthritis. Autoimmun. Rev.4, 130–136. 10.1016/j.autrev.2004.09.002 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Smolen, J. S., Aletaha, D. & McInnes, I. B. rheumatoid arthritis. Lancet388, 2023–2038. 10.1016/S0140-6736(16)30173-8 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Cross, M. et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis.73, 1316–1322. 10.1136/annrheumdis-2013-204627 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Sharp, J. & Purser, D. W. Spontaneous atlanto-axial dislocation in Ankylosing spondylitis and rheumatoid arthritis. Ann. Rheum. Dis.20, 47–77. 10.1136/ard.20.1.47 (1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martel, W. The occipito-atlanto-axial joints in rheumatoid arthritis and ankylosing spondylitis. Am. J. Roentgenol. Radium Ther. Nucl. Med.86, 223–240 (1961). [PubMed] [Google Scholar]
- 6.Lourie, H. & Stewart, W. A. Spontaneous atlantoaxial dislocation. A complication of rheumatoid disease. N. Engl. J. Med.265, 677–681. 10.1056/NEJM196110052651405 (1961). [DOI] [PubMed] [Google Scholar]
- 7.Mathews, J. A. Atlanto-axial subluxation in rheumatoid arthritis. A 5-year follow-up study. Ann. Rheum. Dis.33, 526–531. 10.1136/ard.33.6.526 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissman, B. N., Aliabadi, P., Weinfeld, M. S., Thomas, W. H. & Sosman, J. L. Prognostic features of atlantoaxial subluxation in rheumatoid arthritis patients. Radiology. 144, 745–751. 10.1148/radiology.144.4.7111719 (1982). [DOI] [PubMed] [Google Scholar]
- 9.Rana, N. A. Natural history of atlanto-axial subluxation in rheumatoid arthritis. Spine 14, 1054–1056, 10.1097/00007632-198910000-00005 (1989). [DOI] [PubMed]
- 10.Halla, J. T. & Hardin, J. G. Jr. The spectrum of atlantoaxial facet joint involvement in rheumatoid arthritis. Arthritis Rheum.33, 325–329. 10.1002/art.1780330304 (1990). [DOI] [PubMed] [Google Scholar]
- 11.Ranawat, C. S. et al. Cervical spine fusion in rheumatoid arthritis. J. Bone Joint Surg. Am.61, 1003–1010 (1979). [PubMed] [Google Scholar]
- 12.Redlund-Johnell, I. & Pettersson, H. Radiographic measurements of the cranio-vertebral region. Designed for evaluation of abnormalities in rheumatoid arthritis. Acta Radiol. Diagn. (Stockh)25, 23–28. 10.1177/028418518402500105 (1984). [DOI] [PubMed] [Google Scholar]
- 13.Yonezawa, T., Tsuji, H., Matsui, H. & Hirano, N. Subaxial lesions in rheumatoid arthritis. Radiographic factors suggestive of lower cervical myelopathy. Spine20, 208–215 (1995). [PubMed] [Google Scholar]
- 14.Casey, A. T. et al. Surgery on the rheumatoid cervical spine for the non-ambulant myelopathic patient-too much, too late? Lancet347, 1004–1007. 10.1016/s0140-6736(96)90146-4 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Zhang, T. & Pope, J. Cervical spine involvement in rheumatoid arthritis over time: results from a meta-analysis. Arthritis Res. Ther.1710.1186/s13075-015-0643-0 (2015). [DOI] [PMC free article] [PubMed]
- 16.Ropes, M. W., Bennett, G. A., Cobb, S., Jacox, R. & Jessar, R. A. Revision of diagnostic criteria for rheumatoid arthritis. Bull. Rheum. Dis. 9, 175–176. (1958). [PubMed]
- 17.Arnett, F. C. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum.31, 315–324. 10.1002/art.1780310302 (1988). [DOI] [PubMed] [Google Scholar]
- 18.Yurube, T. et al. Progression of cervical spine instabilities in rheumatoid arthritis: a prospective cohort study of outpatients over 5 years. Spine36, 647–653. 10.1097/BRS.0b013e3181da21c5 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Yurube, T. et al. Incidence and aggravation of cervical spine instabilities in rheumatoid arthritis: a prospective minimum 5-year follow-up study of patients initially without cervical involvement. Spine37, 2136–2144. 10.1097/BRS.0b013e31826def1c (2012). [DOI] [PubMed] [Google Scholar]
- 20.Yurube, T. et al. Accelerated development of cervical spine instabilities in rheumatoid arthritis: a prospective minimum 5-year cohort study. PLoS One9, e88970. 10.1371/journal.pone.0088970 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terashima, Y. et al. Predictive risk factors of cervical spine instabilities in rheumatoid arthritis: a prospective Multicenter over 10-Year Cohort Study. Spine (Phila Pa. 1976)42, 556–564. 10.1097/BRS.0000000000001853 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Fiehn, C., Belke-Voss, E., Krause, D., Wassenberg, S. & Rau, R. Improved radiological outcome of rheumatoid arthritis: the importance of early treatment with methotrexate in the era of biological drugs. Clin. Rheumatol.32, 1735–1742. 10.1007/s10067-013-2325-0 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Kaito, T. et al. Predictors for progression of two different types of cervical lesions in rheumatoid arthritis treated with biologic agents. J. Orthop. Sci.24, 214–218. 10.1016/j.jos.2018.09.001 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Steinbrocker, O., Traeger, C. H. & Batterman, R. C. Therapeutic criteria in rheumatoid arthritis. J. Am. Med. Assoc.140, 659–662. 10.1001/jama.1949.02900430001001 (1949). [DOI] [PubMed] [Google Scholar]
- 25.Belt, E. A. et al. Assessment of mutilans-like hand deformities in chronic inflammatory joint diseases. A radiographic study of 52 patients. Ann. Rheum. Dis.58, 250–252. 10.1136/ard.58.4.250 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laiho, K., Belt, E. & Kauppi, M. The cervical spine in mutilant rheumatoid arthritis. Rheumatol. Int.20, 225–228. 10.1007/s002960100114 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Ochi, T. et al. Natural course of joint destruction and fluctuation of serum C1q levels in patients with rheumatoid arthritis. Arthritis Rheum.31, 37–43. 10.1002/art.1780310106 (1988). [DOI] [PubMed] [Google Scholar]
- 28.Neva, M. H. et al. Early and extensive erosiveness in peripheral joints predicts atlantoaxial subluxations in patients with rheumatoid arthritis. Arthritis Rheum.48, 1808–1813. 10.1002/art.11086 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Zhu, S., Xu, W., Luo, Y., Zhao, Y. & Liu, Y. Cervical spine involvement risk factors in rheumatoid arthritis: a meta-analysis. Int. J. Rheum. Dis.20, 541–549. 10.1111/1756-185X.13096 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Collins, D. N., Barnes, C. L. & FitzRandolph, R. L. Cervical spine instability in rheumatoid patients having total hip or knee arthroplasty. Clin. Orthop. Relat. Res.272, 127–135 (1991). [PubMed] [Google Scholar]
- 31.Fujiwara, K., Owaki, H., Fujimoto, M., Yonenobu, K. & Ochi, T. A long-term follow-up study of cervical lesions in rheumatoid arthritis. J. Spinal Disord.13, 519–526. 10.1097/00002517-200012000-00010 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara, K. et al. Cervical lesions related to the systemic progression in rheumatoid arthritis. Spine (Phila Pa. 1976)23, 2052–2056. 10.1097/00007632-199810010-00003 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Felson, D. T. et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann. Rheum. Dis.70, 404–413. 10.1136/ard.2011.149765 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Pellicci, P. M., Ranawat, C. S., Tsairis, P. & Bryan, W. J. A prospective study of the progression of rheumatoid arthritis of the cervical spine. J. Bone Joint Surg. Am.63, 342–350 (1981). [PubMed] [Google Scholar]
- 35.Oda, T., Fujiwara, K., Yonenobu, K., Azuma, B. & Ochi, T. Natural course of cervical spine lesions in rheumatoid arthritis. Spine (Phila Pa. 1976)20, 1128–1135. 10.1097/00007632-199505150-00004 (1995). [DOI] [PubMed] [Google Scholar]
- 36.Kaito, T., Ohshima, S., Fujiwara, H., Makino, T. & Yonenobu, K. Predictors for the progression of cervical lesion in rheumatoid arthritis under the treatment of biological agents. Spine (Phila Pa. 1976)38, 2258–2263. 10.1097/BRS.0000000000000066 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Inoue, T. et al. Risk factors Associated with aggravation of cervical spine lesions in patients with rheumatoid arthritis: a retrospective longitudinal cohort study. Spine (Phila Pa. 1976)47, 484–489. 10.1097/BRS.0000000000004220 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Casey, A. T., Crockard, H. A., Geddes, J. F. & Stevens, J. Vertical translocation: the enigma of the disappearing atlantodens interval in patients with myelopathy and rheumatoid arthritis. Part I. Clinical, radiological, and neuropathological features. J. Neurosurg.87, 856–862. 10.3171/jns.1997.87.6.0856 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Casey, A. T., Crockard, H. A. & Stevens, J. Vertical translocation. Part II. Outcomes after surgical treatment of rheumatoid cervical myelopathy. J. Neurosurg.87, 863–869. 10.3171/jns.1997.87.6.0863 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Smolen, J. S. et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann. Rheum. Dis.69, 631–637. 10.1136/ard.2009.123919 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grauer, J. N., Tingstad, E. M., Rand, N., Christie, M. J. & Hilibrand, A. S. Predictors of paralysis in the rheumatoid cervical spine in patients undergoing total joint arthroplasty. J. Bone Joint Surg. Am.86, 1420–1424. 10.2106/00004623-200407000-00009 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Takahashi, S. et al. Current prevalence and characteristics of cervical spine instability in patients with rheumatoid arthritis in the era of biologics. Mod. Rheumatol.24, 904–909. 10.3109/14397595.2014.895123 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Kaito, T. et al. Incidence and risk factors for cervical lesions in patients with rheumatoid arthritis under the current pharmacologic treatment paradigm. Mod. Rheumatol.27, 593–597. 10.1080/14397595.2016.1253649 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Uchino, Y. et al. Risk factors associated with cervical spine lesions in patients with rheumatoid arthritis: an observational study. BMC Musculoskelet. Disord. 22, 408. 10.1186/s12891-021-04285-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rasker, J. J. & Cosh, J. A. Radiological study of cervical spine and hand in patients with rheumatoid arthritis of 15 years’ duration: an assessment of the effects of corticosteroid treatment. Ann. Rheum. Dis.37, 529–535. 10.1136/ard.37.6.529 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobs, J. W., Bijlsma, J. W. & van Laar, J. M. Glucocorticoids in early rheumatoid arthritis: are the benefits of joint-sparing effects offset by the adverse effect of osteoporosis? The effects on bone in the utrecht study and the CAMERA-II study. Neuroimmunomodulation. 22, 66–71. 10.1159/000362729 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Doi, K. et al. Oral steroid decreases the progression of joint destruction of large joints in the lower extremities in rheumatoid arthritis. Med. (Baltim).98, e17968. 10.1097/MD.0000000000017968 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aletaha, D., Alasti, F. & Smolen, J. S. Rheumatoid arthritis near remission: clinical rather than laboratory inflammation is associated with radiographic progression. Ann. Rheum. Dis.70, 1975–1980. 10.1136/ard.2011.153734 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Kaito, T. et al. Effect of biological agents on cervical spine lesions in rheumatoid arthritis. Spine (Phila Pa. 1976)37, 1742–1746. 10.1097/BRS.0b013e318256b584 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Morita, O. et al. Changes in the incidence of cervical lesions owing to the development of rheumatoid arthritis treatment and the impact of cervical lesions on patients’ quality of life. Mod. Rheumatol.30, 495–501. 10.1080/14397595.2019.1621428 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.