Abstract
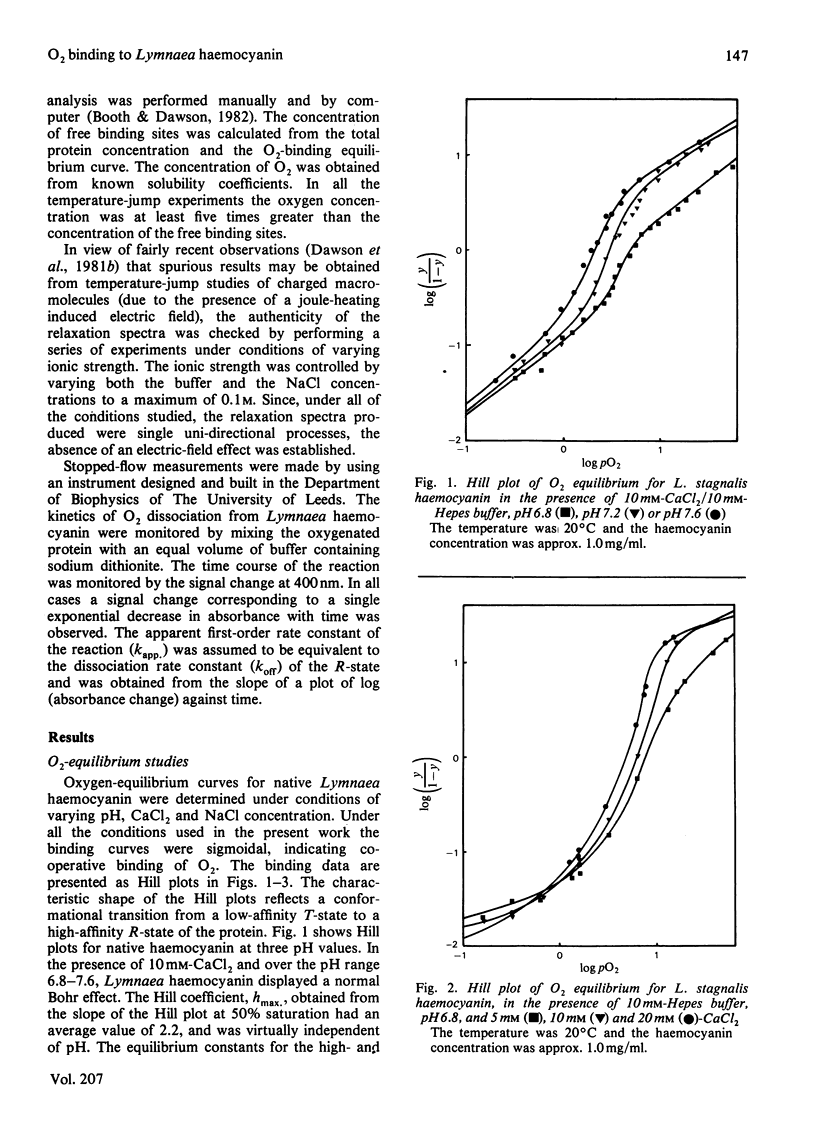
The binding of oxygen by the haemocyanin of the gastropod Lymnaea stagnalis was studied by equilibrium and kinetic methods. The studies were performed under conditions in which the haemocyanin molecule was in the native state. Over the pH range 6.8-7.6, in the presence of 10mM-CaCl2 the haemocyanin bound O2 cooperatively. Over this pH range the haemocyanin molecule displayed a normal Bohr effect whereby the O2 affinity of the molecule decreased with a fall in the pH of the solution. The maximum slope of the Hill plot (hmax.) was 3.5, obtained at pH 7.5. An increase in the CaCl2 concentration from 5 to 20 mM at pH 6.8 resulted in a slight increase in the oxygen affinity, with hmax. remaining virtually unchanged. At constant pH and CaCl2 concentration, an increase in NaCl concentration from 0 to 50 mM resulted in a small decrease in O2 affinity, but a significant increase in the value of hmax. from 3.5 to 8.6. Temperature-jump relaxation experiments over a range of O2 concentrations produced single relaxation times. The dependence of the relaxation time on the reactant concentrations indicated a simple bimolecular binding process. The calculated association and dissociation rate constants for this process at pH 7.5 are 29.5 X 10(6) M-1 X S-1 and 49 S-1 respectively. The association rate constant kon was found to be essentially independent of pH and CaCl2 concentration. The dissociation rate constant, koff, however, increased with a decrease in the pH, but was also independent of CaCl2 concentration. These results indicate that the stability of the haemocyanin-O2 complex is determined by the dissociation rate constant.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brouwer M., Bonaventura C., Bonaventura J. Oxygen binding by Limulus polyphemus hemocyanin: allosteric modulation by chloride ions. Biochemistry. 1977 Aug 23;16(17):3897–3902. doi: 10.1021/bi00636a027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosimo A., Brunori M., Wyman J. Concerted changes in an allosteric macromolecule. Biophys Chem. 1974 Dec;2(4):338–344. doi: 10.1016/0301-4622(74)80060-8. [DOI] [PubMed] [Google Scholar]
- Eigen M. New looks and outlooks on physical enzymology. Q Rev Biophys. 1968 May;1(1):3–33. doi: 10.1017/s0033583500000445. [DOI] [PubMed] [Google Scholar]
- Er-El Z., Shaklai N., Daniel E. Oxygen binding properties of haemocyanin from Levantina hierosolima. J Mol Biol. 1972 Mar 14;64(2):341–352. doi: 10.1016/0022-2836(72)90502-5. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Herries D. G., Wood E. J. Characterization of domains obtained from a mollusc haemocyanin by limited proteolytic digestion. Biochem J. 1979 Jun 1;179(3):593–602. doi: 10.1042/bj1790593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. L., Pearson J. S., Wood E. J. The haemocyanin of Lymnaea stagnalis L. (Gastropoda: Pulmonata). Comp Biochem Physiol B. 1975 Oct 15;52(2):211–218. doi: 10.1016/0305-0491(75)90054-1. [DOI] [PubMed] [Google Scholar]
- Kuiper H. A., Antonini E., Brunori M. Kinetic control of co-operativity in the oxygen binding of Panulirus interruptus hemocyanin. J Mol Biol. 1977 Nov 5;116(3):569–576. doi: 10.1016/0022-2836(77)90084-5. [DOI] [PubMed] [Google Scholar]
- Kuiper H. A., Forlani L., Chiancone E., Antonini E., Brunori M., Wyman J. Multiple linkage in Panulirus interruptus hemocyanin. Biochemistry. 1979 Dec 25;18(26):5849–5854. doi: 10.1021/bi00593a015. [DOI] [PubMed] [Google Scholar]
- Kuiper H. A., Gaastra W., Beintema J. J., van Bruggen E. F., Schepman A. M., Drenth J. Subunit composition, x-ray diffraction, amino acid analysis and oxygen binding behaviour of Panulirus interruptus hemocyanin. J Mol Biol. 1975 Dec 25;99(4):619–629. doi: 10.1016/s0022-2836(75)80176-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lontie R., De Ley M., Robberecht H., Witters R. Isolation of small functional subunits of Helix pomatia haemocyanin after subtilisin treatment. Nat New Biol. 1973 Apr 11;242(119):180–182. doi: 10.1038/newbio242180a0. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mellema J. E., Klug A. Quaternary structure of gastropod haemocyanin. Nature. 1972 Sep 15;239(5368):146–150. doi: 10.1038/239146a0. [DOI] [PubMed] [Google Scholar]
- RIGGS A. F., WOLBACH R. A. Sulfhydryl groups and the structure of hemoglobin. J Gen Physiol. 1956 Mar 20;39(4):585–605. doi: 10.1085/jgp.39.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklai N., Klarman A., Daniel E. Oxygen binding by hemocyanin from Levantina hierosolima. II. Interpretation of cooperativity in terms of ligand-ligand linkage. Biochemistry. 1975 Jan 14;14(1):105–108. doi: 10.1021/bi00672a018. [DOI] [PubMed] [Google Scholar]
- Sick H., Gersonde K. Method for continuous registration of O2-binding curves of hemoproteins by means of a diffusion chamber. Anal Biochem. 1969 Dec;32(3):362–376. doi: 10.1016/s0003-2697(69)80002-3. [DOI] [PubMed] [Google Scholar]
- Sundsmo J. S., Wood L. M. Activated factor B (Bb) of the alternative pathway of complement activation cleaves and activates plasminogen. J Immunol. 1981 Sep;127(3):877–880. [PubMed] [Google Scholar]
- Wood E. J., Cayley G. R., Pearson J. S. Oxygen binding by the haemocyanin from Buccinum undatum. J Mol Biol. 1977 Jan 5;109(1):1–11. doi: 10.1016/s0022-2836(77)80042-9. [DOI] [PubMed] [Google Scholar]
- Wood E. J., Peacocke A. R. Murex trunculus haemocyanin. I. Physical properties and pH-induced dissociation. Eur J Biochem. 1973 Jun 15;35(3):410–420. doi: 10.1111/j.1432-1033.1973.tb02853.x. [DOI] [PubMed] [Google Scholar]
- Wood E. J. The oxygen transport and storage proteins of invertebrates. Essays Biochem. 1980;16:1–47. [PubMed] [Google Scholar]
- Zolla L., Kuiper H. A., Vecchini P., Antonini E., Brunori M. Dissociation and oxygen-binding behaviour of beta-hemocyanin from Helix pomatia. Eur J Biochem. 1978 Jul 3;87(3):467–473. doi: 10.1111/j.1432-1033.1978.tb12397.x. [DOI] [PubMed] [Google Scholar]
- van Driel R., Kuiper H. A., Antonini E., Brunori M. Kinetics of the co-operative reaction of Helix pomatia hemocyanin with oxygen. Oxygen binding at low and intermediate oxygen saturations. J Mol Biol. 1978 Jun 5;121(4):431–439. doi: 10.1016/0022-2836(78)90392-3. [DOI] [PubMed] [Google Scholar]




