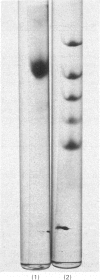
Abstract
A Ca2+-ATPase (Ca2+- and Mg2+-requiring ATPase) was purified from a synaptic plasma-membrane fraction of rat brain. This enzyme had properties similar to those of plasma-membrane Ca2+-ATPases from other organs: its splitting of ATP was dependent on both Ca2+ and Mg2+, it bound in a Ca2+-dependent fashion to calmodulin-Sepharose and it cross-reacted with specific antibodies raised against human erythrocyte-membrane Ca2+-ATPase. It had an apparent Mr of 138 000, similar to those of plasma-membrane ATPases from human erythrocyte and from dog heart sarcolemma. Previous high-Ca2+-affinity ATPases observed in brain had Mr 100 000; in at least one case, such an ATPase probably represented a different type of enzyme, derived from coated vesicles.
Full text
PDF

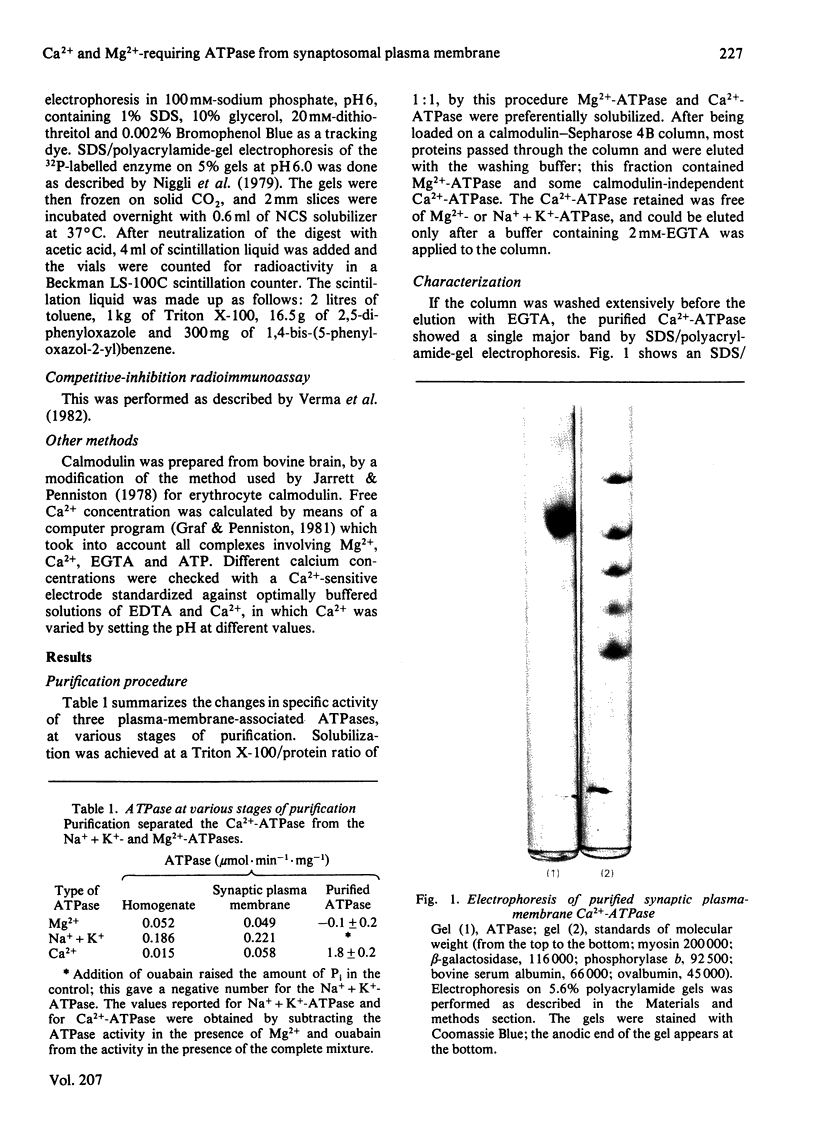
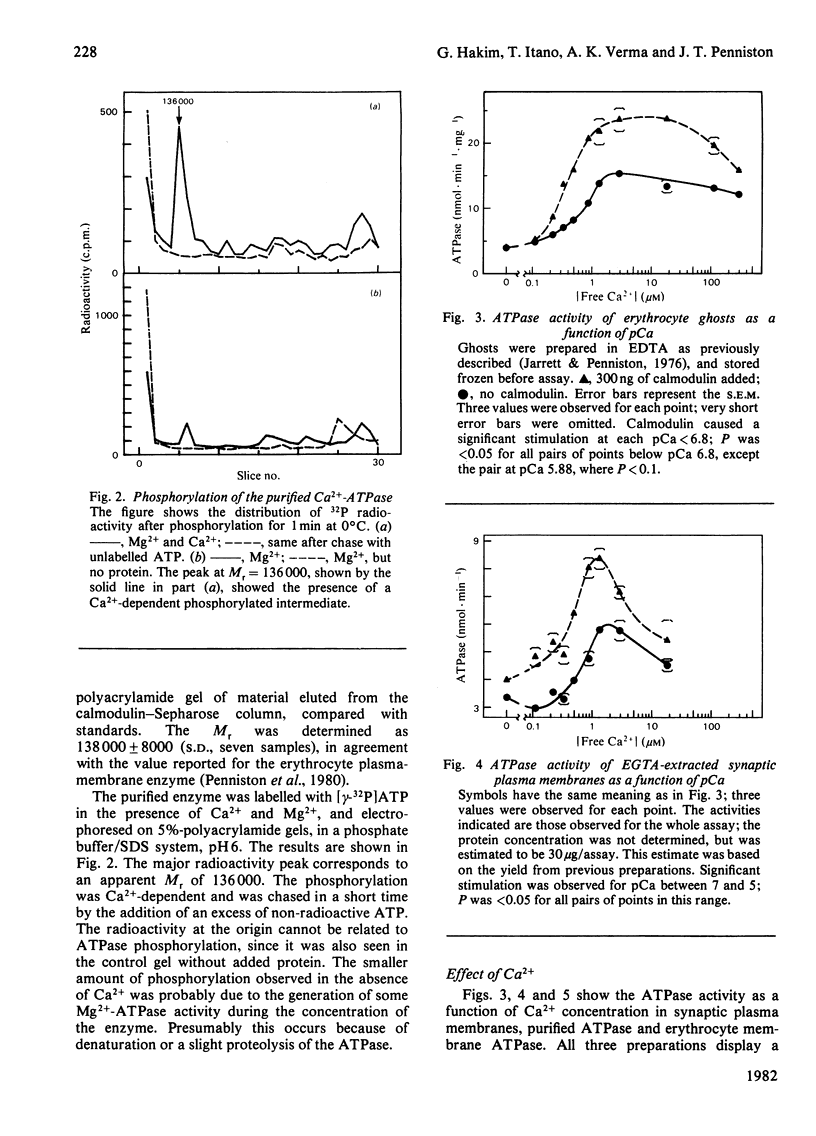
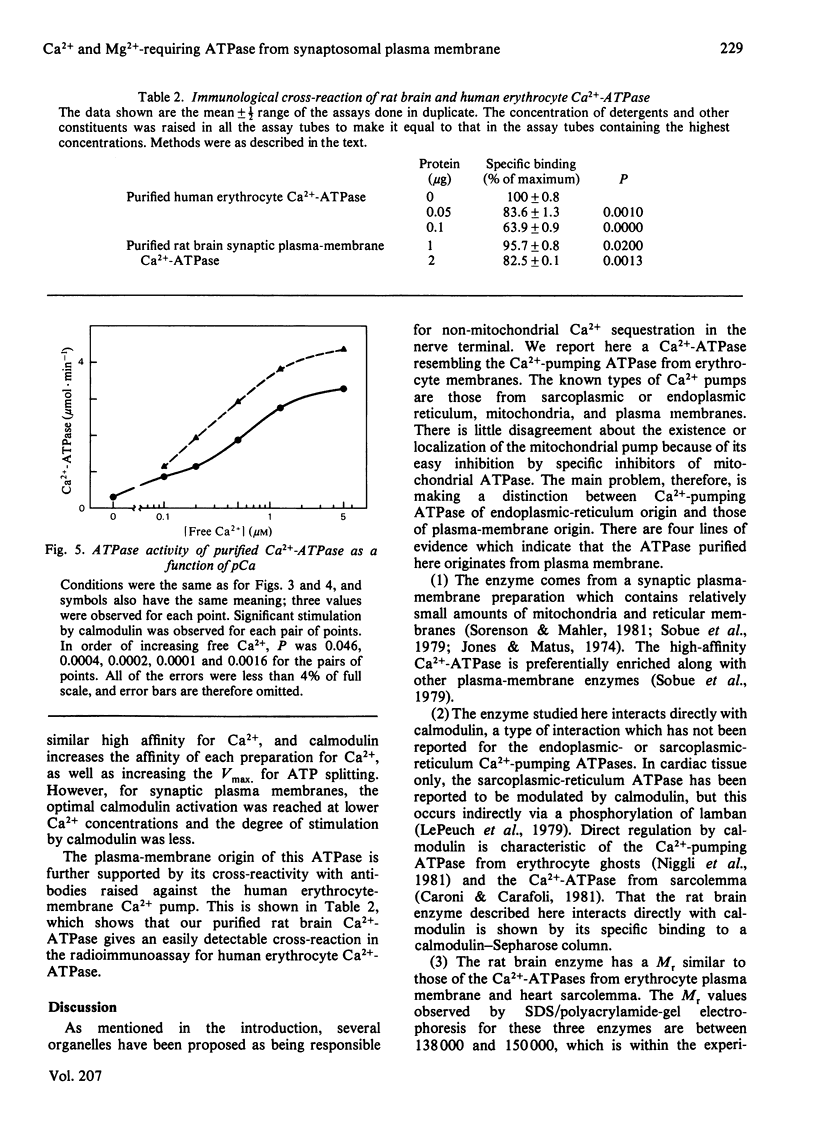


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Ratzlaff R. W., Kendrick N. C., Schweitzer E. S. Calcium buffering in presynaptic nerve terminals. I. Evidence for involvement of a nonmitochondrial ATP-dependent sequestration mechanism. J Gen Physiol. 1978 Jul;72(1):15–41. doi: 10.1085/jgp.72.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz A. L., Fine R. E., Toselli P. A. Evidence that coated vesicles isolated from brain are calcium-sequestering organelles resembling sarcoplasmic reticulum. J Cell Biol. 1977 Oct;75(1):135–147. doi: 10.1083/jcb.75.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Graf E., Penniston J. T. CaATP: the substrate, at low ATP concentrations, of Ca2+ ATPase from human erythrocyte membranes. J Biol Chem. 1981 Feb 25;256(4):1587–1592. [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. A new assay for endocytosis in erythrocyte ghosts based on loss of acetylcholinesterase activity. Biochim Biophys Acta. 1976 Oct 5;448(2):314–324. doi: 10.1016/0005-2736(76)90245-5. [DOI] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: its similarity to the activator of 3':5' - cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1210–1216. doi: 10.1016/s0006-291x(77)80108-3. [DOI] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Purification of the Ca2+-stimulated ATPase activator from human erythrocytes. Its membership in the class of Ca2+-binding modulator proteins. J Biol Chem. 1978 Jul 10;253(13):4676–4682. [PubMed] [Google Scholar]
- Jones D. H., Matus A. I. Isolation of synaptic plasma membrane from brain by combined flotation-sedimentation density gradient centrifugation. Biochim Biophys Acta. 1974 Aug 9;356(3):276–287. doi: 10.1016/0005-2736(74)90268-5. [DOI] [PubMed] [Google Scholar]
- Kendrick N. C., Blaustein M. P., Fried R. C., Ratzlaff R. W. ATP-dependent calcium storage in presynaptic nerve terminals. Nature. 1977 Jan 20;265(5591):246–248. doi: 10.1038/265246a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Peuch C. J., Haiech J., Demaille J. G. Concerted regulation of cardiac sarcoplasmic reticulum calcium transport by cyclic adenosine monophosphate dependent and calcium--calmodulin-dependent phosphorylations. Biochemistry. 1979 Nov 13;18(23):5150–5157. doi: 10.1021/bi00590a019. [DOI] [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Penniston J. T., Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem. 1981 Jan 10;256(1):395–401. [PubMed] [Google Scholar]
- Niggli V., Penniston J. T., Carafoli E. Purification of the (Ca2+-Mg2+)-ATPase from human erythrocyte membranes using a calmodulin affinity column. J Biol Chem. 1979 Oct 25;254(20):9955–9958. [PubMed] [Google Scholar]
- Robinson J. D. Calcium-stimulated phosphorylation of a brain (Ca + Mg)-ATPase preparation. FEBS Lett. 1978 Mar 15;87(2):261–264. doi: 10.1016/0014-5793(78)80347-0. [DOI] [PubMed] [Google Scholar]
- Sobue K., Ichida S., Yoshida H., Yamazaki R., Kakiuchi S. Occurrence of a Ca2+- and modulator protein-activatable ATPase in the synaptic plasma membranes of brain. FEBS Lett. 1979 Mar 1;99(1):199–202. doi: 10.1016/0014-5793(79)80278-1. [DOI] [PubMed] [Google Scholar]
- Sorensen R. G., Mahler H. R. Calcium-stimulated adenosine triphosphatases in synaptic membranes. J Neurochem. 1981 Dec;37(6):1407–1418. doi: 10.1111/j.1471-4159.1981.tb06309.x. [DOI] [PubMed] [Google Scholar]
- Tada M., Yamamoto T., Tonomura Y. Molecular mechanism of active calcium transport by sarcoplasmic reticulum. Physiol Rev. 1978 Jan;58(1):1–79. doi: 10.1152/physrev.1978.58.1.1. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Gorski J. P., Penniston J. T. Antibodies directed toward human erythrocyte Ca2+-ATPase: effect on enzyme function and immunoreactivity of Ca2+-ATPases from other sources. Arch Biochem Biophys. 1982 May;215(2):345–354. doi: 10.1016/0003-9861(82)90095-9. [DOI] [PubMed] [Google Scholar]