Abstract
During COVID-19 pandemic, international pharmaceutical companies put effort to build global manufacturing networks for vaccines. Soberana Plus vaccine, a recombinant protein based vaccine (RBD dimer), with the trade name of PastoCovac Plus in Iran, is based on a protein subunit platform in Cuba and completed preclinical and toxicological assessments. This study aimed at presenting the steps of vaccine technology transfer from Cuba to Iran. This study provides the first practical comparability results in Iran to ensure the quality, safety and efficacy of a protein subunit vaccine against COVID-19 after a successful technology transfer from Cuba. PastoCovac Plus was transferred to Iran at the formulation stage. The assessment of the active ingredient pharmaceutical (API) was achieved through physicochemical and clinical data collection and tests to assure if there was any adverse impact on the vaccination results. In order to assess the quality of the vaccine product after technology transfer, we sought different properties including regulatory features, physicochemical quality, vaccine potency and stability as well as its immunogenicity and safety. Following the evaluation of the clinical quality attributes (CQAs) based on the standard protocols, the results showed that the two vaccines are highly similar and comparable, with no considerable effect on safety or efficacy profiles. The CQAs were all in the acceptance limits in terms of safety and efficacy as well as clinical evaluation results. The immunogenicity evaluation also confirmed no significant differences between the vaccines regarding reinfection (P = 0.199) or vaccine breakthrough (P = 0.176). Furthermore, the level of anti-spike and neutralizing antibodies in the both vaccine groups was not significantly different indicating the equality of performance between the two vaccines. According to the results of the quality and clinical assessment of this study, we achieved an acceptable quality attributes and acceptant limits in terms of safety and efficacy of the vaccines pre and post technology transfer.
Keywords: PastoCovac Plus, Soberana Plus, RBD dimer, Protein-based vaccine, Iran
Subject terms: Business strategy in drug development, Drug regulation, Drug safety, Biotechnology, Drug discovery, Immunology
Introduction
Vaccines have always been considered as the most effective approach to control infectious diseases worldwide. Given the urgent need for COVID-19 (Coronavirus disease 2019) vaccination and its potential far-reaching impact on global health, a massive race to achieve a candidate vaccine took an extraordinary urgency across the world1. However, research and development (R&D) in terms of vaccines is normally a costly and high risk process which may take many years to reach an approved formulation with an appropriate dosage form2. Therefore, vaccine developers generally seek a continuous sequence of steps and pauses to data analysis and process assessment. Nevertheless, this pathway substantially changed in response to COVID-19 pandemic3,4.
COVID-19 imposed different sets of hardships, owing to the unique features of the virus, urgent need of quick action and the technological complications of the emerging new vaccines. To address this global crisis, technology transfer for local production has been widely regarded by international organizations5,6.
Vaccine technology transfer to low-income countries from multinational pharmaceutical companies to small scale facilities has more obstacles on the way including lack of financial resources and skillfully trained personnel for vaccine production, limitation of appropriate physical infrastructure, and poor R&D investment capacity7,8. Consequently, the recent pandemic mounted enormous interests regarding the role of international technology transfer of vaccines. In order to provide the proceeding demands on vaccination, there was a need of manufacturing networks for COVID-19 vaccines through which the developers could transfer the technology to the global partners9.
Vaccine supply network also was subjected to severe disruptions in 2021, according to import and export restrictions10. Iran is one of few Middle Eastern countries with the capacity to develop vaccines. Pasteur Institute of Iran (PII) intended to acquire the technology of Soberana Plus vaccine due to the previous experiences in international technological collaborations11 and the infrastructure of the industrial vaccine production besides Cuba’s prestigious biotech sector leading to developed different COVID-19 vaccines to that date. It is worth mentioning that pre-clinical, phase 1 and phase 2 of clinical studies of the vaccines were performed in Cuba12. The vaccine underwent clinical studies phase III in May 202113–15, and in July 2021 an emergency approval was achieved and set in public vaccination program of Iran16.
CQAs (Critical Quality Attributes) are the physical, chemical, biological and microbiological properties or characteristics of a vaccine that are essential to ensure its quality and safety. This study describes a hierarchy of sequential tests in analytical and clinical testing for comparing the CQAs of Soberana Plus, the produced vaccine at the original site, to the CQAs of the manufactured vaccine, PastoCovac Plus, at the new site.
Results
Soberana Plus/PastoCovac Plus immunogenicity feature
The initial binding of SARS-Cov-2 viral particles is mediated by Spike (S)-glycoprotein trimer via its Receptor Binding Domain (RBD) to the host’s cell surface receptor, the angiotensin-converting enzyme 2 (ACE2)17. This process was targeted by most of the 200 COVID-19 vaccines being currently developed18–20. By focusing on the whole S-protein or the RBD as antigen, the primarily goal lies at the induction of anti-RBD antibodies interfering with the RBD-ACE2 interaction, blocking the first step of infection and usually not participating in antibody dependent enhancement (ADE)21. RBD fragments in the S-glycoprotein trimer can adopt two different conformations on the virus surface: The “down” conformation with a well-camouflaged critical receptor-binding motif (RBM), and the “up” conformation with the RBM exposed and ready to bind to the ACE2 receptor in the human host cells. However, the “up” conformation also exposes the RBM epitopes to the immune system, allowing the induction of potent neutralizing antibodies22–24. Recombinant low-molecular weight RBD exposes not only the RBM, but also other protein epitopes that might become more exposed, and thus deflecting the immune response against less relevant epitopes in term of neutralization22,25.
Justifications of the physicochemical CQAs results and Potency analytical testing
The results of the quality analytical testing are summarized in Table 1. As summarized data are indicative, the pH values as a numerical index by which the greater or lesser acidity is determined depending on the activity of the hydronium ions (H3O+) are in the defined range [6.0–7.2] with no statistically difference between both vaccines. The stability of the antigen that constitutes the main CQA of the vaccine, is susceptible to basic or acidic pH values, the conservation of this parameter is evaluated within a slightly acidic to neutral pH range.
Table 1.
Results of the quality control assays.
| CQA | Method | Specification | Soberana Plus | PastoCovac Plus |
|---|---|---|---|---|
| Appearance | Direct Observation | White opaque suspension | Passed | Passed |
| pH | Potentiometry | 6.0-7.2 | 7.09 | 6.78 |
| Aluminum hydroxide content | Complexometry | 1.5–3.5 mg/mL | 2.31 mg/mL | 2.57 mg/mL |
| Total protein content | Modified Lowry1 | (100 ± 30) µg/mL | 74 µg/mL | 88 µg/mL |
| Adsorption percent | Modified Lowry | ≥ 70% | 91% | 84% |
| Osmolality | Osmometer | – | 284 mOsmol/Kg | 281 mOsmol/Kg |
| Volume | Using separate syringes | Not less than stated amount | Passed | Passed |
| RBD identity | SDS-PAGE and Immonoblotting | Positive recognition | Passed | Passed |
| Endotoxin content | Chromogenic LAL2 Test | >100 EU/mL | 0.4 EU/mL | 0.45 EU/mL |
| Sterility | Culture | Absence of viable microorganism | Passed | Passed |
| Abnormal toxicity | Injection into a laboratory animal | None of the animals show any weight loss and toxic signs at the end of the observation | Passed | Passed |
| Immunogenicity | Injection into mice & ELISA | At least 70% of the animals showing a seroconversion with a titre 4 times higher than the non-immunized animals | 90% | 100% |
1Lowry Protein Assay.
2LAL: limulus amebocyte lysate.
Total protein content was another critical quality parameter that indicates the amount of total protein in the final product. Taking this into account, the total protein content in each vaccine is within the defined range of protein content for final product to be immunogenic.
Adjuvant content and the antigen percent adsorption are indicators of the active ingredients adsorption to the adjuvant and in turn optimum efficacy of the vaccines. As calculated the adsorption percentage were equal to or greater than 70% with no significant difference in the vaccines. This is also a quality parameter indicative of the consistency of formulations.
Immunogenicity assay was performed to evaluate the biological activity of the product. A mouse immunization model is selected as a sensitive species and an ELISA (enzyme-linked immunosorbent assay) is selected to evaluate anti-RBD IgG antibody response, taking into account its high specificity, sensitivity and capacity to analyze a large number of samples simultaneously (Table 1).
Qualitative determination of the active pharmaceutical ingredient
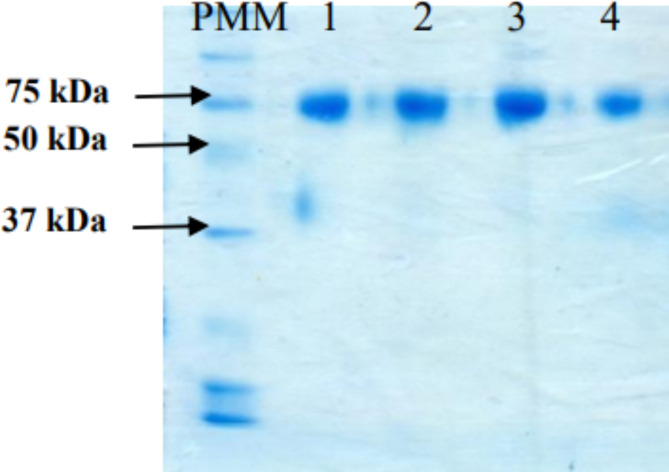
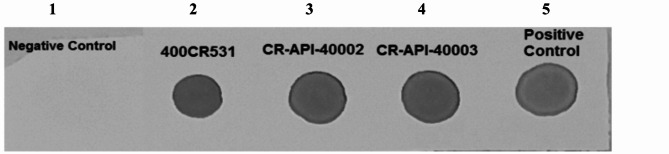
The SDS-PAGE, the molecular weight of API of the RBD dimer his (aa 319–541) was in the similar range of the RBD dimer from the original site (Fig. 1). Furthermore, the immunoassay was passed successfully in which the API applying in PastoCovac Plus is in the acceptable range comparing to RBD dimer as the positive control (Fig. 2).
Fig. 1.
SDS-PAGE for the API of the (D) RBD his (aa 319–541). PMM: Molecular Mass Pattern, 1and 2: PastoCovac Plus API, 4 and 5 Soberana Plus API.
Fig. 2.
Dot Blot Assay. 1: Preparation buffer as the negative control, 2: API batch (400CR531) of Soberana Plus, 3 & 4: API batches of PastoCovac Plus, 5: RBD dimer as the positive control. The immunoassay result shows that the dots belonging to two different batches of the produced API (CR-API-40002 and CR-API-40003) are in a similar strength range of the positive control.
Immunogenicity and safety evaluation
To compare the booster types, a total of 94 individuals including 39 males and 45 females were investigated who received two doses of AstraZeneca vaccine. Of this population, 30 received the Soberana Plus whereas 37 who received PastoCovac Plus boosters (Table 2). (The rest got a booster shot of AstraZeneca vaccine). According to the demographics, 6 individuals had a history of underlying diseases, 27 ones had a history of COVID-19 infection, and 11 people experienced re-infection. Vaccine breakthrough was defined as COVID-19 infection 4 weeks post the booster injection which was detected in 13 subjects. The comorbidities were hypertension (n = 2), thyroid problems (n = 3), diabetes mellitus (n = 3), polycystic ovary syndrome (n = 1) and allergy (n = 2). Nevertheless, there was no significant differences between the three groups regarding reinfection (P = 0.199) or vaccine breakthrough (P = 0.176).
Table 2.
Demographic data of Soberana Plus or PastoCovac Plus booster recipients.
| Astra-Astra | Soberana plus | PastoCovac Plus | P value | |
|---|---|---|---|---|
| n = 27 | n = 30 | n = 37 | ||
| Age | ||||
| Mean (SD) | 43.0 (12.4) | 39.9 (1.6) | 45.7 (2.0) | 0.034 |
| Min, Max | 26, 73 | |||
| Sex | ||||
| Female | 15 (55.6) | 21 (70) | 19 (51.4) | 0.122 |
| Male | 12 (44.4) | 9 (30) | 18 (48.7) | |
| BMI* | ||||
| Mean (SD) | 26.1 (4.2) | 25.1 (0.6) | 25.7 (0.7) | 0.556 |
| COVID-19 history | ||||
| Before vaccination | 12 (44.4) | 14 (46.67) | 13 (35.14) | 0.339 |
| Between vaccination | 4 (14.8) | 3 (10.0) | 3 (8.1) | 0.558 |
| 2 weeks after Booster | 2 (7.4) | 0 (0) | 0 (0) | – |
| Reinfection | 4 (14.8) | 7 (23.3) | 4 (10.8) | 0.199 |
| Vaccine breakthrough | 3 (11.1) | 8 (26.6) | 5 (13.5) | 0.176 |
| Comorbidity | ||||
| No | 22 (81.5) | 26 (86.67) | 35 (94.59) | 0.396 |
| Yes | 5 (18.5) | 4 (13.3) | 2 (5.4) | |
Significant values are in bold.
BMI*: Body mass index according to WHO definition as < 18.5 = underweight; ≥25 = overweight.
The results show that the level of anti-spike and neutralizing antibodies in the both vaccine groups was not significantly different indicating the equality of performance between the two vaccines (Table 3).
Table 3.
Assessment of anti-SARS-CoV-2 antibodies before and after the booster shots.
| Sobarana Plus (n = 30) |
PastoCovac Plus (n = 37) |
P value | |
|---|---|---|---|
| Anti-Spike IgG | |||
| Before GMT (95% CI) | 45.8 (34.6, 60.7) | 84.1 (50.4, 140.4) | 0.1919§ |
| After GMT (95% CI) | 255.3 (182.8, 356.6) | 363.8 (231.5, 571.9) | 0.4801§ |
| Rise GMT (95% CI) | 186.2 (127.9, 270.9) | 209.7 (130.2, 337.8) | 0.6408§ |
| Fold Rise GMT (95% CI) | 5.6 (3.8, 8.2) | 4.3 (3.2, 5.8) | 0.4569§ |
| Seroconversion, % | 18 (60.0) | 18 (48.7) | 0.354* |
| Seronegative to Seropositive | 1 (3.3) | 1 (2.7) | 0.699** |
| Neutralizing Ab | |||
| Before GMT (95% CI) | 11.6 (7.5, 17.8) | 9.5 (5.4, 16.7) | 0.7622§ |
| After GMT (95% CI) | 33.1 (31.0, 35.4) | 31.7 (28.6, 35.2) | 0.7574§ |
| Rise GMT (95% CI) | 9.0 (5.3, 15.1) | 7.0 (4.1, 12.0) | 0.5202§ |
| Fold Rise GMT (95% CI) | 2.9 (1.9, 4.3) | 3.3 (1.9, 5.7) | 0.6096§ |
| Seroconversion, % | 9 (30.0) | 9 (24.3) | 0.602* |
| Seronegative to Seropositive | 4 (13.3) | 7 (18.9) | 0.742* |
* Pearson Chi-Square; ** Fisher’s Exact Test; § Mann-Withney U.
Upon adjustment of age, history of COVID-19 and underlying diseases, the immunogenicity of Cuban and Iranian vaccines were still similar and no significant difference was detected (Table 4).
Table 4.
The effect of age, COVID-19 history and underlying disease on specific antibodies.
| Pasto Covac Plus vs. Soberana Plus | ||
|---|---|---|
| OR | P value | |
| Ig-S Seroconversion(Yes/No) | ||
| Crude | 0.6 (0.2, 1.7) | 0.355 |
| Model 1 | ||
| Seroconversion (Yes/No) | 0.7 (0.3, 1.9) | 0.480 |
| Age (Years) | 1.0 (0.9, 1.0) | 0.458 |
| Model 2: | ||
| Seroconversion (Yes/No) | 0.5 (0.2, 1.5) | 0.246 |
| COVID-19 (Yes/ No) | 0.4 (0.1, 1.1) | 0.062 |
| Model 3 | ||
| Seroconversion (Yes/No) | 0.6 (0.2, 1.7) | 0.337 |
| Comorbidity (Yes/ No) | 0.8 (0.1, 4.2) | 0.745 |
| Model 4 | ||
| Seroconversion (Yes/No) | 0.6 (0.2, 1.7) | 0.321 |
| Age (Years) | 1.0 (0.9, 1.0) | 0.632 |
| COVID-19 (Yes/ No) | 0.4 (0.1, 1.1) | 0.075 |
| Comorbidity (Yes/ No) | 0.8 (0.1, 5.1) | 0.858 |
| Neutralizing Ab Seroconversion(Yes/No) | ||
| Crude | 0.8 (0.3, 2.2) | 0.603 |
| Model 1 | ||
| Seroconversion (Yes/No) | 0.7 (0.3, 1.9) | 0.480 |
| Age (Years) | 1.0 (0.9, 1.0) | 0.458 |
| Model 2: | ||
| Seroconversion (Yes/No) | 0.5 (0.2, 1.5) | 0.246 |
| COVID-19 (Yes/ No) | 0.4 (0.1, 1.1) | 0.062 |
| Model 3 | ||
| Seroconversion (Yes/No) | 0.8 (0.3, 2.3) | 0.635 |
| Comorbidity (Yes/ No) | 1.3 (0.2, 8.1) | 0.763 |
| Model 4 | ||
| Seroconversion (Yes/No) | 0.7 (0.2, 2.7) | 0.585 |
| Age (Years) | 1.0 (0.9, 1.1) | 0.632 |
| COVID-19 (Yes/ No) | 0.4 (0.1, 1.1) | 0.075 |
| Comorbidity (Yes/ No) | 2.2 (0.2, 28.0) | 0.532 |
In order to compare the vaccine safety, any adverse event post the booster shots were recorded (Table 5). The frequency of unsolicited systemic side effects after the booster dose injection did not show a significant difference between the vaccine groups, while the pain in the injection site (local side effects) was significantly higher in the Soberana Plus receivers than PastoCovac Plus ones (p = 0.03).
Table 5.
The frequency of adverse events between the groups.
| Feature | AstraZeneca/PastoCovac Plus (%) | AstraZeneca/Soberana Plus (%) | P Value§ |
|---|---|---|---|
| Local | |||
| Local Pain | 7/37 (18.9) | 13/30 (43.3) | 0.030 |
| Systemic | |||
| Weakness | 3/37 (8.1) | 3/30 (10.0) | 0.558 |
| Fever | 1/37 (2.7) | 0/30 (0) | 0.552 |
| Nausea | 0/37 (0) | 0/30 (0) | – |
| Myalgia | 2/37 (5.4) | 2/30 (6.7) | 0.610 |
| Headache | 1/37 (2.7) | 2/30 (6.7) | 0.421 |
| Chills | 0/37 (0) | 0/30 (0) | – |
| Anorexia | 0/37 (0) | 0/30 (0) | – |
| Earache | 1/37 (2.7) | 0/30 (0) | 0.552 |
| Sore throat | 0/37 (0) | 0/30 (0) | – |
Significant values are in bold.
§ Fisher’s Exact Test; £ Pearson Chi Square.
Discussion
During COVID-19 outbreak, different approaches were adopted to achieve a vaccine against SARS-CoV-2 worldwide. Pasteur Institute of Iran was also among the organizations which put effort to reach the vaccine technology based on a 20-year successful collaboration with Cuba. After successful technology transfer to reveal an account of the partnership between PII and FVI, clinical trials were conducted in collaboration with Cuba to assess the immunogenicity and safety of the discussed vaccines and prove the comparability of the vaccines manufactured in different production platforms13. In order to determine the vaccine quality changes after the technology transfer, the acceptor of the technology should assess the comparability of the original product with the vaccine produced in its site to ensure that possible manufacturing changes have not affected the safety, identity, purity, or efficacy of the vaccine. Depending on the nature of the active immunogen ingredient, this assessment might include sequential analytical tests and/or clinical studies. Differences in analytical test results between pre and post change products may require functional testing to detect any biological or clinical significance of the observed difference. An underlying principle of comparability is that under certain conditions, protein products may be considered comparable on the basis of analytical testing results alone. However, the ability to compare biological materials is fully dependent on the set of applied tests, since no single analytical method is able to compare every aspect of protein structure or function.
According to the published guidelines regarding comparability assessment of Biotechnological/Biological Products, developed by the ICH guideline Q5E26, European Medicines Agency (EMA, London)27 and the U.S. Food and Drug Administration guidance on protocols to assist the comparability process. There is a strong emphasis that no single test can definitively determine if there has been a quality change in a vaccine after a technology transfer.
A virtual workshop was conducted on March 24–25, 2021 by the Stability Community of the American Association of Pharmaceutical Scientists (AAPS). The purpose of the workshop was to facilitate the discussion about the latest developments in vaccine stability strategies with a particular emphasis on COVID-19 vaccines that had to be developed quickly. The need for fast track timelines rendered the traditional ICH Q5C and real-time expiry dating study approach introduced to be insufficient for the urgency of COVID-19 vaccine need28. On the other hand, according to the recombinant platform technology that was used for these vaccines development and manufacture, PII and FVI previous knowledge of platform-based stability data were helpful for shelf-life projection, scientific justification that accelerate vaccine development. These scientific rationales were sufficient to achieve Iranian regulatory authorization for market entrance. These data partially supported the vaccine comparability after technology transfer in the pandemic situation.
Following CQAs, we proved that the two vaccines are highly similar and comparable, predicting no adverse impact on safety profile.
Comparability of these two vaccines could be deduced from quality (and stability) studies alone, but according to the emergency use market authorization, we found it advisable to perform a clinical study in parallel. The extent of the studies was necessary to demonstrate comparability though there were no significant difference in the purity as well as the physicochemical and biological properties.
The clinical evaluation of PastoCovac Plus in comparison with the other vaccine showed similar immunogenicity strength even upon age and comorbidity adjustment as well as a great safety profile. Anti-Spike and neutralizing antibodies mean titer rises and fold rises after either Soberana Plus or PastoCovac were in similar ranges as well as the seroconversion percentage.
Local pain as a clinical presentation was more frequent in Soberana Plus recipients. This result could possibly stem from different osmolality and pH between the two products29. Nevertheless, these chemical properties were not significantly different between the vaccines. The small sample size (number of the participants) and more female population in Soberana Plus group might have affected the results as women normally seek for medical cares and so report the signs. In total, the local pain difference was considered as a non-serious adverse event. In the future follow-up studies, the acceptance limit would be revised if the local pain reports frequently as a corrective action.
In addition to the present data, the other investigations on PastoCovac Plus booster in Iran, showed excellent results as a booster dose on primed individuals with COVAXIN. Interestingly, our follow-up schedule showed that 47.9 and 24.3% of the vaccinated subjects with COVAXIN were seronegative for anti-N and anti-S antibodies three months after the last dose, respectively. Following the booster injection, there were fold-rises of 70 and 93 regarding neutralizing antibody and quantitative anti-Spike antibody30. PastoCovac Plus boosting is strongly recommended in combination with inactivated vaccine platforms against SARS-CoV-2. Moreover, the other investigation on primed individuals with Sinopharm vaccine showed that all the seronegative cases became seropositive after receiving PastoCovac Plus booster on day 21. Anti-Spike IgG and neutralizing antibody rises were significantly comparable between those who got the same booster shot as Sinopharm and those who got PastoCovac Plus. Furthermore, the virus neutralizing assay indicated that collected samples from PastoCovac Plus recipients significantly neutralized the virus of both Wuhan and Omicron variants compared to homologous Sinopharm group31.
Conclusion
In order to achieve a successful firm to firm technology transfer, a skillful clinical trained team is acquired as well as well-defined and documented manufacturing process and validated quality control procedures. These elements in place contribute to minimize the risk of quality changes in the vaccine after the technology transfer. Taken together, the results of the quality and clinical evaluation of our investigation showed the acceptable quality attributes and acceptant limits in terms of safety and efficacy of the vaccines pre and post technology transfer. The results confirmed that PastoCovac Plus as the transferred vaccine technology is in the acceptable product limit in comparison with Soberana Plus vaccine. Furthermore, the clinical evaluation of PastoCovac Plus/ Soberana Plus showed that the recombinant protein-based vaccine against COVID-19 is a suitable booster choice after an Adenovirus-based vaccine.
Methods
This study was performed in Pasteur Institute of Iran. The investigated participants were provided with the written informed consent form prior to the participation. The study protocol was performed according to the Declaration of Helsinki (Fortaleza, 13th Oct, 2013) and was approved by the Ethics Committee of Pasteur Institute of Iran (ethics code number for animal study IR.PII.REC.1399.057 and R.PII.REC.1400.076 for human investigation). Animal setting and procedures were done according to ARRIVE guidelines 2.032.
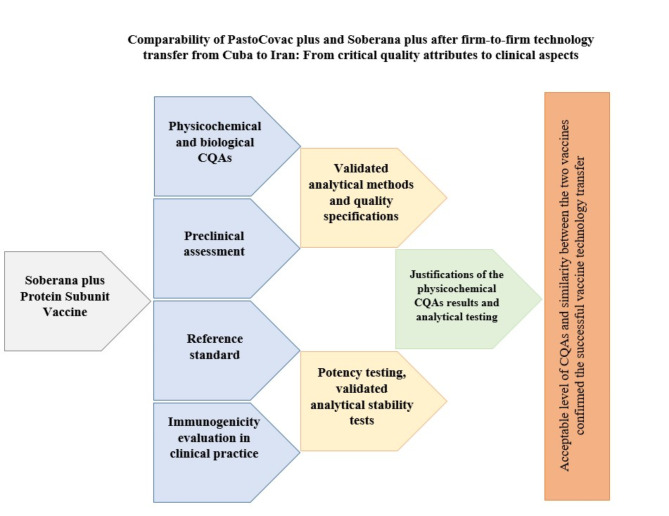
The taken steps in this study are simply shown in Fig. 3.
Fig. 3.
A schematic view on the process to evaluate and compare PastoCovac Plus with Soberana Plus after firm-to-firm technology transfer.
Soberana Plus/PastoCovac Plus composition
The human ACE2 receptor-binding domain (RBD) of the SARS-Cov2 virus is located in the envelope glycoprotein “Spike” which has been proposed as the antigen of several vaccine candidates to prevent COVID-1933. Using recombinant DNA technology, the RBD molecule was expressed in the CHO K1 cell line, located from Arg 319 to Phe 541 residues with 6 additional His residues at its C terminal end to facilitate the purification process. This region also contains 4 intramolecular disulfide bonds and 4 glycosylation sites (2 N and 2 O). In addition, the amino acidic sequence has the peculiarity of having a free cysteine at position 538, which allows the formation of RBD dimers.
This protein-based vaccine is composed of a dimer of recombinant RBD with sequence 319–541 dimerized from an inter-chain disulfide bridge between a cysteine at position 538 of each monomer, adsorbed on aluminum hydroxide to form an opalescent white suspension that slowly tends to form a white deposit, which is easily re-suspended with gentle shaking, generated in genetically modified in CHO cells34,35. CHO expression system was used to ensure proper glycosylation of amino acids 331, 343, 323 and 325 to resemble that of RBD in the virus described elsewhere36. Evidence in the literature indicates that RBD expressed as a recombinant protein is not toxic, regardless of its exact sequence and expression system37.
Soberana Plus, is being used as a third dose through different schedules depending on the priming vaccine platforms. In addition, a single dose of this vaccine is an excellent booster of natural immunity in convalescent through a mechanism named hybrid immunity38,39.
The storage condition for this product is 2–8 °C.
Regulatory situation of the products
At the time of the study, both Soberana Plus/PastoCovac Plus vaccines had completed preclinical and clinical evaluations and were produced and controlled under GMP and Good Laboratory Practice (GLP) condition in both countries, Cuba and Iran. According to the clinical studies, these vaccines were evaluated in children and adult populations in both countries and got the emergency use authorization13,40.
Evaluation of the physicochemical CQAs
Characterization of a biotechnological derived vaccine product using appropriate techniques, as described in International Committee for Harmonization (ICH) Q6B, includes the determination of physicochemical and organoleptic characteristics as well as biological and microbiological properties. Therefore, the following critical quality attributes were assessed via validated analytical methods and according to the defined quality specifications and acceptance limits, as described in Table 6.
Table 6.
Critical physicochemical and biological attributes, validated analytical methods and quality specifications.
| CQA | Method | Quality specification (acceptance limit) |
|---|---|---|
| Appearance | Organoleptic inspection | Opalescent white suspension that slowly tends to form a white deposit, easily re-suspended by shaking. Characteristic odor of Thimerosal |
| pH | Potentiometry (USP 42) | 6.0–7.2 |
| Adjuvant content (Al3+) | Complexometry | 1.5–3.5 mg/mL |
| Thiomersal content | Spectrophotometry, Biologicals (1995) 23, 65–69 | 0.07–0.13 mg/mL |
| Total protein content | Modified Lowry1 | (100 ± 30) µg/mL, total protein content must be within a value ± 30% of the theoretical calculated value |
| Adsorption percent | Modified Lowry, Lowry O.H. et al. J. Biol. Chem. 193:265–275, 1951 | ≥ 70% |
| Osmolality | Osmometer | – |
| Volume | Using separate syringes | Not less than stated amount |
| RBD identity | SDS-PAGE and Immonoblotting (immunoenzymatic assay) | Positive recognition, i.e. dot intensity of sample of final product equal or higher than positive control |
| Endotoxin content | Chromogenic LAL Test2 | > 100 EU/mL |
| Sterility | Culture- Membrane filtration method (USP 29) | Absence of viable microorganism |
| Abnormal toxicity | Injection into laboratory animals | None of the animals show any weight loss and toxic signs at the end of the observation |
1Lowry Protein Assay.
2LAL: limulus amebocyte lysate.
As two different quality control laboratories used the established analytical methods, an assessment of the method performance was used to ensure each quality control laboratory is able to appropriately control the vaccine. The probable statistically differences between the results of the analytical test concluded after comparing the mean of the results from three consequent batches, produced in Finlay Vaccine Institute (FVI) and PII which were analyzed by the validated methods according to the ICH guidelines. Moreover, the evaluation of reproducibility methods was included in the validation protocols.
Determination of Anti-RBD IgG in mice sera by ELISA
BALB/c mice (age: 6–8 weeks) were purchased from the animal center of the Pasteur Institute of Iran. The animal study was reviewed and approved by the ethical committee of the Pasteur Institute of Iran under Ethical Number: IR.PII.REC.1399.057. Furthermore, we confirm that all the applied methods which involved the animals followed the recommendations in the ARRIVE guidelines, and all experimental procedures were carried out in accordance with the relevant guidelines and regulations.
Briefly, 0.1 mL of the API-RBD vaccine product (50 µg RBD in 0.5 mL) was injected to each animal intramuscularly and blood samples were collected from the venous sinus on days 0, 14 and 28. Sera isolation was carried out by centrifugation and the separated sera were kept at – 20 °C. A non-immunized group of mice was also considered as the control group. For each group 10 mice were considered. The cut-off of seroconversion was considered ≥ negative samples OD (average) × 4.
The ELISA assay was performed to determine the anti-RBD IgG of the SARS-CoV-2 virus in mouse serums according to the provided guidelines [SOP 12–667, Finlay Vaccine Institute] under Regulation No. 16/2012 “Guidelines on Good Practice of production of Pharmaceutical Products” (CECMED). For determination of anti-RBD IgG antibody titer in mice sera, an indirect imunoenzymatic assay was developed in which the induced antibodies (Abs) bind to the RBD. Briefly, 96-well plates (NUNC Maxisorp) were used. The required volume of the covering dissolution (RBD) according to the number of the plates was prepared (having in mind 50 µL per well is added). The volume of the sample required to obtain a final RBD concentration of 3 µg/mL through the Volumetric Law was calculated. The samples (serums) required to be evaluated, were diluted 1/50. 150 µL of the dilution 1/50 of the serum of each mouse was added to the associated labelled well. The validated sample containing anti-RBD antibody was used as the control. Post the washing and incubation steps, the samples were read at 450 nm after stop solution adding.
For the determination of the protein content, a Bovine Serum Albumin working standard (coded as BSA5/18) calibrated against a NIBSC (National Institute for Biological Standards and Control) international standard, was applied. In order to assess the preservative content, a thimerosal working standard (coded as TR5/15) calibrated against a USP international Standard, was used (Table 7).
Table 7.
Potency testing, validated analytical method and acceptance limit.
| CQA | Method | Quality specification (acceptance limit) |
|---|---|---|
| Immunogenicity | Injection into mice & ELISA | At least 70% of the animals showing a seroconversion with a titer, 4 times higher than the non-immunized animals |
Dimer-RBD as an internal control for identity assays and reference sera (anti-RBD IgG) for generating calibration curves, positive and negative controls in potency assay were all developed by the FVI and used in all of the assays.
Stability studies
Stability testing is a series of tests that are performed to determine how the vaccine works over time. If the produced vaccine at the new site does not exhibit the same stability characteristics of the original site, this could indicate a quality change. Therefore, stability studies for approval of the both vaccines were fully characterized before initiation of phase III clinical trials in the both countries separately to collect sufficient stability data. Therefore, we designed stability studies supporting possible manufacturing changes that were suspected to have an impact on vaccine stability after firm to firm technology transfer. CQAs of three consequent batches of the vaccine manufactured in Iran were evaluated side-by-side and the results were compared to the data from three consequent batches produced from the original production process at different temperatures. Accelerated stability studies was designed to obtain a reliable estimates of change in the stability characteristic41 .
Immunogenicity evaluation in clinical practice
To evaluate any probable immunogenicity trends, the individuals who received two doses of AstraZeneca vaccine were investigated. In fact, the recombinant protein-based vaccines, Soberana Plus and PastoCovac Plus were applied as a booster dose after AstraZeneca (ChAdOx1-S) vaccine which is a replication-deficient adenoviral vector vaccine against COVID-19. This vaccine expresses the SARS-CoV-2 spike protein gene, which makes the host cells generate the protein of the S-antigen unique to SARS-CoV-2 and therefore induce the immune response.
The study population mainly included healthcare workers (HCWs) from PII during a period of time in which AstraZeneca was recommended for HCWs. The recommended schedule is 2 doses (0.5 ml) and can be administered with an interval of 4–12 weeks according to the manufacturer’s product information42. The booster shot of Soberana Plus or PastoCovac Plus was then provided as well as the group who got a booster shot of AstraZeneca vaccine. The demographic data including COVID-19 history and comorbidities were documented (Table 4).
Sera samples were collected before booster injection and also on day 28 ± 5 after it. The evaluation of vaccine immunogenicity was explored through Anti-SARS-CoV-2 Quantivac ELISA (IgG) (Euroimmun, Lübeck, Germany) and SARS-CoV-2 Neutralizing antibody (Pishtazteb, Iran) titer assessment.
In order to compare the vaccine adverse events, all the local and systemic outcomes were recorded in the appropriate questionnaire.
Antibody rise was calculated by subtracting antibody titers 28 ± 5 after the booster shot from the baseline. Fold rise was calculated by dividing antibody titers at the same window time to the baseline. The Geometric Mean Titer (GMT) of anti-SARS-CoV-2-Spike IgG and neutralizing antibodies, titer rise, and fold rise were calculated in the immunogenicity analysis. 95% confidence interval (CI) was considered.
Pearson Chi Square test, Fisher exact test and logistic regression analysis were used to evaluate the impact of booster types on antibody features.
Acknowledgements
This study was supported by Pasteur Institute of Iran (Grant No. 2060) and was approved by the ethical board of the Pasteur Institute of Iran (Reference number: IR.PII.REC.1399.057 & IR.PII.REC.1400.076).
Author contributions
Delaram Doroud, Mona Sadat Larijani, Amitis Ramezani and Alireza Biglari contributed to study design, conceptualization, writing the original draft and revision; Fatemeh Ashrafian, Talieh Sabooni and Sana Eybpoosh contributed to methodology and data analysis; Vicente Verez-Bencomo, Yury Valdés-Balbín, Dagmar García-Rivera, Yaneli Herrera-Rojas, Yanet Climent-Ruiz and Darielys Santana-Mederos provided scientific advice and contributed to data collection.
Data availability
The data which support the findings are included in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Delaram Doroud and Mona Sadat Larijani.
Contributor Information
Alireza Biglari, Email: biglari63@hotmail.com.
Amitis Ramezani, Email: amitisramezani@hotmail.com.
References
- 1.Koirala, A., Joo, Y. J., Khatami, A., Chiu, C. & Britton, P. N. Vaccines for COVID-19: the current state of play. Paediatr. Respir. Rev.35, 43–49 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funk, C. D., Laferrière, C. & Ardakani, A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front. Pharmacol.11, 937 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lurie, N., Saville, M., Hatchett, R. & Halton, J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med.382(21), 1969–1973 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Carneiro, D. C., Sousa, J. D. & Monteiro-Cunha, J. P. The COVID-19 vaccine development: A pandemic paradigm. Virus Res.301, 198454 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamey, G. et al. It is not. too late to achieve global covid-19 vaccine equity. BMJ376, o812 (2022). [DOI] [PMC free article] [PubMed]
- 6.Krishtel, P. & Hassan, F. Share Vaccine Know-How 379 (American Association for the Advancement of Science, 2021). [DOI] [PubMed]
- 7.Friede, M. et al. WHO initiative to increase global and equitable access to influenza vaccine in the event of a pandemic: Supporting developing country production capacity through technology transfer. Vaccine29, A2–A7 (2011). [DOI] [PubMed]
- 8.Bown, C. P. & Bollyky, T. J. How COVID-19 vaccine supply chains emerged in the midst of a pandemic. World Econ.45(2), 468–522 (2022). [DOI] [PMC free article] [PubMed]
- 9.Fonseca, E. M. D., Shadlen, K. C. & Achcar, H. M. Vaccine technology transfer in a global health crisis: Actors, capabilities, and institutions. Res. Policy52(4), 104739 (2023). [DOI] [PMC free article] [PubMed]
- 10.Forman, R., Shah, S., Jeurissen, P., Jit, M. & Mossialos, E. COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy125(5), 553–567 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maslehat, S., Doroud, D. & Mostafavi, E. A leading institute in the production and development of vaccines in Iran. Vacres6(1), 33–42 (2019). [Google Scholar]
- 12.Optimism as Cuba set to test its own Covid vaccine [Internet]. BBC News. https://www.bbc.com/news/world-latin-america-56069577 (2023).
- 13.Mostafavi, E. et al. Efficacy and safety of a protein-based SARS-CoV-2 vaccine: A randomized clinical trial. JAMA Netw. Open.6(5), e2310302 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Rodríguez, S. et al. A randomized, double-blind phase I clinical trial of two recombinant dimeric RBD COVID-19 vaccine candidates: Safety, reactogenicity and immunogenicity. Vaccine40(13), 2068–2075 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toledo-Romani, M. E. et al. Safety and immunogenicity of anti-SARS-CoV-2 heterologous scheme with SOBERANA 02 and SOBERANA Plus vaccines: Phase IIb clinical trial in adults. Med. (New York NY)3(11), 760–773 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghanei, M. et al. Exploring the experience of developing COVID-19 vaccines in Iran. Clin. Exp. Vacc. Res.12(1), 1–12 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang, J. et al. Structural basis of receptor recognition by SARS-CoV-2. Nature581(7807), 221–224 (2020). [DOI] [PMC free article] [PubMed]
- 18.Lan, J. et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature581(7807), 215–220 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Krammer, F. SARS-CoV-2 vaccines in development. Nature586(7830), 516–527 (2020). [DOI] [PubMed]
- 20.Valdes-Balbin, Y. et al. Molecular aspects concerning the use of the SARS-CoV-2 receptor binding domain as a target for preventive vaccines. ACS Cent. Sci.7(5), 757–767 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zang, J. et al. Immunization with the receptor-binding domain of SARS-CoV-2 elicits antibodies cross-neutralizing SARS-CoV-2 and SARS-CoV without antibody-dependent enhancement. Cell. Discov.6(1), 61 (2020). [DOI] [PMC free article] [PubMed]
- 22.Brouwer, P. J. M. et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Sci. (New York NY)369(6504), 643–650 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers, T. F. et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Sci. (New York NY)369(6506), 956–963 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zost, S. J. et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature584(7821), 443–449 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutten, L. et al. Impact of SARS-CoV-2 spike stability and RBD exposure on antigenicity and immunogenicity. Sci. Rep.14(1), 5735 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.http://www.emea.eu.int/pdfs/human/bwp/320702en.pdf. https://www.ema.europa.eu/en/documents/scientific-guideline/comparability-medicinal-products-containing-biotechnology-derived-proteins-active-substance-quality/ich/5721/03_en.pdf (2003).
- 27.http://www.emea.eu.int/pdfs/human/bwp/320700en.pdf. https://www.ema.europa.eu/en/documents/scientific-guideline/comparability-medicinal-products-containing-biotechnology-derived-proteins-active-substance-quality/ich/5721/03_en.pdf (2003).
- 28.Alasandro, M. et al. Meeting report: Vaccine stability considerations to enable rapid development and deployment. AAPS Open.7(1), 6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Usach, I., Martinez, R., Festini, T. & Peris, J-E. Subcutaneous injection of drugs: Literature review of factors influencing pain sensation at the injection site. Adv. Therapy36(11), 2986–2996 (2019). [DOI] [PMC free article] [PubMed]
- 30.Farahmand, B. et al. Evaluation of PastoCovac plus vaccine as a booster dose on vaccinated individuals with inactivated COVID-19 vaccine. Heliyon9(10), e20555 (2023). [DOI] [PMC free article] [PubMed]
- 31.Ramezani, A. et al. PastoCovac and PastoCovac Plus as protein subunit COVID-19 vaccines led to great humoral immune responses in BBIP-CorV immunized individuals. Sci. Rep.13(1), 8065 (2023). [DOI] [PMC free article] [PubMed]
- 32.Percie du Sert, N. et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol.18(7), e3000411 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valdes-Balbin, Y. et al. Molecular aspects concerning the use of the SARS-CoV-2 receptor binding domain as a target for preventive vaccines. ACS Central Sci.7(5), 757–767 (2021). [DOI] [PMC free article] [PubMed]
- 34.Chang-Monteagudo, A. et al. A single dose of SARS-CoV-2 FINLAY-FR-1A vaccine enhances neutralization response in COVID-19 convalescents, with a very good safety profile: An open-label phase 1 clinical trial. Lancet Reg. Health Am.2021(4), 100079 (2021). [DOI] [PMC free article] [PubMed]
- 35.Santana-Mederos, D. et al. A COVID-19 vaccine candidate composed of the SARS-CoV-2 RBD dimer and Neisseria meningitidis outer membrane vesicles. RSC Chem. Biol.. 3(2), 242–249 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan, P. M. et al. Potency, toxicity and protection evaluation of PastoCoAd candidate vaccines: Novel preclinical mix and match rAd5 S, rAd5 RBD-N and SOBERANA dimeric-RBD protein. Vaccine40(20), 2856–2868 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pichichero, M. E. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum. Vacc. Immunother.9(12), 2505–2523 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang-Monteagudo, A. et al. A single dose of SARS-CoV-2 FINLAY-FR-1A vaccine enhances neutralization response in COVID-19 convalescents, with a very good safety profile: An open-label phase 1 clinical trial. Lancet Reg. Health Am.4, 100079 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochoa-Azze, R. et al. Safety and immunogenicity of the FINLAY-FR-1A vaccine in COVID-19 convalescent participants: an open-label phase 2a and double-blind, randomised, placebo-controlled, phase 2b, seamless, clinical trial. Lancet Respiratory Med.10(8), 785–795 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toledo-Romaní, M. E. et al. Safety and efficacy of the two doses conjugated protein-based SOBERANA-02 COVID-19 vaccine and of a heterologous three-dose combination with SOBERANA-Plus: a double-blind, randomised, placebo-controlled phase 3 clinical trial. Lancet Reg. Health Americas2033, 45 (2023). [DOI] [PMC free article] [PubMed]
- 41.Annex 3 Guidelines on stability evaluation of vaccines. WHO Expert Committee on Biological Standardization (2011).
- 42.Interim recommendations for use of the ChAdOx1-S. [recombinant] vaccine against COVID-19 (AstraZeneca COVID-19 vaccine AZD1222 Vaxzevria™, SII COVISHIELD™) [Internet]. World Health Organization. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1 (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data which support the findings are included in the manuscript.