Abstract
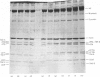
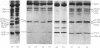
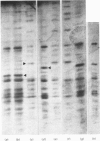
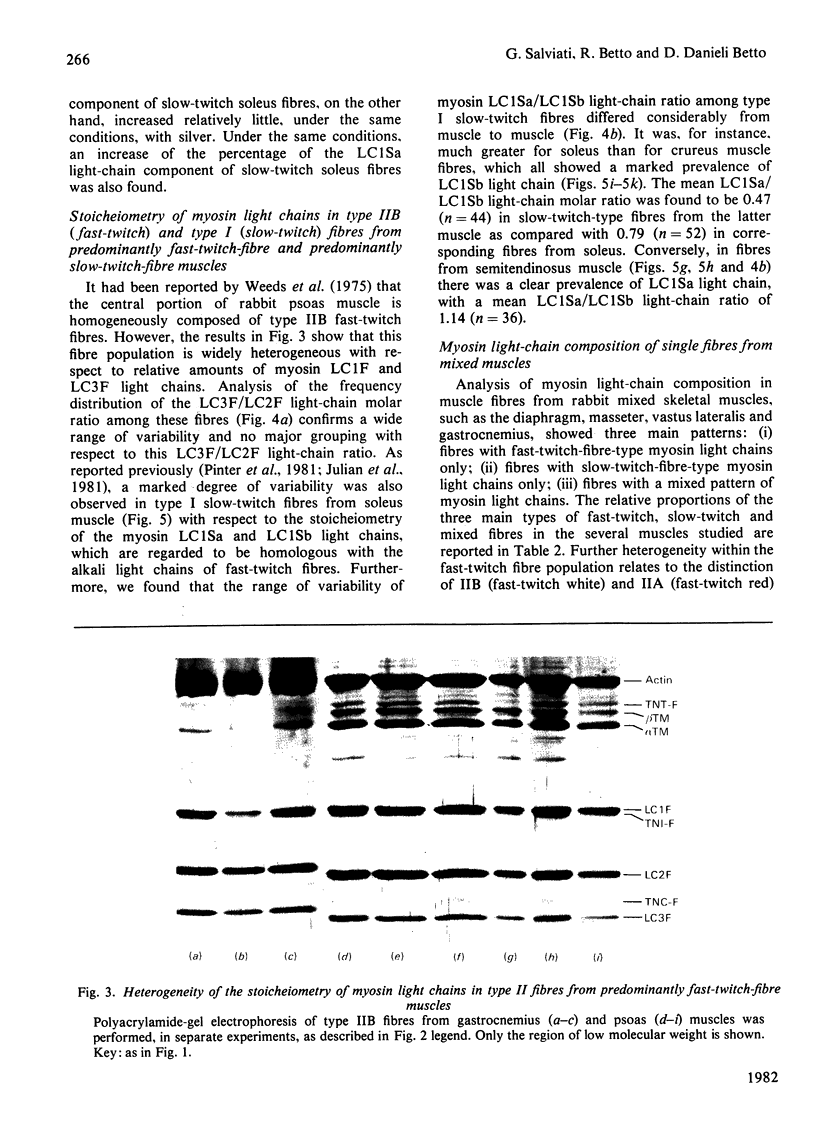
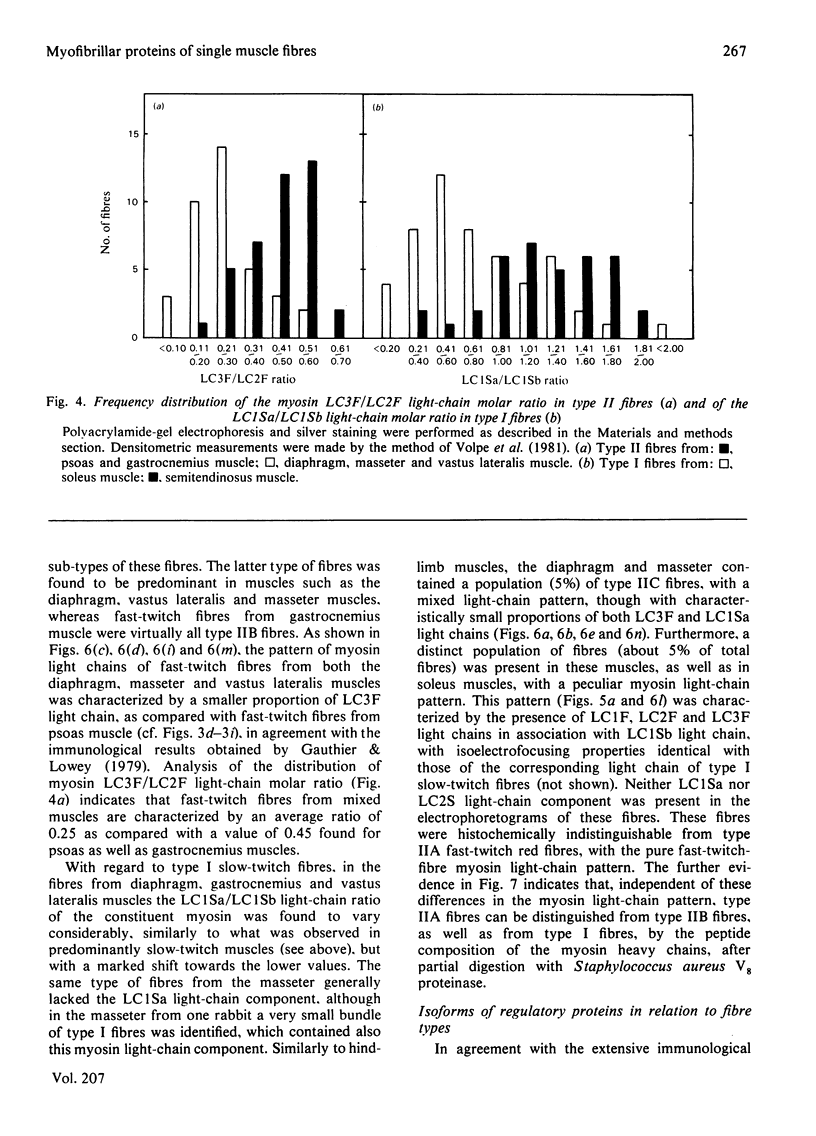
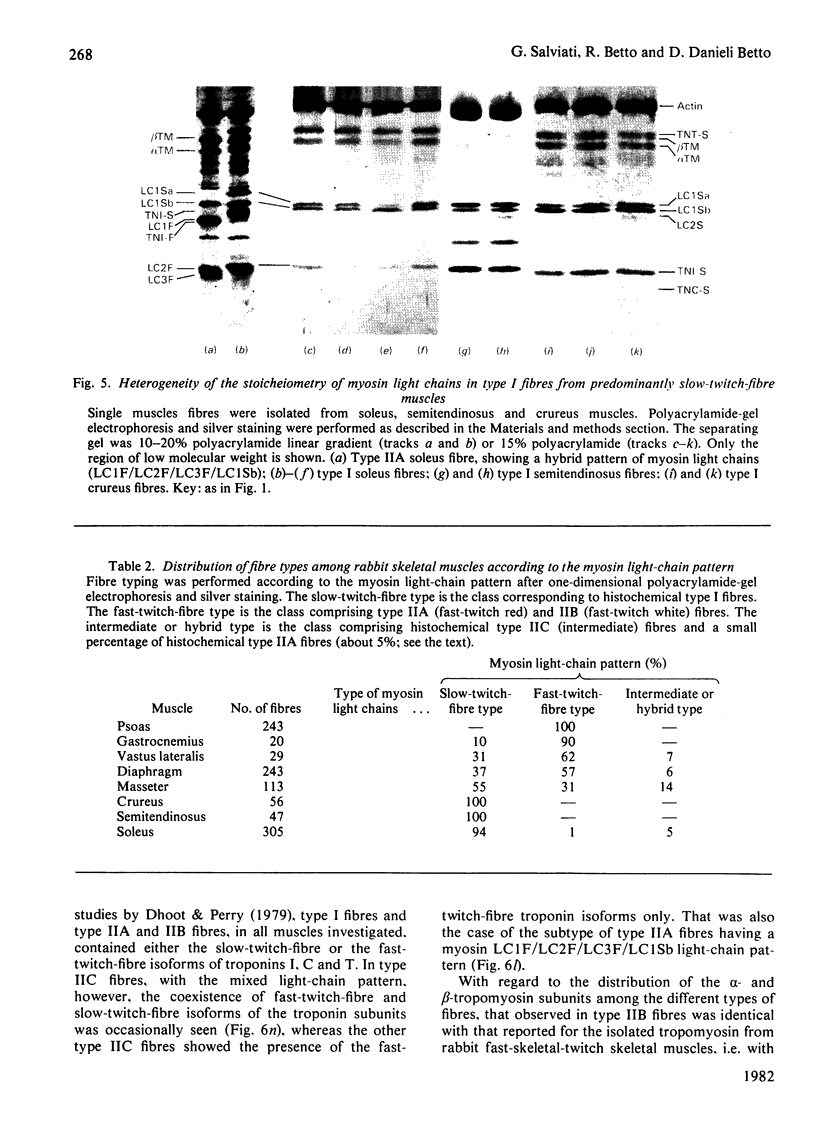
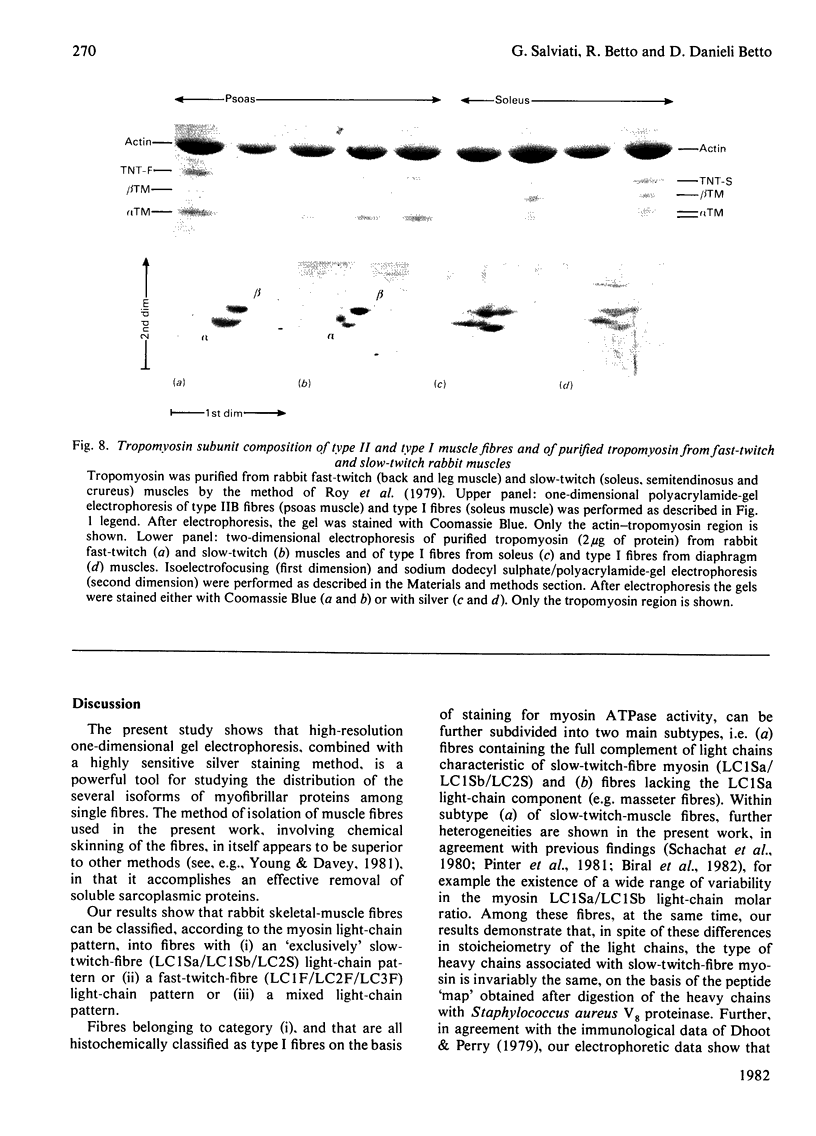
Rabbit predominantly fast-twitch-fibre and predominantly slow-twitch-fibre skeletal muscles of the hind limbs, the psoas, the diaphragm and the masseter muscles were fibre-typed by one-dimensional polyacrylamide-gel electrophoresis of the myofibrillar proteins of chemically skinned single fibres. Investigation of the distribution of fast-twitch-fibre and slow-twitch-fibre isoforms of myosin light chains and the type of myosin heavy chains, based on peptide 'maps' published in Cleveland. Fischer, Kirschner & Laemmli [(1977) J. Biol. Chem. 252, 1102-1106], allowed a classification of muscle fibres into four classes, corresponding to histochemical types I, IIA, IIB and IIC. Type I fibres with a pure slow-twitch-type of myosin were found to be characterized by a unique set of isoforms of troponins I, C and T, in agreement with the immunological data of Dhoot & Perry [(1979) Nature (London) 278, 714-718], by predominance of the beta-tropomyosin subunit and by the presence of a small amount of an additional tropomyosin subunit, apparently dissimilar from fast-twitch-fibre alpha-tropomyosin subunit. The myofibrillar composition of type IIB fast-twitch white fibres was the mirror image of that found for slow-twitch fibres in that the fast-twitch-fibre isoforms only of the troponin subunits were present and the alpha-tropomyosin subunit predominated. Type IIA fast-twitch red fibres showed a troponin subunit composition identical with that of type IIB fast-twitch white fibres. On the other hand, a unique type of myosin heavy chains was found to be associated with type IIA fibres. Furthermore, the myosin light-chain composition of these fibres was invariably characterized by a small amount of LC3F light chain and by a pattern that was either a pure fast-twitch-fibre light-chain pattern or a hybrid LC1F/LC2F/LC3F/LC1Sb light-chain pattern. By these criteria type IIA fibres could be distinguished from type IIC intermediate fibres, which showed coexistence of fast-twitch-fibre and slow-twitch-fibre forms of myosin light chains and of troponin subunits.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass A., Brdiczka D., Eyer P., Hofer S., Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969 Sep;10(2):198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Billeter R., Heizmann C. W., Howald H., Jenny E. Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur J Biochem. 1981 May 15;116(2):389–395. doi: 10.1111/j.1432-1033.1981.tb05347.x. [DOI] [PubMed] [Google Scholar]
- Biral D., Damiani E., Volpe P., Salviati G., Margreth A. Polymorphism of myosin light chains. An electrophoretic and immunological study of rabbit skeletal-muscle myosins. Biochem J. 1982 Jun 1;203(3):529–540. doi: 10.1042/bj2030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M., Close R. I. The transformation of myosin in cross-innervated rat muscles. J Physiol. 1971 Mar;213(2):455–474. doi: 10.1113/jphysiol.1971.sp009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro U., Catani C., Dalla Libera L. Myosin light and heavy chains in rat gastrocnemius and diaphragm muscles after chronic denervation or reinnervation. Exp Neurol. 1981 May;72(2):401–412. doi: 10.1016/0014-4886(81)90232-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dalla Libera L. Myosin heavy chains in fast skeletal muscle of chick embryo. Experientia. 1981 Dec 15;37(12):1268–1270. doi: 10.1007/BF01948352. [DOI] [PubMed] [Google Scholar]
- Dalla Libera L., Sartore S., Pierobon-Bormioli S., Schiaffino S. Fast-white and fast-red isomyosins in guinea pig muscles. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1662–1670. doi: 10.1016/0006-291x(80)91365-0. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. Distribution of polymorphic forms of troponin components and tropomyosin in skeletal muscle. Nature. 1979 Apr 19;278(5706):714–718. doi: 10.1038/278714a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg B. R., Kuda A. M. Discrimination between fiber populations in mammalian skeletal muscle by using ultrastructural parameters. J Ultrastruct Res. 1976 Jan;54(1):76–88. doi: 10.1016/s0022-5320(76)80010-x. [DOI] [PubMed] [Google Scholar]
- Etlinger J. D., Zak R., Fischman D. A. Compositional studies of myofibrils from rabbit striated muscle. J Cell Biol. 1976 Jan;68(1):123–141. doi: 10.1083/jcb.68.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S. Distribution of myosin isoenzymes among skeletal muscle fiber types. J Cell Biol. 1979 Apr;81(1):10–25. doi: 10.1083/jcb.81.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969 Sep;25(1):138–152. doi: 10.1016/0014-4886(69)90077-6. [DOI] [PubMed] [Google Scholar]
- Heilmann C., Brdiczka D., Nickel E., Pette D. ATPase activities, Ca2+ transport and phosphoprotein formation in sarcoplasmic reticulum subfractions of fast and slow rabbit muscles. Eur J Biochem. 1977 Dec 1;81(2):211–222. doi: 10.1111/j.1432-1033.1977.tb11943.x. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L., Waller G. S. Mechanical properties and myosin light chain composition of skinned muscle fibres from adult and new-born rabbits. J Physiol. 1981 Feb;311:201–218. doi: 10.1113/jphysiol.1981.sp013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Mikawa T., Takeda S., Shimizu T., Kitaura T. Gene expression of myofibrillar proteins in single muscle fibers of adult chicken: micro two dimensional gel electrophoretic analysis. J Biochem. 1981 Jun;89(6):1951–1962. doi: 10.1093/oxfordjournals.jbchem.a133397. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. Factors affecting the activity of adenosine triphosphatase and other phosphatases as measured by histochemical techniques. J Histochem Cytochem. 1955 May;3(3):161–169. doi: 10.1177/3.3.161. [DOI] [PubMed] [Google Scholar]
- Pette D., Schnez U. Myosin light chain patterns of individual fast and slow-twitch fibres of rabbit muscles. Histochemistry. 1977 Oct 22;54(2):97–107. doi: 10.1007/BF00489668. [DOI] [PubMed] [Google Scholar]
- Pinter K., Mabuchi K., Sreter F. A. Isoenzymes of rabbit slow myosin. FEBS Lett. 1981 Jun 15;128(2):336–338. doi: 10.1016/0014-5793(81)80111-1. [DOI] [PubMed] [Google Scholar]
- Porzio M. A., Pearson A. M. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1977 Jan 25;490(1):27–34. doi: 10.1016/0005-2795(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- Roy R. K., Mabuchi K., Sarkar S., Mis C., Sreter F. A. Changes in tropomyosin subunit pattern in chronic electrically stimulated rabbit fast muscles. Biochem Biophys Res Commun. 1979 Jul 12;89(1):181–187. doi: 10.1016/0006-291x(79)90961-6. [DOI] [PubMed] [Google Scholar]
- Salviati G., Sorenson M. M., Eastwood A. B. Calcium accumulation by the sarcoplasmic reticulum in two populations of chemically skinned human muscle fibers. Effects of calcium and cyclic AMP. J Gen Physiol. 1982 Apr;79(4):603–632. doi: 10.1085/jgp.79.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore S., Pierobon-Bormioli S., Schiaffino S. Immunohistochemical evidence for myosin polymorphism in the chicken heart. Nature. 1978 Jul 6;274(5666):82–83. doi: 10.1038/274082a0. [DOI] [PubMed] [Google Scholar]
- Schachat F. H., Bronson D. D., McDonald O. B. Two kinds of slow skeletal muscle fibers which differ in their myosin light chain complements. FEBS Lett. 1980 Dec 15;122(1):80–82. doi: 10.1016/0014-5793(80)80406-6. [DOI] [PubMed] [Google Scholar]
- Sorenson M. M., Reuben J. P., Eastwood A. B., Orentlicher M., Katz G. M. Functional heterogeneity of the sarcoplasmic reticulum within sarcomeres of skinned muscle fibers. J Membr Biol. 1980 Mar 31;53(1):1–17. doi: 10.1007/BF01871168. [DOI] [PubMed] [Google Scholar]
- Spamer C., Pette D. Activity patterns of phosphofructokinase, glyceraldehydephosphate dehydrogenase, lactate dehydrogenase and malate dehydrogenase in microdissected fast and slow fibres from rabbit psoas and soleus muscle. Histochemistry. 1977 Jun 8;52(3):201–216. doi: 10.1007/BF00495857. [DOI] [PubMed] [Google Scholar]
- Steinbach J. H., Schubert D., Eldridge L. Changes in cat muscle contractile proteins after prolonged muscle inactivity. Exp Neurol. 1980 Mar;67(3):655–669. doi: 10.1016/0014-4886(80)90134-x. [DOI] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Volpe P., Biral D., Damiani E., Margreth A. Characterization of human muscle myosins with respect to the light chains. Biochem J. 1981 Apr 1;195(1):251–258. doi: 10.1042/bj1950251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Hall R., Spurway N. C. Characterization of myosin light chains from histochemically identified fibres of rabbit psoas muscle. FEBS Lett. 1975 Jan 1;49(3):320–324. doi: 10.1016/0014-5793(75)80776-9. [DOI] [PubMed] [Google Scholar]
- Wood D. S. Human skeletal muscle: analysis of Ca2+ regulation in skinned fibers using caffeine. Exp Neurol. 1978 Jan 15;58(2):218–230. doi: 10.1016/0014-4886(78)90135-8. [DOI] [PubMed] [Google Scholar]
- Wood D. S., Zollman J., Reuben J. P., Brandt P. W. Human skeletal muscle: properties of the "chemically skinned%" fiber. Science. 1975 Mar 21;187(4181):1075–1076. doi: 10.1126/science.187.4181.1075. [DOI] [PubMed] [Google Scholar]
- Young O. A., Davey C. L. Electrophoretic analysis of proteins from single bovine muscle fibres. Biochem J. 1981 Apr 1;195(1):317–327. doi: 10.1042/bj1950317. [DOI] [PMC free article] [PubMed] [Google Scholar]