Abstract
Background
This study compared the costs associated with transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) in Korea by utilizing the National Health Insurance Service database.
Methods
Between June 2015 and May 2019, 1,468 patients underwent primary isolated transfemoral TAVI, while 2,835 patients received primary isolated SAVR with a bioprosthesis. We assessed the costs of index hospitalization and subsequent healthcare utilization, categorizing the cohort into 6 age subgroups <70, 70–74, 75–79, 80–84, 85–89, and ≥90 years. The median follow-up periods were 2.5 and 3.0 years in the TAVI and SAVR groups, respectively.
Results
The index hospitalization costs were 41.0 million Korean won (KRW) (interquartile range [IQR], 39.1–44.7) for the TAVI group and 24.6 million KRW (IQR, 21.3–30.2) for the SAVR group (p<0.001). The TAVI group exhibited relatively constant index hospitalization costs across different age subgroups. In contrast, the SAVR group showed increasing index hospitalization costs with advancing age. The healthcare utilization costs were 5.7 million KRW per year (IQR, 3.3–14.2) for the TAVI group and 4.0 million KRW per year (IQR, 2.2–9.0) for the SAVR group (p<0.001). Healthcare utilization costs were higher in the TAVI group than in the SAVR group for the age subgroups of <70, 70–74, and 75–79 years, and were comparable in the age subgroups of 80–84, 85–89, and ≥90 years.
Conclusion
TAVI had much higher index hospitalization costs than SAVR. Additionally, the overall healthcare utilization costs post-discharge for TAVI were also marginally higher than those for SAVR in younger age subgroups.
Keywords: Transcatheter aortic valve implantation, Aortic valve disease, Heart valve prosthesis, Cost
Introduction
Transcatheter aortic valve implantation (TAVI) was initially introduced as an alternative to surgical aortic valve replacement (SAVR) for symptomatic patients with aortic stenosis who were at prohibitive or high surgical risk [1,2]. However, TAVI is now supplanting SAVR as the first-line treatment for severe aortic stenosis in patients with low surgical risk [3,4]. Additionally, the annual volume of TAVI procedures now surpasses that of SAVR in Western countries [5-7].
In Korea, TAVI was first included in the national health insurance coverage in 2015, with the National Health Insurance Service (NHIS) beginning to partially reimburse the costs associated with the procedure. Since then, the number of TAVI procedures has risen sharply, now exceeding 1,000 cases annually [8]. Despite the Korean government’s 2015 regulation restricting TAVI to low- and intermediate-risk patients, interventional cardiologists have frequently performed it on this group, guided by emerging evidence and updated guidelines [9,10]. Moreover, in 2022, a significant amendment to the healthcare policy on TAVI broadened the scope and depth of insurance coverage for the procedure. As a result, the economic burden on the NHIS and its impact on national finances are expected to increase significantly.
The aim of this study was to evaluate the costs associated with TAVI and SAVR during the index hospitalization and post-discharge in a real-world Korean population, utilizing the NHIS database.
Methods
The study protocol underwent review by the Institutional Review Board of Seoul National University Hospital and received approval as a minimal-risk retrospective study (approval date: 10/07/2021, approval number: H-2110-009-1259). Based on the institutional guidelines for waiving consent, individual consent was not required.
Data source and patient characteristics
The present study utilized a database from the NHIS, a government-managed single insurer that provides public health insurance to over 97% of residents in Korea [11]. This database contains demographic data such as age and sex, as well as diagnoses; it also includes information on procedures performed and medical claims per patient. Diagnoses within this database are recorded according to the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10) [11].
The first TAVI in Korea was performed in 2010 [12]. However, there was no NHIS dataset available for TAVI prior to May 2015, as it was only included in the NHIS insurance coverage starting in June 2015. In this study, we analyzed the initial 5-year period of TAVI data, from June 2015 to May 2019, following its inclusion under insurance coverage. We also enrolled patients who underwent SAVR during the same period.
Of the 1,735 patients who underwent TAVI from June 2015 to May 2019, 208 underwent additional procedures (203 coronary interventions, 3 mitral valvuloplasties, and 2 catheter ablations for arrhythmia). Six patients had unidentifiable data, and 1,521 underwent primary isolated TAVI. After excluding those who received transapical and transaortic TAVI, isolated transfemoral TAVI was performed on 1,497 patients. Following the exclusion of 29 patients with previous cardiac surgery, 1,468 patients were ultimately included in the TAVI group.
Of the 10,288 patients who underwent SAVR between June 2015 and May 2019, 4,418 underwent primary isolated SAVR. After excluding 1,303 patients who received mechanical valves, primary isolated SAVR with a bioprosthetic valve was performed on 3,115 patients. Further exclusions were made for 24 patients with previous cardiac surgery, 3 patients with prior TAVI, and 253 patients with endocarditis, leaving a total of 2,835 patients ultimately enrolled in the SAVR group (Fig. 1).
Fig. 1.
(A, B) Flow diagram of patient enrollment. SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Preoperative comorbidities were assessed within 1 year prior to surgery using the diagnosis codes listed in Supplementary Table 1. The Charlson comorbidity index was determined by summing the weights assigned to 17 comorbidities, as outlined by the diagnosis codes in Supplementary Table 2 [13].
Evaluation of early and mid-term mortality
To evaluate the impact of mortality differences between the groups on costs, we analyzed both early and mid-term mortality. Early mortality was defined as any death occurring within 30 days post-surgery or during the initial hospital admission. Mid-term mortality included any all-cause death that occurred after the surgery. Mortality status was determined by cross-referencing the discharge date of the index procedure with the date of death listed on the death certificate, as provided by Statistics Korea, a central statistical organization under the Ministry of Strategy and Finance. Additionally, the dates of mortality during follow-up were obtained from the death certificates issued by Statistics Korea.
Clinical follow-up ended on December 31, 2020, with a completeness rate of 100.0%, as mortality data were recorded in the Statistics Korea database by the government. The median follow-up duration was 2.5 years (interquartile range [IQR], 1.8–3.5 years) for the TAVI group and 3.0 years (IQR, 2.1–4.1 years) for the SAVR group.
Evaluation of costs
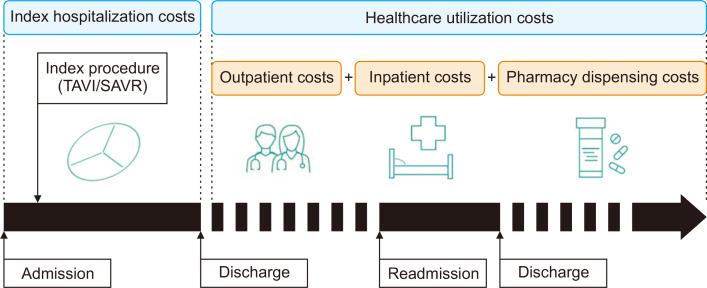
The total costs were categorized based on the timing of the expenses incurred, specifically into index hospitalization costs and healthcare utilization costs. Index hospitalization costs encompassed the total expenses accrued during the initial admission for TAVI or SAVR procedures. This included fees for doctors, hospitalization, prescriptions, injections, anesthesia, operations, examinations, radiologic diagnoses, medical materials, rehabilitation and physical therapy, transfusions, sonographic diagnoses, meals, and certificates. Healthcare utilization costs represented the annual costs post-discharge and consisted of 3 main components: outpatient costs, inpatient costs, and pharmacy dispensing costs (Fig. 2).
Fig. 2.
Schematic diagram of the costs associated with transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) during the index hospitalization and after discharge.
The costs were calculated based on who bore the expense; they were categorized into out-of-pocket costs, paid directly by the patient, and insurance coverage costs, paid by the NHIS. The term “out-of-pocket” referred to the copayment (i.e., the part of the healthcare service costs paid by patients to the medical service provider during a hospital visit). The copayment rate varied depending on whether the service was inpatient or outpatient, as well as the type of service and the healthcare institution’s level. The out-of-pocket and insurance coverage costs were calculated using the classification tables from the National Health Insurance database. The currency unit used in the cost analysis was the Korean won (KRW).
Statistical analysis
Statistical analyses were conducted using IBM SPSS software ver. 25.0 (IBM Corp., Armonk, NY, USA) and SAS Enterprise Guide ver. 7.1 (SAS Institute Inc., Cary, NC, USA). Continuous variables are reported as either the mean±standard deviation for normally distributed data or the median with interquartile range for data that are not normally distributed. Categorical variables are expressed as the number and percentage of subjects. To compare baseline characteristics between the 2 groups, we used the chi-square test or the Fisher exact test for categorical variables and the Student t-test or the Wilcoxon rank-sum test for continuous variables.
To focus on the age criteria associated with the healthcare policy, cost analyses were conducted by dividing the patient cohort into 6 age subgroups at 5-year intervals: <70, 70–74, 75–79, 80–84, 85–89, and ≥90 years. Costs of index hospitalization and subsequent healthcare utilization were compared using a generalized linear model with a gamma distribution.
Early and mid-term mortality rates were compared between the groups to supplement the cost analysis. The risk of early mortality was assessed using multivariable logistic regression analysis. Mid-term mortality was analyzed using a Cox proportional hazard model, which accounted for the effect of covariates to adjust for baseline characteristic differences between the groups [14]. The analyses included adjustments for variables that were not balanced (standardized mean difference >0.1) between the groups. All tests were 2-tailed, and a p-value <0.05 was deemed statistically significant.
Results
Baseline characteristics
The average age of the TAVI group was higher than that of the SAVR group (80.0±5.9 versus 72.6±7.8 years, p< 0.001). Additionally, a greater proportion of women was observed in the TAVI group than in the SAVR group (52.5% versus 47.2%, p<0.001). The TAVI group also exhibited a higher incidence of risk factors, including diabetes mellitus, hypertension, chronic lung disease, cerebrovascular disease, renal disease, liver disease, cancer, atrial fibrillation, and coronary artery disease. As a result, the Charlson comorbidity index was higher in the TAVI group (Table 1). However, when subgroup analyses were conducted for each age group, the differences in baseline characteristics between the groups were mostly nonsignificant, with the exception of patients aged under 70 years. In specific age subgroups, higher prevalence rates in the TAVI group were observed only for renal disease and cancer in the 70–74 years age subgroup, coronary artery disease in the 75–79 years age subgroup, dyslipidemia in the 80–84 years age subgroup, and cancer in the 85–89 years age subgroup (Supplementary Tables 3–8).
Table 1.
Preoperative characteristics and risk factors for the study patients
| Characteristic | TAVI (n=1,468) | SAVR (n=2,835) | SMD | p-value |
|---|---|---|---|---|
| Sex, female | 771 (52.5) | 1,337 (47.2) | 0.107 | <0.001 |
| Mean age (yr) | 80.0±5.9 | 72.6±7.8 | -1.076 | <0.001 |
| Age group (yr) | 1.129 | <0.001 | ||
| <70 | 63 (4.3) | 861 (30.4) | ||
| 70–74 | 157 (10.7) | 749 (26.4) | ||
| 75–79 | 430 (29.3) | 743 (26.2) | ||
| 80–84 | 509 (34.7) | 382 (13.5) | ||
| 85–89 | 253 (17.2) | 89 (3.1) | ||
| ≥90 | 56 (3.8) | 11 (0.4) | ||
| Charlson comorbidity index | 4 (2–6) | 3 (2–5) | -0.230 | <0.001 |
| Risk factors | ||||
| Diabetes mellitus | 735 (50.1) | 1,328 (46.8) | -0.065 | 0.044 |
| Hypertension | 1,317 (89.7) | 2,391 (84.3) | -0.161 | <0.001 |
| Dyslipidemia | 1,160 (79.0) | 2,178 (76.8) | -0.053 | 0.102 |
| Chronic lung disease | 194 (13.2) | 294 (10.4) | -0.088 | 0.005 |
| Cerebrovascular disease | 247 (16.8) | 343 (12.1) | -0.135 | <0.001 |
| Renal disease | 203 (13.8) | 270 (9.5) | -0.134 | <0.001 |
| Liver disease | 40 (2.7) | 48 (1.7) | -0.070 | 0.023 |
| Cancer | 228 (15.5) | 326 (11.5) | -0.118 | <0.001 |
| Atrial fibrillation | 250 (17.0) | 267 (9.4) | -0.226 | <0.001 |
| Coronary artery disease | 503 (34.3) | 806 (28.4) | -0.126 | <0.001 |
| Peripheral vascular disease | 92 (6.3) | 148 (5.2) | -0.045 | 0.156 |
| Diagnosis | ||||
| Aortic stenosis | 1,239 (84.4) | 2,034 (71.7) | -0.310 | <0.001 |
| Aortic regurgitation | 61 (4.2) | 438 (15.4) | 0.387 | <0.001 |
| Aortic stenoinsufficiency | 246 (16.8) | 286 (10.1) | -0.197 | <0.001 |
| Bicuspid aortic valve | 36 (2.5) | 187 (6.6) | 0.200 | <0.001 |
| Year of the index procedure | 0.312 | |||
| 2015 (from June) | 83 (5.7) | 304 (10.7) | ||
| 2016 | 237 (16.1) | 642 (22.7) | ||
| 2017 | 376 (25.6) | 739 (26.1) | ||
| 2018 | 486 (33.1) | 807 (28.5) | ||
| 2019 (to May) | 286 (19.5) | 343 (12.1) |
Continuous variables are presented as the mean±standard deviation for normally distributed variables and as the median (interquartile range) for nonnormally distributed variables. Categorical variables are presented as number (%).
TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement; SMD, standardized mean difference.
Early and mid-term mortality
Early mortality was higher in the SAVR group than in the TAVI group (4.0% for SAVR versus 3.2% for TAVI, p=0.009). However, mortality rates were similar between the TAVI and SAVR groups across the age subgroups of <70, 70–74, 75–79, 80–84, and 85–89 years. In contrast, for the subgroup aged ≥90 years, early mortality was significantly higher in the SAVR group (Table 2).
Table 2.
Early mortality stratified by age subgroups
| Variable | Mortality rate (no./total no.) | Adjusted ORa) (95% CI) | p-value | |
|---|---|---|---|---|
|
| ||||
| TAVI | SAVR | |||
| Overall population | 3.2 (47/1,468) | 4.0 (114/2,835) | 0.58 (0.39–0.87) | 0.009 |
| Age subgroups (yr) | ||||
| <70 | 3.2 (2/60) | 3.0 (26/861) | 0.45 (0.09–2.36) | 0.348 |
| 70–74 | 1.9 (3/157) | 2.9 (22/749) | 0.62 (0.17–2.33) | 0.479 |
| 75–79 | 3.3 (14/430) | 4.4 (33/743) | 0.80 (0.39–1.63) | 0.531 |
| 80–84 | 2.8 (14/509) | 5.0 (19/382) | 0.54 (0.25–1.19) | 0.125 |
| 85–89 | 4.3 (11/253) | 11.2 (10/89) | 0.37 (0.12–1.11) | 0.076 |
| ≥90 | 5.4 (3/56) | 36.4 (4/11) | 0.10 (0.02–0.54) | 0.007 |
TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement; OR, odds ratio; CI, confidence interval.
a)Adjusted odds ratios were calculated using a multivariable logistic model for the variables that were not balanced (absolute standardized difference >0.1) between the groups. ORs were calculated for the TAVI group using the SAVR group as a reference.
In terms of mid-term mortality, the cumulative incidence of all-cause mortality was higher in the TAVI group compared to the SAVR group (p<0.001) (Supplementary Fig. 1). However, subgroup analyses for each age category showed similar outcomes between the groups, with the exception of patients younger than 70 years, where the incidence of all-cause mortality was higher in the TAVI subgroup (Supplementary Fig. 2).
Cost
Index hospitalization costs
The index hospitalization costs amounted to 41.0 million KRW (IQR, 39.1–44.7) for the TAVI group and 24.6 million KRW (IQR, 21.3–30.2) for the SAVR group (p<0.001). Regarding these costs, the out-of-pocket expenses were 27.6 million KRW (IQR, 27.1–28.2) for the TAVI group and 1.5 million KRW (IQR, 1.2–2.3) for the SAVR group. Conversely, the insurance coverage costs were 13.4 million KRW (IQR, 11.7–16.8) for the TAVI group and 22.1 million KRW (IQR, 19.7–26.4) for the SAVR group.
In the subgroup analyses for different age categories, the TAVI group incurred higher index hospitalization costs compared to the SAVR group across all age subgroups, with the exception of those aged 90 years and above. The costs for the TAVI group remained relatively stable, varying from 40.0 to 42.2 million KRW across the different age groups. In contrast, the SAVR group showed a trend of increasing costs with age, ranging from 23.1 to 37.2 million KRW (Table 3, Fig. 3).
Table 3.
Index hospitalization costs in patients who underwent TAVI or SAVR
| Variable | No. of patients | Total cost (A+B) | Out-of-pocket (A) | Insurance coverage (B) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||
| TAVI | SAVR | TAVI | SAVR | p-value | TAVI | SAVR | p-value | TAVI | SAVR | p-value | ||||
| Overall population | 1,468 | 2,835 | 41.0 (39.1–44.7) | 24.6 (21.3–30.2) | <0.001 | 27.6 (27.1–28.2) | 1.5 (1.2–2.3) | <0.001 | 13.4 (11.7–16.8) | 22.1 (19.7–26.4) | <0.001 | |||
| Age subgroups (yr) | ||||||||||||||
| <70 | 63 | 861 | 41.1 (39.2–47.2) | 23.1 (20.3–28.3) | <0.001 | 27.9 (27.3–28.5) | 1.4 (1.1–1.9) | <0.001 | 13.1 (11.6–18.8) | 21.0 (19.0–24.5) | <0.001 | |||
| 70–74 | 157 | 749 | 40.0 (38.6–42.5) | 24.0 (21.0–29.4) | <0.001 | 27.5 (27.1–28.1) | 1.4 (1.1–2.0) | <0.001 | 12.3 (11.3–15.0) | 21.8 (19.6–25.4) | <0.001 | |||
| 75–79 | 430 | 743 | 40.6 (38.9–43.4) | 25.6 (21.8–31.0) | <0.001 | 27.6 (27.0–28.1) | 1.5 (1.2–2.7) | <0.001 | 13.1 (11.5–15.8) | 22.7 (20.2–26.9) | <0.001 | |||
| 80–84 | 509 | 382 | 41.0 (39.1–44.0) | 27.2 (22.9–33.4) | <0.001 | 27.6 (27.1–28.2) | 1.7 (1.3–6.7) | <0.001 | 13.4 (11.6–16.5) | 24.1 (20.8–28.9) | <0.001 | |||
| 85–89 | 253 | 89 | 42.2 (40.1–47.3) | 28.4 (23.6–35.2) | <0.001 | 27.8 (27.2–28.4) | 1.6 (1.3–5.2) | <0.001 | 14.6 (12.3–19.1) | 24.3 (21.7–31.3) | <0.001 | |||
| ≥90 | 56 | 11 | 42.0 (39.9–47.3) | 37.2 (32.1–42.9) | 0.423 | 27.6 (27.1–28.5) | 8.3 (1.9–9.3) | <0.001 | 14.4 (12.3–20.1) | 32.1 (25.9–40.5) | <0.001 | |||
Values are presented as number of patients or medians (interquartile range) unless otherwise stated. The unit of cost is 1,000,000 Korean won.
TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement.
Fig. 3.
Index hospitalization costs of transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) in Korea by age subgroup. The unit of cost is 1,000,000 Korean won.
Healthcare utilization costs
The annual healthcare utilization costs were 5.7 million KRW (IQR, 3.3–14.2) in the TAVI group and 4.0 million KRW (IQR, 2.2–9.0) in the SAVR group. Within these costs, the out-of-pocket expenses amounted to 1.6 million KRW (IQR, 1.0–3.0) for the TAVI group and 1.0 million KRW (IQR, 0.5–1.8) for the SAVR group. The insurance coverage costs were 4.0 million KRW (IQR, 2.2–10.7) per year for the TAVI group and 2.9 million KRW (IQR, 1.5–7.1) for the SAVR group. In subgroup analyses by age, the TAVI group incurred higher costs than the SAVR group in the age subgroups of <70, 70–74, and 75–79 years. However, costs were comparable between the TAVI and SAVR groups in the age subgroups of 80–84, 85–89, and ≥90 years (Table 4, Fig. 4A)
Table 4.
Healthcare utilization costs after discharge in patients who underwent TAVI or SAVR
| Variable | No. of patients | Total cost (A+B) | Out-of-pocket (A) | Insurance coverage (B) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| TAVI | SAVR | TAVI | SAVR | p-value | TAVI | SAVR | TAVI | SAVR | ||||
| Overall population | 1,421 | 2,721 | 5.7 (3.3–14.2) | 4.0 (2.2–9.0) | <0.001 | 1.6 (1.0–3.0) | 1.0 (0.5–1.8) | 4.0 (2.2–10.7) | 2.9 (1.5–7.1) | |||
| Age subgroups | ||||||||||||
| <70 | 61 | 835 | 11.7 (4.1–38.1) | 3.1 (1.7–7.1) | <0.001 | 1.8 (0.7–3.6) | 0.8 (0.4–1.4) | 8.8 (3.0–33.9) | 2.2 (1.2–5.4) | |||
| 70–74 | 154 | 727 | 5.3 (3.3–17.5) | 4.2 (2.3–8.7) | <0.001 | 1.6 (1.0–2.9) | 1.0 (0.5–1.7) | 3.8 (2.1–13.6) | 3.0 (1.6–7.0) | |||
| 75–79 | 416 | 710 | 5.2 (3.3–12.3) | 4.4 (2.5–9.9) | <0.001 | 1.5 (1.0–2.5) | 1.0 (0.6–2.0) | 3.9 (2.2–9.1) | 3.2 (1.8–7.8) | |||
| 80–84 | 495 | 363 | 5.5 (3.3–11.1) | 4.7 (2.8–11.2) | 0.955 | 1.6 (1.0–2.9) | 1.1 (0.6–2.2) | 3.9 (2.2–8.7) | 3.5 (1.9–9.2) | |||
| 85–89 | 242 | 79 | 6.4 (3.5–15.3) | 6.2 (3.9–18.8) | 0.629 | 1.7 (1.0–3.5) | 1.3 (0.6–2.3) | 4.6 (2.3–12.9) | 4.8 (2.7–14.4) | |||
| ≥90 | 53 | 7 | 5.6 (3.3–20.4) | 3.7 (2.6–19.3) | 0.958 | 1.7 (1.0–5.0) | 1.2 (0.7–4.5) | 4.4 (2.0–15.2) | 2.5 (1.8–14.8) | |||
Values are presented as number of patients or medians (interquartile range) unless otherwise stated. The unit of cost is 1,000,000 KRW.
TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement.
Fig. 4.
Healthcare utilization costs of transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) after discharge in Korea by age subgroup. (A) Overall healthcare utilization costs after discharge, (B) outpatient costs after discharge, (C) inpatient costs after discharge, and (D) pharmacy dispensing costs after discharge. The unit of cost is 1,000,000 Korean won.
The annual outpatient costs were 1.4 million KRW (IQR, 0.9–2.3) for the TAVI group and 1.1 million KRW (IQR, 0.7–1.9) for the SAVR group. The TAVI group incurred slightly higher outpatient costs than the SAVR group across all age subgroups (Supplementary Table 9, Fig. 4B).
The annual inpatient costs were 1.5 million KRW (IQR, 0.0–8.2) in the TAVI group and 0.9 million KRW (IQR, 0.0–4.7) in the SAVR group. In the subgroup of patients younger than 80 years, the TAVI group incurred higher outpatient costs compared to the SAVR group. Conversely, in the subgroup of patients aged 80 years and older, the SAVR group incurred higher costs (Supplementary Table 10, Fig. 4C).
The annual pharmacy dispensing costs were 1.7 million KRW (IQR, 1.1–2.3) in the TAVI group and 1.2 million KRW (IQR, 0.7–1.7) in the SAVR group. The TAVI group consistently showed slightly higher pharmacy dispensing costs than the SAVR group across all age subgroups (Supplementary Table 11, Fig. 4D).
Discussion
The present study demonstrated 2 main findings. First, the index hospitalization costs, which were defined as the total costs incurred during the index hospitalization to perform TAVI or SAVR, were much higher for TAVI than for SAVR. Second, the healthcare utilization costs, which were defined as the maintenance costs per year during the follow-up after discharge, were also slightly higher in the TAVI group than in the SAVR group.
In 2010, the first TAVI in Korea was classified as a “non- benefit” item by the national health insurance system, meaning it was not covered by insurance, and patients were responsible for the full cost [12]. This made the procedure very expensive. However, in 2015, the NHIS reclassified TAVI as a “selective healthcare benefit” item. Consequently, the government established a fixed price for the materials and procedures involved in TAVI, and the NHIS began to cover 20% of these costs. This change significantly reduced the financial burden on patients and led to a marked increase in the number of TAVI procedures performed.
In 2022, the Ministry of Health and Welfare of the Korean government announced an expansion of the reimbursement indications and an increase in the proportion of costs covered by the NHIS. The NHIS now reimburses 95% of the costs for patients who meet at least 1 of the following 3 criteria: (1) a Society of Thoracic Surgeons (STS) score greater than 8%, (2) age 80 years or older, or (3) classified as “inoperable” by consensus of 2 cardiac surgeons within a multidisciplinary TAVI team. Patients who do not meet any of these criteria receive NHIS coverage of only 20% of the costs if they are low-risk (STS score less than 4%) or 50% if they are intermediate-risk (STS score between 4% and 8%). Following this amendment in 2022, there was a significant increase in both the proportion of costs borne by the NHIS per patient and the total number of patients undergoing TAVI. Given these developments, it is necessary to assess the costs associated with TAVI and SAVR to guide future directions of TAVI insurance policy in Korea.
Several international studies have reported the superior cost-effectiveness of TAVI compared to SAVR. A recent study highlighted significant cost savings with the latest generation TAVI device over a 10-year period for patients with intermediate surgical risks [15]. For patients with low surgical risk and severe aortic stenosis, multiple studies from various countries have consistently suggested that TAVI is a cost-effective option [16-19]. These cost savings are primarily attributed to lower long-term management costs, which include expenses related to postsurgical rehabilitation, atrial fibrillation, and disabling stroke [18]. However, these studies have inherent limitations as they rely on efficacy inputs from The Placement of Aortic Transcatheter Valves (PARTNER) 3 trial and base their cost-effectiveness models on this trial. The conclusions drawn from these studies cannot be generalized to the entire aortic stenosis population, particularly those with bicuspid valves or other high-risk anatomical features such as annular calcification and unfavorable coronary anatomy. This is because the PARTNER 3 trial excluded patients who were at a high risk of postinterventional complications [4]. Additionally, these cost-effectiveness models are limited by assumptions made with the “best fit” data or a scarcity of data, extrapolations beyond the existing data range, and potential underestimations or overestimations due to differences in healthcare systems [18].
Another study evaluated the costs associated with TAVI and SAVR using real-world data, adjusted for STS risk and key sociodemographic factors. It found that both the cost of the initial procedure and the total costs up to 30 days post-procedure were lower for TAVI than for SAVR, achieving a cost savings of 13% during the 30-day follow-up period [20]. However, the generalizability of this study is limited due to significant differences in the index hospitalization costs compared to other countries and its nature as a single-center study with a brief follow-up period. Additionally, the study did not account for community-incurred costs, such as visits to general practitioners, prescription medications, or other nonsubsidized health services costs.
In contrast to previous international cost-effectiveness studies, our research was grounded in the actual costs incurred by all patients across various institutions in Korea. The findings revealed that both the procedural and index admission costs—collectively referred to as the initial costs—were significantly higher for TAVI than for SAVR. Additionally, the healthcare utilization costs, or maintenance costs, were marginally higher for TAVI than for SAVR. Typically, the higher initial cost of a procedure could be justified if its subsequent maintenance costs are lower than those of an alternative procedure. However, our study indicated that TAVI not only demands greater expenditure initially but also incurs higher costs during both short-term and follow-up management.
In terms of clinical outcomes, there is no doubt that the outcomes of TAVI will continue to improve and that the demand for TAVI will increase in the coming years. The 2020 American College of Cardiology/American Heart Association guidelines recommend TAVI for severe aortic stenosis, taking into account only the patient’s age, life expectancy, and suitability for transfemoral TAVI, regardless of the risk score [9]. The most recent 2021 European guidelines recommend TAVI for all patients aged 75 years and older, irrespective of their surgical risk [10]. The indications for TAVI are likely to expand further in the near future, and the long-term outcomes are also expected to be satisfactory. From a societal perspective, TAVI may offer additional benefits over SAVR that have not yet been fully recognized, such as a quicker return to normal activities, which could reduce the need for caregivers.
However, further cost reductions of TAVI will be limited, even though TAVI has become more efficient due to operator learning, procedural modifications, advances in postprocedural care, and a consequent reduction in the average length of hospital stays [21]. The primary obstacle to lowering TAVI costs is the high price of the TAVI valve itself, which costs approximately 30,000,000 KRW and represents about 75% of the total index hospitalization cost. In contrast, SAVR is significantly less expensive than TAVI. This is because the government-set cost for SAVR is relatively low compared to that in Western countries. The procedure cost for SAVR, including the prosthetic valve, is approximately 17,000,000 KRW, with national health insurance covering up to 95% of the index hospitalization cost.
The reasons behind the higher post-discharge costs for TAVI compared to SAVR remain unclear. One hypothesis is that patients undergoing TAVI may experience more complications such as paravalvular leakage, heart failure, and the need for pacemaker implantation. As a result, cardiologists may prescribe more medications than cardiac surgeons typically do. Furthermore, despite similar ages and comparable risk factors between the groups within the same age subgroups, TAVI patients may have additional undisclosed comorbidities not captured in the database. These could include conditions like porcelain aorta, post-chest radiation status, severe aortic annulus calcifications, or diminished left ventricular function.
In Korea’s healthcare system, careful policy-making is essential to ensure both outstanding clinical results and the equitable distribution of resources for patients with severe aortic stenosis. Given the costs associated with TAVI and SAVR, healthcare policies should guide low-risk patients, who are likely to recover well from SAVR, towards this option due to its proven long-term benefits and lower costs. Additionally, for patients under 70 years old with a high likelihood of having a bicuspid aortic valve, healthcare policies should strongly discourage the use of TAVI, not only because of its cost but also due to its less optimal clinical outcomes [22,23]. TAVI in patients with a bicuspid aortic valve is known to carry a higher risk of device malposition and underexpansion, which can compromise the success of the procedure [24]. Furthermore, long-term durability data for TAVI in patients with bicuspid valves remain inadequate [25], and a previous study indicated a time-varying risk that favors SAVR over TAVI for these patients at later timepoints [26].
In addition, considering the aging population and the increasing number of patients with aortic stenosis who require aortic valve intervention, the NHIS covering up to 95% of the total cost will undoubtedly impose a significant financial burden. To ensure the sustainability of the national healthcare insurance system, the reimbursement policy for TAVI needs to be stricter and more restrictive. It is essential to engage in public discussions and reach a social consensus on various issues, including the appropriate percentages for copayments and insurance coverage, limitations on performing TAVI in patients with very short life expectancies, and regulations on performing TAVI in extremely frail patients who are unlikely to benefit from the procedure.
It is widely understood that healthcare policy, especially in terms of costs, significantly impacts real-world practice. It is crucial to develop a nuanced policy that considers the costs of procedures, long-term management, and overall budgets comprehensively. This approach will prevent the wastage of national healthcare resources and ensure that patients receive the necessary treatments.
Limitations
Several limitations of the current study must be recognized. First, the study relied on the NHIS database, which only includes diagnosis codes with ICD-10 classifications, and did not allow for the collection of STS scores. Second, the retrospective analysis involved nonrandomized groups with varying baseline characteristics. Although subgroup analyses by age partially adjusted for these differences, they may still have impacted the findings. The higher healthcare utilization costs observed in the TAVI group may not be solely due to the costs associated with the aortic valve prosthesis. The increased prevalence of comorbidities among TAVI patients suggests a broader need for medical care beyond treating aortic valve disease. Third, this study did not include a long-term cost evaluation. In a long-term follow-up, factors such as the durability of transcatheter and surgical valves, and the potential need for subsequent interventions like TAVI after TAVI, SAVR after TAVI, and TAVI after SAVR, could significantly influence the conclusions of this type of cost analysis. Fourth, the study did not conduct a cost-effectiveness evaluation that included quality of life. From another perspective, a higher cost might be justified if it results in significant improvements in quality of life and secondary socioeconomic benefits, such as an early return to work. Fifth, the out-of-pocket costs reported in this study may differ significantly from those encountered in everyday practice due to several factors. These include non-benefit items not captured in the database, varying costs across different hospitals, and individual patient copayment rates.
Conclusions
The index hospitalization costs for TAVI were much higher than those for SAVR, and the overall healthcare utilization costs of TAVI after discharge were also slightly higher than those for SAVR. Based on the results of this study, the cost of the index procedures during the index admission and after discharge should be considered when establishing a national health insurance policy regarding procedures for aortic valve diseases.
Supplementary materials
Supplementary materials can be found via https://doi.org/10.5090/jcs.24.048. Supplementary Table 1. Diagnosis codes used for the evaluation of preoperative comorbidities. Supplementary Table 2. Medical conditions, disease codes, and weighted values for the calculation of the Charlson comorbidity index. Supplementary Table 3. Preoperative characteristics and risk factors for the subgroup aged under 70 years. Supplementary Table 4. Preoperative characteristics and risk factors for the subgroup aged from 70 to 74 years. Supplementary Table 5. Preoperative characteristics and risk factors for the subgroup aged from 75 to 79 years. Supplementary Table 6. Preoperative characteristics and risk factors for the subgroup aged from 80 to 84 years. Supplementary Table 7. Preoperative characteristics and risk factors for the subgroup aged from 85 to 89 years. Supplementary Table 8. Preoperative characteristics and risk factors for the subgroup aged 90 years or more. Supplementary Table 9. Outpatient costs after discharge for TAVI and SAVR. Supplementary Table 10. Inpatient costs after discharge for TAVI and SAVR. Supplementary Table 11. Pharmacy dispensing costs after discharge for TAVI and SAVR. Supplementary Fig. 1. The cumulative incidence of all-cause mortality between the transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) groups in the entire population. Supplementary Fig. 2. The cumulative incidence of all-cause mortality between transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) in the age subgroups of (A) <70, (B) 70–74, (C) 75–79, (D) 80–84, (E) 85–89, and (F) ≥90 years.
Acknowledgments
The authors thank the Medical Research Collaborating Center of Seoul National University Hospital for the statistical support.
Funding Statement
Funding This research received grants from the Patient-centered Clinical Research Coordinating Center (PACEN) in the National Evidence-based Healthcare Collaborating Agency (NECA) of Korea as a project for health policy research (no., HC21C0180010021). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Article information
Author contributions
Conceptualization: SH Sohn, KHK, YK, SHL, SH Shinn, CL. Data curation: SH Sohn, YK. Formal analysis: SH Sohn. Methodology: SH Sohn, KHK, JWC, SHL, SH Shinn, JSY, CL. Visualization: SH Sohn. Writing–original draft: SH Sohn. Writing–review & editing: KHK, JWC, SHL, SH Shinn, JSY, CL. Final approval of the manuscript: SH Sohn, KHK, JWC, SHL, SH Shinn, JSY, CL.
Conflict of interest
Jae Woong Choi serves as an Editorial Board member of the Journal of Chest Surgery but has no role in the decision to publish this article. Except for that, no potential conflict of interest relevant to this article was reported.
References
- 1.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. https://doi.org/10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Mack MJ, Leon MB, Smith CR, et al. 5-Year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–84. doi: 10.1016/S0140-6736(15)60308-7. https://doi.org/10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–20. doi: 10.1056/NEJMoa1514616. https://doi.org/10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 4.Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–705. doi: 10.1056/NEJMoa1814052. https://doi.org/10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 5.Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:2492–516. doi: 10.1016/j.jacc.2020.09.595. https://doi.org/10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen V, Michel M, Eltchaninoff H, et al. Implementation of transcatheter aortic valve replacement in France. J Am Coll Cardiol. 2018;71:1614–27. doi: 10.1016/j.jacc.2018.01.079. https://doi.org/10.1016/j.jacc.2018.01.079. [DOI] [PubMed] [Google Scholar]
- 7.Reinohl J, Kaier K, Reinecke H, et al. Effect of availability of transcatheter aortic-valve replacement on clinical practice. N Engl J Med. 2015;373:2438–47. doi: 10.1056/NEJMoa1500893. https://doi.org/10.1056/NEJMoa1500893. [DOI] [PubMed] [Google Scholar]
- 8.Sohn SH, Kim KH, Kang Y, et al. Aortic valve replacement in the era of transcatheter aortic valve implantation: current status in Korea. J Korean Med Sci. 2023;38:e404. doi: 10.3346/jkms.2023.38.e404. https://doi.org/10.3346/jkms.2023.38.e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto CM, Nishimura RA, et al. Writing Committee Members, author. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:e25–197. doi: 10.1016/j.jacc.2020.11.018. https://doi.org/10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. https://doi.org/10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 11.Sohn SH, Kang Y, Kim JS, Hwang HY, Kim KH, Choi JW. Long-term clinical outcomes of tricuspid valve replacement using bovine versus porcine valves: a nationwide population-based study. Eur J Cardiothorac Surg. 2023;64:ezad151. doi: 10.1093/ejcts/ezad151. https://doi.org/10.1093/ejcts/ezad151. [DOI] [PubMed] [Google Scholar]
- 12.Kim WJ, Kim YH, Lee JY, et al. Transcatheter aortic valve implantation: early experience in Korea. Korean Circ J. 2012;42:684–91. doi: 10.4070/kcj.2012.42.10.684. https://doi.org/10.4070/kcj.2012.42.10.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–94. doi: 10.1016/j.jclinepi.2004.03.012. https://doi.org/10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36:4391–4400. doi: 10.1002/sim.7501. https://doi.org/10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Liew D, Duffy SJ, Walton A, Htun N, Stub D. Cost-effectiveness of transcatheter aortic valve implantation compared to surgical aortic valve replacement in the intermediate surgical risk population. Int J Cardiol. 2019;294:17–22. doi: 10.1016/j.ijcard.2019.06.057. https://doi.org/10.1016/j.ijcard.2019.06.057. [DOI] [PubMed] [Google Scholar]
- 16.Galper BZ, Chinnakondepalli KM, Wang K, et al. Economic outcomes of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis and low surgical risk: results from the PARTNER 3 Trial. Circulation. 2023;147:1594–605. doi: 10.1161/CIRCULATIONAHA.122.062481. https://doi.org/10.1161/CIRCULATIONAHA.122.062481. [DOI] [PubMed] [Google Scholar]
- 17.Tam DY, Azizi PM, Fremes SE, Chikwe J, Gaudino M, Wijeysundera HC. The cost-effectiveness of transcatheter aortic valve replacement in low surgical risk patients with severe aortic stenosis. Eur Heart J Qual Care Clin Outcomes. 2021;7:556–63. doi: 10.1093/ehjqcco/qcaa058. https://doi.org/10.1093/ehjqcco/qcaa058. [DOI] [PubMed] [Google Scholar]
- 18.Gilard M, Eltchaninoff H, Iung B, et al. Cost-effectiveness analysis of SAPIEN 3 transcatheter aortic valve implantation procedure compared with surgery in patients with severe aortic stenosis at low risk of surgical mortality in France. Value Health. 2022;25:605–13. doi: 10.1016/j.jval.2021.10.003. https://doi.org/10.1016/j.jval.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Mennini FS, Meucci F, Pesarini G, et al. Cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement in low surgical risk aortic stenosis patients. Int J Cardiol. 2022;357:26–32. doi: 10.1016/j.ijcard.2022.03.034. https://doi.org/10.1016/j.ijcard.2022.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Shah KK, Elder D, Nguyen MT, et al. Transcatheter aortic valve implantation (TAVI) versus surgical aortic valve replacement for aortic stenosis (SAVR): a cost-comparison study. Heart Lung Circ. 2021;30:1918–28. doi: 10.1016/j.hlc.2021.05.088. https://doi.org/10.1016/j.hlc.2021.05.088. [DOI] [PubMed] [Google Scholar]
- 21.Baron SJ, Wang K, House JA, et al. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at intermediate risk. Circulation. 2019;139:877–88. doi: 10.1161/CIRCULATIONAHA.118.035236. https://doi.org/10.1161/CIRCULATIONAHA.118.035236. [DOI] [PubMed] [Google Scholar]
- 22.Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol. 2017;69:2579–89. doi: 10.1016/j.jacc.2017.03.017. https://doi.org/10.1016/j.jacc.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Moriyama N, Miyashita H, Lehtola H, et al. Device failure in bicuspid aortic stenosis following transcatheter aortic valve implantation. Am J Cardiol. 2022;176:96–104. doi: 10.1016/j.amjcard.2022.04.037. https://doi.org/10.1016/j.amjcard.2022.04.037. [DOI] [PubMed] [Google Scholar]
- 24.Philip F, Faza NN, Schoenhagen P, et al. Aortic annulus and root characteristics in severe aortic stenosis due to bicuspid aortic valve and tricuspid aortic valves: implications for transcatheter aortic valve therapies. Catheter Cardiovasc Interv. 2015;86:E88–98. doi: 10.1002/ccd.25948. https://doi.org/10.1002/ccd.25948. [DOI] [PubMed] [Google Scholar]
- 25.Montarello NJ, Willemen Y, Tirado-Conte G, et al. Transcatheter aortic valve durability: a contemporary clinical review. Front Cardiovasc Med. 2023;10:1195397. doi: 10.3389/fcvm.2023.1195397. https://doi.org/10.3389/fcvm.2023.1195397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sa MP, Jacquemyn X, Tasoudis PT, et al. Immediate and late outcomes of transcatheter aortic valve implantation versus surgical aortic valve replacement in bicuspid valves: meta-analysis of reconstructed time-to-event data. J Card Surg. 2022;37:3300–10. doi: 10.1111/jocs.16840. https://doi.org/10.1111/jocs.16840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.