Abstract
Background and Objectives
Few studies have addressed the predictive implications of right ventricular (RV) and pulmonary arterial (PA) coupling as assessed by echocardiography in patients with acute heart failure (AHF). This study aimed to ascertain the prognostic importance of RV-PA coupling in AHF cases and discern any divergence in its prognostic efficacy based on different heart failure (HF) phenotypes.
Methods
We evaluated RV-PA coupling by measuring the ratio of right ventricular global longitudinal strain (RVGLS) to pulmonary arterial systolic pressure (PASP), termed the RVGLS/PASP ratio, and assessed its prognostic role using the STrain for Risk Assessment and Therapeutic Strategies in Patients with Acute Heart Failure registry.
Results
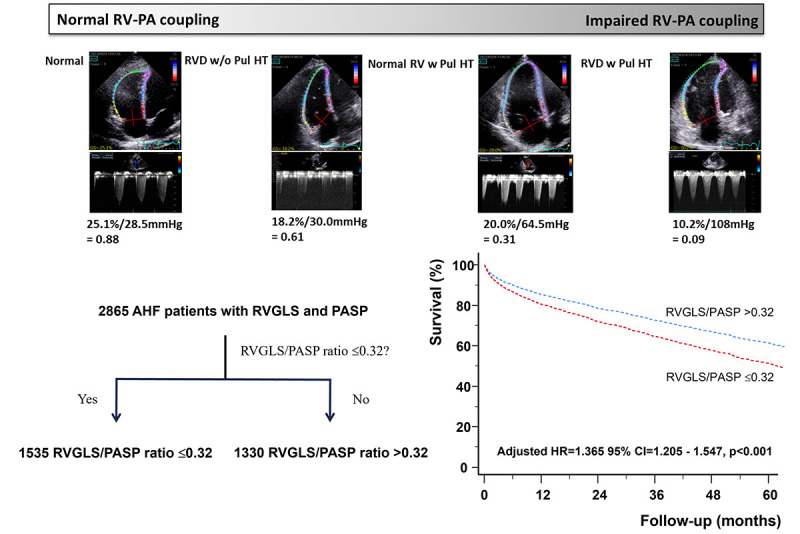
From an AHF registry of 4312 patients, we analyzed the RVGLS/PASP ratio in 2,865 patients (1,449 men; age, 71.1±13.5 years). At a median follow-up of 35.0 months, 1,199 (41.8%) patients died. Remarkably, PASP (hazard ratio [HR], 1.012; p<0.001), RVGLS (HR, 1.019; p<0.001), and the RVGLS/PASP ratio (HR, 2.426; p<0.001) were statistically significant predictors of all-cause mortality in the univariate analysis. The RVGLS/PASP ratio was a significant predictor of all-cause mortality in all the HF phenotypes, including HF with reduced ejection fraction (HR, 2.124; p=0.002), HF with mildly reduced ejection fraction (HR, 2.733; p=0.021), and HF with preserved ejection fraction (HR, 2.134; p=0.006). Multivariate analysis after adjusting for clinical and echocardiographic variables revealed that the RVGLS/PASP ratio ≤0.32 was associated with a 36% increase in all-cause mortality (HR, 1.365; p<0.001).
Conclusions
Impaired RV-PA coupling, defined as an RVGLS/PASP ratio (≤0.32) was associated with an increased risk of mortality in patients with AHF across all HF phenotypes.
Trial Registration
ClinicalTrials.gov Identifier: NCT03513653
Keywords: Heart failure; Echocardiography, strain; Prognosis; Right ventricle; Pulmonary artery
Graphical Abstract
INTRODUCTION
Right ventricular (RV)-pulmonary arterial (PA) coupling is an index of RV contractility in relation to RV afterload and describes a hemodynamic state in which mechanical stroke work is most efficiently transferred to the pulmonary vasculature.1) This coupling is often quantified through invasive right heart catheterization (RHC). Notably, while the pulmonary circulation receives an equivalent cardiac output to that of the systemic circulation, it operates at a pressure that is only one-fifth that of the systemic circulation. Consequently, the pressure-volume loop of the RV contrasts with that of the left ventricle (LV) in terms of pressure fluctuation amplitude, typically adopting a more triangular or trapezoidal shape.2) As in the LV, the slope of the RV end-systolic pressure-volume relationship, or end-systolic elastance (Ees), should be equal to the effective arterial elastance (Ea), resulting in an Ees/Ea ratio greater than 1.0 in a healthy individual.3)
Nonetheless, the invasive evaluation of RV-PA coupling necessitates the use of RHC, a procedure that entails potential complications. Therefore, there are several echocardiographic markers of RV-PA coupling. The most prevalent among these is the tricuspid annular plane systolic excursion (TAPSE)/pulmonary arterial systolic pressure (PASP). This ratio has been studied in patients with pulmonary hypertension and heart failure (HF).4,5,6,7) However, it does not reflect the intrinsic myocardial performance of the RV. Recently, strain analysis has emerged as a method to provide an objective marker of intrinsic myocardial mechanics, and its application has been extended to evaluate RV systolic function.8) Specifically, right ventricular global longitudinal strain (RVGLS) is a well-known marker of RV systolic function and reduced RVGLS values have been associated with unfavorable prognosis in patients with acute heart failure (AHF).9,10) However, few studies have shown the prognostic effect of RV-PA coupling assessed by RVGLS/PASP in patients with AHF. Thus, we evaluated the prognostic value of RV-PA coupling assessed using the RVGLS/PASP in patients with AHF.
METHODS
Study population
We calculated the RVGLS/PASP ratio in the STrain for Risk Assessment and Therapeutic Strategies in Patients with Acute Heart Failure (STRATS-AHF) registry (ClinicalTrials.gov Identifier: NCT03513653), in which we consecutively included 4,312 patients with AHF from three tertiary university hospitals in Korea from January 2009 to December 2016.11) AHF was defined as rapidly developing or worsening HF symptoms with or without objective signs requiring urgent medical evaluation and treatment. We included all admitted patients with symptoms and/or signs of AHF with either pulmonary congestion, objective findings of abnormal LV function, or structural heart disease.12) Exclusion criteria were severe primary valvular heart disease. We identified deaths from all causes and hospitalizations for HF from the medical records of all patients who underwent regular medical follow-ups. For patients without regular hospital visits or those lost to follow-up, death data were obtained from the Ministry of Public Administration and Security of the Republic of Korea. The study protocol was approved by the Institutional Review Board (IRB) of each hospital. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Definitions of study variables
The body mass index (BMI) was calculated based on height and weight. Hypertension was defined as the use of antihypertensive medication for >6 months, or lifestyle modifications only after the diagnosis of hypertension. Patients receiving active treatment with oral hypoglycemic agents or insulin were defined as having diabetes mellitus (DM). Patients with abnormal fasting glucose levels (≥126 mg/dL) or abnormal 2-hour postprandial glucose levels (≥200 mg/dL), in those treated with diet alone were also considered to have DM. Atrial fibrillation (AF) was defined as having been documented on electrocardiography or if the patient had previously been diagnosed with AF (International Classification of Diseases, 10th Revision [ICD-10] code I48). Ischemic heart disease (IHD) was identified if there was a history of coronary intervention and an IHD diagnosis (ICD-10 codes I20, I21, and I25). Patients were categorized as having heart failure with reduced ejection fraction (HFrEF), heart failure with mildly reduced ejection fraction (HFmrEF), and heart failure with preserved ejection fraction (HFpEF) based on the left ventricular ejection fraction (LVEF; ≤40%, 41–49%, and ≥50%, respectively) obtained from the echocardiography upon admission for AHF.
Echocardiographic evaluation
All echocardiographic images were obtained and stored on commercial echocardiographic machines with a 2.5 MHz probe using standard echocardiographic techniques with M-mode, 2-dimensional, and Doppler echocardiographic modalities as recommended by the American Society of Echocardiography.13) We measured LV dimensions from the parasternal long-axis views at end-systole and end-diastole. Mitral inflow and tissue Doppler-derived peak systolic and early and late diastolic velocities of the mitral annular septum were calculated using pulsed-wave Doppler echocardiography. LVEF was calculated from the end-systolic and end-diastolic LV volumes using the 2-dimensional Simpson’s method from apical 4- and 2-chamber views. PASP was calculated from the peak tricuspid regurgitation velocity jet (TR Vmax) using the modified Bernoulli equation, and the right atrial pressure was estimated from the size of the inferior vena cava and its collapsibility. The left atrial (LA) diameter was measured from the parasternal long-axis view at the end of systole.
Strain analysis
We analyzed strain values from stored echocardiographic images using TomTec software (ImageArena version 4.6; TomTec Imaging Systems GmbH, Munich, Germany), which is vendor-independent.14,15)
To analyze RV myocardial deformation, the endocardial border was manually traced on the end-systolic frame of the selected image. The end-systole frame was defined as the QRS complex or the minimum RV volume during the cardiac cycle. Speckles along the endocardial border and myocardium during the cardiac cycle were automatically tracked using specific software. A negative peak value during the cardiac cycle was defined as peak longitudinal systolic strain. The global longitudinal strain (GLS) was calculated as the average of the six segments. The RVGLS was measured in the apical 4-chamber view or focused RV view. GLS was analyzed in a single cardiac cycle in patients with sinus rhythm and averaged over 3 cardiac cycles in patients with AF. The RVGLS values were measured independently by an echocardiography specialist who was blinded to the clinical data. To avoid unnecessary misunderstanding, we used the absolute value of RVGLS.
Statistical analyses
Continuous variables are presented as means ± standard deviation and categorical variables as frequencies. For comparisons between groups, we used the Student’s t-test or one-way analysis of variance for continuous variables and the χ2 test for categorical variables. Pearson’s correlation coefficient was used to calculate the correlation between the RVGLS/PASP ratio and other variables. To determine the independent predictors of all-cause mortality at the time of the first adverse clinical event, we used a multivariate Cox proportional hazards analysis. In our analysis, given the adequate number of observed adverse clinical events, all variables that demonstrated significance in the univariate assessment were incorporated as covariates, and those showing multicollinearity in the multivariate analysis were excluded. Thus, we included the LA diameter as an LV diastolic parameter instead of the mitral E/E′ ratio in the multivariate analysis.
To compare the discriminative powers of the models with the addition of the RVGLS/PASP ratio, we calculated Uno’s concordance statistics for each model. Data were analyzed using SPSS (version 25; IBM Corp., Armonk, NY, USA) and MedCalc version 12.3.0.0 (MedCalc Software, Mariakerke, Belgium). Statistical significance was defined as a 2-tailed p value <0.05.
RESULTS
Patient characteristics
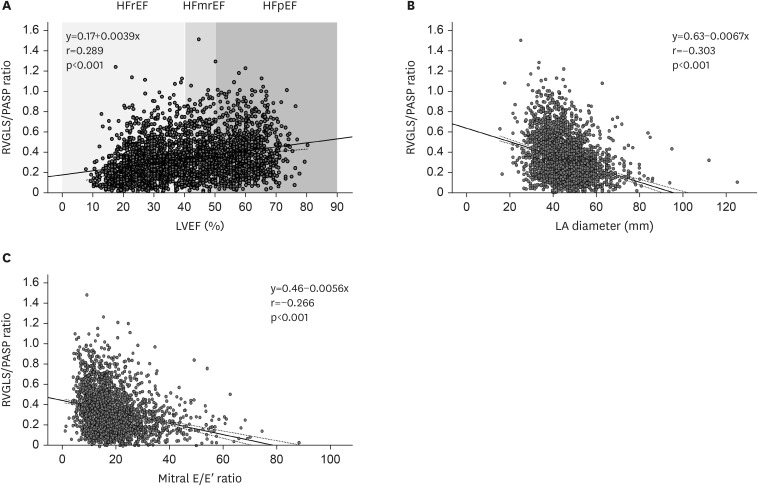
From the STRATS-AHF registry, comprising a consecutive cohort of 4,312 patients diagnosed with AHF, we included 2,865 patients (1,449 men; mean age, 71.1±13.5 years) after exclusion of 335 patients without available RVGLS and 1,112 patients without measurable TR Vmax in this study. The baseline characteristics of the patients are presented in Table 1. Hypertension was the most common cardiovascular risk factor, observed in 62.5% of the patients, and DM was documented in 965 patients (33.8%). The mean LVEF was 40.2±15.6%, delineating 1,515 (52.9%), 447 (15.7%), and 903 (31.4%) patients categorized into HFrEF, HFmrEF, and HFpEF, respectively. RVGLS exhibited a mean of 12.6±6.2%, while the mean PASP was 41.9±14.3 mmHg, and the RVGLS/PASP ratio was 0.34±0.21. The RVGLS/PASP ratio showed significant correlations with LVEF (r=0.289, p<0.001), LA diameter (r=−0.303, p<0.001), and mitral E/E′ ratio (r=−0.266, p<0.001) as illustrated in Figure 1.
Table 1. Baseline characteristics and comparison of characteristics according to ratio of RVGLS/PASP.
| Characteristics | Total (n=2,865) | RVGLS/PASP ratio | p value | ||
|---|---|---|---|---|---|
| ≤0.32 (n=1,535) | >0.32 (n=1,330) | ||||
| Baseline clinical characteristics | |||||
| Male sex | 1,449 (50.6) | 813 (53.0) | 636 (47.8) | 0.006 | |
| Age (years) | 71.1±13.5 | 70.6±13.9 | 71.7±13.0 | 0.020 | |
| BMI (kg/m2) | 23.28±4.10 | 23.3±4.3 | 23.3±3.8 | 0.964 | |
| NYHA functional class IV (%) | 1,216 (43.0) | 675 (44.3) | 541 (41.5) | 0.137 | |
| Physical examination | |||||
| SBP (mmHg) | 129.0±27.6 | 127.4±26.9 | 130.1±28.3 | 0.002 | |
| DBP (mmHg) | 74.4±17.1 | 74.9±17.3 | 73.9±17.0 | 0.142 | |
| Heart rate (/min) | 89.4±25.5 | 95.0±25.9 | 83.0±23.3 | <0.001 | |
| Past medical history | |||||
| AF | 965 (33.8) | 640 (41.7) | 325 (24.6) | <0.001 | |
| Hypertension | 1,790 (62.5) | 935 (60.9) | 855 (64.3) | 0.063 | |
| DM | 993 (34.7) | 544 (35.4) | 449 (33.8) | 0.365 | |
| IHD | 926 (32.3) | 447 (29.1) | 479 (36.0) | <0.001 | |
| Laboratory findings | |||||
| Hemoglobin (g/dL) | 12.2±2.3 | 12.3±2.4 | 12.1±2.2 | <0.001 | |
| BUN (mg/dL) | 26.3±17.0 | 27.5±18.2 | 24.8±15.4 | <0.001 | |
| Creatinine (mg/dL) | 1.53±1.66 | 1.56±1.74 | 1.48±1.57 | 0.200 | |
| Total cholesterol (mg/dL) | 152.5±42.5 | 147.1±41.5 | 158.8±44.9 | <0.001 | |
| NT-proBNP (pg/mL) | 9,725±11,580 | 12,132±12,885 | 7,279±9,497 | <0.001 | |
| Echocardiographic findings | |||||
| LVEDD (mm) | 53.2±9.5 | 54.6±9.9 | 51.6±8.9 | <0.001 | |
| LVESD (mm) | 40.9±11.6 | 43.01±11.9 | 38.4±10.8 | <0.001 | |
| LVEDV (mL) | 117.5±62.1 | 128.0±68.3 | 106.0±52.1 | <0.001 | |
| LVESV (mL) | 76.3±54.9 | 87.8±60.5 | 63.7±44.7 | <0.001 | |
| LVEF (%) | 40.2±15.6 | 36.7±15.5 | 41.3±14.9 | <0.001 | |
| LA diameter (mm) | 45.4±9.6 | 47.7±9.7 | 42.7±8.7 | <0.001 | |
| Mitral E-velocity (m/sec) | 0.91±0.36 | 1.01±0.37 | 0.81±0.32 | <0.001 | |
| Mitral A-velocity (m/sec) | 0.76±0.31 | 0.67±0.31 | 0.82±0.29 | 0.404 | |
| E′ velocity (cm/sec) | 5.2±2.2 | 5.2±2.1 | 5.3±2.2 | 0.105 | |
| E/E′ ratio | 19.3±10.6 | 21.6±11.1 | 16.8±8.4 | <0.001 | |
| PASP (mmHg) | 41.9±14.3 | 50.7±15.3 | 35.3±10.3 | <0.001 | |
| RVFAC (%) | 37.9±14.8 | 32.1±14.4 | 43.1±13.1 | <0.001 | |
| RVGLS (%) | 12.6±6.2 | 8.7±4.2 | 17.0±4.9 | <0.001 | |
| RVGLS/PASP ratio | 0.34±0.21 | 0.18±0.08 | 0.51±0.18 | <0.001 | |
| Definition of HF | <0.001 | ||||
| HFrEF | 1,515 (52.9) | 960 (62.5) | 555 (41.7) | ||
| HFmrEF | 447 (15.6) | 200 (13.0) | 247 (18.6) | ||
| HFpEF | 903 (31.5) | 375 (24.4) | 528 (39.7) | ||
| Medication at discharge | |||||
| RAAS-inhibitor | 2,066 (72.1) | 1,074 (70.0) | 992 (74.6) | 0.007 | |
| Beta-blocker | 1,836 (64.3) | 951 (62.0) | 885 (66.5) | 0.011 | |
| MRA | 1,393 (48.6) | 767 (50.0) | 626 (47.1) | 0.125 | |
Continuous variables are presented as means ± standard deviation and categorical variables as frequencies.
RVGLS = right ventricular global longitudinal strain; PASP = pulmonary arterial systolic pressure; BMI = body mass index; NYHA = New York Heart Association; SBP = systolic blood pressure; DBP = diastolic blood pressure; AF = atrial fibrillation; DM = diabetes mellitus; IHD = ischemic heart disease; BUN = blood urea nitrogen; NT-proBNP = N-terminal pro-B-type natriuretic peptide; LVEDD = left ventricular end-diastolic dimension; LVESD = left ventricular end-systolic dimension; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; LVEF = left ventricular ejection fraction; LA = left atrial; RVFAC = right ventricular fractional area change; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HFmrEF = heart failure with mildly-reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; RAAS = renin-angiotensin-aldosterone system; MRA = mineralocorticoid receptor antagonist.
Figure 1. Correlations between RVGLS/PASP ratio and LVEF, LA diameter and mitral E/E′ ratio.
RVGLS = right ventricular global longitudinal strain; PASP = pulmonary arterial systolic pressure; HFrEF = heart failure with reduced ejection fraction; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; LVEF = left ventricular ejection fraction; LA = left atrial.
All-cause death and its determinants
A total of 1,199 patients (42.0%) died during the median follow-up duration of 35.0 months. Supplementary Table 1 illustrates a comparison of the variables affecting all-cause mortality. The deceased patients exhibited advanced age (p<0.001), a lower BMI (p<0.001), and a greater prevalence of severe symptoms with New York Heart Association functional class IV (p<0.001). The prevalence of hypertension (p<0.001), DM (p<0.001), and IHD (p<0.001) was higher in the deceased group than in the survivor group.
LVEF was not significantly different between the 2 groups (p=0.512). Regarding LV diastolic parameters, the LA diameter (p=0.001) and mitral E/E′ ratio (p<0.001) were higher in the death group. RV systolic function, as assessed by RVGLS, was significantly lower in the death group (p<0.001). PASP was significantly higher in the deceased group (p<0.001).
In the univariate analysis for predicting all-cause death (Table 2), PASP (hazard ratio [HR], 1.012; p<0.001), RVGLS (HR, 1.019; p<0.001), and RVGLS/PASP ratio (HR, 2.426; p<0.001) were significant determinants of all-cause mortality. In the multivariate analysis (Table 3), the RVGLS/PASP ratio was a significant determinant of all-cause mortality (HR, 2.231; p<0.001). The addition of this ratio also demonstrated an incremental prognostic value with a maximal C-statistic 0.714 and χ2 value 610 (Table 3).
Table 2. Univariate analysis in the prediction of all-cause death within 5 years.
| Variable | HR | 95% CI | p value | |
|---|---|---|---|---|
| Age (per 1 year) | 1.053 | 1.047–1.058 | <0.001 | |
| Male sex | 0.983 | 0.878–1.100 | 0.761 | |
| BMI (per 1 kg/m2) | 0.926 | 0.911–0.941 | <0.001 | |
| SBP (per 1 mmHg) | 0.999 | 0.997–1.001 | 0.444 | |
| DBP (per 1 mmHg) | 0.992 | 0.989–0.995 | <0.001 | |
| Heart rate (per 1/min) | 1.001 | 0.998–1.003 | 0.601 | |
| NYHA functional class IV | 1.358 | 1.212–1.521 | <0.001 | |
| AF | 0.95 | 0.843–1.070 | 0.399 | |
| Hypertension | 1.415 | 1.252–1.598 | <0.001 | |
| DM | 1.343 | 1.196–1.507 | <0.001 | |
| IHD | 1.293 | 1.150–1.454 | <0.001 | |
| Hemoglobin (per 1 g/dL) | 0.855 | 0.835–0.875 | <0.001 | |
| Creatinine (per 1 mg/dL) | 1.053 | 1.032–1.076 | <0.001 | |
| Total cholesterol (per 1 mg/dL) | 0.996 | 0.995–0.998 | <0.001 | |
| LVEDD (per 1 mm) | 0.988 | 0.982–0.995 | <0.001 | |
| LVESD (per 1 mm) | 0.993 | 0.988–0.999 | 0.012 | |
| LVEDV (per 1 mL) | 0.999 | 0.998–1.000 | 0.031 | |
| LVESV (per 1 mL) | 0.999 | 0.998–1.000 | 0.161 | |
| LVEF (per 1 %) | 0.998 | 0.995–1.002 | 0.528 | |
| LA diameter (per 1 mm) | 1.008 | 1.002–1.138 | 0.008 | |
| E/E′ ratio (per 1) | 1.019 | 1.014–1.025 | <0.001 | |
| PASP (per 1mmHg) | 1.012 | 1.009–1.016 | <0.001 | |
| HF phenotype | ||||
| HFrEF | Reference | 0.372 | ||
| HFmrEF | 0.887 | 0.751–1.048 | 0.160 | |
| HFpEF | 0.972 | 0.856–1.104 | 0.664 | |
| RVGLS (per 1% decrease) | 1.019 | 1.009–1.029 | <0.001 | |
| RVGLS/PASP ratio (per 1%/mmHg decrease) | 2.426 | 1.821–3.232 | <0.001 | |
| Use of RAAS-inhibitor at discharge | 0.616 | 0.547–0.694 | <0.001 | |
| Use of beta-blocker at discharge | 0.621 | 0.554–0.695 | <0.001 | |
| Use of MRA at discharge | 0.844 | 0.753–0.945 | 0.003 | |
HR = hazard ratio; CI = confidence interval; BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; NYHA = New York Heart Association; AF = atrial fibrillation; DM = diabetes mellitus; IHD = ischemic heart disease; LVEDD = left ventricular end-diastolic dimension; LVESD = left ventricular end-systolic dimension; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; LVEF = left ventricular ejection fraction; LA = left atrial; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HFmrEF = heart failure with mildly-reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; RVGLS = right ventricular global longitudinal strain; PASP = pulmonary arterial systolic pressure; RAAS = renin-angiotensin-aldosterone system; MRA = mineralocorticoid receptor antagonist.
Table 3. Multivariate analysis in the prediction of all-cause death within 5 years.
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Male sex | 1.292 | 1.144–1.460 | <0.001 | |||||||||
| Age (per 1 year) | 1.054 | 1.047–1.060 | <0.001 | |||||||||
| NYHA functional class IV | 1.120 | 0.997–1.258 | 0.056 | |||||||||
| Hypertension | 1.018 | 0.892–1.161 | 0.794 | |||||||||
| DM | 1.219 | 1.078–1.379 | 0.002 | |||||||||
| IHD | 1.082 | 0.957–1.225 | 0.208 | |||||||||
| RAAS inhibitor at discharge | 0.664 | 0.585–0.754 | <0.001 | |||||||||
| Beta-blocker at discharge | 0.732 | 0.649–0.826 | <0.001 | |||||||||
| MRA at discharge | 0.991 | 0.879–1.118 | 0.886 | |||||||||
| Hemoglobin (per 1g/dL) | 0.902 | 0.877–0.928 | <0.001 | |||||||||
| Creatinine (per 1 mg/dL) | 1.068 | 1.035–1.102 | <0.001 | |||||||||
| LVEF (per 1%) | 0.990 | 0.986–0.994 | <0.001 | |||||||||
| LA diameter (per 1 mm) | 1.006 | 0.999–1.012 | 0.074 | |||||||||
| RVGLS/PASP ratio | 2.231 | 1.616–3.080 | <0.001 | |||||||||
| C-statistics | 0.688 | 0.702 | 0.710 | 0.714 | ||||||||
| Global χ2 | 460 | 529 | 582 | 610 | ||||||||
Model 1 included clinical variables such as sex, age, functional status, cardiovascular risk factors, and medications. Model 2 included the clinical variables in model 1 and laboratory variables, including hemoglobin and creatinine concentrations. Model 3 included variables from Models 1 and 2 and echocardiographic variables indicating LV systolic and diastolic function. Model 4 included variables in Models 1, 2, and 3 and the RVGLS/PASP ratio.
HR = hazard ratio; CI = confidence interval; NYHA = New York Heart Association; DM = diabetes mellitus; IHD = ischemic heart disease; RAAS = renin-angiotensin-aldosterone system; MRA = mineralocorticoid receptor antagonist; LVEF = left ventricular ejection fraction; LA = left atrial; RVGLS = right ventricular global longitudinal strain; PASP = pulmonary arterial systolic pressure.
In the receiver operating curve analysis, the optimal threshold value for the RVGLS/PASP ratio in predicting all-cause mortality was determined to be 0.32 (p<0.001). In the group with an RVGLS/PASP ratio of ≤0.32, a higher proportion of males was observed (p=0.006), and the mean age was lower (p=0.020). Furthermore, this group exhibited increased LV dimensions and volumes, an increased LA diameter, and a higher mitral E/E′ ratio. The RVGLS was significantly lower (p<0.001), the PASP was significantly higher (p<0.001), and the percentage of patients with HFrEF was higher (p<0.001) than in the rest of the cohort (Table 1).
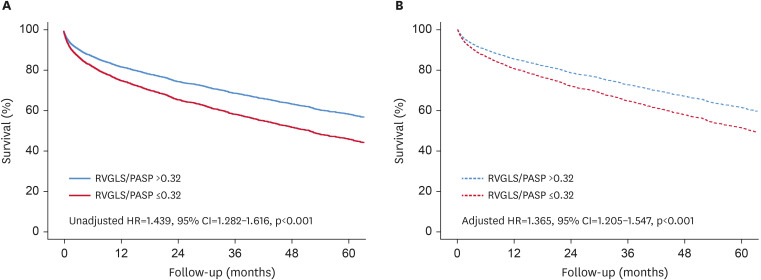
Patients with an RVGLS/PASP ratio of ≤0.32 had significantly higher all-cause mortality in both univariate (unadjusted HR, 1.439; 95% confidence interval [CI], 1.282–1.616; p<0.001) and multivariate analyses (adjusted HR, 1.365; 95% CI, 1.205–1.547; p<0.001) (Figure 2).
Figure 2. Survival analysis according to RVGLS/PASP ratio ≤0.32. Patients with low RVGLS/PASP ratio ≤0.32 have worse survival than controls in the univariate (A) and the multivariate analysis (B).
RVGLS = right ventricular global longitudinal strain; PASP = pulmonary arterial systolic pressure; HR = hazard ratio; CI = confidence interval.
Prediction of all-cause mortality according to HF phenotypes
Supplementary Table 1 shows a comparison of variables based on HF phenotypes. There were more males (p<0.001), younger patients (p<0.001), and patients with higher diastolic blood pressure (p<0.001) in the HFrEF group. In contrast, the HFpEF group had more females (p<0.001), a higher proportion of patients with hypertension (p<0.001), and a lower proportion of patients with IHD (p<0.001). The RVGLS/PASP ratio was significantly lower in the HFrEF group (p<0.001).
Supplementary Table 2 reveals the results of the multivariate analysis based on the HF phenotype. In all HF phenotypes, an RVGLS/PASP ratio of ≤0.32 was a significant factor for all-cause mortality along with male sex, age, and use of renin-angiotensin-aldosterone system (RAAS) inhibitors at discharge.
An RVGLS/PASP ratio of ≤0.32 was significantly associated with increased all-cause mortality in all HF phenotypes and demonstrated the greatest HR in the HFpEF group (Supplementary Table 2).
The RVGLS/PASP ratio showed a significant interaction with the use of beta-blockers and RAAS inhibitors at discharge (Supplementary Table 3).
DISCUSSION
We found that the RVGLS/PASP ratio showed a weak correlation with LVEF, LA diameter, and mitral E/E′ ratio. Regardless of HF phenotype, a low RVGLS/PASP ratio (≤0.32) was significantly associated with increased all-cause mortality in patients with AHF.
The pressure-volume loop in the RV has a different triangular shape from the pressure-volume loop in the LV because the pulmonary circulation must receive the same cardiac output at one-fifth of the systemic circulation pressure. Consequently, the slope of the RV end-systolic pressure-volume relationship or Ees should be equal to the effective Ea, resulting in an Ees/Ea ratio greater than 1.0 in healthy persons.3,16) When PA pressure is increased, the RV initially adapts by increasing its contractile force, and RV hypertrophy rather than RV dilatation is the primary mechanism. In this adaptation period, RV-PA coupling, cardiac output, and exercise capacity are preserved and the Ees/Ea ratio is approximately 0.7–1.5.16) If PA pressure rises further, RV contractility cannot cope with the increased afterload and decoupling may occur. As PA pressure increases further, RV myocytes are unable to compensate and the RV dilates, leading to an increase in RV wall stress and RV interstitial fibrosis, which makes the RV stiffer and leads to RV dysfunction.16)
RV-PA coupling is a quantitative marker of the adaptation of RV systolic function to its afterload and can detect pending RV failure.17) Fundamentally, this RV-PA coupling can be measured by an invasive method during RHC. As a result of its noninvasiveness, echocardiography has recently replaced invasive catheterization. TAPSE is an objective echocardiographic marker of RV systolic function. The PASP can be estimated from TR Vmax. Thus, RV-PA coupling can be estimated by measuring the TAPSE/PASP ratio using echocardiography.7) This TAPSE/PASP ratio has been studied and a reduced value is known to be a poor prognostic factor in patients with HF or pulmonary hypertension (PH).4,5,6,7,17) The TAPSE/PASP ratio was validated using gold standard Ees/Ea measurements in precapillary PH and a cut-off value of 0.31 is used to discriminate between preserved coupling and RV failure.6) Moreover, the TAPSE/PASP ratio was an independent prognostic marker in patients with severe mitral regurgitation, and a ratio of ≤0.35 was a poor prognostic marker.17)
In patients with AHF, LA pressure can be elevated by decreasing ventricular pumping or filling from the HF. Elevated LA pressure can sequentially elevate pulmonary capillary pressure and PA pressure. This increase in PASP levels can impair the RV systolic function. Consequently, PH can manifest in patients with AHF. Notably, the onset of PH can be linked to RV dysfunction, which is recognized as a harbinger of adverse outcomes in patients with HF.9,18,19) Because RVGLS can represent the intrinsic myocardial property of the RV, it has been used as an objective marker of RV systolic function.8) In a study of 315 patients with congestive heart failure, an RVGLS/PASP ratio less than 0.36 was a poor prognostic marker for death.20) They also showed that the RVGLS/PASP ratio was more accurate than the TAPSE/PASP ratio in detecting 1-year mortality.
We showed that a low RVGLS/PASP ratio of ≤0.32 was a good prognostic marker in patients with AHF regardless of the phenotype, and its HR was the highest in patients with HFpEF. It is unclear why an impaired RVGLS/PASP ratio seems to be more predictive in patients with HFpEF. One possible explanation is that there is a different pathophysiology involved in the development of this type of HF. Thus, the LA pathology in HFpEF may differ from that in HFrEF. The major LA pathologies in HFpEF are fibrosis and stiffness of the LA, whereas those in HFrEF are enlargement, eccentric hypertrophy, and loss of LA compliance.21) In patients with HFpEF, the onset of pulmonary hypertension and RV dysfunction occurs earlier, with potential variability in treatment reversibility, rendering the RV coupling index an important marker. However, these parameters occur at a later stage in patients with HFrEF. Given the intrinsic progression of RV myocardial pathology, which is independent of pulmonary hypertension, the importance of the RV coupling index is diminished. Moreover, patients with HFrEF have significantly reduced RVGLS and impaired systolic and diastolic function of the LV compared with those with HFpEF or HFmrEF. This suggests that patients with HFrEF are at a relatively more advanced stage of HF and that LV function plays a pivotal role in determining their prognosis. Consequently, the RVGLS/PASP HR was lower in the HFmrEF and HFrEF groups.
The prognostic significance of echocardiographic RV-PA coupling indices in patients with HF has been investigated in several studies. However, these studies had limitations such as a small sample size and a low number of events. There are also a few studies on the RVGLS that can represent intrinsic RV myocardial properties. In evaluating the RVGLS/PASP ratio as a predictor of all-cause mortality, our study was comprehensive in both clinical and echocardiographic aspects. Given our large sample size and number of events observed, we were well-positioned for rigorous statistical analyses, even after controlling for potential confounders.
However, this study also had several limitations. First, given its retrospective design using a cohort dataset, the inherent observational nature could have introduced residual confounding factors, potentially influencing our findings. Second, we analyzed the strain values in the digitally stored echocardiographic images. Some of these methods are not suitable for strain analysis. Finally, because we enrolled only East Asian patients admitted for AHF, our results may not be generalizable to other clinical settings or patients of other ethnicities.
A low RVGLS/PASP ratio (≤0.32) was significantly associated with increased mortality in patients with AHF. This indicator may be useful for prognosticating patients with AHF admitted to hospitals.
ACKNOWLEDGEMENTS
We sincerely thank Prof. Ju Mi Lee, MD, PhD, Department of Preventive Medicine, Chungnam National University College of Medicine, for her help with statistical analysis.
Footnotes
Conflict of Interest: Jin Joo Park serves as an Editor-in-Chief of the International Journal of Heart Failure but has no role in the decision to publish this article. Except for that, no potential conflict of interest relevant to this article was reported.
- Conceptualization: Park JH, Kim M, Park JJ, Park JB, Cho GY.
- Data curation: Park JH, Park JJ, Park JB, Cho GY.
- Formal analysis: Park JJ, Cho GY.
- Supervision: Cho GY.
- Writing - original draft: Park JH, Kim M.
- Writing - review & editing: Cho GY.
SUPPLEMENTARY MATERIALS
Comparison of baseline and echocardiographic characteristics according to heart failure phenotype
Multivariate analysis of the prediction of all-cause death within 5 years according to heart failure phenotypes
The p values for interaction
References
- 1.Brener MI, Lurz P, Hausleiter J, et al. Right ventricular-pulmonary arterial coupling and afterload reserve in patients undergoing transcatheter tricuspid valve repair. J Am Coll Cardiol. 2022;79:448–461. doi: 10.1016/j.jacc.2021.11.031. [DOI] [PubMed] [Google Scholar]
- 2.Hsu S, Fang JC, Borlaug BA. Hemodynamics for the heart failure clinician: a state-of-the-art review. J Card Fail. 2022;28:133–148. doi: 10.1016/j.cardfail.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vonk Noordegraaf A, Chin KM, Haddad F, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019;53:1801900. doi: 10.1183/13993003.01900-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorter TM, van Veldhuisen DJ, Voors AA, et al. Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs. post-capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2018;19:425–432. doi: 10.1093/ehjci/jex133. [DOI] [PubMed] [Google Scholar]
- 5.Guazzi M, Dixon D, Labate V, et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10:1211–1221. doi: 10.1016/j.jcmg.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Tello K, Wan J, Dalmer A, et al. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging. 2019;12:e009047. doi: 10.1161/CIRCIMAGING.119.009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bok Y, Kim JY, Park JH. Prognostic role of right ventricular-pulmonary artery coupling assessed by TAPSE/PASP ratio in patients with acute heart failure. J Cardiovasc Imaging. 2023;31:200–206. doi: 10.4250/jcvi.2023.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Park JH. Strain Analysis of the Right Ventricle Using Two-dimensional Echocardiography. J Cardiovasc Imaging. 2018;26:111–124. doi: 10.4250/jcvi.2018.26.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Park JJ, Park JB, Cho GY. Prognostic value of biventricular strain in risk stratifying in patients with acute heart failure. J Am Heart Assoc. 2018;7:e009331. doi: 10.1161/JAHA.118.009331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shukla M, Park JH, Thomas JD, et al. Prognostic value of right ventricular strain using speckle-tracking echocardiography in pulmonary hypertension: a systematic review and meta-analysis. Can J Cardiol. 2018;34:1069–1078. doi: 10.1016/j.cjca.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71:1947–1957. doi: 10.1016/j.jacc.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 12.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Sun BJ, Park JH. Echocardiographic measurement of left atrial strain - a key requirement in clinical practice. Circ J. 2021;86:6–13. doi: 10.1253/circj.CJ-21-0373. [DOI] [PubMed] [Google Scholar]
- 15.Park JH. Two-dimensional echocardiographic assessment of myocardial strain: important echocardiographic parameter readily useful in clinical field. Korean Circ J. 2019;49:908–931. doi: 10.4070/kcj.2019.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rako ZA, Kremer N, Yogeswaran A, Richter MJ, Tello K. Adaptive versus maladaptive right ventricular remodelling. ESC Heart Fail. 2023;10:762–775. doi: 10.1002/ehf2.14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trejo-Velasco B, Estevez-Loureiro R, Carrasco-Chinchilla F, et al. Prognostic role of TAPSE to PASP ratio in patients undergoing MitraClip procedure. J Clin Med. 2021;10:1006. doi: 10.3390/jcm10051006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiéry JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37:942–954. doi: 10.1093/eurheartj/ehv512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J, Choi HM, Hwang IC, et al. Prognostic implications of mechanical phenotypes in heart failure characterized by 3-chamber strain echocardiography. J Am Heart Assoc. 2022;11:e028040. doi: 10.1161/JAHA.122.028040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iacoviello M, Monitillo F, Citarelli G, et al. Right ventriculo-arterial coupling assessed by two-dimensional strain: A new parameter of right ventricular function independently associated with prognosis in chronic heart failure patients. Int J Cardiol. 2017;241:318–321. doi: 10.1016/j.ijcard.2017.04.051. [DOI] [PubMed] [Google Scholar]
- 21.Guazzi M, Ghio S, Adir Y. Pulmonary hypertension in HFpEF and HFrEF: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1102–1111. doi: 10.1016/j.jacc.2020.06.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of baseline and echocardiographic characteristics according to heart failure phenotype
Multivariate analysis of the prediction of all-cause death within 5 years according to heart failure phenotypes
The p values for interaction