Abstract
This study has explored the sustainable solution after designing an economical metal-free biomass-derived nanocarbon for the selective sensing of lead. The nitrogen and sulfur-rich mesoporous nanocarbon is designed through a facile hydrothermal-assisted thermal annealing method. The high-temperature treatment gave nanocarbon unique carbon dot decorated layered morphology, while nitrogen and sulfur precursor thiourea and melamine strengthened the nanomaterial stability, sensitivity, and selectivity toward lead metal ions. The high specific surface area of mesoporous nanocarbon viz., 1671.93 m2/g with the pore width and pore volume of 2.02 nm and 0.476 cm3/g has enhanced the conductivity of as-synthesized sensor, which helps in increasing sensitivity toward lead. The high conductivity was also confirmed through cyclic voltammetry, where an 80 % increment in current was observed in the case of the modified electrode when compared with bare GCE. The differential pulse normal voltammetry and differential pulse anodic stripping voltammetry were performed to calculate the detection limit, where an excellent detection limit of 22 nM was obtained from the DPASV technique. Moreover, the nanomaterial was also tested for detecting lead in tap water. The as-synthesized nanocomposite is highly efficient and selective for the detection of lead. This study will motivate the researchers to engineer sustainable and efficient devices for sensing metal pollutants.
Keywords: Coconut husk, Nitrogen functionalized mesoporous carbon, Cyclic voltammetry, Lead (II) ions
Highlights
-
•
Metal-free sustainable electrochemical sensor is developed.
-
•
N and S-rich biomass-derived mesoporous carbon is successfully developed through hydrothermal carbonization.
-
•
Mesoporous carbon material is used for the detection of lead through DPASV.
-
•
It shows an excellent detection limit of 22 nM.
1. Introduction
The persistent demand for lead (Pb) in various industrial and consumer products poses a significant challenge to environmental quality and human well-being. Lead is one of the most toxic heavy elements that accumulate in the environment due to its non-biodegradable nature. The contamination occurs as lead leaches into soil and water sources, where plants and aquatic organisms subsequently absorb it. It eventually makes its way up the food chain to affect human and animal populations. Human functions are also affected by lead toxicity. According to the United States Center for Disease Control and Prevention and the World Health Organization, a blood lead level of 10 μg/dL or above is of serious concern; even a lower level may lead to harmful health effects and there is no known safe exposure level. The common adverse health effects of lead concentration in the human body are neurological and organ impairments. Therefore, it is crucial to monitor the lead flow in waste however, its detection at open sources such as rivers, ponds, and seas is still a major challenge. Therefore, an industrial-based sensor for monitoring lead leakage at its point sources is the need of the hour. Despite the pressing need, most of the previously reported sensors, such as metal-organic frameworks (MOFs) [1], DNA-mediated sensors [2,3], metal-based nanocomposite [[4], [5], [6], [7], [8], [9], [10]], transition metal dichalcogenides [11,12] and molecularly imprinted polymers (MIPs) [13,14], often suffer from complexity associated with their synthesis procedure, interaction mechanism, sensing stability, high cost, and toxicity of waste generated from metal ions. This discourages their commercialization [15] and motivates the researchers to explore sustainable and highly efficient sensors that can meet the requirements of commercialization [16].
Carbon-based nanomaterials are among the best alternatives for costly and complicated metal-based sensors due to their high conductivity and specific surface area. Moreover, the abundance of carbon makes it an economical and sustainable nanomaterial [17]. The functionalization of carbon-based nanomaterial has also been reported to enhance its catalytic and electrochemical applications [[18], [19], [20], [21], [22]]. The widespread applications of heteroatom-doped/functionalized carbon-based nanomaterial in adsorption [23, 24], sensing [25], supercapacitor [26], carbon sequestration [27], and hydrogen evolution reaction [28] underscore its potential and versatility. The synthetic routes reported in the literature for synthesizing carbon-based nanomaterial are mechanical or chemical exfoliation of graphite [29] pyrolysis and chemical activation of biomass. The pyrolysis or carbonization of biomass for extraction of carbon rich material is a new method that is not only gaining wide attention among researchers but also offers easy synthesis, cost-effectiveness and sustainability.
Biomass-derived nanocarbon offers several advantages such as abundance, renewability, sustainability, high specific surface area, easy tunability, biocompatibility and natural heteroatom functionalities [30]. Moreover, the use of biomass waste promotes environmental cleaning and waste management practices. The wide applicability of biomass-derived carbon in fuel cells, electrocatalytic oxidation-reduction reactions, water splitting, hydrogen evolution reactions, supercapacitors, lithium-ion batteries, and sensing makes it more demanding in today's world [25,30,31]. The applicability of biomass-based sensors for lead ions is reported in previous literature. Zeinu et al. have synthesized the composite of biomass-derived carbon and bismuth, and the obtained detection limit was 1.72 pM [32]. Zhu et al., have designed the electrochemical sensor Bi@BAC for lead from Platanus seeds. The detection limit obtained in the case was 0.13 μg/L [33]. Chen et al., have coated the bismuth nanoparticles on to the hierarchical porous tubular biochar and the obtained detection limit of the resultant nanocomposite was 0.02 μg/L [34]. Zeng et al., have prepared the ordered mesoporous carbon doped hollow spherical bismuth oxide nanocomposite as an electrochemical sensor for lead ion and the obtained detection limit was 0.025 nM [35]. Agustini et al., have anchored the bismuth nanoparticles on to the biochar for detection of lead and the obtained detection limit was 1.41 nM [36]. Jin et al., have used porous carbon derived from soyabean straw to load platinum nanoparticles and the obtained detection limit from composite was 18 pM [37]. Although bismuth and platinum-decorated biomass-derived carbon offer many advantages regarding excellent detection limit and high sensitivity, its cost and associated toxicity discourage its long-term or commercial uses. Dali et al. have functionalized the biomass with SWCNT, and although the study has obtained an excellent detection limit of 0.01 μM, the complexity and cost associated with the SWCNT limit its further applications [38]. In the study by Xu et al., for nitrogen functionalization milk is used [39]. As milk is edible and used as a dietary product, its use as a nitrogen precursor questions the sustainability agendas. The chemical activation approaches with excellent detection limits for the development of sensors were also achieved by Oliveira and Guan [40,41]. Chemical activation methods suffer from some limitations, such as toxicity associated with the use of high quantities of chemicals, structural stability, and impurities, as the chances of obtaining pure carbon are less in these cases. Thermal annealing methods are also advantageous over chemical activation for the degree of graphitization, conductivity, and carbon richness of nanocomposite. Metal-free functionalization of biomass-derived carbon often suffers from high detection limits and less sensitivity; little or no study is reported on this.
Motivated by the above facts, the present study has successfully designed a sustainable metal-free biomass-derived sensor from coconut husk. Coconut husk is a prevalent secondary biomass waste, a residue of processed food products [42]. The two-step synthetic approach viz., hydrothermal carbonization followed by pyrolysis, was adopted for developing non-metal functionalized carbon nanomaterial. The mesoporous structure offers numerous advantages, such as high specific surface area, high conductivity and more active sites for functionalization. Mesopores of the nanostructure can be easily engineered for different applications. The functionalization of mesoporous nanostructure was done through hydrothermal carbonization with thiourea and melamine. Hydrothermal carbonization is advantageous for retaining the integrity of organic compounds, which aids in increasing the sensitivity and selectivity of carbon nanomaterial as a sensor. Melamine and thiourea are the cheapest precursors for the source of nitrogen and sulfur. The thiourea is an organosulfur compound with amino and C=S bonds. The C=S bond in thiourea provides active sites for binding, which makes the resultant nanocomposite selective for sensing the analyte. Additionally, melamine is also a triazine ligand with three amino groups. The structure of melamine is helpful in providing stability to the resultant nanoparticles, and it aids in the synthesis of graphitic nitrogen. Both melamine and thiourea make excellent choices for designing sustainable sensors due to their cheap cost, easy availability and chemical stability. The novelty of the study lies in the simplicity of designing a sustainable sensor for the efficient detection of lead.
2. Experimental details
2.1. Chemicals
Coconut husk waste was collected from the local market. Concentrated sulfuric acid (H2SO4), with 99.9 % purity, sodium acetate (ACS reagent, >99 %), lead nitrate (ACS reagent, >99 %), mercuric chloride (ACS reagent, >99 %), cadmium chloride (99.99 %), cobalt chloride (ACS reagent, 98 %), sodium chloride (ACS reagent, >99 %), aluminum chloride (reagent grade, 98 %), calcium chloride (ACS reagent, >99 %), potassium chloride (ACS reagent, 99–105 %), nickel chloride (98 %), silver nitrate (ACS reagent, >99 %), iron(II) chloride (98 %), iron(III) chloride (ACS reagent, 97 %), potassium hexacyanoferrate(II) trihydrate (ACS reagent, 98.5–102 %) and potassium hexacyanoferrate(III) ACS reagent, >99 % were bought from Sigma Aldrich. All the chemicals were used as received without any further purification. Distilled water (18.2 MΩ cm) was obtained from Milli-Q Ultrapure Water System.
2.2. Instruments
The geometric structure of nitrogen functionalized nanocarbon is studied through an X-ray diffraction pattern with Cu Kα radiation (λ = 1.54178 Å) on the instrument Rigaku Japan XRD. Field emission scanning electron microscope (FE-SEM) and high-resolution transmission electron microscope (HR-TEM) images were obtained from the instrument Carl Zeiss Model Supra 55 Germany and JEOL JEM 2100, 200 Kev, respectively. The chemical composition and connectivity were obtained through an X-ray photoelectron spectroscopy (XPS) analysis technique using Thermo Fischer Scientific ESCALAB Xi+. All the electrochemical sensing experiments were performed with the electrochemical workstation, Corrtest CS2350.
2.3. Synthesis of N-rich nanocarbon
The synthesis process of N-rich nanocarbon is a step-wise process. The dried coconut waste was first treated with 50 ml of concentrated sulfuric acid for 24 h to remove the moisture, oxygen-containing functional groups, and other impurities, then after consecutive washing, black carbon powder underwent thermal treatment at 1200 °C under N2 atmosphere with the flow rate of 0.2 mL/min. This treatment removes the volatile impurities and aids in the synthesis of a pure carbon matrix with a mesoporous structure. Afterward, the resultant carbon powder (200 mg) was mixed with thiourea and melamine in equal proportion, and the mixture was sonicated in ethanol. The sonicated solution mixture was transferred into a Teflon-lined autoclave. The hydrothermal treatment was given for 6 h at 200 °C. The black mass collected after hydrothermal treatment is then dried in the hot air oven at 70 °C.
2.4. Electrochemical sensing
All the electrochemical sensing experiments were performed using an electrochemical workstation (Corrtest CS2350). The three-electrode setup was prepared, where platinum wire, KCl saturated Ag/AgCl, and glassy carbon electrode (GCE) were used as counter, reference, and working electrode respectively. The slurry of nanomaterial was prepared in DI water (1mg/2 ml). Before modification of the working electrode, it was polished with 0.3 μm alumina powder. After 30 min of sonication, 5 μL slurry is dropped onto the surface of the working electrode and allowed to dry under a 100 W tungsten filament bulb. The dried electrode was then used as a sensor. The electrochemical techniques, viz., cyclic voltammetry, differential pulse normal voltammetry (DPNV) and differential pulse anodic stripping voltammetry (DPASV) are used where 0.1 M acetate buffer of pH 5 is used as an electrolyte.
3. Results and discussion
3.1. Characterizations and properties
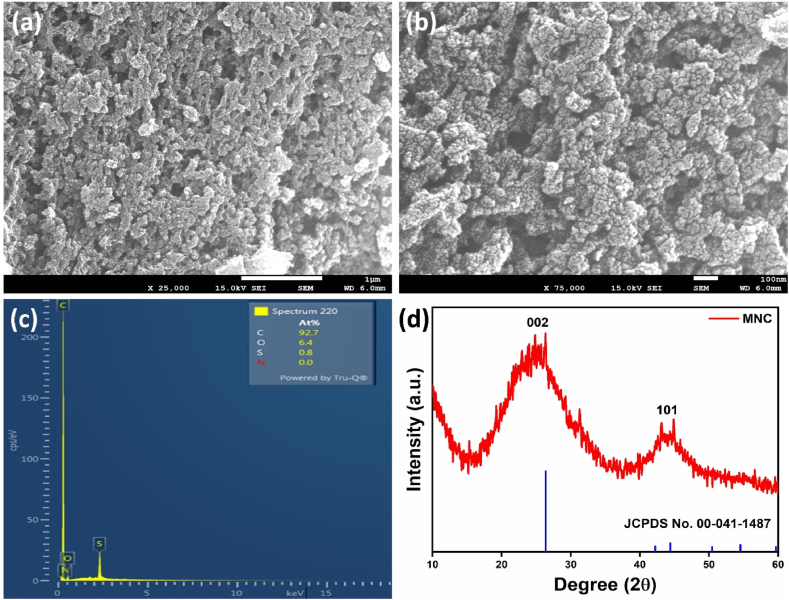
The low magnification FE-SEM image (Fig. 1(a)) of mesoporous nanocarbon (MNC) has shown a dense and fluffy porous net-like structure. The detailed examination of the high-magnification image (Fig. 1(b)) illustrates that the entire structure is intricately constructed with dot-like nanostructures. The energy dispersive X-ray spectroscopy (EDS) indicates the prevalence of carbon followed by oxygen, nitrogen and sulfur, with no additional impurities detected within the EDS detection limit, as shown in Fig. 1(c). The XRD spectra of MNC are shown in Fig. 1(d), where two distinguished peaks at 24.8o and 44.1o correspond to 002 and 101 crystal facets [23]. These peaks are assigned to the graphitic plane of the mesoporous nanocarbon structure.
Fig. 1.
(a,b) Low and high magnification FESEM images, (c) EDS and (d) XRD of nanocarbon structure.
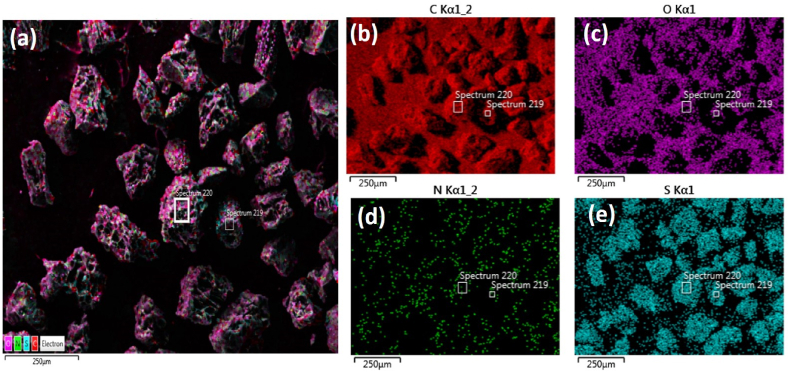
Fig. 2 shows the elemental mapping images of the as-synthesized mesoporous nanocarbon. Fig. 2(a) shows the presence of all the elements in a single image where the differently coloured fluorescent dot represents the presence of C, N, S, and O elements. Fig. 2(b–e), represents the presence of these atoms individually. It is observed from the EDS map that carbon is abundantly spread in the matrix, followed by oxygen. Nitrogen appears to be sprinkled over the carbon bed, while sulfur patches are a little darker, making the composite nitrogen and sulfur-rich.
Fig. 2.
EDS elemental mapping pattern of (a) mesoporous nanocarbon showing the presence of (b) C (c) O (d) N and (e) S element.
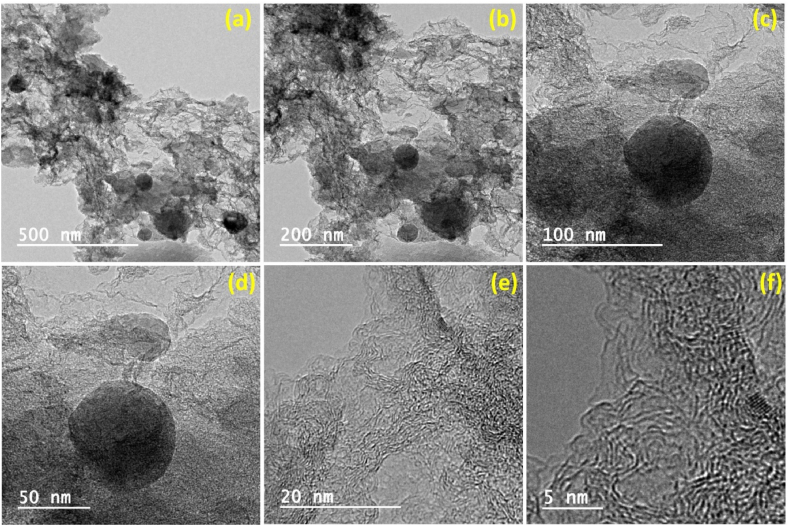
Fig. 3(a–f) represents the HR-TEM images of N-nanocarbon at different magnifications. The small fibrous transparent layers of carbon matrix, with round shaped dots, conclude that at high temperatures, a sheet of carbon breaks at some place and the small round shaped sheet accumulates on the surface of thin layers of carbon matrix. This appeared to be a mixture of carbon quantum dots and graphene-like layers of nanocomposite. These dots-like structure plays the role of defects and helps in increasing the conductivity and surface area of the nanostructure. The as-synthesized nanostructure appeared to be a hybrid structure, where small crystalline patches appeared in the amorphous network of the carbon structure.
Fig. 3.
HR-TEM images of mesoporous nanocarbon at different magnifications viz., (a) 500 nm (b) 200 nm (c) 100 nm (d) 50 nm (e) 20 nm (f) 5 nm.
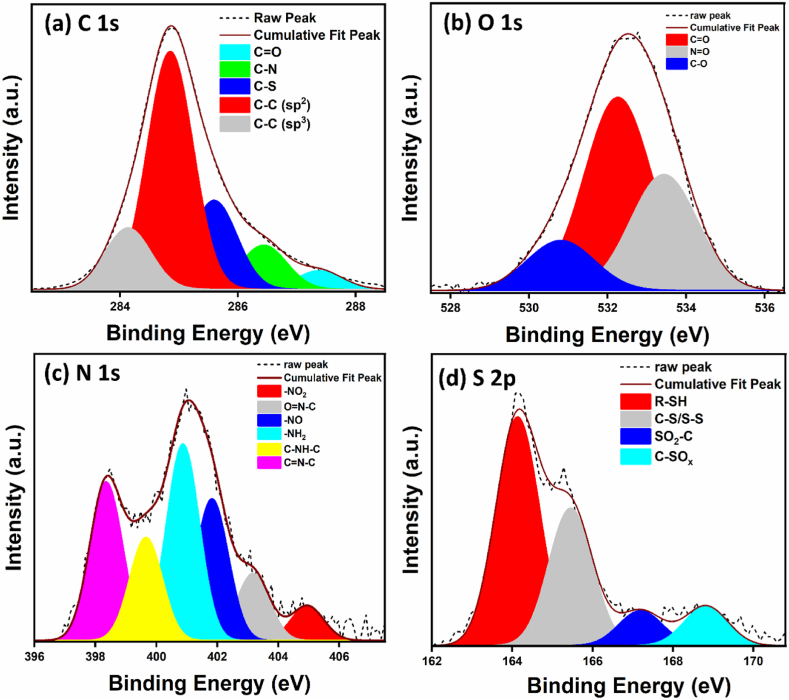
The XPS spectra of as-synthesized mesoporous nanocarbon have shown the highest amount of carbon, followed by oxygen, nitrogen, and sulfur (Fig. S1). Moreover, Fig. 4(a–d) has shown the deconvoluted spectra of C 1s, O 1s, N 1s and S 2p. The deconvoluted spectra of C 1s have different peaks at locations 284.1, 284.8, 285.6, 286.4, and 287.4 eV, which corresponds to various functional groups present in the nanocarbon viz., C-C (sp3), C-C (sp2), C-S, C-N, and C=O respectively. Different functional groups were present due to the nanostructure's addition of thiourea and melamine. In addition to these, the oxygen-containing functional groups, viz., carbonyl and oxides of nitrogen and carbon, are also present and are verified from the XPS spectra of O 1s, where the peaks are located at 530.7, 532.2 and 533.4 eV. The different bonds of nitrogen viz., -NO2 (404.9 eV), O=N-C (403.2 eV), -NO (401.8 eV), -NH2 (400.8 eV), pyrrolic C-NH-C (399.6 eV) and pyridinic C=N-C (398.3eV) bonds represents the diversified richness of N, S in mesoporous nanocarbon. Moreover, sulfur functional groups from thiourea are also indicated in Fig. 4(d), where peaks at 164.1, 165.4167.2 and 168.8 eV represent the R-SH, C-S/S-S, SO2-C, and C-SOx functional groups. The XPS data matches the NIST database well. This XPS data is also consistent with the information obtained from EDS.
Fig. 4.
XPS deconvoluted spectra of (a) C 1s, (b) O 1s, (c) N 1s, and (d) S 2p of mesoporous nanocarbon.
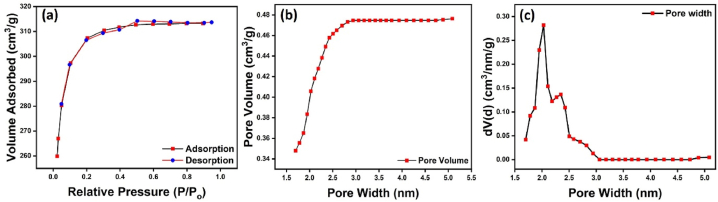
Fig. 5(a–c) has summarized the Brunauer-Emmett-Teller (BET) study in the form of adsorption-desorption isotherm, pore volume, and pore width, respectively. The nanocarbon has shown the type 1 adsorption isotherm according to IUPAC classification. The obtained surface area of the nanostructure is 1671.93 m2/g with the pore width and pore volume of 2.02 nm and 0.476 cm3/g. The obtained pore width of the nanostructure concludes that the nanostructure is mesoporous, while the pore width of nanoparticles appears to be in the range of 2–5 nm. The small range of pore width shows the uniformity in the nanostructure, which is known to increase the catalytic activity and sensing application of the nanostructure. The annealing at high temperatures reduces the pore size, and as the size decreases, the material goes to the nanoscale, due to which surface area increases.
Fig. 5.
(a) Adsorption-desorption isotherm, (b) pore volume and (c) pore width spectra of mesoporous nanocarbon.
3.2. Electrochemical behavior of mesoporous nanocarbon-modified GCE
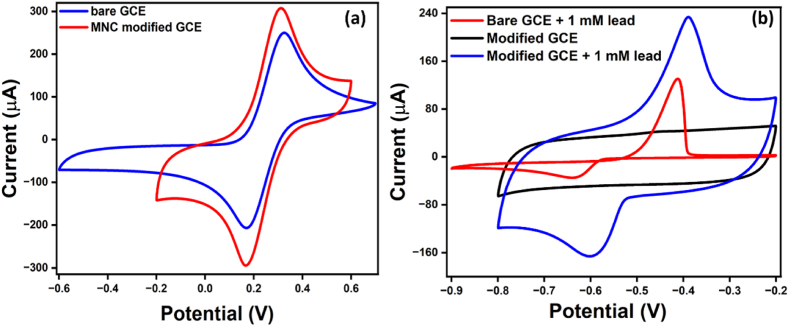
Our study compared the electrochemical behavior of the mesoporous nanocarbon-modified electrode with the bare glassy carbon electrode. Fig. 6(a) illustrates the superior performance of the mesoporous nanocarbon-modified GCE in the presence of 10 mM K3[Fe(CN)6]/K4[Fe(CN)6] in 0.1 M KCl, showing a remarkable ∼24 % increase in current, and an even more impressive ∼80 % increase in the case of lead. This significant enhancement in conductivity and sensitivity towards lead, attributed to the presence of nitrogen and sulfur-containing functional groups in the nanostructure, not only demonstrates the potential of our research in lead sensing but also in sustainable waste management.
Fig. 6.
Cyclic voltammogram of bare and mesoporous nanocarbon-modified GCE in the presence of (a) 10 mM K3[Fe(CN)6]/K4[Fe(CN)6] in 0.1 M KCl and (b) 1 mM lead.
3.2.1. Scan rate study
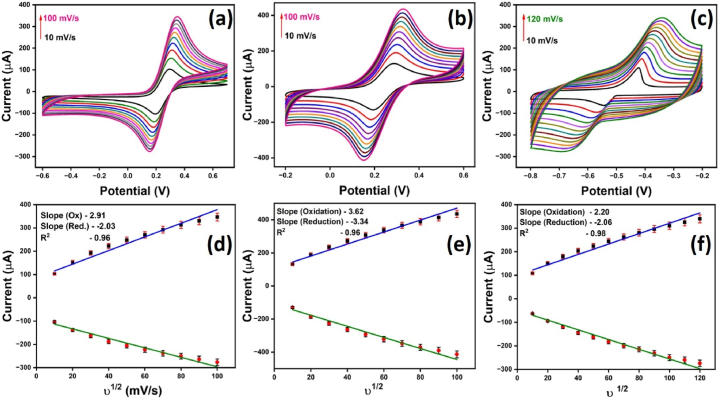
The scan rate study, a significant research method, was conducted to observe the effect of current with respect to variation in scan speed. This study is instrumental in determining the reaction mechanism and electroactive surface area of the modified GCE. Fig. 7(a–f) presents the scan rate study of 10 mM K3[Fe(CN)6]/K4[Fe(CN)6] in 0.1 M KCl and lead. Fig. 7(a) and (d) show the current response of bare electrodes with ferroferri, where the current is observed to increase linearly with increased scan rate, indicating that the analyte is freely diffusing in the solvent. The diffusion coefficient value is determined after applying the Randles-Sevcik equation.
| (1) |
Fig. 7.
Cyclic voltammogram of (a) bare and (b) mesoporous nanocarbon-modified GCE in the presence of 10 mM K3[Fe(CN)6]/K4[Fe(CN)6] in 0.1 M KCl (c) 1 mM lead with mesoporous nanocarbon modified GCE at the scan rate ranging from 10 to 100 mV/s respectively and (d–f) respective linear relationship of oxidation and reduction peak current of corresponding to the above graph.
The diffusion coefficient calculated from the equation for 10 mM K3[Fe(CN)6]/K4[Fe(CN)6] in 0.1 M KCl is 2.1 × 10−6 cm2/s. The obtained electroactive surface area of N, S-rich modified electrode after applying equation (1) is 0.35 cm2. The relatively high electroactive surface area of N, S- nanocarbon modified electrode compared to the geometric surface of bare electrode was also added to the enhanced conductivity. Similarly, the scan rate study was done with 1 mM lead with modified GCE. A linear relationship with the observed current peak and scan rate also indicated a diffusion-controlled reaction mechanism of lead. The broadness of the peak with increasing scan rate also indicates a diffusion-controlled reaction. The calculated value of the diffusion coefficient after applying equation (1) for a lead solution is 7.5 × 10−6 cm2/s.
3.2.2. Differential pulse anodic stripping voltammetry
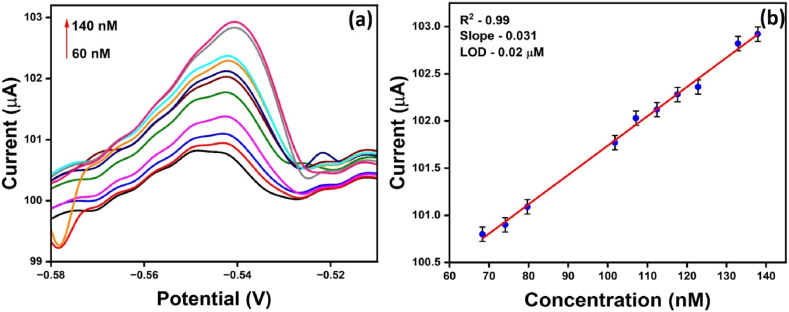
Differential pulse anodic stripping voltammetry (DPASV) is one of the highly sensitive techniques known for its high selectivity and quantification of trace metal ions. For the sensing of lead ions, the DPASV study was conducted, where the current of different lead concentrations were recorded. Fig. 8(a) shows the respective current response of N, S- rich nanocarbon modified electrode in the 60–140 nM range. Fig. 8(b) displays the observed linear relationship between the recorded current and associated concentration. The obtained detection limit after applying equation (2) is 0.022 μM. The obtained limit of quantification and sensitivity after using equations (3), (4)) is 0.075 μM and 0.11 nM/μA cm2. The achievement of excellent detection limit can be due to high specific surface area, and presence of nitrogen and sulfur functional group on to the carbon substrate. A comparison of DPASV techniques with differential pulse normal voltammetry (DPNV) was studied, and a DPNV study was also conducted for sensing lead. Fig. S2 shows the DPNV curve of lead at the oxidation potential −0.4 V. The study has shown that the current peak increased linearly in the 50–200 μM concentration range. The detection limit of MNC obtained from this technique is 50.67 μM, which is relatively high compared to the DPASV technique. It has been concluded that DPASV is a highly sensitive technique that can detect lead at the nanomolar level. Table 1 has added all the previously used nanoparticles to detect lead.
| (2) |
| (3) |
| (4) |
Fig. 8.
(a) Differential pulse anodic stripping voltammogram of N, S-nanocarbon modified GCE recorded with different concentrations of lead 60–140 nM in acetate buffer and (b) linear relationship of current and concentration in the range 60–140 nM.
Table 1.
Comparative response of different nanomaterials previously used for the detection of lead with reference to their detection methods, linear detection range, pH, detection limit, LOQ and sensitivity.
| S. No. | Nanomaterial | Detection Method | Linear Detection Range | pH | Detection Limit | LOQ | Sensitivity | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 1-dodecanoyl-3-phenylthiourea modified GCE | SWASV | 100–1000 μM | 3 | 0.695 μg/L | – | – | [43] |
| 2 | MWCNT-βCD(Phys)/SPE MWCNT-βCD(SE)/SPE |
DPV | 0.015–0.5 μM 6.2–103.5 ppb |
5 | 0.9 ppb 2.3 ppb |
– | 98 nA/ppb 38.6 nA/ppb |
[44] |
| 3 | Cu-based Sensor | ASV | 0.025–10 μM | 5.5 | 21 nM | – | – | [45] |
| 4 | PPy NPs | DPV | 0.1–50 μM | 5 | 55 nM | – | – | [46] |
| 5 | GR-5/(Fe-P)n-MOF | CA | 0–200 nM | 5.5 | 0.034 nM | – | – | [2] |
| 6 | NHAP/Ionophore/Nafion-Modified Electrode | ASDPV | 0.005–0.8 μM | 4.4 | 1 nM | – | 13 μA/μM | [47] |
| 7 | ZYMCPE | CV | 0.0025–0.1 μM | 5.9 | 17 nM | – | [48] | |
| 8. | GO-Fc | CV | 0.1–1000 μg/L | – | 0.168 μg/L | – | 250 μg/L | [49] |
| 9. | IP/NPs | DPSV | 0.1–10 nM | 5 | 30 pM | – | 49.179 nA/nM | [13] |
| 10. | Fe3O4@SiO2@IIP | DPV | 0.1–80 ng/mL | 5.6 | 0.05 ng/mL | 0.16 ng/mL | 7962 μA/μmol dm−3 | [50] |
| 11. | porph@MOF | SWV | 50 pM −5 μM | – | 5 pM | – | – | [1] |
| 12. | Pb2+-specific DNAzyme-based sensor | SWV | 0.05–50 nM | 4.3 | 32 pM | – | – | [3] |
| 13. | G/PANI/PS | ASV | 10–500 μg/L | 1 | 3.30 μg/L | – | – | [51] |
| 14. | MWCNT-IIP | DPV | 1–5 ppm | 5 | 0.02 μM | – | – | [52] |
| 15. | MIP | DPV | 0.3–50 μM | – | 0.2 μM | – | – | [14] |
| 16. | MWCNTs/GNP | DPV | 5 × 10−11-1 × 10−14 M | – | 4.3 fM | – | – | [4] |
| 17. | GO-imi-(CH2)2-NH2) | DPASV | 5–300 nM | 5 | 0.30 nM | – | – | [53] |
| 18. | N, S-rich nanocarbon | DPASV | 60–140 nM | 5 | 22 nM | 75 nM | 0.00011 μA/μM cm2 | This work |
3.3. Selectivity study
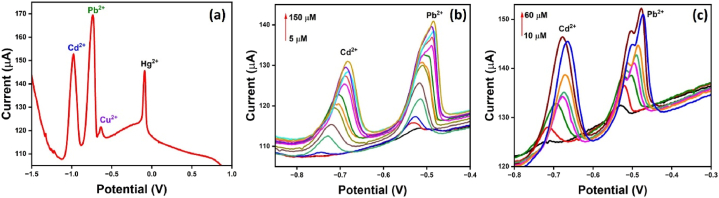
Selectivity study is one of the critical parameters to test the overall performance and affinity of as-synthesized nanocomposite. It is also helpful in selecting analytes based on the sensitivity of the modified electrode. The study was conducted in the presence of different metal ions (Ca2+, K+, Cd2+, Fe2+, Ag+, Ni2+, Fe3+, Al3+, Pb2+, Co2+, Na+, and Hg2+) 1 mM in 0.1 M acetate buffer electrolyte solution. Fig. 9(a) has displayed the current response of N, S- rich nanocarbon GCE, wherein the potential window of −1.5 to 1.5 V, only four metal ions (Cd2+, Pb2+, Cu2+ and Hg2+) has displayed the current response, where the peaks are distinguished. The current reaction of lead is higher among all the heavy metal ions. This has shown the high selectivity and affinity of as-synthesized nano sensors toward lead. Consequently, the current response was also recorded after varying the concentration of metal ions in acetate buffer (Fig. 9(b)) and tap water (Fig. 9(c)). It is well confirmed from the recorded results that an N, S-rich nanocarbon-modified electrode is highly efficient in detecting lead in the mixed metal component, even at low concentrations. Moreover, the modified electrode has also detected lead in the tap water mixture. This indicates that the as-synthesized nanocomposite is highly sensitive and selective toward lead in diverse environments and can be applied in real-time sensing.
Fig. 9.
(a) DPASV graph of N, S-nanocarbon in the presence of different metal ions viz., Ca, K+, Cd2+, Fe2+, Ag+, Ni2+, Fe3+, Al3+, Pb2+, Co2+, Na+ and Hg2+ at a concentration (a) 1 mM (b) 5–150 μM in acetate buffer and (c) 10–60 μM in tap water.
3.4. Real sample-based sensing
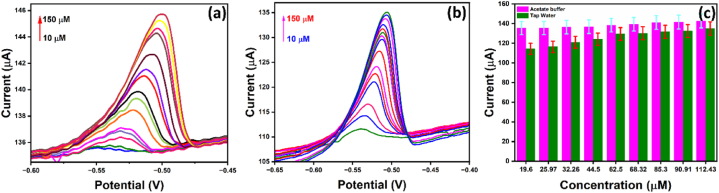
To determine the reliability of the N, S-rich nanocarbon-modified electrode, the current response of lead in acetate buffer and tap water at varied concentrations is compared. Fig. 10(a) and Fig. 10(b) have shown the current response of varied concentrations of lead ions in different solutions. Although both graphs show a linear current response with respective concentrations, the current response of lead in tap water is relatively less. The bar graph in Fig. 10(c) shows the difference in current response at specific lead concentrations. The difference in current response can be due to the solubility issue of lead in tap water.
Fig. 10.
DPASV response of N, S- nanocarbon modified GCE with the different concentrations of lead (10–150 μM) (a) in acetate buffer, (b) Tap water and (c) Bar graph showing a comparison of current response at the same concentration of lead in different solution viz., 0.1 M acetate buffer and tap water.
Although the difference in current response is minute, this can interfere with sensing accuracy in real time. In this case, lead calibration in different water sources can be successful before sensing an unknown concentration in an actual application.
3.5. Repeatability, reproducibility and stability study
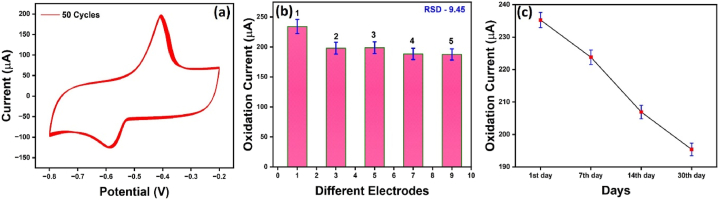
The reliability and performance of the sensor can also be tested through repeatability, reproducibility, and stability studies. In the repeatability study, the current response of 1 mM lead was repeated under the same experimental conditions, while in the reproducibility study, the whole experiment, viz., solution and modification of electrode, is repeated. Fig. 11(a) shows the 50 cycles of CV with 1 mM lead, where the stable current response indicates the stability of N, S-rich nanocarbon-modified electrodes. Fig. 11(b) shows the current response of 1 mM lead after repetition of the experiment; the relative standard deviation calculated from the current response of 5 studies was 9.45 %. Fig. 11(c) represents the current response of N, S-rich nanocarbon after its storage in a room environment. The current response was recorded after interval periods of 7 days, where no significant change in the current response is recorded.
Fig. 11.
Cyclic voltammetric response of 1 mM lead (a) 50 cycles, (b) with different N, S-nanocarbon modified GCE and (c) after successive intervals of 7 days of storage.
4. Conclusion
This study has explored the cheap and sustainable waste management approach, where coconut husk waste was treated and converted into nitrogen and sulfur-rich mesoporous carbon nanomaterials. All the characterization studies have indicated that graphene-like thin and transparent layers with the nonuniform scattering of the quantum dot-like structure were well developed with a high surface area of 1671.93 m2/g along with pore width and pore volume of 2.02 nm and 0.476 cm3/g, respectively. The diversity of nitrogen and sulfur functional groups in the carbon matrix has indicated the chemically active site for facilitating electron transfer and sensing of lead ions. The as-synthesized nanostructure was applied to the surface of the glassy carbon electrode, and the modified electrode was then used as a sensor for efficient detection of lead. The nitrogen and sulfur enrichment of the carbon matrix makes the nanostructure highly sensitive toward lead, while the morphological geometry aids in increasing the conductivity. The obtained detection limit, the limit of quantification and sensitivity were 22 nM, 75 nM, and 0.00011 μA/μM cm2, respectively. Moreover, the as-prepared nanostructure also shows high selectivity toward lead, even in the presence of other metal ions in distilled or real water samples. The as-prepared nanomaterial has achieved the very low detection limit, showing its high efficiency and performance over costly metal-based nanoparticles.
CRediT authorship contribution statement
Ritika Sharma: Writing – original draft, Formal analysis, Data curation. Dharmender Singh Rana: Writing – original draft, Formal analysis, Data curation. Abhishek Awasthi: Formal analysis, Data curation. Dilbag Singh: Writing – review & editing, Formal analysis, Data curation. Ahmed A. Ibrahim: Formal analysis, Data curation. Ahmad Umar: Writing – review & editing, Data curation. Sotirios Baskoutas: Writing – review & editing, Data curation.
Ethical approval
This research did not involve human or animal samples.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
RS is thankful to UGC-New Delhi for the award of SRF and Central University of Himachal Pradesh for providing necessary research facilities. Ahmad Umar and Ahmed A. Ibrahim would like to acknowledge the Research, Development and Innovation Authority (RDIA), Saudi Arabia for granting and financially supporting the "STEM Pioneers Training Lab" at Najran University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39090.
Contributor Information
Dilbag Singh, Email: dilbagrana@hpcu.ac.in.
Ahmad Umar, Email: ahmadumar786@gmail.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Zhang X., Huang X., Xu Y., Wang X., Guo Z., Huang X., Li Z., Shi J., Zou X. Single-step electrochemical sensing of ppt-level lead in leaf vegetables based on peroxidase-mimicking metal-organic framework. Biosens. Bioelectron. 2020;168 doi: 10.1016/j.bios.2020.112544. [DOI] [PubMed] [Google Scholar]
- 2.Kamble T.P., Shingte S.R., Chavan V.D., Kim D.K., Chougale A.D., Dongale T.D., Patil P.B. MOF derived CoFe2O4@Carbon nanofibers for supercapacitor application. ChemSci Adv. 2024;1(1):34–40. doi: 10.69626/csa.2024.0034. [DOI] [Google Scholar]
- 3.Pandey P., Yadav S., Dwivedi D.K., Lohia P. Development and modelling of a photonic crystal fiber sensor for detecting harmful chemicals in polycarbonate plastics. MatSci Express. 2024;1(4):253–261. doi: 10.69626/mse.2024.0253. [DOI] [Google Scholar]
- 4.Zhu Y., Zeng G., Zhang Y., Tang L., Chen J., Cheng M., Zhang L., He L., Guo Y., He X., Lai M., He Y. Highly sensitive electrochemical sensor using a MWCNTs/GNPs-modified electrode for lead (II) detection based on Pb2+-induced G-rich DNA conformation. Analyst. 2014;139(19):5014–5020. doi: 10.1039/C4AN00874J. [DOI] [PubMed] [Google Scholar]
- 5.Mourya V., Yadav S., Dwivedi D.K., Lohia P. Design and evaluation of a twin-core photonic crystal fiber sensor for human blood biomolecules. SciEngg Adv. 2024;1(1):20–29. doi: 10.69626/sea.2024.0020. [DOI] [Google Scholar]
- 6.Salunke R.S., Nakate Y., Umar A., Patil A.M., Nakate U., Baskoutas S., Shirale D.J. Selective recognition of lead and cadmium in potable water using single polypyrrole nanowire decorated with cobalt oxide nanoparticles electrode. MatSci Express. 2024;1(2):69–80. doi: 10.69626/mse.2024.0069. [DOI] [Google Scholar]
- 7.Bhosale T.R., Wategaonkar S.B., Patil P.P., Anbhule P.V., Deshmukh M.B. Crown ether functionalized barbituric acid for colorimetric determination of Au3+ ChemSci Adv. 2024;1(3):147–154. doi: 10.69626/csa.2024.0147. [DOI] [Google Scholar]
- 8.Mariyappan V., Manavalan S., Chen S.-M., Jaysiva G., Veerakumar P., Keerthi M. Sr@FeNi-S nanoparticle/carbon nanotube nanocomposite with superior electrocatalytic activity for electrochemical detection of toxic mercury(II) ACS Appl. Electron. Mater. 2020;2(7):1943–1952. doi: 10.1021/acsaelm.0c00248. [DOI] [Google Scholar]
- 9.Ganie A.S., Hussain S., Shah S., Liaqat M.J., Begi A.N., Miao Z., Han Y. In2O3 doped ZnO nanosheets for ultra-trace detection of NO2. SciEngg Adv. 2024;1(2):56–65. doi: 10.69626/sea.2024.0056. [DOI] [Google Scholar]
- 10.Pawar S.K., Wadodkar N.A., Salunke R.S., Patil A.M., Shirale D.J. Eco-friendly electrochemical sensor for accurate soil nitrate detection using ZnOx/PANI nanocomposite on nickel foam electrode. MatSci Express. 2024;1(3):170–178. doi: 10.69626/mse.2024.0170. [DOI] [Google Scholar]
- 11.Sharma R., Kumar S., Rana D.S., Thakur S., Gupta N., Singh D. Molybdenum disulfide nanostructure grown on multi-walled carbon nanotube for the electrochemical detection of ofloxacin. J. Environ. Chem. Eng. 2024;12(2) doi: 10.1016/j.jece.2024.112413. [DOI] [Google Scholar]
- 12.Rana D.S., Sharma R., Kumar S., Gupta N., Thakur S., Thakur K.K., Singh D. Molybdenum disulfide (MoS2) and reduced graphene oxide (rGO) nanocomposite based electrochemical sensor for detecting mercury(II) ions. Nano-Struct. Nano-Objects. 2023;36 doi: 10.1016/j.nanoso.2023.101041. [DOI] [Google Scholar]
- 13.Bojdi M.K., Mashhadizadeh M.H., Behbahani M., Farahani A., Davarani S.S.H., Bagheri A. Synthesis, characterization and application of novel lead imprinted polymer nanoparticles as a high selective electrochemical sensor for ultra-trace determination of lead ions in complex matrixes. Electrochim. Acta. 2014;136:59–65. doi: 10.1016/j.electacta.2014.05.095. [DOI] [Google Scholar]
- 14.Wang Z., Qin Y., Wang C., Sun L., Lu X., Lu X. Preparation of electrochemical sensor for lead(II) based on molecularly imprinted film. Appl. Surf. Sci. 2012;258(6):2017–2021. doi: 10.1016/j.apsusc.2011.05.005. [DOI] [Google Scholar]
- 15.Soni A., Sharma R., Rana D.S., Singh D., Gupta N. Structural designs of functional metal organic frameworks for the detection of mercury in contaminated water sources. Coord. Chem. Rev. 2023;494 doi: 10.1016/j.ccr.2023.215343. [DOI] [Google Scholar]
- 16.Sharma R., Rana D.S., Gupta N., Thakur S., Thakur K.K., Singh D. Parthenium hysterophorus derived nanostructures as an efficient carbocatalyst for the electrochemical sensing of mercury(II) ions. Chemosphere. 2024;354 doi: 10.1016/j.chemosphere.2024.141591. [DOI] [PubMed] [Google Scholar]
- 17.Gusain R., Kumar N., Ray S.S. Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020;405 doi: 10.1016/j.ccr.2019.213111. [DOI] [Google Scholar]
- 18.Sahil, Kumar S., Barot Y.B., Mishra R., Singh D., Gupta N. Nitrogen-doped fluorescent active fullerenes as a fluorescent probe for the detection of Hg2+ ions in aqueous solutions. Environ. Nanotechnol. Monit. Manag. 2023;20 doi: 10.1016/j.enmm.2023.100845. [DOI] [Google Scholar]
- 19.Kumar S., Sharma R., Singh D., Awasthi A., Kumar V., Singh K. Tungsten sulphide decorated carbon nanotube based electroanalytical sensing of neurotransmitter dopamine. Electrochim. Acta. 2024;475 doi: 10.1016/j.electacta.2023.143584. [DOI] [Google Scholar]
- 20.Kalia S., Kumar R., Sharma R., Kumar S., Singh D., Singh R.K. Two-dimensional layered rGO-MoS2 heterostructures decorated with Fe3O4 nanoparticles as an electrochemical sensor for detection of para-nitrophenol. J. Phys. Chem. Solid. 2024;184 doi: 10.1016/j.jpcs.2023.111719. [DOI] [Google Scholar]
- 21.Kumar S., Singh D., Pathania D., Awasthi A., Singh K. Molybdenum disulphide-nitrogen doped reduced graphene oxide heterostructure based electrochemical sensing of epinephrine. Mater. Chem. Phys. 2023;297 doi: 10.1016/j.matchemphys.2023.127446. [DOI] [Google Scholar]
- 22.Rana D.S., Kalia S., Thakur N., Singh R.K., Kumar R., Singh D. Synthesis of reduced graphene oxide-molybdenum disulfide nanocomposite as potential scaffold for fabrication of efficient hydrazine sensor. Mater. Chem. Phys. 2023;294 doi: 10.1016/j.matchemphys.2022.127048. [DOI] [Google Scholar]
- 23.Sharma R., Thakur J., Jaryal V.B., Rana D.S., Thakur S., Gupta N., Singh D. Nitrogen and sulfur functionalized microporous carbon nanomaterial derived from waste coconut husk for the efficient detection and removal of ofloxacin. Chemosphere. 2024;346 doi: 10.1016/j.chemosphere.2023.140653. [DOI] [PubMed] [Google Scholar]
- 24.Farooq N., Rehman Z., Hareem A., Masood R., Ashfaq R., Fatimah I., Hussain S., Ansari S.A., Parveen N. Graphene oxide and based materials: synthesis, properties, and applications – a comprehensive review. MatSci Express. 2024;1(4):185–231. doi: 10.69626/mse.2024.0185. [DOI] [Google Scholar]
- 25.Rana D.S., Sharma R., Gupta N., Sharma V., Thakur S., Singh D. Development of metal free carbon catalyst derived from parthenium hysterophorus for the electrochemical detection of dopamine. Environ. Res. 2023;231 doi: 10.1016/j.envres.2023.116151. [DOI] [PubMed] [Google Scholar]
- 26.Ji L., Wang B., Yu Y., Wang N., Zhao J.N. S Co-doped biomass derived carbon with sheet-like microstructures for supercapacitors. Electrochim. Acta. 2020;331 doi: 10.1016/j.electacta.2019.135348. [DOI] [Google Scholar]
- 27.Sreekanth N., Nazrulla M.A., Vineesh T.V., Sailaja K., Phani K.L. Metal-free boron-doped graphene for selective electroreduction of carbon dioxide to formic acid/formate. Chem. Commun. 2015;51(89):16061–16064. doi: 10.1039/C5CC06051F. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L., Xiao J., Wang H., Shao M. Carbon-based electrocatalysts for hydrogen and oxygen evolution reactions. ACS Catal. 2017;7(11):7855–7865. doi: 10.1021/acscatal.7b02718. [DOI] [Google Scholar]
- 29.Novoselov K.S., Geim A.K., Morozov S.V., Jiang D., Zhang Y., Dubonos S.V., Grigorieva I.V., Firsov A.A. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 30.Deng J., Li M., Wang Y. Biomass-derived carbon: synthesis and applications in energy storage and conversion. Green Chem. 2016;18(18):4824–4854. doi: 10.1039/C6GC01172A. [DOI] [Google Scholar]
- 31.Coconut Husk Waste‐Derived Nitrogen‐Doped Mesoporous Carbon Nanomaterial as an Efficient and Sustainable Supercapacitor - Sharma - 2024 - Energy Technology - Wiley Online Library. https://onlinelibrary.wiley.com/doi/abs/10.1002/ente.202400294 (accessed 2024-08-07).
- 32.Zeinu K.M., Hou H., Liu B., Yuan X., Huang L., Zhu X., Hu J., Yang J., Liang S., Wu X. A novel hollow sphere bismuth oxide doped mesoporous carbon nanocomposite material derived from sustainable biomass for picomolar electrochemical detection of lead and cadmium. J. Mater. Chem. A. 2016;4(36):13967–13979. doi: 10.1039/C6TA04881A. [DOI] [Google Scholar]
- 33.Zhu X., Liu B., Chen S., Wu L., Yang J., Liang S., Xiao K., Hu J., Hou H. Ultrasensitive and simultaneous electrochemical determination of Pb2+ and Cd2+ based on biomass derived Lotus root-like hierarchical porous carbon/bismuth composite. J. Electrochem. Soc. 2020;167(8) doi: 10.1149/1945-7111/ab82f7. [DOI] [Google Scholar]
- 34.Chen X., Lu K., Lin D., Li Y., Yin S., Zhang Z., Tang M., Chen G. Hierarchical porous tubular biochar based sensor for detection of trace lead (II) Electroanalysis. 2021;33(2):473–482. doi: 10.1002/elan.202060148. [DOI] [Google Scholar]
- 35.Zeng Z., Fang S., Tang D., Xiao R., Tang L., Peng B., Gong J., Long B., Ouyang X., Zeng G. Ultrasensitive sensor based on novel bismuth carbon nanomaterial for lead and cadmium determination in natural water, contaminated soil and human plasma. Microporous Mesoporous Mater. 2019;284:177–185. doi: 10.1016/j.micromeso.2019.04.045. [DOI] [Google Scholar]
- 36.Agustini D., Mangrich A.S., Bergamini M.F., Marcolino-Junior L.H. Sensitive voltammetric determination of lead released from ceramic dishes by using of bismuth nanostructures anchored on biochar. Talanta. 2015;142:221–227. doi: 10.1016/j.talanta.2015.04.052. [DOI] [PubMed] [Google Scholar]
- 37.Jin H., Zhang D., Liu Y., Wei M. An electrochemical aptasensor for lead ion detection based on catalytic hairpin assembly and porous carbon supported platinum as signal amplification. RSC Adv. 2020;10(11):6647–6653. doi: 10.1039/D0RA00022A. [DOI] [Google Scholar]
- 38.Dali M., Zinoubi K., Chrouda A., Abderrahmane S., Cherrad S., Jaffrezic-Renault N. A biosensor based on fungal soil biomass for electrochemical detection of lead (II) and cadmium (II) by differential pulse anodic stripping voltammetry. J. Electroanal. Chem. 2018;813:9–19. doi: 10.1016/j.jelechem.2018.02.009. [DOI] [Google Scholar]
- 39.Xu C., Liu J., Bi Y., Ma C., Bai J., Hu Z., Zhou M. Biomass derived worm-like nitrogen-doped-carbon framework for trace determination of toxic heavy metal lead (II) Anal. Chim. Acta. 2020;1116:16–26. doi: 10.1016/j.aca.2020.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira G.A., Gevaerd A., Mangrich A.S., Marcolino-Junior L.H., Bergamini M.F. Biochar obtained from spent coffee grounds: evaluation of adsorption properties and its application in a voltammetric sensor for lead (II) ions. Microchem. J. 2021;165 doi: 10.1016/j.microc.2021.106114. [DOI] [Google Scholar]
- 41.Guan J., Fang Y., Zhang T., Wang L., Zhu H., Du M., Zhang M. Kelp-derived activated porous carbon for the detection of heavy metal ions via square wave anodic stripping voltammetry. Electrocatalysis. 2020;11(1):59–67. doi: 10.1007/s12678-019-00568-9. [DOI] [Google Scholar]
- 42.Tripathi N., Hills C.D., Singh R.S., Atkinson C.J. Biomass waste utilisation in low-carbon products: harnessing a major potential resource. Npj Clim. Atmospheric Sci. 2019;2(1):1–10. doi: 10.1038/s41612-019-0093-5. [DOI] [Google Scholar]
- 43.Shah A., Zahid A., Khan A., Iftikhar F.J., Nisar J., Fernandez C., Akhter M.S., Almutawah A.A., Kraatz H.-B. Development of a highly sensitive electrochemical sensing platform for the trace level detection of lead ions. J. Electrochem. Soc. 2019;166(9):B3136–B3142. doi: 10.1149/2.0271909jes. [DOI] [Google Scholar]
- 44.Alam A.U., Howlader M.M.R., Hu N.-X., Deen M.J. Electrochemical sensing of lead in drinking water using β-cyclodextrin-modified MWCNTs. Sensor. Actuator. B Chem. 2019;296 doi: 10.1016/j.snb.2019.126632. [DOI] [Google Scholar]
- 45.Kang W., Pei X., Rusinek C.A., Bange A., Haynes E.N., Heineman W.R., Papautsky I. Determination of lead with a copper-based electrochemical sensor. Anal. Chem. 2017;89(6):3345–3352. doi: 10.1021/acs.analchem.6b03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu T., Dai H., Jin Y. Electrochemical sensing of lead(II) by differential pulse voltammetry using conductive polypyrrole nanoparticles. Microchim. Acta. 2019;187(1):23. doi: 10.1007/s00604-019-4027-z. [DOI] [PubMed] [Google Scholar]
- 47.Pan D., Wang Y., Chen Z., Lou T., Qin W. Nanomaterial/ionophore-based electrode for anodic stripping voltammetric determination of lead: an electrochemical sensing platform toward heavy metals. Anal. Chem. 2009;81(12):5088–5094. doi: 10.1021/ac900417e. [DOI] [PubMed] [Google Scholar]
- 48.Senthilkumar S., Saraswathi R. Electrochemical sensing of cadmium and lead ions at zeolite-modified electrodes: optimization and field measurements. Sensor. Actuator. B Chem. 2009;141(1):65–75. doi: 10.1016/j.snb.2009.05.029. [DOI] [Google Scholar]
- 49.Ashwin Karthick N., Thangappan R., Arivanandhan M., Gnanamani A., Jayavel R. A facile synthesis of ferrocene functionalized graphene oxide nanocomposite for electrochemical sensing of lead. J. Inorg. Organomet. Polym. Mater. 2018;28(3):1021–1028. doi: 10.1007/s10904-017-0744-0. [DOI] [Google Scholar]
- 50.Dahaghin Z., Kilmartin P.A., Mousavi H.Z. Novel ion imprinted polymer electrochemical sensor for the selective detection of lead(II) Food Chem. 2020;303 doi: 10.1016/j.foodchem.2019.125374. [DOI] [PubMed] [Google Scholar]
- 51.Promphet N., Rattanarat P., Rangkupan R., Chailapakul O., Rodthongkum N. An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sensor. Actuator. B Chem. 2015;207:526–534. doi: 10.1016/j.snb.2014.10.126. [DOI] [Google Scholar]
- 52.Sebastian M., Mathew B. Ion imprinting approach for the fabrication of an electrochemical sensor and sorbent for lead ions in real samples using modified multiwalled carbon nanotubes. J. Mater. Sci. 2018;53(5):3557–3572. doi: 10.1007/s10853-017-1787-x. [DOI] [Google Scholar]
- 53.Eshlaghi M.A., Kowsari E., Ehsani A., Akbari-Adergani B., Hekmati M. Functionalized graphene oxide GO-[imi-(CH2)2-NH2] as a high efficient material for electrochemical sensing of lead: synthesis surface and electrochemical characterization. J. Electroanal. Chem. 2020;858 doi: 10.1016/j.jelechem.2019.113784. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.