Abstract
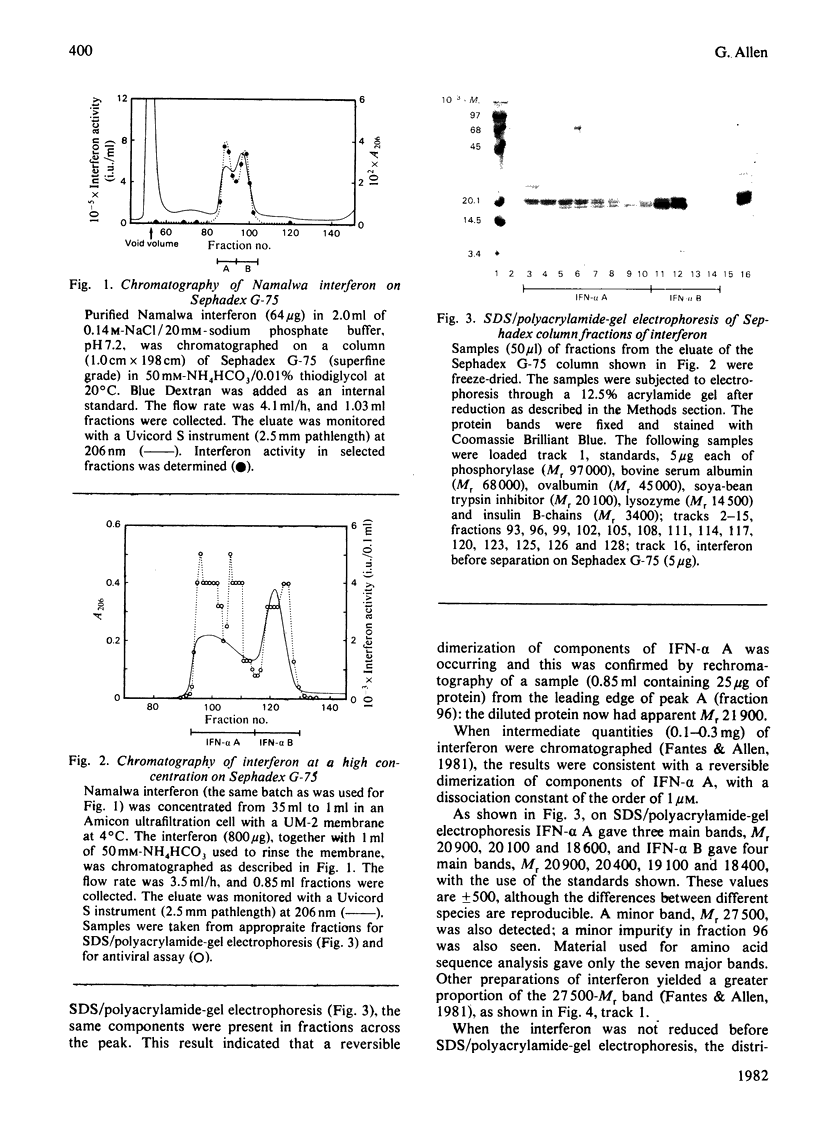
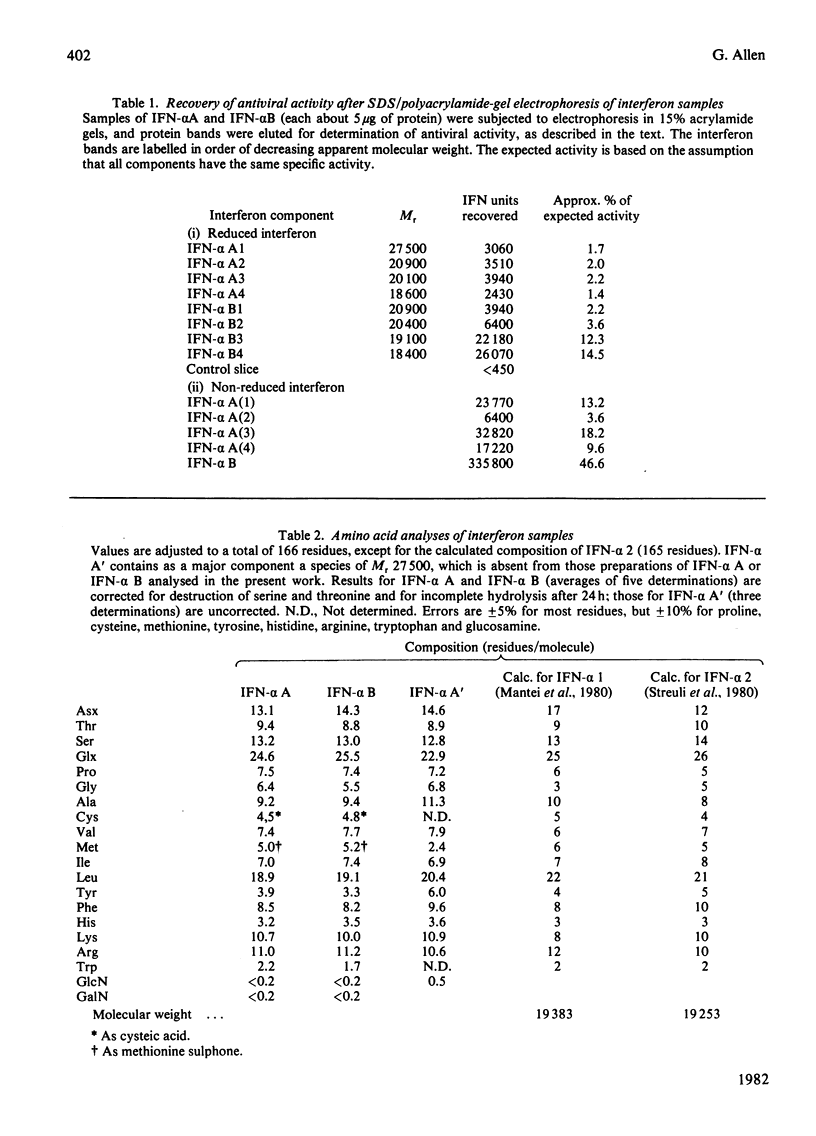
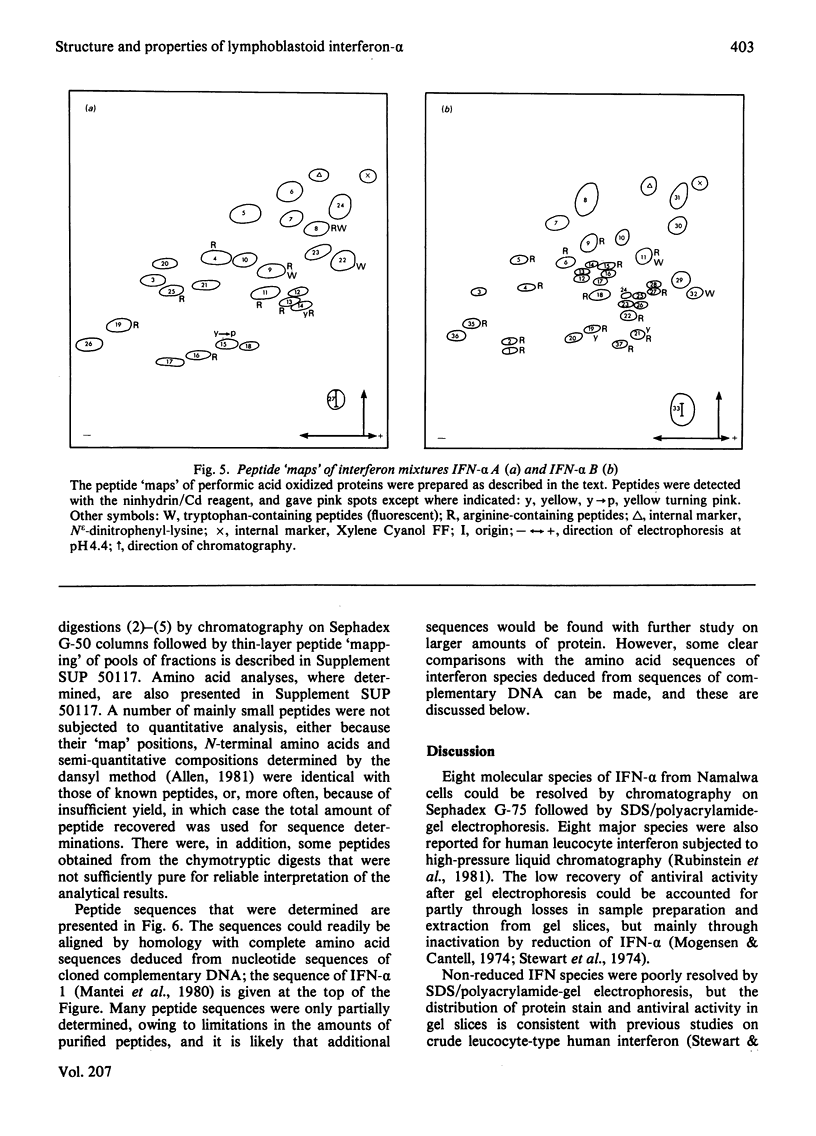
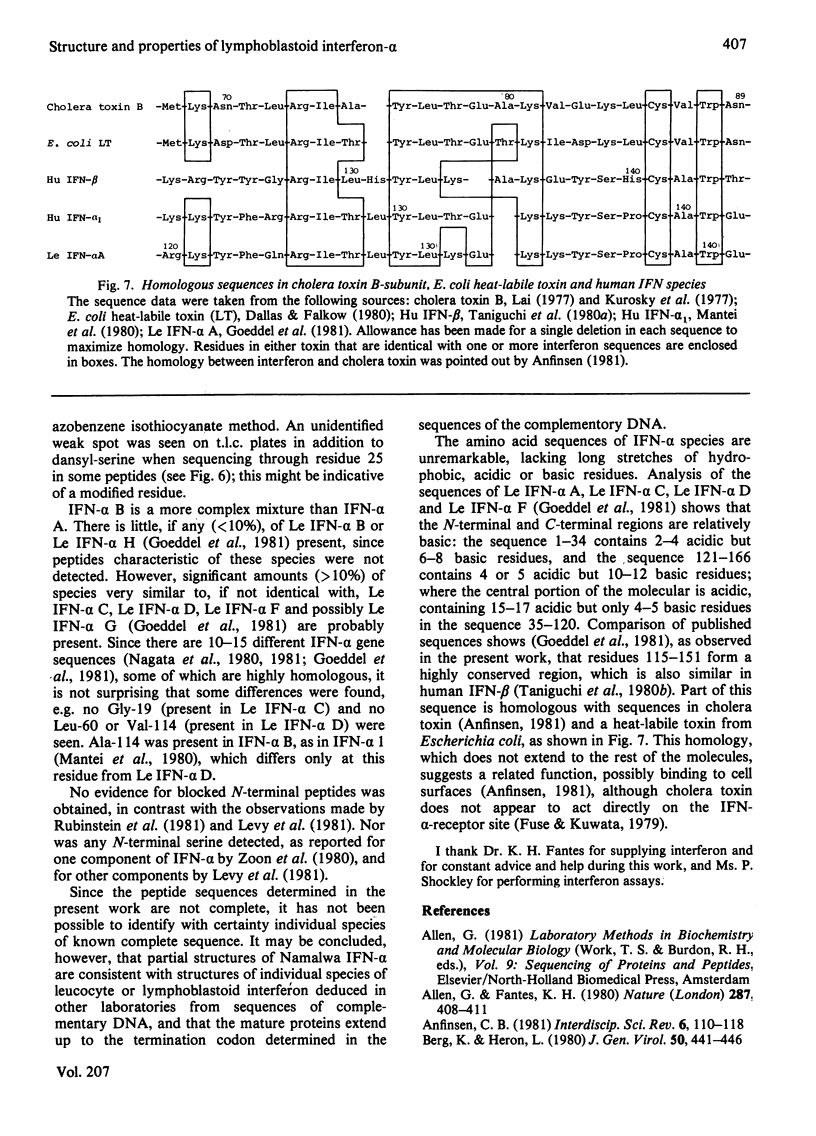
The chromatographic properties of human interferon-alpha from Namalwa lymphoblastoid cells on Sephadex G-75 are described. The interferons are separated into two groups of four, with apparent molecular weights 19050 and 22000. Some of the latter form dimers at high concentrations. Fractions containing interferon were studied by polyacrylamide-gel electrophoresis in the presence of sodium dodecyl sulphate. Seven of the components had apparent molecular weights in this system, after reduction, of between 18400 and 20900: one component is probably glycosylated and has an apparent molecular weight of 27500. Amino acid sequences of peptides derived from interferon mixtures were determined and are related to published sequences deduced from the nucleotide sequences of cloned complementary DNA coding for interferon-alpha. The results show that the major interferon-alpha species isolated from Namalwa cells do not undergo C-terminal processing. Amino acid analyses of peptides are presented in Supplementary Publication SUP 50117 (28 pages), which has been deposited with the British Library Lending Division, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1981) 193, 5.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Fantes K. H. A family of structural genes for human lymphoblastoid (leukocyte-type) interferon. Nature. 1980 Oct 2;287(5781):408–411. doi: 10.1038/287408a0. [DOI] [PubMed] [Google Scholar]
- Berg K., Heron I. SDS-polyacrylamide gel electrophoresis of purified human leucocyte interferon and the antiviral and anticellular activities of the different interferon species. J Gen Virol. 1980 Oct;50(2):441–446. doi: 10.1099/0022-1317-50-2-441. [DOI] [PubMed] [Google Scholar]
- Bose S., Gurari-Rotman D., Ruegg U. T., Corley L., Anfinsen C. B. Apparent dispensability of the carbohydrate moiety of human interferon for antiviral activity. J Biol Chem. 1976 Mar 25;251(6):1659–1662. [PubMed] [Google Scholar]
- Bridgen P. J., Anfinsen C. B., Corley L., Bose S., Zoon K. C., Rüegg U. T., Buckler C. E. Human lymphoblastoid interferon. Large scale production and partial purification. J Biol Chem. 1977 Oct 10;252(19):6585–6587. [PubMed] [Google Scholar]
- Bruton C. J., Hartley B. S. Chemical studies on methionyl-tRNA synthetase from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):165–178. doi: 10.1016/0022-2836(70)90023-9. [DOI] [PubMed] [Google Scholar]
- Chadha K. C., Grob P. M., Hamill R. L., Sulkowski E. Glycosylation of human leukocyte interferon: effects of tunicamycin. Arch Virol. 1980;64(2):109–117. doi: 10.1007/BF01318014. [DOI] [PubMed] [Google Scholar]
- Chadha K. C., Sclair M., Sulkowski E., Carter W. A. Molecular size heterogeneity of human leukocyte interferon. Biochemistry. 1978 Jan 10;17(1):196–200. doi: 10.1021/bi00594a029. [DOI] [PubMed] [Google Scholar]
- Chen J. K., Jankowski W. J., O'Malley J. A., Sulkowski E., Carter W. A. Nature of the molecular heterogeneity of human leukocyte interferon. J Virol. 1976 Aug;19(2):425–434. doi: 10.1128/jvi.19.2.425-434.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofinis G. J. Biological characteristics of cell line GL-V3 derived from the kidney of a vervet monkey (Cercopithecus aethiops). J Med Microbiol. 1970 May;3(2):251–258. doi: 10.1099/00222615-3-2-251. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980 Dec 4;288(5790):499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- Desmyter J., Stewart W. E., 2nd Molecular modification of interferon: attainment of human interferon in a conformation active on cat cells but inactive on human cells. Virology. 1976 Apr;70(2):451–458. doi: 10.1016/0042-6822(76)90286-5. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fantes K. H., Allen G. Specific activity of pure human interferons and a non-biological method for estimating the purity of highly purified interferon preparations. J Interferon Res. 1981;1(4):465–471. doi: 10.1089/jir.1981.1.465. [DOI] [PubMed] [Google Scholar]
- Fuse A., Kuwata T. Effect of cholera toxin on the antiviral and anticellular activities of human leukocyte interferon. Infect Immun. 1979 Oct;26(1):235–239. doi: 10.1128/iai.26.1.235-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeddel D. V., Leung D. W., Dull T. J., Gross M., Lawn R. M., McCandliss R., Seeburg P. H., Ullrich A., Yelverton E., Gray P. W. The structure of eight distinct cloned human leukocyte interferon cDNAs. Nature. 1981 Mar 5;290(5801):20–26. doi: 10.1038/290020a0. [DOI] [PubMed] [Google Scholar]
- Grob P. M., Chadha K. C. Separation of human leukocyte interferon components by concanavalin A-agarose affinity chromatography and their characterization. Biochemistry. 1979 Dec 25;18(26):5782–5786. doi: 10.1021/bi00593a006. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Yip Y. K., Vilcek J. Correlation of physicochemical and antigenic properties of human leukocyte interferon subspecies. Arch Virol. 1977;55(1-2):121–129. doi: 10.1007/BF01314485. [DOI] [PubMed] [Google Scholar]
- Heiland I., Brauer D., Wittmann-Liebold B. Primary structure of protein L10 from the large subunit of Escherichia coli ribosomes. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(12):1751–1770. doi: 10.1515/bchm2.1976.357.2.1751. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr Heterogeneity of purified mouse interferons. J Biol Chem. 1975 Jun 10;250(11):4139–4144. [PubMed] [Google Scholar]
- Knight E., Jr Interferon: purification and initial characterization from human diploid cells. Proc Natl Acad Sci U S A. 1976 Feb;73(2):520–523. doi: 10.1073/pnas.73.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosky A., Markel D. E., Peterson J. W. Covalent structure of the beta chain of cholera enterotoxin. J Biol Chem. 1977 Oct 25;252(20):7257–7264. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. Y. Determination of the primary structure of cholera toxin B subunit. J Biol Chem. 1977 Oct 25;252(20):7249–7256. [PubMed] [Google Scholar]
- Levy W. P., Rubinstein M., Shively J., Del Valle U., Lai C. Y., Moschera J., Brink L., Gerber L., Stein S., Pestka S. Amino acid sequence of a human leukocyte interferon. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6186–6190. doi: 10.1073/pnas.78.10.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantei N., Schwarzstein M., Streuli M., Panem S., Nagata S., Weissmann C. The nucleotide sequence of a cloned human leukocyte interferon cDNA. Gene. 1980 Jun;10(1):1–10. doi: 10.1016/0378-1119(80)90137-7. [DOI] [PubMed] [Google Scholar]
- Mogensen K. E., Cantell K. Human leukocyte interferon: a role for disulphide bonds. J Gen Virol. 1974 Jan;22(1):95–103. doi: 10.1099/0022-1317-22-1-95. [DOI] [PubMed] [Google Scholar]
- Mogensen K. E., Pyhälä L., Törmä E., Cantell K. No evidence for a carbohydrate moiety affecting the clearance of circulating human leukocyte interferon in rabbits. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):305–310. doi: 10.1111/j.1699-0463.1974.tb02331.x. [DOI] [PubMed] [Google Scholar]
- Moore G. E., Gerner R. E., Franklin H. A. Culture of normal human leukocytes. JAMA. 1967 Feb 20;199(8):519–524. [PubMed] [Google Scholar]
- Nagata S., Mantei N., Weissmann C. The structure of one of the eight or more distinct chromosomal genes for human interferon-alpha. Nature. 1980 Oct 2;287(5781):401–408. doi: 10.1038/287401a0. [DOI] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Paucker K., Dalton B. J., Törmä E. T., Ogburn C. A. Biological properties of human leukocyte interferon components. J Gen Virol. 1977 May;35(2):341–351. doi: 10.1099/0022-1317-35-2-341. [DOI] [PubMed] [Google Scholar]
- Roth M., Hampaï A. Column chromatography of amino acids with fluorescence detection. J Chromatogr. 1973 Aug 29;83:353–356. doi: 10.1016/s0021-9673(00)97051-1. [DOI] [PubMed] [Google Scholar]
- Rubinstein M., Levy W. P., Moschera J. A., Lai C. Y., Hershberg R. D., Bartlett R. T., Pestka S. Human leukocyte interferon: isolation and characterization of several molecular forms. Arch Biochem Biophys. 1981 Aug;210(1):307–318. doi: 10.1016/0003-9861(81)90194-6. [DOI] [PubMed] [Google Scholar]
- Rubinstein M., Rubinstein S., Familletti P. C., Miller R. S., Waldman A. A., Pestka S. Human leukocyte interferon: production, purification to homogeneity, and initial characterization. Proc Natl Acad Sci U S A. 1979 Feb;76(2):640–644. doi: 10.1073/pnas.76.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit M. G., Ogburn C. A. Human leukocyte interferon: separation of biologically different species by modification of carbohydrate moieties. Arch Virol. 1980;63(2):133–142. doi: 10.1007/BF01320770. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, De Clercq E., De Somer P. Stabilisation of interferons by defensive reversible denaturation. Nature. 1974 May 31;249(456):460–461. doi: 10.1038/249460a0. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Desmyter J. Molecular heterogeneity of human leukocyte interferon: two populations differing in molecular weights, requirements for renaturation, and cross-species antiviral activity. Virology. 1975 Sep;67(1):68–73. doi: 10.1016/0042-6822(75)90403-1. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Lin L. S., Wiranowska-Stewart M., Cantell K. Elimination of size and charge heterogeneities of human leukocyte interferons by chemical cleavage. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4200–4204. doi: 10.1073/pnas.74.10.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Wiranowska-Stewart M., Koistinen V., Cantell K. Effect of glycosylation inhibitors on the production and properties of human leukocyte interferon. Virology. 1979 Sep;97(2):473–476. doi: 10.1016/0042-6822(79)90359-3. [DOI] [PubMed] [Google Scholar]
- Streuli M., Nagata S., Weissmann C. At least three human type alpha interferons: structure of alpha 2. Science. 1980 Sep 19;209(4463):1343–1347. doi: 10.1126/science.6158094. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Barakat F., Berthold W., Smith-Johannsen H., Tan C. The isolation and amino acid/sugar composition of human fibroblastoid interferon. J Biol Chem. 1979 Aug 25;254(16):8067–8073. [PubMed] [Google Scholar]
- Taniguchi T., Mantei N., Schwarzstein M., Nagata S., Muramatsu M., Weissmann C. Human leukocyte and fibroblast interferons are structurally related. Nature. 1980 Jun 19;285(5766):547–549. doi: 10.1038/285547a0. [DOI] [PubMed] [Google Scholar]
- Thang M. N., Thang D. C., Chelbi-Alix M. K., Robert-Galliot B., Commoy-Chevalier M. J., Chany C. Human leukocyte interferon: relationship between molecular structure and species specificity. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3717–3721. doi: 10.1073/pnas.76.8.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore A., Anséhn S., Lundin A., Bergman S. Detection of bacteriuria by luciferase assay of adenosine triphosphate. J Clin Microbiol. 1975 Jan;1(1):1–8. doi: 10.1128/jcm.1.1.1-8.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törmä E. T., Paucker K. Purification and characterization of human leukocyte interferon components. J Biol Chem. 1976 Aug 25;251(16):4810–4816. [PubMed] [Google Scholar]
- Wetzel R. Assignment of the disulphide bonds of leukocyte interferon. Nature. 1981 Feb 12;289(5798):606–607. doi: 10.1038/289606a0. [DOI] [PubMed] [Google Scholar]
- Zoon K. C., Smith M. E., Bridgen P. J., Anfinsen C. B., Hunkapiller M. W., Hood L. E. Amino terminal sequence of the major component of human lymphoblastoid interferon. Science. 1980 Feb 1;207(4430):527–528. doi: 10.1126/science.7352260. [DOI] [PubMed] [Google Scholar]
- Zoon K. C., Smith M. E., Bridgen P. J., zur Nedden D., Anfinsen C. B. Purification and partial characterization of human lymphoblast interferon. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5601–5605. doi: 10.1073/pnas.76.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong W. W., Zweers A., Cohen L. H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem Biophys Res Commun. 1978 May 30;82(2):532–539. doi: 10.1016/0006-291x(78)90907-5. [DOI] [PubMed] [Google Scholar]




