Abstract
Study Design
A retrospective cohort study.
Purpose
To examine the effect of cannabis use history on postoperative opioid utilization in patients undergoing one- to three-level lumbar fusion for degenerative spine disease.
Overview of Literature
Strategies to minimize dosing and chronic opioid use are needed for spine surgery given their widespread prescription for postsurgical pain management.
Methods
In this database study, medical coding was used to identify patients who had undergone one- to three-level lumbar fusions between 2012 and 2021. Propensity score matching was used to create two equal cohorts with respect to cannabis use history. Opioid utilization rates (morphine milligram equivalents [MME]/day) and overuse rates at 6 months post-index procedure were assessed. All p-values <0.05 were considered statistically significant.
Results
Following examination of 153,500 patient records, 1,216 patients were matched into cannabis user and non-cannabis user cohorts. Cannabis users had lower rates of opioid utilization compared to non-cannabis users as early as 2 months after fusion (47.7% vs. 41.1%, p<0.05), a relationship which persisted at 6 months (46.2% vs. 37.7%, p<0.01). Additionally, cannabis users had lower rates of high-dose opioid utilization (≥100 MME per day) during the initial 14–30 days following surgery (6.91% vs. 3.79%, p<0.05).
Conclusions
Patients with a history of cannabis use were less likely to be using opioids as early as 2 months postoperatively and had lower rates of high-dose opioid utilization in the immediate postoperative period. Physicians operating on these patients should consider their cannabis use patterns to provide appropriate titration of pain medication over time.
Keywords: Cannabis, Lumbar fusion, Opioid, Opioid epidemic, Pain management
Introduction
Opioids are among the most widely prescribed analgesics for managing postoperative pain in the United States [1]. Opioid use is ubiquitous in fields like spine surgery because of highly invasive and painful surgeries. Lumbar fusions, for example, are notorious as prolonged muscle retraction and stripping of attachments from the vertebrae causes painful paraspinal muscle instability [2]. Consequently, despite advances in surgical techniques and perioperative care, spine patients often require opioids for adequate pain management.
The opioid epidemic has raised awareness among both patients and healthcare providers about the risks and adverse effects of opiate prescription. However, there is currently a lack of consensus guidelines on the optimal duration of opioid prescription for patients undergoing lumbar fusion surgery to minimize the potential for abuse. Therefore, surgeons typically rely on factors such as demographics, preoperative substance use history, the type and complexity of surgical procedure (such as the number of levels involved in a spine procedure), and a patient’s risk for overuse to inform their prescription: a decision essentially based on clinical gestalt [3].
Research suggests that supplementation with medical cannabis may be an effective risk mitigation strategy for pain management in lumbar fusion patients. Studies have shown that cannabis can prevent hyperalgesia and minimize unfavorable side effects of opioid use [4,5]. This phenomenon, referred to as the “opioid-sparing effect of cannabinoids,” is attributed to the shared signal transduction pathways between cannabinoids and opioids [6]. Patients who use cannabis in conjunction with opioids report lower opioid usage and improved pain relief compared to those using opioids alone [7,8]. While states that have legalized cannabis have seen a reduction in deaths related to opioid overdose, there is no clear consensus on the effect of cannabis on postoperative opioid utilization. This study sought to evaluate opioid utilization trends in patients with a history of cannabis use to guide the appropriate prescription of opioids during the postoperative period after lumbar fusion surgery.
Materials and Methods
Data source
The MARINER database (PearlDiver Technologies, Colorado Springs, CO, USA) was queried for patients who underwent transforaminal lumbar interbody fusion (TLIF) over one to three spinal levels. This is a commercially available, deidentified database of healthcare encounters and insurance claims billed to all payers from 2010 to 2020. MARINER pools data from several sources including the National Inpatient Sample, Medicare, and private insurance companies, allowing for large longitudinal analyses. The accuracy and completeness of the data are ensured through PearlDiver’s rigorous processes, which include regular audits of claims, internal reviews, and third-party adjudications [9]. Data were extracted using relevant International Classification of Disease (ICD)-9 and 10 codes, and Current Procedural Terminology (CPT) codes.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Illinois at Chicago (UIC) Institutional Review Board and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the UIC Institutional Review Board, with a waiver of patient informed consent because the analysis posed minimal risk to participants.
Study design
Patients who underwent TLIF for degenerative spine disease involving one to three levels from 2012 to 2021 were identified using relevant ICD-9, ICD-10, and CPT codes as previously defined in our work (Supplement 1) [10]. Inclusion criteria were as follows: (1) patients aged between 18 and 85 years; (2) no missing demographic information. Patient demographic characteristics (age, sex, levels of opioid use prior to surgery) and comorbidities (anxiety, depression, and disparities in social determinants of health [SDoH]) were identified using medical billing codes. Comorbidities were assessed based on ICD codes from encounters within the 12 months leading to TLIF [11,12].
Once the patients were identified and categorized into cannabis user and non-cannabis user groups, a 1:1 propensity score match adjusting for age, sex, levels of fusion, comorbidities (anxiety, depression, and disparities in SDoH), and preoperative opioid use within 30 days of surgery was conducted. The variables included for matching were those that showed an independent association with higher rates of opioid use on logistic regression analyses (Supplement 2).
Social determinants of health
The disparities in SDoH were included as a variable for propensity score matching to capture the nuances of patient socioeconomic status and their impact on health. The United States government’s Healthy People 2030 Initiative 8 identifies five key domains of SDoH that contribute to health disparities: (1) economic, which includes employment status, poor occupational environment, housing instability, and financial hardship; (2) education, including early childhood developmental issues and inadequate education and literacy; (3) social context, which includes cultural, race, incarceration, legal, and psychosocial issues; (4) health access, which includes unavailability, inaccessibility, or unspecified problem of healthcare/medical facilities; and (5) physical environment and neighborhood, which includes disaster, lead or mold exposure, and safety (such as assault, sexual, physical, or psychological abuse) [13].
Outcomes
Prescription data was used to measure opioid use (morphine milligram equivalent [MME]/day) at various time points after the index procedure: 14–30 days, 31–60 days, 61–90 days, 0–3 months, and 0–6 months. High opioid utilization was defined as ≥100 MME/day, a previously defined threshold. The Centers for Disease Control and Prevention guidelines for prescribing opioids for chronic pain, published in 2016, identified dosages above 100 MME per day as significantly increasing the risk of opioid overdose by a factor of 2.0 to 8.0, compared to dosages below 20 MME per day [14].
Statistical analysis
Mean (±standard deviation) values for age, sex, and comorbidities were compared between cannabis and non-cannabis users. Linear regression analysis was performed to identify variables independently associated with high opioid utilization; these variables were included as confounding variables to match cannabis and non-cannabis user cohorts in a 1:1 ratio. Between-group differences with respect to categorical and continuous variables were assessed for statistical significance using the chi-square test and Student t-test, respectively. All p-values <0.05 were considered indicative of statistical significance. All statistical analyses were performed using R ver. 4.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
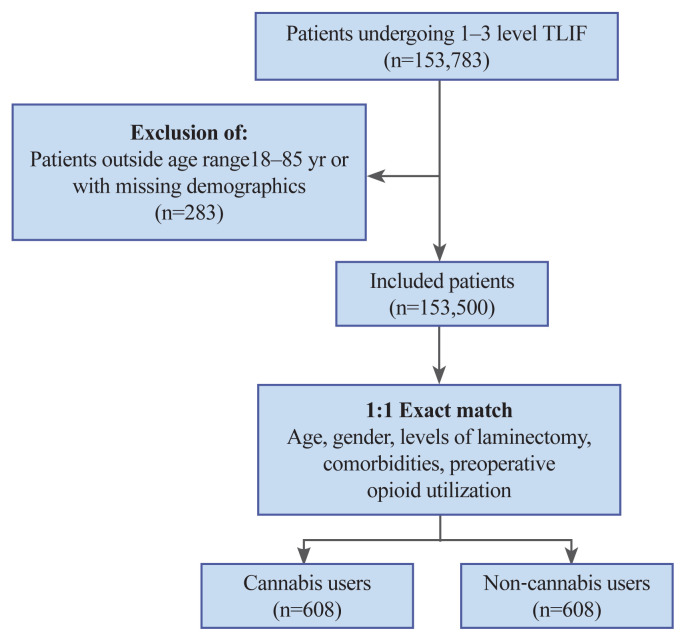
A total of 153,783 patients were identified to have undergone one- to three-level TLIF. Of these, 283 were excluded because they did not meet the required age criteria or had a history of opioid use within the 6 months leading to surgery (Fig. 1).
Fig. 1.
Patient selection procedure. TLIF, transforaminal lumbar interbody fusion.
Following 1:1 propensity matching to adjust for preoperative opioid utilization, demographics, comorbidities, and disparities in SDoH, 1,216 patients (51% male) were divided into two groups of 608 each: cannabis users and non-cannabis users. Patients aged 55–59 were the most common in our cohort (18.59%). The percentage of patients with comorbid anxiety and depression was 58.23% (n=354) and 57.07% (n=347), respectively. Single-level fusion was the most common surgical procedure in this cohort. Cannabis and non-cannabis users were also matched according to their history of opioid utilization prior to surgery. The percentage of patients with disparities in SDoH in the cannabis user group (39.64%, n=241) was significantly higher than that in the non-cannabis user group (26.32%, n=160). Demographic characteristics of the unmatched population and matched populations are summarized in Tables 1 and 2, respectively.
Table 1.
Descriptive characteristics of unmatched patients (n=153,500)
| Characteristic | Cannabis users (n=642) | Cannabis non-users (n=152,858) | p-value |
|---|---|---|---|
| Age (yr) | <0.001 | ||
| 15–19 | 0 | 128 (0.08) | |
| 20–24 | 0 | 572 (0.37) | |
| 25–29 | 20 (3.23) | 1,341 (0.88) | |
| 30–34 | 39 (6.30) | 3,237 (2.12) | |
| 35–39 | 59 (9.53) | 5,438 (3.56) | |
| 40–44 | 72 (11.63) | 8,108 (5.30) | |
| 45–49 | 94 (15.19) | 11,655 (7.62) | |
| 50–54 | 101 (16.32) | 16,248 (10.63) | |
| 55–59 | 114 (18.42) | 20,846 (13.64) | |
| 60–64 | 77 (12.44) | 23,128 (15.13) | |
| 65–69 | 43 (6.95) | 23,302 (15.24) | |
| 70–74 | 0 | 21,975 (14.38) | |
| 75–79 | 0 | 14,627 (9.57) | |
| 80–85 | 0 | 2,253 (1.47) | |
| Gender | <0.001 | ||
| Female | 312 (48.60) | 88,430 (57.85) | <0.001 |
| Male | 330 (51.40) | 64,428 (42.15) | |
| Comorbidity | |||
| Depression | 375 (58.41) | 25,936 (16.97) | <0.001 |
| Anxiety | 378 (58.88) | 22,778 (14.90) | <0.001 |
| SDoH disparity | 253 (39.41) | 19,810 (12.96) | <0.001 |
| Levels of fusion | |||
| Single | 425 (66.20) | 104,028 (68.06) | 0.34 |
| Two | 101 (15.73) | 20,288 (13.27) | 0.08 |
| Three | 116 (18.07) | 28,542 (18.67) | 0.73 |
| Insurance type | <0.001 | ||
| Cash | 0 | 118 (0.08) | |
| Commercial | 369 (63.84) | 101,922 (66.68) | |
| Government | 0 | 3,615 (2.36) | |
| Medicaid | 131 (22.66) | 6,613 (4.33) | |
| Medicare | 78 (13.49) | 39,326 (25.73) | |
| Unknown | 0 | 1,264 (0.83) | |
| Preoperative opioid usea) | |||
| Opioid naïve | 315 (49.07) | 97,307 (63.66) | <0.001 |
| ≤34 MME | 13 (2.02) | 2,021 (1.32) | 0.17 |
| 35–74 MME | 12 (1.87) | 2,014 (1.32) | 0.29 |
| 75–89 MME | 0 | 662 (0.43) | 0.67 |
| 90–100 MME | 0 | 810 (0.53) | 0.26 |
| ≥100 MME | 0 | 595 (0.39) | >0.999 |
Values are presented as number (%).
SDoH, social determinants of health; MME, morphine milliequivalents.
Preoperative opioid use within 30 days of surgery.
Table 2.
Demographics of the matched cohorts
| Variable | Total (N=1,216) | Non-cannabis users (N=608) | Cannabis users (N=608) | p-value |
|---|---|---|---|---|
| Age group (yr) | >0.99 | |||
| 25–29 | 32 (2.63) | 16 (2.63) | 16 (2.63) | |
| 30–34 | 66 (5.43) | 33 (5.43) | 33 (5.43) | |
| 35–39 | 114 (9.38) | 57 (9.38) | 57 (9.38) | |
| 40–44 | 140 (11.51) | 70 (11.51) | 70 (11.51) | |
| 45–49 | 180 (14.80) | 90 (14.80) | 90 (14.80) | |
| 50–54 | 200 (16.45) | 100 (16.45) | 100 (16.45) | |
| 55–59 | 226 (18.59) | 113 (18.59) | 113 (18.59) | |
| 60–64 | 148 (12.17) | 74 (12.17) | 74 (12.17) | |
| 65–69 | 82 (6.74) | 41 (6.74) | 41 (6.74) | |
| 70–85 | 28 (2.30) | 14 (2.30) | 14 (2.30) | |
| Gender | >0.99 | |||
| Female | 600 (49.00) | 300 (49.00) | 300 (49.00) | |
| Male | 616 (51.00) | 308 (51.00) | 308 (51.00) | |
| Comorbidity | ||||
| Depression | 694 (57.07) | 347 (57.07) | 347 (57.07) | >0.99 |
| Anxiety | 708 (58.23) | 354 (58.23) | 354 (58.23) | >0.99 |
| SDoH | 401 (32.98) | 160 (26.32) | 241 (39.64) | <0.01 |
| Levels of fusion | >0.99 | |||
| Single | 802 (65.95) | 401 (65.95) | 401 (65.95) | |
| Two | 194 (15.96) | 97 (15.96) | 97 (15.96) | |
| Three | 220 (18.10) | 110 (18.10) | 110 (18.10) | |
| Preoperative opioid usea) | ||||
| Opioid-naïve | 710 (50.16) | 305 (50.16) | 305 (50.16) | >0.99 |
| ≤34 MME | 0 | 0 | 0 | >0.99 |
| 35–74 MME | 2 (1.64) | 1 (1.64) | 1 (1.64) | >0.99 |
| 75–89 MME | 0 | 0 | 0 | >0.99 |
| 90–100 MME | 0 | 0 | 0 | >0.99 |
| ≥100 MME | 0 | 0 | 0 | >0.99 |
Values are presented as number (%).
SDoH, social determinants of health; MME, morphine milliequivalents.
Preoperative opioid use within 30 days of surgery.
Opioid utilization following surgery
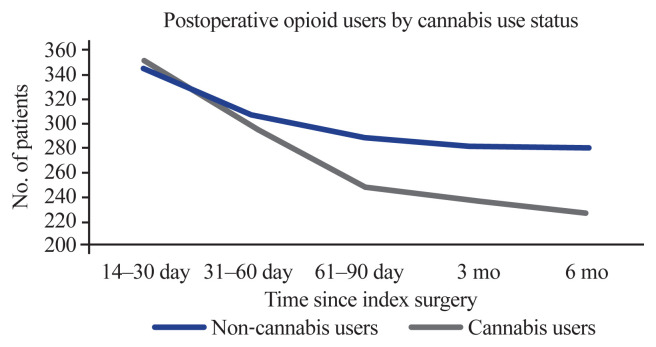
Opioid utilization (in MME/day) was measured at five different intervals ranging from 2 weeks to 6 months following the index procedure. The mean utilization among cannabis users and non-cannabis users increased over time (Table 3). Cannabis users utilized lower doses in comparison to their counterparts, although the difference was not statistically significant. Over time, the number of patients who continued to use opioids decreased in both cohorts, with a lower percentage of cannabis users using opioids in comparison to non-cannabis users (Fig. 2).
Table 3.
Mean and standard deviation of opioid utilization (measured in MME/day) among cannabis users and non-cannabis users
| Time period | Non-cannabis users (n=608) | Cannabis users (n=608) | p-value |
|---|---|---|---|
| MME (/day) | |||
| 14–30 day | 111.98±129.3 | 96.41±98.4 | 0.075 |
| 31–60 day | 123.69±131.4 | 118.04±137.9 | 0.607 |
| 61–90 day | 143.99±157.5 | 133.04±157.9 | 0.423 |
| 3 mo | 168.97±183.9 | 140.51±154.9 | 0.06 |
| 6 mo | 183.39±202.4 | 154.55±175.3 | 0.092 |
| Opioid users | |||
| 14–30 day | 344 (56.6) | 351 (57.7) | 0.72 |
| 31–60 day | 307 (50.5) | 297 (48.9) | 0.6 |
| 61–90 day | 290 (47.7) | 250 (41.1) | <0.05* |
| 3 mo | 282 (46.4) | 237 (39.0) | <0.05* |
| 6 mo | 281 (46.2) | 229 (37.7) | <0.01* |
Values are presented as mean±standard deviation or number (%).
MME, morphine milliequivalents.
p<0.05.
Fig. 2.
Opioid users stratified by cannabis use.
High opioid utilization
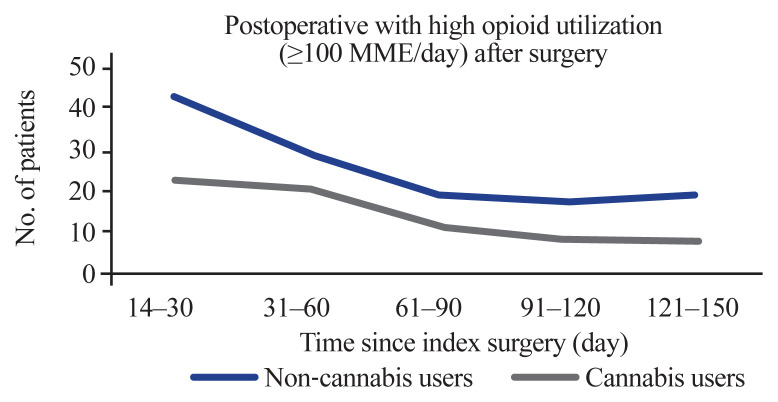
The number of patients with high opioid utilization (≥100 MME/day) decreased over time in both cohorts (Fig. 3). Lower rates of high-dose opioid utilization were found in the cannabis user cohort during the initial period of 14–30 days following surgery (6.91% versus 3.79%, p<0.05). This difference also approached statistical significance 121–150 days after surgery (3.13% versus 1.32%, p=0.051) (Table 4).
Fig. 3.
Patients with high opioid utilization defined as greater than or equal to 100 morphine milliequivalents (MME)/day after surgery.
Table 4.
Temporal trends in high opioid utilization (>100 MME/day) in users and non-users
| Time period (day) | Total | Non-cannabis users (n=608) | Cannabis users (n=608) | p-value |
|---|---|---|---|---|
| 14–30 | 65 (5.35) | 42 (6.91) | 23 (3.79) | <0.05 |
| 31–60 | 49 (8.04) | 29 (4.76) | 20 (3.29) | 0.24 |
| 61–90 | 31 (2.55) | 19 (13.13) | 12 (1.98) | 0.27 |
| 91–120 | 25 (2.06) | 17 (2.80) | 8 (1.32) | 0.10 |
| 121–150 | 27 (2.23) | 19 (3.13) | 8 (1.32) | 0.051 |
Values are presented as number (%).
MME, morphine milliequivalents.
Discussion
The devastating consequences of the opioid epidemic have become increasingly clear as our understanding of it continues to evolve. Overdose deaths began rising in 1999, and by 2020, the total number of fatalities had approached one million [15,16]. Despite their risks, opioids remain a critical tool in the modern pain management arsenal. In the field of spine surgery, opioid use is especially prevalent as they facilitate early mobility and restore quality of life postoperatively [17].
This study analyzed temporal trends in opioid utilization among matched cohorts of cannabis and non-cannabis users after one- to three-level TLIF for degenerative spine disease. We found lower rates of opioid utilization among cannabis users in comparison to non-cannabis users as early as 2 months after TLIF (p<0.05), a phenomenon that persisted at 6 months as well (p<0.01). In addition, the cannabis user cohort had lower rates of high-dose opioid utilization (≥100 MME per day) during the initial period of 14–30 days following surgery (p<0.05). This relationship also approached significance 121–150 days after surgery (p=0.051).
Consistent with these results, numerous preclinical studies have suggested an “opioid-sparing” effect of cannabinoids, wherein the synergistic effects of cannabis and opioids enable the use of lower opioid doses to achieve comparable analgesic effect [18]. The clinical translation of this relationship has not been well-established in part due to heterogeneity in study populations, limited sample sizes, variations in endpoints, and differences in cannabinoid types [6,19].
A few studies published within the last 2 years have examined the relationship between cannabis use and opioid utilization following spine surgery. First, our previous work examined postoperative opioid utilization among 454 cannabis users and nonusers following single-level lumbar fusion in a matched analysis. The opioid utilization rates in opioid-naïve cannabis users were comparable to those in nonusers, but cannabis users utilized a smaller daily dosage when compared to nonusers [10]. Moon et al. [20] retrospectively analyzed 301 patients who underwent one or two-level posterior spinal fusion and found that cannabis users had increased postoperative opioid usage. However, their sample size was small with only 42 cannabis users. Furthermore, they did not perform propensity score matching to adjust for the confounding influence of age and psychiatric comorbidities. D’Antonio et al. [21] performed a propensity score-matched analysis to investigate the effect of marijuana use on lumbar fusion outcomes and found no association between marijuana use and opioid utilization. A similar study by Lambrechts et al. [22] used a 3:1 propensity-matched cohort to investigate the effect of preoperative marijuana use on the number of perioperative opioid prescriptions in patients undergoing one- to four-level anterior cervical decompression and fusion (ACDF). While preoperative marijuana was found to increase the risk of reoperation, fewer cannabis users were using opioids at 1 year postoperatively in their cohort, although this did not quite reach clinical significance (p=0.050) [22]. Razzouk et al. [23] retrospectively examined the association between preoperative cannabis use and postoperative opioid use following ACDF in a cohort of 198 patients. They found that a history of cannabis was associated with an increased likelihood of using opioids at the 1-year postoperative mark. However, this study had a very small population of cannabis users (24 out of 198) and did not adjust for any known confounders of cannabis and opioid use [23]. readmission, or reoperation rates following anterior cervical discectomy and fusion (ACDFLastly, in a recent large (n=2,678), retrospective propensity-matched study by Silver et al. [24], patients who had a previous history of cannabis use were found to have filled fewer prescriptions 3 days postoperatively (p<0.001) and required lower doses of MME for pain control at 60 days (p=0.018) when compared to the control group.
Thus, the existing literature on opioid prescription and cannabis use in spine surgery is limited and controversial, making it challenging to establish consensus on best practices. In contrast to the aforementioned studies, our study has several strengths. We adjusted for confounders including comorbidities, disparities in SDoH, and levels of fusion based on results from the literature [12,25]. Additionally, our sample size is greater than any other previous study. Third, this study provides the finest temporal granularity to date, with comparisons of opioid usage at 5-time points over 6 months. Fourth, this is the first study to investigate the relationship between cannabis use and high opioid utilization (defined as >100 MME/day) over time in this patient population.
We found that cannabis users tended to wean off opioid analgesics earlier than non-cannabis users postoperatively, offering support for the opioid-sparing effect of cannabis theory. However, more robust studies are required to carefully examine the side effects and risks of marijuana use before recommending adjunctive cannabis prescriptions. A point in favor of cannabis in this study was the decreased rates of high opioid utilization in the immediate postoperative period which again approached significance at the 6-month postoperative mark. The dose-dependent risks of opioid use have been well-studied, and marijuana may prove to be a useful tool in decreasing this critical factor for opioid abuse [14]. When prescribing opioids for pain control after spine surgery, physicians should account for cannabis use and minimize prescription through a shared decision-making approach with each patient.
Some limitations of this study should be considered while interpreting the results. First, our study employed propensity score matching to achieve improved covariate balance, but this technique has limitations. As the number of covariates increases, the sample size decreases, since patients with matching characteristics become less common. In our analysis, this resulted in a sample comprising only 1% of eligible patients. However, using a large dataset helped mitigate this issue, allowing us to match over 1,216 patients and maintain sufficient statistical power while focusing on a specific research question. Second, all clinical information from this database including diagnoses, demographics, and comorbidities were identified through medical coding and were thus subject to misclassification errors. Third, cannabis use was measured on a self-reporting basis. The variable legal and social status of cannabis across the United States may have led patients to underreport their usage. However, because cannabis use status in a clinical setting is most often determined on a self-reported basis, our results likely apply to most postoperative patients who are known cannabis users. Fourth, since we measured opioid use with prescription data, any opioids obtained without a prescription would not be included in our dataset. Finally, there were significant differences in our matched cohort regarding disparities of SDoH. Cannabis users were more likely to experience SDoH disparities than non-cannabis users (39.62% versus 26.32%, p<0.01). Nonetheless, our findings were significant even after adjusting for SDoH. Finally, neither cannabis quantity nor type was quantified, precluding the investigation of a dose-dependent opioid-sparing effect. It is still unclear whether this effect is homogenous between heavy and light cannabis users. To better tailor postoperative pain regimens to each patient, future research should attempt to delineate the differences in opioid usage in heavy and light cannabis users.
Conclusions
Effective pain management with opioids is crucial for restoring quality of life after a TLIF procedure. In this retrospective propensity-matched study, patients with a history of cannabis use were found less likely to rely on opioids just 2 months after surgery and had lower rates of high-dose opioid use in the immediate postoperative period. Knowledge of cannabis use patterns may potentially allow physicians to cater to individual needs and provide appropriate dose titration over time. This may help reduce opioid dependence and improve patient outcomes.
Key Points.
Patients with a history of cannabis use were found less likely to be using opioids as early as 2 months postoperatively.
Patients with a history of cannabis use had lower rates of high-dose opioid utilization postoperatively.
Physicians must be aware of their patient’s canna-bis use patterns given the implications for opioid utilization.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: PM, SK, OA. Data curation: PM, SK. Formal analysis: PM, SK. Funding acquisition: SK, OA. Methodology: PM, SK, PK, JR. Project administration: SK, AM, OA. Visualization: PM. Writing–original draft: PM, SK, PK, JR. Writing–review & edit: AE, AM, OA. Final approval: all authors.
Supplementary Materials
Supplementary materials can be available from https://doi.org/10.31616/2024.0194
Supplement 1. ICD-9 & 10 codes and CPT codes used to identify patient population, comorbidities, and outcomes.
Supplement 2. Logistic regression model assessing factor-association with odds of high opioid utilization at 6 months following surgery.
References
- 1.Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33:383–91. doi: 10.1002/phar.1223. [DOI] [PubMed] [Google Scholar]
- 2.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1:2–18. doi: 10.3978/j.issn.2414-469X.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–65. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844–51. doi: 10.1038/clpt.2011.188. [DOI] [PubMed] [Google Scholar]
- 5.Lucas P. Cannabis as an adjunct to or substitute for opiates in the treatment of chronic pain. J Psychoactive Drugs. 2012;44:125–33. doi: 10.1080/02791072.2012.684624. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S, Sabioni P, Trigo JM, et al. Opioid-sparing effect of cannabinoids: a systematic review and meta-analysis. Neuropsychopharmacology. 2017;42:1752–65. doi: 10.1038/npp.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degenhardt L, Lintzeris N, Campbell G, et al. Experience of adjunctive cannabis use for chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend. 2015;147:144–50. doi: 10.1016/j.drugalcdep.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Kral AH, Wenger L, Novak SP, et al. Is cannabis use associated with less opioid use among people who inject drugs? Drug Alcohol Depend. 2015;153:236–41. doi: 10.1016/j.drugalcdep.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolognesi MP, Habermann EB. Commercial claims data sources: PearlDiver and individual payer databases. J Bone Joint Surg Am. 2022;104(Suppl 3):15–7. doi: 10.2106/JBJS.22.00607. [DOI] [PubMed] [Google Scholar]
- 10.Khalid SI, Jiang S, Khilwani H, Thomson K, Mirpuri P, Mehta AI. Postoperative opioid use among opioid-naive cannabis users following single-level lumbar fusions. World Neurosurg. 2023;175:e644–52. doi: 10.1016/j.wneu.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Khalid SI, Maasarani S, Shanker RM, Becerra AZ, Omotosho P, Torquati A. Social determinants of health and their impact on rates of postoperative complications among patients undergoing vertical sleeve gastrectomy. Surgery. 2022;171:447–52. doi: 10.1016/j.surg.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Khalid SI, Maasarani S, Nunna RS, et al. Association between social determinants of health and postoperative outcomes in patients undergoing single-level lumbar fusions: a matched analysis. Spine (Phila Pa 1976) 2021;46:E559–65. doi: 10.1097/BRS.0000000000003829. [DOI] [PubMed] [Google Scholar]
- 13.Healthy People 2030 Social Determinants of Health [Internet] Washington (DC): U.S. Department of Health and Human Services [date unknown]; [cited 2024 Aug 4]. Available from: https://health.gov/healthypeople/priority-areas/social-determinants-health. [Google Scholar]
- 14.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain: United States, 2016. JAMA. 2016;315:1624–45. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention . Understanding the opioid overdose epidemic [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2022. [cited 2024 May 19]. Available from: https://stacks.cdc.gov/view/cdc/119116/cdc_119116_DS1.pdf. [Google Scholar]
- 16.Hedegaard H, Miniño AM, Spencer MR, Warner M. Drug overdose deaths in the United States, 1999–2020. NCHS Data Brief. 2021;428:1–8. [PubMed] [Google Scholar]
- 17.Chakravarthy V, Yokoi H, Manlapaz MR, Krishnaney AA. Enhanced recovery in spine surgery and perioperative pain management. Neurosurg Clin N Am. 2020;31:81–91. doi: 10.1016/j.nec.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Babalonis S, Walsh SL. Therapeutic potential of opioid/cannabinoid combinations in humans: review of the evidence. Eur Neuropsychopharmacol. 2020;36:206–16. doi: 10.1016/j.euroneuro.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noori A, Miroshnychenko A, Shergill Y, et al. Opioid-sparing effects of medical cannabis or cannabinoids for chronic pain: a systematic review and meta-analysis of randomised and observational studies. BMJ Open. 2021;11:e047717. doi: 10.1136/bmjopen-2020-047717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon AS, LeRoy TE, Yacoubian V, Gedman M, Aidlen JP, Rogerson A. Cannabis use is associated with increased use of prescription opioids following posterior lumbar spinal fusion surgery. Global Spine J. 2024;14:204–10. doi: 10.1177/21925682221099857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Antonio ND, Lambrechts MJ, Heard JC, et al. The effect of preoperative marijuana use on surgical outcomes, patient-reported outcomes, and opioid consumption following lumbar fusion. Global Spine J. 2024;14:568–76. doi: 10.1177/21925682221116819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambrechts MJ, D’Antonio ND, Toci GR, et al. Marijuana use and its effect on clinical outcomes and revision rates in patients undergoing anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2022;47:1558–66. doi: 10.1097/BRS.0000000000004431. [DOI] [PubMed] [Google Scholar]
- 23.Razzouk J, Chung JH, Lindsey W, Ramos O, Cheng W, Danisa O. Preoperative cannabis use associated with an increased rate of reoperation and postoperative opioid use following anterior cervical decompression and fusion. Cureus. 2022;14:e31285. doi: 10.7759/cureus.31285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silver J, Pavano C, Bellas N, et al. Cannabis use is associated with decreased opioid prescription fulfillment following single level anterior cervical discectomy and fusion (ACDF) N Am Spine Soc J. 2023;14:100226. doi: 10.1016/j.xnsj.2023.100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menendez ME, Neuhaus V, Bot AG, Ring D, Cha TD. Psychiatric disorders and major spine surgery: epidemiology and perioperative outcomes. Spine (Phila Pa 1976) 2014;39:E111–22. doi: 10.1097/BRS.0000000000000064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. ICD-9 & 10 codes and CPT codes used to identify patient population, comorbidities, and outcomes.
Supplement 2. Logistic regression model assessing factor-association with odds of high opioid utilization at 6 months following surgery.