Abstract
Background
Calcified nodule (CN) is a phenotypic feature of calcified plaques which causes acute coronary syndrome (ACS). Recent studies reported that culprit lesions harboring CN has been shown to increase a risk of repeat revascularization after percutaneous coronary intervention (PCI) with the implantation of newer-generation drug-eluting stent (DES) or debulking device. Mechanistically, a re-protrusion of CN into the lumen has been considered as an important cause associated with repeat revascularization after PCI. These observations suggest the need for additional therapeutic approach to mitigate a risk of repeat revascularization at CN lesions. Here we report a case who received the implantation of one covered stent due to coronary artery perforation after stent implantation at coronary lesion exhibiting CN. This case is unique in terms of preventing restenosis by using covered stent which could physically hinder protrusion of CN through the stent strut.
Case Description
A 79-year-old man presented to the emergency department with his prolonged chest pain. Although he had a history of hypertension and adrenal hypertrophy, he was not taking any medication prior to his admission. He was diagnosed as ST-segment elevation myocardial infarction. Emergent coronary angiography revealed one severe stenosis in the middle segment of his right coronary artery (RCA). Primary PCI was performed under the guidance of intravascular ultrasound (IVUS) imaging. IVUS imaging prior to PCI revealed a protruding shape of calcification and its irregular surface at his culprit lesion, suggesting the presence of a CN. Following one DES implantation, coronary artery perforation occurred at the segment receiving DES implantation. We implanted one covered stent for the coronary artery perforation. This procedure resulted in successfully sealing coronary artery perforation. Seven months later, follow-up coronary angiography and optical coherence tomography (OCT) imaging were conducted to evaluate his RCA. Any in-stent restenosis (ISR) was not observed. Furthermore, OCT imaging elucidated a small amount of neointimal proliferation without any re-protruding feature of CN through the segment receiving a covered stent. Of note, he did not experience any clinically-driven target lesion revascularization (TLR) for 2 years after PCI.
Conclusions
Our case indicates the use of covered stent as an effective approach to physically hinder the re-protrusion of calcification tissues into the lumen, potentially mitigating a risk of ISR.
Keywords: Calcified nodule (CN), covered stent, acute coronary syndrome (ACS), in-stent restenosis (ISR), case report
Highlight box.
Key findings
• In our case, coronary artery perforation occurred after drug-eluting stent (DES) implantation at one calcified nodule (CN) lesion in a patient presenting acute coronary syndrome. The implantation of one covered stent enabled to seal coronary artery perforation. Seven months later, follow-up coronary angiography and optical coherence tomography (OCT) imaging did not identify any in-stent restenosis (ISR). In addition, re-protrusion of CN was not observed.
What is known and what is new?
• Recent studies reported that culprit lesions harboring CN has been shown to increase a risk of repeat revascularization after percutaneous coronary intervention (PCI) with the implantation of newer-generation DES or debulking device. Mechanistically, a re-protrusion of CN into the lumen has been considered as an important cause associated with repeat revascularization after PCI.
• In our case, following the use of covered stent, any ISR did not occur at one CN lesion receiving DES implantation. OCT imaging did not show any re-protrusion of CN 7 months after PCI.
What is the implication, and what should change now?
• Our case indicates the use of covered stent as an effective approach to physically hinder the re-protrusion of calcification tissues into the lumen, potentially mitigating a risk of ISR.
Introduction
Background
Calcified nodule (CN) is a phenotypic feature of calcified plaques which cause acute coronary syndrome (ACS). Despite the use of newer-generation drug-eluting stent (DES), ACS patients attributable to CN have been shown to present an increased risk of target lesion revascularization (TLR) after percutaneous coronary intervention (PCI) (Table 1) (1-6). Sugane et al. reported that the presence of CN was associated with a 7.9-fold greater likelihood of experiencing TLR in ACS patients receiving newer-generation DES (3). Of note, the recurrence of ACS due to in-stent restenosis (ISR) at CN lesion more likely occurred within 12 months after the implantation of newer-generation DES.
Table 1. Summary of published papers about the efficacy of PCI in patients with CN.
| Authors | Study subjects | The use of DES | Imaging modality | Outcomes |
|---|---|---|---|---|
| Nakamura et al. 2020 (1) | 29 ISR lesions | 76% | Histopathological analysis | • This study included 8 and 21 ISR lesions receiving directional coronary atherectomy with and without dialysis |
| • Subjects with hemodialysis had significantly higher prevalence of in-stent calcified nodule compared with the non-hemodialysis group (75% vs. 5%, P<0.01) | ||||
| Watanabe et al. 2020 (2) | 204 lesions with CN | 97% | IVUS | • This study included 73 and 131 CN lesions receiving PCI with and without RA, respectively |
| • After propensity-score matching, there was no significant difference in one-year ischemia-driven TVR rate between two groups in before and after propensity-score matching (P=0.82, 0.87, respectively) | ||||
| Sugane et al. 2021 (3) | 657 ACS subjects | 100% | IVUS | • On IVUS imaging analysis, 5.3% of ACS subjects exhibited CN at their culprit lesions |
| • During the observational period (median =1,304 days), CN was associated with a 7.68-, 12.32- and 10.48-fold increased risks of MACE (95% CI: 4.61–12.80, P<0.001), ACS recurrence (95% CI: 6.05–25.11, P<0.001), and TLR (95% CI: 5.80–18.94, P<0.001), respectively | ||||
| Tada et al. 2022 (4) | 651 ISR lesions | 87% | OCT | • 4.9% of ISR lesions had CN |
| • ISR and TLR rates were significantly higher in lesions with CN compared with those without CN (ISR rate: 43.8% vs. 25.0%, P=0.023, TLR rate: 37.5% vs. 18.8%, P=0.020) | ||||
| Takahashi et al. 2022 (5) | 118 lesions with stent thrombosis | 100% | IVUS | • In-stent CN was observed in 13% of analyzed lesions |
| • The cumulative 5-year incidence of TLR was significantly higher in the in-stent CN group compared with that in the non-in-stent CN group (62.7% vs. 21.5%, HR =3.01, 95% CI: 1.16–7.85, P=0.02) | ||||
| Hamana et al. 2023 (6) | 108 patients with CNs | – | OCT | • The 5-year cumulative incidence of TLR was 32.6% |
| • The prevalence of in-stent CNs observed at follow-up OCT was significantly higher in the TLR group than in the non-TLR group |
PCI, percutaneous coronary intervention; CN, calcified nodule; DES, drug-eluting stent; ISR, in-stent restenosis; IVUS, intravascular ultrasound; RA, rotational atherectomy; TVR, target vessel revascularization; ACS, acute coronary syndrome; MACE, major adverse cardiovascular events; CI, confidence interval; TLR, target lesion revascularization; OCT, optical coherence tomography; HR, hazard ratio.
Rationale and knowledge gap
Calcified lesions more likely hinder stent expansion, which worsens cardiovascular outcomes after PCI. Debulking devices have been used to modify calcific tissues for achieving optimal acute gain. One observational study investigated the efficacy of rotational atherectomy on TLR rate in patients exhibiting CN. In this study, modification of CN with rotational atherectomy did not reduce TLR rate compared to PCI without rotational atherectomy (2). Mechanistically, pathological and intravascular imaging studies reported the re-protruding nodules of calcification as a potential mechanism causing ISR after DES implantation (7,8). These observations suggest the need for another therapeutic approach to prevent this dynamic protrusion of calcification tissue through struts of an implanted stent. The covered stent has been developed to seal coronary artery perforation due to PCI procedure. Given that the covered stent is surrounded by circumferential membrane (9), it may be effective to prevent the protruding of CN after stent implantation.
Objective
We report a case who received the implantation of one covered stent due to coronary artery perforation after stent implantation at coronary lesion exhibiting CN. Since this case does not experience any clinically-driven TLR after covered stent implantation, this case report will discuss the potential benefit of covered stent to treat CN by analyzing intravascular ultrasound (IVUS) and optical coherence tomography (OCT) imaging. We present this case in accordance with the CARE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-216/rc).
Case presentation
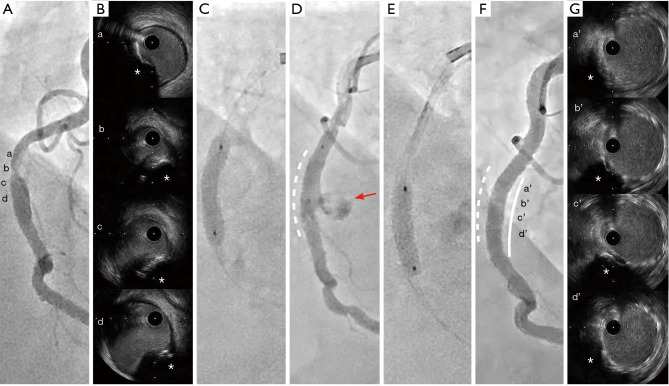
A 79-year-old man presented to the emergency department with his prolonged chest pain. He had a history of hypertension and adrenal hypertrophy, however he did not take any medications prior to his admission. He did not take hemodialysis. His initial blood pressure was 163/127 mmHg and heart rate was 82 beats per minute at the emergency department. There was no remarkable physical examination including cardiac murmurs and respiratory sounds. The electrocardiogram showed atrial fibrillation and ST-segment elevation in leads I, II, aVL and V4–6. In addition, echocardiography showed a reduced wall motion at posterolateral region. On laboratory data, his creatinine was 1.02 mg/dL, hemoglobin A1c was 5.9%, low density lipoprotein cholesterol was 112 mg/dL, and no evidence of elevation of cardiac enzyme at the presentation. He was diagnosed as ST-segment elevation myocardial infarction, and therefore emergent coronary angiography was conducted. Coronary angiogram revealed one severe stenosis at the middle segment of his right coronary artery (RCA) (Figure 1A), moderate stenosis at middle segment of left anterior descending artery and middle segment of left circumflex artery (Videos S1,S2). Primary PCI was performed under the guidance of IVUS imaging (AltaViewTM, Terumo, Tokyo, Japan). IVUS imaging prior to PCI revealed a convex shape of the luminal surface, convex shape of the luminal side of calcium, an irregular luminal surface, and an irregular leading edge of calcium at his culprit lesion, suggesting the presence of type 1 eccentric CN (Figure 1B, Video 1). The device delivery was difficult due to the coronary artery tortuosity and moderate stenosis at the proximal segment of his RCA, we implanted one 4.0 mm × 15 mm DES (Resolute OnyxTM, Medtronic, Dublin, Ireland) at the proximal segment of his RCA. And then, we conducted the intervention to the culprit lesion. Following balloon angioplasty with 3.0 mm × 15 mm non-compliant balloon catheter at 14 atm (Sapphire NC24TM, Orbusneich Medical, Hong Kong, China), one 4.0 mm × 15 mm DES (Resolute OnyxTM, Medtronic) was implanted at 12 atm (Figure 1C). However, stent underexpansion was observed by coronary angiography after stent implantation. The post-dilatation of the implanted stent was undergone by using the stent balloon catheter at 16 atm. After this procedure, he complained chest pain and then his hemodynamic status was suddenly deteriorated. Coronary angiography revealed the occurrence of coronary artery perforation at the segment receiving DES implantation (Figure 1D). A 4.0 mm × 15 mm non-compliant balloon (Sapphire NC24TM, Orbusneich Medical) was used to seal coronary artery perforation site (10 atm for 120 seconds). However, coronary perforation was still observed. After the insertion of intra-aortic balloon pumping catheter, we decided to implant one 3.5 mm × 15 mm covered stent (PK PapyrusTM, Biotronik, Berline, Germany) (Figure 1E,1F). Then, post-dilatation of the implanted covered stent was undergone by using the 4.0 mm × 8 mm non-compliant balloon (Sapphire NCproTM, Orbusneich Medical) at 14 atm. These procedures resulted in successfully sealing coronary artery perforation site. The IVUS imaging after post dilatation of covered stent, demonstrated optimal stent apposition and stent expansion (Figure 1G, Video 2). The stent was dilated almost to a regular circle. Because of successful PCI, his symptom was recovered and ST segment elevation at electrocardiogram was resolved. He started taking 100 mg of aspirin and 3.75 mg prasugrel and 30 mg edoxaban once daily. Aspirin was stopped when he discharged, the others had been kept 1 year after the PCI.
Figure 1.
Coronary angiography and IVUS images before and after PCI. (A) Severe stenosis at the middle segment of his RCA [a-d correspond to IVUS images in (B)]. (B) IVUS images of CN (asterisks). (C) Implantation of drug-eluting stent. (D) Coronary artery perforation (red arrow) at segment receiving drug-eluting stent (white dotted line). (E) Covered stent implantation. (F) Final coronary angiography (white line = implanted covered stent; white dotted line = implanted drug eluting stent) [a’-d’ correspond to IVUS images in (G)]. (G) IVUS images at segment receiving covered stent. CNs (asterisks) did not erupt into the stent. IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention; RCA, right coronary artery; CN, calcified nodule.
Video S1.
Coronary angiography of his LCA (cranial view). Coronary angiogram revealed one moderate stenosis at the middle segment of his left anterior descending artery. LCA, left coronary artery.
Video S2.
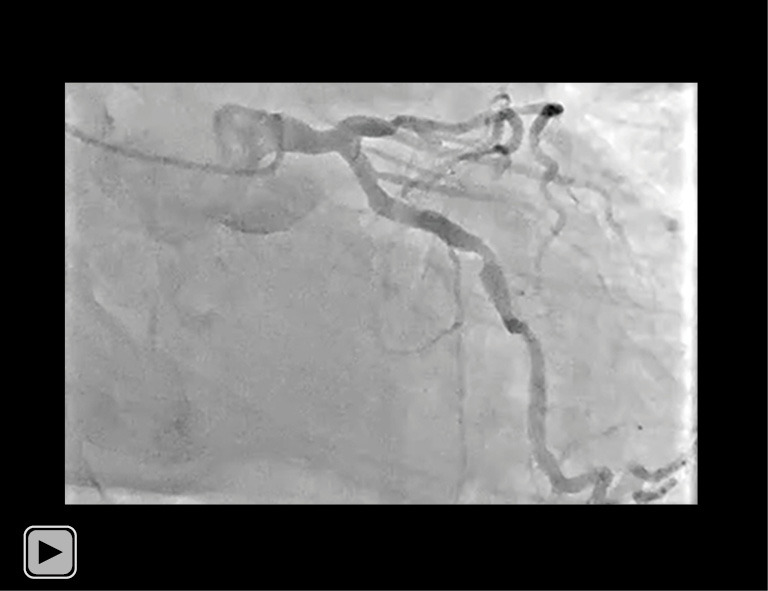
Coronary angiography of his LCA (caudal view). Coronary angiogram revealed one moderate stenosis at the middle segment of his left circumflex artery. LCA, left coronary artery.
Video 1.
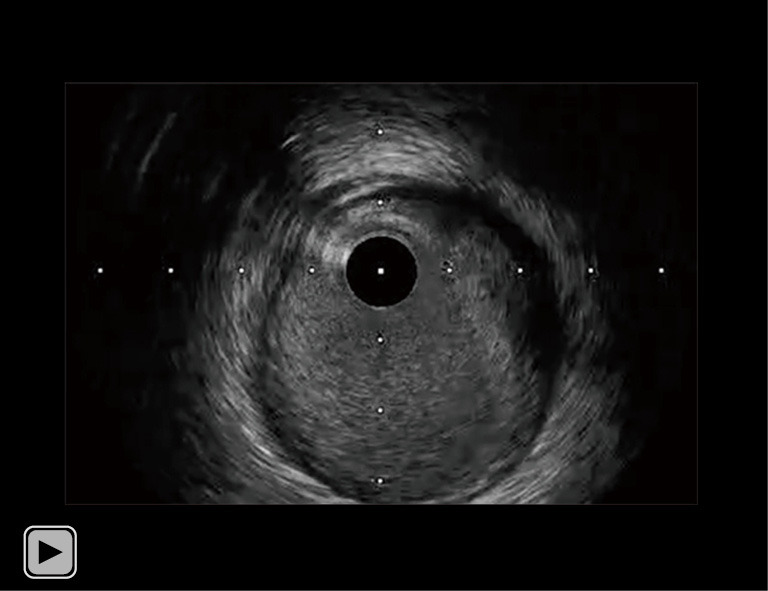
IVUS imaging of his RCA prior to PCI. IVUS imaging revealed a convex shape of the luminal surface, convex shape of the luminal side of calcium, an irregular luminal surface, and an irregular leading edge of calcium at his culprit lesion, suggesting the presence of type 1 eccentric CN. IVUS, intravascular ultrasound; RCA, right coronary artery; PCI, percutaneous coronary intervention; CN, calcified nodule.
Video 2.
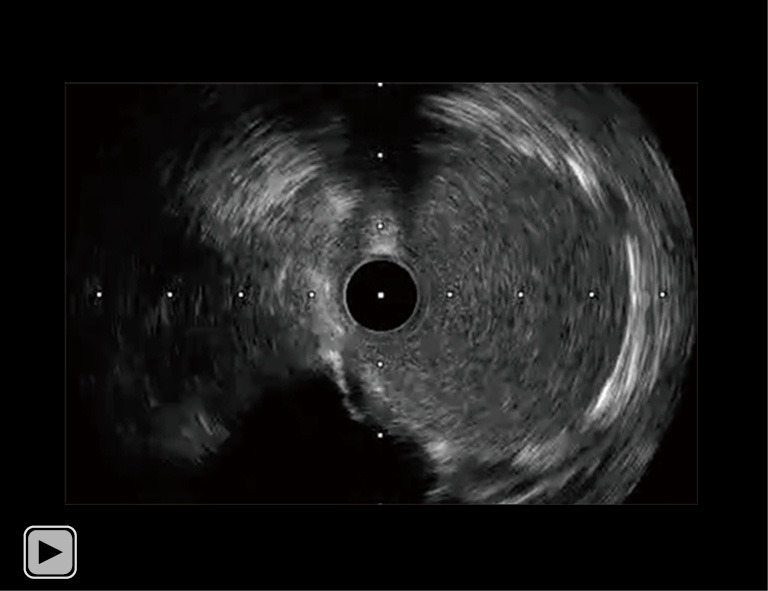
IVUS imaging of his RCA after PCI. The IVUS imaging after post dilatation of covered stent, demonstrated optimal stent apposition and stent expansion. IVUS, intravascular ultrasound; RCA, right coronary artery; PCI, percutaneous coronary intervention.
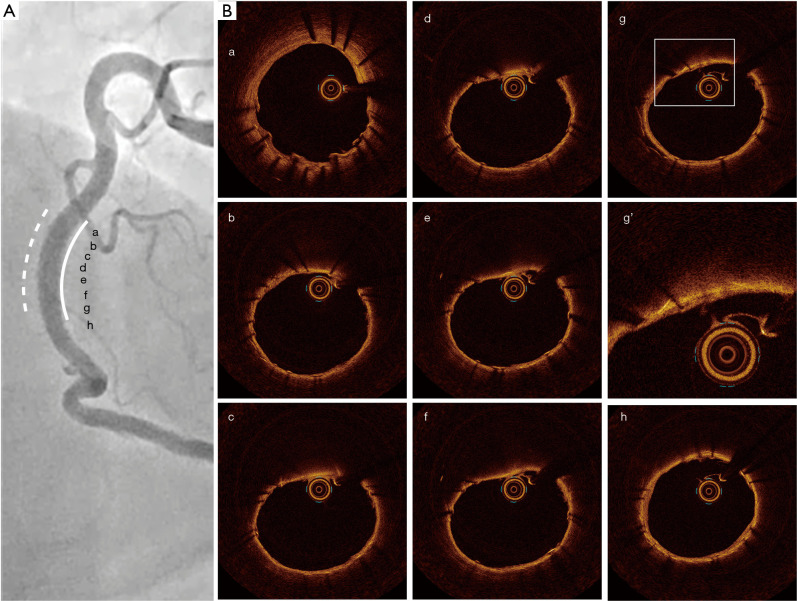
Seven months after the PCI, follow-up coronary angiography and OCT (Dragonfly OpstarTM, Abott Vascular, Chicago, IL, USA) imaging were conducted to evaluate his RCA. Any ISR was not observed in his RCA (Figure 2A). Furthermore, OCT imaging elucidated a small amount of neointimal proliferation without any protruding feature of CN through the segment receiving a covered stent (Figure 2B, Video 3). One year after the PCI, he discontinued taking 3.75 mg of prasugrel. Of note, he did not experience any clinically-driven TLR or no adverse events for 2 years after PCI. The timeline of the imaging and treatment was summarized in Figure 3.
Figure 2.
Follow-up coronary angiography and OCT imaging at 7 months after PCI. (A) In-stent restenosis did not occur at segment receiving covered stent (white line) [a-h correspond to OCT images in (B)]. Dotted white line indicates the implanted drug-eluting stent. (B) Protruding of CN was not observed at segment receiving covered stent by OCT imaging (g’ is an enlargement of the frame of g). OCT, optical coherence tomography; PCI, percutaneous coronary intervention; CN, calcified nodule.
Video 3.
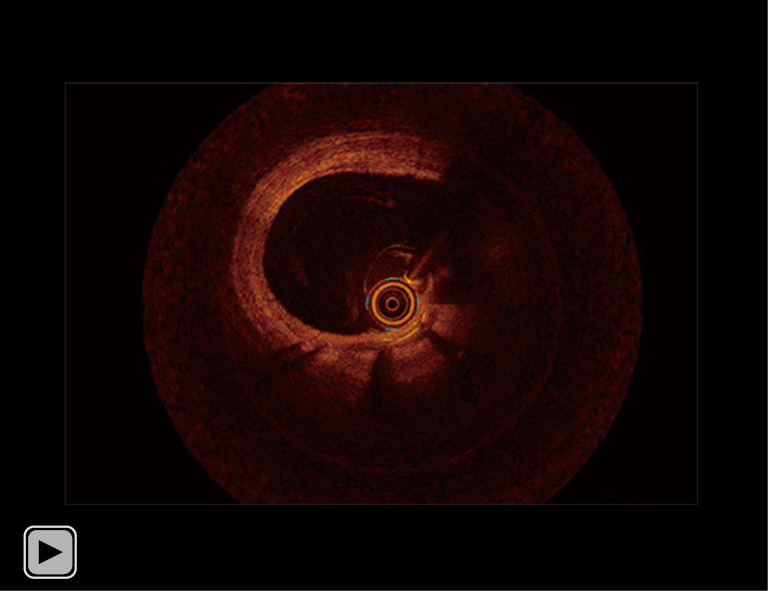
OCT imaging 7 months after PCI. OCT imaging 7 months after PCI elucidated a small amount of neointimal proliferation without any protruding feature of CN through the segment receiving a covered stent. OCT, optical coherence tomography; PCI, percutaneous coronary intervention; CN, calcified nodule.
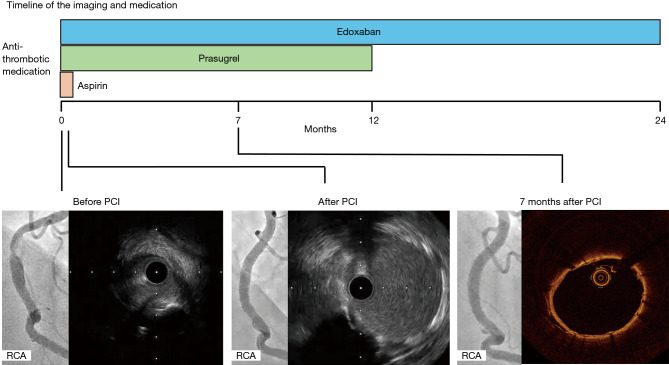
Figure 3.
The timeline of the imaging and treatment. The x-axis displays the clinical course, with coronary angiogram, intravascular imaging, and medications. One covered stent was implanted to the coronary perforation in lesion with calcified nodule of his RCA. He started taking 100 mg of aspirin and 3.75 mg prasugrel and 30 mg edoxaban once daily. Aspirin was stopped when he discharged, the others had been kept one year after the PCI. In-stent restenosis did not occur 7 months after the PCI. One year after the PCI, he discontinued taking 3.75 mg of prasugrel. He did not experience any clinically-driven target lesion revascularization or no adverse events for 2 years after PCI. PCI, percutaneous coronary intervention; RCA, right coronary artery.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
In our case presenting ACS attributable to CN, one covered stent successfully sealed coronary artery perforation caused by DES implantation. OCT imaging 7 months after PCI did not demonstrate any protrusion of CN within the implanted covered stent. Furthermore, this case did not experience any clinically-driven TLR for 2 years after PCI even in the presence of the eccentric CN which associated with worse clinical outcome (10). The current case may suggest the potential of covered stent to prevent the continuing protrusion of CN through stent struts.
Strengths and limitations
The strength of this case report is to serially evaluate lesions with CN by using intravascular imaging modality. OCT imaging visualized that the protrusion of CN did not occur 7 months after PCI. These observations suggest the use of covered stent as another therapeutic approach to treat CN. Since this is a case report, future dedicated study is required to investigate whether the use of covered stent reduces TLR rate at CN lesions. We did not conduct OCT imaging at PCI. Therefore, we did not detect whether the fibrous cap was disrupted or not. However, balloon and DES was well dilatated. By the favourable balloon and DES response, this CN might be the eruptive CN. In most case of CN, a greater amount of calcification is often recognized at the adjacent lesion. However, this case had less calcification in the adjacent lesion. It might affect the favourable clinical course. We do not conduct additional follow-up evaluation of CN lesion receiving covered stent by intravascular imaging. Therefore, it remains unknown whether the protrusion of CN occurred later or not. This is only one case report. Further clinical follow-up is required to monitor whether the use of covered stent continues to prevent CN-related ISR.
Comparison with similar researches
As shown in Table 1, published studies investigated the efficacy of newer-generation DES and rotational atherectomy on TLR rate at CN lesions, respectively (1-6). To date, these devices do not necessarily reduce TLR rate at coronary lesions exhibiting CN. Moreover, there are no studies and case reports to evaluate the efficacy of covered stent for this high-risk calcified lesion.
Explanations of findings
Pathophysiologically, protruding of CN through implanted stent struts has been considered as a potential mechanism causing ISR (7). PK PapyrusTM is covered by polyurethane membrane, which seals coronary perforation site (9). Given that follow-up angiography and OCT did not show any protrusion of CN within the implanted covered stent, polyurethane membrane of this covered stent may be effective to physically hinder the protrusion of calcification tissues into the implanted stent.
Implications and action needed
Covered stent may be effective to physically hinder the re-protrusion of calcification tissues into the lumen. Further clinical follow-up is required to monitor whether the use of covered stent continues to prevent CN-related ISR.
Conclusions
In the current case, coronary perforation occurred after the implantation of DES at culprit lesion exhibiting CN. One covered stent was successfully implanted, which enabled to seal coronary artery perforation. Seven months later, coronary angiography and OCT imaging did not show any ISR, accompanied by the absence of protruding calcification tissues within the implanted covered stent. Furthermore, he did not experience any clinically-driven TLR for 2 years after PCI. Polyurethane membrane of the covered stent may be effective to physically hinder the re-protrusion of calcification tissues into the lumen. Further clinical follow-up is required to monitor whether the use of covered stent continues to prevent CN-related ISR.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-216/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-216/coif). Y.K. serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2023 to August 2025. Y.K. has received research support from Kowa, Nipro and Abbott, and honoraria from Nipro, Abbott, Kowa, Amgen, Sanofi, Astellas, Takeda and Daiichi-Sankyo. The other authors have no conflicts of interest to declare.
References
- 1.Nakamura N, Torii S, Tsuchiya H, et al. Formation of Calcified Nodule as a Cause of Early In-Stent Restenosis in Patients Undergoing Dialysis. J Am Heart Assoc 2020;9:e016595. 10.1161/JAHA.120.016595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe Y, Sakakura K, Taniguchi Y, et al. Comparison of clinical outcomes of intravascular ultrasound-calcified nodule between percutaneous coronary intervention with versus without rotational atherectomy in a propensity-score matched analysis. PLoS One 2020;15:e0241836. 10.1371/journal.pone.0241836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugane H, Kataoka Y, Otsuka F, et al. Cardiac outcomes in patients with acute coronary syndrome attributable to calcified nodule. Atherosclerosis 2021;318:70-5. 10.1016/j.atherosclerosis.2020.11.005 [DOI] [PubMed] [Google Scholar]
- 4.Tada T, Miura K, Ikuta A, et al. Prevalence, predictors, and outcomes of in-stent restenosis with calcified nodules. EuroIntervention 2022;17:1352-61. 10.4244/EIJ-D-21-00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi Y, Otake H, Kuramitsu S, et al. Prevalence and outcomes of stent thrombosis with in-stent calcified nodules: substudy from the REAL-ST registry. EuroIntervention 2022;18:749-58. 10.4244/EIJ-D-21-00976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamana T, Kawamori H, Toba T, et al. Predictors of target lesion revascularisation after drug-eluting stent implantation for calcified nodules: an optical coherence tomography study. EuroIntervention 2023;19:e123-33. 10.4244/EIJ-D-22-00836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori H, Finn AV, Atkinson JB, et al. Calcified Nodule: An Early and Late Cause of In-Stent Failure. JACC Cardiovasc Interv 2016;9:e125-6. 10.1016/j.jcin.2016.03.036 [DOI] [PubMed] [Google Scholar]
- 8.Shin D, Karimi Galougahi K, Spratt JC, et al. Calcified Nodule in Percutaneous Coronary Intervention: Therapeutic Challenges. JACC Cardiovasc Interv 2024;17:1187-99. 10.1016/j.jcin.2024.03.032 [DOI] [PubMed] [Google Scholar]
- 9.Barbero U, Cerrato E, Secco GG, et al. PK Papyrus coronary stent system: the ultrathin struts polyurethane-covered stent. Future Cardiol 2020;16:405-11. 10.2217/fca-2020-0022 [DOI] [PubMed] [Google Scholar]
- 10.Pengchata P, Pongakasira R, Wongsawangkit N, et al. Characteristics and Pattern of Calcified Nodule and/or Nodular Calcification Detected by Intravascular Ultrasound on the Device-Oriented Composite Endpoint (DoCE) in Patients with Heavily Calcified Lesions Who Underwent Rotational Atherectomy-Assisted Percutaneous Coronary Intervention. J Interv Cardiol 2023;2023:6456695. 10.1155/2023/6456695 [DOI] [PMC free article] [PubMed] [Google Scholar]